Summary
The clinical correlation between adiponectin (APN) signal and hypertrophic scar (HS) remains unclear. Here, we found significantly reduced expression of APN receptors (AdipoR1/2) in HS tissues and derived fibroblasts (HFs), suggesting that HS formation may be associated with APN/AdipoR1/2 decline. RNA sequencing and RT-PCR validation revealed that APN significantly elevated the expression of SIRT1. Both in vitro and in vivo experiments confirmed that SIRT1 plays important role in APN inhibiting the fibrotic phenotype transformation and proliferation of scar fibroblasts and improving skin fibrosis. Mechanistically, SIRT1 inhibited the acetylation of C/EBPβ K39, histone H3K27, and H3K9, resulting in impaired transcription activity of C/EBPβ and compact chromatin conformation, thus preventing C/EBPβ from activating the transcription of YAP. Moreover, we found that YAP was critical for the transcriptional regulation of CTGF, CCND1, and CCNE1 by TEAD4. In conclusion, our study revealed the role of APN in antagonizing HS fibrosis by regulating the SIRT1/C/EBPβ/YAP pathway.
Subject areas: Biological sciences, molecular biology, molecular mechanism of gene regulation
Graphical abstract

Highlights
-
•
The APN-AdipoR1/2 signal is attenuated in hypertrophic scars (HS)
-
•
APN treatment inhibits HS fibrosis by upregulating the level of SIRT1
-
•
SIRT1 deacetylates C/EBPβK39, H3K27, and H3K9, thereby inhibiting YAP transcription
-
•
YAP assists TEAD4 in regulating the expression of CTGF, CCND1, and CCNE1
Biological sciences; Molecular biology; Molecular mechanism of gene regulation.
Introduction
Hypertrophic scar (HS) is a kind of skin fibrosis lesion caused by excessive hyperplasia repair of new connective tissue after the dermis or deep tissue is damaged. Cytology shows that fibroblasts transform into myofibroblasts with enhanced α-SMA expression, leading to abnormal proliferation and collagen synthesis (Zhang et al., 1995). HS not only affects a patient’s appearance, but also results in joint dysfunction, which has some serious impacts on a patient’s quality of life. At present, the exact cause of HS remains unclear, so there is no specific treatment currently used as a standard of care. Therefore, exploring the pathogenesis of HS and finding tools to correct or compensate for its genetic defects has become a popular research topic in the field of burn plastic surgery.
Adiponectin (APN) has been found to be locally secreted by a variety of cell types, and it acts as protective hormone to maintain cellular homeostasis. Decreased levels of APN or its receptors (AdipoR1 and R2) are the driving force for many diseases. Restoring APN signaling has been reported to exert significant anti-diabetes, anti-cardiovascular disorder, and anti-fibrosis effects (Kim et al., 2018; Dhandapany et al., 2021; Kamada et al., 2003; Fang et al., 2012). For example, overexpression of APN in the mouse liver can effectively prevent liver fibrosis induced by CCl4, which may occur because APN inhibits the entry of Smad2 into the nucleus (Kamada et al., 2003). In addition, studies on systemic sclerosis have shown that the serum APN levels in these patients were significantly reduced compared to healthy patients, and APN supplementation promotes AMPK activation and inhibits TGFβ1-induced Col1 and α-SMA expression (Fang et al., 2012). However, the effect of APN on HS has received little attention.
Silent information regulator (SIRT1) is a nicotinamide adenine dinucleotide (NAD+)-dependent class III histone deacetylase, which is involved in inflammation, oxidative stress, cell metabolism, and other cellular physiological activity by regulating the deacetylation of histone and nonhistone substrates (He et al., 2020; Chen et al., 2021; Sathyanarayan et al., 2017). The regulation of organ fibrotic diseases by SIRT1 has been reported (Li et al., 2018; Chu et al., 2018). The level of SIRT1 is decreased in patients with cirrhosis, and SIRT1 re-enhancement can repress the trans-differentiation of hepatic stellate cells to myofibroblasts by regulating the EZH2-PPAR pathway (Li et al., 2018). In patients with pulmonary fibrosis, the activation or overexpression of SIRT1 reduces the acetylation of NF-κB and inhibits TGFβ1 and mTOR-related signaling, thereby suppressing the inflammatory response and collagen production in lung fibroblasts (Chu et al., 2018). Therefore, a fibrosis treatment strategy targeting SIRT1 may be beneficial and effective for the prevention and treatment of HS.
The Hippo pathway is highly conserved across evolution and plays an important role in organ development regulation, tissue regeneration, and homeostasis (Isago et al., 2020; Verboven et al., 2021; Pepe-Mooney et al., 2019; Elbediwy et al., 2016). The transcriptional co-activator Yes-associated protein (YAP) is the main effector molecule of the Hippo pathway. In recent years, many reports have illustrated the crosstalk between YAP and other established signaling pathways. For instance, YAP has been shown to promote TGFβ signaling by promoting the nuclear translocation of Smad2/3 (Pefani et al., 2016). Research on rhabdomyosarcoma found that there is a positive feedback loop regulation between Notch signaling and YAP (Slemmons et al., 2017). Moreover, it has been reported that YAP is regulated by SIRT1. In HeLa cells, SIRT1 mediates YAP deacetylation to promote YAP phosphorylation and inhibit YAP activity (Hata et al., 2012). We then pondered what is the regulatory relationship between SIRT1 and YAP in HS. This may be a mechanism for the pathogenesis of HS.
Here, we showed that APN targets SIRT1 to inhibit the fibrotic state and proliferative ability of scar fibroblasts, which was also demonstrated in a mouse model of bleomycin-induced skin fibrosis. In addition, we revealed a mechanism by which SIRT1 regulates fibrosis. SIRT1 inhibits the acetylation levels of C/EBPβ K39 and histone H3K27 and H3K9, thus preventing the direct transcriptional activation of the YAP promoter by the transcription factor C/EBPβ. As a co-transcriptional activator, YAP plays a critical role in the transcriptional activation of the TEAD4-mediated pro-fibrotic factor CTGF and the cyclins CCND1 and CCNE1.
Results
Adiponectin treatment can inhibit the fibrotic state and cell proliferation of hypertrophic scar-derived fibroblasts (HFs)
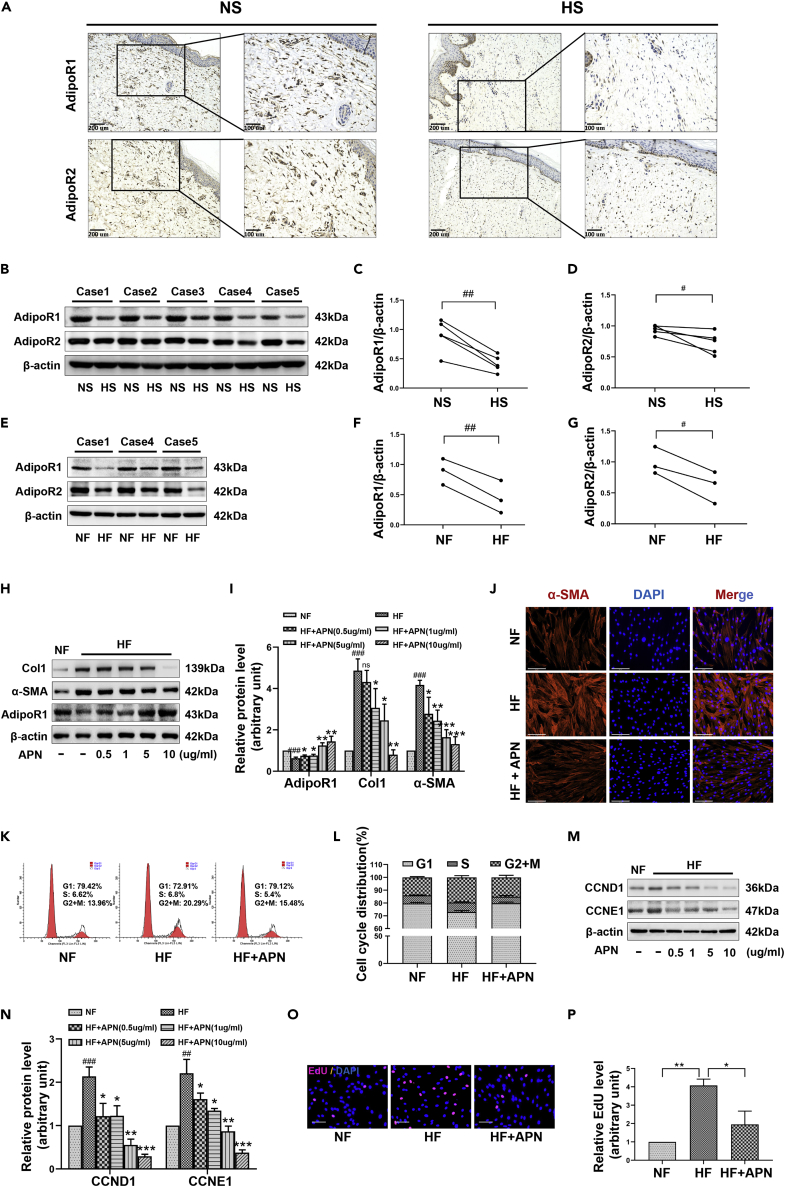
The abnormal expression of APN and its receptor AdipoR1/2 has been shown to be closely related to the development of diseases such as cancer, diabetes, and fibrosis, so they are expected to become important indicators for future disease detection. We collected five pairs of hypertrophic scar (HS) tissues and counterpart full-thickness normal skin (NS) tissues, and isolated three sets of HS-derived fibroblasts (HFs) and NS-derived fibroblasts (NFs) to assess the clinical relevance of adipoR1/2 to HS. Immunohistochemical results showed that compared with NS, the distribution of type I collagen (Col1) was dense and the α-SMA positively stained fibroblasts (α-SMA+) were significantly elevated in HS (Figure S1A), while the number of adipoR1+ and adipoR2+ fibroblasts was all reduced (Figure 1A). Consistently, Western blot also showed high levels of Col1 and α-SMA in HS and HFs (Figures S1B–S1D and S1G–S1I), but the expression of adipoR1 and adipoR2 was significantly decreased (Figures 1B–1G). These results showed that HS occurrence may be related to the impairment of APN signaling. AMP-activated protein kinase (AMPK) is activated by elevated AMP/ATP ratio caused by cellular and environmental stress, and the phosphorylation at Thr172 of the AMPKα subunit is required for AMPK activation (Shaw et al., 2004). Several studies have shown that the regulation of APN on diabetes, tumor, and organ fibrosis depends on the activation of AMPK (Li et al., 2020; Chung et al., 2017; Alzahrani et al., 2018). Our assessment of P-AMPK (Thr172) in NS/HS and NFs/HFs demonstrated a loss of AMPK activity in HS tissue fibroblasts (Figures S1A, S1B, S1E–S1G, S1J, and S1K), this further reflects the hindered transmission of APN-adipoR1/2 signaling in HS skin fibrosis.
Figure 1.
Restoration of APN-AdipoR signaling inhibits the fibrotic state and cell proliferation of scar fibroblasts (HFs)
(A) Representative images of immunohistochemical staining for the detection of AdipoR1 and AdipoR2 in hypertrophic scar (HS) and counterpart normal skin (NS) tissues, scale bar = 200 and 100μm.
(B–D) The protein levels of AdipoR1 and AdipoR2 from five pairs of NS and HS tissues were detected by Western blot.
(E–G) Representative Western blot images of AdipoR1 and AdipoR2 in three pairs of hypertrophic scar-derived fibroblasts (HFs) and counterpart normal skin-derived fibroblasts (NFs).
(H and I) The expression of Col1, α-SMA, and AdipoR1 in HFs treated with different concentrations of APN for 48h was detected by Western blot.
(J) Immunofluorescence showing the effect of APN at 10 μg/ml for 48h on the expression of α-SMA in HFs, scale bar = 125μm.
(K and L) The cell cycle distribution of HFs treated with 10 μg/ml of APN was analyzed by flow cytometry. Cell numbers at G0/G1, S, and G2/M phases were counted and the percentage was calculated.
(M and N) The expression of cyclins CCND1 and CCNE1 in HFs treated with different concentrations of APN for 48h was detected by Western blot.
(O and P) Nuclear EdU staining was conducted to assess the DNA replication activity of HFs treated with APN at 10 μg/ml for 48h. scale bar = 100μm. Five random views of 1,000 cell nuclei per each condition were used to calculate the average EdU ratio (% vs. DAPI, same for all EdU studies). Data are represented as mean ± SD. #p < 0.05, ##p < 0.01 and ###p < 0.001, compared to NS or NFs group. ns, p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared to the HFs group. The differences were analyzed by Paired-samples t-tests (C, D, F, G) or One-way ANOVA with Tukey’s multiple-comparison tests (I, L, N, P).
Next, HFs were treated with APN at different concentrations (0.5-10 μg/ml) for 48h. Western blot results showed that the expression of AdipoR1 increased in a concentration-dependent manner, and this was accompanied by a decrease in the production of Col1 and the impairment of the transformation of fibroblasts into myofibroblasts (marked by the decreased expression of α-SMA) (Figures 1H–1J). Comparing the cell cycle distribution of NFs and HFs from the same individual by flow cytometry, we found that the proportion of cells in the S and G2+M phases in HFs was significantly higher than that in NFs. However, when HFs were treated with 10 μg/ml of APN for 48h, the cell cycle was arrested in prophase G1, suggesting that the cell proliferation ability was weakened by this treatment (Figures 1K and 1L). Cyclin D1 (CCND1) and cyclin E1 (CCNE1) are members of the highly conserved cyclin family, and they form complexes with CDK2/4/6 and act as their regulatory subunits to promote the cell cycle progression from the G1 phase to the S phase. Consistent with our flow cytometry results, we found that the expression of CCND1 and CCNE1 in HFs was significantly increased compared with that in control NFs, while APN at a concentration above 0.5 μg/ml could inhibit their dominance in HFs (Figures 1M and 1N). In addition, EdU incorporation results showed that APN (10 μg/ml) treatment significantly reduced the positive rate for EdU incorporation in HFs, which indicated that APN inhibited the DNA replication activity of HFs (Figures 1O and 1P). Taken together, these results clearly demonstrated that APN inhibited the expression of fibrosis-related proteins and key proteins for G1-S transition in HFs, thereby inhibiting the fibrotic phenotype of cells and reducing their proliferative abilities.
