Abstract
Improving feed efficiency is one of the main goals of chicken breeding and production. The function of the digestive system, where feed is digested and nutrients are absorbed, is closely related to feed efficiency. However, the association between feed efficiency and the development of different digestive organs in chickens remains unclear. Here, we investigated the individual feed efficiency of 207 broilers during the fast-growing period with an electronic feeder and examined the characteristics of 8 organs of their digestive system (the liver, bile, proventriculus, gizzard, duodenum, jejunum, ileum, and cecum) at market age. Both the feed conversion ratio (FCR) and residual feed intake (RFI) were significantly negatively correlated with the gizzard weight (GW) and significantly positively correlated with the relative weight of the liver (RLW). Additionally, we found an obvious negative relationship between the FCR and cecal length (CL). A two-tailed t test further confirmed these correlation analysis results. Specifically, compared to birds with the lowest feed efficiencies, the GW of broilers with the highest feed efficiencies (the lowest FCR or RFI) was 22.74% and 17.97% higher, respectively. The RLW of chickens with the highest feed efficiencies was 10.82 to 13.73% less than that of chickens with the lowest feed efficiencies. In addition, we found that increased CL (5.42–12.09%) was significantly associated with better feed efficiency. Thus, our study showed that the feed efficiency of broilers was related to the development of the gizzard, liver, and cecum. These findings provide new insight into the genetic and physiological regulation of feed efficiency in broilers.
Key words: broiler, feed conversion ratio, residual feed intake, digestive organs, developmental features
INTRODUCTION
As an important source of animal protein, chicken meat is the most popular meat product in the world. Chicken meat accounted for greater than 89% of world poultry production (Zampiga et al., 2021), and it is predicted that the yield of poultry meat production will account for half of future meat product growth from 2021 to 2030 (OECD-FAO, 2022). However, the increasing demand for meat products imposes an increasing burden on limited land and cereal feed resources (Vgsholm et al., 2020). The cost of feed accounts for more than 60% of the total cost of modern broiler production; and fluctuates, which makes it difficult to predict profits (Sell-Kubiak et al., 2017; Zampiga et al., 2021). Therefore, improving feed efficiency can save natural resources and reduce production costs.
The feed conversion ratio (FCR), which is calculated as the change in feed intake divided by the change in body weight, is a common index for measuring feed efficiency (Hess et al., 1941); increases in efficiency are associated with lower FCR values. Another index is the residual feed intake (RFI), which refers to the difference between the actual feed intake of animals and the desired intake for maintenance and production and was first proposed by Koch et al. (1963). Chickens with lower RFI values require less feed to achieve the same growth rate and are therefore more efficient. Selective breeding and improved dietary formulations have effectively improved feed efficiency, resulting in more products produced with the same amount of feed or the same amounts of products produced with less feed (Aggrey et al., 2010; Yi et al., 2015; Wen et al., 2018). Indeed, the feed efficiency of modern broiler chickens has dramatically improved over the last decade, with current FCR values of 1.5 or less, due to genetic selection and improved nutritional management (Siegel, 2014).
The digestive tract of chickens, which consists of the crop, proventriculus, gizzard, small intestine (including the duodenum, jejunum, and ileum), large intestine (the cecum and rectum), and cloaca, considerably differs from that of other animals. The crop is an enlarged portion of the alimentary tract that is involved in the storage and moistening of food (Inês and Mingan, 2018). The chyme is then mixed with pepsin, hydrochloric acid, and mucus in the proventriculus, crushed by the strong shrinkage and squeezing of the gizzard and moved to the small intestine, where it is chemically resolved into micromolecules by specific digestive enzymes secreted by the digestive glands (Uni et al., 1999; Feye et al., 2020). These nutrients are fully absorbed by the mucous membrane of the small intestine and transported to other tissues through the blood.
However, the intense selection pressure on broiler performance traits, such as increased body weight and feed efficiency, has resulted in decreased maturity at slaughter, which affects the size of organs, such as digestive organs, at all ages. Rougiere et al. (2009) observed that improvements in feed efficiency significantly increased the weight of the proventriculus and gizzard and decreased the weight of the small intestine in chickens. Schmidt et al. (2009) found that the jejunum and ileum of modern commercial broilers were 20% longer than those of an unselected heritage line established in the 1950s. Despite the rapid maturation of the digestive system in modern lines compared to that in unselected heritage lines, the digestive system in modern lines is smaller relative to body weight at the same ages (Schmidt et al., 2009; Rougière and Carré, 2010). In addition, chickens with better feed efficiency exhibit several interesting changes in the histological characteristics of the small intestine, such as increases in villus height and the number of goblet cells per villus (De Verdal et al., 2010).
Previous studies have reported the effect of genetic selection for feed efficiency on the characteristics of digestive organs in chickens, yet the relationship between the developmental characteristics of the digestive organs and feed efficiency remains largely unknown. Therefore, the main objectives of the present study were to evaluate the phenotypic associations between feed efficiency and the development of digestive organs. The results of this study provide an important biological and theoretical basis for future breeding strategies to improve broiler feed efficiency.
MATERIALS AND METHODS
Animals and Housing
The experiment was conducted in accordance with the guidelines of the Animal Care and Use Committee of China Agricultural University.
