Abstract
The increasing prevalence of obesity is a worldwide health concern. Although obesity primarily affects the development of cardiometabolic disorders, it has also been closely linked to chronic kidney disease (CKD). However, potential causal relationships between obesity and CKD remain unclear, as obesity and CKD share a number of common risk factors. Accordingly, the risk of incident CKD in obese people without metabolic abnormalities, also called “metabolically healthy obesity” (MHO), has been a matter of interest. Recent investigations found that MHO was related to increased risk of incident CKD; however, the conclusions were based primarily on the static state. According to previous publications, approximately one-half of people initially identified as MHO became metabolically unhealthy, while one-tenth reduced their body weight to normal range while remaining metabolically healthy. It is essential to consider these transitions in obese-metabolic health status when analyzing obesity-related outcomes. This review discusses research on obesity and metabolic health in patients with CKD. Furthermore, we summarize recent reports on the implications of obesity and metabolic health in CKD and discuss the possible mechanisms of their relationship with CKD.
Keywords: Chronic kidney disease, Metabolic syndrome, Obesity
INTRODUCTION
The rapidly increasing prevalence of obesity is a global health concern. The World Health Organization declared obesity a major public health problem and a global epidemic in 1997.1 Furthermore, the prevalence of obesity has increased dramatically during the last four decades. More alarming is that if this trend continues, the majority of the world’s adult population will be either overweight or obese by 2030. Obesity carries a considerable health burden as it contributes to numerous comorbidities, including diabetes mellitus, hypertension, cardiovascular disease (CVD), and some cancers.2 Researchers and healthcare providers have proposed a range of interventions to combat the obesity epidemic.3 They have urged people, particularly children and students, not to consume unhealthy foods by imposing new taxes and by providing subsidies for healthy foods.4 However, the success of this worldwide mission is uncertain; in fact, no major population success has been shown. Despite several efforts to curb the obesity pandemic and its consequences, obesity continues to be a serious public health problem on a global scale.5-7 This review describes recent evidence on the implications of obesity on chronic kidney disease (CKD) in consideration of metabolic health status.
HEALTH OUTCOMES OF OBESITY
The concept of obesity as a disease describes two kinds of medical problems: those caused by the mass of adipose tissue itself and those caused by the metabolic actions of adipose tissue.2 The former includes social difficulties caused by obesity stigma,8 sleep apnea caused partly by excessive parapharyngeal adipose tissue,9 and joint problems caused by carrying excess body weight.10 The second group is largely related to insulin resistance and the long-term impacts of products generated by larger fat cells.2 Insulin resistance is common in obesity, which is mainly caused by increased release of fatty acids from adipocytes and subsequent fat accumulation in muscle or liver.11 Finally, diabetes occurs when the pancreas’ secretory function is exhausted by the fight against insulin resistance.11 Cytokines, notably interleukin-6 and tumor necrosis factor-alpha (TNF-α), released by fat cells may contribute to the proinflammatory state associated with obesity.2,12 Secretion of prothrombin activator inhibitor-1 from adipocytes is increased in obese people, which contributes to obesity’s procoagulant state and may lead to increased risk of hypertension and CVD in combination with changes in endothelial function.13 In the case of cancer, increased estrogen production by the enlarged stromal mass contributes to the risk of breast cancer.14 Increased cytokine release and chronic inflammation in obese patients may also contribute to various types of proliferation and eventual development of several types of malignancies.15 When the pathogenic implications of greater fat deposits are combined, the result is an increased chance of premature death.2
OBESITY AND KIDNEY DISEASE
