Abstract
Summary: Opioid use and misuse in the United States has been at epidemic proportions and is predicted to increase further in the setting of the Coronavirus disease 19 pandemic. Acute kidney injury is a condition associated with significant morbidity and increased mortality. We review the literature on the effect of opioids on kidney function and critically examine the association between opioid use and acute kidney injury and identify at-risk populations in whom opioids should be used with caution. We also discuss the role of biomarkers in elucidating this condition and propose preventive measures, novel therapeutic options, and research directions.
Keywords: Acute kidney injury, opioid use, Covid 19, biomarkers
Opioids are a class of drug that bind to specific receptors in the central and peripheral nervous systems and interrupt the transmission of pain signals via second messenger G-protein pathways. Opioids can be classified as endogenous and exogenous (natural, semisynthetic, and synthetic).1 Binding at the receptor may result in agonistic, partial agonistic, or antagonistic outcomes. (Table 1 ). The three central nervous system opioid receptors are μ, κ, δ; in addition, opioid receptors are found in the peripheral nervous system as well as various organs.2 The opioid receptors can affect complex outcomes, based on the ligand, location of the receptor, and the end-organ or system it influences. For example, opioids can enhance the parasympathetic effect of the vagus nerve, which in the myocardium can result in bradycardia and a decrease in cardiac inotropy.3 , 4 When central opioid receptors are activated leading to increased renal sympathetic nerve effect, it leads to vasoconstriction and renal ischemia. This renal ischemia promotes further sympathetic activity, and if not resolved can lead to ischemic acute kidney injury (AKI). Renal ischemia can occur with morphine, owing to such increased renal sympathetic activation, and can be decreased with naloxone (a central opioid antagonist).5
Table 1.
Classification, Potency, and Characteristics of Opioids and Opioid Antagonists
| Opioid | Type | Morphine 10 mg SC Equivalence | Comments |
|---|---|---|---|
| Naturally derived | |||
| Codeine | Agonist | 60 orally | Prodrug of morphine |
| Morphine | Agonist | 10 SC or IM | |
| Semisynthetic | |||
| Hydrocodone | Agonist | 1 IM, 2 orally | |
| Hydromorphone | Agonist | 1.3 SC | |
| Oxycodone | Agonist | 5 orally | |
| Oxymorphone | Agonist | 1 SC | |
| Naloxone | Antagonist | Nonanalgesic | Short-acting antagonist (0.5 h) |
| Naltrexone | Antagonist | Nonanalgesic | Very-long-acting antagonist (24 h) |
| Buprenorphine | Partial agonist | 0.3 IM | Medication-assisted therapy requires 6-16 mg/d (contains naloxone) |
| Synthetic | |||
| Fentanyl | Agonist | 0.75 IM | Very short acting (<1 h) |
| Loperamide | Agonist | Nonanalgesic | Antidiarrheal, abuse, P-glycoprotein substrate |
| Meperidine | Agonist | 75 SC or IM | Seizures caused by metabolite accumulation |
| Methadone | Agonist | 10 IM | Very long acting (24 h) |
| Tramadol | Agonist | 50-100 orally | Seizures possible with therapeutic dosing |
| Methylnaltrexone | Antagonist | Nonanalgesic | Peripherally acting antagonist; reverses opioid-induced constipation |
IM, intramuscularly; SC, subcutaneously.
Goldfrank's Toxicologic Emergencies 11e, Nelson et al 2019.
TRENDS IN OPIOID USE AND ABUSE
In 2008, Manchikanti and Singh6 noted that although Americans comprise 4.6% of the world's population, they consume 80% of the world's opioid supply, most notably 99% of the hydrocodone supply. Outside of the prescribed use of opioids, their euphoric effect leads to abuse of the drug with unfortunate consequences.76 From 2000 to 2014, the Centers for Disease Control reported that nearly half a million people in the United States died from opioid overdose.7 Reports also have suggested increases in local, national, and worldwide abuse of opioids over the past 2 to 3 decades.8 , 9 Opioid abuse was reported to be increasing nationally, and across several age groups including pediatric and young adults.10, 11, 12 In the United States, opioid overdose, intensive care unit admission, and deaths between 2001 and 2015 have increased significantly.13 Intensive care unit admissions for opioid overdose have a high in-hospital mortality rate.14 In 2018, the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act, also known as the SUPPORT for Patients and Communities Act, was passed15 to help end the opioid crises via continuing medical education for best pain management practices, prescribing limits, prescription drug monitoring programs, and systematic support for recovery from addiction. Systematic support is comprised of medication-assisted recovery, monitoring outlier prescribers of opioids to Medicare part D based on geography, and supporting communities of recovery, which also helps with housing. Since then, data have shown a decrease in opioid use,16 however, the Coronavirus disease 19 pandemic is predicted to diminish or reverse system-based gains.17 Opioid use has increased in ventilated patients and those in hospice, leading to temporary fentanyl shortages of titratable agents during the Coronavirus disease 19 pandemic.18 , 19
EPIDEMIOLOGY OF AKI
AKI is defined as any of the following: an increase in serum creatinine level by 0.3 mg/dL within 48 hours; or an increase in serum creatinine level 1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or urine volume less than 0.5 mL/kg per hour for 6 hours.20 A large US population (n = 3,787,410) study estimated that the community-based incidence of non–renal replacement therapy (RRT)-requiring AKI and RRT-requiring AKI were 384.1 and 24.4 per 100,000 persons-years, respectively.21 AKI increasingly is recognized as an important contributor to morbidity, mortality, and economic loss worldwide22; the estimated unadjusted mortality associated with an episode of AKI recently was estimated at 23.9% in adults and 13.8% in children.23
EPIDEMIOLOGY AND ASSOCIATION OF AKI WITH OPIOID USE
Adverse effects of opioids include nausea, constipation, and urinary retention, which can contribute to the development of AKI. In addition, the pitfalls of inappropriate opioid dosage in the setting of impaired renal function is well documented.24 , 25 Despite these adverse effects, the incidence of AKI stemming from opioids is unclear.