A paternal line of yellow-feathered chickens from Guangdong Wen's Nanfang Poultry Breeding Co., Ltd. (China) was used in the present study. This strain was a slow-growing broiler, that was selected over 6 generations based on feather color and body weight but not on feed efficiency. The market age of this line is 76 days old when the body weight is about 2.2 kg. The broilers could not be transferred to semienclosed testing coops early, because they are too small to measure accurately. Therefore, the measurement period was set at 3 wk before the slaughter age. We used only male chicks from a brood provided with appropriate temperature and illumination in a closed coop during the brooding period (following Wen's broiler management handbook). At 35 d of age, all chickens were transferred to semienclosed coops that were 24 × 5.5 m at ground level. The floor of the coops was covered with fresh wood chips, and clean water was provided from 70 nipple drinkers. The corn–soybean meal-type diet was automatically provided at 30 electronic feeders. To ensure the accuracy and reliability of the subsequent results, chickens that did not meet breed regulations (e.g., were weak, handicapped, or had defects in feather color or appearance) were eliminated at 55 d of age. A total of 223 chickens were selected for the subsequent feeding trial and provided with an ankle ring containing a radio frequency identification (RFID) chip. These chickens were fed a diet containing 2,900 kcal/kg metabolizable energy and 190 g/kg crude protein from 55 to 76 d of age; during this experimental feeding period, the broilers had free access to feed and water. The light/dark ratio was held constant at 20:4 h.
The design of the feed intake automatic recording machine was similar to a previous report by Howie et al. (2009). This equipment measures the feed intake of chickens and greatly reduces the labor and material costs of assessing chicken feed efficiency compared to individual cage measurements, which have been extensively used in poultry breeding programs (Yan et al., 2018; Zhu et al., 2018; Li et al., 2021). The feeder had a barrier and was set to allow access to only one chicken at a time. The system automatically identified the chickens according to their RFID chip and recorded bird entry and exit as well as individual identification number and feed intake. This information was immediately transferred to the computer terminal. To ensure that every chicken could freely eat, the feed barrel automatically replenished the feed when the amount in the trough fell below 20 g. The feeding data were exported from the feeding record system, and data from chicks that later died, incorrect RFIDs and individuals with abnormal feeding patterns were excluded, resulting in feeding records for 207 chickens. Further details of this device and the growth curve of daily feed intake and average body weight of this population at 56 to 76 d of age were reported in our previous studies (Wen et al., 2018; Wen et al., 2019).
Body Weight Measurement
Body weight was measured at the beginning (d 56; BW56) and end (d 76; BW76) of the feeding experiment with an electronic scale (accuracy to 5 g). The body weight gain (BWG) and metabolic mid-weight (MMW) were calculated with the following equations:
Determination of Feed Efficiency
The total feed intake (TFI) of each individual during the fast-growing period was calculated based on the feed intake data recorded by the automatic feed intake recording equipment. The FCR and RFI were also calculated based on feed intake and body weight. Outliers more than 3 standard deviations (SD) from the mean in terms of body weight or the TFI were excluded. The specific formulae for calculating the FCR and RFI are as follows:
where the TFI encompassed that from 56 to 76 d of age, b0 is the intercept, and b1 and b2 are partial regression coefficients.
Characteristics of Digestive Organs
All broilers were euthanized by cervical dislocation followed by decapitation after the feeding experiment. Each digestive organ in the abdominal cavity was retrieved, and the following traits were measured: liver weight (LW), bile weight (cut open the gallbladder and weighed the bile, BiW), proventriculus weight (PW, contents removed), gizzard weight (GW, contents and surrounding abdominal fat removed), and the lengths of the duodenum (DL), jejunum (JL), ileum (IL), and left cecum (CL). All digestive organ weights were measured with an electronic balance with an accuracy of 0.1 g. The length of each intestinal segment was measured with a measuring tape to the nearest 1 mm. The length of the small intestine (SL) was calculated as the sum of the lengths of the duodenum, jejunum, and ileum. To correct for the effect of body weight, the relative weight, and length of digestive organs were obtained (Juanchich et al., 2020). Specifically, the relative weights of the bile (RBiW), liver (RLW), proventriculus (RPW), gizzard (RGW), duodenum (RDW), jejunum (RJW), ileum (RIW), and cecum (RCW) were calculated as the weight of the specific organ divided by the body weight, and the relative lengths of the duodenum (RDL), jejunum (RJL), ileum (RIL), and cecum (RCL) were calculated as the length of the specific organ divided by the body weight.
Statistical Analysis
After collating the data, statistical outliers were identified as values beyond 3 SD of the mean level and were eliminated from analyses. The phenotypic correlations (Pearson correlations) between feed efficiency and the characteristics of various digestive organs were calculated with SPSS 26.0 software. A P value of < 0.05 was considered statistically significant. To determine the specific digestive organs that influenced feed efficiency, a one-way analysis of variance (ANOVA) was conducted to determine the difference in digestive organ characteristics between the highest 15% and lowest 15% of FCR-ranked broilers (N = 30). P values of less than 0.05 and 0.10 were considered statistically significant and trending toward significance, respectively. Two-tailed t tests were conducted to investigate differences between chickens with the highest and lowest RFI values. A digestive organ was considered to have a large contribution to feed efficiency if the P values from the two-tailed t tests were both less than 0.05.
RESULTS
Descriptive Statistics of Chicken Phenotypes
The descriptive statistics of all phenotypic traits, including the mean, SD, coefficient of variation (CV), minimum, and maximum are summarized in Table 1. The average weight of chickens at 56 d of age was 1.57 kg. After 21 d of growth, the average weight (average slaughter weight) reached 2.32 kg. Notably, the CV of digestive organs had a wider range (from 12.64 to 37.63%) than that of body weight (7.83–10.00%). The CV of digestive organ weights ranged from 20.30 to 37.63%, and the CV of gut lengths ranged from 12.64 to 20.58%. The CV of digestive organ characteristics remained high after correcting for weight, indicating that artificial selection largely did not influence the developmental uniformity of internal digestive organs. The results also indicated large differences between the maximum and minimum values of phenotypic traits in broiler digestive organs. The maximum value (68.10 g) of liver weight was nearly 2 to 3 times the minimum value (24.10 g), and the maximum value (88.80 g) of gizzard weight was more than four times higher than the minimum value (20.60 g).
Table 1.
