Abstract
Introduction
Fucosyl-GM1 is a monosialoganglioside with limited expression in healthy tissues and high expression on SCLC cells. BMS-986012 is a nonfucosylated, first-in-class, fully human immunoglobulin G1 monoclonal antibody that binds to fucosyl-GM1.
Methods
CA001-030 is a phase 1/2, first-in-human study of BMS-986012 as monotherapy or in combination with nivolumab for adults with relapsed or refractory SCLC. Safety is the primary end point. Additional end points include objective response rate, duration of response, progression-free survival, pharmacokinetics, and overall survival.
Results
Patients (BMS-986012 monotherapy, n = 77; BMS-986012 + nivolumab, n = 29) were predominantly of male sex (58%), 63 years old (mean), current or past tobacco users (97%), and treated previously with first-line systemic therapy (99%). The most common treatment-related adverse event was pruritus (n = 95 [90%]). Grade 4 treatment-related adverse events were reported in 2% (n = 2) of patients. The objective response rate (95% confidence interval [CI]) was higher with BMS-986012 plus nivolumab (38% [20.7%–57.7%]) than with monotherapy (4% [0.8%–11.0%]). Median (95% CI) duration of response with BMS-986012 plus nivolumab was 26.4 (4.4–not reached) months. Progression-free survival (95% CI) at 24 weeks with monotherapy and BMS-986012 plus nivolumab was 12.2% (6.0%–20.7%) and 39.3% (21.7%–56.5%), respectively. The pharmacokinetics profile of monotherapy and BMS-986012 plus nivolumab suggested dose proportionality across the tested dose range. Median overall survival (95% CI) with monotherapy and BMS-986012 plus nivolumab was 5.4 (4.0–7.3) and 18.7 (8.2–37.3) months, respectively.
Conclusions
BMS-986012 in combination with nivolumab represents a well-tolerated, potential new therapy for relapsed or refractory SCLC. BMS-986012 is currently being explored in combination with carboplatin, etoposide, and nivolumab as a first-line therapy in extensive-stage SCLC (NCT04702880).
Keywords: BMS-986012, Nivolumab, Combination therapy, SCLC, Fucosyl-GM1
Introduction
Lung cancer is the most common cause of death from cancer.1 SCLC is an aggressive disease with limited treatment options, accounting for 15% of all lung cancer cases worldwide.2,3 Approximately 60% of patients with SCLC present with extensive-stage (ES) disease,4 and the 5-year survival rate is less than 7% for patients with SCLC.5 Although SCLC is a chemotherapy-sensitive disease, historically, patients with ES-SCLC have a poor prognosis with standard-of-care platinum and etoposide chemotherapy.3,6 Most patients experience disease relapse within the first year of treatment,3 and median overall survival (OS) ranges from 9.4 to 9.6 months.7 Addition of an anti–programmed death-(ligand) 1 (PD-[L]1) therapy, such as durvalumab or atezolizumab, to first-line (1L) chemotherapy was found to have a clinically modest OS benefit (2.0–2.7 mo longer median OS compared with chemotherapy alone).8,9 Therefore, SCLC remains a difficult-to-treat disease, and most phase 3 trials have failed in this setting.10, 11, 12, 13, 14 Few drugs are approved for second-line (2L) treatment of SCLC. Topotecan is a standard 2L choice; however, owing to its modest efficacy and relevant toxicity profile, topotecan is not an option for all patients, including those with disease that was refractory to 1L platinum-based regimens.2 In patients with relapsed SCLC, the median survival was approximately 26 weeks with topotecan plus chemotherapy and approximately 14 weeks with chemotherapy alone.15 Combination regimens including novel therapies with improved activity and less toxicity are urgently needed to improve patient outcomes.
Carbohydrate antigens are highly expressed on the surface of cancer cells.16 Gangliosides, which contain a carbohydrate chain, are complex glycosphingolipids that have been implicated in promoting cell proliferation, angiogenesis, and immune cell evasion in tumors.17 Notably, antibodies to gangliosides have inhibited tumor growth and induced apoptosis in antigen-positive cells.18,19 Fucosyl-GM1 (FucGM1) is a monosialoganglioside highly expressed on the surface of SCLC cells,20, 21, 22 but it has limited expression in normal tissues, including a subset of peripheral sensory neurons and dorsal root ganglia.17
BMS-986012 is a nonfucosylated, first-in-class, fully human immunoglobulin G1 monoclonal antibody that binds to FucGM1 with high affinity and specificity (Supplementary Fig. 1).23 BMS-986012 was designed to have enhanced effector functions, notably antibody-dependent cellular cytotoxicity (ADCC), by elimination of the fucosylation on the fragment crystallizable domain. The absence of this fucosyl group in BMS-986012 confers higher affinity for fragment crystallizable receptors, resulting in enhanced ADCC.23
Clinical experience with targeting FucGM1 comes from early studies in patients with SCLC who were vaccinated with keyhole limpet hemocyanin-conjugated FucGM1 and developed antibody titers to this antigen.24,25 A preclinical study revealed binding of BMS-986012, which resulted in tumor cell death by means of ADCC, antibody-dependent cellular phagocytosis, and complement-dependent cytotoxicity (Supplementary Fig. 1).23 Modification of BMS-986012 leading to a lack of fucosylation on the antibody was associated with greater binding on natural killer cells and increased ADCC.23 BMS-986012 was found to have significant efficacy in SCLC mouse xenograft and syngeneic models. In addition, BMS-986012 has antitumor activity as monotherapy with synergistic effects in combination with chemotherapy, anti-CD137 agonist antibodies, or immunomodulating agents, including anti–programmed cell death protein-1 (anti–PD-1) antibodies.23 The addition of nivolumab, an anti–PD-1 antibody and immune checkpoint inhibitor that was found to have activity in ES-SCLC,26 to BMS-986012 may function synergistically to induce antigen-presenting cells to activate T cells and reduce tumor cell-derived immune inhibition.
In this phase 1/2 first-in-human (FIH) study (NCT02247349), we evaluated the safety and preliminary efficacy of BMS-986012 as monotherapy and in combination with nivolumab in patients with relapsed or refractory (R/R) SCLC.