The true incidence of AKI from opioid use likely is under-reported because only hospitalized events are known. Most reports that draw a clear association between opioids and AKI are related to heroin, which is a Schedule I illicit drug in the United States.26 In patients with illicit drug use leading to rhabdomyolysis, 46% developed AKI with a 3.4% mortality rate.27 The association between AKI and prescription opioids is less clear. Intriguingly, in a large prospective cohort study, the lifetime opioid use in people with chronic kidney disease (CKD) was associated with reduced estimated glomerular filtration rate (GFR) and albuminuria, but not with the rapid kidney function decline of AKI.28 On the other hand, another study involving 55,747 elderly individuals (mean age, 82.14 y) from New Zealand identified opioids as a risk factor for AKI.29
Fassio et al16 noted that after the implementation of the opioid safety initiative, opioid use decreased; however, patients continued to be at greater risk of adverse events with opioids, including for AKI, even when compared with nonsteroidal anti-inflammatory (NSAID) use. During the study, the investigators noted a decrease in opioid use incidence rates, whereas NSAID use stayed the same. Despite this, AKI, cardiovascular events, and all-cause mortality were higher with opioid use than with NSAID use.
The incidence of AKI from urinary retention is not well defined and observational reports have suggested 10% of urinary retention cases may be owing to medications such as opioids.30 In the elderly and in patients with renal dysfunction, most opioids, except buprenorphine, have a longer half-life of the active drug and metabolites.31 Elderly patients additionally can be at greater risk for AKI because of comorbid conditions such as benign prostatic hypertrophy.
EFFECTS OF OPIOIDS AND MECHANISM OF AKI
Although opioids may be used safely for pain control and anesthesia, inappropriate use with higher doses, the presence of toxins, drug interactions, and other conditions such as dehydration, chronic liver or kidney dysfunction, or benign prostatic hypertrophy may increase the risk of AKI. Opioid use may result in AKI owing to systemic effects such as dehydration and hypotension, with resultant ischemic-reperfusion injury and free radical generation. Other possible mechanisms include rhabdomyolysis and urinary retention. Administration methods such as intravenous drug use also may result in the spread of hepatitis B and C and human immunodeficiency virus (HIV). These chronic viral infections can result in kidney injury over varying periods of time with AKI or CKD. Opioids can exert direct cellular effects such as renal lipidosis32 as noted on kidney biopsy specimens of patients on methadone. However, many of these cases include confounding factors, such as hepatitis B and C infections.33 Opioids also can induce AKI in susceptible individuals via indirect mechanisms such as modulation of the parasympathetic and sympathetic nervous systems, renin-angiotensin-aldosterone system (RAAS), and the antidiuretic hormone (ADH) axis as discussed later.
PRERENAL ISCHEMIA AND REPERFUSION INJURY
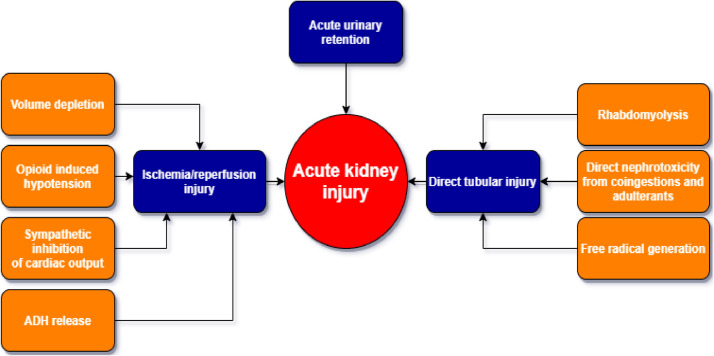
Dehydration, changes in parasympathetic and sympathetic activation, and polypharmacy can result in ischemic AKI and subsequent reperfusion injury. (Fig. 1 ). Recurrent ischemia can result in AKI that may be irreversible and progress to CKD.
Figure 1.
How opioids cause acute kidney injury.
Dehydration
Dehydration resulting from opioid-induced changes in mentation or nausea can result in dehydration and ischemic AKI. Another less well-known contributor to opioid-related dehydration is opioid effects on human chemosensory function, especially olfactory perception. Limited data have shown that use of opioids reduced olfactory perception by affecting the trigeminal ganglia nuclei.34 Olfactory input contributes largely to taste perception, and opioid users report alterations in taste.35 This in turn leads to dehydration from reduced dietary intake.
