Abstract
Background and Objectives
Experiencing structural racism over the life course contributes to disproportionate pain-related disability among African American older adults. Positive STEPS, delivered by community health workers, is a culturally congruent chronic pain self-management intervention that incorporates positive psychology principles and gives attention to social determinants of pain and pain management.
Research Design and Methods
We conducted a randomized pilot trial among older adults with chronic musculoskeletal pain in an underserved, primarily African American community (Detroit, Michigan). The 7-week intervention included weekly telephone sessions with a community health worker; web-based videos teaching pain self-management skills; positive activities (e.g., Life Review, Gratitude Jar); and use of wearable activity trackers. Outcomes were measured at baseline and 8-week follow-up. We assessed participant retention, engagement, and satisfaction.
Results
Study completers (n = 46; 90% retention) were 93% African American, 89% female, mean 72 years, and completed 5.7 of 7 sessions. Intervention participants versus controls showed greater improvement in PROMIS Pain Interference (4.3-point T-score decrease vs. 0.4-point increase; p = .01) and the Pain Self-Efficacy Questionnaire (p = .007). Furthermore, compared with controls, significantly more intervention participants reported “better” or “much better” global functioning (86% vs. 25%; p = .000) and pain (67% vs. 21%; p = .003) since baseline. Improvements in physical functioning, social participation, and resilience were noted, but differences were not significant. Participant feedback on the intervention was overwhelmingly positive.
Discussion and Implications
A community health worker-led chronic pain self-management intervention combining positive activities with self-management skills training demonstrated the potential to enhance pain-related functioning among a vulnerable group of older adults.
Clinical Trial Number
Keywords: African Americans, Community-engaged research, Mobile health
Learning chronic pain self-management (CPSM) skills is a key component of optimal chronic pain treatment (Interagency Pain Research Coordinating Committee, 2016). CPSM interventions include psychological and behavioral elements, such as pain education, pain-coping skills training, and encouragement to engage in physical activity and goal-setting, and are intended to bring about improvements in functioning and quality of life while avoiding medication-associated risks (Clauw et al., 2019). Increasing uptake of CPSM interventions is particularly urgent among marginalized groups. African American older adults, for example, bear an extraordinarily high burden of disabling pain, linked to the health-damaging impact of structural racism and associated disadvantage, and are more likely to receive suboptimal pain care (Bailey et al. 2017; Booker et al., 2020, 2021; Maly & Vallerand, 2018; Morales & Yong, 2021). Yet only a small number of behavioral interventions for pain have been designed around the strengths and needs of this population subgroup (Allen et al., 2019; Janevic et al., 2021; Schrubbe et al., 2016).
Community Health Workers and Chronic Pain Care
Community health workers (CHWs) may be uniquely suited to provide CPSM support to marginalized older adults. CHWs are frontline public health workers who are from or have close connections to the communities they serve. We previously demonstrated the feasibility of a CHW-led, technology-supported CPSM intervention for African American older adults, “STEPS 2” (Seniors using Technology to Engage in Pain Self-management; Janevic et al., 2021). The rationale for engaging CHWs to improve pain care within underserved groups is that their shared community identity and life experiences foster a uniquely trusting provider–client relationship and a natural and profound cultural congruence (Barnett, Gonzalez et al., 2018; Barnett, Lau et al., 2018; Perry et al. 2014). By providing tailored social support and credible, relevant information, CHWs are well equipped to address the psychological and social elements that can contribute to persistent pain and suffering, per the widely accepted biopsychosocial model of chronic pain (Gatchel et al., 2007). Furthermore, adverse conditions linked to structural racism, such as residential segregation and economic deprivation, mean that many older adults in underserved areas struggle with adverse social determinants of health, such as food insecurity and health care access. These hardships not only affect pain, health, and functioning directly but can also form obstacles to the practice of new self-management skills—particularly within the present coronavirus disease 2019 (COVID-19) pandemic (Garcia et al., 2021). CHWs have deep knowledge of local resources and are trained to connect individuals to formal health and social services and other community resources, to address unmet social needs.
Positive Psychology for Pain Management
Positive, or resilience-building, activities are a promising approach to enhancing pain self-management (Hassett, 2018). While standard cognitive-behavioral approaches tend to focus on reducing negative affect, targeting positive affective states instead, or in addition, may enhance treatment outcomes (Ong et al., 2020). In a recent meta-analysis, Ong et al. (2020) found that positive affect is associated with less chronic pain severity. According to Frederickson’s Broaden and Build theory of positive psychology, positive emotions expand “thought–action repertoires,” making people more willing to practice new skills and invest in relationships (Fredrickson, 2001). Even though positive affective states may be transient, these personal resources accrue over time, building psychological resilience. Positive activity interventions based on the Broaden and Build theory attempt to induce positive emotions through simple, enjoyable activities (e.g., savoring everyday moments; Greenawalt et al. 2019; Smith & Hanni, 2019). Such activities may improve pain outcomes via a variety of pathways; for example, improved mental health, increased behavioral activation, decreased catastrophizing, decreased fear-avoidance, and increased social support (Hanssen et al., 2017; Hassett & Finan, 2016; Hausmann, Ibrahim et al., 2018). These processes can counteract the sense of helplessness and depression often reported by older adults living with persistent pain and help them find ways to persevere and thrive during this life stage (Robinson-Lane et al., in press; Stensland, 2021). While there is some overlap with formal Behavioral Activation therapies including the promotion of pleasant activities, positive awareness, and social functioning (Lejuez et al., 2001), positive activities themselves can be used to support Behavioral Activation treatment.
Positive activity interventions are engaging, easily taught, and well-suited for older adults, who have highly developed emotional regulation abilities (Smith & Hanni, 2019). In the meta-analysis by Ong et al. (2020), effect sizes between positive affect and pain severity were larger in older compared with younger samples. Positive activity interventions may also have particular utility in African American older adults with chronic pain (Hausmann, Ibrahim et al., 2018). A prior study among people with knee osteoarthritis found that psychosocial resilience had a stronger negative association with movement-evoked pain in African American adults compared with non-Hispanic Whites (Bartley et al., 2019). The strengths-based approach of positive activity interventions is consistent with African American cultural preferences (Hausmann, Ibrahim et al., 2018; Hausmann, Youk et al., 2018) and may help offset discrimination-related stress (Hausmann, Ibrahim et al., 2018). In nursing research, maintaining a positive frame of mind has been identified as a major way that African American older adults strive to live well despite chronic pain (Robinson-Lane, 2020).
Positive STEPS: Aims of Randomized Pilot Trial
In a randomized pilot trial, we assessed the feasibility and preliminary effects of a new version of the STEPS 2 CPSM intervention called “Positive STEPS,” which blends positive activities with core CPSM skills and is tailored for cultural relevance to African American older adults. Our first aim was to assess the intervention’s feasibility, as indicated by retention, engagement, and acceptability (participant satisfaction). Our second aim was to assess the preliminary effects of Positive STEPS on the primary outcomes of pain interference and physical functioning, and on secondary outcomes of global impression of change in pain and functioning, resilience, social participation, and pain self-efficacy.
Method
Study Design/Setting
A randomized, parallel-group pilot and feasibility trial was conducted in Detroit, Michigan, from May to December 2020. Participants were randomized after completion of the baseline assessment (preventing assessor bias) to intervention or control conditions using a computer-generated block randomization scheme (sealedenvelope.com). At the 8-week follow-up, assessors were aware of group assignment because intervention group members were administered additional questions regarding their experience. This trial was registered on clinicaltrials.gov (NCT04321239) and was approved by the University of Michigan Institutional Review Board (HUM00162275).
