Abstract
In this study, the influence of a partial substitution of corn with carob (Ceratonia siliqua L.) pulp powder on broiler performance, intestinal microbiota, carcass traits, and meat quality, was investigated. Two dietary treatments were compared: the control group received a diet containing basically corn, soybean meal, and wheat bran for all the breeding periods while the experimental group received the same starter diet as the control group and grower and finisher diets containing 3% and 7% of carob pulp powder, respectively. Growth performance (weight gain, feed intake, and feed conversion ratio), carcass traits, lactic acid bacteria, and total coliforms were determined. Physicochemical parameters, proximate composition, fatty acid profiles, lipid oxidation index, and sensory characteristics of broiler thigh and breast muscles were determined. Dietary supplementation with carob pulp powder did not show an effect (P > 0.05) on growth performance and carcass traits except for slaughter yield and breast relative weight. An increase in sum lactic acid bacteria count and a decrease in total coliforms were observed in the experimental group. No effect (P > 0.05) of dietary carob was observed on pH, cooking losses, ash, mineral, and protein contents in breast and thigh muscles from broilers. However, the experimental diet decreased (P < 0.05) moisture and fat contents in thigh muscles but not in breast muscles. Both muscles from the experimental group of birds exhibited lower palmitic and oleic acids contents, and higher linoleic and linolenic acids levels than those from the control group. Whereas the polyunsaturated fatty acid contents in broiler breast and thigh meat was increased by dietary carob, the concentration of lipid oxidation products decreased in this group. The dietary intervention had no significant effect on the sensory profile of breast and thigh muscles. Thus, carob pulp powder can be applied to broiler diet at 7% as a nonconventional feed with no negative impact on growth performance and carcass traits and to increase the degree of unsaturation with no negative impact on oxidative stability or sensory traits.
Key words: broiler, carob pulp powder, growth performance, intestinal microbiota, meat quality
INTRODUCTION
The consumption of chicken meat has become very popular worldwide owing to its desirable nutritional characteristics, such as high protein, low fat, and relatively high concentrations of polyunsaturated fatty acids (PUFA) compared to beef or pork (Ahmed et al., 2015). Further to its image of being a healthy animal-sourced protein food, chicken meat consumption is not constrained by religious restrictions, is commonly appreciated by its sensory properties and is suitable for the production of numerous commodities (Estévez, 2015).
In 2020, poultry constituted the second livestock production in Algeria behind sheep meat (35.6% vs. 42.1%). Indeed, over 284,020 tons of poultry meat (91.64% of broiler meat) was produced (FAO, 2022). The short duration of breeding, the solvent market for poultry products, the remuneration for this activity, and the lower cost of production compared to traditional breeding (sheep and cattle) are the main factors guiding the choice of this activity by the breeders (Mahmoudi et al., 2019). Nevertheless, the poultry industry in Algeria is directly affected by global commodity prices and the availability of corn and soybean, as principal components of feed (90% of the imports), on the international market. This is still the main limiting factor for the development of the poultry sector (Alloui and Bennoune, 2013). In this respect, local raw material, such us carob, could be one of the interesting alternatives to these imported components.
In the current global scenario of promotion of circular economy as a strategy to reduce potentially pollutant waste materials, using surplus of carob materials or fruits unsuitable for human consumption to feed livestock seems to be an interesting and economically viable decision. As recently, reported by Estévez (2021), using natural sources of nutrients and bioactive compounds may be an inexpensive, feasible, and effective means to improve meat quality.
Carob tree (Ceratonia siliqua L.), an evergreen tree belonging to the Fabaceae family, is native to the Mediterranean area, but is also grown in some regions of the United States, Latin America, and Australia (Custodio et al., 2011; Ben Ayache et al., 2020). In 2020, Algeria participated by 6.6% in the world production of carob fruit, which is estimated at about 49,693 tons; with Portugal, Morocco, and Greece being the major producers of this fruit (FAO, 2022). The carob is one of the most useful native Mediterranean trees. In producing countries, carob pods have traditionally been used as animal and human food and currently the main use is the seed for gum extraction (Batlle and Tous, 1997).
Carob pulp has a high sugar content of between 30 and 60%; the main sugars are sucrose (65–75% of total sugars), fructose and glucose (15 and 25% of total sugars, respectively). In addition, it contains a considerable fiber content (about 8%), appreciable amounts of protein (3%), and low levels of fat (0.6%) (Avallone et al., 1997; Kotrotsios et al., 2011; El Bouzdoudi et al., 2017). Furthermore, the presence of biomolecules such us phenolic compounds, flavonoids, tannins, and anthocyanins in carob pods may have beneficial effects on human and animal health. Numerous studies reported that the extracts of carob exhibit an antioxidant, antidiarrheal, antibacterial, antifungal, anti-inflammatory, antidiabetic activities, and also hepatoprotective and antiproliferative effects (Custodio et al., 2011; Kotrotsios et al., 2012; Lakkab et al., 2018; Ben Ayache et al., 2020). Carob and carob byproducts have been studied previously as a local alternative feed and to prevent and treat diarrhea and as anthelmintic in geese (Sahle et al., 1992), broiler chicken (Ortiz et al., 2004), fish (Fadel et al., 2017), quail (Calislar and Kaplan, 2017) and sheep (Saratsi et al., 2020) diets. It is however, ignored, the extent to which the incorporation of carob pulp powder to broiler diets could have an impact on carcass traits, intestinal microbiota, meat quality, and sensory features.
The aim of the present study was to investigate the effects of carob pulp powder supplement in broiler chicken diet on the growth performance, carcass traits and intestinal microbiota. It also aimed to study their impact on the physicochemical, nutritional, oxidative stability and sensory characteristics of chicken breast and thigh meats.
MATERIALS AND METHODS
Experimental Settings
A total of 160 male and female Cobb 500 one-day old broilers (♂:♀1:1) were randomly assigned to one of these 2 dietary treatments (control and experimental diet) with 4 replicates each (20 animals per treatment in independent pens) and fed for 45 d. The control diet was based on corn, soybean meal, and wheat bran and the experimental diet was elaborated by a partial substitution of corn with carob powder in the growing and finishing diets (3 and 7%, respectively; Table 1). The diets and water were provided ad libitum. The experimental protocol was approved by the University of Blida 1 (Algeria) animal care and ethics committee and the broilers were handled by competent and educated staff.
Table 1.
Feed ingredients and nutrient analysis of the used diets during the starter, grower, and finisher periods.
| Ingredients % | Starter (1–21 d) |
Grower control (22–32 d) |
Grower experimental (22–32 d) |
Finisher control (33–45 d) |
Finisher experimental (33–45 d) |
|---|---|---|---|---|---|
| Corn | 62.1 | 66.3 | 63.3 | 69.4 | 64 |
| Soybean meal | 28.7 | 24 | 24 | 19.5 | 19.5 |
| Wheat bran | 6 | 6.5 | 6.5 | 8 | 6.5 |
| Carob powder | 0 | 0 | 3 | 0 | 7 |
| Limestone | 0 | 0.7 | 0.7 | 0.6 | 0.5 |
| Dicalcium phosphate | 2.2 | 1.5 | 1.5 | 1.5 | 1.5 |
| Vitamin and mineral premix1 | 1 | 1 | 1 | 1 | 1 |
| Nutrient analysis | |||||
| Metabolizable energy (kcal/kg)2 | 2,936 | 2,977 | 2,930 | 3,002 | 2,923 |
| Crude proteins (%) | 21.9 | 19.7 | 19.7 | 17.5 | 16 |
| Ether extracts (%) | 4.78 | 3.13 | 2.61 | 9.06 | 7.12 |
| Fibers (%) | 2.85 | 2.77 | 2.72 | 3.55 | 3.17 |
| Calcium (%) | 1.04 | 0.95 | 1.04 | 0.92 | 1.10 |
| Phosphorus (%) | 0.38 | 0.37 | 0.40 | 0.33 | 0.35 |
Vitamine and mineral premix provided per kilogram of diet: Starter: vitamin A: 350,000 UI; vitamin D3: 75,000 UI; vitamin E: 2,000 UI; vitamin B1: 70 mg; vitamin B2: 190 mg; vitamin B5: 300 mg; Niacin: 1 300; Ca: 190 g; Na: 42 g; P: 80 g; Methionine: 45 g; lysine: 20 g. Grower and Finisher: vitamin A: 350,000 UI; vitamin D3: 75,000 UI; vitamin E: 2,000 UI; vitamin B1: 70 mg; vitamin B2: 190 mg; vitamin B5: 300 mg; Niacin: 1,300; Ca: 250 g; Na: 42 g; P: 65 g; Methionine: 41 g; lysine: 16 g.