Basic descriptive statistics for all phenotypic traits.
| Categories | Traits | N | Mean ± SD | CV, % | Minimum | Maximum |
|---|---|---|---|---|---|---|
| General traits | BW56 | 207 | 1.57 ± 0.12 | 7.83 | 1.17 | 1.83 |
| BW76 | 207 | 2.32 ± 0.23 | 10.00 | 1.65 | 2.89 | |
| FCR | 204 | 3.22 ± 0.77 | 23.92 | 2.27 | 8.72 | |
| RFI | 204 | 0.00 ± 0.20 | — | -0.65 | 0.98 | |
| TFI | 204 | 2.30 ± 0.36 | 15.81 | 1.33 | 3.26 | |
| BWG | 207 | 0.75 ± 0.18 | 23.78 | 0.18 | 1.15 | |
| Organ weight | LW | 205 | 38.32 ± 7.78 | 20.30 | 24.10 | 68.10 |
| BiW | 205 | 2.06 ± 0.78 | 37.63 | 0.50 | 5.00 | |
| PW | 205 | 9.71 ± 3.63 | 37.42 | 4.90 | 30.50 | |
| GW | 205 | 39.44 ± 9.44 | 23.94 | 20.60 | 88.80 | |
| Relative weight of organ | RLW | 205 | 16.58 ± 3.14 | 18.94 | 11.86 | 32.12 |
| RBiW | 205 | 0.89 ± 0.33 | 37.08 | 0.23 | 2.20 | |
| RPW | 205 | 4.21 ± 1.58 | 37.49 | 2.28 | 12.74 | |
| RGW | 205 | 17.10 ± 4.56 | 26.69 | 9.01 | 37.08 | |
| Intestinal length | DL | 205 | 26.42 ± 3.34 | 12.64 | 19.00 | 37.00 |
| JL | 205 | 50.32 ± 10.36 | 20.58 | 21.00 | 84.50 | |
| IL | 205 | 47.38 ± 10.04 | 21.19 | 25.10 | 99.90 | |
| SL | 205 | 124.20 ± 18.00 | 14.49 | 93.50 | 187.80 | |
| CL | 203 | 17.30 ± 2.58 | 14.89 | 10.00 | 22.00 | |
| Relative length of intestine | RDL | 205 | 11.51 ± 1.77 | 15.39 | 7.17 | 18.93 |
| RJL | 205 | 21.86 ± 4.56 | 20.85 | 8.83 | 33.87 | |
| RIL | 205 | 20.56 ± 4.28 | 20.83 | 11.82 | 40.92 | |
| RSL | 205 | 53.98 ± 8.32 | 15.41 | 38.03 | 78.41 | |
| RCL | 203 | 7.52 ± 1.24 | 16.44 | 4.95 | 11.88 |
N: the number of observations which phenotype falling within mean ± 3 SD.
Abbreviations: BW56, body weight at 56 d of age (kg); BW76, body weight at 76 d of age (kg); BWG, body weight gain (kg); FCR, feed conversion ratio (kg); RFI, residual feed intake (kg); TFI, total feed intake from 56 to 76 days of age (kg); LW, BiW, PW and GW = the weight of liver, bile, proventriculus, and gizzard (g); RLW, RBiW, RPW and RGW, relative weight of the liver, bile, proventriculus and gizzard in 76 days of age (g/kg); DL, JL, IL, SL and CL, the length of duodenum, jejunum, ileum, small intestine and cecum (cm); RDL, RJL, RIL, RSL and RCL, relative length of the duodenum, jejunum, ileum, small intestine and cecum in 76 days of age (cm/kg).
Correlations Between Feed Efficiency and Characteristics of Digestive Organs
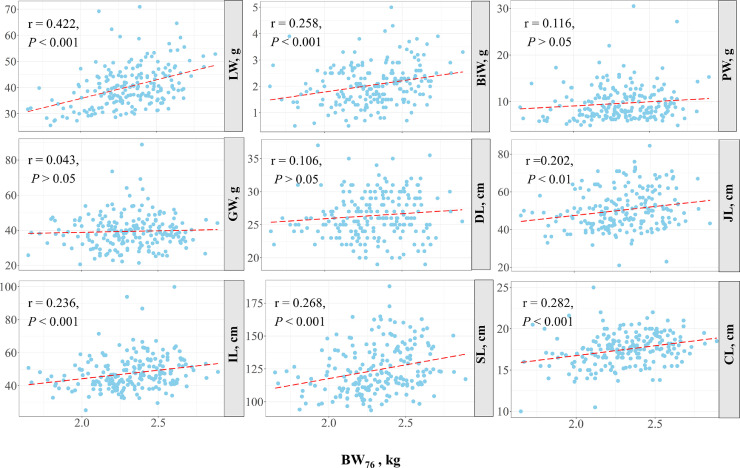
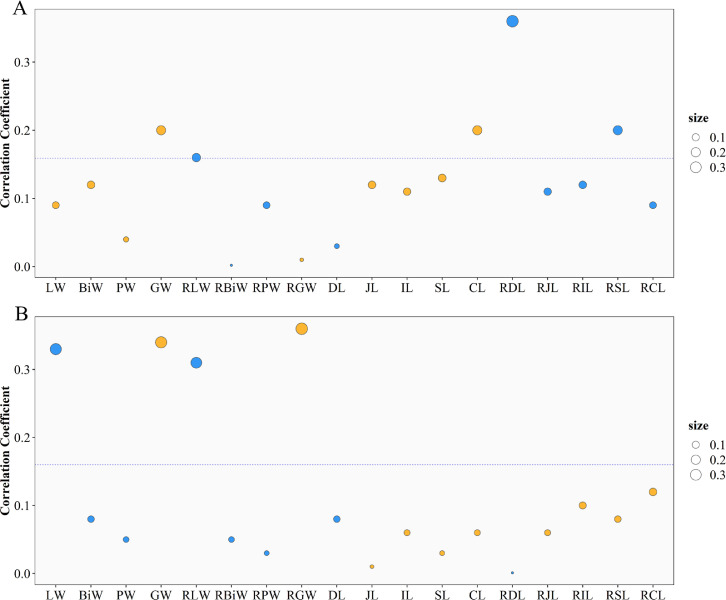
The correlations between body weight at market age and the characteristics of digestive organs are shown in Figure 1. Most of the digestive organ characteristics, including LW, BiW, JL, IL, SL, and CL, had a significant (P < 0.05) positive correlation with the BW76. These results indicate that the characteristics of digestive organs are influenced by body weight. Thus, we used the relative characteristics (which corrected for the effect of body weight) in the following analysis. The phenotypic correlation between the FCR and RFI was moderate and positive (0.34, P < 0.01). The phenotypic correlation coefficients between the FCR and digestive organ characteristics are shown in Figure 2A and Supplementary Table S1. The FCR was significantly positively correlated with the GLW, RDL, and RSL (0.16, 0.36, and 0.20, respectively, P < 0.05). In contrast, the correlation between the FCR and GW was significant and negative (−0.20, P < 0.05). A significant negative correlation was also found between the FCR and CL (-0.20, P < 0.05). As shown in Figure 2B, the RFI was positively correlated with the LW and RLW (0.33 and 0.31, respectively, P < 0.01) and negatively correlated with the GW and RGW (−0.34 and −0.36, respectively, P < 0.01). These results indicate that the development of the digestive organs was significantly associated with feed efficiency.