Intervention development
Positive STEPS was adapted from our “STEPS 2” pilot intervention (Janevic et al., 2021), which was based on a prior successful internet CPSM intervention (Williams et al., 2010). Because we had recently developed culturally responsive content and procedures for STEPS 2, when developing Positive STEPS we focused on selecting and refining the new positive activity content.
Focus groups
We conducted two focus groups (n = 16; 100% identified as female and African American, with chronic musculoskeletal pain), in order to (a) determine which potential positive activities were most appealing, and (b) elicit examples and capture appropriate ways of describing these activities that could be incorporated into intervention materials to enhance their relevance to the priority population. We presented eight potential activities, drawn primarily from the ongoing PRISM trial (R01-AR070296 NIH/NIAMS) led by coauthor A. L. Hassett. We described each activity and gauged participants’ reactions. Where possible, participants tried out the activity (e.g., by thinking of things they could savor or acts of kindness). The activities that received the most uniformly positive feedback were Life Review, Savoring, Random Acts of Kindness, Gratitude Jar, and Music as Medicine. Other activities, including Best Possible Self, Forgiveness Letters, and Legacy Building, received mixed feedback; we opted not to include these in the intervention.
CHW input
Three CHWs (who had also delivered the STEPS 2 intervention) played an essential role in the development of the new positive activity content. In a series of working meetings, they reviewed iterative versions of the CHW manual, participant workbook, and program website, which were modified per their suggestions. Materials incorporated culturally familiar examples throughout; these elements also reflected CHW input.
Intervention Description
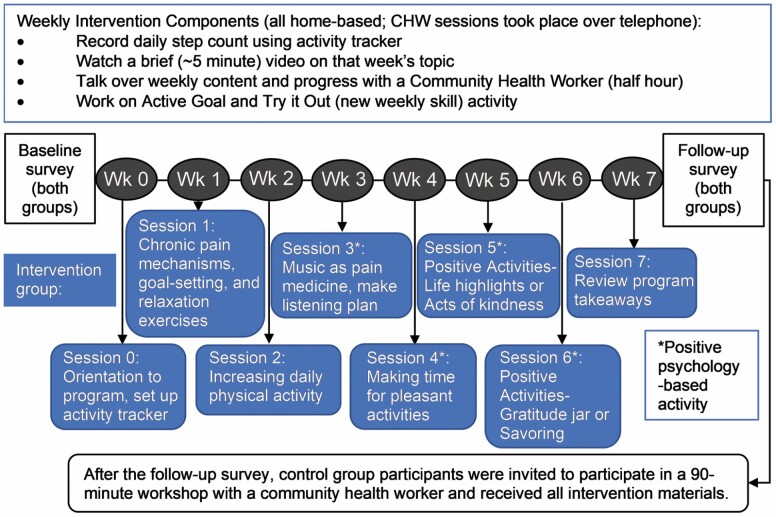
Figure 1 depicts the study flow and intervention content. This was a completely remote intervention: program materials were mailed; all sessions including the orientation session took place over the phone, and participants completed program tasks including watching videos on their own. For each of the 7 weeks following the orientation, participants watched a web-based video addressing a specific cognitive-behavioral pain management skill or positive activity and/or read about these in their workbook. They also had a weekly 30-min, structured telephone session with their designated CHW (details below).
Figure 1.
Positive STEPS sequence and content. CHW = community health worker.
Cultural adaptations
Adaptations to program materials reflected “surface” changes such as adjusting some of the language used to reflect the vernacular of African American older adults and integrating race-concordant presenters in videos, as well as “deep” changes that embedded cultural values such as spirituality and community engagement into the program (Resnicow et al., 1999). In addition, the program was tailored to the local context. For example, some of the “enjoyable activity” suggestions in the workbook drawn from focus groups included local options (e.g., jewelry-making, virtual Bible study, classes at senior centers or community colleges, virtual trips to the Detroit Zoo).
Core pain self-management skills
The following STEPS 2 modules were used: Understanding Chronic Pain, SMART Goal-Setting, Staying Active, and Doing What You Love.
Positive activities
Four sessions were devoted to positive activities. All participants completed the Doing What You Love (engaging in pleasant activities; also considered a core CPSM skill) and Music as Medicine modules. Then, CHWs presented a menu of four additional positive activities (Savoring, Life Review, Gratitude Jar, and Random Acts of Kindness), briefly describing each. Participants were told that they would choose one of the four activities to complete during the current session and one during the next session. In the final intervention session, participants were reminded that they could try the two remaining activities following program completion, if they wished. The rationale for allowing participant choice was that a given positive activity was most likely to benefit a participant if it was appealing to them.
CHW-led component
CHW training.
The CHWs who delivered the STEPS 2 intervention (Janevic et al., 2021) also delivered Positive STEPS, following a half-day training in the newly developed content. All three CHWs had previously completed the Michigan Community Health Worker Alliance training, which teaches core competencies including communication skills, healthy lifestyle components, and legal and ethical responsibilities.
Session format.
In each session, CHWs reinforced key points related to the weekly topic, discussed the participant’s situation, and formulated a “Try it Out” plan related to that week’s topic. Participants also set an “Active Goal” (a pain self-management goal chosen by the participant that focused on increasing activity—whether physical, social, self-care or otherwise) in SMART format. CHWs reviewed daily step counts with participants, using this as a springboard to discuss activity patterns and barriers. The Participant Workbook included a summary of the key points from each week’s video and accompanying worksheets. CHWs referred participants to community resources as needed. CHWs recorded all information about sessions, including case notes, using REDCap electronic data capture tools hosted at the University of Michigan (Harris et al., 2019).
Positive STEPS website
The simple program website (https://sites.google.com/umich.edu/positivesteps/) included videos introducing each of the three CHWs and a separate page for each of the seven program modules. Most modules included a brief didactic video presented by a University of Michigan expert presenting a particular skill, along with additional links to useful articles and videos.
Activity trackers
A commercially available, wrist-worn activity tracker was provided to participants. They were asked to wear the tracker during waking hours for the duration of the intervention. Each evening, participants received an automated text message asking them to input that day’s step count, visible on their activity tracker. (Five participants chose to log their step counts on paper instead.) Project staff regularly input texted step count data into REDCap, so that it could be accessed by CHWs. Step counts were used as a helpful source of information for CHWs and participants alike to assess activity levels. Participants could choose to incorporate step counts into their Active Goal, but they were not required to. Step count data are not analyzed in the current report.
Control condition
Between the baseline and follow-up surveys, participants in the control condition did not take part in any study activities. After completing the follow-up telephone survey, control group members were given a link to the website, the workbook, and the activity tracker and were invited to take part in a one-time session of approximately 75 min with a CHW covering key intervention content.
Participants
Inclusion criteria
Eligible adults were aged 60 and older, ambulatory with or without an assistive device, with musculoskeletal pain of at least 3 months’ duration, with at least one day in the last 30 days when pain made it difficult to do usual activities. Participants had to have a smartphone or other means of watching online videos.
Exclusion criteria
Serious acute illness or hospitalization in the last month or planned major surgery in the next 3 months. Participants were asked if they had significant memory difficulties that interfered with daily activities. If they answered yes, they were read a description of the program and asked if their memory challenges would get in the way of being able to do the program; participants responding no were eligible.