Enhanced Parasympathetic Effect and Reduced Sympathetic Effect
Activation of opioid peptide receptors can inhibit myocardial excitation-contraction coupling. In an animal study, stimulation of peripheral opioid peptide receptors with a synthetic μ opioid agonist in normotensive animals resulted in a prolonged decrease in arterial pressure.36 Although the findings of cardiac endorphin effects in decreasing myocardial contractility and attenuating the sympathetic response are relatively new, earlier work has shown similar effects with opioid anesthetics; it was noted that renal blood flow was lower with the opioid fentanyl when compared with other anesthetics such as ketamine.37 Usually, in the range of renal autoregulation with intact renal innervation, renal blood flow is a reflection of cardiac output. The decrease in sympathetic input from fentanyl resulted in a decrease in cardiac output and renal blood flow with a compensatory increase in plasma renin.
Combining opioids with benzodiazepines can decrease cardiac function with bradycardia and vasodilation, further leading to orthostatic hypotension, hypotension, and syncope.38 Overall, opioids increase parasympathetic nervous system effects, resulting in a decrease in heart rate and mean arterial pressure (MAP). Systemic hypotension impairs renal hemodynamics when renal arterial pressure decreases to less than 70 mm Hg, and glomerular filtration ceases when the renal arterial blood pressure decreases to less than 50 mm Hg.39
The kidney is sensitive to ischemia-reperfusion injury, albeit to a lesser degree than the brain or heart.40 Oxygen levels decrease across a gradient starting at the renal cortex and going deeper toward the medulla. Correspondingly, renal cortical cells are more affected by ischemia than renal medulla cells. The outer medullary cells have the capacity to switch to anaerobic metabolism in the setting of hypoxia, and the inner medulla uses anaerobic glycolysis.41 In the native kidney, ischemia-reperfusion injury presents as AKI, and in the transplanted kidney as early graft failure.42
Renal ischemia, commonly called prerenal, traditionally has been considered to be a reversible cause of AKI, and rehydration leads to improvement in GFR. However, recurrent dehydration can cause AKI to progress to CKD, as noted in the kidney injury model seen in Meso-American nephropathy, an entity reported among farm workers in the setting of repeated dehydration and AKI that led to CKD. Further contributing to the pathology are chronically activated vasopressin, RAAS, and polyol-fructokinase pathways in the kidney, along with subclinical rhabdomyolysis and nephrotoxin exposure.43 Kidney biopsy specimens in patients with Meso-American nephropathy have glomerular ischemia, glomerulosclerosis, tubular atrophy, interstitial fibrosis, and progressive renal failure.44 Although the exact mechanism is yet to be determined, urinary tubular markers neutrophil gelatinase–associated lipocalin and interleukin 18 are increased.45
Free Radical Generation
Oxidative stress results in the formation of free radicals, which can damage cells. Antioxidant enzymes and substances such as superoxide dismutase, glutathione peroxidase, catalase, and glutathione help with redox processes that combat the damage caused by oxidative stress. Opioids can contribute to oxidative stress either by increasing the production of free radicals or decreasing antioxidant function. Morphine demonstrably can cause oxidative stress with the production of free radicals and a decrease in the levels of anti-oxidants.46, 47, 48 In an in vivo model, mice dosed with morphine had increased albuminuria after 72 hours with effacement of podocyte foot processes; in vitro examination of human podocytes showed that morphine decreased the slit diaphragm constituting molecules such as nephrin, podocin, and cluster of differentiation-2–associated protein; this suggested podocyte injury as a possible mechanism for morphine to alter the glomerular filtration barrier.49
Rhabdomyolysis
Rhabdomyolysis is a well-delineated cause of AKI and its pathophysiologic mechanisms are renal vasoconstriction from dehydration, intraluminal cast formation from myoglobin precipitation, and direct heme-protein–induced cytotoxicity. In rhabdomyolysis, AKI with acute urate nephropathy is related to the high rates of generation and urinary excretion of uric acid and direct nephrotoxicity of free iron released from myoglobin.50 Opioids have been reported to cause rhabdomyolysis in excessive doses,51 but the exact causative mechanism is unclear.