Adiponectin upregulates the expression of SIRT1
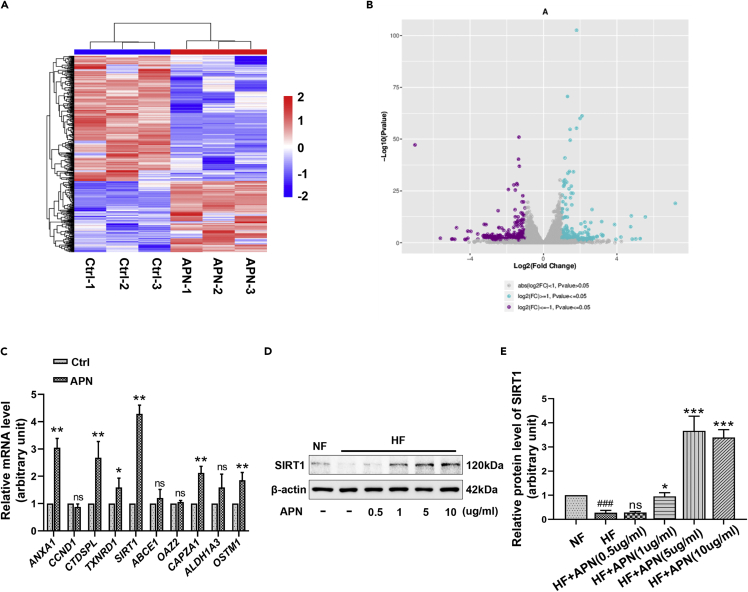
To investigate the potential mechanism of APN regulation, we initiated comprehensive mRNA sequencing to identify differentially expressed genes (DE-Gs) in HFs after exposure to 10 μg/ml of APN for 24h. Heatmaps were used to cluster DE-Gs according to their expression profiles, which indicated that the repeatability between samples from the same treatment group was good (Figure 2A). Volcano plots revealed that 281 genes were upregulated and 498 genes were downregulated after APN treatment (Figure 2B). We performed RT-qPCR validation on the top ten up-regulated genes with the most significant statistical significance obtained by RNA-sequencing, and found that the expression trend of ANXA1, CTDSPL, TXNRD1, SIRT1, CAPZA1, and OSTM1 was consistent with the results of RNA sequencing, among which the upregulation of histone deacetylase SIRT1 was the most obvious (Figure 2C). Western blot analysis (Figures 2D and 2E) showed that APN could significantly promote the expression of SIRT1 at protein levels. Gene ontology (GO) enrichment analysis was performed to further explore the underlying function of DE-Gs. As shown in Figure S2, the results showed that the top 20 potential functions regulated by APN on HFs mainly focus on cell periphery, cellular response to chemical stimulus and homeostatic process, and so forth.
Figure 2.
APN upregulates the expression of SIRT1
(A) HFs treated with 10 μg/ml of APN for 24h (APN group) or not (Ctrl group) were subjected to RNA-sequencing. The heatmap is an analysis of the two groups with clustering.
(B) The volcano plots showing the differentially expressed genes (DE-Gs) [log2(FC) ≥ 1 or ≤ −1, p ≤ 0.05] from the RNA sequencing data.
(C) The expression of the top 10 up-regulated genes with the most significant statistical significance obtained by RNA-sequencing was assessed by RT-qPCR.
(D and E) The effect of APN at different concentrations for 48h on the expression of SIRT1 in HFs was evaluated by Western blot. Data are represented as mean ± SD. ns, p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared to the group of HFs without APN treatment. ###p < 0.001, compared to NFs group. The differences were analyzed by One-way ANOVA with Tukey’s multiple-comparison tests.
SIRT1 inhibits the expression of fibrosis-related proteins and the cell proliferation of hypertrophic scar-derived fibroblasts
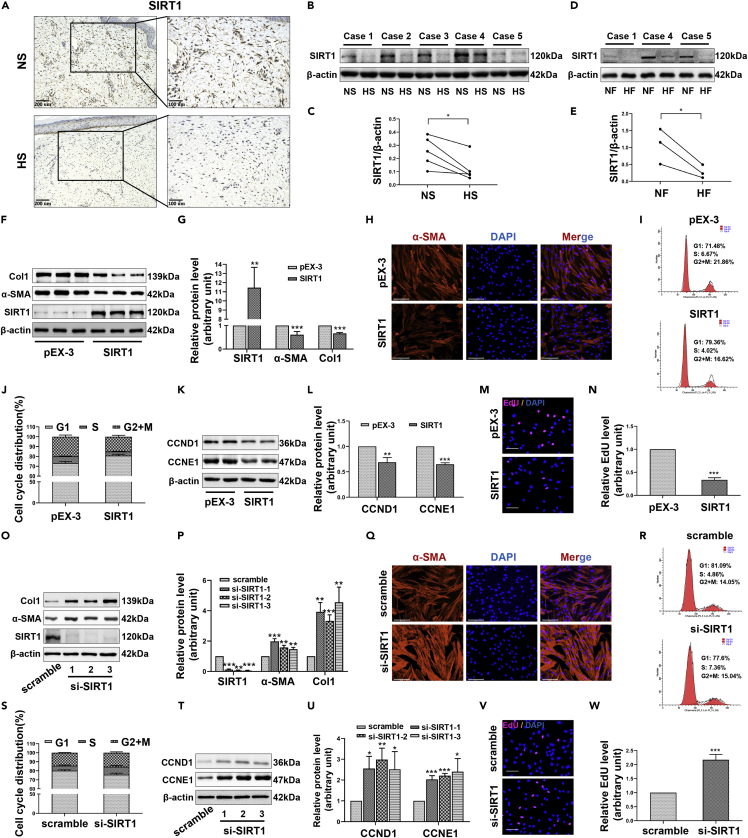
Having identified SIRT1 above, we then asked what role do SIRT1 play in skin fibrosis. The results of immunohistochemistry showed that the positive detection rate of SIRT1 in fibroblasts from HS samples was obviously lower than that from NS samples (Figure 3A). Similarly, the protein levels of SIRT in HS/HFs by Western blot were also significantly lower than that in NS/NFs (Figures 3B–3E). As NAD+ is a substrate for SIRT1, reduced levels of NAD + trigger consequent SIRT1 dysfunction (Cantó et al., 2012; Zhang et al., 2022). The content of NAD+ in another 6 pairs of fresh HS tissues and corresponding NS tissues (cases 6-11) was detected by WST-8 method. The results showed that the content of NAD+ in HS was significantly lower than that in NS (Figure S3A). Similarly, NAD + levels in HFs were significantly lower than those in NFs (Figure S3B). The changes in NAD+ and SIRT1 levels during the development of HS fibrosis are consistent. We next transfected HFs with overexpression plasmids to increase the level of SIRT1 and found that SIRT1 overexpression significantly inhibited the expression of the fibrosis-related proteins Col1 and α-SMA (Figures 3F–3H). In addition, this enhancement of SIRT1 significantly increased the proportion of cells in the G1 phase (Figures 3I and 3J), indicating that the progression of the cell cycle to the S phase and G2/M phase was hindered. The expression changes in CCND1 and CCNE1 further confirmed the inhibitory effect of SIRT1 on cell cycle progression (Figures 3K and 3L). A significant negative correlation between SIRT1 overexpression and DNA replication activity was also observed by EdU incorporation assays (Figures 3M and 3N). To confirm our results, we used small interfering RNA (siRNA) to further deplete SIRT1 in HFs. The results showed that continued SIRT1 deletion increased the expression of Col1, α-SMA (Figures 3O–3Q), CCND1, and CCNE1 (Figures 3T and 3U) in HFs, thus increasing the cell ratio in S and G2/M phases (Figures 3R and 3S) and enhancing the DNA replication activity (Figures 3V and 3W). Based on the above results, our experiments demonstrate that SIRT1 is strongly inversely correlated with the transformation of fibroblasts into myofibroblasts, collagen deposition, and cell hyperproliferation. The decreased expression of SIRT1 may thus be an important factor for skin hyperplasia after trauma.
Figure 3.
SIRT1 inhibits the fibrotic state and proliferation of HFs
(A) The expression of SIRT1 in NS and HS tissues was detected by immunohistochemistry. scale bar = 200 and 100μm.
(B and C) Western blot showing the expression of SIRT1 in five pairs of NS and HS tissues.
(D and E) Western blot showing the expression of SIRT1 in three pairs of NFs and HFs.
(F and G) Western blot showing the changes in the expression of Col1 and α-SMA when SIRT1 was overexpressed by plasmids (pEX-3-SIRT1).
(H) Immunofluorescence staining for α-SMA when SIRT1 was overexpressed. scale bar = 125μm.
(I and J) Flow cytometry showing the cell cycle distribution when SIRT1 was overexpressed.
(K and L) Western blot to detect the effect of SIRT1 overexpression on the expression of CCND1 and CCNE1.
(M and N) Nuclear EdU staining was used to assess the effect of SIRT1 overexpression on the DNA replication activity of HFs. scale bar = 100μm.
(O and P) The expression changes in Col1 and α-SMA when SIRT1 was knocked down by small interfering RNA (si-SIRT1-1/2/3).
(Q) Immunofluorescence staining for α-SMA in HFs transfected with si-SIRT1-1. scale bar = 125μm. (R, S) The effects of SIRT1 silencing on cell cycle distribution.
(T and U) The effects of SIRT1 silencing on the expression of CCND1 and CCNE1.
(V and W) Changes in DNA replication activity when SIRT1 was silenced. scale bar = 100μm. Data are represented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001, compared to NS, NFs or corresponding control group. The differences were analyzed by Paired-samples t-tests (C, E) or One-way ANOVA with Tukey’s multiple-comparison tests (G, J, L, N, P, S, U, W).
SIRT1 mediates the regulation of adiponectin on fibrosis in vitro and in vivo
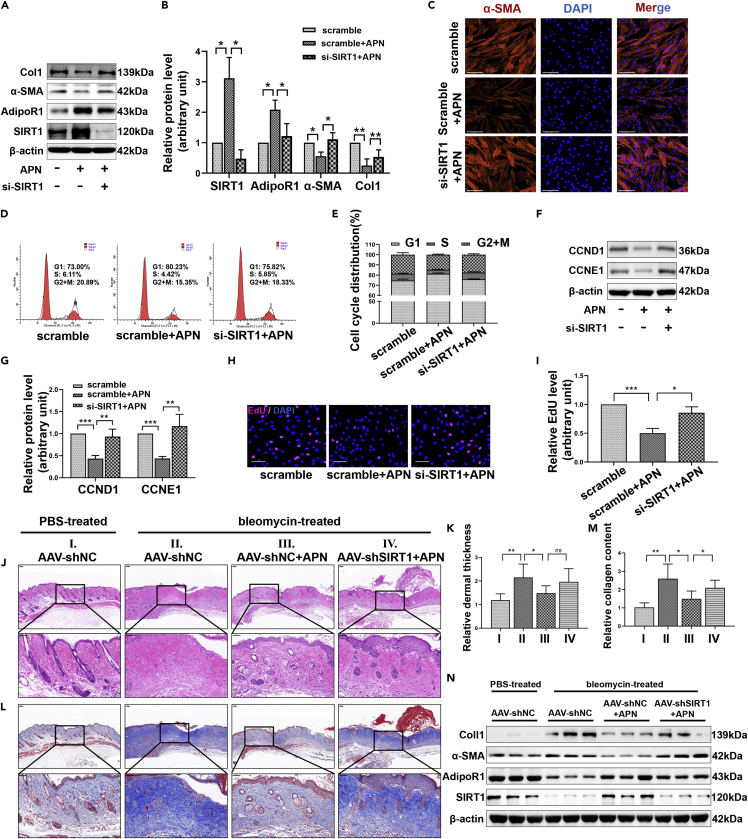
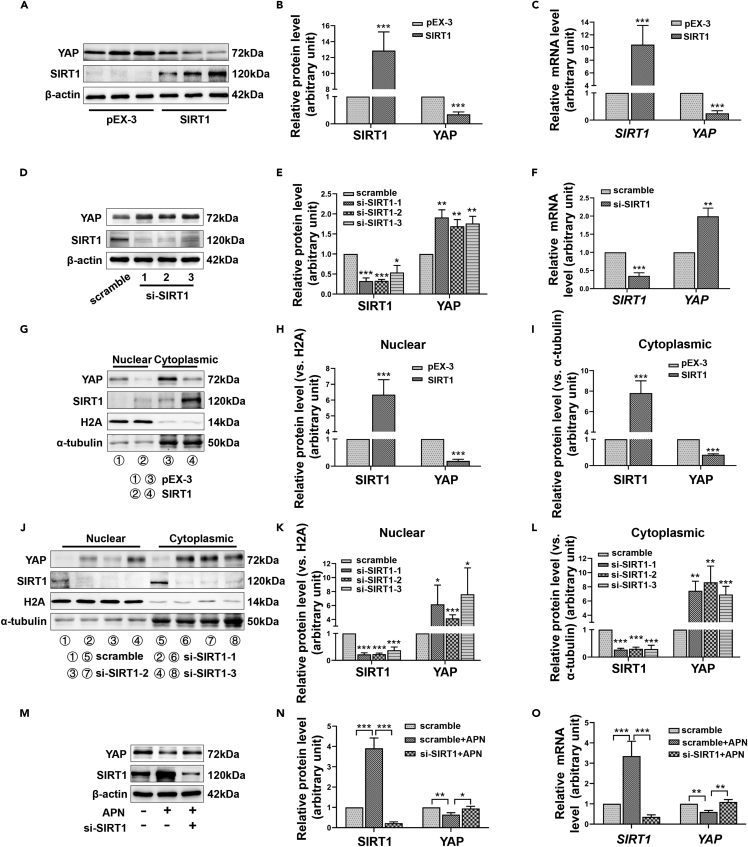
To determine whether the inhibition of fibrosis by APN was related to its regulation of SIRT1, we used siRNA to disrupt the upregulation of SIRT1 expression induced by APN. Using this model, the fibrosis state and proliferation ability of HFs were evaluated. Western blot showed that once SIRT1 was silenced, the ability of APN to inhibit the expression of Col1 and α-SMA was significantly reduced (Figures 4A and 4B). Immunofluorescence also reflected that APN-induced α-SMA expression inhibition depended on SIRT1 (Figure 4C). Flow cytometry results indicated that the absence of SIRT1 weakened the inhibitory effect of APN on the cell cycle transition from the G1 phase to the S and G2/M phases (Figures 4D and 4E). Moreover, the expression changes in CCND1 and CCNE1 were consistent with our flow cytometry results (Figures 4F and 4G). Similarly, EdU incorporation experiments also showed that SIRT1 knockdown antagonized the inhibition of DNA replication by APN (Figures 4H and 4I).