Metabolizable energy was calculated using the INRAE-CIRAD-AFZ feed tables (2004).
Proximate Composition of Feed
The nutritional composition (Tables 1 and 2) of the diets and carob pulp powder was determined as follow: crude proteins (AOAC 934.13), ether extracts (AOAC 2003.05), total fiber (AFNOR NF V03-40 (1993)), total carbohydrates (Dubois et al., 1956), calcium (Welcher, 1958), and phosphorus (Youshida et al., 1976). Phenolic compounds in mg GAE/g DM (Gallic Acid Equivalent/ Dry Matter), tannins in mg CE/g DM (Catechol Equivalent/Dry Matter), and the antioxidant capacity in IC50 (amount of extract required to scavenge 50% of radicals present in the reaction mixture) of carob pulp powder were also determined (Mahmoudi et al., 2018).
Table 2.
Nutrient analysis, phenolics, tannins, and antioxidant capacity of carob pulp powder.
| Nutrients | Dry matter | Crude ashes | Crude proteins (%) | Total sugar (%) | Ether extracts (%) | Fibers (%) | Ca (%) | P (%) | Phenolics1 | Tannins2 | IC50 (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amounts | 87.4 ± 0.12 | 2.92 ± 0.19 | 6.57 ± 1.45 | 40.7 ± 1.06 | 1.82 ± 0.1 | 3.78 ± 0.13 | 0.26 ± 0.01 | 0.25 ± 0.01 | 29.4 ± 1.21 | 1.44 ± 0.05 | 6.82 ± 0.16 |
Each value represents the mean of three measurements ±SEM. The IC50 values were obtained by linear regression analysis.
Result was expressed as mg equivalent gallic acid/g of dry matter.
Result was expressed as mg equivalent catechol/g of dry matter.
Growth Performance
The broilers were weighed individually on the day they were received and at the end of each phase of the breeding process: 1, 21, 32, and 45 d. Feed intake, weight gain, and feed conversion ratio of each lot were determined.
Carcass Traits
At the d 45 of the experiment, four broilers per treatment were weighted then slaughtered. Relative weight was determined for eviscerated carcass, breast, legs, liver, heart, intestine, gizzard, and abdominal fat. Slaughter yield was estimated as eviscerated carcass weight × 100/broiler live weight.
Intestinal Microbiota
At the end of the experiment, four broilers from each treatment were chosen for intestinal collection. Intestinal samples were placed in sterile glace containers. About 25 g of sample were introduced in stomacher plastic bags, which contain 225 mL of TSW (Tryptone Salt water) and homogenized for 8 min. Serial dilutions (10−2 to 10−7) were prepared by removing one mL from the prepared suspension and adding it into 9 mL buffer TSW in another tube. One hundred microliter was removed from 10−5, 10−6, and 10−7 dilutions and poured into empty petri dishes and then 15 mL of MRS and M17 mediums were poured for lactic acid bacteria count. Lactic acid bacteria were incubated for 48 h at 37°C in in anaerobic conditions. Total coliforms were counted using VRBL medium and incubated for 24 h at 37°C. Bacterial counts were reported as CFU (Colony-Forming Unit) of bacteria per 1 g sample.
Meat Sampling
At the end of the breeding cycle (45 d), 3 chickens were randomly selected from each replicate (12 broilers per experimental group and 24 birds in total) and slaughtered. After feathering, evisceration and cleaning, chicken breast, and thigh muscles were separated from the carcasses, grinded, packed in plastic boxes, and stored at 4°C for the physicochemical analysis (to be performed in the following 24 h) and at −20°C for the other analyses.
Chemicals and Reagents
All chemicals and reagents used in this study were of analytical grade and purchased from PanReac, GPR Rectapur, Biochem Chemopharma (France), Sigma-Aldrich (Germany), Honeywell Fluka (France), and VWR Prolabo.
Physicochemical and Nutritional Analyses
The following parameters were determined in the breast and thigh muscles: pH (Journal, 2006) and cooking losses (Lyon et al., 2004). For the proximate composition, moisture was assessed by dehydration of sample in oven at 102°C (AOAC 950.46 B), ashes by calcination furnace at 550°C (AOAC 920.153), proteins by Kjeldahl method (AOAC 981.10) and lipids were extracted according to the method of Folch et al. (1957). The minerals: sodium and potassium were analyzed by a JENWAY PFP7 flame spectrophotometer (Mahmoudi et al., 2018). Calcium and magnesium were determined by titration (Welcher, 1958) and phosphorus concentration was estimated by the phospho-vanado-molybdic method described by Youshida et al. (1976).
Fatty Acid Profile
Fatty acid profiles were determined as described by Benamirouche et al. (2020). The fatty acid methyl esters were injected in a gas chromatograph (Hewlett Packard Agilent 6890-USA) coupled with a mass spectrometer (Hewlett Packard Agilent 5973-USA). The gas chromatograph was equipped with a flame-ionization detector and a capillary column (30 m × 0.25 mm, 0.25-μm film thickness) with helium as the carrier gas running at a constant flow of 1 mL min-1. The injector was set at 250°C and operates in split mode 1:50. The detector temperature was set at 270°C. The temperature profile was programmed as follows: the initial oven temperature was set at 130°C, increased at a rate of 2°C min−1 to 180°C, then increased at a rate of 4°C min−1 to 225°C; the final temperature was held for 7 min. The identification of fatty acids was achieved by comparing mass spectral data with those of the NIST 2.0 library.
Determination of Lipid Oxidation Index
The lipid oxidation was determined using the method described by Klein (1970) and Babincová et al. (1999). Absorption spectra of conjugated dienes were recorded in the wavelength range 215 to 320 nm using a SHIMADZU UV-1800 (Japan) 240 V spectrophotometer. The increase of the absorption at 233 nm was considered as evidence of the formation of conjugated dienes, and the oxidation index was calculated from the ratio of the absorbances (A233/A215).
Sensory Analysis
The protocol of Kim et al. (2009) was adopted for the sensory analysis with slight modifications. The breast and thigh meat samples were cut in about 2 cm3 pieces and boiled in water at 85°C for 25 min. The samples were cooled at room temperature and each piece was placed in a plastic cup, codded with tree-digit numbers and presented to the panelists. Twenty trained panelists were selected based on their previous experience in sensory testing, frequency of meat consumption, and willingness to participate in the test. The panelists were asked to evaluate the samples for tenderness, juiciness, flavor, after taste, and fat using a 5-point hedonic scale (1 = dislike extremely to 5 = like extremely).
Statistical Analysis
The data collected from the animals (n = 8 for carcass traits and intestinal microbiota; n = 24 for meat quality traits) and analytical procedures (all done in triplicate) were subjected to statistical analysis. Results were shown as mean ± SEM. Statistical significance at P < 0.05 was determined by t test and ANOVA (one-way) followed by Tukey's multiple comparison tests using GraphPad Prism 7 statistics program.
RESULTS AND DISCUSSION
Proximate Composition of Carob Pulp Powder
The nutritional value, phenolic, and tannin composition and the antioxidant capacity of carob pulp powder are represented in Table 2. According to Batlle and Tous (1997), the 2 main carob pod constituents are (by weight): pulp (90%) and seed (10%). Chemical composition of the pulp depends on cultivar, origin, climate, and harvesting time.
The crude protein content in our study was higher than that reported by Calislar and Kaplan (2017) (4%) and by Kyriacou et al. (2021) for Italian carobs (4.21 ± 0.22%). However, a similar protein level was reported by Vekiari et al. (2012) for Greek select genotypes (6.4 ± 0.18%) and by Youssef et al. (2013) for Egyptian genotype (6.34%). Carob pulp powder contains a high average content of sugars. Total sugar content of carob in our study is within the interval (30.03–81.93%) cited by Dikkaya et al. (2020) for 90 selected Turkish carob genotypes. These authors reported that sucrose, glucose, and fructose are the major sugars in carob pods. Our data revealed that carob powder is as a good source of Ca and P, which is in agreement with Youssef et al. (2013).
Phenolic compounds are non-nutrient but biologically active secondary plant metabolites, which can act as antioxidants (Vekiari et al., 2012). In our study, carob pulp powder contained significantly higher total phenolic content (29.37 ± 1.21 mg GAE/g DM) compared to the range (17–19 mg GAE/g DM) reported by Custodio et al. (2011) and Kyriacou et al. (2021). The main constituents of carob polyphenols were found to be condensed tannins (Vekiari et al., 2012). Tannin level in our sample (1.44 ± 0.05 mg CE/g DM) was less than the amount found previously by Ben Ayache et al. (2020) (0.3 ± 0.0 g CE/100 g DM). According to Ben Ayache et al. (2020), the antiradical activity of carob increases in a dose-dependent way, which is in good agreement with our study. However, these researchers found a much lower IC50 (1.04 ± 0.0 mg/mL) than ours (6.82 ± 0.16 mg/mL). Lower values of IC50 indicate more effectiveness. This variation could be related to variety, the solvent system and the method used to extract antioxidant compounds.