Urinary Retention
Urinary retention can lead to renal injury by way of backward flow pressure resulting in hydronephrosis and irreversible renal scarring. Rawal et al52 performed urodynamic studies on 30 male volunteers aged 20 to 28 years after administration of epidural, intramuscular, and intravenous morphine and found that irrespective of dose, all subjects receiving epidural morphine showed marked relaxation of the detrusor muscle and a corresponding increase in the maximal bladder capacity leading to urinary retention, whereas intramuscular and intravenous morphine had minimal effect. The presence of opiate receptors in the micturition center in the pons and the urinary bladder may contribute to this effect. Relief of bladder pressure also may contribute to sequelae such as postobstructive diuresis and electrolyte derangements.53
Effect on RAAS and ADH Systems
RAAS responds primarily to changes in mean arterial pressure, which may explain the complexity of the overall effects of opioids on the RAAS. Opioids increase thirst after being administered for pain control and with reduced urine flow.54 Animal research has indicated that opioids partly function in the hypothalamic thirst center, which can result in alterations in thirst sensation.55 Limited data suggest that morphine results in nonosmotic ADH release.56 , 57 Rats nephrectomized before administration of morphine no longer show morphine-induced water intake, pointing to involvement of the kidneys in the dipsogenic effect of morphine. Rats who are renin-depleted via clipping of one renal artery and then removal of the clipped kidney a month later showed an opioid-induced water intake linearly related to the basal plasma renin level, an effect not seen in rats with normal renin levels. Interestingly, captopril, an inhibitor of the angiotensin-converting enzyme that increases circulating renin and angiotensin I levels, increased morphine-induced water intake, whereas the competitive antagonist of angiotensin II, saralasin, had no such effect. These results suggest a permissive interaction between morphine and the RAAS at the circulating angiotensin I or renin level, with the dipsogenic effect of morphine dependent on an intact RAAS.58
PREVENTION AND TREATMENT OF AKI IN THE SETTING OF OPIOID USE
Hydration
Early hydration is essential to limit vasoconstriction, which is a physiologic response to maintain adequate MAP, usually at the expense of a decrease in GFR. (Table 2) . Timely rehydration therapy increases MAP and reverses vasoconstriction, allowing GFR and renal tubular flow to return to normal, followed by an appropriate increase in urine output. Physicians should monitor both fluid intake and urine output. Patients on chronic opioids should be educated to maintain an adequate amount of urine by staying well hydrated. Patients with altered mentation or nausea require intravenous fluids. No clear evidence suggests optimal intravenous fluid doses, but prescribers should balance adequate MAP requirements while avoiding adverse events such as pulmonary edema. In rhabdomyolysis-induced AKI, animal studies suggest a prophylactic benefit to intravenous fluid with bicarbonate, but this benefit is less clear in human studies. A large study of patients with trauma and rhabdomyolysis comparing 0.9% sodium chloride intravenous solution with intravenous bicarbonate with mannitol found no significant differences between the groups.59
Table 2.
Methods to Prevent AKI When Using Opioids
| Intervention | Mechanism |
|---|---|
| Hydration | Improve renal perfusion |
| Naloxone | Sympathetic inhibition |
| HIF prolyl-hydroxylase inhibitors | Promote HIF-related mechanisms to protect against ischemia |
| TLR2 blockade | Inhibition of inflammatory response to ischemia |
| Thymoquinone | Reduce free radical generation |
| κ-opioid receptor agonist | Reduce free radical generation |
AKI, acute kidney injury; HIF, hypoxia-inducible factor; TLR2, Toll like receptor 2.
Monitoring and Preventing Urinary Retention in the Setting of Opioid Use
Time-limited perioperative use of opioids may cause transient renal function changes, and are managed by keeping patients well hydrated and closely monitoring urine output, frequently with a bladder catheter.
Meperidine, a long-acting opioid, has been shown to be an independent risk factor for urinary difficulties after elective cholecystectomy.60 Perioperative use of morphine and fentanyl reportedly decrease GFR and urine output, suggesting value to monitoring urine output in this setting.61
Medication Combinations That Could Increase the Risk of AKI
In the management of chronic pain, anti-inflammatory medications such as NSAIDs often are used before, or combined with, opioids. The combination of the two may increase the risk for AKI in a vulnerable population. NSAIDs decrease the effects of renal prostaglandin and could lead to AKI by causing renal tubular damage, interstitial nephritis, or nephrotic syndrome, while opioids may decrease MAP and cause urinary retention.62 Although the combination of these medications may be used safely, patients should be advised to be well hydrated and monitor adequate urine output, and have their renal function monitored regularly.
Drug Choice and Dosing
Opioid pharmacokinetics can be affected by renal function. A select few opioids are metabolized by the kidney, such as meperidine. Although many opioids are metabolized in the liver, clearance of the metabolites may be via the kidney, as in the case of morphine and codeine. When a prodrug such as codeine is metabolized, the drug and the intermediary metabolites can build up in the various compartments, resulting in unwanted side effects.
Rational dose adjustments of opioids is crucial to prevent adverse effects including AKI in high-risk populations. Few opioids are metabolized by the kidney, an example being meperidine. Although many opioids are metabolized in the liver, clearance of the metabolites may be via the kidney, as in the case of morphine and codeine. Certain opioids such as buprenorphine appear to be less dependent on kidney clearance, and have a lower risk of accumulation. These opioids would be the drug of choice for the elderly.
Future Therapeutics
Known blockers of harmful side effects could help reduce the kidney injury caused by opioids. Morphine triggers apoptosis of mesangial cells in both HIV transgenic mice and controls via the production of superoxide, and in the HIV mice the pro-apoptotic effect of morphine could be blocked by the protease inhibitor saquinavir via reduction of superoxide.63
In the mouse model, Liu et al64 found pretreatment with κ opioid–receptor agonist u50448h, used to prevent renal ischemia-reperfusion injury in cardiac myocytes and neuronal cells, significantly decreased apoptosis, as well as the serum levels of creatinine and blood urea nitrogen. A possible mechanism included the significantly increased superoxide dismutase activity and nitric oxide levels by activating the phosphatidylinositol 3-kinase and protein kinase B pathways, which may offer protection against renal ischemia-reperfusion injury.
Morphine, in addition to its analgesic function, has antiangiogenic effects.65 Hill et al66 noted in a mouse model that l-mimosine and dimethyloxalylglycine, molecules that activate hypoxia inducible factor (HIF) by the inhibition of HIF hydroxylases, helped protect the kidneys from ischemia-reperfusion injury. Possible pharmacologic activation of HIF may be an option to protect the kidney from ischemia. Ongoing clinical trials of HIF stabilizers for the treatment of anemia in CKD hopefully will shed light on this.