Figure 4.
SIRT1 mediates the regulation of APN on fibrosis in vitro and in vivo
HFs were treated with 10 μg/ml of APN for 48h after transfected with scramble (50nM) or si-SIRT1 (50nM). The in vitro results presented in this figure were all obtained under the above treatment conditions.
(A and B) The expression of SIRT1, AdipoR1, α-SMA, and Col1 in each group was assessed by Western blot.
(C) Representative immunofluorescence images of α-SMA in different groups. scale bar = 125μm.
(D and E) The cell cycle distribution in HFs of different treatments was assessed by flow cytometry.
(F and G) The expression of CCND1 and CCNE1 in different treatment groups was detected by Western blot.
(H and I) The DNA replication activity of different treatment groups was determined by nuclear EdU staining. scale bar = 100μm.
(J) HE staining images showing the thickness of the dermis of mice in different treatment groups. scale bar = 100 and 50μm.
(K) The thickness of the dermis was measured and analyzed by Image pro plus software.
(L) Masson’s trichrome staining shows the deposition and arrangement of collagen fibers in different treatment groups. scale bar = 100 and 50μm.
(M) The relative collagen content was analyzed by Image pro plus software.
(N) The skin of the dorsal treatment area of mice was collected for protein extraction. Western blot was used to detect the expression of SIRT1, AdipoR1, α-SMA, and Col1 in three randomly selected samples from each group. AAV-shNC, adeno-associated virus (AAV) carrying negative control short hairpin RNA (shNC), AAV-shSIRT1, AAV carrying short hairpin RNA targeting SIRT1 (shSIRT1). Data are represented as mean ± SD. ns, p > 0.05, ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001. The differences were analyzed by One-way ANOVA with Tukey’s multiple-comparison tests.
Continuous subcutaneous injection of bleomycin in mice has been shown to strongly stimulate the expression of a series of pro-inflammatory and pro-fibrotic mediators such as IL-1β, TGFβ1, and PDGF, and to also promote inflammatory cell infiltration, myofibroblast activation and collagen secretion (Yamamoto and Nishioka, 2005; Moseley et al., 1986). These histopathological features are comparable to human HS. Therefore, we used this animal model to further explore the effect of APN on HS. HE and Masson staining results showed that bleomycin promoted the thickening of the dermis, increased collagen deposition, and led to disordered collagen arrangement. The application of APN significantly improved this fibrosis state, while short hairpin RNA targeting SIRT1 (shSIRT1) delivered by adeno-associated virus (AAV) reversed the anti-fibrosis effect of APN, restoring the collagen deposition and disordered collagen arrangement (Figures 4J–4M). Protein extraction from skin at the injection site was performed, and Western blot was conducted. We found that the expression of Col1 and α-SMA under bleomycin stimulation increased significantly as expected, accompanied by the decrease of adipoR1 and SIRT1. APN treatment restored the adipoR1 and SIRT1 levels, and inhibited bleomycin-induced Col1 and α-SMA expression (Figure 4N). SIRT1 depletion successfully disrupted APN-induced SIRT1 upregulation, making APN less effective against fibrosis (Figure 4N).
Based on the results of these in vitro and in vivo experiments, we believe that APN represents a promising tool in the treatment of skin fibrosis, and SIRT1 is an important target in its regulation of scar fibrosis.
Yes-associated protein is an important downstream molecule of SIRT1 in the regulation fibrosis in hypertrophic scar-derived fibroblasts
YAP is an important effector of the hippo pathway and an important molecule in the field of tumor research, but studies about its functions in HS are scant. Previously, we noticed that SIRT1 mediates the deacetylation of YAP in HeLa cells, promoting the phosphorylation of YAP and inhibiting YAP activity. We, therefore, sought to understand whether YAP was also involved in SIRT1’s regulation of HS. We enhanced or silenced SIRT1 in HFs by transfecting overexpression plasmids or interfering RNA. Interestingly, we found that the protein level of YAP decreased significantly when SIRT1 was overexpressed (Figures 5A and 5B). Furthermore, RT-qPCR revealed that the regulation of YAP expression by SIRT1 occurred at the transcriptional level (Figure 5C). Consistent with this, SIRT1 silencing promoted the expression of YAP (Figures 5D–5F). As is well known, the entry of YAP into the nucleus is crucial to its function. Nuclear and cytoplasmic proteins, therefore, were, respectively, extracted, and as is shown in Figures 5G–5I, the enhancement of SIRT1 significantly reduced the level of YAP in the nucleus. However, SIRT1 silencing had the opposite effect (Figures 5J–5L). Moreover, we found that APN inhibited YAP expression through SIRT1 at both the protein and mRNA levels (Figures 5M−5O).
Figure 5.
SIRT1 represses YAP expression at the transcriptional level
(A–C) The effect of SIRT1 overexpression on YAP expression was assessed by Western blot (A, B) or RT-qPCR (C).
(D–F) The effect of SIRT1 silencing on YAP expression was assessed by Western blot (D, E) or RT-qPCR (F).
(G–L) The changes in SIRT1 and YAP levels in the cytoplasm and nucleus of HFs after SIRT1 overexpression (G-I) or knockdown (J-L) were detected by Western blot.
(M−O) HFs were treated with 10 μg/ml of APN after transfected with scramble or si-SIRT1. The expression of SIRT1 and YAP was detected by Western blot (M, N) or RT-qPCR (O). Data are represented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001, compared to corresponding control group or APN treatment group. The differences were analyzed by One-way ANOVA with Tukey’s multiple-comparison tests.
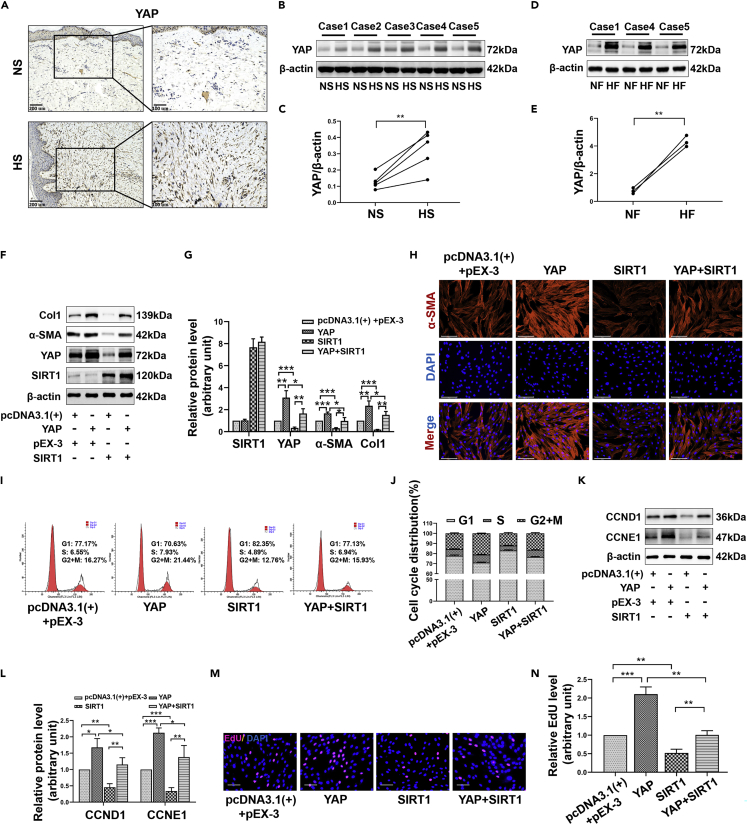
We next evaluated the expression of YAP in NS/HS and NFs/HFs. In histological examination, as shown in Figure 6A, there were more YAP + nuclear-stained fibroblasts in the HS dermis than in the NS dermis. Moreover, Western blot showed that the expression level of YAP in HS/HFs was significantly higher than the corresponding NS/NFs (p < 0.05) (Figures 6B–6E). To explore whether SIRT1 regulates fibrosis through YAP, we conducted separate transfection or co-transfection of YAP and SIRT1 overexpression plasmids into HFs. The results demonstrated that the inhibition of Col1 and α-SMA expression by SIRT1 decreased once the overexpression plasmid enhanced the expression of YAP (Figures 6F–6H). In addition, YAP overexpression attenuated the inhibitory effect of SIRT1 on the progression of the cell cycle to S and G2/M phases (Figures 6I and 6J). Correspondingly, expression changes in CCND1 and CCNE1 also verified this (Figures 6K and 6L). EdU incorporation assays showed that SIRT1-induced DNA replication suppression depended on its regulation of YAP (Figures 6M and 6N). These results all indicated that YAP is an important downstream target of SIRT1 in regulating fibrotic transformation and cell proliferation.
Figure 6.
SIRT1 overexpression inhibits YAP-induced fibrosis and proliferation of HFs
(A) The expression of YAP in HS and NS tissues was detected by immunohistochemical staining. scale bar = 200 and 100μm.
(B and C) Western blot showing the expression of YAP in five pairs of HS and counterpart NS tissues.
(D and E) Representative images of YAP Western blot from three pairs of HFs and NFs.
(F and G) HFs were transfected with SIRT1 overexpression plasmids (pEX-3-SIRT1) or/and YAP overexpression plasmids (pcDNA3.1(+)-YAP). Representative Western blot images showing the expression of Col1 and α-SMA in different treatment groups.
(H) Representative immunofluorescence images of α-SMA in different groups. scale bar = 125μm.
(I and J) The cell cycle distribution of differently treated HFs was assessed by flow cytometry.
(K and L) The expression of CCND1 and CCNE1 in different treatment groups was assessed by Western blot.
(M and N) The DNA replication activity of different treatment groups was detected by nuclear EdU staining. scale bar = 100μm. Data are represented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001. The differences were analyzed by Paired-samples t-tests (C, E) or One-way ANOVA with Tukey’s multiple-comparison tests (G, J, L, N).
SIRT1 inhibits the transcription activation of YAP by suppressing the acetylation of C/EBPβ K39 and histone 3 (H3) K9 and K27 in hypertrophic scar-derived fibroblasts
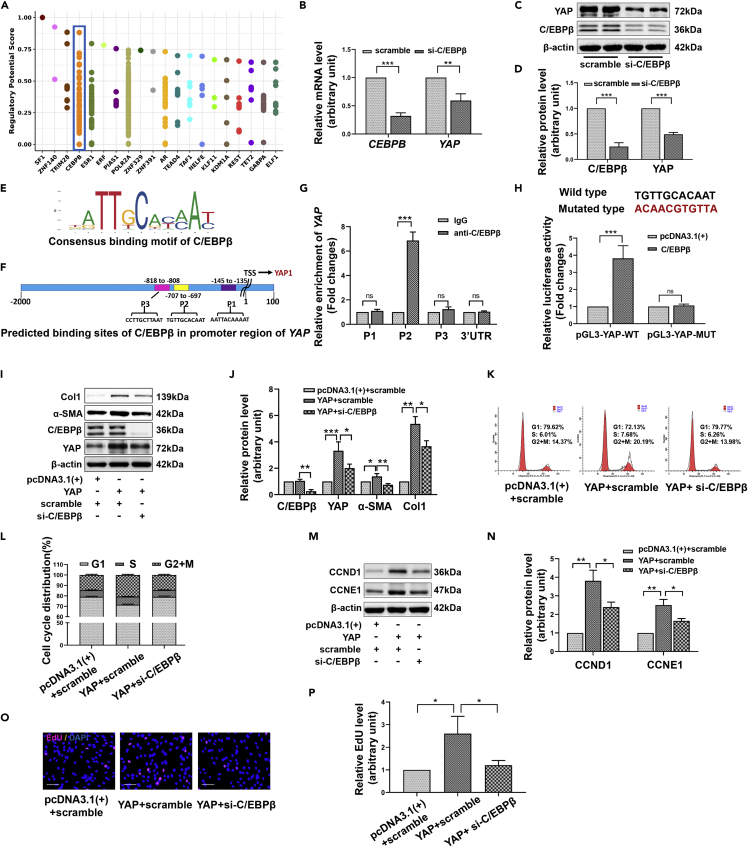
Next, we sought to explore how SIRT1 regulates YAP expression at the transcriptional level. Based on the published ChIP-Seq data, we searched the enriched transcription factors or chromatin regulators in the upstream 1kb sequence of the YAP transcription start site (TSS) through the Toolkit module of the Cistrome Data Browser (Figure 7A). Combined with the above regulatory potential score and data support (each point on the y axis represents a ChIP-seq dataset supporting this regulation factor) and JASPAR database prediction, transcription factor CCAAT enhancer binding protein β (C/EBPβ) appeared to be very likely to participate in the transcriptional activation of YAP (Figure 7A). RT-qPCR and Western blot showed that C/EBPβ silencing conspicuously downregulated YAP expression at both the mRNA and protein levels in HFs (Figures 7B–7D). Binding sites for C/EBPβ in the YAP promoter were predicted using the JASPAR database, and the results showed that three regions upstream of TSS might favor C/EBPβ binding: P1 (−145 to −135), P2 (−707 to −697), and P3 (−818 to −808) (Figures 7E and 7F). ChIP-qPCR demonstrated that C/EBPβ was significantly enriched in the P2 region of the YAP promoter (Figure 7G). We, therefore, designed reporter gene vectors containing either the YAP wild-type promoter (pGL3-YAP-WT) or a YAP P2 site mutant promoter (pGL3-YAP-MUT) for dual-luciferase experiments. We found that in pGL3-YAP-WT transfected HFs, C/EBPβ overexpression significantly enhanced luciferase activity, while in pGL3-YAP-MUT transfected cells, this effect disappeared (Figure 7H). These results indicated that C/EBPβ directly bound to the YAP promoter to activate YAP transcription. Further research found that C/EBPβ silencing weakened the expression ability of the YAP overexpression plasmid, and inhibited the expression induction of fibrosis-related proteins (Figures 7I and 7J). As expected, when C/EBPβ was depleted, the progression of cell cycles (Figures 7K and 7L), the expression of cyclins (Figures 7M and 7N) and the activation of DNA replication (Figures 7O and 7P) induced by the YAP overexpression plasmid were significantly attenuated. From the above results, it can be concluded that C/EBPβ regulates the fibrosis state and cell proliferation activity of skin fibroblasts by transcriptionally activating the expression of YAP.