Recruitment
Participants were primarily recruited through a registry for African American older adults interested in participating in research maintained by the Healthier Black Elders Center at the Wayne State University Institute of Gerontology (Chadiha et al., 2011). Individuals meeting broad study criteria (age, indication of back pain, or arthritis) were initially contacted by letter describing the study, followed by a phone call to further assess eligibility, answer questions, and to invite to participate, if appropriate. Participants were also recruited via word of mouth at community locations in Detroit serving older adults and from a list maintained by the researchers of people who wished to be contacted for future pain-related studies.
Data Collection
Telephone interviews lasting approximately one hour were conducted by trained research assistants at baseline and 8 weeks (immediately postprogram). The assessment included self-reported health, psychosocial, and demographic measures. For intervention group members, the follow-up interview also included a series of closed- and open-ended questions about their experience in Positive STEPS. Participants received a small financial incentive for each research interview. They were also invited to keep the activity tracker.
Measures
Primary outcomes
Pain interference.
This is measured with the six-item PROMIS-43 Adult Profile subscale. All PROMIS measures have demonstrated high reliability and construct validity (Cella et al., 2019) and evidence of equivalent item functioning supports their use in sociodemographically diverse groups (Jones et al., 2016; Teresi & Reeve, 2016). Items ask how much pain has interfered with daily activities such as household chores and social activities in the last 7 days (1 = not at all to 5 = very much).
Physical functioning.
This is measured with the four-item PROMIS-29 Adult Profile subscale. Items ask how much difficulty the participant has with daily activities such as household chores, using stairs, and walking for 15 min (1 = unable to do to 5 = without any difficulty; Cella et al., 2019).
Secondary outcomes
Participant global impression of change.
Participants were asked at follow-up how their functioning and pain (in separate items) had changed from baseline, using a 7-point scale from “much worse” to “much better” (Dworkin et al., 2005). As an exploratory outcome, we asked “How does the amount of pain medication you are taking now compare to what you were taking at the time of the first interview?” on a 7-point scale from “a lot more” to “a lot less.”
Pain intensity.
A numeric rating scale ranging from 0 (no pain at all) to 10 (worst pain you can imagine; Dworkin et al., 2005).
Pain self-efficacy.
An eight-item scale assessing confidence to participate in various life activities despite pain (0 = not at all confident to 6 = completely confident), which has high internal consistency reliability (Cronbach’s alpha > 0.90) and strong construct validity (Nicholas, 2007).
Social participation.
A four-item PROMIS-29 subscale rating difficulty participating in family activities, leisure activities, and work (5 = never to 1 = always; Cella et al., 2019).
Resilience.
The 10-item Connor–Davidson Resilience Scale rates the degree to which respondents can be resilient in various situations (0 = not true at all to 4 = true nearly all the time; Connor & Davidson, 2003). This unidimensional scale has good construct validity and internal consistency (Campbell-Sills & Stein, 2007).
Health and demographic variables.
We collected data on gender, racialized group/ethnicity, educational attainment, employment status, health literacy (Chew et al., 2004), education, financial strain (indicated by difficulty paying bills each month, from “not at all” to “extremely” difficult), and health insurance status.
Feasibility and acceptability
Retention.
The proportion of participants who completed the study out of those enrolled.
Engagement.
The number of sessions completed out of 7, and the proportion of participants who reported watching all the program videos. We established a threshold of 60% of sessions completed as a minimum to indicate feasibility.
Acceptability.
In a series of closed- and open-ended questions, participants were asked to give feedback on each intervention component as well as the overall program. The impact of each specific module was assessed by tallying the number of times the module was stated to be useful to participants (as part of a response to any item).
Data Analysis
IBM SPSS Statistics (version 24; Armonk, NY) was used for data analysis. We calculated descriptive statistics for baseline characteristics and compared groups at baseline using independent-sample t-tests for continuous variables and chi-squared tests for categorical variables. We converted each PROMIS subscale score to a T-score (a standardized score with a mean of 50 and SD of 10) by summing the scales and using the conversion tables provided at HealthMeasures.net.
To assess the effect of being in the intervention group, we estimated a univariate analysis of variance model for each continuous primary and secondary outcome. The dependent variable in each model was the value of the outcome at 8-week follow-up; the independent factor was the treatment group (intervention vs. control), with the baseline value of the outcome used as a continuous covariate. Effect size was indicated by partial eta squared for the treatment group variable. In order to assess the potential clinical relevance of the intervention, we used chi-squared tests to compare the proportion of participants in the intervention versus control groups who achieved the Minimally Important Difference of ≥ 3 T-score points in PROMIS pain interference (Chen et al., 2018). Finally, we calculated frequencies for closed-ended participant satisfaction items and categorized open-ended responses about each major intervention element (CHW sessions, web-based videos, activity trackers, workbooks, and overall) into subthemes under each element, tallying the number of positive and negative comments for each.
Results
Sample Characteristics
As given in Table 1, participants (mean age 72 years, range 60–90 years) were 89% female, 100% identified as African American or multiracial, and most (59%) lived alone. More than half the sample (57%) had at least a bachelor’s degree; 24% reported difficulty paying bills each month and one fifth received Medicaid. The most common chronic conditions were arthritis (91%), hypertension (85%), and low back pain (78%), with an average of six chronic conditions per person. Mean pain intensity at baseline was 6.2 (SD 1.7).
Table 1.
Positive STEPS Study: Sample Characteristics at Baseline (n = 46)a
| Variable | All participants (n = 46) | Positive STEPS group (n = 22) | Control group (n = 24) | |||
|---|---|---|---|---|---|---|
| M (SD) | % (n) | M (SD) | % (n) | M (SD) | % (n) | |
| Age, in years (range 60–90) | 72.1 (7.2) | 72.0 (6.9) | 72.3 (7.6) | |||
| Female | 89% (41) | 91% (20) | 88% (21) | |||
| African Americanb | 93% (43) | 91% (20) | 96% (23) | |||
| Lives alone | 59% (27) | 59% (13) | 58% (14) | |||
| Bachelor’s degree or more | 57% (26) | 50% (11) | 63% (15) | |||
| Difficulty paying billsc | 24% (11) | 27% (6) | 21% (5) | |||
| High health literacyd | 89% (41) | 86% (19) | 92% (22) | |||
| Chronic conditions | ||||||
| Arthritis | 91% (42) | 91% (20) | 92% (22) | |||
| Low back pain | 78% (36) | 77% (17) | 79% (19) | |||
| Hypertension | 85% (39) | 91% (20) | 79% (19) | |||
| Diabetes | 28% (13) | 23% (5) | 33% (8) | |||
| Heartburn/acid reflux | 61% (28) | 59% (22) | 63% (15) | |||
| Depression | 37% (17) | 46% (10) | 29% (7) | |||
| Count of chronic conditions | 6.0 (2.2) | 6.2 (2.3) | 5.6 (2.0) | |||
| Pain | ||||||
| Pain intensity in last week (0 = no pain to 10 = worst imaginable pain) | 6.2 (1.7) | 6.1 (1.8) | 6.3 (1.5) |
aParticipants with baseline and 8-week follow-up data.
bThe remaining participants were identified as multiracial.
c“In a typical month, how hard is it for you to cover your expenses and pay all your bills?” (proportion answering moderate or greater).
d“Extremely” or “quite” confident in filling out medical forms by self (Chew et al., 2008).
