Abstract
Nature has abundant source of drugs that need to be identified/purified for use as essential biologics, either individually or in combination in the modern medical field. These drugs are divided into small bio-molecules, plant-made biologics, and a recently introduced third category known as phytopharmaceutical drugs. The development of phytopharmaceutical medicines is based on the ethnopharmacological approach, which relies on the traditional medicine system. The concept of ‘one-disease one-target drug’ is becoming less popular, and the use of plant extracts, fractions, and molecules is the new paradigm that holds promising scope to formulate appropriate drugs. This led to discovering a new concept known as polypharmacology, where natural products from varying sources can engage with multiple human physiology targets. This article summarizes different approaches for phytopharmaceutical drug development and discusses the progress in systems biology and computational tools for identifying drug targets. We review the existing drug delivery methods to facilitate the efficient delivery of drugs to the targets. In addition, we describe different analytical techniques for the authentication and fingerprinting of plant materials. Finally, we highlight the role of biopharming in developing plant-based biologics.
Keywords: Natural products, Phytopharmaceutical drugs, Ethnopharmacology, Bioavailability, Biopharming
Introduction
Plants are a source of a wide range of natural products that possess various therapeutic properties and are continuously explored to develop novel drugs [78]. For ages, traditional medicines have depended on these natural products to treat many diseases. Today, most of the pharmaceutical medications are processed from these natural products. Natural products are made up of many bioactive compounds. These bioactive compounds impart biological activity against several disease-causing agents. To date, numerous secondary metabolites with diverse structures and pharmacological properties have been identified from plants [31, 78]. Knowledge adhered by the traditional medicine system has paved the way for the ongoing exploration of medicinal plants for manufacturing pharmaceutical products [59]. More than 85–90% of the world’s population depends on the traditional medicine system for combating various diseases [93].
The isolation of morphine, the first natural and pure plant-derived compound, from Papaver somniferum in 1803 marked the beginning of the era of drug discovery [44]. About 70,000 herbal plants have been used for medicinal applications, mainly in Asian medicines. About 20% of the available plants are used for medicinal purposes in India. These medicinal plants are the storehouse of unlimited ethnobotanical compounds, which are being utilized today for various drug delivery programs (Table 1). The advancement in genomics, proteomics, transcriptomics and metabolomics has enhanced the contribution of natural products in drug discovery. Metabolomic studies are progressively employed to identify novel drugs and drug targets, interpret drug action mechanisms and maintain records of developed drugs and their therapeutic effects.
Table 1.
Important natural products derived from plant sources
| Botanical source | Ethnobotanical compounds | Therapeutic application | References | |
|---|---|---|---|---|
| Single molecules | Hyperian perfotum | Hypericin | Immunogenic cell death inducer | [45] |
| Lithospermum erythrorhizon | Shikonin | Immunogenic cell death inducer | [100] | |
| Scutellaria baicalensis | Wogonine | Immunogenic cell death inducer | [51] | |
| Piper nigrum | Piperine | Nanotheranostic agent for cancer treatment | [11] | |
| Phytopharmaceutical drugs | Berberis vulgaris L | Berberine, Jatrorrhizine, Palmatine, Ceptisine | Antidiabetic, Anticancer, Antibacterial, Analgesic, Antiinflammatory, and Cardiovascular | [79] |
| Cinchona spp | Quinine | Antimalarial drugs | [57] | |
| Artemisia annua | Artemisinin | Type I diabetes and cancer | [47] | |
| Salvia divinorum | Salvinorin A | Neuro-psychopharmacotherapeutic plant-based drugs | [53] | |
| Cleome | Pinocembrin, Kaempferol, Kaempferitrin | Anti-cancer | [10] | |
| Silybum marianum | Silymarin | Hepatoprotective activities | [26] | |
| Taxus brevifolia | Taxol | Lung, ovarian and breast cancer | [55] | |
| Coleus forskohlii | Forskolin | Antiglaucoma drug | [89] | |
| Curcuma longa L. (Turmeric) | Curcumin | Antioxidant, anti-inflammatory, arthritis, metabolic syndrome and pain | [38] | |
| Galanthus nivalis | Galantamine | Alzhemer | [15] | |
| Capsicum annuum | Capsaicin | Pain relievers | [67] | |
| Artemisia glabella | Arglabin | Anti-tumor | [54] | |
| Genista tinctoria L | Genistein | Anticancer, Alzheimer’s disease | [86] | |
| Vitis vinifera L | Resveratrol | Chemotherapeutic, antidiabetic, antioxidant | [24] | |
| Azadirachta indica A. Juss (Neem) | Azadirachtin | Insecticidal and antimicrobial | [84] | |
| Panax ginseng (Black ginseng) | Extract | Anticancer; anti-inflammatory | [56] | |
| Althaea officinalis | Extract | Anti-inflammatory | [37] | |
| Punica granatum (Pomegranate) | Extract | Antidiarrheal activity | [72] | |
| Averrhoa carambola | Extract | CNS depressant and hypnotic properties | [1] | |
| Ganoderma lucidum, Glycyrrhiza uralensis and Sophora flavescens | Extracts | Anti-asthma | [95] | |
| Trigonella foenum-graceum L | Trigonelline, Diaszhenin | Antidiabetic, Anti-conception | [6] | |
| Capsicum annuum | Capsaicin | Antilithogenic effect, Antiinflammatory | [80] | |
| Plant-made biologics | Genetically engineered carrot cells produce enzyme Taliglucerase Alfa | – | Gaucher´s disease | [27] |
Types of plant-based molecules
Conceptually, plants can be utilized in many ways to extract their therapeutic potential. The most implied usage is in the form of homemade remedies such as herbal teas. Plant extracts in crude form or standardized fractions are used in various pharmaceutical products such as powders, tinctures, pills, etc. Several bioactive compounds have also been extracted from plants and are directly used as drugs.
Small molecules
Plants produce various signalling molecules (auxin, abscisic acid, cytokinin, gibberellic acid, salicylic acid, ethylene, jasmonate and brassinosteroid) and secondary metabolites (alkaloids, terpenoids and phenylpropanoids) which play a crucial role in various developmental and defence processes. These molecules play a vital role in regulating the plants' life cycle and are often referred to as small molecules. These small molecules are released in the state of stress to protect the plant from pathogens, cold, or UV light. Because of their small size (< 500 Da) and diverse mechanism of action, they have dominated the traditional system of medicine and remain the primary component of an ever-expanding therapeutic toolbox [104].