Figure 1.
Correlation scatter plot of the association between body weight and digestive organs traits at 76 days of age. The red dotted lines in each plot represent the linear regression. The r and P indicate Pearson correlation coefficients and P value, respectively. BW76 = body weight at 76 days of age (kg); LW, BiW, PW and GW = the weight of liver, bile, proventriculus, and gizzard, respectively; DL, JL, IL, SL and CL = the length of duodenum, jejunum, ileum, small intestine and cecum, respectively.
Figure 2.
Correlations between feed efficiency and digestive organ characteristics. (A) Pearson correlation coefficients between the FCR and digestive organ characteristics. (B) Pearson correlation coefficients between the RFI and digestive organ characteristics. For both A and B, the dashed line indicates the significance threshold (P = 0.05). Each point represents a digestive organ characteristic. The orange and sky-blue points indicate negative and positive correlations, respectively, between feed efficiency and digestive organ characteristics. The size of each point indicates the absolute value of the Pearson correlation coefficients. Abbreviations: FCR, feed conversion ratio; RFI, residual feed intake.
Phenotypic Differences Between Broilers With the Highest and Lowest Feed Efficiencies
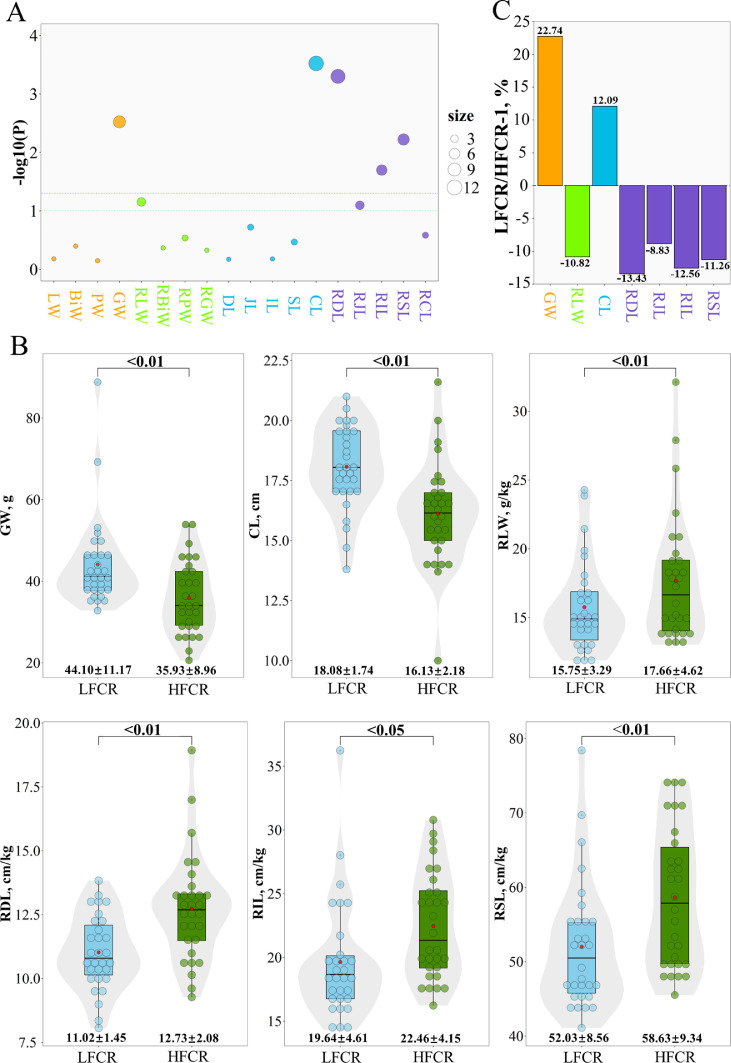
The highest 15% of FCR-ranked and RFI-ranked chickens had 9 (30%) replicates in the two-tailed t tests, while the lowest 15% of FCR-ranked and RFI-ranked chickens had 3 (10%) replicates. The differences in feed efficiency and digestive organ characteristics between the highest FCR (HFCR) and lowest FCR (LFCR) groups are shown in Figure 3. These groups obviously differed (P < 0.05) in the GW, CL, RDL, RIL, and RSL (Figure 3A). RLW and RJL showed a trend toward differing (P < 0.10) between the HFCR and LFCR groups. However, there were no differences (P > 0.05) in the LW, BW, PW, RBW, RPW, RGW, DL, JL, IL, SL, or RCL between the 2 groups. Regarding digestive organ characteristics (Figure 3B, Supplementary Table S2), we found that the GW (44.10 vs. 35.93 g) and CL (18.08 vs. 16.13 cm) were significantly higher in the LFCR group (P < 0.01), but the RLW (15.75 vs. 17.66 g/kg), RDL (11.02 vs. 12.73 cm/kg), RIL (19.64 vs. 22.46 cm/kg), RJL (21.37 vs. 23.44 cm/kg), and RSL (52.03 vs. 58.63 cm/kg) were obviously lower in the LFCR group (P < 0.05). In addition, GW and CL were 22.74% and 12.09% (Figure 3C) higher, respectively, in the LFCR group than in the HFCR group. The RLW, RDL, RJL, RIL, and RSL were 10.82, 13.43, 8.83, 12.56, and 11.26% lower, respectively, in the LFCR group than in the HFCR group.