Growth Performance
Data of the effect of feeding Cobb 500 broilers with 0%, 3%, and 7% of carob pulp powder on growth performance during starter (1st–21st days), grower (1st–32th days), and finisher (1st–45th days) are presented in Table 3.
Table 3.
The impact of carob pulp powder on broiler growth performance.
| Treatments | Feed intake (g/chick/ period) | Weight gain (g/chick/ period) | Weight gain (g/chick/ day) | Feed conversion ratio | |
|---|---|---|---|---|---|
| Starter (1–21 days) | Control | 1,030 ± 43.0a | 649 ± 15.5 | 30.9 ± 0.7 | 1.59 ± 0.03 |
| Experimental | 1,153 ± 38.1b | 659 ± 9.1 | 31.4 ± 0.4 | 1.75 ± 0.08 | |
| Grower (1–32 days) | Control | 2,434 ± 52.6 | 1,433 ± 85.7 | 44.8 ± 2.7 | 1.70 ± 0.12 |
| Experimental | 2,535 ± 91.1 | 1,435 ± 36.3 | 44.8 ± 1.0 | 1.77 ± 0.04 | |
| Finisher (1–45 days) | Control | 4,437 ± 90.8 | 2,175 ± 93.4 | 48.3 ± 2.1 | 2.05 ± 0.13 |
| Experimental | 4,349 ± 49.5 | 2,151 ± 106 | 47.8 ± 2.4 | 2.04 ± 0.12 | |
Data are represented as means ± standard error.
Mean values in the same column within each dietary period and treatment are significantly different at P > 0.05 by the mean of t test.
In the starter period, feed intake is in favor of the experimental group (P < 0.05) compared to the control one, a difference of 123 g/chick/period. However, in the growth period, feed intake decreased by 11.5 g/chick/period in the experimental group fed with a diet of 3% carob pulp powder compared to the control; this difference is more accentuated (162.6 g/chick/period) with the increase of carob rate incorporation in the finisher diet (7%). Indeed, this difference remains nonsignificant for the 2 periods (P > 0.05).
These feed intakes, influence chick weight gain. The daily weight gains are slightly to the advantage of the experimental group (31.4 ± 0.4 g) vs. the control group (30.9 ± 0.7 g) in the starter period; while in the grower and finisher periods, they are in favor (P > 0.05) of the control group with a difference of 3.3 and 0.7 g/chick/day in the 2 periods. It seems that the difference of weight gain in the finisher is less than in the grower period despite the increase in the carob powder rate, which could be explained by the adaptation of the broilers to carob powder.
Overall, the weight gain and the feed conversion ratio, at slaughter age (45 d), of the 2 groups are close with a slight superiority for the control (P > 0.05); they are evaluated at 48.3 ± 2.1 g against 47.8 ± 2.4 g/chick/day and 2.05 ± 0.13 against 2.04 ± 0.12, respectively for the control and the experimental group.
Álvarez-Fuentes et al. (2012) had recorded a significant decrease in feed intake and weight gain and a height increase in conversion ratio in chicks fed with a diet containing 30% of carob seeds compared to the control. In this respect, Ortiz et al. (2004) observed an important reduction (12.7 and 29.5%) of the apparent digestibility coefficients in chicks fed diets containing 6 and 9% of carob seeds in the starter period compared to the control. These authors explained that carob increased digesta viscosity, which lower digestibility and nutrient absorption. Moreover, Lizardo et al. (2002) reported that carob tannins could reduce feed palatability and consumption in chicks. These findings disagree with our work probably because we included carob powder progressively in the grower and finisher diets and not in the starter one. In addition, the amount of tannins registered in our study (1.44 mg/g) is less than the amount reported by Ortiz et al. (2004) (8 mg/g) and according to Iji et al. (2004) and Schiavone et al. (2008) studies, the negative impact of tannins on chick performance is related to the concentration of the later in the diet. In their study on Cobb 500 broilers of 14 d, Schiavone et al. (2008) observed a significant increase in daily feed intakes and daily weight gain in broilers fed 0.15 and 0.20% of tannins. On the contrary, Iji et al. (2004) recorded a decline in feed intake and body weight gain of broilers fed diets containing an increased levels of tannins (0, 0.5, 1.5, 2, and 2.5% diet) in the starter period. Vohra and Kbatzee (1964) concluded that part of the inhibition in the growth of chickens when ground carobs are used in their diets is due to a deficiency of metabolizable energy of this product. Along with fats, carobs can be successfully used at a 20% level in chicken rations in those areas where there is a shortage of cereals. Our results are in agreement with the reports of Yıldırım and Kaya (2011), Sahle et al. (1992), Calislar and Kaplan (2017), in which ground carob supplement in broiler (5–20%), geese (up to 200 g/kg), and quail (3–15%) diets did not affect significantly growth performance. According to Calislar and Kaplan (2017) despite the presence of tannins in carob powder, which can reduce feed intake and body weight, the sweet taste of the carob powder supplement may result in higher feed intake.
Carcass Traits
After 45 d of fattening period a total number of 8 broilers (4 broilers per diet), with an average live weight (2,308 ± 60 g for the experimental group and 2,350 ± 35 g for the control group) were slaughtered after 12 h feed withdrawal. The effect of feeding broilers with a diet containing carob pulp powder on the carcass characteristics is represented in Table 4. No effect of the diet types was observed (P > 0.05) on eviscerated carcass, legs, liver, heart, intestine, and gizzard relative weights. However, higher (P < 0.05) slaughter yield and breast relative weights were found in broilers fed carob pulp powder diet.
Table 4.
Carcass characteristics of broilers fed control and experimental diets.
| Treatments | Traits (relative weight %) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Slaughter yield | Eviscerated carcass | Breast | Legs | Liver | heart | Intestine | Gizzard | Abdominal fat | |
| Control | 57.1 ± 5.6a | 82.5 ± 2.0 | 26.8 ± 0.7A | 24.1 ± 1.0 | 2.2 ± 0.4 | 0.6 ± 0.1 | 5.9 ± 0.8 | 2.0 ± 0.3 | 2.6 ± 0.7 |
| Experimental | 64.3 ± 4.3b | 85 ± 3.8 | 34.2 ± 0.7B | 24.2 ± 1.3 | 2.1 ± 0.2 | 0.5 ± 0.06 | 6.2 ± 0.6 | 2.0 ± 0.3 | 1.1 ± 0.7 |
Data are represented as means ± standard error of mean.
Mean values in the same column with different letters are significantly different at P < 0.05 by the mean of t test.
Our data of the relative weight of eviscerated carcass, breast, and legs are higher compared to the Cobb 500 standards for 2381g (73.56, 24.49, and 23.07%, respectively) (Cobb, 2019). In a study carried out on the impact of the incorporation of carob pods (20%) in rabbit diet, a similar carcass yields were found in the experimental and control groups (Hadj Ayed et al., 2019). According to these authors carob pods can be incorporated in growing rabbit's diets at 20% without any negative incidence on carcass traits.
In the present work, a decrease in abdominal fat relative weight was observed in the group of broilers fed a diet supplemented with carob pulp powder compared to the control group (1.1 vs. 2.6% respectively). A similar effect was observed by Sour et al. (2019) who found that consumption of a diet enriched with carob pulp for 2 months, lead to a decrease in adipose tissue weight in rat model. These authors explained that this effect is related to carob fiber and phenolic compounds. Different anti-obesity mechanisms for carob polyphenol were proposed, such as the suppression of fat absorption from the gut, inhibition of differentiation of pre-adipocytes to adipocytes, and stimulation of apoptosis of mature adipocytes (Meydani and Hasan, 2010).
Intestinal Microbiota
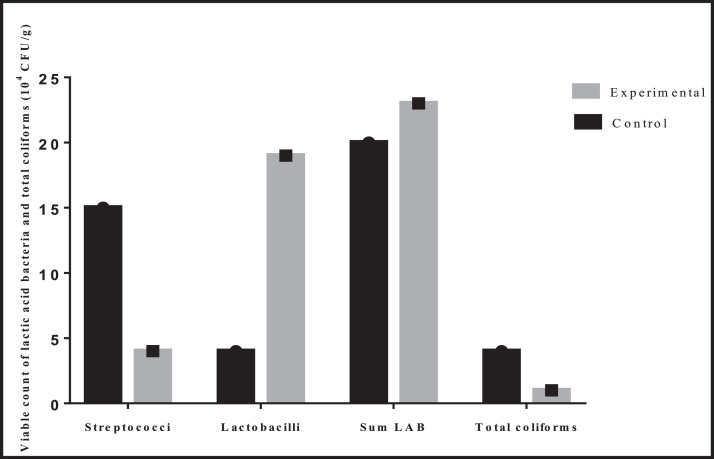
There were an increase in Lactobacilli and sum lactic acid bacteria counts in the group of broilers fed with a diet containing carob pulp powder. However, a higher Streptococci and total coliform counts were registered in the control group compared to the experimental one (Figure 1).