Plant-made biologics
Biotechnological advancement has enabled the use of plants to produce therapeutic proteins for manufacturing medicines and biotech drugs for treating fatal diseases such as cancer, diabetes, HIV, cystic fibrosis, heart disease, and Alzheimer's disease. These plant-made biologics (PMBs) or plant-made pharmaceuticals (PMPs) provide an efficient, safer, and cost-effective platform to produce therapeutic proteins compared to traditional tools based on animal cell cultures and microbial fermentation, which are dependent on expensive facilities. Further, there is a minimum chance of animal or human pathogen infection in plants, making them a competent platform and one of the fastest-growing classes of pharmaceutical products. PMBs have also facilitated the patient’s access to medicines. Many life-saving drugs can be manufactured through these plant-produced proteins [12]. The first approved PMB Elelyso (taliglucerase alfa) is a carrot made enzyme engineered in carrot cells and used to treat Gaucher´s disease [27]. Vaccines for the influenza virus are under clinical trials [71], whereas plant-derived lectins are in the pipeline to produce novel anti-cancer biologics [20]. Under the current global pandemic caused by COVID-19, there is an urgent need to adapt low-budget technologies for manufacturing PMBs against COVID-19. In this context, a promising biopharmaceutical candidate is anticipated, and vaccines based on Virus-like particles (VLPs) have been announced [74].
Phytopharmaceutical drugs
Phytopharmaceutical drug (PPD) is a new class of herbal drugs that are prepared according to the guidelines issued by AYUSH (Department of Ayurveda, Unani, Siddha, and Homeopathy) and CDSCO (Central Drugs Standards Control Organization), in India. These drugs are prepared from herbal plants having a long history of being used as traditional medicines, but proper documentation is not available. PPD is defined as a standardized and purified fraction of a medicinal plant extract consisting of a minimum of four bio-active phytoconstituents and is used to cure and prevent diseases [9]. Usually, the herbal drug manufacturing process lacks proper control and regulation. Hence, guidelines have been incorporated for the analytical analysis and standardization of these herbal drugs for their safe consumption. PPDs are enriched extracts composed of phytomolecules, flavonoids, carotenoids, polyphenols, lycopene, anthocyanidins, omega-3 fatty acids, phytoestrogens, and glucosinolates having distinct pharmacological properties against many human health problems such as allergy, inflammation, diabetes, and many more [66].
Need for production of plant-based drugs
Natural products have always attracted the pharmaceutical industry, with interest in plant-derived drugs and alternative therapies for many reasons. Though synthetic medicines provide quick relief, many adverse effects accompany them. Synthetic medicine is costly due to its manufacturing process and may be inaccessible to a large section of the world’s population. On the other hand, traditional medicines are by and large harmless, more effective with minimum side effects, and easily metabolized and absorbed in the body. Due to the cultural and social belief of the people, they are widely accepted, affordable and easily accessible to the people. Increased scientific studies and clinical trials by researchers and pharmaceutical companies have provided evidence-based medicines [93]. Furthermore, the purification and standardization of a single compound is more convenient, thereby facilitating its use in the modern drug delivery system.
Challenges in production of phytopharmaceutical drugs
Despite several advantages, there exist a few challenges associated with the production of PPD. Plant-derived products sometimes lack quality and are ineffective due to India's poor regulation of natural products. As a result, there is a decline in trade and reluctance in prescribing PPDs. Other hurdles include (i) low yield of the plant material used, (ii) solubility level of plant extracts in water and other solvents, (iii) presence of cytotoxic components in the extract, (iv) limited bio-availability of the sample, (v) inappropriate use of available phytomedicines leading to toxic accidents, (vi) error in botanical identification of plants and their use, (vii) unauthorized usage of popular remedies, (viii) domestic accidents due to consumption of decorative plants having cardiotonic components, (ix) haemorrhagic accidents and hypertensive accidents due to coumarin derivatives present in some plants, (x) presence of oestrogenic components in plants, (xi) use of plants causing allergic reactions due to pollens or volatile components [66].
Approaches for phytopharmaceutical drug development
Many approaches have been developed for drug development depending on the aim and desired end-product used as a herbal medicine or a part of different formulations.
Ethnopharmacology
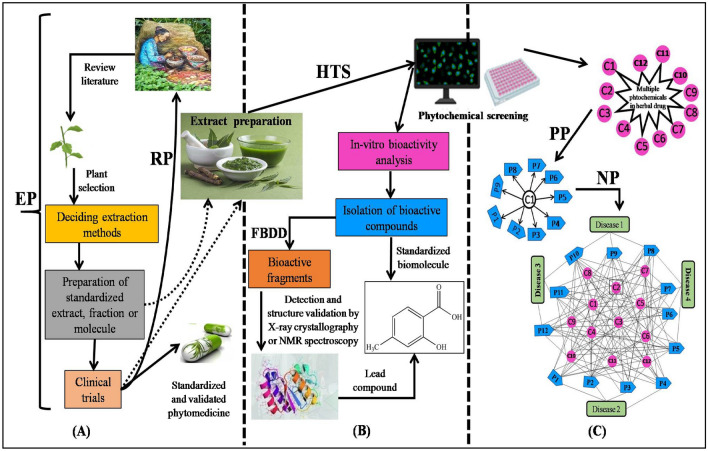
The most important and decisive step for any pharmacological study is selecting the plant. Usually, plants with a history of being used in traditional medicines by different ethnic groups are preferred and such type of approach is known as ethnobotany or ethnopharmacology [81] (Fig. 1A). Various extraction methods and herbal formulae used by the ethnic groups form the base of this approach. Herbal formulations provide concise information regarding the medicinal properties possessed by the herbal formula. Details on how the drug is consumed and the amount used are also acknowledged. However, proper screening of the herbal drug is needed as different ethnic groups have varied health concepts and healthcare systems. Hence, the symptoms should be properly interpreted before using any herbal formulation therapeutically. Ethnopharmacological approach coupled with random high throughput screening has also been employed and is known as the biorational approach. The long history of therapeutic uses increases the hit rate of bioactivity for a new drug candidate. It thus simplifies drug selection, making it the most effective search engine for identifying drugs from nature [93].