Toll-like receptors are receptors that help with recognizing pathogens and host substances released during injury. In the kidney, Toll-like receptor 2 messenger RNA from renal tubular cells increased with renal ischemia-reperfusion injury, and in the mouse model Toll-like receptor 2 blockade is a possible mechanism for prevention of ischemic kidney injury.67
Limited data have suggested that morphine could increase the generation of free radicals, with possible renal free radical injury, which may be prevented with thymoquinone; but no clinical evidence yet supports this.68
AKI Requiring RRT
Myoglobin is a large molecule that ideally should be filtered well with continuous RRT (CRRT) membranes. However, studies using CRRT for rhabdomyolysis are inconclusive about any benefit over conventional dialysis therapy for those with AKI resulting from rhabdomyolysis.47 Uncontrollable events such as filter clotting may result in variations in dialysis dose, which prevents finding any clear advantage of CRRT in this setting.69
DO BIOMARKERS OF AKI CHANGE WITH OPIOID USE?
The timely and accurate diagnosis of AKI with the use of serum creatinine has many pitfalls. The delay between the increase in serum creatinine and changes in GFR can impair accurate estimates of onset of injury.70 In addition, there is considerable variability among patients in the correlation between serum creatinine and baseline GFR, owing to differences in functional renal reserve and in creatinine synthesis rates.70 , 71 Creatinine kinetics also cannot pinpoint the etiology of AKI or site of injury (ie, glomeruli, interstitium, or tubules).
Identification of a specific biomarker of injury will be challenging given the multifaceted mechanisms of opioid-associated AKI. Few established biomarkers of kidney injury have been identified72 in the context of opioid-associated AKI (Table 3) .
Table 3.
Biomarkers in AKI
| Name (Abbreviation) | Known Inciting Factors | Proposed Functional Role in AKI |
|---|---|---|
| Neutrophil gelatinase–associated lipocalin (NGAL) | Ischemia, toxin | Protective: apoptosis inhibition |
| Kidney injury molecule 1 (KIM-1) | Ischemia-reperfusion | Protective: promotes removal of apoptotic and necrotic products |
| Interleukin 18 | Ischemia-reperfusion, toxin | Proinjury: inflammatory cytokine |
| Liver-type fatty acid–binding protein (L-FABP) | Ischemia, cardiac surgery | Protective: antioxidant |
| Angiotensinogen (Atg) | Cardiac surgery, others | Proinjury: RAAS activation |
| Tissue inhibitor of metalloproteinase-2 (TIMP-2) | Cardiac surgery, critical illness | Protective: unclear mechanisms |
| Insulin like growth factor binding protein 7 (IGFBP-7) | Critical illness | Protective: cell-cycle arrest |
AKI, acute kidney injury; RAAS, renin-angiotensin-aldosterone system.
Alge and Arthur.78
In an analysis of the National Health and Nutrition Examination Survey 2009 to 2010 involving 3,980 participants, prescription opioid use (β = 0.19; P = .002) was associated with higher urinary albumin-creatinine ratio values in comparison with NSAIDs (β = 0.17; P = .105).73
Are certain opioid medications better at preventing AKI? The evidence is unclear. Although retrospective human data in 130 patients during cardiopulmonary bypass showed remifentanil use did not increase the incidence of postoperative AKI after cardiac surgery, in the mouse model, a study showed lower levels of the AKI biomarker neutrophil gelatinase–associated lipocalin in the renal ischemia-reperfusion injury model with dexmedetomidine as compared with the group treated with remifentanil.74
Sakai et al75 conducted a retrospective study on 80 patients to clarify the effect of the use of remifentanil during cardiopulmonary bypass on the incidence of postoperative AKI and reported that the incidence of AKI was not significantly different in the remifentanil group and controls (51% versus 36%; P = .10).
Opioid-induced increases in central sympathetic tone may worsen AKI owing to renal ischemia. In a mouse model, naloxone blockade of central opioid receptors attenuated ischemic AKI through the inhibition of renal sympatho-excitation.5
Methadone is a long-acting weak opioid agonist with limited euphoric effects. Methadone was first used to treat opioid dependence in the mid-1960s, and was officially approved for this in 1972 by the US Food and Drug Administration. Methadone generally is considered safe in renal disease owing to a lack of renally cleared metabolites. Because a methadone prescription has significant restrictions, office-based treatment of opioid dependence uses the partial agonist buprenorphine, which has fewer regulatory controls.77
CONCLUSIONS
Opioid use and AKI are two rapidly emerging health issues. Opioids have direct and indirect interactions with the kidney that may impair renal function. However, the incidence of opioid-associated AKI remains under-reported and its mechanisms remain to be clearly elucidated. Injury-specific biomarker studies involving opioids will be illuminating. Novel therapeutic options for opioid-associated AKI are intriguing but glaringly lack randomized clinical data at this time. More research in this area is needed to address the knowledge gap in this critical unmet medical need.
Footnotes
Financial disclosure and conflict of interest statements: none.