Table 2 presents that the pain interference T-score at baseline was 59.4 (SD = 6.7) in the intervention group and 58.9 (SD = 8.2; control group); physical function scores were 41.5 (SD = 7.4) for the intervention group and 40.8 (SD = 6.7) for controls. These T-scores indicate a level of pain interference approximately 1 SD higher and physical function 1 SD lower, than their respective means in the general population. There were no significant differences between intervention and control groups at baseline on any health, demographic, or outcome variables.
Table 2.
Baseline and Follow-Up Means and Effect Size for Primary and Secondary Outcomes
| Outcome | Positive STEPS group (n = 22) | Control group (n = 24) | Treatment effect size (partial eta squared)a | P value for treatment effecta | ||
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | |||||
| Baseline | Follow-up | Baseline | Follow-up | |||
| Pain Interferenceb | 59.4 (6.7) | 55.1 (7.5) | 58.9 (8.2) | 59.3 (6.8) | 0.166 | .000 |
| Physical Functioningb | 41.5 (7.4) | 43.7 (7.7) | 40.8 (6.7) | 40.9 (6.3) | 0.075 | .068 |
| Social Participationb | 50.6 (9.1) | 51.9 (7.2) | 48.3 (9.1) | 48.1 (7.6) | 0.014 | .464 |
| Pain Self-Efficacyc | 4.8 (1.2) | 5.1 (0.9) | 4.4 (1.3) | 4.2 (1.4) | 0.155 | .007 |
| Resilienced | 3.1 (0.6) | 3.2 (0.6) | 3.2 (0.5) | 3.2 (0.5) | 0.009 | .531 |
aBased on univariate analysis of variance.
b T-scores are normed so that a score of 50 is the mean for the reference population and 1 SD = 10 T-score points.
cPain Self-Efficacy Questionnaire (Nicholas, 2007).
dConnor–Davidson Resilience Scale (Connor & Davidson, 2003).
Retention
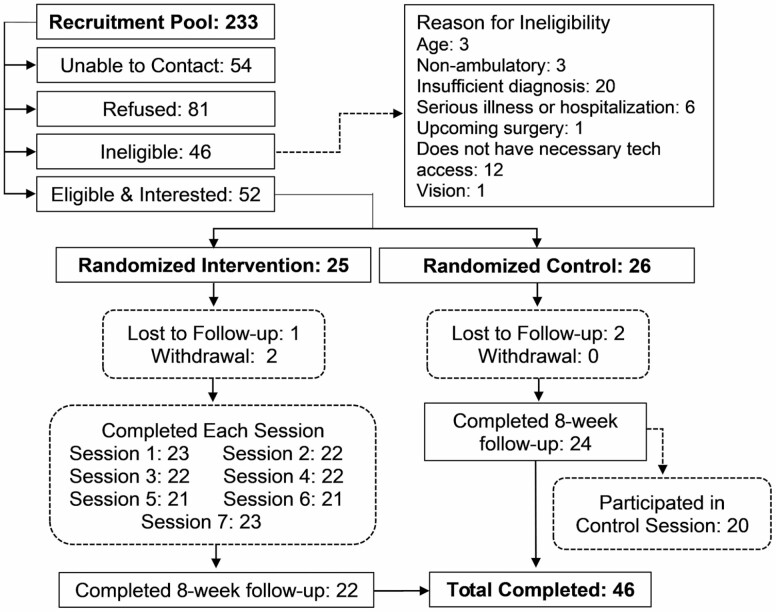
As shown in Figure 2, five participants (three in the intervention group and two in the control group) were lost to follow-up or withdrew. Noncompleters compared with completers were younger (68 vs. 72 years) and had higher baseline pain intensity (7.8 vs. 6.2 on a 0–10 scale), greater pain interference (T-score 62.1 vs. 59.1), worse physical functioning (T-score 39.0 vs. 41.1), worse social participation (T-score 42.7 vs. 49.4), lower pain self-efficacy (4.3 vs. 4.6), and lower resilience (3.1 vs. 3.2). These differences were not statistically significant.
Figure 2.
Positive STEPS flowchart.
Primary and Secondary Outcomes
Intervention group participation was significantly associated with greater improvement in pain interference and pain self-efficacy (Table 2). Improvements in physical functioning, social participation, and resilience were greater among intervention participants but did not reach statistical significance. The Minimally Important Difference of 2.5 points for the PROMIS pain interference subscale (Chen et al., 2018) was achieved by 53% of intervention participants and 17% of controls (χ 2 [1, 42] = 5.8, p = .02).
Significantly more intervention participants (86%) versus controls (25%) reported “better” or “much better” function (χ 2 [1, 45] = 16.6, p = .000) since baseline in the global impression of change item; for pain these proportions were 67% and 21%, respectively (χ 2 [1, 45] = 9.6, p = .003). For medication use (exploratory outcome), more intervention (48%) participants versus controls (12%) reported taking “less” or “much less” medication since baseline (χ 2 [1, 45] = 6.72, p = .019; not shown in table).
Participant engagement
Participants completed a mean of 5.7 of the seven CHW telephone sessions offered. Out of 21 intervention participants, 20 (95%) reported watching all program videos.
Acceptability/participant feedback
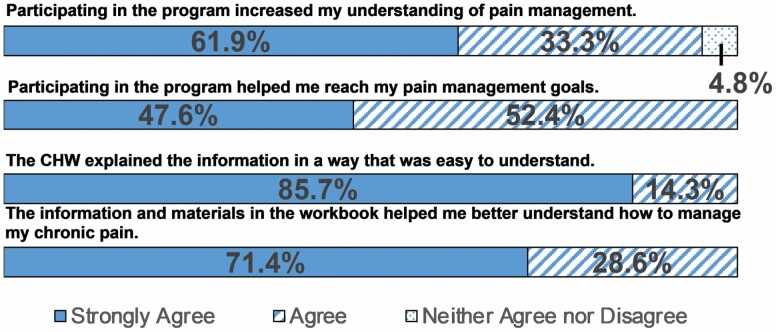
Participants provided favorable feedback about the Positive STEPS intervention, in both clsoed- and open-ended questions. Nearly all participants strongly agreed or agreed that they increased their understanding of pain management (n = 20; 95%) and that the program helped them reach their pain management goals (n = 21; 100%; Figure 3). Responses from open-ended items are summarized in Table 3. Major themes included that the program helped participants think more positively, reduced their chronic pain, and taught them new strategies and activities. The activity tracker was the element that received the largest proportion of negative comments. Participants reported either forgetting to wear or charge the device or technical problems.
Figure 3.
Participant satisfaction with Positive STEPS intervention. CHW = community health worker.
Table 3.