Materials and Methods
Study Design
CA001-030 is an ongoing, open-label, phase 1/2, multicenter, FIH dose-escalation and -expansion trial of BMS-986012 administered as a monotherapy or in combination with nivolumab to patients with R/R SCLC (Supplementary Fig. 2A and B). The study was conducted in four parts, as follows: (1) BMS-986012 monotherapy dose escalation to identify the maximum tolerated dose (MTD) or maximum administered dose (MAD) if no MTD is determined; (2) BMS-986012 monotherapy dose expansion (two dose levels of BMS-986012 at or below the MTD or MAD) to confirm safety and evaluate antitumor activity; (3) BMS-986012 plus nivolumab combination therapy dose escalation; and (4) BMS-98012 plus nivolumab dose expansion (treated at or below MTD identified in part 3) to confirm safety and evaluate antitumor activity. Hereafter, results will be described as BMS-986012 monotherapy (parts 1 and 2) and BMS-986012 plus nivolumab (parts 3 and 4) unless otherwise noted.
Patient Population
All patients in this study were adults with histologically or cytologically confirmed SCLC. Among these patients, those enrolled in parts 1 and 3 must have received more than or equal to one previous line of therapy. Patients in parts 2 and 4 were R/R to 1L therapy and could have only received one previous line of therapy. Patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1. Other inclusion criteria were as follows: more than or equal to 4 weeks must have elapsed from the last exposure of anticancer therapy and patients must have had more than or equal to 1 measurable lesion per Response Evaluation Criteria in Solid Tumors version 1.1 that is not amenable to resection. Key exclusion criteria included known or suspected brain metastasis or nonpulmonary small cell cancer. If enrolled in the BMS-986012 plus nivolumab cohort, additional exclusion criteria included any previous treatment with anti–PD-1, anti–PD-L1, anti–programmed death ligand-2, anti-CD137, anti–cytotoxic T lymphocyte-associated protein 4 antibodies, or any other antibody therapy specifically targeting T-cell costimulation or checkpoint pathways.
This study was conducted in accordance with Good Clinical Practice, as defined by the International Conference on Harmonisation, and the ethical principles underlying European Union Directive 2001/20/EC and the U.S. Code of Federal Regulations, Title 21, Part 50 (21CFR50). Each study site’s independent ethics committee or institutional review board approved the protocol before study initiation. Informed consent was obtained before any study-related procedure in adherence to the ethical principles described in the Declaration of Helsinki.
Treatments and Dosing
Parts 1 to 4 all included the following four periods in the study: screening (within 28 d before administration of study medication), treatment (until meeting protocol-specified discontinuation criteria), clinical follow-up (≈100 d), and survival follow-up (up to ≈3 y after the end of treatment).
In part 1 (monotherapy dose escalation), patients received intravenous (IV) BMS-986012 every 3 weeks (Q3W) at one of the following four dose levels: 70, 160, 400, and 1000 mg. BMS-986012 70 mg was chosen based on results from preclinical toxicologic and pharmacologic studies. This dose was predicted to result in human exposure that is 12 times lower than the no-observed-adverse-effect level in cynomolgus monkeys. Subsequent doses (160, 400, and 1000 mg) were escalated in approximate half-log units (2.5-fold increases). BMS-986012 monotherapy dose levels selected for part 2 (monotherapy dose expansion) were 400 and 1000 mg. SCLC tumors are considered to have high expression levels of FucGM1, with little or no expression in normal tissues. Higher BMS-986012 doses were hypothesized to achieve potentially higher intratumoral receptor occupancy and therefore could achieve greater efficacy in SCLC. BMS-986012 doses of 400 mg and 1000 mg Q3W were predicted to provide consistently high levels of target engagement. Given the lack of receptor occupancy assays and other pharmacodynamic data available during the conduct of CA001030, doses were selected on the basis of clinical safety and activity alone. In addition, part 2 evaluated BMS-986012 as 2L treatment in patients with disease who had relapsed after 1L chemotherapy as follows: cohort A, less than or equal to 90-day response duration (refractory) at or below the MTD or MAD (400 mg BMS-986012); cohort B, less than or equal to 90-day response duration (refractory) at a dose level below the MTD or MAD (1000 mg BMS-986012); cohort C, more than 90-day response duration (sensitive) at or below the MTD/MAD (400 mg BMS-986012); and cohort D, more than 90-day response duration (sensitive) at a dose level below the MTD/MAD (1000 mg BMS-986012). Disease was considered “refractory” or “sensitive” if the response duration after 1L chemotherapy was less than or equal to 90 days or more than 90 days, respectively.
In part 3 (dose escalation; BMS-986012 + nivolumab), patients received BMS-986012 (400 mg IV escalated to 1000 mg Q3W) and nivolumab (360 mg IV Q3W). Part 4 (dose expansion; BMS-986012 + nivolumab) evaluated BMS-986012 (400 mg IV Q3W) and nivolumab (360 mg Q3W) as 2L treatment in patients with disease who relapsed after 1L chemotherapy.
Study End Points
The primary end point of this phase 1/2 study was safety as measured by the incidence of adverse events (AEs), serious AEs (SAEs), AEs leading to discontinuations, and AEs related to study treatment. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA version 24.1) and were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). AEs were documented as early as time of enrollment until 100 days after discontinuation of the study treatment. Patients discontinued treatment if there was evidence of disease progression, clinical deterioration as assessed by the investigator, grade 3 infusion reaction, toxicity that met dose-limiting criteria, or any dose interruption lasting more than 6 weeks. Secondary end points included efficacy on the basis of Response Evaluation Criteria in Solid Tumors version 1.1 (objective response rate [ORR] by investigator, duration of response [DOR], progression-free survival [PFS], and PFS rate), pharmacokinetics (PK), and immunogenicity. Exploratory end points included OS and OS rate.
The following PK parameters were evaluated for BMS-986012 as monotherapy or in combination with nivolumab: maximum observed serum concentration (Cmax), time to achieve Cmax (Tmax), observed serum concentration at the end of a dosing interval (Cτ), area under the serum concentration-time curve from time zero to time t (AUC(0-t)), and area under the serum concentration-time curve in one dosing interval (AUC(τ)).
Immunogenicity of BMS-986012 was measured by assessment of the presence of specific antidrug antibodies (ADAs) to BMS-986012. ADA assessments were defined as follows: baseline ADA-positive patients (baseline ADA-positive sample), ADA-positive patients postbaseline (≥1 ADA-positive samples relative to baseline; ADA negative at baseline, or ADA titer to be more than or equal to fourfold higher than baseline positive titer at any time after initiation of treatment during the defined observation period), and ADA-negative patients (no ADA-positive samples after the initiation of treatment). Postbaseline assessments are reported between first dose and 100 days after last dose of BMS-986012.