Figure 7.
C/EBPβ transcriptionally activates YAP to facilitate the fibrosis transformation and proliferation of HFs
(A) Transcription factors or chromatin regulators enriched in the 1kb sequence upstream of the YAP transcription start site (TSS) were arranged according to regulatory potential score. This is provided by the Cistrome data browser based on the analysis of published ChIP-Seq data.
(B–D) RT-qPCR (B) or Western blot (C, D) assays showing the effect of C/EBPβ silencing on the mRNA or protein levels of YAP.
(E) Consensus binding motif of C/EBPβ.
(F) The binding sites of C/EBPβ in the promoter of YAP were predicted using the JASPAR database.
(G) ChIP-qPCR was used to detect the binding of C/EBPβ to different sites of the YAP promoter.
(H) Dual-luciferase assay was used to detect the effect of C/EBPβ overexpression plasmid (pcDNA3.1(+)-C/EBPβ) on the luciferase activity of the reporter gene vectors containing wild-type YAP promoter (pGL3-YAP-WT) or P2 site mutant YAP promoter (pGL3-YAP-MUT).
(I and J) Western blot showing the expression of YAP, C/EBPβ, α-SMA and Col1 in HFs transfected with YAP overexpression plasmid (pcDNA3.1(+)-YAP) or/and C/EBPβ specific siRNA (si-C/EBPβ).
(K and L) The cell cycle distribution in different groups was assessed by flow cytometry.
(M and N) The expression of CCND1 and CCNE1 in different treatment groups.
(O and P) The DNA replication activity of different treatment groups was detected by nuclear EdU staining. Data are represented as mean ± SD. ns, p>0.05, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. The differences were analyzed by One-way ANOVA with Tukey’s multiple-comparison tests.
Further study illustrated that SIRT1 inhibited the YAP expression induced by C/EBPβ at the transcriptional and translational levels (Figures 8A–8C). Consistent with this, dual-luciferase assays showed that SIRT1 overexpression attenuated the activation of the YAP promoter by C/EBPβ (Figure 8D). ChIP-qPCR also demonstrated that the overexpression of SIRT1 resulted in decreased binding of C/EBPβ to the P2 motif of the YAP promoter in HFs (Figure 8E). The lysine 39 (K39) site of C/EBPβ is located at its N-terminal transcriptional activation domain (aa 22-104). It is acetylated by the acetyltransferase p300, which is related to the abrogation of its self-repression, thereby activating its transcriptional activity (Lee et al., 2010; Sin et al., 2019). IP results showed that SIRT1 silencing enhanced the overall acetylation level of C/EBPβ; however, this acetylation-promoting effect was also significantly weakened once K39 was mutated to an acetylation-defective arginine (R), suggesting that K39 was an important acetylation regulatory target of SIRT1 (Figures 8F and 8G). Moreover, we found that the SIRT1 silencing-induced YAP upregulation was also reduced along with the loss of acetylation capacity at K39 (Figures 8F and 8H). These results indicated that SIRT1 could reduce the transcriptional activity of CEBPβ by inhibiting the K39 acetylation, thereby suppressing the expression of YAP.
Figure 8.
SIRT1 inhibits the transcription activation of YAP by suppressing the acetylation of C/EBPβ K39 and histone 3 (H3) K9 and K27 in HFs
(A) RT-qPCR results showing the mRNA levels of CEBPB, SIRT1, and YAP in HFs transfected with C/EBPβ overexpression plasmids (pcDNA3.1(+)-C/EBPβ) or/and SIRT1 overexpression plasmids (pEX-3-SIRT1).
(B and C) Western blot results show the protein levels of C/EBPβ, SIRT1, and YAP in different groups.
(D) Dual-luciferase assay was used to detect the luciferase activity of the reporter gene vectors containing wild-type YAP promoter (pGL3-YAP-WT) in different groups.
(E) ChIP-qPCR to detect the effect of SIRT1 overexpression on the enrichment of C/EBPβ at the YAP promoter.
(F–H) The effect of SIRT1 silencing on C/EBPβ acetylation level (F, G) and YAP expression (F, H) in HFs transfected with C/EBPβ overexpression plasmid (pcDNA3.1(+)-C/EBPβ) or C/EBPβ K39 acetylation-defective plasmid (pcDNA3.1(+)-C/EBPβ K39R) was evaluated by IP combined with Western blot.
(I and J) ChIP-qPCR assay to detect the effect of SIRT1 overexpression on the acetylation levels of H3K9 and H3K27 at the C/EBPβ binding region of the YAP promoter. Data are represented as mean ± SD. ns, p>0.05, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. The differences were analyzed by One-way ANOVA with Tukey’s multiple-comparison tests.
It was worth noting that analysis from the Cistrome database revealed that there might be H3K27Ac and H3K9Ac histone modifications in the 1kb region upstream of the TSS of YAP (Figure S4). Histone modification patterns can be used to predict the type of chromatin (heterochromatin or euchromatin). The significant enrichment of H3K27Ac and H3K9Ac in the enhancer or promoter usually indicates that the gene is in a transcriptional active state. To examine whether these modifications in the YAP promoter were influenced by SIRT1, we used anti-H3K27Ac and H3K9Ac antibodies to conduct the chromosome immunoprecipitation (ChIP) of HFs transfected with a control plasmid or a SIRT1 overexpression plasmid, and qPCR was performed using primers specific to the P2 site of YAP promoter. The results showed that SIRT1 overexpression inhibited the enrichment of H3K27Ac and H3K9Ac in the P2 region (Figures 8I and 8J). Collectively, our study indicated that SIRT1 promotes the deacetylation of C/EBPβ K39, H3K27, and H3K9, resulting in decreased transcriptional activity of C/EBPβ and condensation of chromatin at the YAP promoter, thus preventing the transcriptional activation of YAP promoter by C/EBPβ.
Yes-associated protein assists TEAD4 to directly activate the transcription of CTGF, CCND1, and CCNE1 in hypertrophic scar-derived fibroblasts
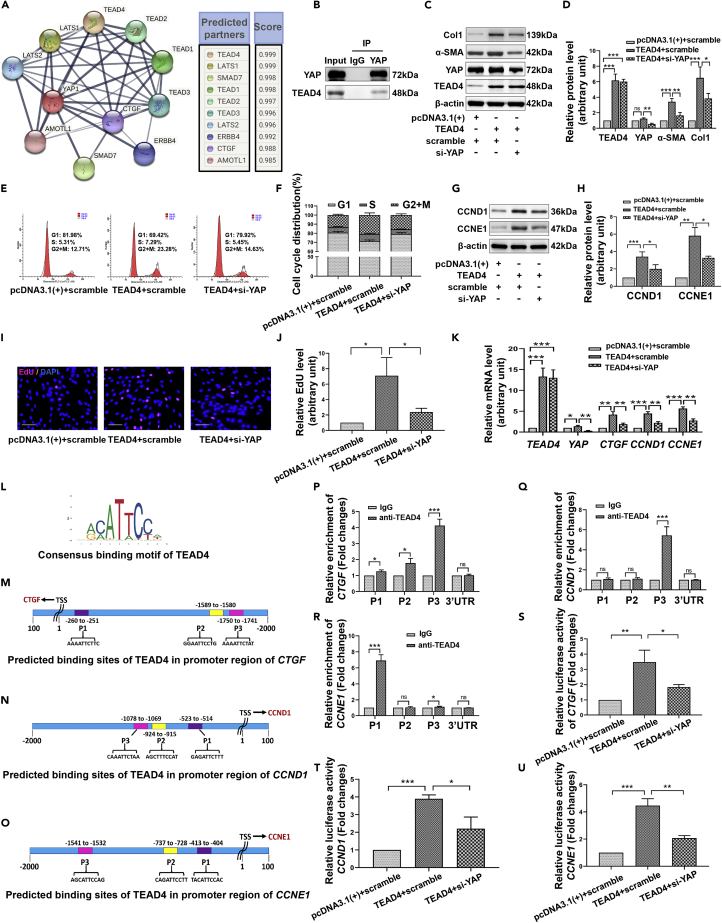
At present, the role of YAP in tumors has been widely studied, but there have not been many studies on its role in HS. Therefore, we further explored the mechanism of YAP affecting skin fibrosis. As shown in Figure 9A, the molecules interacting with YAP were identified using the STRING database, and TEAD4 had the highest score. Co-IP experiments further confirmed the interaction between endogenous YAP and TEAD4 in HFs (Figure 9B). Moreover, YAP deficiency obviously hindered the upregulation of fibrotic proteins induced by TEAD4 (Figures 9C and 9D), and limited the promotion of TEAD4 on cell cycle progression (Figures 9E and 9F), cyclins expression (Figures 9G and 9H) and DNA replication activity (Figures 9I and 9J). Connective tissue growth factor (CTGF) is an important cytokine that promotes fibroblast proliferation and collagen deposition, and is a classic target of YAP. RT-qPCR results showed that TEAD4 overexpression significantly enhanced the mRNA levels of CTGF, CCND1, and CCNE1, but YAP silencing inhibited the transcriptional regulation of TEAD4 on these genes (Figure 9K). As shown in Figures 9L–9O, JASPAR database was used to predict the binding sites of TEAD4 to the CTGF, CCND1, and CCNE1 promoters. The actual binding ability of TEAD4 to these promoter regions in HFs was examined by ChIP-qPCR. The results showed that the enrichment of TEAD4 could be detected at the P1 (−260 to −251), P2 (−1589 to −1580), and P3 (−1750 to −1741) sites upstream of the TSS of CTGF, especially at the P3 site (Figure 9P). In addition, significant TEAD4 enrichment appeared at the P3 site (−1078 to −1069) upstream of the TSS of CCND1 (Figure 9Q) and the P1 site (−413 to −404) upstream of the TSS of CCNE1 (Figure 9R). To further clarify the influence of YAP and TEAD4 on the activity of these gene promoters, we conducted dual-luciferase experiments. The results showed that TEAD4 overexpression dramatically increased the luciferase activity of the reporter gene containing a wild-type CTGF, CCND1, or CCNE1 promoter, but in the case of co-transfection with siYAP, the luciferase activity of these reporter genes decreased substantially (Figures 9S–9U). These results suggested that YAP regulates fibrotic phenotype and proliferation activity of fibroblasts by assisting TEAD4 in the transcriptional activation of CTGF, CCND1, and CCNE1.
Figure 9.
YAP is important for the transcriptional activation of CTGF, CCND1, and CCNE1 by TEAD4 in HFs
(A) Molecules that interact with YAP predicted by the STRING database.
(B) The interaction between YAP and TEAD4 was detected by Co-IP combined with Western blot.
(C and D) Western blot showing the protein levels of TEAD4, YAP, α-SMA, and Col1 in HFs transfected with TEAD4 overexpression plasmids (pcDNA3.1(+)-TEAD4) or/and YAP-specific siRNA (si-YAP).
(E and F) Flow cytometry assay to detect the cell cycle distribution after different treatments.
(G and H) The protein levels of CCND1 and CCNE1 in different groups were assessed by Western blot.
(I and J) The DNA replication activity of different treatment groups was detected by nuclear EdU staining. scale bar = 100μm.
(K) RT-qPCR assay showing the mRNA levels of TEAD4, YAP, CTGF, CCND1, and CCNE1 in different treatment groups.
(L) Consensus binding motif for TEAD4.
(M−O) The binding sites of TEAD4 in the promoters of CTGF (M), CCND1 (N), and CCNE1 (O) were predicted using the JASPAR database.
(P–R) ChIP-qPCR was used to detect the binding of TEAD4 to different sites of CTGF (P), CCND1 (Q), and CCNE1 (R) promoters.
(S–U) Dual-luciferase assay was used to assess the effect of different treatments on the luciferase activity of the reporter gene vectors containing wild-type CTGF, CCND1, or CCNE1 promoter. Data are represented as mean ± SD. ns, p > 0.05, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. The differences were analyzed by One-way ANOVA with Tukey’s multiple-comparison tests.
Discussion
When the skin encounters severe burns or trauma, pathological wound healing often occurs, resulting in skin fibrosis, that is the formation of hypertrophic scar (HS). Cytological examination has shown that fibroblasts transform into myofibroblasts with strong expression of α-SMA and proliferate abnormally, and collagen is secreted vigorously and arranged in disorder (Zhang et al., 1995). At present, the formation mechanism of HS has not been clarified, so there are no specific and effective drug treatments available in the clinic. Therefore, elucidating the relevant molecular mechanisms of HS and finding effective therapeutic targets are of great significance for the prevention and treatment of HS.