Obesity is a well-known risk factor for kidney diseases. A meta-analysis showed that individuals who were overweight had a 40% increased risk of kidney diseases compared to individuals with normal weight (pooled risk ratio [RR], 1.40; 95% confidence interval [CI], 1.30–1.50) after adjusting for conventional risk factors, while individuals with obesity are at even greater risk (RR, 1.83; 95% CI, 1.57–2.13).16 The kidney diseases included in the abovementioned analysis were CKD, end-stage renal disease, kidney stones, kidney cancer, and renal cell carcinoma.16 A recent meta-analysis focusing on CKD included 39 cohorts totaling 630,677 individuals with an average follow-up of 6.8 years.17 The authors showed that the probability of developing new-onset low estimated glomerular filtration rate (GFR; under 60 mL/min/1.73 m2) and albuminuria was 28% and 51% higher, respectively, in participants with obesity.17
Obesity is related to several cardiometabolic derangements such as diabetes mellitus, hypertension, and dyslipidemia. Metabolic abnormalities (i.e., metabolic syndrome components) are linked to the development of kidney disease. In 2004, the National Health and Nutrition Examination Survey (NHANES) III, a cross-sectional study that included 6,217 participants, found that the risk of CKD and microalbuminuria rose gradually as the number of metabolic syndrome components increased.18 Elevated blood pressure, low high-density lipoprotein cholesterol, high triglyceride levels, and abdominal obesity were related to a higher odds ratio of chronic renal disease. Participants with 2, 3, 4, and 5 metabolic syndrome components exhibited higher odds for CKD (2.21, 3.38, 4.23, and 5.85, respectively) compared to those with none or only one component.18 Compared to those without metabolic syndrome, people with metabolic syndrome were at 2.60-fold greater risk of CKD. The results were constant even after adjusting for body mass index.18 However, the link between obesity and kidney disease may be mediated by metabolic risk factors. To better understand the impact of obesity on kidney disease, recent research including ours has focused on a more specific kind of obesity distinguished by metabolic abnormalities.19-23
CONCEPT OF METABOLICALLY HEALTHY OBESITY
A distinct population of people with obesity has recently been reported in the medical literature as being more resistant to developing the metabolic problems associated with obesity. Despite having excessive body adiposity, these individuals, now defined as “metabolically healthy obesity” (MHO), have a positive metabolic profile characterized by high levels of insulin sensitivity, no hypertension, and good lipid, inflammatory, hormonal, liver enzyme, and immunological profiles.24 However, it remains to be determined whether MHO is a distinct and persistent phenotype and whether MHO has clinical implications for the prediction of cardiometabolic disease risk and other obesity-related comorbidities.25-30 Despite its uncertain clinical relevance, the concept of MHO could be utilized as a model to better understand the pathways that link obesity to cardiometabolic diseases.31
The primary hurdle to understanding the MHO phenotype and its long-term metabolic consequences is the disparity in study definitions of metabolic health and obesity.32-35 When exploring the question of whether people with MHO are actually healthy, it is critical to acknowledge that the criteria used to determine MHO vary from study to study.32-36 These diverse MHO definitions pose a substantial barrier to interpreting study findings on the association of this phenotype with cardiometabolic outcomes and mortality.30,31,36-39 For example, more than 40% of the participants in the NHANES III program were classified as MHO using the National Cholesterol Education Program Adult Treatment Panel III metabolic syndrome criteria.31,40 However, only 20% were classified as MHO using more stringent insulin sensitivity parameter cutoffs.31,41 Because of the difficulty identifying MHO, it is possible that MHO might not constitute a separate biologically defined subset of people with obesity. Recent evidence showing that the MHO phenotype is not a benign state appears to support the idea that MHO should not be treated differently from obesity with established type 2 diabetes, CVD, or both.28,42-44 Regarding the risk of CKD, the definition of MHO across research was less heterogeneous; however, there is still discordance regarding the definition (Table 1). For instance, the inclusion of homeostasis model assessment of Insulin resistance and the number of metabolic abnormalities used to define metabolic health are different among studies. These disparities could eventually limit the interpretation of this phenotype’s influence on CKD risk (Table 1).
Table 1.