Figure 1.
Intestinal microflora count of Cobb 500 broilers fed with a diet containing carob pulp powder.
Chicken gut microbiota are complex microbial communities with bacteria the most abundant among all these (Clostridium, Lactobacillus, Bacteroides, Escherichia coli, Streptococcus) (Gabriel et al., 2005). The maintenance of this population in the gut is responsible for the digestion and absorption of nutrients, the development of immunity and disease resistance. A deviation in the normal gut microbiota can lead to translocation of infectious bacterial species, and inflammatory response. This can affect feed conversion ratio, productivity, performance, and overall health of poultry birds (Iqbal et al., 2020).
According to Gabriel et al. (2005) the digestive flora can be modified by feed type, in particular the presence of soluble non-starch polysaccharides. In their study, Benguiar et al. (2015) found that carob extract enhances the growth of Lactobacillus, which is attributed to carbohydrates (glucose, fructose, and sucrose) and dietetic fibers. These findings are in agreement with our results. Rodríguez-Solana et al. (2021) explained that some of the carob's galacto-oligosaccharides are readily fermented by intestinal microbes in the small intestine, and others escape fermentation in the small intestine and enter the large intestine, where they may exert a prebiotic effect, increasing the bifidobacterial population, stimulating the immune system, and reducing diarrhea or constipation.
Similarly, Abu Hafsa et al. (2017) reported that 5 to 10% of carob pods in growing rabbit diet increased significantly Lactobacillus count and decreased Escherichia coli count. Lactobacillus species have been usually correlated with higher performance and better health (Ocejo et al., 2019). Other studies suggested a prebiotic effect of carob due to the increase of lactic acid bacteria count when this product was used (Guler-Akin et al., 2016; Rodríguez-Solana et al., 2021). According to these authors, such a prebiotic effect could be attributed to bioactive compounds such as phenolics. A possible explanation for the stimulatory effect of polyphenolic compounds on bacterial growth is that some microorganisms are able to use these compounds as nutritional substrates (Viveros et al., 2011). Lactobacilli possess the ability to metabolize phenolic compounds supplying energy to cells and positively affecting the bacterial metabolism (García-Ruíz et al., 2008).
The inhibitory effect of carob on coliforms in our study could be attributed partially to the polyphenolic constituents. The bacteriostatic and/or bactericidal action of phenolics could be explained by their ability to adsorb onto cell membranes and to disrupt their permeability barrier, through their interaction with enzymes and effectors or the deprivation of substrates and metal ions (Rodríguez-Solana et al., 2021). These compounds could act to inhibit adhesion of infection-causing bacteria within cells of the intestinal tract and decrease ATP production and inhibit the DNA gyrase that involves the mechanism of DNA and RNA synthesis of bacteria. Moreover, phenolic causes cell homeostasis and resulted in cell death by losing ions, as the denaturation of cellular protein is responsible for bacterial cell death (Viveros et al., 2011; Mahfuz, et al., 2021).
In our study, an important phenolic content was registered in carob powder (29.37 mg GAE/g DM). Many researchers had reported the positive effect of dietary polyphenol-rich products on the intestinal microbiota and broiler performance (Viveros et al., 2011; Ahmed et al., 2015; Iqbal et al., 2020). Ahmed et al. (2015) suggested that plant polyphenols might improve utilization of diet energy by manipulating the gut microbiota to achieve better performance.
Physicochemical and Nutritional Analyses of Meat
Dietary supplementation with carob pulp powder did not show an effect (P > 0.05) on the pH of breast and thigh meat from broilers fed control and experimental diets (Table 5).
Table 5.
Physicochemical parameters of chicken breast and thigh meat samples.
| Parameters | Control |
Experimental |
||
|---|---|---|---|---|
| Breast | Thigh | Breast | Thigh | |
| pH | 5.71 ± 0.11ac | 6.04 ± 0.08ab | 5.90 ± 0.02a | 5.99 ± 0.03a |
| Cooking losses (%) | 19.4 ± 1.74AB | 21.6 ± 1.82B | 14.9 ± 0.80A | 16.4 ± 1.12AB |
Each value represents the mean of three measurements ± SEM.
Mean values in the same line with different letters are significantly different at P < 0.05 by mean of the Tukey's multiple comparison test.
The technological quality of meat has become a major issue for the poultry industry. This largely depends on the ultimate pH. Two major defects exist: “acid” meats with low ultimate pH (< 5.7) which are characterized by a pale color, hard texture after cooking and poor processability, and “DFD” «Dark, Firm, Dry» meats with high ultimate pH (> 6.2) which are darker and tender, dry, and more sensitive to conservation issues (Le Bihan-Duval et al., 2014; Berri, 2015). Our pH data are within the range mentioned by the above-cited authors.
Similarly, no effect of diet type on the cooking losses of both breast and thigh meat samples were observed (Table 5). Cooking losses in this study were lower than those found in some previous studies on Cobb 500 broiler (25.3% and 24.7%) and on commercial chicken hybrid (21.05% and 26.23%) breast muscles (Petracci et al., 2013; Benamirouche et al., 2020). Cooking loss is one of the important determinants of meat quality, because it is related to the amount of water lost and nutrients that dissolve in water due to the influence of the cooking process (Abubakar et al., 2021). According to these authors, meat with low cooking losses has better physical quality when compared to meats that have higher cooking losses, because the loss of nutrients during the cooking process is less.
The nutritional composition of broiler breast and thigh meats is summarized in Table 6. Moisture content was slightly higher in thigh meat than in breast meat (P > 0.05) of broilers feed control and experimental diets. The diet type had no effect on the moisture in breast muscles, but the experimental diet decreased (P < 0.05) the moisture level in the thigh muscle. Our data of moisture content are within the ranges cited by Brunel et al. (2010) (71.5–78.4% for breast muscle and 70.8–77.4% for thigh muscle).
Table 6.
Nutritional value of breast and thigh muscles of broilers fed diet containing carob pulp powder.
| Parameters | Control |
Experimental |
||
|---|---|---|---|---|
| Breast | Thigh | Breast | Thigh | |
| Moisture (%) | 73.6 ± 0.29a | 75.9 ± 0.13b | 73.5 ± 0.33a | 74.2 ± 0.10a |
| Ashes (%) | 1.24 ± 0.09a | 1.41 ± 0.17a | 1.20 ± 0.09a | 1.10 ± 0.05a |
| Proteins (%) | 20.4 ± 0.78a | 22.8 ± 1.17a | 23.3 ± 1.95a | 19.8 ± 0.78a |
| Fates (%) | 1.51 ± 0.33a | 7.42 ± 0.30c | 1.48 ± 0.07a | 4.28 ± 0.01b |
| Na (mg/100 g) | 66.6 ± 6.37ab | 61.8 ± 4.78a | 43.8 ± 3.59ac | 51 ± 3.59a |
| K (mg/100 g) | 241 ± 4.59ab | 224 ± 3.45a | 224 ± 3.45a | 213 ± 6.89ac |
| Ca (mg/100 g) | 13.4 ± 0.83a | 14.5 ± 0.74ab | 12.7 ± 0.70ac | 13.5 ± 0.42a |
| P (mg/100 g) | 219 ± 6.51ac | 131 ± 5.19b | 229 ± 9.84ac | 157 ± 4.96ab |
| Mg (mg/100 g) | 25.3 ± 0.65a | 22.8 ± 0.24ab | 26 ± 0.61ac | 20.6 ± 0.47b |
Each value represents the mean of three measurements ± SEM.
Mean values in the same line with different letters are significantly different at P < 0.05 by mean of the Tukey's multiple comparison test.
Crude protein levels in our samples ranged between 19.84 ± 0.78% and 22.76 ± 1.17% for thigh muscles and between 20.43 ± 0.78% and 23.34 ± 1.95% for breast muscles. These results are comparable to those of Petracci et al. (2013) (23.48% and 22.82%) on commercial chicken hybrids breast. No effect (P > 0.05) of diet or muscle types on ash and protein contents was observed in meat samples.