Previous studies have confirmed that APN is effective in the treatment of a variety of skin diseases. For example, the levels of serum APN in patients with diffuse cutaneous systemic sclerosis (SSc) are reduced (Masui et al., 2012), and APN administration can effectively inhibit the fibrotic response of skin fibroblasts in patients with SSc (Fang et al., 2012). In addition, studies have found that APN levels in the skin tissue and subcutaneous fat of patients with psoriasis are significantly reduced, and APN supplementation can act on dermal T cells and inhibit the production of IL-17, thereby alleviating skin inflammation (Shibata et al., 2015). To date, the effect of APN on HS has not been reported. Our research found that compared with counterpart normal skin (NS) tissues and derived fibroblasts (NFs), the expression of AdipoR1 and AdipoR2 in HS tissues and their fibroblasts (HFs) was all significantly reduced (Figures 1A–1G). We speculated that the occurrence of HS might be related to the attenuation of the APN-AdipoR1/2 signal. We found that stimulating HFs with APN could restore the AdipoR1 levels in a concentration-dependent manner. The enhancement of this signal inhibited the expression of Col1 and α-SMA (Figures 1H–1J). In addition, the flow cytometry results showed that APN treatment caused cell-cycle arrest in the G1 phase and significantly reduced the proportion of cells in S and G2/M phases (Figures 1K and 1L), which was related to the decreased expression of key cyclins CCND1 and CCNE1 that regulate the cell cycle transition from G1 phase to S phase (Figures 1M and 1N). Moreover, EdU incorporation assays showed the inhibitory effect of APN on DNA replication activity (Figures 1O and 1P). In conclusion, we demonstrated in vitro that APN can antagonize the fibrotic phenotype transformation and abnormal proliferation of scar fibroblasts.
To determine the downstream targets of APN in regulating fibrotic phenotype and cell proliferation of scar fibroblasts, we used RNA-sequencing to identify the changes in genome expression induced by APN. Results showed that compared with the control group, the APN treatment group had 281 upregulated genes and 498 downregulated genes (Figure 2B). We performed RT-qPCR validation on the top ten up-regulated genes with the most significant statistical significance obtained by RNA-sequencing, and found that the expression trend of ANXA1, CTDSPL, TXNRD1, SIRT1, CAPZA1, and OSTM1 was consistent with the results of RNA sequencing, among which the upregulation of histone deacetylase SIRT1 was the most obvious (Figure 2C). Subsequent Western blot results showed that APN treatment could, indeed, significantly enhance the expression of SIRT1 at the translational levels (Figures 2D and 2E), which furtherly verified our RNA sequencing results.
SIRT1 is an NAD+-dependent deacetylase. It has been reported that it regulates the activity of a variety of signal molecules or transcription factors through deacetylation, such as NLRP3, EZH2, NF-κB, Smad2/3, and so on, and is widely involved in inflammatory response, insulin secretion, extracellular matrix synthesis, apoptosis and other physiological activities (He et al., 2020; Li et al., 2018, 2019; Chu et al., 2018; Ryu et al., 2019; Bugyei-Twum et al., 2018). The disorder of these physiological activities is closely related to the occurrence of diseases such as diabetes, organ fibrosis, and cancer. Therefore, in recent years, SIRT1 has become a popular drug design target for the treatment of various diseases (Chen et al., 2021; Li et al., 2019). In our study, the results of immunohistochemical staining and Western blot showed that the levels of SIRT1 in HS/HFs were significantly lower than that of NS/NFs (Figures 3A–3E). In addition, the lack of NAD+ in HS/HFs also indicated the inhibition of SIRT1 function during the development of HS fibrosis (Figure S3). We re-enhanced the expression of SIRT1 in HFs by transfection with overexpression plasmids, which resulted in the expression of fibrosis-related proteins Col1 and α-SMA being significantly suppressed (Figures 3F and 3H). The flow cytometry results showed that SIRT1 overexpression significantly reduced the proportion of cells in S and G2/M phases (Figures 3I and 3J). Consistent with this, the expression of cyclins CCND1 and CCNE1 was inhibited (Figures 3K and 3L) and the number of cells with active DNA replication was reduced (Figures 3M and 3N). These results all demonstrated that during HS fibrosis the persistent activation and hyperproliferation of fibroblasts and the abnormal secretion of collagen are associated with reduced SIRT1 expression. We silenced SIRT1 with siRNA and conducted the above-mentioned tests, and the results further supported this conclusion (Figures 3O–3W).
The regulation of SIRT1 by APN has been reported, but most of the studies focus on inflammation, mitochondrial homeostasis, lipid metabolism, and insulin resistance (Zhang et al., 2017; Hong et al., 2019; Shah et al., 2019; Guo et al., 2020), and research in the field of fibrosis is scarce. Based on the fact that the expression of SIRT1 was significantly enhanced in HFs treated with APN, we further studied whether the inhibition of fibrotic phenotype transformation and cell proliferation of HFs by APN was related to its regulation of SIRT1. SiRNAs were transfected into HFs to interfere with the upregulation of SIRT1 by APN. It was found that the inhibitory effects of APN on fibrosis-related proteins expression, cell cycle progression, cyclins expression, and DNA replication were all attenuated by SIRT1 deletion (Figures 4A–4I). The results of HE and Masson staining showed that continuous subcutaneous injection of bleomycin in the murine back resulted in thickening of the dermis, severe deposition of collagen, and the formation of skin fibrosis lesions (Figures 4J–4M). Proteins from damaged skin were extracted and detected by Western blot. Results showed that the enhancement of collagen and α-SMA expression was accompanied by a marked decrease of AdipoR1 and SIRT1 expression (Figure 4N). APN treatment for 14 consecutive days restored AdipoR1 and SIRT1 levels and improved skin fibrosis (Figures 4J–4N). However, in the course of treatment, the anti-fibrotic effect of APN was significantly attenuated if short hairpin RNA was used to disrupt APN-induced SIRT1 expression (Figures 4J–4N). Combining the results of in vitro and in vivo studies, we confirmed that APN inhibits the fibrotic phenotype transformation and proliferation of fibroblasts and improves skin fibrosis by promoting the expression of SIRT1. Interestingly, we found that interference with SIRT1 expression often resulted in a decrease in AdipoR1 expression, with significant statistical differences (Figures 4A and 4N). Therefore, we believe that there is a positive feedback loop between APN-AdipoR1 and SIRT1. The treatment of APN makes the signal cascade of this loop amplify, and has a significant effect on inhibiting HS fibrosis.
The Hippo pathway is a kinase chain composed of a series of protein kinases and transcription factors, and YAP is its main effector. YAP is involved in regulating a variety of physiological and pathological processes of skin tissues (Lee et al., 2014; Debaugnies et al., 2018; Zhang et al., 2020). In the process of skin wound healing, the expression of YAP in the dermal and epidermal cells around the wound is increased and the nuclear localization is enhanced, which can promote wound healing by regulating the expression of TGFβ1 (Lee et al., 2014). In addition, YAP also plays important role in the initiation and progression of skin malignancies such as basal cell carcinoma, squamous cell carcinoma, and melanoma (Debaugnies et al., 2018; Zhang et al., 2020). In the field of HS research, there is still a lack of evaluation of YAP expression at NS/HS tissue and NFs/HFs primary cell, and studies on the role and related mechanisms of YAP in regulating the fibrotic phenotype transformation and proliferation of scar fibroblasts are relatively limited. We noticed that in HeLa cells, SIRT1 mediates the deacetylation of YAP, thereby enhancing its phosphorylation levels, inhibiting its activity and sensitivity to DNA damage (Hata et al., 2012). In the study of nicotine-induced atherosclerosis, it was also found that nicotine inhibits the expression of SIRT1, thereby activating YAP, resulting in increased secretion of extracellular matrix (Ding et al., 2019). Therefore, we wanted to explore whether the regulation of HFs by SIRT1 is also related to YAP. Interestingly, unlike previous reports that SIRT1 regulates YAP activity, we found that SIRT1 regulated the expression of YAP. Our results showed that SIRT1 overexpression decreased the expression of YAP starting from the transcriptional level (Figures 5A–5C). Western blot of nuclear proteins showed that SIRT1 overexpression significantly suppressed the enrichment of YAP in the nucleus (Figures 5G–5I). Consistently, knockdown of SIRT1 promoted YAP expression and nuclear transfer (Figures 5D–5F and 5J–5L). Further studies found that SIRT1 mediated the inhibitory effect of APN on YAP expression (Figures 5M−5O). Immunohistochemical staining of NS/HS tissues and Western blot results of tissues and cells showed that the levels of YAP in HS and HFs were significantly higher than that in NS and NFs (Figures 6A–6E). In addition, we found that the inhibitory effects of SIRT1 on fibrotic proteins expression, cell cycle progression, and DNA replication activity depended on its inhibition of YAP expression. The anti-fibrosis effect of SIRT1 was attenuated once the overexpression plasmid resisted SIRT1-induced YAP downregulation (Figures 6F–6N). In conclusion, our study revealed the role and mechanism of APN in regulating HS fibrosis by inhibiting YAP expression through SIRT1.
We used the Cistrome database to identify the transcription factors or chromatin regulators that are tightly bound to the YAP promoter from published human ChIP-Seq data. Among them, the regulatory potential score and data support of C/EBPβ are prominent (Figure 7A). As an important transcription factor, C/EBPβ has been reported to participate in physiological activities such as neural signal transduction, cell proliferation, and epidermal-mesenchymal transition by regulating the expression of APOE, TGFβ1, and α-SMA (Xia et al., 2021; Cao et al., 2021; Ding et al., 2021). This is related to the pathogenesis of Alzheimer’s disease, cancer, fibrosis, and other diseases. Our study found that interfering with C/EBPβ in HFs resulted in a significant decrease in YAP expression at both mRNA and protein levels (Figures 7B–7D). ChIP-qPCR results showed that C/EBPβ was significantly enriched at the P2 site (−707 to −697) of the YAP promoter predicted using the JASPAR database (Figure 7G). Moreover, dual-luciferase assays showed that C/EBPβ overexpression significantly enhanced the luciferase activity of a reporter gene vector containing YAP wild-type promoter, but this did not occur for a reporter gene vector with P2 site mutation (Figure 7H). These experiments confirmed that C/EBPβ is a transcription factor regulating YAP expression. Further studies showed that C/EBPβ silencing impaired the expression ability of YAP overexpression plasmid, as well as attenuated its induction of fibrosis proteins expression and cell proliferation (Figures 7I–7P).
Moreover, we found that SIRT1 overexpression inhibited C/EBPβ-induced YAP expression at both the transcriptional and translational levels (Figures 8A–8C). Combining dual-luciferase (Figure 8D) and ChIP-qPCR (Figure 8E) experiments, it was clearly shown that SIRT1 prevented the binding and activation of C/EBPβ to the YAP promoter. Previous reports indicated that acetyltransferase p300-mediated acetylation of C/EBPβ at the K39 site of the N-terminal transcriptional activation domain (aa 22-104) alleviates the inhibition of C/EBPβ on self-activity (Lee et al., 2010; Sin et al., 2019). This may be because the acetylation of K39 changes the conformation of the domain, thereby favoring C/EBPβ to contact and regulate target genes. Our study found that when K39 was mutated to an acetylation-defective arginine, the induction of C/EBPβ acetylation by SIRT1 knockdown was significantly inhibited (Figures 8F and 8G). In addition, the promotion effect of SIRT1 silencing on YAP expression was weakened accordingly (Figures 8F and 8H). These results indicated that SIRT1 inhibits the acetylation of C/EBPβ K39, thereby reducing the ability of C/EBPβ to activate YAP transcription.
Histone acetylation is a significant histone modification, and it reduces the positive charge of histones and thus weakens the interaction between histones and negatively charged DNA, leading to the relaxation of chromatin structure and facilitating the binding of transcription complexes to gene promoters (Grunstein, 1997). Genome-wide studies have shown that the levels of histone acetylation in the promoter regions of actively transcribed genes are higher than that of inactive genes (Heintzman et al., 2007). The epigenetic markers H3K9Ac, H3K27Ac and H4K16Ac are well-characterized acetylation markers that are enriched in the promoter and enhancer regions of transcriptional active genes and play clearly defined roles in regulating chromatin structure (Kouzarides, 2007; Wang et al., 2008). In contrast, their deacetylation leads to the formation of a more compact chromosomal structure and blocks gene transcription (Ishijima et al., 2019). It has been shown that SIRT1 regulates gene expression by deacetylating H1K26Ac, H3K9Ac, and H4K16Ac (Vaquero et al., 2004; Bosch-Presegué and Vaquero, 2015; Ferguson et al., 2015; Jang et al., 2017). We wondered whether SIRT1’s regulation of YAP transcription is also associated with changes in histone acetylation levels in the YAP promoter region. Analysis from the Cistrome database revealed significant H3K27Ac and H3K9Ac histone modification in the 1kb region upstream of the TSS of YAP (Figure S4). We speculated that the binding of C/EBPβ to the YAP promoter may have been affected by acetylation levels of H3K27 and H3K9 to some extent. Chromosome immunoprecipitation was performed on HFs transfected with control plasmids or SIRT1 overexpression plasmids using anti-H3K27Ac or H3K9Ac antibodies, and PCR detection was conducted on the YAP promoter P2 site using specific primers. The results showed that the overexpression of SIRT1 inhibited the enrichment of H3K27Ac or H3K9Ac in the P2 region (Figures 8I and 8J). Taken together, our studies indicated that SIRT1 deacetylates C/EBPβ K39 and histones H3K27 and H3K9, which not only represses the transcriptional activity of C/EBPβ but also leads to chromatin compaction, thereby preventing the transcriptional activation of YAP promoter by C/EBPβ.
Previous studies have revealed the role of YAP as a transcriptional co-activator in regulating the activity of transcription factors such as STAT3 and TEAD (He et al., 2018; Zhao et al., 2008), but in the field of HS research, the regulatory mechanism of YAP has not been reported. Therefore, we did further exploration. The STRING database provides molecules that may interact with YAP, and TEAD4 has the highest score (Figure 9A). Co-IP assay showed that anti-YAP antibody could successfully precipitate TEAD4 from HFs (Figure 9B). Importantly, silencing of YAP significantly suppressed the promoting effect of TEAD4 on fibrosis-related proteins expression and cell proliferation (Figures 9C–9J). Further studies found that TEAD4 transcriptionally regulated the expression of CTGF, CCND1, and CCNE1, but its transcription activity was dependent on YAP (Figures 9K–9U). Therefore, our study elucidated the mechanism by which YAP regulates the fibrotic state and cell proliferation of fibroblasts by assisting TEAD4 to promote the expression of CTGF, CCND1, and CCNE1.