Criteria used to define metabolically healthy obesity in investigations on its impact on chronic kidney disease
| Variable | Jung et al. (2015)22 | Hashimoto et al. (2015)20 | Chang et al. (2016)21 | Wang et al. (2022)23 |
|---|---|---|---|---|
| Metabolic component | ||||
| BP (mmHg) | ≥ 130/85 or treatment | ≥ 130/85 or treatment | ≥ 130/85 or treatment | Diagnosis of HTN |
| FPG (mg/dL) | ≥ 100 or treatment | ≥ 100 or treatment | ≥ 100 or treatment | Diagnosis of DM |
| TG (mg/dL) | ≥ 150 or treatment | ≥ 150 or treatment | ≥ 150 or treatment | ≥ 200 |
| HDL-C (mg/dL) | < 40 (M)/50 (F) | < 40 (M)/50 (F) | < 40 (M)/50 (F) | < 40 |
| HOMA-IR | - | - | ≥ 2.5 | - |
| Other | Diagnosis of dyslipidemia TC ≥ 240 mg/dL LDL-C ≥ 160 mg/dL |
|||
| Metabolic health criteria | < 2 of the above | < 2 of the above | None of the above | None of the above |
| Obesity component | ||||
| BMI (kg/m2) | ≥ 25 | ≥ 25 | ≥ 25 | ≥ 30 |
BP, blood pressure; HTN, hypertension; FPG, fasting plasma glucose; DM, diabetes mellitus; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of Insulin resistance; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; BMI, body mass index.
MHO AND CKD RISK
Prior studies have shown that people with MHO are not at elevated risk of cardiometabolic diseases or death when compared to people of normal weight.33,34,36,45 However, the predictive utility of MHO is debatable and faces considerable challenges.46,47 Furthermore, the implications of MHO may be affected by the health outcomes being studied.47 Thus far, inconsistent and conflicting results have been reported in terms of CKD risk in people with MHO.20-23 A Japanese study conducted in 3,136 individuals with an 8-year follow-up demonstrated that those with MHO were not at increased risk of CKD.20 According to their findings, when compared to the metabolically healthy non-obese (MHNO) phenotype, the odds ratio for incident CKD was only 0.83 (95% CI, 0.36–1.72; P=0.64) for MHO.20 On the other hand, recent research has found a link between the MHO phenotype and incident CKD.21-23 In a large cohort study of metabolically healthy Korean adults, the researchers revealed that metabolically healthy individuals who were overweight or obese had an elevated risk of CKD compared with normal weight participants.21 The multivariable-adjusted differences in the 5-year cumulative incidence of CKD in individuals who were underweight, overweight, and obese were –4.0 (95% CI, –7.8 to –0.3), 3.5 (95% CI, 0.9 to –6.1), and 6.7 (95% CI, 3.0 to –10.4) cases per 1,000 people, respectively.21 These correlations were found in all clinically different categories.21 Another Korean longitudinal research indicated that the incidence of CKD was higher in those who were MHO than in those without obesity.22 When compared to the MHNO group, the MHO group had a multivariate-adjusted hazard ratio (HR) of 1.38 (95% CI, 1.01–1.87).22 Furthermore, in fully adjusted analyses, no differences were seen between MHO individuals and their metabolically unhealthy non-obese (MUNO) counterparts.22 These data imply that a good metabolic profile does not protect persons with obesity from developing CKD.
A recent population-based cohort study in a British primary-care population discovered that individuals who were overweight or obese without metabolic abnormalities had a higher risk of incident CKD than those with normal body weight and no metabolic abnormalities, with an adjusted HR compared to metabolically healthy normal weight of 1.30 (95% CI, 1.28 to –1.33) in metabolically healthy overweight and 1.66 (95% CI, 1.62–1.70) in MHO.23 This discordant results among studies might be caused, in part or entirely, by the previously described discordant definitions of MHO. Different research population variables, such as study cohort ethnicity, might be another reason; nevertheless, studies limited to Asian populations also reported inconsistent findings.20-23 Potential misclassification due to delayed detection, as well as residual confounding due to unmeasured factors, might all have influenced study results.