REFERENCES
- 1.Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012;6:11–16. doi: 10.1177/2049463712438493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghelardini C, Di Cesare Mannelli L, Bianchi E. The pharmacological basis of opioids. Clin Cases Miner Bone Metab. 2015;12:219–221. doi: 10.11138/ccmbm/2015.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz JJ, Hsu AK, Gross GJ. Ischemic preconditioning and morphine-induced cardioprotection involve the delta (delta)-opioid receptor in the intact rat heart. J Mol Cell Cardiol. 1997;29:2187–2195. doi: 10.1006/jmcc.1997.0454. [DOI] [PubMed] [Google Scholar]
- 4.Pepe S, van den Brink OW, Lakatta EG, Xiao RP. Cross-talk of opioid peptide receptor and beta-adrenergic receptor signalling in the heart. Cardiovasc Res. 2004;63:414–422. doi: 10.1016/j.cardiores.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Mutoh J, Ohsawa M, Hisa H. Effect of naloxone on ischemic acute kidney injury in the mouse. Neuropharmacology. 2013;71:10–18. doi: 10.1016/j.neuropharm.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11:S63–S88. [PubMed] [Google Scholar]
- 7.2020 [cited 2020 January 2]. Increases in drug and opioid overdose deaths - United States, 2000-2014.https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6450a3.htm Available from: [Google Scholar]
- 8.Cerda M, Ransome Y, Keyes KM, et al. Prescription opioid mortality trends in New York City, 1990-2006: examining the emergence of an epidemic. Drug Alcohol Depend. 2013;132:53–62. doi: 10.1016/j.drugalcdep.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martins SS, Sampson L, Cerda M, Galea S. Worldwide prevalence and trends in unintentional drug overdose: a systematic review of the literature. Am J Public Health. 2015;105:e29–e49. doi: 10.2105/AJPH.2015.302843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havens JR, Young AM, Havens CE. Nonmedical prescription drug use in a nationally representative sample of adolescents: evidence of greater use among rural adolescents. Arch Pediatr Adolesc Med. 2011;165:250–255. doi: 10.1001/archpediatrics.2010.217. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez SH, Nelson LS. Prescription drug abuse: insight into the epidemic. Clin Pharmacol Ther. 2010;88:307–317. doi: 10.1038/clpt.2010.154. [DOI] [PubMed] [Google Scholar]
- 12.Ihongbe TO, Masho SW. Prevalence, correlates and patterns of heroin use among young adults in the United States. Addict Behav. 2016;63:74–81. doi: 10.1016/j.addbeh.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Parthvi R, Agrawal A, Khanijo S, Tsegaye A, Talwar A. Acute opiate overdose: an update on management strategies in emergency department and critical care unit. Am J Ther. 2019;26:e380–e3e7. doi: 10.1097/MJT.0000000000000681. [DOI] [PubMed] [Google Scholar]
- 14.Pfister GJ, Burkes RM, Guinn B, et al. Opioid overdose leading to intensive care unit admission: epidemiology and outcomes. J Crit Care. 2016;35:29–32. doi: 10.1016/j.jcrc.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Substance Use–Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act, H. R.6. 2018. [cited 2020 April 13]. Available from:https://www.finance.senate.gov/imo/media/doc/930%20AM%20Edits%2009.26.18% 20Final%20Opioid%20Sec-by-Sec%20BIPART%20BICAM.pdf.
- 16.Fassio V, Aspinall SL, Zhao X, et al. Trends in opioid and nonsteroidal anti-inflammatory use and adverse events. Am J Manag Care. 2018;24:e61–e72. [PubMed] [Google Scholar]
- 17.Becker WC, Fiellin DA. When epidemics collide: coronavirus disease 2019 (COVID-19) and the opioid crisis. Ann Intern Med. 2020;173:59–60. doi: 10.7326/M20-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.[cited 2020 June 25]. Available from: https://www.asahq.org/about-asa/newsroom/news-releases/2020/06/critical-care-drug-recommendations-for-covid-19-during-times-of-drug-shortages.
- 19.Lovell N, Maddocks M, Etkind SN, et al. Characteristics, symptom management and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manage. 2020;60:e77–e81. doi: 10.1016/j.jpainsymman.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease Improving Global Outcomes. KDIGO clinical practice guidelines on acute kidney injury. Kidney Int Suppl. 2020;2:8-12.