Fig. 1.
Schematic representation of herbal drug discovery showing how different approaches are applied based on desired product A Workflow of procedures involved in Ethnopharmacology (EP) and reverse pharmacology (RP) for development of plant-based drugs B Phytochemical evaluation of prepared extracts by high throughput screening (HTS) and fragment-based drug discovery (FBDD) for identification of lead molecules and their subsequent utilization in drug development C Integration of polypharmacology (PP) and network pharmacology (NP) approaches for modern drug discovery. C1-C12 in pink color represents different drug compounds and P1-P12 in blue are different protein targets
Biologically active constituents which possess pharmaceutical properties are isolated from the plant extracts during the drug development process. The whole plant extract is more active than an individual compound in some cases. Plant extracts consist of several structurally diverse chemical components that may be present in low or high concentrations and are responsible for the herbal extract’s overall quality. Bioactivity-guided fractionation of these extracts is needed to isolate and identify bioactive compounds. Bioactive standardized extracts are essential when the pharmacological effect is due to the synergistic effect of many compounds and is not governed by a single component. For instance, the “standardized extract” of Gingko contains ginkgolides A, B, C, and M that can inhibit platelet aggregation factor (PAF)-induced platelet aggregation [3]. On the other hand, bioactive standardized saponin fractions of Panax ginseng were found to be more active than isolated compounds [94]. Several bioactive standardized molecules have also been reported [19, 65].
Reverse pharmacology
Conventional drug development has opened new paths for drug discovery, but sometimes it can be inefficient and expensive. A trans-disciplinary approach has recently emerged, which is cost-effective with reduced time and toxicity levels compared to the conventional method. This new approach is called reverse pharmacology (RP) (Fig. 1A) [69]. RP is based on the experimental validation of the documented findings leading to identifying effective drugs. It includes the documentation of the clinical studies done for herbal formulations used in folk medicine. This is followed by studies on drug dose, drug tolerance, and in vitro and in vivo analysis of the formulation for drug target activity. The last phase includes the clinical and experimentation studies at different levels of biological organization. This leads to the proper identification and validation of RP study in correlation with the safety and efficacy of the herbal drug. Therefore, RP has replaced the common route of “laboratory-to-clinic” with the “clinic-to-laboratories” pathway [82]. RP is the bridge between modern technologies and traditional medicines and has improved their collaboration. RP approach is based on targeted screening of the potential compounds with functional activity and can further be used for drug discovery. RP based drug discovery starts and ends with humans, thereby assuring their safety and efficacy [5].
High throughput screening
For decades pharmaceutical screening of natural products has been carried out for identifying potential drugs. However, high throughput screening (HTS) is the latest approach applied widely for drug delivery programs. HTS incorporates high-quality components and assays used to explore the biological activity of many samples (Fig. 1B). Various bioactive natural compounds and their derivatives have been identified with anti-cancer, anti-diabetic, and anti-inflammatory activities, whereas over a hundred natural compounds are under clinical screening. However, there is an increased interest in the possibility of assaying these natural compounds from traditionally used medicines. With the advancement of analytical tools and fractionation techniques for identifying, isolating, and purifying natural products, screening of these natural compounds is now in accordance with the HTS [49].
Fragment-based drug discovery
The fragment-based drug discovery (FBDD) approach is a new concept used as an alternative to HTS in the pharmaceutical industry. This approach is based on the structure-based drug design and uses X-ray crystallography or NMR spectroscopy to identify potent drug molecules (Fig. 1B). FBDD can reduce attrition and can locate leads for the biological targets which were previously intractable. It can identify very small molecules (fragments) with low-molecular-weight (∼150 Da), which bind to macromolecules or drug leads. To extend FBDD to more laboratories, new and improved computational tools and biophysical methods are being developed and new fragment libraries are being designed [21].
Polypharmacology
In the past few years, drug research has witnessed several significant transformations. Of late, many drugs are withdrawn from the market after a few days of release. Thus, developing novel drug discovery methods has become a great challenge [68]. Several bioactive molecules (alkaloids, phyllanthins, piperidines, bacosides, curcumin) from medicinal plants have successfully treated many human diseases. Moreover, complex diseases such as cancer, heart diseases, multiple sclerosis, and diabetes require a multi-targeted approach. Hence, a new technique known as polypharmacology has emerged, which is based on a multi-target approach (Fig. 1C). This approach involves designing drugs that can modulate multiple targets compared to the traditional concept of one gene, one drug, one disease [23]. The advances in omics technologies and bioinformatics further enabled the identification of key targets in these diseases.
The multitarget drug approaches offer several advantages in comparison to existing combinational therapies. Single molecule acting on several targets offers greater efficacy and reduces toxicity than drug combinations. In addition, there are chances of adverse synergistic effects in combined drugs which pose challenges during testing. However, the regulatory issues which delay clinical trials, are minimum with single compounds [4]. Besides, natural products are also known to have higher polypharmacological profiles than synthetic molecules [23]. Different studies have employed the polypharmacology approach for understanding the mechanisms involved in Traditional Chinese Medicines (TCM) [99]. Fang et al. [23] illustrated the polypharmacological profile of five natural compounds (curcumin, epigallocatechin gallate, quercetin, resveratrol, berberine) and presented different methods for studying drug-target interactions. Similarly, a machine learning-based virtual screening approach was utilized to identify the polypharmacological profile of a natural product galantamine [36]. Construction of databases and development of new bioinformatics tools will accelerate and improve polypharmacology-based studies [90, 92, 98, 103]. More recently, Polypharm-DB has been developed to identify drug candidates for COVID19 [42]. Thus, polypharmacology offers an excellent solution for drug repurposing in the future.
Network pharmacology
With the advancement in system biology, the concept of ‘one-disease one-target drug’ is becoming less popular and comprehends difficulties in treating complex diseases. Hence, new concepts of multiple targets, i.e., polypharmacology and network pharmacology, are gaining impetus (Fig. 1C). The concept of network pharmacology is based on systems biology, network analysis, redundancy, connectivity, and pleiotropy [50]. It offers ways to improve drugs' clinical efficacy by monitoring the side effects and toxicity level by studying the drug’s kinetic and biological profile [39]. According to network biology theory, bioactive compounds that can act on two or more targets are more efficient than those working on single targets [39]. Hence, network pharmacology is the next paradigm in drug discovery because of its cost-effective structure and efficiency in explaining the principles of network theory and systems biology. Many case studies for traditional medicines are based on this network pharmacology approach [16, 91, 96]. The network pharmacology approach is also applied for studying different biological systems, diseases, drugs, and “compound-proteins/genes-disease” pathways based on network biology [102].