DYNAMIC NATURE OF OBESE METABOLIC HEALTH PHENOTYPES AND CKD RISK
A participant’s health status can fluctuate between metabolically healthy and metabolically unhealthy.32 According to Soriguer et al.,48 the baseline prevalence of the MHO phenotype was between 3.0% and 16.9%, depending on the criteria used. Additionally, 30% to 40% of persons with MHO at initial assessment progressed to an unhealthy state after 6 years of follow-up. Similarly, a prospective community-based cohort study in Korea reported that a number of individuals who were classified as MHO at baseline transitioned to unhealthy states eventually.49 Furthermore, a prospective cohort study of 4,056 adults free from atherosclerotic CVD events found that approximately one-third of obese healthy subjects evolved to a metabolically unhealthy obesity (MUO) phenotype, and subjects who experienced this deterioration of metabolic health were at a higher risk for type 2 diabetes compared to people who maintained their MHO status.50
Because there is mounting evidence that MHO is not a permanent state, some investigators have focused on predictors for metabolic deterioration or improvement. As expected, increases in anthropometric measurements such as waist-to-hip ratio, as well as conventional measurements including body mass index and waist circumference, predicted a shift from MHO to MUO.51 In contrast, a healthy lifestyle, such as a balanced diet, a high level of physical exercise, or not smoking, protected against this shift.51 Higher levels of plasma insulin, excessive visceral fat, and lower high-density lipoprotein cholesterol levels at baseline were risk factors for the development of metabolic unhealth,52 while a healthier lifestyle, less abdominal or ectopic adiposity, less chronic inflammation, better insulin sensitivity and greater incretin response to meals protected against MUO.33,34,36,45 Maintaining these characteristics may help people with MHO avoid transitioning to MUO.
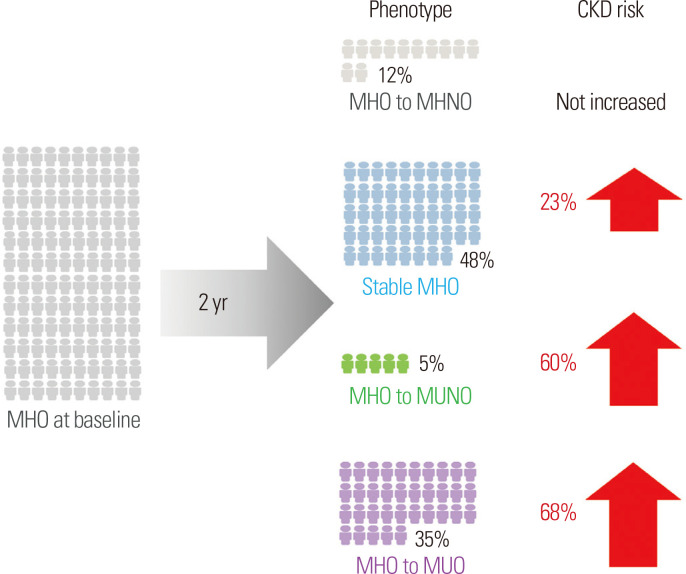
Given this background, our research team explored the influence of phenotypic transitions on the risk of developing CKD among individuals with MHO using a Korean national health screening examination cohort dataset.19 Even after full adjustment, the risk of incident CKD was significantly higher in the MHO group than in the MHNO group (multivariate-adjusted HR, 1.23; 95% CI, 1.12–1.36). However, the majority of MHO participants exhibited phenotypic shifts on the following biennial health examination (12.1%, MHO to MHNO; 5.5%, MHO to MUNO; and 34.8%, MHO to MUO) (Fig. 1).19 Furthermore, we demonstrated that phenotypic change was a predictor of the likelihood of incident CKD.19 More specifically, the transition from MHO to MHNO, which entails losing weight while maintaining metabolic fitness, protects persons from developing CKD (multivariate-adjusted HR for CKD in the MHO to MHNO group vs. the stable MHNO group, 0.98; 95% CI, 0.72–1.32). People who remained obese or progressed to a metabolically unhealthy state (stable MHO, MHO to MUNO, and MHO to MUO groups) were all at elevated risk of CKD (Fig. 1).19 Our data show that MHO is a dynamic disease in a diverse patient population. Although the MHO phenotype has been linked to incident CKD, maintaining metabolic health and decreasing body weight may reduce the risk of CKD. As a result, risk evaluation for incident CKD is necessary in MHO patients, and physicians should encourage them to maintain a healthy lifestyle to preserve metabolic fitness and lower body weight.