- 21.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li PK, Burdmann EA, Mehta RL. Acute kidney injury: global health alert. Kidney Int. 2013;83:372–376. doi: 10.1038/ki.2012.427. [DOI] [PubMed] [Google Scholar]
- 23.Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 25.Tomaszewski D, Balkota M, Truszczynski A, Machowicz A. Intrathecal morphine increases the incidence of urinary retention in orthopaedic patients under spinal anaesthesia. Anaesthesiol Intensive Ther. 2014;46:29–33. doi: 10.5603/AIT.2014.0006. [DOI] [PubMed] [Google Scholar]
- 26.Hossain MA, Mahida H, Haroon A, et al. 2017. [cited 2020 April 9]. Available from: https://www.journalmc.org/index.php/JMC/article/view/2898/2228. Heroin epidemic and acute kidney injury: an under-recognized but important consequence of opioid overdose. [Google Scholar]
- 27.Melli G, Chaudhry V, Cornblath DR. Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore) 2005;84:377–385. doi: 10.1097/01.md.0000188565.48918.41. [DOI] [PubMed] [Google Scholar]
- 28.Novick T, Liu Y, Alvanzo A, Zonderman AB, Evans MK, Crews DC. Lifetime cocaine and opiate use and chronic kidney disease. Am J Nephrol. 2016;44:447–453. doi: 10.1159/000452348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishtala PS, Chyou TY. Identifying drug combinations associated with acute kidney injury using association rules method. Pharmacoepidemiol Drug Saf. 2020;29:467–473. doi: 10.1002/pds.4960. [DOI] [PubMed] [Google Scholar]
- 30.Verhamme KM, Sturkenboom MC, Stricker BH, Bosch R. Drug-induced urinary retention: incidence, management and prevention. Drug Saf. 2008;31:373–388. doi: 10.2165/00002018-200831050-00002. [DOI] [PubMed] [Google Scholar]
- 31.Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 32.Yatsynovich Y, Souza D, Maroz N. Oxymorphone hydrochloride extended-release (OPANA®) associated with acute kidney injury in a chronic pain patient: a case report. A A Case Rep. 2017;9:324–327. doi: 10.1213/XAA.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 33.Porubsky S, Kuppe C, Maier T, et al. Renal lipidosis in patients enrolled in a methadone substitution program. Arch Pathol Lab Med. 2014;138:689–693. doi: 10.5858/arpa.2013-0075-CR. [DOI] [PubMed] [Google Scholar]
- 34.Mizera L, Gossrau G, Hummel T, Haehner A. Effects of analgesics on olfactory function and the perception of intranasal trigeminal stimuli. Eur J Pain. 2017;21:92–100. doi: 10.1002/ejp.903. [DOI] [PubMed] [Google Scholar]
- 35.Green A, Kaul A, O'Shea J, et al. Opiate agonists and antagonists modulate taste perception in opiate-maintained and recently detoxified subjects. J Psychopharmacol. 2013;27:265–275. doi: 10.1177/0269881112472567. [DOI] [PubMed] [Google Scholar]
- 36.Kuczeriszka M, Lipkowski AW, Sadowski J, Kompanowska-Jezierska E. An endomorphine analog ([d-Ala(2)]-endomorphin 2, TAPP) lowers blood pressure and enhances tissue nitric oxide in anesthetized rats. Pharmacol Rep. 2016;68:616–619. doi: 10.1016/j.pharep.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Schenk HD, Radke J, Ensink FB, et al. [Interactions between renal and general hemodynamics in fentanyl, droperidol, ketamine, thiopental and in peridural anesthesia–animal studies] Anaesthesiol Reanim. 1995;20:60–70. [PubMed] [Google Scholar]
- 38.Chen A, Ashburn MA. Cardiac effects of opioid therapy. Pain Med. 2015;16(Suppl 1):S27–S31. doi: 10.1111/pme.12915. [DOI] [PubMed] [Google Scholar]
- 39.Hall JE, Guyton AC, Jackson TE, Coleman TG, Lohmeier TE, Trippodo NC. Control of glomerular filtration rate by renin-angiotensin system. Am J Physiol. 1977;233:F366–F372. doi: 10.1152/ajprenal.1977.233.5.F366. [DOI] [PubMed] [Google Scholar]
- 40.Palomino J, Echavarria R, Franco-Acevedo A, Moreno-Carranza B, Melo Z. Opioids preconditioning upon renal function and ischemia-reperfusion injury: a narrative review. Medicina (Kaunas) 2019:55:522. doi: 10.3390/medicina55090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madero M, Garcia-Arroyo FE, Sanchez-Lozada LG. Pathophysiologic insight into MesoAmerican nephropathy. Curr Opin Nephrol Hypertens. 2017;26:296–302. doi: 10.1097/MNH.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 44.Wijkstrom J, Leiva R, Elinder CG, et al. Clinical and pathological characterization of Mesoamerican nephropathy: a new kidney disease in Central America. Am J Kidney Dis. 2013;62:908–918. doi: 10.1053/j.ajkd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Laws RL, Brooks DR, Amador JJ, et al. Biomarkers of kidney injury among Nicaraguan sugarcane workers. Am J Kidney Dis. 2016;67:209–217. doi: 10.1053/j.ajkd.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goudas LC, Langlade A, Serrie A, et al. Acute decreases in cerebrospinal fluid glutathione levels after intracerebroventricular morphine for cancer pain. Anesth Analg. 1999;89:1209–1215. [PubMed] [Google Scholar]
- 47.Zhang YT, Zheng QS, Pan J, Zheng RL. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin Pharmacol Toxicol. 2004;95:53–58. doi: 10.1111/j.1742-7843.2004.950202.x. [DOI] [PubMed] [Google Scholar]
- 48.Ozmen I, Naziroglu M, Alici HA, Sahin F, Cengiz M, Eren I. Spinal morphine administration reduces the fatty acid contents in spinal cord and brain by increasing oxidative stress. Neurochem Res. 2007;32:19–25. doi: 10.1007/s11064-006-9217-5. [DOI] [PubMed] [Google Scholar]