Statistical Analyses
CA001-030 is an ongoing clinical trial; however, enrollment has been completed and the primary analyses have been conducted. All recorded AEs were listed and tabulated by system organ class, MedDRA preferred term, and treatment. The ORR confidence intervals (CIs) were based on the Clopper-Pearson method. The DOR and PFS were estimated by Kaplan-Meier methodology, with the Greenwood formula for PFS rates. Individual changes in tumor burden over time are presented graphically by dose level within each cohort. Geometric means and coefficients of variation, CV (%), are presented for Cmax, AUC, and Cτ. Medians and ranges are presented for Tmax. All available immunogenicity data for BMS-986012 as monotherapy or in combination with nivolumab are listed. OS was assessed as part of exploratory efficacy analysis by Kaplan-Meier plots including median OS and OS rates at specified times (e.g., at 6, 12, and 24 mo).
Results
Baseline Characteristics and Patient Disposition
Across treatment groups, the median age was 63 years (BMS-986012 monotherapy, 63 y [range: 26–81 y]; BMS-986012 + nivolumab, 65 y [range: 46–79 y]; Table 1). Most patients in the BMS-986012 monotherapy group were white (91%) and were either current (33%) or former (64%) smokers. In the monotherapy group, 78% of patients had an ECOG PS of 1. All patients who received BMS-986012 monotherapy had previous systemic therapy; 81% and 16% of patients had received 1L or 2L systemic therapy previously, respectively. In addition, 42 patients (55%) were sensitive to previous 1L therapy and 35 patients (45%) were refractory to previous 1L therapy.
Table 1.
Baseline Characteristics of Each Patient Cohort
| Baseline Characteristics and Patient Disposition | BMS-986012 Monotherapy (n = 77) | BMS-986012 + Nivolumab (n = 29) |
|---|---|---|
| Age, median (range), y | 63 (26–81) | 65 (46–79) |
| Male, n (%) | 46 (60) | 15 (52) |
| White, n (%) | 70 (91) | 29 (100) |
| Tobacco use, current/former, n (%) | 25 (33)/49 (64) | 7 (24)/22 (76) |
| ECOG PS, 0/1, n (%) | 17 (22)/60 (78) | 11 (38)/18 (62) |
| 1L platinum response, sensitive/refractory, n (%) | 42 (55)/35 (45) | 20 (69)/9 (31) |
| Previous lines of therapy, 1/2/3, n (%) | 62 (81)/12 (16)/1 (1) | 25 (86)/2 (7)/2 (7) |
1L, first line; ECOG PS, Eastern Cooperative Oncology Group performance status.
Similarly, all patients who received BMS-986012 plus nivolumab were either current (24%) or former (76%) smokers, and almost all were white (93.4%). In the combination group, 62% of patients had an ECOG PS of 1. Most patients (86%) had one previous line of therapy, two patients (7%) had two lines of previous therapy, and two other patients had three lines of previous therapy (7%). More patients were platinum sensitive (69% [n = 20]) than platinum refractory (31% [n = 9]).
Safety Profile of BMS-986012 Monotherapy or Combination Treatment With Nivolumab
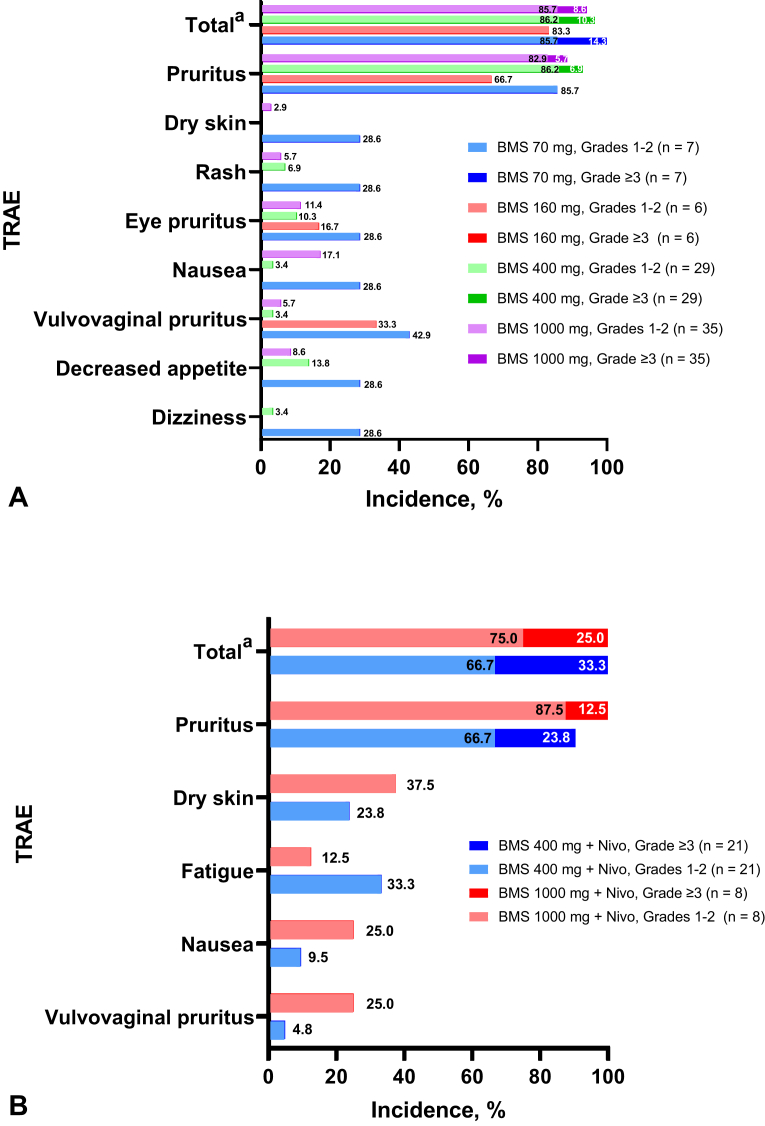
Treatment-related AEs (TRAEs) were reported in 73 participants (94.8%) treated with BMS-986012 monotherapy (by BMS-986012 dose group: 70 mg, n = 7 [100%]; 160 mg, n = 5 [83.3%]; 400 mg, n = 28 [96.6%]; and 1000 mg, n = 33 [94.3%]). TRAEs reported in more than or equal to 10% of patients were pruritus (n = 68 [88.3%]), eye pruritus (n = 10 [13%]), fatigue (n = 10 [13.0%]), nausea (n = 9, [11.7%]), decreased appetite (n = 9 [11.7%]), and vulvovaginal pruritus (8 [10.4%]) (Fig. 1A). Most TRAEs were grade 1 or 2. Grade 3 TRAEs included pruritus (n = 4 [5.2%]), fatigue, hypokalemia, lower respiratory tract infection, and myalgia (n = 1 [1.3%] each). There were no grade 4 or 5 TRAEs in patients treated with BMS-986012 monotherapy.