Phytopharmaceutical drug delivery systems
Herbal drugs have gained popularity because they are less toxic and possess better therapeutic properties. But due to the unstable acidic pH and solubility issues, the drug concentration in the blood plasma can decrease, leading to reduced healing effects. Though the plant metabolites such as flavonoids, glycosides, etc., possess therapeutic properties, their polar nature and large molecular size restrict their absorption through the lipid rich biological membranes reducing their bioavailability. The introduction of a novel drug delivery system for plants has minimized the drug loss and degradation in the target tissues. It has narrowed down the side effects with enhanced therapeutic efficacy and improved drug bioavailability [20].
Available approaches for efficient drug delivery include nanoparticles, bioadhesive microspheres, chitosan-based hydrogels, pulsatile drug delivery system, self-emulsifying drug delivery systems, liposomes, phytosomes etc. (Fig. 2).
Fig. 2.
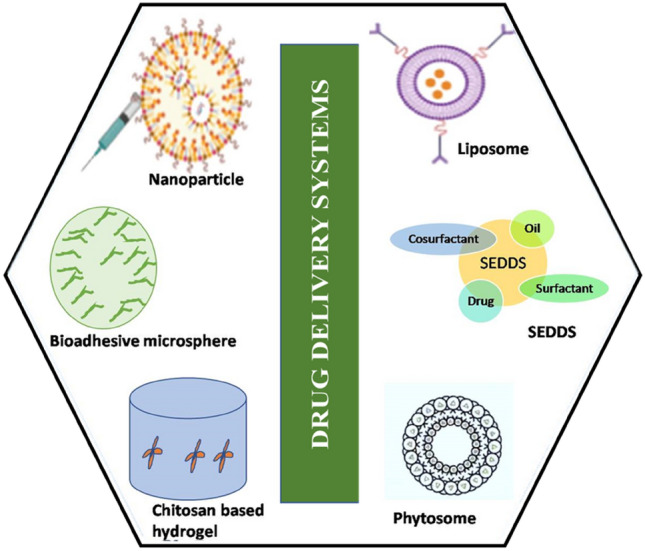
Different herbal drug delivery systems
Nanoparticles
Nanotechnology has emerged as an efficient system in resolving the issues related to herbal drugs’ stability, solubility, and bioavailability [7]. The system employs surface-engineered nanoparticles to increase the therapeutic efficiency of phytochemicals in targeting specific body sites. Nanoparticles derived from plant viruses (tobacco mosaic virus) are effectively used as drug carriers in immunotherapeutic and chemotherapeutic stimulation of tumour-associated immune cells [20]. The introduction of Nanoparticles in the drug delivery system has eased phytochemical transportation beyond the biological membranes with their precise target delivery with minimum degradation. Different nano formulated phytochemicals include hypericin, curcumin, silymarin, etc. [75].
Bioadhesive microspheres
Bioadhesive microspheres (BMs) are unique drug delivery systems that provide intimate contact of the drug with the biological membrane. It comprises micro-particles and microcapsules which are in the range of 1–1000 μm in diameter. BMs are tailored by combining microspheres with bio-adhesive properties. This coupling enhances the bioavailability and target specificity of the drug at the absorption site. Different polymers used to customize the BMs influence their surface properties, bioadhesion force, drug release pattern, and clearance. These polymers include biodegradable, non-biodegradable, insoluble, and soluble polymers. BMs have been produced for eye tissues, mucosal tissues, oral and respiratory tissues, gastrointestinal and urinary tract. They are used to control the release of the drug and targeted drug delivery to specific sites in the body [88].
Chitosan-based hydrogels
Hydrogels are swelled cross-linked networks of polymers that can absorb large amount of water [48]. The characteristic features of hydrogel include swelling potential, mechanical strength similar to host tissues and biodegradability. Hydrogels are made up of either natural or synthetic polymers. Biopolymers like chitosan have been mainly used for hydrogel preparation as they can structurally modify themselves. Chitosan has hydrophilic nature and possesses biocompatibility and biodegradability properties. Hydrogels can carry small drug molecules, reduce their side effects, and enhance their concentration at the site of action. Chitosan-based hydrogels are mainly used for the controlled delivery of therapeutic components. The mucoadhesive characteristics of chitosan facilitate tissue binding capacity for specific drug delivery [70].
Pulsatile drug delivery system
Controlled drug delivery systems deliver the drugs at a constant rate and continuous release. However, some conditions require intermittent drug delivery, i.e., a time lag. Such delivery is achieved by the pulsatile drug delivery system (PDDS). PDDS closely imitates the body’s mechanism of releasing insulin in a controlled way as and when needed. PDDS can effectively deliver the drug in the optimum amount at the right place and time. This system has been successfully used for hypercholesterolemia, asthma, hypertension, arthritis, and peptic ulcer cardiovascular diseases. For pulsatile delivery, time-dependent systems and pH-dependent systems, etc., are used, which have polymers sensitive to temperature, pH change and light [40]. PDDS offers many advantages over conventional drug delivery systems including the persistent amount of drug at the site of action, reduced drug dose, preventing fluctuations, controlling side effects, and improving patient compliance. Thus, this pulsatile drug delivery with coordinated biological rhythms and therapeutic needs provides minimum harm and maximum health benefit to the patient [14].
Self-emulsifying drug delivery systems
The self-emulsifying drug delivery approach is very promising for herbal drug formulations with poor water solubility and lipophilic plant actives [13]. A self-emulsifying drug delivery system (SEDDS) is a thermodynamically stable solution composed of drug, oil, surfactant and cosurfactant. When the solution is mixed with water and gently stirred, it immediately forms oil-in-water micro/nano emulsion. These emulsions range from a few nanometres to several microns. ‘‘Self-micro emulsifying drug delivery systems’’ (SMEDDS) form oil droplets in the range of 100–250 nm, whereas ‘‘Self-nano emulsifying drug delivery systems’’ (SNEDDS) range 5100 nm [43]. SEDDS has been effectively used to enhance the bioavailability of poorly absorbed plant metabolites such as patchouli alcohol [101], mangiferin [97]. SEDDS is preferred over other drug delivery methods because of its simple and easy nature, and it also can be stored in liquid and solid forms. Hence, SEDDS can be efficiently used to improve herbal drugs’ bioavailability and solubility.