Figure 3.
Comparisons of digestive organ characteristics between the chickens with the highest and lowest FCR values. (A) ANOVA for all digestive organ phenotypic differences between the chickens with the lowest and the highest FCR values. The green and purple dashed lines show trends toward significance (P = 0.10) and significance (P = 0.05), respectively. The orange, green, blue, and purple points represent the weight, relative weight, length, and relative length of digestive organs, respectively. The P values obtained from the significance test are plotted as log10(P). The size of the point indicates the absolute value of log2(P). (B) Relative difference in digestive organs between the chickens with the lowest and the highest FCR values. The orange, green, blue, and purple bars represent the weight, relative weight, length, and relative length of digestive organs, respectively. (C) Phenotypic differences in digestive organs between the chickens with the lowest and the highest FCR values. The red points in the center of the bars indicate the group means. Abbreviation: FCR, feed conversion ratio.
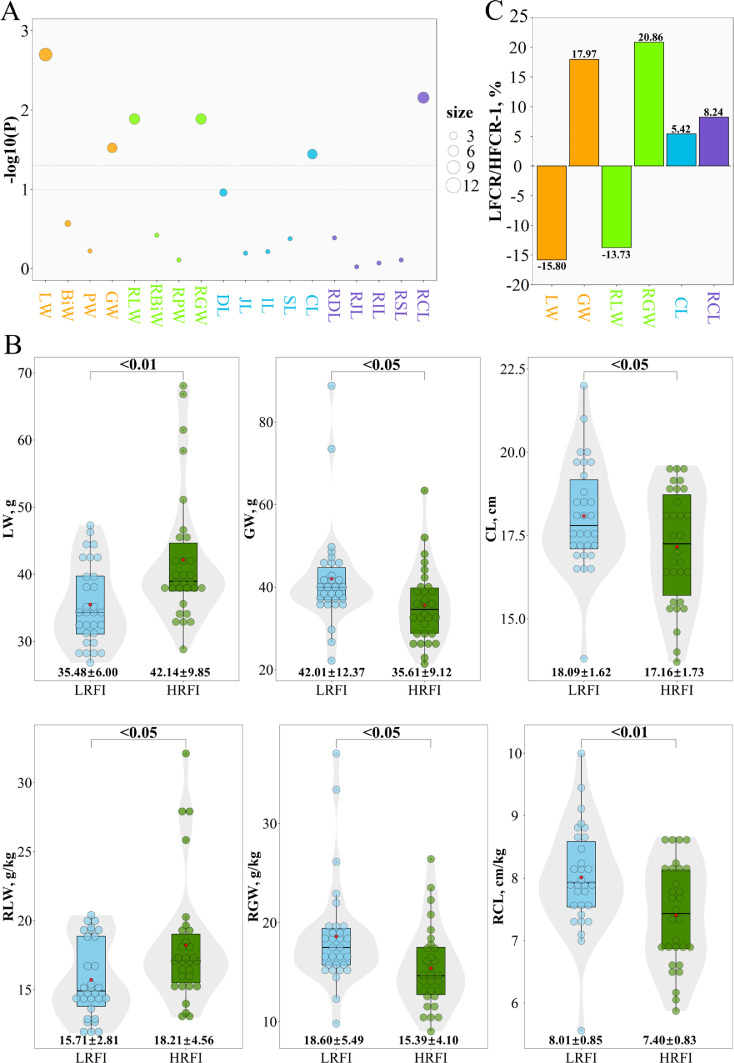
The differences in feed efficiency and digestive organ characteristics between the groups with the highest RFI (HRFI) and the lowest RFI (LRFI) are presented in Figure 4. The LW, GW, RLW, RGW, and CL significantly differed (P < 0.05) between the HRFI and LRFI groups (Figure 4A). Of the digestive organ characteristics (Figure 4B, Supplementary Table S3), the GW (42.01 vs. 35.61 g), RGW (18.60 vs. 15.39 g/kg), CL (18.09 vs. 17.16 cm), and RCL (8.01 vs. 7.40 cm/kg) were clearly higher (P < 0.05) in the LRFI group. However, the opposite pattern was observed in liver development; the LW (34.48 vs. 42.14 g) and RLW (15.71 vs. 18.21 g/kg) were obviously lower (P < 0.05) in the LRFI group. The GW, RGW, CL, and RCL were 17.97, 20.86, 5.42, and 8.24% higher, respectively, in the LRFI group than in the HRFI group (Figure 3C). The LW and RLW were 15.8% and 13.73% lower, respectively, in the LRFI group.
Figure 4.