Figure 1.
Phenotypic transitions in individuals with MHO and the risk of chronic kidney disease.19 CKD, chronic kidney disease; MHO, metabolically healthy obesity; MHNO, metabolically healthy non-obesity; MUNO, metabolically unhealthy non-obesity; MUO, metabolically unhealthy obesity.
MECHANISMS LINKING OBESITY AND CKD
Obesity may indirectly increase CKD development by raising the risk of hypertension, atherosclerosis, and diabetes. Metabolic syndrome, a collection of metabolic derangements, is also a well-known contributor to CKD development.22,53-56 Because people with obesity frequently have several components of metabolic syndrome, the independent impact of obesity on renal function is sometimes difficult to pin down. Our prior investigation demonstrated that patients with a persistent MHO status had a higher risk of developing CKD than those with a stable MHNO status, suggesting that obesity plays a role in the incidence of CKD.19 Whereas the relationship between obesity and CKD is mainly derived from combined metabolic unfitness, alternative pathways that directly connect excessive adiposity to kidney injury may exist.19
Obesity-induced kidney injury has been linked to alterations in renal hemodynamics.57-59 In 2020, a Japanese research team measured single-nephron GFR (SNGFR) and single-nephron urinary protein excretion to investigate the pathophysiology of obesity-related glomerulopathy (ORG).59 Patients with ORG had enlarged glomeruli with lowered glomerular density and glomerulosclerosis, in spite of their preserved kidney function.59 Interestingly, patients with ORG exhibited greater estimated total GFR than controls, resulting in increased SNGFR and considerably increased 24-hour urine protein excretion.59 As a result, as obesity persists, the glomeruli widen, and podocyte hypertrophy develops because they cannot indefinitely divide and de-differentiate to fill the increased glomerular capillary loops.59 Eventually, the podocytes are unable to perform hypertrophy, resulting in a devastating cycle involving foot process effacement, podocyte detachment, and increased glomerular permeability.59 The glomerular tuft collapses after extensive podocyte separation, resulting in global glomerulosclerosis and loss of function.59 Aside from the alterations in renal hemodynamics, current research suggests that hormones and cytokines released by adipose tissue also contribute to CKD.19 Leptin and adiponectin are adipokines related to renal function, and TNF-α, interleukin-6, and plasminogen activator inhibitor-1 are also adipose tissue-derived molecules that have been shown to impair renal function.60,61 Although it remains unclear whether MHO individuals actually have altered levels of these factors, these mechanisms are possible pathophysiologies of CKD in obese people and merit further studies.62-66
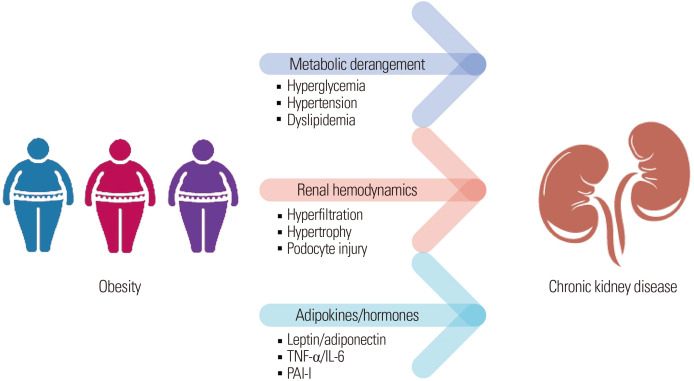
In summary, the first mechanism between obesity and CKD is unavoidably the metabolic derangements associated with obesity, since our results indicate that metabolic unhealthiness is a key contributor to CKD risk in patients with obesity.19 However, some studies, including ours, revealed that patients with stable MHO had slightly greater risk of developing CKD as well, indicating that obesity itself plays a role in CKD development. Alternative mechanisms that directly relate obesity to CKD may exist; possible explanations include hemodynamic changes that contribute to hyperfiltration as well as alterations in adipokines and hormones. The possible pathomechanisms between obesity and renal disease are outlined in Fig. 2.