Liposomes
Liposomes are non-toxic, biodegradable drug delivery vehicles that can accommodate hydrophobic and hydrophilic materials. They are spherical, with one or multiple concentric membranes and a solvent for their free diffusion. They are made up of polar lipids and are used to alter the pharmacokinetics profile of drugs. Liposomes can accelerate the drug solubility, stability, bioavailability, intracellular uptake and biodistribution. They can improve and maintain the drugs’ therapeutic features and their level for a long duration and thus are used as a drug delivery system. Liposomes have been used as drug carriers for proteins, small drug molecules, viruses, nucleotides, and other biologically active compounds [76]. Recently, a herbal drug loaded in nano liposomal vesicles has been used to deliver plant-derived bioactive molecules with anti-cancer properties [32].
Phytosomes
Bioactive compounds mostly have less bioavailability due to their oral intake. Lipid-rich biomembranes pose a hindrance in the crossing of water-soluble phytoconstituents. Thus, herbal extracts that are insoluble in lipids can be dissolved in phospholipids in a specific ratio and converted into lipid-compatible molecular complexes with therapeutic properties. This technology is based on the phospholipid complex procedure which involves a chemical reaction between polyphenolic plant actives and phospholipids containing phosphatidylcholine known as phytosome. The technique also produces cellular vesicles, which protect these water-soluble phytocomponents (flavonoids, terpenoids, phenolics) from getting destroyed by the gut microflora and gastric secretions. This procedure enhances the therapeutic index of the plants’ active compounds [7]. It ensures better quality and efficient target delivery of active plant components. This technology has provided better chemical linkage of the drug and accelerated its penetration through the skin in reduced doses. Thus, the phytophospholipid complex technique has provided an advanced and systemic absorption of herbal extracts. Hence, these phytophospholipid complexes are promising candidates for better drug dosage therapy with anti-inflammatory, cardiovascular, anticancer, and hepatoprotective applications [2].
Authentication of plant-derived molecules
Herbal drugs have been widely accepted globally and are in high demand because of their claimed health benefits. This has led to their massive adulteration for which many authentication tools have been developed to evaluate their quality and authenticity. Herbal formulations consist of many bioactive compounds in minimal concentration, which may significantly affect the overall quality of the phytomedicine [31]. Herbal drugs being mixtures of various components, need certain qualitative and quantitative analysis. For the quality of an herbal drug, standardization is the prerequisite. The drug quality is affected by multiple factors such as inter or intraspecies variation, environmental factors, season, time and methods of harvesting, geographical location of the herb, plant part used, storage and processing practices, etc. [22, 73].
In recent times, chromatographic fingerprinting is one of the most important and powerful techniques used to evaluate the quality of herbal drugs [41]. In 1991, chromatographic fingerprinting was accepted by WHO as a technique for the identification and consistency evaluation of the herbal drugs. American Food and Drug Administration (FDA), European Medicine Evaluation Agency (EMEA) and Chinese State Food and Drug Administration (SFDA) also accepted the chromatographic fingerprint of traditional medicines as standards and chromatographic fingerprinting technology as an alternative method for the quality check of herbal drugs [34, 85].
The criterion for assessing the individual herbal material is the common pattern obtained from the chromatographic fingerprinting from various samples of the same species. To ensure the safety and efficacy of an herbal drug, a chemical fingerprint (CF) is developed which represents a unique profile of the phytochemical composition of the sample [52]. This chemical fingerprint has specific features. The first feature is the intactness of the CF having a specific profile for identification which is constituted by all the detectable components of the sample. Second, two levels of significance should be present, i.e., ‘elementary’ quality control which includes the identification and quantification of the herbal medicine, and the other is ‘intensive’ quality control which serves the in-depth studies of the CF with chemometrics, information theory and other sophisticated technologies. Thus, a CF of a product can be accepted economically and technologically for its official and industrial specifications [52]. Other identification methods include DNA barcoding which uses short DNA sequences from the sample plant genome for species identification [58]. The acceptance of a herbal drug is based on the principles of safety, consistency and efficacy [35]. Thus, chemical fingerprinting should be the top priority as it is the fundamental level for the quality check of herbal drugs.
Several chromatographic fingerprinting techniques have been developed for the quality check and authenticity of herbal medicine. In general, fingerprints can be developed by various spectroscopic and chromatographic techniques. Spectroscopic fingerprints can be developed by using Raman or Nuclear Magnetic Resonance (NMR) spectroscopy or Infrared (IR) spectroscopy [30]. Mass spectrometric (MS) fingerprints also can be developed. Chromatographic fingerprints can be obtained using Thin-layer chromatography (TLC) [77], High-performance thin-layer chromatography (HPTLC) [18], High performance liquid chromatography (HPLC) [17], Ultra-high performance liquid chromatography (UHPLC) [105], Capillary electrophoresis (CE) [29], Gas chromatography (GC) [64], Gas chromatography-mass spectrometry (GC–MS) [61], Two-dimensional gas chromatography-time-of-flight mass spectrometry (GCxGC-TOFMS) [63].
HPLC is analytical equipment widely used for checking the authenticity of herbal products. HPLC coupled with multivariate analysis is used for differentiating two closely related herbs [31]. HPTLC is an easily operated tool with low cost and high sample throughput. It can analyze many samples parallelly and give accurate results. It is widely used for detecting adulterants in herbal samples [18]. UPLC is an advanced liquid chromatographic technique requiring less solvent as a mobile phase and completes the analysis in minimal time. It is also more efficient in separating and resolving analyte mixtures. It is broadly used for pharmaceutical and biomedical analysis of various samples [60]. GC is a dynamic analytical technique well known for detecting and quantifying volatile components. The stability, improved visualization, efficient separation and sensitivity for detection by Flame ionization detector (FID) or Mass spectrometry (MS), makes this instrument a robust tool for the study of essential oils and herbal formulations [61]. GCMS is one of the most widely accepted tools for identifying and qualitatively evaluating herbal drugs' volatile components. It has been used widely by many workers to analyze various phytoconstituents because of its high efficiency, reproducibility, sensitive detection, simplicity and stability [61], GC × GC-TOFMS is the most efficient separation tool for analyzing complex mixtures due to its high resolution and high peak capacity. Using two columns with varying separation methodology makes this technique more advantageous by increasing resolution, sensitivity, and identification of more unknown compounds [63]. This technique can be used to detect minor components, develop comprehensive fingerprints and detect unknown volatile constituents of the herbal drug.