Otherwise, compared to control diet, experimental diet decreased (P < 0.05) fat contents in thigh muscles but not in breast muscles. In addition, fat contents were higher (P < 0.05) in thigh muscle than in breast muscle in both experimental and control groups. These data are consistent with other published data (Cortinas et al., 2004; Kralik et al., 2018; Benamirouche et al., 2020). The difference in lipid content could result from the low metabolic energy content in the experimental diet compared to the control one (3,002 vs. 2,923 kcal/kg, respectively; Table 1). Cherian et al. (2002) claimed that the energy content of the ration and the feed consumption are the most important factors in altering the lipid content of birds. The extra energy consumed by the birds could have been deposited as lipids. Moreover, Fadel et al. (2017) suggested that tannins could be responsible for the decrease in the whole-body lipid in red hybrid tilapia fed 30 and 40% carob seed germ meal due to an increase in lipid retention. Similarly, a study showed about 10% lower total lipids, triglycerides and about 22% lower cholesterol in broilers offered polyphenol rich grape seed at 10 to 40 g/kg diets (Mahfuz et al., 2021).
With regard to mineral composition, no differences (P > 0.05) were detected in potassium, calcium, phosphorus and magnesium contents in breast and thigh meat of broilers feed both diets; whereas the experimental diet tended to lower (P < 0.05) the sodium content in thigh meats (66.56 ± 6.37 vs. 43.85 ± 3.59 mg/100 g for control and experimental groups, respectively). In their report, Kralik et al. (2018) mentioned that chicken meat is a good source of some minerals. It appears to be higher in Magnesium, Calcium, and Phosphorus values than other raw meats, except fish. In their review, Kralik et al. (2018) claimed that when compared to other types of meat, white chicken meat has better nutritional quality (more protein, less total fat, less saturated fat, and less calories) and therefore, it is recommended for consumption to anyone who takes care of diet and health.
Fatty Acid Profiles
As for fatty acid profiles (Table 7), breast and thigh meat samples of the experimental group exhibited a lower palmitic acid (28.3% vs. 31.6% for breast and 31.3% vs. 34% for thigh) and oleic acid (33.5% vs. 38.9% for breast and 34.8% vs. 40.3% for thigh) contents than these of the control group. In concordance, Calislar and Kaplan (2017) observed that there was a tendency of decrease in palmitic acid (C16:0) and oleic acid (C18:1) concentrations in egg yolk with increasing carob supplementation in quail diet. Otherwise, the experimental diet increased the linoleic acid (11.5% vs. 6.2% for breast and 11.1% vs. 4.3% for thigh) and the linolenic acid (3.9% vs. 3.4% for breast and 4.5% vs. 2.9% for thigh) contents compared to the control diet.
Table 7.
Major fatty acid composition in breast and thigh muscles of broilers fed diet containing carob pulp powder (%).
| Compounds | Control |
Experimental |
||
|---|---|---|---|---|
| Breast | Thigh | Breast | Thigh | |
| Palmitoleic acid (C16:1) | 0.9 | 0.5 | 2.5 | 0.9 |
| Palmitic acid (C16:0) | 31.6 | 34 | 28.3 | 31.3 |
| Linolenic acid (C18:3n3) | 3.4 | 2.9 | 3.9 | 4.5 |
| Linoleic acid (C18:2n6) | 6.2 | 4.3 | 11.5 | 11.1 |
| Oleic acid (C18:1) | 38.9 | 40.3 | 33.5 | 34.8 |
| Stearic acid (C18:0) | 17.2 | 15.7 | 17 | 16.8 |
| Arachidonic acid (C20:4n6) | 1.8 | 2.3 | 3.2 | 0.7 |
| SFA | 48.8 | 49.7 | 45.3 | 48.1 |
| MUFA | 39.8 | 40.8 | 36 | 35.7 |
| PUFA | 11.4 | 9.5 | 18.6 | 16.3 |
| UFA | 51.2 | 50.3 | 54.6 | 52 |
| PUFA/SFA | 0.2 | 0.2 | 0.4 | 0.3 |
Abbreviations: MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, Saturated fatty acids; UFA, Unsaturated fatty acids.
Moreover, it should be noted that both breast and thigh muscles of the experimental group showed lower SFA saturated fatty acid levels (45.3% vs. 48.8% for breast and 48.1% vs. 49.7% for thigh) and tended to have a higher PUFA (54.6% vs. 51.2% for breast and 52% vs. 50.3% for thigh) than the control group. Nevertheless, there was a decrease in monounsaturated fatty acids in breast and thigh meats of broilers fed a diet supplemented with carob pulp powder compared to the control group (36% vs. 39.8% and 35.7% vs. 40.8%, respectively), which corroborate with Calislar and Kaplan (2017) results. This is in agreement with Saleh et al. (2017) who reported that increasing the levels of PUFA leads to decreased MUFA synthesis through inhibiting the activity of 9-desaturase complex, which is the key enzyme needed for the conversion of SFA to MUFA.
Our results also agree with previous studies that stated that increased inclusion rates of dietary carob pods and pulp resulted in a tendency for increased polyunsaturated fatty acid content in the lamb and pork meats, which improve the meat fatty acid composition (Vasta et al., 2007; Kotrotsios et al., 2012; Inserra et al., 2015). Indeed, the lower levels of saturated fatty acids and higher levels of unsaturated fatty acids in muscle from carob-fed animals meet the recommendation of lowering the intake saturated fatty acids and increasing that of unsaturated fatty acids to decrease the risk of cardiovascular diseases (Inserra et al., 2015; Kralik et al., 2018). In our study, carob pulp supplementation tended to increase the PUFA/SFA in breast (0.2–0.4) and thigh (0.2–0.3) meats of the experimental group compared to the control group. These results meet the recommended average ratio PUFA/SFA (0.23–0.45) (Randle, 1985). Overall, the dietary manipulation of the feeds by inclusion of carob pulp improves the fatty acid composition of the broilers’ meat which may be seen as an advantage over red meats (ruminants) in which the occurrence of high saturated fats has been linked to cardiovascular diseases (Delgado et al., 2021).
In our study, the increase of PUFA in meat samples from the experimental group could be related to the phenolic content in the diet containing carob. According to Gravador et al. (2015) a possible effect of dietary carob pulp on PUFA of meat from kids and lambs fed carob-containing diets could be justified taking into account that carob has been reported to contain phenolic compounds, including condensed tannins. Saleh et al. (2017) suggested that the improvement of the PUFA in the breast meat of broilers could be explained by the antioxidant potential of phenolic compounds in the diet. Diet-containing antioxidants may inhibit the oxidation of the PUFA.
Lipid Oxidation Index
The conjugated diene index ranged between 0.142 ± 0.005 and 0.235 ± 0.017 for the breast meat and between 0.185 ± 0.018 and 0.282 ± 0.010 for the thigh meat (Table 8). The conjugated diene index was found to decrease as the diet is supplemented with carob pulp powder. It seems that this index was affected (P < 0.05) by the diet type but not by the muscle type (P > 0.05).
Table 8.
Conjugated diene index values of breast and thigh muscles of broilers fed diet containing carob pulp powder.
| Parameters | Control |
Experimental |
||
|---|---|---|---|---|
| Breast | Thigh | Breast | Thigh | |
| Conjugated dienes A233/A214 | 0.235 ± 0.017ab | 0.282 ± 0.010a | 0.142 ± 0.005bc | 0.185 ± 0.018b |
Each value represents the mean of three measurements ± SEM.
Mean values in the same line with different letters are significantly different at P < 0.05 by mean of the Tukey's multiple comparison test.
Lipid oxidation is a major problem in poultry meat causing loss of nutritional and sensory values as well as the formation of potentially toxic compounds that compromise meat quality and reduce the shelf life. It is due to the high content of polyunsaturated fatty acids (PUFA) (Barroeta, 2007; Estévez, 2015). Therefore, methods that are effective, safe, and low cost for controlling poultry product stability are extremely important to the muscle food industry (Barroeta, 2007; Estévez, 2021). In our experiment, supplementation of broiler diets with carob pulp powder significantly reduced the oxidation of lipids in breast and thigh meat samples, which could be related to the presence of natural antioxidants such as phenolic compounds in the diet (Ahmed et al., 2015; Gravador et al., 2015). The antioxidant capacity of carob phenolic compounds and tannins was previously reported by Seczyk et al. (2016), Amessis Ouchemoukh et al. (2017), and Kyriacou et al. (2021). Ahmed et al. (2015) mentioned that antioxidant activity is directly correlated with reducing power and the presence of reductones. Ahmed et al. (2015) and Saleh et al. (2017) explained that tannins and phenolics might act as reductones by donating electrons to the free radicals produced during the first step in lipid oxidation and then converting them to a more stable product to terminate the free radical chain reaction.