Figure 2.
Summary of possible mechanisms linking obesity and chronic kidney disease. TNF-α, tumor necrosis factor-alpha; IL, interleukin; PAI-I, plasminogen activator inhibitor-1.
CONCLUSION
Considering the rapidly growing prevalence of obesity in Korea and the strong association between obesity and morbidities, assessing and preventing obesity-related complications is critical. Obesity adversely affects renal function through accompanying metabolic unhealthiness and other mechanisms. Prior studies suggested that maintaining metabolic health and losing weight may alleviate CKD risk in patients with obesity. Therefore, physicians should screen patients with obesity for CKD development and make recommendations regarding a healthy weight and lifestyle changes.
Footnotes
CONFLICTS OF INTEREST
Chang Hee Jung is an Associate Editor of the journal. However, he was not involved in peer reviewer selection, evaluation, or the decision process for this article. There are no other potential conflicts of interest relevant to this article to report.
AUTHOR CONTRIBUTIONS
Study concept and design: CHJ; acquisition of data: YKC; analysis and interpretation of data: YKC; drafting of the manuscript: YKC; critical revision of the manuscript: CHJ; and study supervision: CHJ.
REFERENCES
- 1.World Health Organization, author. Obesity: preventing and managing the global epidemic. World Health Organization; Geneva: 2000. [PubMed] [Google Scholar]
- 2.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–9. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 3.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. GBD 2015 Obesity Collaborators, author. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkes C, Smith TG, Jewell J, Wardle J, Hammond RA, Friel S, et al. Smart food policies for obesity prevention. Lancet. 2015;385:2410–21. doi: 10.1016/S0140-6736(14)61745-1. [DOI] [PubMed] [Google Scholar]
- 5.Nam GE, Kim YH, Han K, Jung JH, Rhee EJ, Lee WY, et al. Obesity fact sheet in Korea, 2020: prevalence of obesity by obesity class from 2009 to 2018. J Obes Metab Syndr. 2021;30:141–8. doi: 10.7570/jomes21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017;5:161. doi: 10.21037/atm.2017.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyo-Johnson C, Mincey KD. Obesity epidemiology worldwide. Gastroenterol Clin North Am. 2016;45:571–9. doi: 10.1016/j.gtc.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puhl RM, Heuer CA. Obesity stigma: important considerations for public health. Am J Public Health. 2010;100:1019–28. doi: 10.2105/AJPH.2009.159491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185–92. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons: a scoping review. Obes Rev. 2014;15:578–86. doi: 10.1111/obr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 12.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–56. doi: 10.1079/PNS2001110. [DOI] [PubMed] [Google Scholar]
- 13.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–7. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 14.Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–92. doi: 10.1677/erc.1.00729. [DOI] [PubMed] [Google Scholar]
- 15.Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7:2346–59. doi: 10.7150/jca.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91:1224–35. doi: 10.1016/j.kint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–74. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 19.Cho YK, Lee J, Kim HS, Park JY, Lee WJ, Kim YJ, et al. Impact of transition in metabolic health and obesity on the incident chronic kidney disease: a nationwide cohort study. J Clin Endocrinol Metab. 2020;105:dgaa033. doi: 10.1210/clinem/dgaa033. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto Y, Tanaka M, Okada H, Senmaru T, Hamaguchi M, Asano M, et al. Metabolically healthy obesity and risk of incident CKD. Clin J Am Soc Nephrol. 2015;10:578–83. doi: 10.2215/CJN.08980914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y, Ryu S, Choi Y, Zhang Y, Cho J, Kwon MJ, et al. Metabolically healthy obesity and development of chronic kidney disease: a cohort study. Ann Intern Med. 2016;164:305–12. doi: 10.7326/M15-1323. [DOI] [PubMed] [Google Scholar]