Plant biopharming
Over the past decade, plant biotechnology has advanced exponentially and utilizing plants as an alternative for producing recombinant biomolecules is the latest breakthrough in science. Transgenic and transient systems have been developed vigorously to produce high yields of recombinant molecules like enzymes, hormones, antibodies, vaccines and enhanced protein expression [74]. Plants commonly used as bioreactors include tobacco, tomato, rice, potato, and corn. Tobacco plants are most extensively used as a transgenic platform to produce pharmaceutical products [25]. To date, many transgenic plants have been raised for the production of plant-based vaccines such as viral vaccines, bacterial vaccines, immunocontraceptive vaccines, etc. [46].
Biopharming or molecular pharming could be a safer system for pharmaceutical production than yeast, bacteria or cultured mammalian cells because the produced recombinant biomolecules are free from human pathogens, DNA sequences and endotoxins [87]. The plant system has also erased the post-translational modifications that occur when using bacteria [83]. Though biopharming is a better system, the structural authenticity of the plant-derived human proteins is very important because it affects their behavior in vivo [8]. Plant-derived human proteins have carbohydrate groups but lack the terminal galactose and sialic acid. A minor change in the glycan structure can alter recombinant proteins' activity and distribution and make them immunogenic when delivered to humans. Hence confirming a recombinant protein's authenticity is paramount in biopharming [87].
Transfer and expression of genes in plants can be achieved by agroinfiltration, viral transfection, transient expression, nuclear transformation etc. [62]. Plant viral vectors have also been engineered to produce pharmaceuticals. These viruses do not cause infection to humans or animals and can produce large amounts of heterologous proteins in the plants [33]. The engineered plant virus expresses the desired protein during viral replication in the plant cells. The method is advantageous in producing a high amount of recombinant protein expression. The recombinant protein is then purified before vaccine development [46]. Many plant virus expression systems have been used such as cucumber mosaic virus (CMV), tobacco mosaic virus (TMV), cowpea mosaic virus (CPMV) etc. [28]. Plants are a source of numerous bioactive molecules that possess several pharmaceutical properties such as anti-viral, anti-bacterial, anti-fungal, etc. Many of these compounds might be present in low amounts in the plant. Thus, biotechnology has provided a gateway for the rescue of these components through advanced technologies and their potential utilization in the development of plant-based biologics.
Prospects
Over the years, the biotechnology industry has overcome key challenges such as small-molecule resistance, identifying new phytochemicals with a new mode of action and finding new druggable targets. Natural products are the base of novel therapeutic compounds and pose minimum adverse effects. Though the process of drug discovery is slow and time-consuming, recent advances in the plant-based biomanufacturing system, the production and commercialization of herbal drugs and plant-made biologics have gained impetus. The advantages offered by the biopharming platform have provided scope for the development of plant-made cancer biologic which is the need of the hour. Many other medical conditions can be cured if the traditional and modern medical systems work synchronously through integrated approaches. Almost 80–90% of the world’s biodiversity is under-explored and can be a potential source of novel natural compounds and drug leads that can be efficiently used against emerging infectious diseases. Advanced plant production systems being low-cost systems with high safety and scalability also provide scope to produce plant biologics for controlling pandemic outbreaks. The current pandemic which occurred due to the outbreak of COVID-19 has affected the whole world and there is an urgent need to develop a cure. Presently, a number of vaccines have been approved for clinical trials, many are in the pipeline, and some are already being tested on the patients. At this stage, plant-based biologics hold great potential in providing an efficient system to develop anti-viral vaccines against SARS-CoV-2 for fighting the detrimental effects caused by this pandemic.
Acknowledgements
Author acknowledge the facilities and support provided by the Siksha ‘O’ Anusandhan Deemed University and Rama Devi Women's University, Bhubaneswar.
Author contribution
NN conceived the idea, performed literature search and prepared the first draft, IS helped in literature search, writing and review of the draft and SM provided overall supervision and reviewed of the manuscript.
Footnotes
Corresponding Editor: Umesh C. Lavania; Reviewers: Ram J Singh, Anita Mukherjee.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akter A, Islam F, Bepary S, Al-Amin M, Begh M, Alam Z, Islam M, Ashraf GM, Baeesa SS, Ullah MF. CNS depressant activities of Averrhoa carambola leaves extract in thiopental-sodium model of Swiss albino mice: implication for neuro-modulatory properties. Biologia. 2022;77(5):1337–1346. doi: 10.1007/s11756-022-01057-z. [DOI] [Google Scholar]
- 2.Alharbi WS, Almughem FA, Almehmady AM, Jarallah SJ, Alsharif WK, Alzahrani NM, Alshehri AA. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics. 2021;13(9):1475. doi: 10.3390/pharmaceutics13091475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali P, Chen YF, Sargsyan E. Bioactive molecules of herbal extracts with anti-infective and wound healing properties. In: Kon K, Rai M (eds) Microbiology for surgical infections. Academic Press; 2014. pp. 205–220. 10.1016/B978-0-12-411629-0.00012-X.
- 4.Anighoro A, Bajorath J, Rastelli G. Polypharmacology: challenges and opportunities in drug discovery: miniperspective. J Med Chem. 2014;57(19):7874–7887. doi: 10.1021/jm5006463. [DOI] [PubMed] [Google Scholar]
- 5.Arulsamy A, Kumari Y, Shaikh MF. Reverse pharmacology: fast track path of drug discovery. Pharm Pharmacol Int J. 2016;4(3):00077. doi: 10.15406/ppij.2016.04.00077. [DOI] [Google Scholar]
- 6.Bahmani M, Shirzad H, Mirhosseini M, Mesripour A, Rafieian-Kopaei M. A review on ethnobotanical and therapeutic uses of fenugreek (Trigonella foenum-graceum L.) J Evid Based Complement Altern Med. 2016;21(1):53–62. doi: 10.1177/2156587215583405. [DOI] [PubMed] [Google Scholar]
- 7.Barkat MA, Das SS, Beg S, Ahmad FJ. Nanotechnology-based phytotherapeutics: current status and challenges. In: Beg S, Barkat M, Ahmad F (eds) Nanophytomedicine. Springer, Singapore 2020. pp. 1–7. 10.1007/978-981-15-4909-0_1.