In their study Abu Hafsa et al. (2018), offered grape seed to broilers as a source of total phenol 55.5 g/kg, total flavonoids19.5 g/kg, and total tannins 9.4 g/kg diets. These authors found that the polyphenols present in grape seed has been absorbed sufficiently to enhance the antioxidant function in experimental broilers. In another study, the incorporation of phenolics in the diet of rats enriched with n-3 PUFA showed the ability to recycle or spare the vitamin E, and to enhance the activity of antioxidant enzymes (Gladine et al., 2007). In this respect Fang et al. (2020) explained that dietary grape seed procyanidins in weanling piglets significantly increased the activities of antioxidant enzymes, including glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) in serum, liver, and muscle, increased the expression of GSH-Px, SOD, and CAT genes in the liver, and decreased the level of malondialdehyde (MDA) in serum, liver and muscle. In contrast, the results we found disagree with Inserra et al. (2015) study showing that meat from animals fed with the carob 15% diet overall experienced a more pronounced lipid peroxidation across time of storage compared to meat from animals in the other treatments, which is related to highly unsaturated n-3 PUFA in meat. These authors suggested that there was no antioxidant effect of carob condensed tannins because these compounds are poorly degraded into the gastrointestinal tract and, therefore, not readily bioavailable. Indeed, Du et al. (2002) explained that radiolabeled condensed tannins are not absorbed from the digestive tract of chickens, but non-tannin phenolic compounds are absorbed and distributed in various tissues. The above-cited authors added that this finding suggests that the absorbed phenolic compounds present in muscle could exhibit antioxidant effects and, thus, improve the oxidative stability of meat. On the other hand, the higher linoleic acid content in breast and thigh meat samples of the experimental group compared to the control group could explain the higher antioxidant capacity. According to Mahfuz et al. (2021), linoleic acid level could improve the antioxidant capacity of meat. Overall, the potential contribution of phenolics with antioxidant potential from dietary carob pulp could have increased the oxidative stability of chicken muscle tissues despite of the increase in PUFA, which are in fact, more susceptible to oxidation than SFA. Give the role of lipid oxidation in processed chicken meat products (Estévez, 2015), this antioxidant protection may have benefits in terms of nutritional value, sensory properties and safety of the processed foods.
Sensory Attributes
Results of the effect of the dietary supplementation with carob pulp powder on sensory evaluation of chicken breast and thigh muscles are shown in Figure 2. The breast meat of the experimental group had a slightly higher tenderness (3.75 vs. 3.45), juiciness (3.65 vs. 2.95), and after taste (3.05 vs. 2.75) scores compared with that of the control group (P > 0.05). However, the experimental and control breast muscles registered the same flavor and fat scores (3.2 and 4.9, respectively). Du and Ahn (2002) suggested that the differences in chicken meat tenderness and juiciness could be due to the changes in fatty acid composition of muscle lipids. These authors explained that a high proportion of saturated fatty acids increased the melting point of fat, which would make the meat drier and harder. This is in good agreement with our results since we found less saturated fatty acids in breast of the experimental group than that of the control group. For thigh muscles, slight differences (P > 0.05) were also observed in tenderness (4.1 vs. 3.5), juiciness (3.55 vs. 3.4) and fat (3.5 vs. 3.35) scores for the control and experimental groups respectively.
Figure 2.
Spider plots of mean values of five sensory attributes describing the sensory flavor and texture of broiler breast and thigh muscles. Abbreviations: CB, control breast; CT, control thigh; EB, experimental breast; ET, experimental thigh.
The results demonstrated that the panelists had slightly preference over breast meat of the experimental group than of the control group for its tenderness, juiciness, and after taste. It appears that the diet type had no effect (P > 0.05) on the sensory profile of the breast and thigh muscles, but the muscle type had an effect (P < 0.05) on fat score, where the panelists appreciated breast fat more than thigh fat.
The effect of the diet on the sensory attributes of chicken meat has been investigated by many authors. Literature reveals that dietary manipulations may have, or not, a significant impact on the sensory attributes of chicken meat depending on the nature of the manipulation, the composition and concentration of the supplemented material and the exposure to such dietary treatment (Kim et al., 2009; Escobedo del Bosque et al., 2020). Several sensory attributes are associated with consumers’ satisfaction. According to Sow and Grognet (2010), juiciness, oiliness, sweetness, and hardness are attributes correlated to chicken meat preference. However Horsted et al. (2012) reported that aroma, flavor, and umami taste in broiler breast meat are positively correlated to the product's overall liking.
CONCLUSIONS
The present study suggested that incorporating 3 and 7% of carob pulp powder in the growing and finishing broiler feed had no negative impact on growth performance and carcass traits. It could improve the intestinal microbiota and broiler meat quality. It reduced fat content and conjugated diene index and increased the polyunsaturated fatty acid contents in broiler breast and thigh meats. Hence, carob is a local resource that represents a good alternative to imported raw materials in broiler breeding. Using carob pulp in broilers diets is proposed as a feasible, sustainable and effective means to improve chicken meat quality.
ACKNOWLEDGMENTS
This work was financed by the Algerian Ministry of Higher Education and Scientific Research.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Abu Hafsa S.H., Ibrahim S.A., Hassan A.A. Carob pods (Ceratonia siliqua L.) improve growth performance, antioxidant status and caecal characteristics in growing rabbits. J. Anim. Physiol. Anim. Nutr. 2017;101:1307–1315. doi: 10.1111/jpn.12651. [DOI] [PubMed] [Google Scholar]
- Abu Hafsa S.H., Ibrahim S.A. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microbiota in broiler chicks. J. Anim. Physiol. Anim. Nutri. 2018;102:268–275. doi: 10.1111/jpn.12688. [DOI] [PubMed] [Google Scholar]
- Abubakar A., Fitri C.A., Koesmara H., Mudatsir Ardatami S. Analysis of pH and cooking losses of chicken meat due to the use of different percentages of turmeric flour. IOP Conf. Ser. Earth Environ. Sci. 2021;667 [Google Scholar]
- Ahmed S.T., Islam M., Bostami A.B.M.R., Mun H.S., Kim Y.J., Yang C.J. Meat composition, fatty acid profile and oxidative stability of meat from broilers supplemented with pomegranate (Punica granatum L.) by-products. Food Chem. 2015;188:481–488. doi: 10.1016/j.foodchem.2015.04.140. [DOI] [PubMed] [Google Scholar]
- Alloui N., Bennoune O. Poultry production in Algeria: current situation and future prospects. Worlds Poult. Sci. J. 2013;69:613–620. [Google Scholar]
- Álvarez-Fuentes G., García-López J.C., Pinos-Rodríguez J.M., Aguirre-Rivera J.R., Jasso-Pineda Y., Celestino-Santillán S.G. Effects of feeding the seeds of Prosopis laevigata, Acacia schaffneri and Ceratonia siliqua on the performance of broiler chicks. S. Afr. J. Anim. Sci. 2012;42:354–359. [Google Scholar]
- Amessis Ouchemoukh N., Ouchemoukh S., Meziant N., Idiri Y., Hernanz D., Stinco C.M., Rodríguez-Pulido F.J., Heredia F.J., Madani K., Luise J. Bioactive metabolites involved in the antioxidant, anticancer and anticalpain activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. extracts. Ind. Crops. Prod. 2017;95:6–17. [Google Scholar]
- Avallone R., Plessi M., Baraldi M., Monzani A. Determination of chemical composition of carob (Ceratonia siliqua): Protein, fat, carbohydrates, and tannins. J. Food Compos. Anal. 1997;10:166–172. [Google Scholar]