Figure 1.
Incidence of most common TRAEs experienced in patients in any cohort (per dose) treated with (A) BMS-986012 monotherapy or (B) in combination with nivolumab. Stacked bars (grade 1/2 and 3/4 TRAEs) represent total percentages of TRAEs reported. Lack of stacked bar indicates that no grade 3/4 TRAEs occurred. Numbers within bars represent the incidence of TRAEs experienced. aTotal represents total number of patients with an event. BMS, BMS-986012; nivo, nivolumab; TRAE, treatment-related adverse event.
Six treatment-related SAEs were reported in the BMS-986012 monotherapy group. Grade 3 fatigue lasting 8 days was reported in one participant who had received BMS-986012 70 mg. Three SAEs were reported in the 400 mg dose cohort (grade 1 pyrexia [n = 1], grade 3 pruritus [n = 1], and grade 2 infusion-related reaction [n = 1]; all events lasted ≤2 d). The grade 1 pyrexia occurred 8 hours after drug administration and was considered an SAE because the patient was hospitalized; however, the patient was observed (no additional treatment) and discharged in the next morning. Two SAEs in the 1000 mg BMS-986012 treatment group were reported in one patient (grade 3 pruritus and grade 3 lower respiratory tract infection), both of which lasted 3 to 4 days.
There were 63 deaths reported in the BMS-986012 monotherapy cohort unrelated to BMS-986012 treatment. Of these, 61 were considered related to malignant neoplasm progression, one was owing to pneumonia, and one was due to respiratory failure (chronic obstructive pulmonary disease).
All patients treated with BMS-986012 plus nivolumab experienced a TRAE (pruritus, n = 27 [93.1%]; fatigue and dry skin, n = 8 [27.6%] each; hypothyroidism, n = 5 [17.2%]; infusion-related reaction and nausea, n = 4 [13.8%] each; and increased amylase, increased lipase, arthralgia, dry eye, hyperthyroidism, dry mouth, vomiting, and vulvovaginal pruritus, n = 3 [10.3%] each; Fig. 1B). Three grade 4 TRAEs were reported (increased amylase, n = 1 [3%]; increased lipase, n = 1 [3%]; and hyponatremia, n = 1 [3%]).
Three patients reported treatment-related SAEs in the BMS-986012 plus nivolumab group. One patient receiving 1000 mg BMS-986012 plus nivolumab had grade 3 hepatic failure. As a result, therapy was discontinued, and the patient ultimately died of an unrelated event; the hepatic failure was attributed to nivolumab by the investigator. Two SAEs were reported during follow-up: grade 3 pancreatitis and grade 3 diarrhea (both events lasted 4 d).
There were 16 deaths in the combination therapy group unrelated to BMS-986012 plus nivolumab treatment (disease progression, n = 15; respiratory failure [owing to pain medication administered as part of palliative care], n = 1).
Efficacy of BMS-986012 Monotherapy or Combination Treatment With Nivolumab
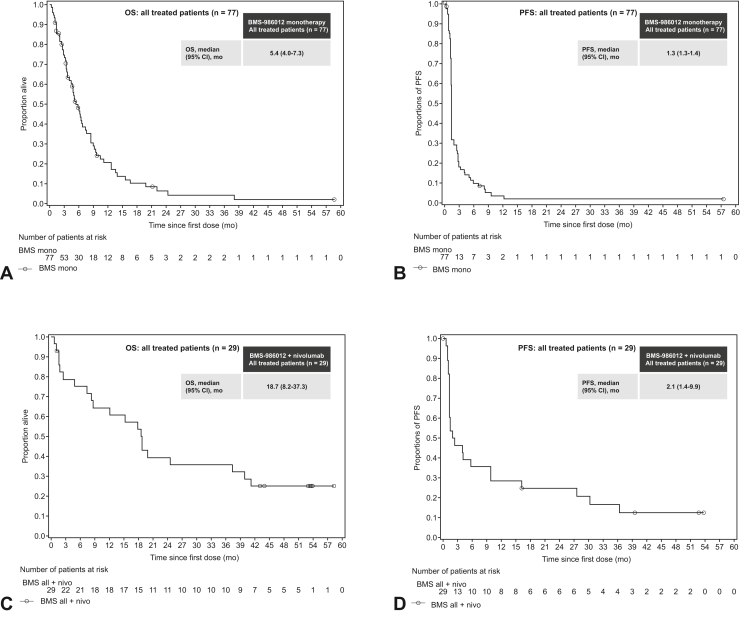
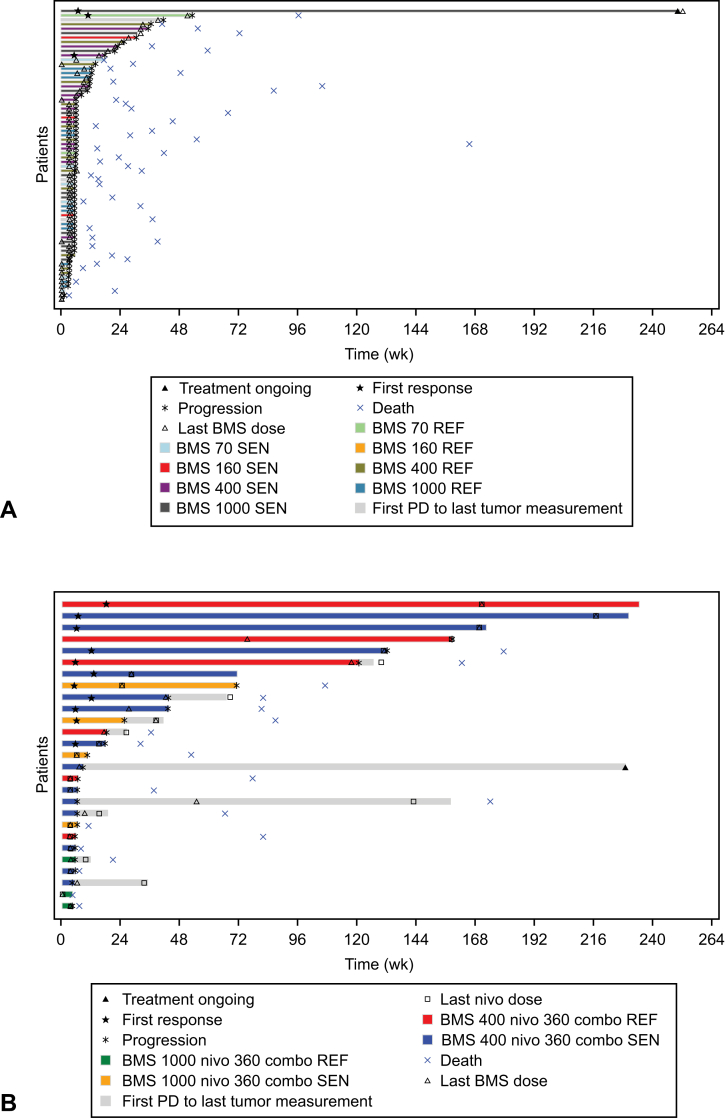
The ORR was 4% (95% CI: 0.8–11.0) in the BMS-986012 monotherapy group; 18 patients (23%) achieved stable disease (BMS-986012 160 mg [n = 2], 400 mg [n = 8], 1000 mg [n = 8]; Table 2). Median OS in the BMS-986012 monotherapy group was 5.4 (95% CI: 4.01–7.33) months (Fig. 2A), with a 12-month OS rate of 21.2% (95% CI: 12.0%–32.0%). Median PFS was 1.3 (95% CI: 1.3–1.4) months (Fig. 2B) with a PFS rate of 12.2% (95% CI: 6.0%–20.7%) at 24 weeks. Two patients achieved a partial response (PR), and one patient achieved complete response (CR; Fig. 3A). At the end of the study, one patient with SCLC refractory to 1L carboplatin plus etoposide was treated with BMS-986012 70 mg and had a CR lasting approximately 47 weeks. One patient with SCLC sensitive to 1L carboplatin plus etoposide was treated with BMS-986012 400 mg and had a PR lasting approximately 12 weeks. Another patient with SCLC sensitive to 1L carboplatin plus etoposide had a PR of 243 weeks at the time of final analysis and was still receiving treatment (BMS-986012 1000 mg).