Comparisons of digestive organ characteristics between the chickens with the highest and lowest RFI values. (A) ANOVA on all digestive organ phenotypic differences between the chickens with the lowest and the highest RFI values. The green and purple dashed lines show the significance threshold (P value of 0.10 and 0.05, respectively). The orange, green, blue, and purple points represent the weight, relative weight, length, and relative length of digestive organs, respectively. The P values obtained from the significance test are plotted as log10(P). The size of the point represents the absolute value of log2(P). (B) Relative difference in digestive organs between the chickens with the lowest and the highest RFI values. The orange, green, blue, and purple bars represent the weight, relative weight, length, and relative length of digestive organs, respectively. (C) Phenotypic difference in digestive organs between the chickens with the lowest and highest RFI values. The red points in the center of the bars indicate the group means.Abbreviation: RFI, residual feed intake.
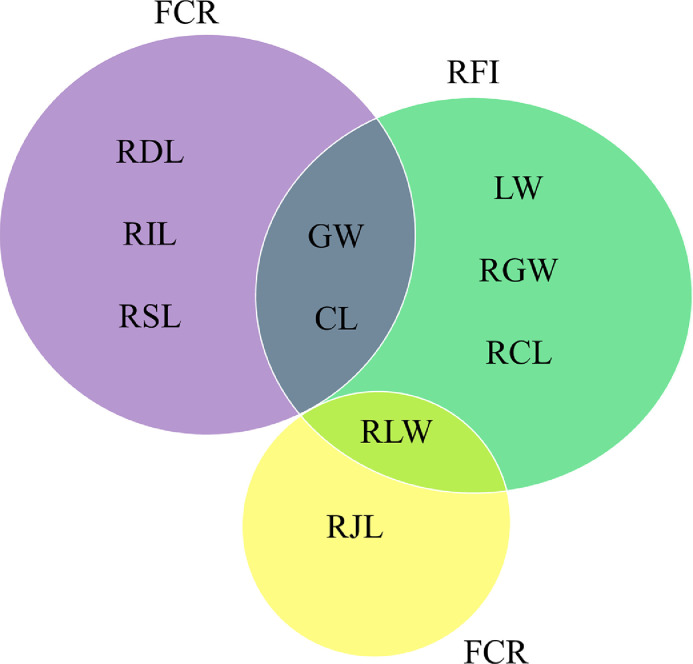
Shared Traits of Chickens With Extreme FCR and RFI Values
The above results showed that the correlations between the digestive organ characteristics and the 2 feed efficiency indexes differed. To investigate which digestive organs were most closely associated with feed efficiency, we performed an overlap analysis of the results of the two-tailed t tests. As shown in Figure 5, significant differences in the GW and CL were found between the chickens with the highest and the lowest feed efficiencies. In addition, the RLW displayed a trend toward significance when analyzing the FCR, so it was also included in the overlap analysis. We found that the gizzard, liver, and cecum may be related to feed efficiency in chickens based on the results from the phenotypic analysis of the digestive organs characteristics.
Figure 5.
Shared significant or potentially significant differences in digestive organ characteristics among individuals with different feed efficiency index values. The digestive organ characteristics in purple and green areas indicate a P value of < 0. 05. The digestive organ characteristics in the yellow area indicate a P value of < 0.10.
DISCUSSION
During the past few decades of genetic selection and improvements in nutritional management, the feed efficiency of chickens has dramatically improved. The FCR of commercial broilers is close to 1.5 or even lower (Zuidhof et al., 2014; Brameld and Parr, 2016; Sell-Kubiak et al., 2017). However, the FCR of the breed we used was 3.22. Worse feed efficiency is the major flaw of slow-growing chickens; thus, production of these chickens requires continued improvements in feed efficiency.
The major site for feed digestion and nutrient absorption is the digestive tract. Therefore, the functional and histological characteristics of digestive organs substantially influence feed efficiency (De Verdal et al., 2010; Tallentire et al., 2016). We found substantial phenotypic variation in the characteristics of the developing digestive organs, indicating that these characteristics have the potential to be improved. Digestive organ traits are possibly affected by genetic selection, especially when improving body weight is a breeding goal. The digestive organs have been subjected to many physical changes due to selection pression in broilers (Tallentire et al., 2016). A previous study found a strong negative correlation between BW and FCR in this population (Wen et al., 2018). In addition, our results found that BW was also positively correlated with LW, BiW, SL, and CL in terms of phenotype and weakly correlated with GW. Therefore, selection for higher BW can significantly increase these digestive traits and feed efficiency, but it had no distinct effect on GW. However, feed efficiency is not regarded as the major breeding goal of this population in our study, so in future work selected populations in this regard could be used to test the generalizability of our results.
Both the FCR and RFI are reverse indicators, in that a lower value indicates a higher feed efficiency. In this study, we found that both the FCR and RFI were negatively correlated with the GW and that larger gizzards were significantly associated with better feed efficiency. The development of gizzards is associated with feed efficiency amelioration and numerous previous studies have indicated that chickens selected for high feed efficiency exhibit larger gizzards (higher weight and relative weight) (Péron et al. 2006; Rougiere et al., 2009; De Verdal et al., 2010; Juanchich et al., 2020). This phenomenon is more pronounced if the feed is a difficult-to-digest grain (Péron et al., 2007; Rougiere et al., 2009). The gizzard is a crucial place for the physical processing of feed in chickens. In general, a well-developed gizzard is connected with mighty shrinkage of the muscular layers and retention duration of chyme (Rougière and Carré, 2010; Rougiere et al., 2012), which improves feed efficiency by increasing muscular control and not only ensures the complete trituration of the feed but also increases intestinal fluidity, promoting the mixing of the chyme with digestive enzymes. These findings suggest that the processes of extrusion and trituration in the gizzard may be key to improving feed efficiency in broilers.