- Babincová M., Machováb E., Kogan G. Carboxymethylated glucan inhibits lipid peroxidation in liposomes. Z. Naturforsch. C. 1999;54:1084–1088. doi: 10.1515/znc-1999-1213. [DOI] [PubMed] [Google Scholar]
- Barroeta A.C. Nutritive value of poultry meat: relationship between vitamin E and PUFA. Worlds Poult. Sci. J. 2007;63:277–284. [Google Scholar]
- Batlle I., Tous J. Page 79 in Ceratonia siliqua L. Promoting the Conservation and Use of Underutilized and Neglected Crops. ed. IPGCPR. Gatersleben/IPGRI; Rome, Italy: 1997. Carob tree. [Google Scholar]
- Ben Ayache S., Saafi E.B., Emhemmed F., Flamini G., Achour L., Muller C.D. Biological activities of aqueous extracts from carob plant (Ceratonia siliqua L.) by antioxidant, analgesic and proapoptotic properties evaluation. Molecules. 2020;25:3120. doi: 10.3390/molecules25143120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamirouche K., Baazize-Ammi D., Hezil N., Djezzar R., Niar A., Guetarni D. Effect of probiotics and Yucca schidigera extract supplementation on broiler meat quality. Acta Sci. - Anim. Sci. 2020;42:1–9. [Google Scholar]
- Benguiar R., Benaraba R., Riazi A. Effet de l'extrait de caroube sur la croissance de deux candidats probiotiques: Lactobacillus fermentum et Lactobacillus rhamnosus. Nat. Technol. B- Sci. Agron. Biol. 2015;13:22–27. [Google Scholar]
- Berri C. La viande de volaille: des attentes pour la qualité qui se diversifient et des défauts spécifiques à corriger. INRA Prod. Anim. 2015;28:115–118. [Google Scholar]
- Brunel V., Jehl N., Drouet L., Portheau M.C. Viande de volailles sa valeur nutritionnelle présente bien des atouts. Viandes Produits Carnés. 2010;25:18. [Google Scholar]
- Calislar S., Kaplan Y. Effects of carob (Ceratonia siliqua) pod byproduct on quail performance, egg characteristics, fatty acids, and cholesterol levels. R. Bras. Zootec. 2017;46:113–117. [Google Scholar]
- Cherian G., Selvaraj R.K., Goeger M.P., Stitt P.A. Muscle fatty acid composition and thiobarbituric acid-reactive substances of broilers fed different cultivars of sorghum. Poult. Sci. 2002;81:1415–1420. doi: 10.1093/ps/81.9.1415. [DOI] [PubMed] [Google Scholar]
- Cobb. 2019. Cobb 500 performances et recommandations nutritionnelles. Accessed Mar. 2022. https://www.cobb-vantress.com/assets/Cobb-Files/a54d5b4201/Cobb500-BroilerPerformance-and-Nutrition-Supplement-French-v2.pdf
- Cortinas L., Villaverde C., Galobart J., Baucells M.D., Codony R., Barroeta A.C. Fatty acid content in chicken thigh and breast as affected by dietary polyunsaturation level. Poult. Sci. 2004;83:1155–1164. doi: 10.1093/ps/83.7.1155. [DOI] [PubMed] [Google Scholar]
- Custodio L., Fernandes E., Escapa A.L., Fajardo A., Aligue R., Albericio F., Neng NR., Nogueira J.M.F., Romano A. Antioxidant and cytotoxic activities of carob tree fruit pulps are strongly influenced by gender and cultivar. J. Agric. Food Chem. 2011;59:7005–7012. doi: 10.1021/jf200838f. [DOI] [PubMed] [Google Scholar]
- Delgado J., Ansorena D., Van Hecke T., Astiasarán I., De Smet S., Estévez M. Meat lipids, NaCl and carnitine: do they unveil the conundrum of the association between red and processed meat intake and cardiovascular diseases? Invited Rev. Meat Sci. 2021;171 doi: 10.1016/j.meatsci.2020.108278. [DOI] [PubMed] [Google Scholar]
- Dikkaya Y.R., Baydar K., Kafkas E., Comlekcioglu S., Kü den A.B. Sugar content of selected carob fruits. Acta Hortic. 2020;1280:269–274. [Google Scholar]
- Du M., Ahn D.U. Effect of dietary conjugated linoleic acid on the growth rate of live birds and on the abdominal fat content and quality of broiler meat. Poult. Sci. 2002;81:428–433. doi: 10.1093/ps/81.3.428. [DOI] [PubMed] [Google Scholar]
- Du M., Cherian G., Stitt P.A., Ahn D.U. Effect of dietary sorghum cultivars on the storage stability of broiler breast and thigh meat. Poult. Sci. 2002;81:1385–1391. doi: 10.1093/ps/81.9.1385. [DOI] [PubMed] [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugar and related substances. Analyt. Chem. 1956;28:350–356. [Google Scholar]
- El Bouzdoudi B., Saïdi R., Embarch K., El Mzibri M., Nejjar El Ansari Z., El Kbiach M., Badoc A., Patrick M., Lamarti A. Mineral composition of mature carob (Ceratonia siliqua L.) pod: a study. Int. J. Food Sci. Nutr. Eng. 2017;7:91–103. [Google Scholar]
- Escobedo del Bosque C.I., Altmann B.A., Ciulu M., Halle I., Jansen S., Nolte T., Weigend S., Mörlein D. Meat quality parameters and sensory properties of one high-performing and two local chicken breeds fed with Vicia faba. Foods. 2020;9:1052. doi: 10.3390/foods9081052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poult. Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Estévez M. Critical overview of the use of plant antioxidants in the meat industry: opportunities, innovative applications and future perspectives. Meat Sci. 2021;181 doi: 10.1016/j.meatsci.2021.108610. [DOI] [PubMed] [Google Scholar]
- Fadel A.H.I., Kamarudin M.S., Romano N., Ebrahimi M., Saad C.R., Samsudin A.A. Carob seed germ meal as a partial soybean meal replacement in the diets of red hybrid tilapia. Egypt. J. Aquat. Res. 2017;43:337–343. [Google Scholar]
- Fang L., Li M., Zhao L., Han S., Li Y., Xiong B., et al. Dietary grape seed procyanidins suppressed weaning stress by improving antioxidant enzyme activity and mRNA expression in weanling piglets. J. Anim. Physiol. Anim. Nutr. 2020;104:1178–1185. doi: 10.1111/jpn.13335. [DOI] [PubMed] [Google Scholar]
- FAO. 2022. Accessed Mar. 2022. https://www.fao.org/faostat/en/#data/QCL
- Folch J., Lees M., Sloane-Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gabriel I., Mallet S., Sibille P. La microflore digestive des volailles: facteurs de variation et conséquences pour l'animal. INRA Prod. Anim. 2005;18:309–322. [Google Scholar]
- García-Ruíz A., Bartolomé B., Martínez-Rodríguez A.J., Puello E., Martín- Álvarez P.J., Moreno-Arribas M.V. Potential of phenolic compounds for controlling lactic acid bacteria growth in wine. Food Contr. 2008;19:835–841. [Google Scholar]
- Gladine C., Morand C., Rock E., Gruffat D., Bauchart D., Duran D. The antioxidative effect of plant extracts rich in polyphenols differs between liver and muscle tissues in rats fed n-3 PUFA rich diets. Anim. Feed Sci. Technol. 2007;139:257–272. [Google Scholar]
- Guler-Akin M.B., Goncu B., Serdar Akin M. Some properties of probiotic yoghurt ice cream supplemented with carob extract and whey powder. Adv. Microbiol. 2016;6:1010–1020. [Google Scholar]
- Gravador R.S., Luciano G., Jongberg S., Bognanno M., Scerra M., Andersen M.L., Lund M.N., Priolo A. Fatty acids and oxidative stability of meat from lambs fed carob-containing diets. Food Chem. 2015;182:27–34. doi: 10.1016/j.foodchem.2015.02.094. [DOI] [PubMed] [Google Scholar]
- Hadj Ayed M., Aïssa A., Chakroun I. Carcass traits and meat quality of rabbits fed regimes containing carob pods and reared under Tunisian summer conditions. Int. J. Agric. Environ. Bio-res. 2019;4:318–330. [Google Scholar]
- Horsted K., Allesen-Holm B.H., Hermansen J.E., Kongsted A.G. Sensory profiles of breast meat from broilers reared in an organic niche production system and conventional standard broilers. J. Sci. Food Agric. 2012;30:258–265. doi: 10.1002/jsfa.4569. [DOI] [PubMed] [Google Scholar]
- Iji P.A., Khumalo K., Slippers S., Gous R.M. Intestinal function and body growth of broiler chickens on maize-based diets supplemented with mimosa tannins and a microbial enzyme. J. Sci. Food Agric. 2004;84:1451–1458. [Google Scholar]
- Inserra L., Lucianoa G., Bella M., Scerra M., Cilione C., Basile P., Lanz M., Priolo A. Effect of including carob pulp in the diet of fattening pigs on the fatty acid composition and oxidative stability of pork. Meat Sci. 2015;100:256–261. doi: 10.1016/j.meatsci.2014.09.146. [DOI] [PubMed] [Google Scholar]
- Iqbal Y., Cottrell J.J., Suleria H.A.R., Dunshea F.R. Gut microbiota-polyphenol interactions in chicken: a review. Animals. 2020;10:1391. doi: 10.3390/ani10081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Jin S.K., Yang H.S. Effect of dietary garlic bulb and husk on the physicochemical properties of chicken meat. Poult. Sci. 2009;88:398–405. doi: 10.3382/ps.2008-00179. [DOI] [PubMed] [Google Scholar]