Table 2.
Overall Response Summary of Patients Treated With BMS-986012 Monotherapy or BMS-986012 in Combination With Nivolumab
| Therapies, by Dose | n | ORR, n (%) | Stable Disease, n (%) |
|---|---|---|---|
| BMS-986012 monotherapy, all patients | 77 | 3 (4) | 18 (23) |
| 70 mg | 7 | 1 (14) | 0 |
| 160 mg | 6 | 0 | 2 (33) |
| 400 mg | 29 | 1 (3) | 8 (28) |
| 1000 mg | 35 | 1 (3) | 8 (23) |
| BMS-986012 + nivolumab, all patients | 29 | 11 (38) | 3 (10) |
| 400 mg + 360 mg | 21 | 9 (43) | 2 (10) |
| 1000 mg + 360 mg | 8 | 2 (25) | 1 (13) |
ORR, objective response rate.
Figure 2.
Efficacy of BMS-986012 monotherapy or in combination with nivolumab after first dose of treatment. Proportion of surviving patients after BMS-986012 monotherapy for (A) OS and (B) PFS and surviving patients after first dose of combination therapy for (C) OS and (D) PFS. Symbols represent censored observations. Number of patients at risk is before entering the time period. Only cohorts with more than or equal to five events are described. BMS, BMS-986012. CI, confidence interval; mono, monotherapy; OS, overall survival; PFS, progression-free survival.
Figure 3.
Plot of PFS (in wk, all treated patients) for (A) BMS-986012 monotherapy and (B) in combination with nivolumab. BMS dose levels were 70, 160, 400, and 1000 mg; nivolumab dose levels were 360 and 240 mg. Patients with on-treatment tumor assessments are described. Time to response is described for responders only. The length of each bar describes time to progression or death or time to the last tumor assessment in patients who have not progressed. Where illustrated, PD is recorded as a reason for ending treatment. BMS, BMS-986012; combo, BMS-986012 in combination with nivo; nivo, nivolumab; PD, progressive disease; REF, SCLC refractory; SEN, SCLC sensitive.
An ORR of 38% (95% CI: 20.7–57.7) was achieved in the BMS-986012 plus nivolumab group (Table 2) with 10 patients achieving PR and one patient achieving CR. The ORR was higher (43%) in BMS-986012 400 mg plus nivolumab compared with BMS-986012 1000 mg plus nivolumab (25%). Of patients treated with BMS-986012 plus nivolumab, 10% (n = 3) achieved stable disease. At the time of data cutoff, the median DOR was 26.4 months (95% CI: 4.4 mo–not reached) and four patients were still receiving treatment in the BMS-986012 plus nivolumab group. Those who received combination treatment had a median OS of 18.7 months (95% CI: 8.2–37.3 mo; Fig. 2C), with OS rates of 64.5% (95% CI: 44.0%–79.1%) at 12 months and 39.4% (95% CI: 21.7%–56.6%) at 24 months. Median PFS was 2.1 months (95% CI: 1.4–9.9 mo; Fig. 2D), with a PFS rate of 39.3% (95% CI: 21.7%–56.5%) at 24 weeks. Seven patients (24%) received combination treatment for more than 12 months (Fig. 3B).
PK Profile and ADA Interaction Summary in BMS-986012 Monotherapy or Combination Treatment With Nivolumab
Median Tmax was approximately 1 to 2 hours for all doses in the BMS-986012 monotherapy cohort and 4 hours with BMS-986012 plus nivolumab combination therapy cohort (Table 3). The mean area under the concentration-time curve (AUCτ and AUC(0-t)) of BMS-986012 increased with increasing dose in monotherapy or in combination with nivolumab (Table 3). In addition, the mean plasma concentration (Cmax and Cτ) of BMS-986012 alone or in combination with nivolumab increased with increasing dose.