In chickens, the liver is the largest gland organ of the digestive system; it synthesizes bile involved in activating pancreatic lipase and promoting the digestion of feed and the absorption of starch, lipids, and fat-soluble vitamins in the intestine (Arab et al., 2017). In this study, the FCR and RFI were both significant and positively correlated with the RLW, which implies that greater feed efficiency may reduce the relative weight of the liver. An obviously lower LW was observed in the LRFI group, and a similar trend in liver development was also observed when using the FCR as a feed efficiency indicator. Nevertheless, the liver weight was susceptible to body weight; thus, there was no obvious difference in liver weight in the FCR two-tailed test. These results are consistent with previous studies on the digestive organs of high- and low-feed-efficiency broilers at 23 d of age (Verdal et al., 2011). The liver of low-feed-efficiency chickens may compensate for deficiencies in gizzard digestion by enhancing digestion and absorption in the small intestine (Rideau et al., 2014).
Uniquely, the poultry digestive tract (except that of pigeons) contains a pair of outpocketings was called cecum that is located at the junction of the small intestine and rectum (Svihus, 2014). In the current study, a strong negative correlation was observed between the FCR and CL: chickens with the highest feed efficiency had longer CLs than those with the lowest feed efficiency. Unlike the rapid peristalsis in the small intestine, the cecum infrequently empties; thus, the contents of the cecum, the main site of microbial fermentation to break down and absorb nondigestible nutrients, are retained for 24 h or longer before excretion (Warriss et al., 2004; Feye et al., 2020). Therefore, a longer cecum may enhance feed efficiency by increasing the duration of chyme fermentation and digestion by cecal microbiota.
Turning to the other digestive organs, we found a stronger correlation between the FCR and SL than between the RFI and SL in high- and low-feed-efficiency chickens, but there was no obvious difference in the length of each small intestine segment. Additionally, there was no apparent difference in the SL despite the heaver body weight of the LFCR chickens. Thus, selection for LFCR chickens may not affect the SL but may significantly reduce the relative length of the small intestine. This finding aligns with the results of other studies that found differences in the digestive organs of chickens that varied in feed efficiency (Juanchich et al., 2020). The correlations between feed efficiency and the PW and BiW in chickens was relatively weak. However, this does not mean that the function of these digestive organs is less important to feed digestive efficiency, as they may influence feed efficiency through other physiological and biochemical mechanisms, such as enzymatic activity, height and density of intestinal villi, and microbial fermentation.
In conclusion, digestive organ characteristics had different degrees of relationship to the feed efficiency. Among those, GW and RLW had correlated fairly well with the feed efficiency of chicken. Broilers with better feed efficiency had significantly higher GW, lower RLW and longer CL than those with worse feed efficiency. These results indicate that gizzard, liver, and cecum development have a substantial influence on chicken feed efficiency.
ACKNOWLEDGMENTS
We thank Guangdong Wen's Nanfang Poultry Breeding, Co., Ltd. for their assistance in the phenotype collection. This work was supported by the National Natural Science Foundation of China (32102535 and 31930105) and the National Key Research and Development Program of China (2021YFD1200803).
DISCLOSURES
The authors declare that they have no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.102184.
Appendix. Supplementary materials
Table S1: Pearson correlations between the feed efficiency and digestive organ characteristics. Table S2. Mean (±SD) for general traits, and digestive organs traits comparisons between the highest and lowest FCR chickens. Table S3. Mean (±SD) for general traits, and digestive organs traits comparisons between the highest and lowest RFI chickens.
References
- OECD-FAO Agricultural Outlook 2021-2030 | OECD-FAO Agricultural Outlook | OECD iLibrary. 2022.
- Aggrey S.E., Karnuah A.B., Sebastian B., Anthony N.B. Genetic properties of feed efficiency parameters in meat-type chickens. Genet. Sel. Evol. 2010;42:25. doi: 10.1186/1297-9686-42-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab J.P., Karpen S.J., Dawson P.A., Arrese M., Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology. 2017;65:350–362. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brameld J.M., Parr T. Improving efficiency in meat production. Proc. Nutr. Soc. 2016;75:242–246. doi: 10.1017/S0029665116000161. [DOI] [PubMed] [Google Scholar]
- De Verdal H., Mignon-Grasteau S., Jeulin C., Bihan-Duval E.Le, Leconte M., Mallet S., Martin C., Narcy A. Digestive tract measurements and histological adaptation in broiler lines divergently selected for digestive efficiency. Poult. Sci. 2010;89:1955. doi: 10.3382/ps.2010-813. [DOI] [PubMed] [Google Scholar]
- Feye K.M., Baxter M., Tellez-Isaias G., Kogut M.H., Ricke S.C. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020;99:653–659. doi: 10.1016/j.psj.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess C.W., Byerly T.C., Jull M.A. The efficiency of feed utilization by barred plymouth rock and crossbred broilers. Poult. Sci. 1941;20:210–216. [Google Scholar]
- Howie J.A., Tolkamp B.J., Avendano S., Kyriazakis I. The structure of feeding behavior in commercial broiler lines selected for different growth rates. Poult. Sci. 2009;88:1143–1150. doi: 10.3382/ps.2008-00441. [DOI] [PubMed] [Google Scholar]
- Inês R., Mingan C. The foregut and its manipulation via feeding practices in the chicken. Poult. Sci. 2018;97:3188–3206. doi: 10.3382/ps/pey191. [DOI] [PubMed] [Google Scholar]