Summary of Qualitative Feedback on Positive STEPS Program
| Program element | Feedback breakdown (most common themes) | Example quotations |
|---|---|---|
| Community health worker | Positive—73 (Supportive, motivating, informative) Negative—5 (Scheduling sessions) |
“She was very supportive and helpful in explaining the different steps of what I need to do or what I was going to get out of the session.” “She was kind and understanding and she was encouraging, inspirational.” |
| Website/videos | Positive—37 (Informative, accessible, helpful) Negative—10 (Difficulties accessing site the first time, connectivity issues) |
“Gave me a lot of information and I’m still using it.” “I did enjoy the videos because after I listened to that week’s video, I was able to find other videos with more information. I did look at the external links and found some helpful. The videos were good.” “One time I could not get in, it was buffering. One time my phone was acting up and I could not see the video. Other than that it was okay.” |
| Participant workbook | Positive—34 (Writing down goals, reinforcement, resources section) Negative—2 (Workbook organization, resource expansion) |
“It had the pages where you could write notes and your goals so I would do that and leave it open and look at it as I walked by.” “Reinforced what I was learning.” “Don’t limit to activities in Detroit proper.” |
| Activity trackers | Positive—10 (Motivating, useful) Negative—20 (Forget to put it on, forget to charge it, difficulties learning how to use a tracker) |
“I enjoyed the activity tracker, it let me know if I need to be walking more. When it got low I knew I needed to do a little more walking.” “Remembering to put it on, that was my challenge.” “Because it was new I didn’t really understand it as well at first … After a while I got used to it and understood how to use it.” |
| Recommend program to others? | Yes—24 (Improved positive thinking, reduced chronic pain, informative) No—1 (Too elementary) |
“It helps you think about your pain and the positive thinking does help and it helped my needs. It gets you moving if you were not moving and helps you realize that to get rid of the pain you have to do something. Move about, engage yourself and not dwell on your pain.” |
Adverse events
Two hospitalizations were reported over the course of the study; both were determined to be unrelated to the study.
Discussion
The Positive STEPS intervention combined positive activities with training in core pain self-management skills. It was designed to be responsive to older adults in a primarily African American community affected by racialized structural disadvantage. To our knowledge, Positive STEPS is the first positive psychology intervention for chronic pain developed specifically for delivery by CHWs. Converging indicators confirmed the feasibility and acceptability of this intervention for the priority population. Over 90% of participants completed the study; the telephone session completion rate (5.7 of 7, or 80%) exceeded our a priori feasibility threshold of 60%, and feedback from participants showed that the intervention was perceived as both engaging and efficacious.
Despite a small sample size that was chosen for feasibility rather than statistical power, Positive STEPS participants had significantly greater improvements in pain interference, pain self-efficacy, and global impression of change in pain and functioning compared with the randomized control condition. Improvements in physical functioning, social participation, and resilience were greater than those in the control group but did not reach statistical significance. Just over half of the intervention group, compared with less than one fifth of controls, demonstrated a clinically meaningful improvement in the primary outcome of PROMIS pain interference, and a large majority of intervention participants reported improved pain and function since baseline, compared with a small proportion of controls. The improvements in the intervention group are similar in magnitude to our earlier pilot study of the related STEPS 2 intervention (Janevic et al., 2021); however, that study lacked a control group. The intervention’s positive impact on pain self-efficacy raises the possibility that increased self-efficacy mediated improvements in these outcomes.
Themes from participants’ open-ended comments provide clues for how the intervention might have brought about improved pain and function (Table 3). These included feeling more motivated, capable, and knowledgeable about techniques to control pain, and successfully working toward personal goals with CHW encouragement. Indeed, interactions with the CHW were mentioned in comments related to each of these themes. This suggests that the positive and trusting relationships established with CHWs were pivotal for helping change participants’ pain-related thoughts and behaviors. Finally, the expert-presented videos and participant workbook were also cited by many participants as helping them understand and apply a variety of pain-coping skills. The modules most often mentioned as impactful were music, physical activity, and deep breathing/relaxation. Gratitude, goal-setting, life review, and acts of kindness were also listed as impactful by a number of participants.
We did not see an effect of the intervention on social participation; however, at baseline, the mean PROMIS social participation T-score was close to the population mean of 50, suggesting that our participants did not have deficits in social participation, despite living with substantial chronic pain. Indeed, because our sample was recruited from a research volunteer registry and community sites, participants were likely already highly engaged socially, and hence did not have much room for improvement. We also note that because the study took place under fairly strict lockdown conditions due to the pandemic, the examples and options for positive and pleasant activities in the materials and as discussed by CHWs de-emphasized social contact except for virtual. In future iterations, increasing the focus on interpersonal positive activities could enhance this outcome.
The Broaden and Build model posits that more opportunities for positive affect will enhance resilience (Fredrickson, 2001). However, resilience increased only very slightly in the intervention group (and was unchanged in the control group). Participants’ scores on the resilience measure used were similar to the mean scores observed for community samples (Davidson, 2018), suggesting that our sample was made up of relatively resilient individuals already. Not seeing significant changes in resilience could also be due to the relatively small number of positive activities offered, or because they were not effective at building resilience in the short-term, given that it may take longer for these effects to emerge (Hassett, 2018). In open-ended feedback, positive activities were described by some participants as being helpful. “Music as Medicine” was the positive psychology module most often singled out as being helpful for pain management. This module was newly developed by the researchers. It provided simplified explanations of the mechanisms by which music could have a positive effect on pain and encouraged participants to formulate a personalized music listening plan to achieve desired positive mood states (relaxed, energized, reflective). Several participants commented that the playlists we provided, which were selected with input from the CHWs and included Gospel and Motown versions, brought back fond memories and motivated them to incorporate more music into their daily lives. This implies that this module had a positive, enduring effect on at least some participants and that cultural tailoring was successful. One negative comment about positive activity content had to do with it being too elementary. Future iterations could include an additional choice of activities including more advanced options.
Almost half of participants (48%) reported taking less or much less pain medication at follow-up—more than 3 times the proportion of control group participants who did so. This bolsters the finding in our STEPS 2 pilot study (Janevic et al., 2021), in which a similar proportion of participants (40%) reported reduced medication use. In both the STEPS 2 and Positive STEPS interventions, participants were not specifically asked to reduce medication use and were told that the new skills could be helpful whether or not they were also taking pain medication. In future studies, more detailed measures of medication use can elucidate this apparent program effect, with longer-term follow-up to determine whether it persists over time.
Finally, our attempts to make the electronic intervention elements easy-to-use and tailored to the needs of this group were largely successful, as indicated by positive comments on the website and the fact that nearly all participants reported watching all intervention videos. As in our prior studies with this population involving activity trackers, these were perceived by some as motivating and useful; however, participants often had difficulties learning to use the tracker and remembering to charge it and/or put it on in the morning. Given that adherence to activity tracking may be better with smartphones compared with wearable devices (Patel et al., 2020), future interventions should consider this as a more accessible option for tracking.
The most similar prior study identified was Hausmann et al.’s “Staying Positive with Arthritis,” a 6-week telephone-based positive psychological intervention delivered by trained staff (Hausmann, Ibrahim et al., 2018; Hausmann, Youk et al., 2018). This intervention included positive activities similar to those in Positive STEPS. In a randomized controlled trial, Staying Positive with Arthritis was tested against a control condition that offered affectively neutral activities in a parallel format. There was no difference between conditions in their effects on pain and functional difficulty, in contrast to findings favoring the positive activity group in an earlier pilot randomized trial (Hausmann et al., 2017). Half of the sample was African American, and greater improvement was hypothesized in this group, but there were no racialized group differences in intervention effect.