- 22.Jung CH, Lee MJ, Kang YM, Hwang JY, Kim EH, Park JY, et al. The risk of chronic kidney disease in a metabolically healthy obese population. Kidney Int. 2015;88:843–50. doi: 10.1038/ki.2015.183. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Niratharakumar K, Gokhale K, Tahrani AA, Taverner T, Thomas GN, et al. Obesity without metabolic abnormality and incident CKD: a population-based British cohort study. Am J Kidney Dis. 2022;79:24–35.e1. doi: 10.1053/j.ajkd.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–81. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167:642–8. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 26.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–12. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 27.van Vliet-Ostaptchouk JV, Nuotio ML, Slagter SN, Doiron D, Fischer K, Foco L, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9. doi: 10.1186/1472-6823-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magkos F. Metabolically healthy obesity: what's in a name? Am J Clin Nutr. 2019;110:533–9. doi: 10.1093/ajcn/nqz133. [DOI] [PubMed] [Google Scholar]
- 29.Rey-López JP, de Rezende LF, Pastor-Valero M, Tess BH. The prevalence of metabolically healthy obesity: a systematic review and critical evaluation of the definitions used. Obes Rev. 2014;15:781–90. doi: 10.1111/obr.12198. [DOI] [PubMed] [Google Scholar]
- 30.Eckel N, Meidtner K, Kalle-Uhlmann T, Stefan N, Schulze MB. Metabolically healthy obesity and cardiovascular events: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:956–66. doi: 10.1177/2047487315623884. [DOI] [PubMed] [Google Scholar]
- 31.Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41:bnaa004. doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung CH, Lee WJ, Song KH. Metabolically healthy obesity: a friend or foe? Korean J Intern Med. 2017;32:611–21. doi: 10.3904/kjim.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14:219–27. doi: 10.1007/s11154-013-9252-x. [DOI] [PubMed] [Google Scholar]
- 34.Samocha-Bonet D, Dixit VD, Kahn CR, Leibel RL, Lin X, Nieuwdorp M, et al. Metabolically healthy and unhealthy obese: the 2013 Stock Conference report. Obes Rev. 2014;15:697–708. doi: 10.1111/obr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171:1–29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 36.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–62. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 37.Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90,257 women (the Nurses' Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6:714–24. doi: 10.1016/S2213-8587(18)30137-2. [DOI] [PubMed] [Google Scholar]
- 38.Mørkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trøndelag Health Study), Norway. J Am Coll Cardiol. 2014;63:1071–8. doi: 10.1016/j.jacc.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 39.Mongraw-Chaffin M, Foster MC, Anderson CA, Burke GL, Haq N, Kalyani RR, et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2018;71:1857–65. doi: 10.1016/j.jacc.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, author. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 41.Durward CM, Hartman TJ, Nickols-Richardson SM. All-cause mortality risk of metabolically healthy obese individuals in NHANES III. J Obes. 2012;2012:460321. doi: 10.1155/2012/460321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, et al. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70:1429–37. doi: 10.1016/j.jacc.2017.07.763. [DOI] [PubMed] [Google Scholar]
- 43.Rey-López JP, de Rezende LF, de Sá TH, Stamatakis E. Is the metabolically healthy obesity phenotype an irrelevant artifact for public health? Am J Epidemiol. 2015;182:737–41. doi: 10.1093/aje/kwv177. [DOI] [PubMed] [Google Scholar]
- 44.Blüher M. Obesity: the myth of innocent obesity. Nat Rev Endocrinol. 2017;13:691–2. doi: 10.1038/nrendo.2017.146. [DOI] [PubMed] [Google Scholar]
- 45.Blüher M. Are metabolically healthy obese individuals really healthy? Eur J Endocrinol. 2014;171:R209–19. doi: 10.1530/EJE-14-0540. [DOI] [PubMed] [Google Scholar]