- Juanchich A., Urvoix S., Hennequet-Antier C., Narcy A., Mignon-Grasteau S. Phenotypic timeline of gastro-intestinal tract development in broilers divergently selected for digestive efficiency. Poult. Sci. 2020;100:1205–1212. doi: 10.1016/j.psj.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R.M., Swiger L.A., Chambers D., Gregory K.E. Efficiency of feed use in beef cattle. J. Anim. Sci. 1963;22:486–494. [Google Scholar]
- Li G., Zhao Y., Purswell J.L., Magee C. Effects of feeder space on broiler feeding behaviors. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péron A., Svihus B., Gabriel I., Bérot S., Tanguy D., Bouchet B., Gomez J., Carré B. Effects of two wheat cultivars on physico-chemical properties of wheat flours and digesta from two broiler chicken lines (D+ and D-) differing in digestion capacity. Br. Poult. Sci. 2007;48:370–380. doi: 10.1080/00071660701341963. [DOI] [PubMed] [Google Scholar]
- Péron A., Gomez J., Mignon-Grasteau S., Sellier N., Carré B. Effects of wheat quality on digestion differ between the D+ and D- chicken lines selected for divergent digestion capacity. Poult. Sci. 2006;85:462–469. doi: 10.1093/ps/85.3.462. [DOI] [PubMed] [Google Scholar]
- Rideau N., Godet E., Combemorel C., Chaudeau M., Carre B., Mignon-Grasteau S. The gastric isthmus from D+ and D- broiler lines divergently selected for digestion efficiency shows histological and morphological differences. Poult. Sci. 2014;93:1245–1250. doi: 10.3382/ps.2013-03756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougiere N., Malbert C.H., Rideau N., Cognie J., Carre B. Comparison of gizzard activity between chickens from genetic D+ and D- lines selected for divergent digestion efficiency. Poult. Sci. 2012;91:460–467. doi: 10.3382/ps.2011-01494. [DOI] [PubMed] [Google Scholar]
- Rougiere N., Gomez J., Mignon-Grasteau S., Carre B. Effects of diet particle size on digestive parameters in D+ and D- genetic chicken lines selected for divergent digestion efficiency. Poult. Sci. 2009;88:1206–1215. doi: 10.3382/ps.2008-00408. [DOI] [PubMed] [Google Scholar]
- Rougière N., Carré B. Comparison of gastrointestinal transit times between chickens from D + and D- genetic lines selected for divergent digestion efficiency. Animal. 2010;4:1861–1872. doi: 10.1017/S1751731110001266. [DOI] [PubMed] [Google Scholar]
- Schmidt C.J., Persia M.E., Feierstein E., Kingham B., Saylor W.W. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poult. Sci. 2009;88:2610–2619. doi: 10.3382/ps.2009-00055. [DOI] [PubMed] [Google Scholar]
- Sell-Kubiak E., Wimmers K., Reyer H., Szwaczkowski T. Genetic aspects of feed efficiency and reduction of environmental footprint in broilers: a review. J. Appl. Genet. 2017;58:487–498. doi: 10.1007/s13353-017-0392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel P.B. Evolution of the modern broiler and feed efficiency. Annu. Rev. Anim. Biosci. 2014;2:375–385. doi: 10.1146/annurev-animal-022513-114132. [DOI] [PubMed] [Google Scholar]
- Svihus B. Function of the digestive system. J. Appl. Poult. Res. 2014;23:306–314. [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Breeding for efficiency in the broiler chicken: a review. Agron. Sustain. Dev. 2016;36:1–16. [Google Scholar]
- Uni Z., Noy Y., Sklan D. Posthatch development of small intestinal function in the poult. Poult. Sci. 1999;78:215–222. doi: 10.1093/ps/78.2.215. [DOI] [PubMed] [Google Scholar]
- Verdal H.D., Narcy A., Bastianelli D., Chapuis H., Même N., Urvoix S., Bihan-Duval E.L., Mignon-Grasteau S. Improving the efficiency of feed utilization in poultry by selection. 1. Genetic parameters of anatomy of the gastro-intestinal tract and digestive efficiency. BMC. Genet. 2011;12:1–9. doi: 10.1186/1471-2156-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgsholm I., Arzoomand N.S., Boqvist S. Food security, safety, and sustainability—getting the trade-offs right. Front. Sustain. Food Syst. 2020;4:16. [Google Scholar]
- Warriss P.D., Wilkins L.J., Brown S.N., Phillips A.J., Allen V. Defaecation and weight of the gastrointestinal tract contents after feed and water withdrawal in broilers. Br. Poult. Sci. 2004;45:61–66. doi: 10.1080/0007166041668879. [DOI] [PubMed] [Google Scholar]
- Wen C., Yan W., Sun C., Ji C., Zhou Q., Zhang D., Zheng J., Yang N. The gut microbiota is largely independent of host genetics in regulating fat deposition in chickens. ISME. J. 2019;13:1422–1436. doi: 10.1038/s41396-019-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen C., Yan W., Zheng J., Ji C., Zhang D., Sun C., Ning Y. Feed efficiency measures and their relationships with production and meat quality traits in slower growing broilers. Poult. Sci. 2018;97:2356–2364. doi: 10.3382/ps/pey062. [DOI] [PubMed] [Google Scholar]
- Yan W., Sun C., Wen C., Ji C., Zhang D., Yang N. Relationships between feeding behaviors and performance traits in slow-growing yellow broilers. Poult. Sci. 2018;98:548–555. doi: 10.3382/ps/pey424. [DOI] [PubMed] [Google Scholar]
- Yi G., Yuan J., Bi H., Yan W., Yang N., Qu L. In-depth duodenal transcriptome survey in chickens with divergent feed efficiency using RNA-seq. PloS. One. 2015;10 doi: 10.1371/journal.pone.0136765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampiga M., Calini F., Sirri F. Importance of feed efficiency for sustainable intensification of chicken meat production: implications and role for amino acids, feed enzymes and organic trace minerals. Worlds Poult. Sci. J. 2021;77:639–659. [Google Scholar]
- Zhu F., Gao Y., Lin F., Hao J., Yang F., Hou Z. Systematic analysis of feeding behaviors and their effects on feed efficiency in Pekin ducks. J. Anim. Sci. Biotechno. 2018;8:1–9. doi: 10.1186/s40104-017-0212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Pearson correlations between the feed efficiency and digestive organ characteristics. Table S2. Mean (±SD) for general traits, and digestive organs traits comparisons between the highest and lowest FCR chickens. Table S3. Mean (±SD) for general traits, and digestive organs traits comparisons between the highest and lowest RFI chickens.