- 49.Lan X, Rai P, Chandel N, et al. Morphine induces albuminuria by compromising podocyte integrity. PLoS One. 2013;8:e55748. doi: 10.1371/journal.pone.0055748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000;11:1553–1561. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 51.Babak K, Mohammad A, Mazaher G, Samaneh A, Fatemeh T. Clinical and laboratory findings of rhabdomyolysis in opioid overdose patients in the intensive care unit of a poisoning center in 2014 in Iran. Epidemiol Health. 2017;39 doi: 10.4178/epih.e2017050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rawal N, Mollefors K, Axelsson K, Lingardh G, Widman B. An experimental study of urodynamic effects of epidural morphine and of naloxone reversal. Anesth Analg. 1983;62:641–647. [PubMed] [Google Scholar]
- 53.Yap E, Salifu M, Ahmad T, Sanusi A, Joseph A, Mallappallil M. Atypical causes of urinary tract obstruction. Case Rep Nephrol. 2019;2019 doi: 10.1155/2019/4903693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller M. Role of endogenous opioids in neurohypophysial function of man*. J Clin Endocrinol Metab. 1980;50:1016–1020. doi: 10.1210/jcem-50-6-1016. [DOI] [PubMed] [Google Scholar]
- 55.Spencer RL, Deupree D, Hsiao S, et al. Centrally-administered opioid selective agonists inhibit drinking in the rat. Pharmacol Biochem Behav. 1986;25:77–82. doi: 10.1016/0091-3057(86)90233-9. [DOI] [PubMed] [Google Scholar]
- 56.Korinek AM, Languille M, Bonnet F, et al. Effect of postoperative extradural morphine on ADH secretion. Br J Anaesth. 1985;57:407–411. doi: 10.1093/bja/57.4.407. [DOI] [PubMed] [Google Scholar]
- 57.Stotts NA, Arai SR, Cooper BA, Nelson JE, Puntillo KA. Predictors of thirst in intensive care unit patients. J Pain Symptom Manage. 2015;49:530–538. doi: 10.1016/j.jpainsymman.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lal J, Atkinson J. Involvement of the renin-angiotensin system in the dipsogenic effect of morphine. Arch Int Pharmacodyn Ther. 1985;278:273–291. [PubMed] [Google Scholar]
- 59.Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC. Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004;56:1191–1196. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 60.Kulacoglu H, Dener C, Kama NA. Urinary retention after elective cholecystectomy. Am J Surg. 2001;182:226–229. doi: 10.1016/s0002-9610(01)00703-6. [DOI] [PubMed] [Google Scholar]
- 61.Mercatello A. [Changes in renal function induced by anesthesia] Ann Fr Anesth Reanim. 1990;9:507–524. doi: 10.1016/s0750-7658(05)80223-0. [DOI] [PubMed] [Google Scholar]
- 62.Ejaz P, Bhojani K, Joshi VR. NSAIDs and kidney. J Assoc Physicians India. 2004;52:632–640. [PubMed] [Google Scholar]
- 63.Mongia A, Bhaskaran M, Reddy K, Manjappa N, Baqi N, Singhal PC. Protease inhibitors modulate apoptosis in mesangial cells derived from a mouse model of HIVAN. Kidney Int. 2004;65:860–870. doi: 10.1111/j.1523-1755.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 64.Liu LJ, Yu JJ, Xu XL. Kappa-opioid receptor agonist U50448H protects against renal ischemia-reperfusion injury in rats via activating the PI3K/Akt signaling pathway. Acta Pharmacol Sin. 2018;39:97–106. doi: 10.1038/aps.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK pathway. Am J Pathol. 2010;177:984–997. doi: 10.2353/ajpath.2010.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill P, Shukla D, Tran MG, et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19:39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leemans JC, Stokman G, Claessen N, et al. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jalili C, Salahshoor MR, Hoseini M, Roshankhah S, Sohrabi M, Shabanizadeh A. Protective effect of thymoquinone against morphine injuries to kidneys of mice. Iran J Kidney Dis. 2017;11:142–150. [PubMed] [Google Scholar]
- 69.Beitland S, Sunde K, Moen H, Os I. Variability in uremic control during continuous venovenous hemodiafiltration in trauma patients. Crit Care Res Pract. 2012;2012 doi: 10.1155/2012/869237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27:928–937. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 71.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol. 2006;24:971–983. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- 73.Barbosa-Leiker C, McPherson S, Daratha K, et al. Association between prescription opioid use and biomarkers of kidney disease in US adults. Kidney Blood Press Res. 2016;41:365–373. doi: 10.1159/000443436. [DOI] [PubMed] [Google Scholar]
- 74.Erkilic E, Kesimci E, Alaybeyoglu F, et al. Does remifentanil attenuate renal ischemia-reperfusion injury better than dexmedetomidine in rat kidney? Drug Des Devel Ther. 2017;11:677–683. doi: 10.2147/DDDT.S126701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sakai W, Yoshikawa Y, Hirata N, Yamakage M, Sakai W, et al. Effect of remifentanil during cardiopulmonary bypass on incidence of acute kidney injury after cardiac surgery. J Anesth. 2017;31:895–902. doi: 10.1007/s00540-017-2419-y. [DOI] [PubMed] [Google Scholar]
- 76.Rettig RA Yarmolinsky A, editors. Institute of Medicine (US) committee on federal regulation of methadone treatment. Washington, DC: Federal Regulation of Methadone Treatment National Academies Press; 1995. [PubMed]
- 77.Wu LT, Woody GE, Yang C, Blazer DG. Subtypes of nonmedical opioid users: results from the national epidemiologic survey on alcohol and related conditions. Drug Alcohol Depend. 2010;112:69–80. doi: 10.1016/j.drugalcdep.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10:147–155. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]