In contrast with Staying Positive with Arthritis, as well as with other positive activity interventions for pain (e.g., the internet-based Happy Despite Pain [Peters et al., 2017] and Gratitude Enhanced Mindfulness [Swain et al., 2020] interventions), the Positive STEPS curriculum blended positive activities with standard CPSM skills. These included pain psychoeducation, a focus on physical activity and use of an activity tracker, and structured goal-setting. Although our study was not specifically designed to test a booster effect of positive activities on these self-management skills, we can nonetheless speculate that the combination may have been an effective one. The Broaden and Build theory posits an upward spiral in which positive emotions lead to better attention and cognition (Fredrickson, 2001), which could facilitate learning new skills. Positive activities have the potential to “enhance the effectiveness of CBT and other behavioral therapies” for pain (Hassett, 2018), and Hanssen et al. (2017) have suggested that the simplicity and flexibility of positive activities make them suitable for insertion in other pain treatments. Such activities may optimize learning processes, while also making treatments more appealing and preventing dropout. Our study confirmed the feasibility and acceptability of including both positive activities and standard self-management strategies within the same intervention. However, there is much yet to be explored regarding the optimal ways to leverage positive activities in different types of pain therapies.
The Future of CHWs in Pain Care
This study contributes evidence that CHWs have a potentially important role in pain care for underserved populations. The CHW workforce is rapidly growing (Kangovi & Asch, 2018; Peretz et al. 2020), and a 2019 commentary in the New England Journal of Medicine asserts that the current health care environment offers a “historic opportunity” to engage CHWs to improve health care in the United States (Lapidos et al., 2019). A recent study shows that CHWs provide a positive return on investment via addressing the unmet social needs of chronically ill patients in poor neighborhoods (Kangovi et al., 2020). Momentum is growing at state and national levels for sustainable financing mechanisms for CHWs, including third-party and Medicaid reimbursement (George et al., 2020). Craig et al. (2019) argue that culturally safe and trustworthy environments are crucial to promote equity for pain patients who live in marginalized conditions. In our study, CHWs acted as credible providers, providing motivation, support, and connecting participants to other resources when needed. Increased involvement of CHWs in pain care may help to mitigate racialized disparities in pain treatment and outcomes.
Study Strengths and Limitations
Noteworthy strengths of this study include a randomized control condition, a range of validated preliminary efficacy outcomes along with indicators of feasibility, and a sample of older adults from a minoritized group that is frequently overlooked in pain research. Consistent with a community-engaged research approach (Key et al., 2019), the intervention was developed with robust input from the priority population, and results were shared with participants.
Some limitations should also be noted. Our study had a small sample, with short-term follow-up, and lacked an attention-control condition. We were not able to collect usage data on the website, as we did not require participants to log in, which would have been a barrier for some participants. This study took place during a lockdown period due to the COVID-19 pandemic; the level of program engagement and even the outcomes could have been different in more normal circumstances.
Positive affect, a key component of the Broaden and Build theory, was not measured in this pilot and will be essential to include in a larger efficacy trial of Positive STEPS. We also did not have the statistical power to formally examine mediators or a “booster” effect of positive activities on pain self-management skills and could not isolate the specific contributions of positive activities on outcomes. While our PROMIS outcome measures have been validated in diverse groups, we could not find psychometric information on our other outcome measures supporting their use in African American older adults. Additionally, our sample was made up of mostly women. There is evidence of a stronger link between positive affect and pain in women and therefore our findings might not generalize to men (Ong et al., 2020).
As is increasingly common in samples of African American adults, our participants were highly educated—more than half of participants had a bachelor’s degree or higher; therefore, we cannot assess how well the program would work if offered to a sample with less formal education. We note, however, that all program materials were written in plain language such that they were suitable for people with a range of literacy and health literacy levels, and that all-important content was also delivered orally, in the videos and by CHWs. Finally, while our findings were undeniably promising—and may be attributable to combining positive activities with established strategies for managing pain—Hausmann et al. (2018) rightly caution that there are a “growing number of studies suggesting that effects of positive psychological interventions reported in early studies are smaller or nonexistent in later replications.”
Conclusion
A CHW-led CPSM intervention combining positive activities with behavioral self-management skills training demonstrated the potential to enhance pain-related functioning among a vulnerable group of older adults. A larger trial will allow examination of the specific contribution of positive activities to outcomes as well as show how positive activities and self-management training may interact to bring about improvements across a range of pain-related outcomes.
Acknowledgments
We gratefully acknowledge our community health workers: Ms. Philesha Gough, Ms. Felicia Lane, and Ms. Linda Reyes; as well as our student assistants: Sarah Brandstadt, Greta Cheng, Sophia Janevic, Sarah Knapp, and Dr. Shuji Tsuda.
Contributor Information
Mary Janevic, Department of Health Behavior and Health Education, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Sheria G Robinson-Lane, Department of Systems, Populations, and Leadership, University of Michigan School of Nursing, Ann Arbor, Michigan, USA.
Rebecca Courser, Department of Health Behavior and Health Education, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Elizabeth Brines, Department of Health Behavior and Health Education, University of Michigan School of Public Health, Ann Arbor, Michigan, USA.
Afton L Hassett, Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, Michigan, USA.
Funding
This work was supported by several grants from the National Institute on Aging (K01 AG050706-01A1 to M. R. Janevic, TRIPLL [Translational Research Institute on Pain in Later Life] Pilot Grant 2P30AG022845-16, and P30 AG015281 [Michigan Center for Urban African American Aging Research]).
Conflict of Interest
None declared.