- Klein R.A. The detection of oxidation in liposome preparations. Biochim. Biophys. Acta. 1970;210:486–489. doi: 10.1016/0005-2760(70)90046-9. [DOI] [PubMed] [Google Scholar]
- Meydani M., Hasan S.T. Dietary polyphenols and obesity. Nutrients. 2010;2:737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrotsios N., Christaki E., Bonos E., Florou-Paneri P. Dietary carob pods on growth performance and meat quality of fattening pigs. Asian-Aust. J. Anim. Sci. 2012;25:880–885. doi: 10.5713/ajas.2011.11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrotsios N., Christaki E., Bonos E., Florou-Paneri P., Spais A.B. Carobs in productive animal nutrition. J. Hell. Vet. Med. Soc. 2011;62:48–57. [Google Scholar]
- Kralik G., Kralik Z., Grčević M., Hanžek D. In: Pages 63–94 in Animal Husbandry and Nutrition. Banu Y., Turgay T., editors. IntechOpen; London, UK: 2018. Chapter 4: Quality of chicken meat. [Google Scholar]
- Kyriacou M.C., Antoniou C., Rouphael Y., Graziani G., Kyratzis A. Mapping the primary and secondary metabolomes of carob (Ceratonia siliqua L.) fruit and its postharvest antioxidant potential at critical stages of ripening. Antioxidants. 2021;10:57. doi: 10.3390/antiox10010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkab I., El Hajaji H., Lachkar N., El Bali B., Lachkar M., Ciobica A. Phytochemistry, bioactivity: suggestion of Ceratonia siliqua L. as neurodegenerative disease therapy. J. Complement Integr. Med. 2018;29:15. doi: 10.1515/jcim-2018-0013. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Chabault M., Boulay M., Guardia S., Jégo Y., Baéza E., Berri C. Mars, La Rochelle; France: 2014. Sélection Divergente sur le pH ultime du filet de poulet: premier bilan après 4 générations in 10èmes Journées de la Recherche Avicole et Palmipèdes à Foie Gras. ffhal-02748221f. [Google Scholar]
- Lizardo R., Cañellas J., Mas F., Torrallardona D., Brufau J. Pages 97-101 in Utilisation of carob powder in piglet diets and its influence on growth performance and health after weaning in 34èmes Journées de la Recherche Porcine, sous l'égide de l'Association Française de Zootechnie, Paris, France; 5-7 février; 2002. [Google Scholar]
- Lyon B.G., Smith D.P., Lyon C.E., Savage E.M. Effects of diet and feed withdrawal on the sensory descriptive and instrumental profiles of broiler breast fillets. Poult. Sci. 2004;83:275–281. doi: 10.1093/ps/83.2.275. [DOI] [PubMed] [Google Scholar]
- Mahfuz S., Shang Q., Piao X. Phenolic compounds as natural feed additives in poultry and swine diet. J. Anim. Sci. Biotechnol. 2021;12:48. doi: 10.1186/s40104-021-00565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi N., Ikhlef H., Kaci A., Mahmoudi S. Evaluation de la durabilité socio-économique des ateliers avicoles à M'sila (Algérie) New Medit. 2019;4:65–77. [Google Scholar]
- Mahmoudi S., Khali M., Benkhaled A., Boucetta I., Dahmani Y., Attallah Z., Belbraouet S. Fresh figs (Ficus carica L.): Pomological characteristics, nutritional value, and phytochemical properties. Eur. J. Hortic. Sci. 2018;83:104–113. [Google Scholar]
- Ocejo M., Oporto B., Hurtado A. 16S rRNA amplicon sequencing characterization of caecal microbiome composition of broilers and free-range slow-growing chickens throughout their productive lifespan. Sci. Rep. 2019;9:2506. doi: 10.1038/s41598-019-39323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz L.T., Rodriguez M.L., Alzueta C., Rebole A., Centeno C., Trevino J. Pages 239–242 in EAAP Publication 110. Waganingen Academic Publishers; Netherlands: 2004. Effect of carob seed (Ceratonia Siliqua L.) in broiler chick diets on nutrient digestibility and intestinal viscosity. [Google Scholar]
- Petracci M., Sirri F., Mazzoni M., Meluzzi A. Comparison of breast muscle traits and meat quality characteristics in 2 commercial chicken hybrids. Poult. Sci. 2013;92:2438–2447. doi: 10.3382/ps.2013-03087. [DOI] [PubMed] [Google Scholar]
- JORA Journal Officiel de la République Algérienne N° 23. 2006. Pages 4–6 in Arrité du 15 janvier 2006 rendant obligatoire la méthode de mesurage du pH de la viande et des produits de la viande. Accessed October 2022. https://www.joradp.dz/FTP/jo-francais/2006/F2006023.pdf.
- Randle, P. J. 1985. Diet and cardiovascular disease. Department of Health and Social Security Report on Health and Social Subjects. Committee on Medical Aspects of Food Policy Report of the Panel on Diet in Relation to Cardiovascular Disease. [PubMed]
- Rodríguez-Solana R., Romano A., Moreno-Rojas J.M. Carob pulp: a nutritional and functional by-product worldwide spread in the formulation of different food products and beverages. A review. Processes. 2021;9:1146. [Google Scholar]
- Sahle M., Coleou J., Haas C. Carob pod (Ceratonia Siliqua) meal in geese diets. Br. Poult. Sci. 1992;33:531–541. doi: 10.1080/00071669208417492. [DOI] [PubMed] [Google Scholar]
- Saleh H., Golian A., Kermanshahi H., Taher Mirakzehi M. Effects of dietary α-tocopherol acetate, pomegranate peel, and pomegranate peel extract on phenolic content, fatty acid composition, and meat quality of broiler chickens. J. Appl. Anim. Res. 2017;45:629–636. [Google Scholar]
- Saratsi K., Hoste H., Voutzourakis N., Tzanidakis N., Stefanakis A., Thamsborg S., Mueller-Harvey I., Hadjigeorgiou I., Sotiraki S. Feeding of carob (Ceratonia siliqua) to sheep infected with gastrointestinal nematodes reduces faecal egg counts and worm fecundity. Vet. Parasitol. 2020;284 doi: 10.1016/j.vetpar.2020.109200. [DOI] [PubMed] [Google Scholar]
- Schiavone A., Guo K.J., Tassone S., Gasco L., Hernandez E., Denti R., Zoccarato I. Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poult. Sci. 2008;87:521–527. doi: 10.3382/ps.2007-00113. [DOI] [PubMed] [Google Scholar]
- Seczyk Ł., Swieca M., Gawlik-Dziki U. Effect of carob (Ceratonia siliqua L.) flour on the antioxidant potential, nutritional quality, and sensory characteristics of fortified durum wheat pasta. Food Chem. 2016;194:637–642. doi: 10.1016/j.foodchem.2015.08.086. [DOI] [PubMed] [Google Scholar]
- Sour S., Fridi C., Taif A. Beneficial effects of carob pulp (Ceratonia siliqua) on lipids profile and oxidant/antioxidant status in obese rats. Agrobiologia. 2019;9:1200–1206. [Google Scholar]
- Sow T.M.A., Grongnet J.F. Sensory characteristics and consumer preference for chicken meat in Guinea. Poult. Sci. 2010;89:2281–2292. doi: 10.3382/ps.2010-00679. [DOI] [PubMed] [Google Scholar]
- Vasta V., Pennisi P., Lanza M., Barbagallo D., Bella M., Priolo A. Intramuscular fatty acid composition of lambs given a tannin iferous diet with or without polyethylene glycol supplementation. Meat Sci. 2007;76:739–745. doi: 10.1016/j.meatsci.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Vekiari A.S., Ouzounidou G., Gork G., Ozturk M., Asfi M. Compositional changes of major chemical compounds in Greek carob pods during development. Bull. Chem. Soc. Ethiop. 2012;26:343–351. [Google Scholar]
- Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microbiota and gut morphology in broiler chicks. Poult. Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Vohra P., Kbatzee F.H. The use of ground carobs in chicken diets. Poult. Sci. 1964;43:790–792. [Google Scholar]
- Welcher J.F. In: The Analytical Uses of Ethylenediamine Tetraacetic Acid. Van D., editor. Vol. 120. Creative Media Partners; Nostrand, NY: 1958. pp. 103–120. [Google Scholar]
- Yıldırım H., Kaya Ş. Karmayeme farklı oranlarda eklenen keçiboynuzu tozunun (Ceratonia siliqua L.) etlik piliçlerin performansına etkisi. Mustafa Kemal Üniv. Ziraat Fakült. Dergisi. 2011;16:43–50. [Google Scholar]
- Youshida S., Forno D., Cock J., Gomez K. The International Rice Research Institute; Philippines: 1976. Page 82 in Laboratory Manual for Physiological Studies of Rice. [Google Scholar]
- Youssef M.K.E., El-Manfaloty M.M., Ali H.M. Assessment of proximate chemical composition, nutritional status, fatty acid composition and phenolic compounds of carob (Ceratonia siliqua L.) Food Public Health. 2013;3:304–308. [Google Scholar]