In conclusion, our research demonstrates that HS formation is related to the weakening of APN-AdipoR1/2 signaling in skin fibroblasts. APN treatment can enhance the expression of SIRT1 in HFs. SIRT1 inhibits the acetylation levels of C/EBPβ K39, histone H3K27, and H3K9, resulting in the transcriptional activity of C/EBPβ is reduced and the chromatin conformation around the YAP promoter is compact, thus blocking the transcriptional activation of YAP. YAP is an important transcription co-activator that assists TEAD4 in the transcriptional regulation of pro-fibrotic cytokine CTGF and cyclins CCND1 and CCNE1. In short, our study reveals the role and mechanism of APN antagonizing HS fibrosis by regulating the SIRT1/C/EBPβ/YAP-TEAD4 pathway, so as to provide promising ideas for the prevention and treatment of HS.
Limitations of the study
The current study has two limitations. First, although our data suggest that APN treatment can significantly increase SIRT1 expression in scar fibroblasts, the exact mechanism involved is unclear and requires further investigation in the future. Second, the lack of suitable animal models has plagued researchers throughout the history of HS research. This is mainly owing to the fact that phylogenetic differences between different species make it difficult for other animals to develop skin fibrosis similar to human HS. The existing HS animal models all have some defects. Rabbit ear scar model: collagen deposition is caused by cartilage injury, which is distinct from human HS fibrosis caused by dermis injury. Nude mouse transplantation model of human HS tissues: as the immune system of mice is suppressed, it is difficult to accurately simulate the human environment. The ex vivo HS tissues usually represent the final stage of the scarring process, which limits the exploration of the mechanism related to HS. Mechanical load-induced HS model: This model is induced by tension load on the basis of skin injury, so it has the same pathogenic background as HS fibrosis. However, the experimental device needs to be disinfected frequently, resulting in damp around the wound surface and easy to rupture. Moreover, the mechanical stretch is not well controlled, which leads to low scar hyperplasia rate and poor repeatability. Bleomycin-induced skin fibrosis model in mice: Bleomycin does not provide sustained inflammatory stimulation, which generally represents the effect of acute inflammation on skin fibrosis. Therefore, although our study was conducted in a bleomycin-induced mouse skin fibrosis model, it still needs to be comprehensively verified by multiple animal models in the future.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-β-actin | Beyotime | Cat#AF5003; RRID: N/A |
| Rabbit anti-AdipoR1 | Abcam | Cat#ab126611; RRID: AB_11129655 |
| Mouse anti-AdipoR2 | Santa Cruz | Cat#sc-514045; RRID: AB_2895612 |
| Rabbit anti-Collagen I | Abcam | Cat#ab138492; RRID: AB_2861258 |
| Rabbit anti-α-SMA | Abcam | Cat#ab124964; RRID: AB_11129103 |
| Rabbit anti- AMPKα1 | Abcam | Cat#ab32047; RRID: AB_722764 |
| Rabbit anti-SIRT1 | Abcam | Cat#ab189494; RRID: AB_2864311 |
| Rabbit anti-YAP | Abcam | Cat#ab52771; RRID: AB_2219141 |
| Rabbit anti-Phospho-AMPKα (Thr172) | Cell signaling Technology | Cat#2535; RRID: AB_331250 |
| Rabbit anti-CCND1 | Abcam | Cat#ab16663; RRID: AB_443423 |
| Rabbit anti-CCNE1 | Abcam | Cat#ab33911; RRID: AB_731787 |
| Rabbit anti-H2A | Abcam | Cat#ab177308; RRID: N/A |
| Mouse anti-α-tubulin | Abcam | Cat#ab7291; RRID: AB_2241126 |
| Rabbit anti-C/EBPβ | Thermo Scientific | Cat#PA5-27244; RRID: AB_2544720 |
| Mouse anti-TEAD4 | Santa Cruz | Cat#sc-101184; RRID: AB_2203086 |
| HRP-conjugated goat anti-rabbit IgG | Beyotime | Cat#A0208; RRID: AB_2892644 |
| HRP-conjugated goat anti-mouse IgG | Beyotime | Cat#A0216; RRID: AB_2860575 |
| Cy3-conjugated goat anti-rabbit IgG | Beyotime | Cat#A0516; RRID: AB_2893015 |
| Rabbit anti-H3K27Ac | Abcam | Cat#ab4729; RRID: AB_2118291 |
| Rabbit anti-H3K9Ac | Abcam | Cat#ab4441; RRID: AB_2118292 |
| Mouse anti-YAP | Abcam | Cat#ab56701; RRID: AB_2219140 |
| Rabbit anti-C/EBPβ | Abcam | Cat#32358; RRID: AB_726796 |
| Rabbit anti-acetyl lysine | Abcam | Cat#ab21623; RRID: AB_446436 |
| Bacterial and virus strains | ||
| rAAV-shSIRT1-Mus particles (target sequences:5′-CTGTGGCAGATTGTTATTAAT-3′) | GenePharma | N/A |
| Biological samples | ||
| Hypertrophic scar (HS) tissues and counterpart normal skin (NS) tissues from patients undergoing skin flap and skin graft plastic surgery | Department of Burns and Cutaneous Surgery, Xijing Hospital, The Fourth Military Medical University | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Dispase II (neutral protease, grade II) | Roche | #04942078001 |
| DMEM, Powder, High Glucose | Gibco | #12100046 |
| Fetal Bovine Serum, qualified, Australia origin | Gibco | #10099141 |
| RIPA buffer | Heart | #WB009 |
| Bleomycin sulfate | MedChemExpress | #HY17565 |
| Recombinant human adiponectin | PeproTech | #450-24 |
| Recombinant murine adiponectin | PeproTech | #315-26 |
| Immobilon Western Chemilum HRP Substrate | Millipore | #WBKlS0100 |
| Bovine serum albumin | Sigma | #A3912 |
| DAPI | Beyotime | #C1002 |
| Critical commercial assays | ||
| HistostainTM kit | ZSGB | #SP-9000 |
| PierceTM BCA Protein Assay Kit | Thermo Scientific | #NCI3225CH |
| BD Pharmingen™ PI/RNase staining buffer (for PI/RNase staining) | BD | #550825 |
| BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 555 | Beyotime | #C0075S |
| Lipofectamine 3000 transfection reagent | Invitrogen | #L3000001 |
| Hematoxylin-eosin (HE) staining kit | Solarbio | #G1120 |
| Masson’s trichrome staining kit | Solarbio | #G1340 |
| RNAiso Plus Kit | Takara | #9108 |
| Prime ScriptTM RT Reagent Kit | Takara | #RR037A |
| TB Green® Premix Ex Taq™ Kit | Takara | #RR420A |
| Nuclear and Cytoplasmic Protein Extraction kit | Keygen Biotech | #KGP150 |
| Simple ChIP® Enzymatic Chromatin IP Kit | Cell signaling Technology | #9003 |
| Dual-Luciferase® Reporter Assay System | Promega | #E1910 |
| Pierce™ Classic Magnetic IP/Co-IP Kit | Thermo Scientific | #88804 |
| NAD+/NADH detection kit | Beyotime | #S0175 |
| Deposited data | ||
| RNA Sequencing Data | This paper | SRA: PRJNA822056 |
| Mendeley Data | This paper | https://doi.org/10.17632/85g9c7gs5d.1 |
| Experimental models: Cell lines | ||
| Human normal skin-derived fibroblasts | This paper | N/A |
| Human hypertrophic scar-derived fibroblasts | This paper | N/A |
| Experimental models: Organisms/strains | ||
| BALB/c mouse | Experimental Animal Center of the Fourth Military Medical University | N/A |
| Oligonucleotides | ||
| Hs-SIRT1-siRNA#1(target sequence:5′- GCUAAGAAUUUCAGGAUUA-3′) | GenePharma | N/A |
| Hs-SIRT1-siRNA#2(target sequence:5′- GGAAATATATCCTGGACAA-3′) | GenePharma | N/A |
| Hs-SIRT1-siRNA#3(target sequence:5′- CCATCTCTCTGTCACAAAT-3′) | GenePharma | N/A |
| Hs-YAP-siRNA (target sequence:5′-CACCAGUGCAGCAGAAUAU-3′) | GenePharma | N/A |
| Hs-ANXA1-siRNA (target sequence:5′- AUGCCUCACAGCUAUCGUGAA-3′) | GenePharma | N/A |
| Hs-CTDSPL-siRNA (target sequence:5′- GCAGCAUCCUUAGCUCCUU-3′) | GenePharma | N/A |
| Hs-C/EBPβ-siRNA | Santa Cruz | #sc-29229 |
| See Table S2 for Primers sequence involved in RT-qPCR assay | Accurate Biotechnology | N/A |
| See Table S3 for Primers sequence involved in ChIP-PCR assay | GeneCreate | N/A |
| Recombinant DNA | ||
| pEX-3-SIRT1 | GenePharma | N/A |
| pcDNA3.1(+)-YAP | GenePharma | N/A |
| pGL3-YAP-WT | GenePharma | N/A |
| pGL3-YAP-MUT | GenePharma | N/A |
| pcDNA3.1(+)-C/EBPβ | GenePharma | N/A |
| pcDNA3.1(+)-C/EBPβ K39R | GenePharma | N/A |
| pcDNA3.1(+)-TEAD4 | GenePharma | N/A |
| Software and algorithms | ||
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| Image Pro Plus | Media Cybernetics Corporation | https://www.mediacy.com/support/imageproplus/updates/#whatsnew |
| GraphPad Prism 8.0.2 | GraphPad Software | https://www.graphpad.com/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dahai Hu (xjburnsur@163.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Clinical human samples
Hypertrophic scar (HS) tissues and counterpart normal skin (NS) tissues from patients undergoing skin flap and skin graft plastic surgery. Samples meeting the following conditions were included in our experiment. a. The appearance of the scar was a locally raised pink or purplish red mass, with hyperemia on the surface and hard texture, accompanied by itching and pain symptoms, without progressive or invasive growth. b. Pathological examination showed no significantly thickened eosinophilic (hyalinizing) collagen bundles. c. No obvious signs of atrophy and regression. d. The patient had not used any drugs within 3 months prior to the collection. A total of 11 patients met the above conditions (Table S1). Before surgery, all patients were informed of the purpose and procedure of this study and volunteered to donate their excess tissue. Informed consent was obtained from each patient. All experiments were approved by the Xijing Hospital Ethics Committee affiliated with the Fourth Military Medical University and conducted in accordance with the Helsinki Declaration. The collected tissue samples from case 1-5 were divided into three portions and processed as follows. The first portion was used for immunohistochemical detection, the second portion was used for the extraction of total protein, and the third portion was used for the culture of fibroblasts as described below. Tissue samples from case 6-11 were for NAD+/NADH detection.
Primary skin fibroblasts
Skin tissues were digested with 0.25% Dispase II (#04942078001, Roche) for 6-8h to separate the dermis from epidermis. The dermal portion was washed repeatedly with PBS containing 100U/ml penicillin and 100 μg/ml streptomycin, cut into pieces, and then inoculated in the culture flask. These tissue explants were cultured in DMEM (#12100046, Gibco) containing 10% fetal bovine serum (#10099141, Gibco), 100U/ml penicillin and 100 μg/ml streptomycin and placed in a humidified incubator containing 5% (v/v) CO2 at 37°C. Primary cells were typically long fusiform, with an oval nucleus in the center. Immunofluorescence staining showed that the fibroblast-specific marker Vimentin was positive, and the statistical positive rate was 100%. All experiments were performed using cells of the 3rd to 5th generation.
Animal models
Animal experiments described in the current study were approved by the Animal Care Committee of the Fourth Military Medical University. Animal studies were conducted in accordance with national guidelines and in compliance with the United States Public Health Service’s Policy on the Humane Care and Use of Laboratory Animals. The mice were purchased from the Experimental Animal Center of the Fourth Military Medical University. Male 6-week-old BALB/c mice were fed normal food and water. First, mice received a daily dorsal subcutaneous injection of 100μL bleomycin (1 μg/μl, dissolved in PBS, #HY17565, MedChemExpress) for three weeks (n = 24), as a control, other mice were injected with an equal volume of PBS (n = 8). Then, 200ul adeno-associated virus rAAV-shNC or rAAV-shSIRT1 particles (1×1012vg/mL) were injected into the skin lesions on days 22, 24 and 26. In addition, 100ul recombinant murine adiponectin (50 μg/ml, dissolved in DMEM medium, #315-26, PeproTech) or control DMEM medium was injected daily from day 25. After two weeks, the mice were sacrificed and their skin tissues were processed for histological analysis. According to the guidance of Hematoxylin-eosin (HE) staining kit (#G1120, Solarbio) and Masson trichrome staining kit (#G1340, Solarbio), HE staining was used to detect the thickness of dermis, and Masson trichrome staining was used to examine the deposition and arrangement of collagen fibers in paraffin-embedded tissue sections.
Method details
Immunohistochemistry
The collected NS and HS tissues were fixed with 4% paraformaldehyde, dehydrated with graded ethanol, embedded in paraffin, and then cut into 7μm thick sections. According to the manufacturer’s instructions of HistostainTM kit (#SP-9000, ZSGB), after dewaxing and hydration, antigen retrieval, the tissue sections were immersed in peroxidase blocker for 15min to remove endogenous peroxidase activity. Next, blocked with normal goat serum working solution for 1h to prevent non-specific binding. Tissue sections were incubated with primary antibody against AdipoR1 (#ab126611), Col1 (#ab138492), α-SMA (#ab124964), AMPKα1 (#ab32047), SIRT1 (#ab189494), YAP (#ab52771) (Abcam), AdipoR2 (#sc-514045, Santa Cruz) or Phospho-AMPKα (Thr172) (#2535, Cell signaling Technology) overnight at 4°C, then stained by biotin-labeled goat anti-rabbit/mouse IgG and horseradish enzyme-labeled streptavidin working solution. DAB was used for color rendering. Finally, after counterstained with hematoxylin, dehydrated and transparent, the slices were sealed. Image acquisition was performed with Evos FL Auto2 microscope (Thermo Fisher Scientific).