- 46.Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care. 2013;36:2294–300. doi: 10.2337/dc12-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinnouho GM, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J. 2015;36:551–9. doi: 10.1093/eurheartj/ehu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soriguer F, Gutiérrez-Repiso C, Rubio-Martín E, García-Fuentes E, Almaraz MC, Colomo N, et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J Clin Endocrinol Metab. 2013;98:2318–25. doi: 10.1210/jc.2012-4253. [DOI] [PubMed] [Google Scholar]
- 49.Lee SK, Kim SH, Cho GY, Baik I, Lim HE, Park CG, et al. Obesity phenotype and incident hypertension: a prospective community-based cohort study. J Hypertens. 2013;31:145–51. doi: 10.1097/HJH.0b013e32835a3637. [DOI] [PubMed] [Google Scholar]
- 50.Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, et al. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36:2388–94. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schröder H, Ramos R, Baena-Díez JM, Mendez MA, Canal DJ, Fíto M, et al. Determinants of the transition from a cardiometabolic normal to abnormal overweight/obese phenotype in a Spanish population. Eur J Nutr. 2014;53:1345–53. doi: 10.1007/s00394-013-0635-2. [DOI] [PubMed] [Google Scholar]
- 52.Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, et al. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes (Lond) 2015;39:1365–70. doi: 10.1038/ijo.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prasad GV. Metabolic syndrome and chronic kidney disease: current status and future directions. World J Nephrol. 2014;3:210–9. doi: 10.5527/wjn.v3.i4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nashar K, Egan BM. Relationship between chronic kidney disease and metabolic syndrome: current perspectives. Diabetes Metab Syndr Obes. 2014;7:421–35. doi: 10.2147/DMSO.S45183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas G, Sehgal AR, Kashyap SR, inivas TR, Sr, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6:2364–73. doi: 10.2215/CJN.02180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. The number of metabolic syndrome components is a good risk indicator for both early- and late-stage kidney damage. Nutr Metab Cardiovasc Dis. 2014;24:277–85. doi: 10.1016/j.numecd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550–62. doi: 10.2215/CJN.04071206. [DOI] [PubMed] [Google Scholar]
- 58.Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33:14–22. doi: 10.1016/j.semnephrol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Okabayashi Y, Tsuboi N, Sasaki T, Haruhara K, Kanzaki G, Koike K, et al. Single-nephron GFR in patients with obesity-related glomerulopathy. Kidney Int Rep. 2020;5:1218–27. doi: 10.1016/j.ekir.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garland JS. Elevated body mass index as a risk factor for chronic kidney disease: current perspectives. Diabetes Metab Syndr Obes. 2014;7:347–55. doi: 10.2147/DMSO.S46674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunley TE, Ma LJ, Kon V. Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens. 2010;19:227–34. doi: 10.1097/MNH.0b013e3283374c09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98:E1610–9. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 63.Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 2006;30:1529–34. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 64.Cӑtoi AF, Pârvu AE, Andreicuț AD, Mironiuc A, Crӑciun A, Cӑtoi C, et al. Metabolically healthy versus unhealthy morbidly obese: chronic inflammation, nitro-oxidative stress, and insulin resistance. Nutrients. 2018;10:1199. doi: 10.3390/nu10091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basurto L, Sánchez L, Díaz A, Valle M, Robledo A, Martínez-Murillo C. Differences between metabolically healthy and unhealthy obesity in PAI-1 level: fibrinolysis, body size phenotypes and metabolism. Thromb Res. 2019;180:110–4. doi: 10.1016/j.thromres.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Gómez-Ambrosi J, Catalán V, Rodríguez A, Andrada P, Ramírez B, Ibáñez P, et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care. 2014;37:2813–21. doi: 10.2337/dc14-0937. [DOI] [PubMed] [Google Scholar]