Table 3.
Summary of PK Parameters After First Dose of BMS-986012 Monotherapy or in Combination With Nivolumab
| Dose, mg | Cmax (μg/mL), GM (%CV) [n] | Tmax (h), median (min, max) [n] | AUC(0-t) (μg•h/mL), GM (%CV) [n] | AUC(τ) (μg•h/mL), GM (%CV) [n] | C(τ) (μg/mL), GM (%CV) [n] |
|---|---|---|---|---|---|
| BMS-986012 monotherapy | |||||
| 70 | 27.5 (31) [7] | 2 (1, 8) [7] | 3978 (31) [7] | 4286 (29) [7] | 4.19 (27) [4] |
| 160 | 74.4 (142) [6] | 1.5 (1, 4) [6] | 5937 (52) [6] | 8480 (29) [5] | 7.22 (15) [5] |
| 400 | 121 (26) [28] | 2 (1, 4) [28] | 18,295 (30) [28] | 19,253 (27) [27] | 18.8 (29) [22] |
| 1000 | 277 (37) [33] | 1 (1, 4) [33] | 35,984 (38) [33] | 40,221 (33) [30] | 37.9 (52) [19] |
| BMS-986012 in combination with nivolumab | |||||
| 400 | 103 (17) [15] | 4 (1, 4) [15] | 16,757 (26) [15] | 17,978 (24) [12] | 16.2 (45) [10] |
| 1000 | 295 (21) [5] | 4 (1, 4) [5] | 41,247 (16) [5] | 45,377 (24) [5] | 28.7 (40) [3] |
% CV, coefficients of variation; AUC(0-t), area under the serum concentration-time curve from time zero to time t; AUC(τ), area under the serum concentration-time curve in one dosing interval; Cmax, maximum observed serum concentration; Cτ, observed serum concentration at the end of a dosing interval; GM, geometric mean; min, minimum; max, maximum; PK, pharmacokinetics; Tmax, time to maximum observed serum concentration.
Of the assessable population in the BMS-986012 monotherapy treatment group (n = 64), one patient (1.6%) was baseline ADA positive to BMS-986012 and all patients (n = 64 [100%]) were ADA negative at time of postbaseline assessment. Similarly, all assessable patients in the BMS-986012 plus nivolumab treatment group (n = 21 [100%]) were ADA negative at time of postbaseline assessment.
Discussion
The objective of this study was to evaluate the safety, efficacy, PK, and immunogenicity of BMS-986012 as monotherapy and in combination with nivolumab in patients with R/R SCLC. We concluded that the safety profile of BMS-986012 monotherapy or combination therapy with nivolumab was manageable with no dose-limiting toxicities (DLTs) reported in the monotherapy group and one DLT reported in the BMS-986012 plus nivolumab group, which was considered related to nivolumab. Pruritus was the most common TRAE in these patients. The cause of pruritus was likely neurogenic given that FucGM1 is expressed on peripheral nerves. Some patients reported recurrent episodes for several weeks, although for most patients, pruritus resolved after the second cycle of therapy. In general, pruritus was treated with antihistamines and low-dose corticosteroids, which did not seem to help manage symptoms, and in most patients, the pruritus seemed to be self-limited and did not recur after the second cycle of treatment. Importantly, potential autoimmune toxicity of BMS-986012 did not seem to be increased in the presence of nivolumab except for pruritus. The safety profiles of BMS-986012 monotherapy and or combination therapy with nivolumab are favorable compared with those of approved therapies for previously treated SCLC. After pruritus, nausea and fatigue were the most common TRAEs. Nevertheless, topotecan, which is poorly tolerated, and lurbinectedin both frequently cause nausea, fatigue, and clinically significant cytopenias.27,28 Seven patients received combination therapy for longer than 1 year, indicating BMS-986012 plus nivolumab has the potential for long-term safety and efficacy.
Despite OS improvement as found in the IMpower1339 and CASPIAN8 trials, a high unmet need still exists to provide a clinically meaningful benefit in survival in patients with ES-SCLC. BMS-986012 monotherapy had evidence of single-agent activity, and several patients experienced stable disease. BMS-986012 plus nivolumab had higher clinical activity in patients with R/R SCLC, including an ORR of 38% and a median OS of 18.7 months.
The PK profile of BMS-986012 was consistent with that expected for a monoclonal antibody and suggested that single doses quickly reached Cmax (median Tmax 1–2 h for BMS-986012 monotherapy and 4 h for BMS-986012 + nivolumab), and plasma concentrations were dose proportional after single doses. Notably, no patient across both treatment groups developed ADAs to BMS-986012.
Because this was a FIH study, which are typically nonrandomized, an assessment of potential effects of demographic or clinical characteristics (e.g., number of previous therapies, active or past tobacco use, race) on safety and efficacy was not possible. Nevertheless, the patient demographics were equally distributed between the BMS-986012 monotherapy and BMS-986012 plus nivolumab combination therapy arms. These results reveal that BMS-986012 plus nivolumab represents a safe combination therapy for patients with R/R SCLC not previously treated with checkpoint inhibitors. Future studies with BMS-986012 will require further exploration to determine whether a correlation exists between the degree of FucGM1 expression in SCLC and responsiveness to BMS-986012. Obtaining sufficient tumor biopsy samples is challenging in this patient population, but other blood-based biomarkers (e.g., circulating tumor cells) could be explored. Supported by the results presented here, a phase 2 study is currently evaluating the safety and therapeutic benefit of BMS-986012 when combined with carboplatin, etoposide, and nivolumab in patients with newly diagnosed ES-SCLC (NCT04702880).29
CRediT Authorship Contribution Statement
Quincy Chu: Investigation, Resources, Writing—review and editing.
Natasha B. Leighl: Validation, Investigation, Resources, Writing—review and editing, Project administration.
Veerle Surmont, Carla van Herpen, Stephen Clarke, Charles M. Rudin, Stephanie Snow, Michael Sanatani: Investigation, Writing—review and editing.
Anne Sibille: Resources, Writing—review and editing.
Ben Markman: Investigation, Resources, Writing—review and editing.
Rosalyn A. Juergens: Writing—review and editing.
Mirelis Acosta Rivera: Investigation.