Western blot
Tissues or fibroblasts were lysed with RIPA buffer (#WB009, Heart) containing 1% aprotinin, 1% activated Na3VO4 and 1% PMSF on ice. The protein concentration was determined according to the instructions of the the PierceTM BCA Protein Assay Kit (#NCI3225CH, Thermo Fisher Scientific). Total protein of 30μg was loaded onto the 10% SDS-polyacrylamide gel, separated by electrophoresis, and then transferred to the PVDF membrane (Millipore, Boston). Membranes were blocked with 5% skim milk for 2h at room temperature and incubated with AdipoR1 (#ab126611), Col1 (#ab138492), α-SMA (#ab124964), CCND1 (#ab16663), CCNE1 (#ab33911), SIRT1 (#ab189494), YAP (#ab52771), H2A (#ab177308), α-tubulin (#ab7291), AMPKα1 (#ab32047) (Abcam), AdipoR2 (#sc-514045, Santa Cruz), P-AMPKα (Thr172) (#2535, Cell signaling Technology), C/EBPβ (# PA5-27244, Thermo Scientific) or TEAD4 (#sc-101184, Santa Cruz) antibodies overnight at 4°C. The next day, membranes were incubated with HRP-conjugated goat anti-rabbit IgG (#A0208,1:2000) or HRP-conjugated goat anti-mouse IgG (#A0216, 1:2000) (Beyotime) for 1h at 37°C. Immunoreactive proteins were detected using ECL reagent Immobilon Western Chemilum HRP Substrate (#WBKLS0100, Millipore) and ChemiDocTM Imaging System (Bio-Rad). Bands were quantitated using ImageJ software.
Immunofluorescence
Cells grown on glass slides were fixed with 4% paraformaldehyde for 20min, washed with PBS, permeabilized with 0.1% Triton X-100 at room temperature for 5min, and then blocked with 1% BSA (BSA, #A3912, Sigma) for 30min to inhibit non-specific binding. Next, the cells were incubated overnight with α-SMA antibody (#ab124964, Abcam) at 4°C. The next day, the α-SMA antibody was removed, and cells were incubated with Cy3-conjugated goat anti-rabbit IgG (#A0516, Beyotime) at room temperature for 1h. The nucleus was counter stained with DAPI (#C1002, Beyotime). Images were taken using an Evos FL Auto2 microscope (Thermo Fisher Scientific).
Flow cytometry assay
All procedures were followed with the instructions of BD Pharmingen™ PI/RNase staining buffer (for PI/RNase staining) (#550825, BD). Fibroblasts were harvested and fixed with precooled 75% ethanol at −20°C for 2h. Afterward, cells were washed twice and centrifuged at 1000 rpm for 10 min to remove the ethanol. The cell pellets were finally resuspended in PI/RNase staining buffer and incubated at room temperature for 15min in the dark. Cell cycles distribution was analyzed on a flow cytometer (FACSAria, BD).
EdU assay
All procedures were followed with the instructions of the BeyoClick EdU Cell Proliferation Kit with Alexa Fluor 555 (#C0075S, Beyotime). Briefly, mixed the cell culture medium with 2xEdU working solution in 1:1 ratio. The cells were incubated with this mixed medium at 37°C for 2h, fixed with 4% paraformaldehyde at room temperature for 15min, and then permeabilized with PBS containing 0.3% Triton X-100 for 15min. Washed the cells twice. The cells were next incubated with the click reaction solution for 30 min at room temperature away from light. Washed away the click reaction solution. The fluorescence images were obtained using an Evos FL Auto2 microscope (Thermo Fisher Scientific). Five random views under each condition were used to calculate the average EdU ratio (% vs. DAPI).
RNA sequencing
Hypertrophic scar fibroblasts (HFs) were treated with complete medium (DMEM containing 10% FBS) with or without 10 μg/ml APN for 24h, and total RNA was extracted using RNAiso Plus reagent. The mRNA enrichment, fragmentation, reverse transcription, library construction, sequencing and data analysis were performed by Genergy Biotechnology Co. Ltd.
Cell transfection
SiRNAs or plasmids were transfected into fibroblasts using Lipofectamine 3000 transfection reagent (#L3000001, Invitrogen) according to the manufacturer’s instructions. Small interfering RNA transfection mixture Lipo3000/si-RNA or/and plasmid transfection mixture Lipo3000/p3000/plasmid was added to HFs. The working concentration of siRNA was 50nM, and that of plasmid was 1 μg/ml. The transfection mixture was replaced with complete DMEM medium after 6h. In different experiments, cells were cultured for the corresponding length of time in a humidified incubator containing 5% (v/v) CO2 at 37°C.
Recombinant adeno-associated virus (rAAV)
Firstly, the mouse shRNA-SIRT1 was designed and synthesized and connected to the downstream of the mouse U6 promoter of the interference virus vector pAAV-U6-EGFP, that is, the interference virus plasmid pAAV-U6-SIRT1-shRNA-EGFP (pAAV-shSIRT1). The negative control was pAAV-U6-NC-shRNA-EGFP (pAAV-shNC) inserted with an invalid sequence. All plasmids were sequenced to confirm their correct identity. The interference virus plasmid pAAV-shSIRT1 or pAAV-shNC, co-packaging plasmid pHELPER and helper plasmid pAAV-DJ were co-transfected into AAV-293 packaging cells. 6h after transfection, it was replaced with complete medium, cultured for 72h, then the cells and supernatant were collected and placed in a centrifuge tube, freeze-thawed three times, centrifuged at 2000rpm for 5min, and the supernatant was taken, purified and concentrated to obtain concentrated adeno-associated virus solutions rAAV-shSIRT1 and rAAV-shNC. The titer of the purified rAAV virus was determined to be 1×1012vg/mL.
Hematoxylin-eosin (HE) staining
According to the guidance of Hematoxylin-eosin (HE) staining kit (#G1120, Solarbio), paraffin sections were routinely dewaxed to water, stained with hematoxylin, differentiated with 1% hydrochloric acid, counterstained with eosin. Finally, the slices were quickly dehydrated and transparent, and then sealed. Evos FL Auto2 was used for image acquisition.
Masson trichrome staining
According to the manufacturer’s instructions of Masson’s trichrome staining kit (#G1340, Solarbio), paraffin sections were routinely dewaxed to water, and were stained with hematoxylin, differentiated with 1% hydrochloric acid, stained with compound staining solution, and treated with 1% phosphotungstic acid and aniline blue. Collagen fibers were stained blue. Evos FL Auto2 was used for image acquisition, and the optical density and distribution area of blue collagen fibers were measured with Image Pro Plus software.
RT-qPCR assay
Fibroblasts were lysed using the RNAiso Plus Kit (#9108, Takara) and total RNA was extracted according to the instructions of this kit. 500 ng RNA was reversely transcribed into cDNA using the Prime Script RT Reagent Kit (#RR037A, Takara). The obtained cDNA was amplified using the TB Green® Premix Ex Taq™ Kit (#RR420A, Takara) and a Bio-Rad IQ5 Real-Time system (Bio-Rad). GAPDH was used as an internal loading control. The PCR amplification conditions included four stages: (1) initial denaturation at 95°C for 30 s, (2) denaturation at 95°C for 5 s, (3) extension at 60°C for 30 s, a total of 39 cycles, (4) denaturation at 65°C for 15 s. The sequence information of the primers used is shown in Table S2.
Nuclear and cytoplasmic protein extraction
All operations were conducted in accordance with the instruction of the Nuclear and Cytoplasmic Protein Extraction kit (#KGP150, Keygen Biotech). The extracted cytoplasmic and nuclear proteins were quantified using the BCA method, and then analyzed by western blot.
ChIP-qPCR
The Simple ChIP Enzymatic Chromatin IP Kit (#9003, Cell signaling Technology) was used for ChIP analysis. Briefly, HFs were fixed with 1% formaldehyde for 10min and then lysed. The chromatin was collected and fragmented with micrococcal nuclease. Purified chromatin lysate was incubated with ChIP grade anti-C/EBPβ antibody (#PA5-27244; Thermo Scientific), anti-H3K27Ac antibody (#ab4729, Abcam), anti-H3K9Ac antibody (#ab4441, Abcam), anti-TEAD4 antibody (#sc-101184, Santa Cruz) or rabbit or mouse IgG (negative control) overnight at 4°C. The immunoprecipitated chromatin was captured using ChIP-Grade Protein G Magnetic Beads. After protein-DNA de-crosslinking, the DNA was purified using DNA purification spin columns. The number of specific DNA sequences enriched by chromatin immunoprecipitation was determined by qPCR. Table S3 shows the primers used for genomic amplification at the target sites.
Dual-luciferase reporter gene assay
HFs were inoculated in 24-well culture plates for 24h. The pGL-3 firefly luciferase reporter vector containing the wild-type or mutant motif of the target gene promoter and the overexpression vector or siRNA for the molecules under study were co-transfected into cells using Lipofectamine 3000. The constitutively expressed Renilla luciferase plasmid pRL was used as internal control. The luciferase activity was determined according to the instructions of the Dual-Luciferase Reporter Assay System (#E1910, Promega). The relative reporter gene activity was obtained by normalizing to Renilla luciferase activity (the ratio of firefly luciferase to Renilla luciferase).
Co-immunoprecipitation (Co-IP)
Co-IP was conducted according to the instructions of the Pierce™ Classic Magnetic IP/Co-IP Kit (#88804, Thermo Fisher Scientific). In brief, cell lysates were incubated with anti-YAP antibody (#ab52771, Abcam), anti-C/EBPβ (#PA5-27244, Thermo Scientific) antibody or rabbit IgG overnight at 4°C to form antigen/antibody complexes. These antigen/antibody complexes were incubated with protein A/G magnetic beads at room temperature for 1h. Magnetic beads were then collected using a magnetic scaffold and washed twice with IP lysis/washing buffer and once with PBS. Magnetic bead precipitates were collected by centrifugation and 2×loading buffer was added. These magnetic beads-antigen-antibody mixtures were then boiled at 100°C for 10min. The levels of YAP, TEAD4, C/EBPβ and acetylated lysine were detected by western blot using anti-YAP (#ab56701, Abcam), anti-TEAD4 (#sc-101184, Santa Cruz), anti-C/EBPβ (#ab32358, Abcam) and anti-acetyl lysine (#ab21623, Abcam) antibodies.
NAD + detection
NAD + levels in collected fresh HS tissues and their corresponding NS tissues as well as in paired primary fibroblasts HF and NF were quantified by NAD+/NADH detection kit (#S0175, Beyotime). First, 20mg skin tissue or 1 × 106 fibroblasts were lysed by 200μL NAD+/NADH extract solution, then centrifuged at 12,000 g at 4°C for 5-10min, and the supernatant was collected for the assay. Next, for the determination of the total amount of NAD+ and NADH [NADtotal], pipette 20μL of the supernatant into a 96-well plate, then add 90ul alcohol dehydrogenase working solution, mix well, incubate at 37°C for 10min in the dark. For NADH content [NADH] detection, 60μL supernatant was heated at 60°C for 30min on a PCR machine to decompose NAD+, centrifuged at 10,000 g at 4°C for 5min, and pipette 20μL of the supernatant into a 96-well plate. At last, 10μL chromogenic agent was added to each well, followed by incubated at 37°C for 30min in the dark, and the absorbance was measured at a wavelength of 450nm. The [NADtotal] and [NADH] were quantified based on a standard concentration curve. The content of NAD+ [NAD+] = [NADtotal] - [NADH].
Quantification and statistical analysis
All experiments were repeated at least three times. All data were analyzed using GraphPad 9.0.0 software and were presented as the mean ± SD. Paired-samples t-tests were used to analyze differences between paired groups. Independent-samples t-tests were used to analyze differences between two independent groups. One-way ANOVA with Tukey’s multiple-comparison tests were performed to analyze differences among multiple groups. p < 0.05 indicates a statistically significant difference.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers 81801920, 81901965, 81772071, 82172209, 82172208, and 81871561].
Author contributions
Conceptualization, J.Z. and Y.L.; Methodology, J.Z. and J.Q.L.; Investigation, J.Z., Y.L., J.Q.L., F.H., J.H.S., G.F.W., K.J.W., X.W.G., and C.Y.T; Writing–Original Draft, J.Z., Y.L., and K.S.; Writing–Review & Editing, J.Z., Y.L. and M.Z.; Funding Acquisition, J.Z., D.H.H., Y.L., J.Q.L., J.H.S., and K.T.; Resources, J.Z., D.H.H., and Y.C.W.; Supervision, D.H.H., Y.C.W., and K.T.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: October 21, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105236.
Contributor Information
Yunchuan Wang, Email: yunchuanwang@yeah.net.
Ke Tao, Email: ketao918@yeah.net.
Dahai Hu, Email: xjburnsur@163.com.