References
- Allen, K. D., Somers, T. J., Campbell, L. C., Arbeeva, L., Coffman, C. J., Cené, C. W., Oddone, E. Z., & Keefe, F. J. (2019). Pain coping skills training for African Americans with osteoarthritis: Results of a randomized controlled trial. Pain, 160(6), 1297–1307. doi: 10.1097/j.pain.000000001525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, Z. D., Krieger, N., Agénor, M., Graves, J., Linos, N., & Bassett, M. T. (2017). Structural racism and health inequities in the USA: Evidence and interventions. The Lancet. 389(10077), 1453–1463. doi: 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- Barnett, M. L., Gonzalez, A., Miranda, J., Chavira, D. A., & Lau, A. S. (2018). Mobilizing community health workers to address mental health disparities for underserved populations: A systematic review. Administration and Policy in Mental Health and Mental Health Services Research, 45(2), 195–211. doi: 10.1007/s10488-017-0815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett, M. L., Lau, A. S., & Miranda, J. (2018). Lay health worker involvement in evidence-based treatment delivery: A conceptual model to address disparities in care. Annual Review of Clinical Psychology, 14, 185–208. doi: 10.1146/annurev-clinpsy-050817-084825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley, E. J., Hossain, N. I., Gravlee, C. C., Sibille, K. T., Terry, E. L., Vaughn, I. A., Cardoso, J. S., Booker, S. Q., Glover, T. L., Goodin, B. R., Sotolongo, A., Thompson, K. A., Bulls, H. W., Staud, R., Edberg, J. C., Bradley, L. A., & Fillingim, R. B.(2019). Race/ethnicity moderates the association between psychosocial resilience and movement-evoked pain in knee osteoarthritis. ACR Open Rheumatology, 1(1), 16–25. doi: 10.1002/acr2.1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker, S. Q., Bartley, E. J., Powell-Roach, K., Palit, S., Morais, C., Thompson, O. J., Cruz-Almeida, Y., & Fillingim, R. B. (2021). The imperative for racial equality in pain science: A way forward. The Journal of Pain, 22(12), 1578–1585. doi: 10.1016/j.jpain.2021.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booker, S. Q., Tripp-Reimer, T., & Herr, K. A. (2020). “Bearing the pain”: The experience of aging African Americans with osteoarthritis pain. Global Qualitative Nursing Research, 7. doi: 10.1177/2333393620925793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills, L., & Stein, M. B. (2007). Psychometric analysis and refinement of the Connor–Davidson resilience scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of Traumatic Stress, 20(6), 1019–1028. doi: 10.1002/jts.20271 [DOI] [PubMed] [Google Scholar]
- Cella, D., Choi, S. W., Condon, D. M., Schalet, B., Hays, R. D., Rothrock, N. E., Yount, S., Cook, K. F., Gershon, R. C., Amtmann, D., DeWalt, D. A., Pilkonis, P. A., Stone, A. A., Weinfurt, K., & Reeve, B. B. (2019). PROMIS® adult health profiles: Efficient short-form measures of seven health domains. Value in Health, 22(5), 537–544. doi: 10.1016/j.jval.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadiha, L. A., Washington, O. G., Lichtenberg, P. A., Green, C. R., Daniels, K. L., & Jackson, J. S. (2011). Building a registry of research volunteers among older urban African Americans: Recruitment processes and outcomes from a community-based partnership. The Gerontologist, 51(Suppl. 1), S106–S115. doi: 10.1093/geront/gnr034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. X., Kroenke, K., Stump, T. E., Kean, J., Carpenter, J. S., Krebs, E. E., Bair, M. J., Damush, T. M., & Monahan, P. O. (2018). Estimating minimally important differences for the PROMIS pain interference scales: Results from 3 randomized clinical trials. Pain, 159(4), 775–782. doi: 10.1097/j.pain.0000000000001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, L. D., Bradley, K. A., & Boyko, E. J.(2004). Brief questions to identify patients with inadequate health literacy. Family Medicine, 36(8), 588–594. [PubMed] [Google Scholar]
- Chew, L. D., Griffin, J. M., Partin, M. R., Noorbaloochi, S., Grill, J. P., Snyder, A., Bradley, K. A., Nugent, S. M., Baines, A. D., & Vanryn, M. (2008). Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of General Internal Medicine, 23(5), 561–566. doi: 10.1007/s11606-008-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw, D. J., Essex, M. N., Pitman, V., & Jones, K. D. (2019). Reframing chronic pain as a disease, not a symptom: Rationale and implications for pain management. Postgraduate Medicine, 131(3), 185–198. doi: 10.1080/00325481.2019.1574403 [DOI] [PubMed] [Google Scholar]
- Connor, K. M., & Davidson, J. R. (2003). Development of a new resilience scale: The Connor–Davidson Resilience Scale (CD-RISC). Depression and Anxiety, 18(2), 76–82. doi: 10.1002/da.10113 [DOI] [PubMed] [Google Scholar]
- Craig, K. D., Holmes, C., Hudspith, M., Moor, G., Moosa-Mitha, M., Varcoe, C., & Wallace, B. (2020). Pain in persons who are marginalized by social conditions. Pain, 161(2), 261–265. doi: 10.1097/j.pain.0000000000001719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, J.R. (2018). Connor–Davidson Resilience Scale (CD-RISC) manual. www.cd-risc.com [Google Scholar]
- Dworkin, R. H., Turk, D. C., Farrar, J. T., Haythornthwaite, J. A., Jensen, M. P., Katz, N. P., Kerns, R. D., Stucki, G., Allen, R. R., Bellamy, N., Carr, D. B., Chandler, J., Cowan, P., Dionne, R., Galer, B. S., Hertz, S., Jadad, A. R., Kramer, L. D., Manning, D. C., … IMMPACT. (2005). Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain, 113(1), 9–19. doi: 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Fredrickson, B. L. (2001). The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. American Psychologist, 56(3), 218. doi: 10.1037//0003-066x.56.3.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, M. A., Homan, P. A., Garcia, C., Brown, T. H. (2021). The color of COVID-19: Structural racism and the disproportionate impact of the pandemic on older black and Latinx adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(3), e75–e80. doi: 10.1093/geronb/gbaa114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel, R. J., Peng, Y. B., Peters, M. L., Fuchs, P. N., & Turk, D. C. (2007). The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychological Bulletin, 133(4), 581. doi: 10.1037/0033-2909.133.4.581 [DOI] [PubMed] [Google Scholar]
- George, R., Gunn, R., Wiggins, N., Rowland, R., Davis, M. M., Maes, K., Kuzma, A., & McConnell, K. J. (2020). Early lessons and strategies from statewide efforts to integrate community health workers into Medicaid. Journal of Health Care for the Poor and Underserved, 31(2), 845–858. doi: 10.1353/hpu.2020.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenawalt, K. E., Orsega-Smith, E., Turner, J. L., Goodwin, S., & Rathie, E. J. (2019). The impact of “the art of happiness” class on community dwelling older adults: A positive psychology intervention. Activities, Adaptation & Aging, 43(2), 118–132. doi: 10.1080/01924788.2018.1493898 [DOI] [Google Scholar]
- Hanssen, M. M., Peters, M. L., Boselie, J. J., & Meulders, A. (2017). Can positive affect attenuate (persistent) pain? State of the art and clinical implications. Current Rheumatology Reports, 19(12), 1–9. doi: 10.1007/s11926-017-0703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, P. A., Taylor, R., Minor, B. L., Elliott, V., Fernandez, M., O’Neal, L., McLeod, L., Delacqua, G., Delacqua, F., Kirby, J., Duda, S. N., & REDCap Consortium. (2019). The REDCap Consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett, A. L. (2018). Remaining positive about positive psychological interventions for pain. JAMA Network Open, 1(5), e182531–e182531. doi: 10.1001/jamanetworkopen.2018.2531 [DOI] [PubMed] [Google Scholar]
- Hassett, A. L., & Finan, P. H. (2016). The role of resilience in the clinical management of chronic pain. Current Pain and Headache Reports, 20(6), 39. doi: 10.1007/s11916-016-0567-7 [DOI] [PubMed] [Google Scholar]