- 8.Basaran P, Rodríguez-Cerezo E. Plant molecular farming: opportunities and challenges. Crit Rev Biotechnol. 2008;28(3):153–172. doi: 10.1080/07388550802046624. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt A. Phytopharmaceuticals: a new drug class regulated in India. Perspect Clin Res. 2016;7(2):59. doi: 10.4103/2229-3485.179435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chand J, Panda SR, Jain S, Murty US, Das AM, Kumar GJ, Naidu VG. Phytochemistry and polypharmacology of cleome species: a comprehensive ethnopharmacological review of the medicinal plants. J Ethnopharmacol. 2022;282:114600. doi: 10.1016/j.jep.2021.114600. [DOI] [PubMed] [Google Scholar]
- 11.Chelora J, Zhang J, Wan Y, Cui X, Zhao J, Meng XM, Wang P, Lee CS. Plant-derived single-molecule-based nanotheranostics for photoenhanced chemotherapy and ferroptotic like cancer cell death. ACS Appl Bio Mater. 2019;2(6):2643–2649. doi: 10.1021/acsabm.9b00311. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Davis KR. The potential of plants as a system for the development and production of human biologics. F1000Research 5. 2016 doi: 10.12688/f1000research.8010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chouhan N, Mittal V, Kaushik D, Khatkar A, Raina M. Self emulsifying drug delivery system (SEDDS) for phytoconstituents: a review. Curr Drug Deliv. 2015;12(2):244–253. doi: 10.2174/1567201811666141021142606. [DOI] [PubMed] [Google Scholar]
- 14.Ciancia S, Cafarelli A, Zahoranova A, Menciassi A, Ricotti L. Pulsatile drug delivery system triggered by acoustic radiation force. Front Bioeng Biotechnol. 2020;8:317. doi: 10.3389/fbioe.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle J, Kershaw P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: effects on the course of Alzheimer’s disease. Biol Psychiatry. 2001;49(3):289–299. doi: 10.1016/S0006-3223(00)01101-X. [DOI] [PubMed] [Google Scholar]
- 16.Cui S, Chen S, Wu Q, Chen T, Li S. A network pharmacology approach to investigate the anti-inflammatory mechanism of effective ingredients from Salvia miltiorrhiza. Int Immunopharmacol. 2020;81:106040. doi: 10.1016/j.intimp.2019.106040. [DOI] [PubMed] [Google Scholar]
- 17.da Gomes MV, da Silva JD, Ribeiro AF, Cabral LM, de Sousa VP. Development and validation of a quantification method for α-humulene and trans-caryophyllene in Cordia verbenacea by high performance liquid chromatography. Rev Bras. 2019;29(2):182–190. doi: 10.1016/j.bjp.2019.01.009. [DOI] [Google Scholar]
- 18.Das S, Ray A, Nasim N, Nayak S, Mohanty S. Effect of different extraction techniques on total phenolic and flavonoid contents, and antioxidant activity of betelvine and quantification of its phenolic constituents by validated HPTLC method. 3 Biotech. 2019;9(1):1–8. doi: 10.1007/s13205-018-1565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denaro M, Smeriglio A, Barreca D, De Francesco C, Occhiuto C, Milano G, Trombetta D. Antiviral activity of plants and their isolated bioactive compounds: an update. Phytother Res. 2020;34(4):742–768. doi: 10.1002/ptr.6575. [DOI] [PubMed] [Google Scholar]
- 20.Dent M, Matoba N. Cancer biologics made in plants. Curr Opin Biotechnol. 2020;61:82–88. doi: 10.1016/j.copbio.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlanson DA. Introduction to fragment-based drug discovery. In: Davies T, Hyvönen M (eds) Fragment-based drug discovery and X-ray crystallography. Topics in Current Chemistry. Springer, Berlin, Heidelberg 2011;317:1–32. 10.1007/128_2011_180. [DOI] [PubMed]
- 22.Fan XH, Cheng YY, Ye ZL, Lin RC, Qian ZZ. Multiple chromatographic fingerprinting and its application to the quality control of herbal medicines. Anal Chim Acta. 2006;555(2):217–224. doi: 10.1016/j.aca.2005.09.037. [DOI] [Google Scholar]
- 23.Fang J, Liu C, Wang Q, Lin P, Cheng F. In silico polypharmacology of natural products. Br Bioinform. 2018;19(6):1153–1171. doi: 10.1093/bib/bbx045. [DOI] [PubMed] [Google Scholar]
- 24.Ferraz da Costa DC, Pereira Rangel L, Martins-Dinis MM, Ferretti GD, Ferreira VF, Silva JL. Anticancer potential of resveratrol, β-lapachone and their analogues. Molecules. 2020;25(4):893. doi: 10.3390/molecules25040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer R, Buyel JF. Molecular farming–the slope of enlightenment. Biotechnol Adv. 2020;40:107519. doi: 10.1016/j.biotechadv.2020.107519. [DOI] [PubMed] [Google Scholar]
- 26.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93(2):139–143. doi: 10.1016/S0002-9270(97)00082-8. [DOI] [PubMed] [Google Scholar]
- 27.Fox JL. First plant-made biologic approved. Nat Biotechnol. 2012;30(6):472–473. doi: 10.1038/nbt0612-472. [DOI] [Google Scholar]
- 28.Fujiki M, Kaczmarczyk JF, Yusibov V, Rabindran S. Development of a new cucumber mosaic virus-based plant expression vector with truncated 3a movement protein. Virology. 2008;381(1):136–142. doi: 10.1016/j.virol.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Gackowski M, Przybylska A, Kruszewski S, Koba M, Mądra-Gackowska K, Bogacz A. Recent applications of capillary electrophoresis in the determination of active compounds in medicinal plants and pharmaceutical formulations. Molecules. 2021;26(14):4141. doi: 10.3390/molecules26144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gad HA, Bouzabata A. Application of chemometrics in quality control of Turmeric (Curcuma longa) based on ultra-violet, Fourier transform-infrared and 1H NMR spectroscopy. Food Chem. 2017;237:857–864. doi: 10.1016/j.foodchem.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Gad HA, El-Ahmady SH, Abou-Shoer MI, Al-Azizi MM. Application of chemometrics in authentication of herbal medicines: a review. Phytochem Anal. 2013;24(1):1–24. doi: 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- 32.Giri TK. Breaking the barrier of cancer through liposome loaded with phytochemicals. Curr Drug Deliv. 2019;16(1):3–17. doi: 10.2174/1567201815666180918112139. [DOI] [PubMed] [Google Scholar]