Supplemental information
Data and code availability
-
•
RNA-seq data have been deposited at SRA and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Original western blot images have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is https://doi.org/10.17632/85g9c7gs5d.1. Microscopy data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Alzahrani B., Iseli T., Ramezani-Moghadam M., Ho V., Wankell M., Sun E.J., Qiao L., George J., Hebbard L.W. The role of AdipoR1 and AdipoR2 in liver fibrosis. Biochimica et biophysica acta. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:700–708. doi: 10.1016/j.bbadis.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Bosch-Presegué L., Vaquero A. Sirtuin-dependent epigenetic regulation in the maintenance of genome integrity. FEBS J. 2015;282:1745–1767. doi: 10.1111/febs.13053. [DOI] [PubMed] [Google Scholar]
- Bugyei-Twum A., Ford C., Civitarese R., Seegobin J., Advani S.L., Desjardins J.F., Kabir G., Zhang Y., Mitchell M., Switzer J., et al. Sirtuin 1 activation attenuates cardiac fibrosis in a rodent pressure overload model by modifying Smad2/3 transactivation. Cardiovasc. Res. 2018;114:1629–1641. doi: 10.1093/cvr/cvy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., Fernandez-Marcos P.J., Yamamoto H., Andreux P.A., Cettour-Rose P., et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metabol. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L.J., Zhang Y.J., Dong S.Q., Li X.Z., Tong X.T., Chen D., Wu Z.Y., Zheng X.H., Xue W.Q., Jia W.H., et al. ATAD2 interacts with C/EBPβ to promote esophageal squamous cell carcinoma metastasis via TGF-β1/Smad3 signaling. J. Exp. Clin. Cancer Res. 2021;40:109. doi: 10.1186/s13046-021-01905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Ji S., Jia P., Chen Y., Li Y., Wang T. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free Radic. Biol. Med. 2021;177:1–14. doi: 10.1016/j.freeradbiomed.2021.10.011. [DOI] [PubMed] [Google Scholar]
- Chu H., Jiang S., Liu Q., Ma Y., Zhu X., Liang M., Shi X., Ding W., Zhou X., Zou H., et al. Sirtuin1 protects against systemic sclerosis-related pulmonary fibrosis by decreasing Proinflammatory and Profibrotic processes. Am. J. Respir. Cell Mol. Biol. 2018;58:28–39. doi: 10.1165/rcmb.2016-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.J., Nagaraju G.P., Nagalingam A., Muniraj N., Kuppusamy P., Walker A., Woo J., Győrffy B., Gabrielson E., Saxena N.K., Sharma D. ADIPOQ/adiponectin induces cytotoxic autophagy in breast cancer cells through STK11/LKB1-mediated activation of the AMPK-ULK1 axis. Autophagy. 2017;13:1386–1403. doi: 10.1080/15548627.2017.1332565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaugnies M., Sánchez-Danés A., Rorive S., Raphaël M., Liagre M., Parent M.A., Brisebarre A., Salmon I., Blanpain C. YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 2018;19:e45809. doi: 10.15252/embr.201845809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhandapany P.S., Kang S., Kashyap D.K., Rajagopal R., Sundaresan N.R., Singh R., Thangaraj K., Jayaprakash S., Manjunath C.N., Shenthar J., et al. Adiponectin receptor 1 variants contribute to hypertrophic cardiomyopathy that can be reversed by rapamycin. Sci. Adv. 2021;7:eabb3991. doi: 10.1126/sciadv.abb3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Han Y., Lu Q., An J., Zhu H., Xie Z., Song P., Zou M.H. Peroxynitrite-mediated SIRT (Sirtuin)-1 inactivation contributes to nicotine-induced arterial stiffness in mice. Arterioscler. Thromb. Vasc. Biol. 2019;39:1419–1431. doi: 10.1161/ATVBAHA.118.312346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Chen J., Qin J., Chen R., Yi Z. TGF-β-induced α-SMA expression is mediated by C/EBPβ acetylation in human alveolar epithelial cells. Mol. Med. 2021;27:22. doi: 10.1186/s10020-021-00283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbediwy A., Vincent-Mistiaen Z.I., Spencer-Dene B., Stone R.K., Boeing S., Wculek S.K., Cordero J., Tan E.H., Ridgway R., Brunton V.G., et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Liu L., Yang Y., Tamaki Z., Wei J., Marangoni R.G., Bhattacharyya S., Summer R.S., Ye B., Varga J. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: novel target for fibrosis therapy. Arthritis Res. Ther. 2012;14:R229. doi: 10.1186/ar4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D., Shao N., Heller E., Feng J., Neve R., Kim H.D., Call T., Magazu S., Shen L., Nestler E.J. SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J. Neurosci. 2015;35:3100–3111. doi: 10.1523/JNEUROSCI.4012-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Guo Q., Chang B., Yu Q.L., Xu S.T., Yi X.J., Cao S.C. Adiponectin treatment improves insulin resistance in mice by regulating the expression of the mitochondrial-derived peptide MOTS-c and its response to exercise via APPL1-SIRT1-PGC-1α. Diabetologia. 2020;63:2675–2688. doi: 10.1007/s00125-020-05269-3. [DOI] [PubMed] [Google Scholar]
- Hata S., Hirayama J., Kajiho H., Nakagawa K., Hata Y., Katada T., Furutani-Seiki M., Nishina H. A novel acetylation cycle of transcription co-activator Yes-associated protein that is downstream of Hippo pathway is triggered in response to SN2 alkylating agents. J. Biol. Chem. 2012;287:22089–22098. doi: 10.1074/jbc.M111.334714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Bao Q., Zhang Y., Liu M., Lv H., Liu Y., Yao L., Li B., Zhang C., He S., et al. Yes-associated protein promotes angiogenesis via signal transducer and activator of transcription 3 in endothelial cells. Circ. Res. 2018;122:591–605. doi: 10.1161/CIRCRESAHA.117.311950. [DOI] [PubMed] [Google Scholar]
- He M., Chiang H.H., Luo H., Zheng Z., Qiao Q., Wang L., Tan M., Ohkubo R., Mu W.C., Zhao S., et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metabol. 2020;31:580–591.e5. doi: 10.1016/j.cmet.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman N.D., Stuart R.K., Hon G., Fu Y., Ching C.W., Hawkins R.D., Barrera L.O., Van Calcar S., Qu C., Ching K.A., et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- Hong S.P., Seo H.S., Shin K.O., Park K., Park B.C., Kim M.H., Park M., Kim C.D., Seo S.J. Adiponectin enhances human keratinocyte lipid synthesis via SIRT1 and nuclear hormone receptor signaling. J. Invest. Dermatol. 2019;139:573–582. doi: 10.1016/j.jid.2018.08.032. [DOI] [PubMed] [Google Scholar]
- Isago H., Mitani A., Mikami Y., Horie M., Urushiyama H., Hamamoto R., Terasaki Y., Nagase T. Epithelial expression of YAP and TAZ is sequentially required in lung development. Am. J. Respir. Cell Mol. Biol. 2020;62:256–266. doi: 10.1165/rcmb.2019-0218OC. [DOI] [PubMed] [Google Scholar]
- Ishijima Y., Ohmori S., Uneme A., Aoki Y., Kobori M., Ohida T., Arai M., Hosaka M., Ohneda K. The Gata2 repression during 3T3-L1 preadipocyte differentiation is dependent on a rapid decrease in histone acetylation in response to glucocorticoid receptor activation. Mol. Cell. Endocrinol. 2019;483:39–49. doi: 10.1016/j.mce.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Jang M.J., Park U.H., Kim J.W., Choi H., Um S.J., Kim E.J. CACUL1 reciprocally regulates SIRT1 and LSD1 to repress PPARγ and inhibit adipogenesis. Cell Death Dis. 2017;8:3201. doi: 10.1038/s41419-017-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Tamura S., Kiso S., Matsumoto H., Saji Y., Yoshida Y., Fukui K., Maeda N., Nishizawa H., Nagaretani H., et al. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–1807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Kim Y., Lim J.H., Kim M.Y., Kim E.N., Yoon H.E., Shin S.J., Choi B.S., Kim Y.S., Chang Y.S., Park C.W. The adiponectin receptor agonist AdipoRon ameliorates diabetic nephropathy in a model of type 2 diabetes. J. Am. Soc. Nephrol. 2018;29:1108–1127. doi: 10.1681/ASN.2017060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lee S., Miller M., Shuman J.D., Johnson P.F. CCAAT/Enhancer-binding protein beta DNA binding is auto-inhibited by multiple elements that also mediate association with p300/CREB-binding protein (CBP) J. Biol. Chem. 2010;285:21399–21410. doi: 10.1074/jbc.M110.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Byun M.R., Furutani-Seiki M., Hong J.H., Jung H.S. YAP and TAZ regulate skin wound healing. J. Invest. Dermatol. 2014;134:518–525. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- Li M., Hong W., Hao C., Li L., Wu D., Shen A., Lu J., Zheng Y., Li P., Xu Y. SIRT1 antagonizes liver fibrosis by blocking hepatic stellate cell activation in mice. FASEB J. 2018;32:500–511. doi: 10.1096/fj.201700612R. [DOI] [PubMed] [Google Scholar]
- Li C., Xie N., Li Y., Liu C., Hou F.F., Wang J. N-acetylcysteine ameliorates cisplatin-induced renal senescence and renal interstitial fibrosis through sirtuin1 activation and p53 deacetylation. Free Radic. Biol. Med. 2019;130:512–527. doi: 10.1016/j.freeradbiomed.2018.11.006. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang D., Vatner D.F., Goedeke L., Hirabara S.M., Zhang Y., Perry R.J., Shulman G.I. Mechanisms by which adiponectin reverses high fat diet-induced insulin resistance in mice. Proc. Natl. Acad. Sci. USA. 2020;117:32584–32593. doi: 10.1073/pnas.1922169117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Asano Y., Shibata S., Noda S., Aozasa N., Akamata K., Yamada D., Tamaki Z., Tada Y., Sugaya M., et al. Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis. J. Eur. Acad. Dermatol. Venereol. 2012;26:354–360. doi: 10.1111/j.1468-3083.2011.04077.x. [DOI] [PubMed] [Google Scholar]
- Moseley P.L., Hemken C., Hunninghake G.W. Augmentation of fibroblast proliferation by bleomycin. J. Clin. Invest. 1986;78:1150–1154. doi: 10.1172/JCI112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pefani D.E., Pankova D., Abraham A.G., Grawenda A.M., Vlahov N., Scrace S., E O.N. TGF-Β targets the hippo pathway scaffold RASSF1A to facilitate YAP/SMAD2 nuclear translocation. Mol. Cell. 2016;63:156–166. doi: 10.1016/j.molcel.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Pepe-Mooney B.J., Dill M.T., Alemany A., Ordovas-Montanes J., Matsushita Y., Rao A., Sen A., Miyazaki M., Anakk S., Dawson P.A., et al. Single-cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for YAP in homeostasis and regeneration. Cell Stem Cell. 2019;25:23–38.e28. doi: 10.1016/j.stem.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu D.R., Yu M.R., Kong K.H., Kim H., Kwon S.H., Jeon J.S., Han D.C., Noh H. Sirt1-hypoxia-inducible factor-1α interaction is a key mediator of tubulointerstitial damage in the aged kidney. Aging Cell. 2019;18:e12904. doi: 10.1111/acel.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayan A., Mashek M.T., Mashek D.G. ATGL promotes autophagy/lipophagy via SIRT1 to control hepatic lipid droplet catabolism. Cell Rep. 2017;19:1–9. doi: 10.1016/j.celrep.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D., Torres C., Bhandari V. Adiponectin deficiency induces mitochondrial dysfunction and promotes endothelial activation and pulmonary vascular injury. FASEB J. 2019;33:13617–13631. doi: 10.1096/fj.201901123R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R.J., Kosmatka M., Bardeesy N., Hurley R.L., Witters L.A., DePinho R.A., Cantley L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Tada Y., Hau C.S., Mitsui A., Kamata M., Asano Y., Sugaya M., Kadono T., Masamoto Y., Kurokawa M., et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat. Commun. 2015;6:7687. doi: 10.1038/ncomms8687. [DOI] [PubMed] [Google Scholar]
- Sin T.K., Zhu J.Z., Zhang G., Li Y.P. p300 mediates muscle wasting in lewis lung carcinoma. Cancer Res. 2019;79:1331–1342. doi: 10.1158/0008-5472.CAN-18-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmons K.K., Crose L.E.S., Riedel S., Sushnitha M., Belyea B., Linardic C.M. A novel notch-YAP circuit drives stemness and tumorigenesis in embryonal rhabdomyosarcoma. Mol. Cancer Res. 2017;15:1777–1791. doi: 10.1158/1541-7786.MCR-17-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero A., Scher M., Lee D., Erdjument-Bromage H., Tempst P., Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell. 2004;16:93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Verboven E., Moya I.M., Sansores-Garcia L., Xie J., Hillen H., Kowalczyk W., Vella G., Verhulst S., Castaldo S.A., Algueró-Nadal A., et al. Regeneration defects in Yap and Taz mutant mouse livers are caused by bile duct disruption and cholestasis. Gastroenterology. 2021;160:847–862. doi: 10.1053/j.gastro.2020.10.035. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zang C., Rosenfeld J.A., Schones D.E., Barski A., Cuddapah S., Cui K., Roh T.Y., Peng W., Zhang M.Q., et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat. Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Wang Z.H., Zhang J., Liu X., Yu S.P., Ye K.X., Wang J.Z., Ye K., Wang X.C. C/EBPβ is a key transcription factor for APOE and preferentially mediates ApoE4 expression in Alzheimer's disease. Mol. Psychiatr. 2021;26:6002–6022. doi: 10.1038/s41380-020-00956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nishioka K. Cellular and molecular mechanisms of bleomycin-induced murine scleroderma: current update and future perspective. Exp. Dermatol. 2005;14:81–95. doi: 10.1111/j.0906-6705.2005.00280.x. [DOI] [PubMed] [Google Scholar]
- Zhang K., Garner W., Cohen L., Rodriguez J., Phan S. Increased types I and III collagen and transforming growth factor-beta 1 mRNA and protein in hypertrophic burn scar. J. Invest. Dermatol. 1995;104:750–754. doi: 10.1111/1523-1747.ep12606979. [DOI] [PubMed] [Google Scholar]
- Zhang K., Guo Y., Ge Z., Zhang Z., Da Y., Li W., Zhang Z., Xue Z., Li Y., Ren Y., et al. Adiponectin suppresses T helper 17 cell differentiation and limits autoimmune CNS inflammation via the SIRT1/PPARγ/RORγt pathway. Mol. Neurobiol. 2017;54:4908–4920. doi: 10.1007/s12035-016-0036-7. [DOI] [PubMed] [Google Scholar]
- Zhang X., Yang L., Szeto P., Abali G.K., Zhang Y., Kulkarni A., Amarasinghe K., Li J., Vergara I.A., Molania R., et al. The Hippo pathway oncoprotein YAP promotes melanoma cell invasion and spontaneous metastasis. Oncogene. 2020;39:5267–5281. doi: 10.1038/s41388-020-1362-9. [DOI] [PubMed] [Google Scholar]
- Zhang X., Tian B., Deng Q., Cao J., Ding X., Liu Q., Zhang Y., Ye C., Deng C., Qiu L., Guo C. Nicotinamide riboside relieves the severity of experimental necrotizing enterocolitis by regulating endothelial function via eNOS deacetylation. Free Radic. Biol. Med. 2022;184:218–229. doi: 10.1016/j.freeradbiomed.2022.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J.D., Wang C.Y., Chinnaiyan A.M., et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
RNA-seq data have been deposited at SRA and are publicly available as of the date of publication. Accession numbers are listed in the key resources table. Original western blot images have been deposited at Mendeley and are publicly available as of the date of publication. The DOI is https://doi.org/10.17632/85g9c7gs5d.1. Microscopy data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.