Vladimir Andelkovic: Investigation, Resources, Data curation, Writing—review and editing, Supervision.
Dong-Wan Kim: Investigation, Resources, Writing—review and editing.
Hongxia Lin: Formal analysis, Data curation, Writing—review and editing.
Kinjal Sanghavi: Formal analysis, Writing—original draft, Writing—review and editing.
Sarah Tannenbaum-Dvir: Conceptualization, Formal analysis, Investigation, Writing—review and editing, Supervision.
Paul Basciano: Conceptualization, Investigation, Writing—review and editing.
Deanne Lathers: Conceptualization, Methodology, Investigation, Data curation, Writing—review and editing, Project administration.
Katarzyna Urbanska: Conceptualization, Methodology.
Georgia Kollia, Chunsheng He: Methodology, Formal analysis.
Andrew DiPiero: Writing—review and editing.
Yu Liu: Conceptualization, Validation, Data curation, Writing—review and editing, Supervision, Funding acquisition.
Neal Ready: Resources, Writing—review and editing.
Acknowledgments
This study was sponsored by Bristol Myers Squibb. The authors thank the patients who participated in the study and their family members and the investigators and staff who conducted the study. Sponsorship of this study and article processing charges were funded by Bristol Myers Squibb, Princeton, NJ. Medical writing and editorial assistance were provided by Clara Huesing, PhD, of SciMentum, Inc., a Nucleus Holding Ltd. company, and funded by Bristol Myers Squibb. The authors are fully responsible for all content and editorial decisions for this manuscript.
Footnotes
Disclosure: Dr. Chu reports receiving grants/contracts from AstraZeneca; receiving consulting fees from AbbVie, Amgen, AnHeart, Astellas, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Jazz, Johnson & Johnson, Merck, Novartis, Pfizer, Roche, Sanofi, and Takeda; receiving payment or honoraria from AbbVie, Amgen, AnHeart, Astellas, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Jazz, Johnson & Johnson, Merck, Novartis, Pfizer, Roche, Sanofi, and Takeda; having participation on a data safety monitoring board or advisory board with Merck KGaA; and having leadership or fiduciary role with Lung Cancer Canada. Dr. Leighl reports receiving payment or honoraria from Bristol Myers Squibb. Dr. van Herpen reports receiving support for present manuscript from AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Merck Sharp & Dohme, Merck, Novartis, Philips MPDx, Regeneron, and Sanofi; payment or honoraria from Congress Care, MedTalks, and V&VN; and other financial or nonfinancial interests with ECZA. Dr. Markman reports receiving support for attending meetings and/or travel with Akeso Biopharma; and having participation on a data safety monitoring board or advisory board with Amgen, BeiGene, Bristol Myers Squibb, and Merck. Dr. Clarke reports receiving payment or honoraria from Bristol Myers Squibb. Dr. Juergens reports having contracted research with Amgen, Astellas, AstraZeneca, Bold Therapeutics, Bristol Myers Squibb, Debio Pharmaceuticals, Fusion Pharmaceuticals, Macrogenics, and Merck Sharp & Dohme; receiving consulting fees from Bristol Myers Squibb, Merck Sharp & Dohme, and Pfizer; receiving honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Jazz, Merck Sharp & Dohme, Novartis, and Takeda; and having participation on an advisory board with Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, EMD Serono, Jazz, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, and Takeda. Dr. Rudin reports receiving personal fees from AbbVie, Amgen, AstraZeneca, Daiichi Sankyo, Epizyme, Genentech/Roche, Ipsen, Jazz, Kowa, Eli Lilly, Merck, Syros, Bridge Medicines, Harpoon Therapeutics, and Earli. Dr. Snow reports receiving grants or contracts from AstraZeneca, Bristol Myers Squibb, and Merck; receiving consulting fees from AstraZeneca, Jazz, and Roche; receiving payment or honoraria from AstraZeneca, Jazz, OncologyEducation.com, and PeerVoice; and having leadership or fiduciary role with Lung Cancer Canada. Dr. Kim reports receiving support for present manuscript from Bristol Myers Squibb; receiving grants or contracts from Alpha Biopharma, Amgen, AstraZeneca, Boehringer Ingelheim, BridgeBio, Chong Keun Dang, Daiichi Sankyo, GlaxoSmithKline, Hanmi, Janssen, Merck, Merus, Mirati Therapeutics, Merck Sharp & Dohme, Novartis, ONO Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery, and Yuhan; receiving payment or honoraria from Amgen, Asian Thoracic Oncology Research Group, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Korean Association for Lung Cancer, Korean Cancer Association, Korean Society of Medical Oncology, Merck, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Taiwan Lung Cancer Society, Takeda, and Yuhan; receiving support for attending meetings and/or travel with Amgen, Asian Thoracic Oncology Research Group, Daiichi Sankyo, International Association for the Study of Lung Cancer, and Taiwan Lung Cancer Society; having participation on a data safety monitoring board or advisory board with Amgen, AstraZeneca, Bristol Myers Squibb/ONO Pharmaceuticals, Daiichi Sankyo, GlaxoSmithKline, Janssen, Merck, Merck Sharp & Dohme, Pfizer, SK Biopharm, and Takeda; having leadership or fiduciary role with Asian Thoracic Oncology Research Group, Korean Association for Lung Cancer, Korean Cancer Association, and Korean Society of Medical Oncology; and having other financial interests with Health Insurance Review and Assessment Service, Republic of Korea. Dr. Lin is an employee of Bristol Myers Squibb. Dr. Sanghavi is an employee of Bristol Myers Squibb. Dr. Tannenbaum-Dvir reports having employment with Bristol Myers Squibb; stock ownership in Bristol Myers Squibb; and patent for BMS-986012. Dr. Basciano reports having employment with Bristol Myers Squibb and owning stock in Bristol Myers Squibb. Dr. Lathers reports having employment with Bristol Myers Squibb and owning stock in Bristol Myers Squibb. Dr. Urbanska is an employee of Bristol Myers Squibb. Dr. Kollia reports having employment with Bristol Myers Squibb and owning stock in Bristol Myers Squibb. Dr. He reports having employment with Bristol Myers Squibb and owning stock in Bristol Myers Squibb. Mr. DiPiero is an employee of Bristol Myers Squibb. Dr. Lin reports having employment with Bristol Myers Squibb, stock ownership in Bristol Myers Squibb, and patent for BMS-986012. Dr. Ready reports receiving grants or contracts from Merck and Memgen; consulting fees with AbbVie, AstraZeneca, Bristol Myers Squibb, Genentech, Jazz, Eli Lilly, Merck, Pfizer, Roche, and Sanofi; and payment or honoraria from Bristol Myers Squibb. The remaining authors declare no conflicts of interest.