- Hausmann, L., Ibrahim, S. A., Kwoh, C. K., Youk, A., Obrosky, D. S., Weiner, D. K., Vina, E., Gallagher, R. M., Mauro, G. T., & Parks, A. (2018). Rationale and design of the Staying Positive with Arthritis (SPA) Study: A randomized controlled trial testing the impact of a positive psychology intervention on racial disparities in pain. Contemporary Clinical Trials, 64, 243–253. doi: 10.1016/j.cct.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann, L., Youk, A., Kwoh, C. K., Gallagher, R. M., Weiner, D. K., Vina, E. R., Obrosky, D. S., Mauro, G. T., McInnes, S., & Ibrahim, S. A. (2018). Effect of a positive psychological intervention on pain and functional difficulty among adults with osteoarthritis: A randomized clinical trial. JAMA Network Open, 1(5), e182533–e182533. doi: 10.1001/jamanetworkopen.2018.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann, L., Youk, A., Kwoh, C. K., Ibrahim, S. A., Hannon, M. J., Weiner, D. K., Gallagher, R. M., & Parks, A. (2017). Testing a positive psychological intervention for osteoarthritis. Pain Medicine, 18(10), 1908–1920. doi: 10.1093/pm/pnx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interagency Pain Research Coordinating Committee . (2016). National pain strategy. https://www.iprcc.nih.gov/sites/default/files/documents/NationalPainStrategy_508C.pdf
- Janevic, M., Robinson-Lane, S. G., Murphy, S. L., Courser, R., & Piette, J. D. (2021). A pilot study of a chronic pain self-management program delivered by community health workers to underserved African American older adults. Pain Medicine. Advance online publication. doi: 10.1093/pm/pnaa468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, R. N., Tommet, D., Ramirez, M., Jensen, R., & Teresi, J. A. (2016). Differential item functioning in Patient Reported Outcomes Measurement Information System® (PROMIS®) physical functioning short forms: Analyses across ethnically diverse groups. Psychological Test and Assessment Modeling, 58(2), 371. doi: 10.1037/t27840-000 [DOI] [Google Scholar]
- Kangovi, S., & Asch, D. A. (2018). The community health worker boom. NEJM Catalyst, 4(4). doi: 10.1056/CAT.18.0102 [DOI] [Google Scholar]
- Kangovi, S., Mitra, N., Grande, D., Long, J. A., & Asch, D. A. (2020). Evidence-based community health worker program addresses unmet social needs and generates positive return on investment: A return on investment analysis of a randomized controlled trial of a standardized community health worker program that addresses unmet social needs for disadvantaged individuals. Health Affairs, 39(2), 207–213. doi: 10.1377/hlthaff.2019.00981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key, K. D., Furr-Holden, D., Lewis, E. Y., Cunningham, R., Zimmerman, M. A., Johnson-Lawrence, V., & Selig, S. (2019). The continuum of community engagement in research: A roadmap for understanding and assessing progress. Progress in Community Health Partnerships: Research, Education, and Action, 13(4), 427–434. doi: 10.1353/cpr.2019.0064 [DOI] [PubMed] [Google Scholar]
- Lapidos, A., Lapedis, J., & Heisler, M. (2019). Realizing the value of community health workers—New opportunities for sustainable financing. The New England Journal of Medicine, 380(21), 1990. doi: 10.1056/NEJMp1815382 [DOI] [PubMed] [Google Scholar]
- Lejuez, C. W., Hopko, D. R., & Hopko, S. D. (2001). A brief behavioral activation treatment for depression. Behavior Modification, 25(2), 255–86. doi: 10.1177/0145445501252005 [DOI] [PubMed] [Google Scholar]
- Maly, A., & Vallerand, A. H. (2018). Neighborhood, socioeconomic, and racial influence on chronic pain. Pain Management Nursing, 19(1), 14–22. doi: 10.1016/j.pmn.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, M. E., & Yong, R. J. (2021). Racial and ethnic disparities in the treatment of chronic pain. Pain Medicine, 22(1), 75–90. doi: 10.1093/pm/pnaa427 [DOI] [PubMed] [Google Scholar]
- Nicholas, M. K.(2007). The pain self-efficacy questionnaire: Taking pain into account. European Journal of Pain, 11(2), 153–163. doi: 10.1016/j.ejpain.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Ong, A. D., Thoemmes, F., Ratner, K., Ghezzi-Kopel, K., & Reid, M. C. (2020). Positive affect and chronic pain: A preregistered systematic review and meta-analysis. Pain, 161(6), 1140. doi: 10.1097/j.pain,000000001828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M. S., Polsky, D., Kennedy, E. H., Small, D. S., Evans, C. N., Rareshide, C. A., & Volpp, K. G. (2020). Smartphones vs wearable devices for remotely monitoring physical activity after hospital discharge: A secondary analysis of a randomized clinical trial. JAMA Network Open, 3(2), e1920677–e1920677. doi: 10.1001/jamanetworkopen.2019.2067 [DOI] [PubMed] [Google Scholar]
- Peretz, P. J., Islam, N., & Matiz, L. A. (2020). Community health workers and COVID-19—Addressing social determinants of health in times of crisis and beyond. New England Journal of Medicine, 383, e108. doi: 10.1056/NEJMp2022641 [DOI] [PubMed] [Google Scholar]
- Perry, H. B., Zulliger, R., & Rogers, M. M. (2014). Community health workers in low-, middle-, and high-income countries: An overview of their history, recent evolution, and current effectiveness. Annual Review of Public Health, 35, 399–421. doi: 10.1146/annurev-publhealth-032013-182354 [DOI] [PubMed] [Google Scholar]
- Peters, M. L., Smeets, E., Feijge, M., van Breukelen, G., Andersson, G., Buhrman, M., & Linton, S. J. (2017). Happy despite pain: A randomized controlled trial of an 8-week internet-delivered positive psychology intervention for enhancing well-being in patients with chronic pain. The Clinical Journal of Pain, 33(11), 962. doi: 10.1097/AJP.0000000000000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicow, K., Baranowski, T., Ahluwalia, J. S., & Braithwaite, R. L. (1999). Cultural sensitivity in public health: defined and demystified. Ethnicity & Disease, 9(1), 10–21. [PubMed] [Google Scholar]
- Robinson-Lane, S. G. (2020). Adapting to chronic pain: A focused ethnography of Black older adults. Geriatric Nursing, 41(4), 468–473. doi: 10.1016/j.gerinurse.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Lane, S. G. Hill-Jarrett, T. G., Janevic, M. R. (2021). “Ooh, you got to holler sometime” pain meaning and experiences of Black older adults. In van Rysweyk S (Ed.), Meanings of pain volume 3: Vulnerable or special groups of people. Springer International Publishing AG. [Google Scholar]
- Schrubbe, L. A., Ravyts, S. G., Benas, B. C., Campbell, L. C., Cené, C. W., Coffman, C. J., Gunn, A. H., Keefe, F. J., Nagle, C. T., Oddone, E. Z., Somers, T. J., Stanwyck, C. L., Taylor, S. S., & Allen, K. D. (2016). Pain coping skills training for African Americans with osteoarthritis (STAART): Study protocol of a randomized controlled trial. BMC Musculoskeletal Disorders, 17(1), 359. doi: 10.1186/s12891-016-1217-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. L., & Hanni, A. A. (2019). Effects of a savoring intervention on resilience and well-being of older adults. Journal of Applied Gerontology, 38(1), 137–152. doi: 10.1177/0733464817693375 [DOI] [PubMed] [Google Scholar]
- Stensland, M. (2021). “If you don’t keep going, you’re gonna die”: Helplessness and perseverance among older adults living with chronic low back pain. The Gerontologist, 61(6), 907–916. doi: 10.1093/geront/gnaa150 [DOI] [PubMed] [Google Scholar]
- Swain, N., Lennox Thompson, B., Gallagher, S., Paddison, J., & Mercer, S. (2020). Gratitude Enhanced Mindfulness (GEM): A pilot study of an internet-delivered programme for self-management of pain and disability in people with arthritis. The Journal of Positive Psychology, 15(3), 420–426. doi: 10.1080/17439760.2019.1627397 [DOI] [Google Scholar]
- Teresi, J. A., & Reeve, B. B. (2016). Epilogue to the two-part series: Measurement equivalence of the Patient Reported Outcomes Measurement Information System® (PROMIS®) short forms. Psychological Test and Assessment Modeling, 58(2), 423. [PMC free article] [PubMed] [Google Scholar]
- Williams, D. A., Kuper, D., Segar, M., Mohan, N., Sheth, M., & Clauw, D. J. (2010). Internet-enhanced management of fibromyalgia: A randomized controlled trial. Pain, 151(3), 694–702. doi: 10.1016/j.pain.2010.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]