Cite this article as: Chu Q, Leighl NB, Surmont V, et al. BMS-986012, an anti–fucosyl-GM1 monoclonal antibody as monotherapy or in combination with nivolumab in relapsed/refractory SCLC: results from a first-in-human phase 1/2 study. JTO Clin Res Rep. 2022;3:100400.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2022.100400.
Supplementary Data
References
- 1.Cancer.org About lung cancer. https://www.cancer.org/cancer/lung-cancer/about/what-is.html
- 2.Yang S., Zhang Z., Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12:47. doi: 10.1186/s13045-019-0736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiddinga B.I., Raskin J., Janssens A., Pauwels P., Van Meerbeeck J.P. Recent developments in the treatment of small cell lung cancer. Eur Respir Rev. 2021;30:210079. doi: 10.1183/16000617.0079-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basumallik N., Agarwal M. StatPearls Publishing; Island, FL: 2022. Small Cell Lung Cancer, StatPearls. Treasure. [PubMed] [Google Scholar]
- 5.Cancer.org Lung cancer early detection, diagnosis, and staging. https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/how-diagnosed.html
- 6.Sun A., Durocher-Allen L.D., Ellis P.M., et al. Initial management of small-cell lung cancer (limited- and extensive-stage) and the role of thoracic radiotherapy and first-line chemotherapy: a systematic review. Curr Oncol. 2019;26:e372–e384. doi: 10.3747/co.26.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi A., Di Maio M., Chiodini P., et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30:1692–1698. doi: 10.1200/JCO.2011.40.4905. [DOI] [PubMed] [Google Scholar]
- 8.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 9.Horn L., Mansfield A.S., Szczesna A., et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 10.Owonikoko T.K., Park K., Govindan R., et al. Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J Clin Oncol. 2021;39:1349–1359. doi: 10.1200/JCO.20.02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spigel D.R., Vicente D., Ciuleanu T.E., et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann Oncol. 2021;32:631–641. doi: 10.1016/j.annonc.2021.01.071. [DOI] [PubMed] [Google Scholar]
- 12.Blackhall F., Jao K., Greillier L., et al. Efficacy and safety of rovalpituzumab tesirine compared with topotecan as second-line therapy in DLL3-high SCLC: results from the phase 3 TAHOE study. J Thorac Oncol. 2021;16:1547–1558. doi: 10.1016/j.jtho.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Reck M., Luft A., Szczesna A., et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34:3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 14.Paz-Ares L., Ciuleanu T., Navarro A., et al. PL02.03 lurbinectedin/doxorubicin versus CAV or topotecan in relapsed SCLC patients: phase III randomized ATLANTIS trial. J Thorac Oncol. 2021;16:S844–S845. [Google Scholar]
- 15.O’Brien M.E., Ciuleanu T.E., Tsekov H., et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 16.Wang C.C., Huang Y.L., Ren C.T., et al. Glycan microarray of Globo H and related structures for quantitative analysis of breast cancer. Proc Natl Acad Sci U S A. 2008;105:11661–11666. doi: 10.1073/pnas.0804923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groux-Degroote S., Delannoy P. Cancer-associated glycosphingolipids as tumor markers and targets for cancer immunotherapy. Int J Mol Sci. 2021;22:6145. doi: 10.3390/ijms22116145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vazquez A.M., Rodreguez-Zhurbenko N., Lopez A.M. Anti-ganglioside anti-idiotypic vaccination: more than molecular mimicry. Front Oncol. 2012;2:170. doi: 10.3389/fonc.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wondimu A., Zhang T., Kieber-Emmons T., et al. Peptides mimicking GD2 ganglioside elicit cellular, humoral and tumor-protective immune responses in mice. Cancer Immunol Immunother. 2008;57:1079–1089. doi: 10.1007/s00262-007-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brezicka F.T., Olling S., Bergman B., et al. Coexpression of ganglioside antigen Fuc-GM1, neural-cell adhesion molecule, carcinoembryonic antigen, and carbohydrate tumor-associated antigen CA 50 in lung cancer. Tumour Biol. 1992;13:308–315. doi: 10.1159/000217780. [DOI] [PubMed] [Google Scholar]
- 21.Brezicka F.T., Olling S., Bergman B., et al. Immunohistochemical detection of two small cell lung carcinoma-associated antigens defined by MAbs F12 and 123C3 in bronchoscopy biopsy tissues. APMIS. 1991;99:797–802. doi: 10.1111/j.1699-0463.1991.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S., Cordon-Cardo C., Zhang H.S., et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Ponath P., Menezes D., Pan C., et al. A novel, fully human anti-fucosyl-GM1 antibody demonstrates potent in vitro and in vivo antitumor activity in preclinical models of small cell lung cancer. Clin Cancer Res. 2018;24:5178–5189. doi: 10.1158/1078-0432.CCR-18-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krug L.M., Ragupathi G., Hood C., et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]
- 25.Dickler M.N., Ragupathi G., Liu N.X., et al. Immunogenicity of a fucosyl-GM1-keyhole limpet hemocyanin conjugate vaccine in patients with small cell lung cancer. Clin Cancer Res. 1999;5:2773–2779. [PubMed] [Google Scholar]
- 26.Ready N.E., Ott P.A., Hellmann M.D., et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. 2020;15:426–435. doi: 10.1016/j.jtho.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Kang J.H., Lee K.H., Kim D.W., et al. A randomised phase 2b study comparing the efficacy and safety of belotecan vs. topotecan as monotherapy for sensitive-relapsed small-cell lung cancer. Br J Cancer. 2021;124:713–720. doi: 10.1038/s41416-020-01055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel S., Petty W.J., Sands J.M. An overview of lurbinectedin as a new second-line treatment option for small cell lung cancer. Ther Adv Med Oncol. 2021;13 doi: 10.1177/17588359211020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ClinicalTrials.gov A study of BMS-986012 in combination with carboplatin, etoposide, and nivolumab as first-line therapy in extensive-stage small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT04702880 Accessed September 14, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.