- 33.Gleba Y, Klimyuk V, Marillonnet S. Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol. 2007;18(2):134–141. doi: 10.1016/j.copbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Goodarzi M, Russell PJ, Vander Heyden Y. Similarity analyses of chromatographic herbal fingerprints: a review. Anal Chim Acta. 2013;804:16–28. doi: 10.1016/j.aca.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Govindaraghavan S, Sucher NJ. Quality assessment of medicinal herbs and their extracts: criteria and prerequisites for consistent safety and efficacy of herbal medicines. Epilepsy Behav. 2015;52:363–371. doi: 10.1016/j.yebeh.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Grisoni F, Merk D, Friedrich L, Schneider G. Design of natural-product-inspired multitarget ligands by machine learning. ChemMedChem. 2019;14(12):1129–1134. doi: 10.1002/cmdc.201900097. [DOI] [PubMed] [Google Scholar]
- 37.Hage-Sleiman R, Mroueh M, Daher CF. Pharmacological evaluation of aqueous extract of Althaea officinalis flower grown in Lebanon. Pharm Biol. 2011;49(3):327–333. doi: 10.3109/13880209.2010.516754. [DOI] [PubMed] [Google Scholar]
- 38.Hewlings SJ, Kalman DS. Curcumin: a review of its effects on human health. Foods. 2017;6(10):92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1111. doi: 10.1038/nbt1007-1110. [DOI] [PubMed] [Google Scholar]
- 40.Jain D, Raturi R, Jain V, Bansal P, Singh R. Recent technologies in pulsatile drug delivery systems. Biomatter. 2011;1(1):57–65. doi: 10.4161/biom.1.1.17717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaushik R, Jain J, Mazumder A. Chromatographic fingerprinting of Sarasvata Churna: an ayurvedic polyherbal formulation for epilepsy. J Appl Pharm Sci. 2018;8(4):090–98. doi: 10.7324/JAPS.2018.8413. [DOI] [Google Scholar]
- 42.Keshavarzi Arshadi A, Webb J, Salem M, Cruz E, Calad-Thomson S, Ghadirian N, Collins J, Diez-Cecilia E, Kelly B, Goodarzi H, Yuan JS. Artificial intelligence for COVID-19 drug discovery and vaccine development. Front Artif Intell. 2020 doi: 10.3389/frai.2020.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discov Today. 2010;15(21–22):958–965. doi: 10.1016/j.drudis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurti C, Rao SC. The isolation of morphine by Serturner. Indian J Anaesth. 2016;60(11):861. doi: 10.4103/0019-5049.193696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 46.Laere E, Ling AP, Wong YP, Koh RY, Mohd Lila MA, Hussein S. Plant-based vaccines: production and challenges. J Bot. 2016 doi: 10.1155/2016/4928637. [DOI] [Google Scholar]
- 47.Lai HC, Singh NP, Sasaki T. Development of artemisinin compounds for cancer treatment. Invest New Drugs. 2013;31(1):230–246. doi: 10.1007/s10637-012-9873-z. [DOI] [PubMed] [Google Scholar]
- 48.Lai WF, Rogach AL. Hydrogel-based materials for delivery of herbal medicines. ACS Appl Mater Interfaces. 2017;9(13):11309–11320. doi: 10.1021/acsami.6b16120. [DOI] [PubMed] [Google Scholar]
- 49.Lautie E, Russo O, Ducrot P, Boutin JA. Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol. 2020;11:397. doi: 10.3389/fphar.2020.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Fan TP, Jia W, Lu A, Zhang W. Network pharmacology in traditional Chinese medicine. Evid Based Complement Altern Med. 2014 doi: 10.1155/2014/138460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Dong W, Nalin AP, Wang Y, Pan P, Xu B, Zhang Y, Tun S, Zhang J, Wang LS, He X. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. Oncoimmunology. 2018;7(6):e1431085. doi: 10.1080/2162402X.2018.1431085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang Y, Xie P, Chau F. Chromatographic fingerprinting and related chemometric techniques for quality control of traditional Chinese medicines. J Sep Sci. 2010;33(3):410–421. doi: 10.1002/jssc.200900653. [DOI] [PubMed] [Google Scholar]
- 53.Listos J, Merska A, Fidecka S. Pharmacological activity of salvinorin A, the major component of Salvia divinorum. Pharmacol Rep. 2011;63(6):1305–1309. doi: 10.1016/S1734-1140(11)70694-6. [DOI] [PubMed] [Google Scholar]
- 54.Lone SH, Bhat KA, Khuroo MA. Arglabin: from isolation to antitumor evaluation. Chem Biol Interact. 2015;240:180–198. doi: 10.1016/j.cbi.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Mamadalieva NZ, Mamedov NA. Taxusbrevifolia a high-value medicinal plant, as a source of taxol. In: Máthé Á, editor. Medicinal and aromatic plants of North America. Cham: Springer; 2020. pp. 201–218. [Google Scholar]
- 56.Metwaly AM, Lianlian Z, Luqi H, Deqiang D. Black ginseng and its saponins: preparation, phytochemistry and pharmacological effects. Molecules. 2019;24(10):1856. doi: 10.3390/molecules24101856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohammadi S, Jafari B, Asgharian P, Martorell M, Sharifi-Rad J. Medicinal plants used in the treatment of Malaria: a key emphasis to Artemisia, Cinchona, Cryptolepis, and Tabebuia genera. Phytother Res. 2020;34(7):1556–1569. doi: 10.1002/ptr.6628. [DOI] [PubMed] [Google Scholar]
- 58.Mohammed Abubakar B, Mohd Salleh F, Shamsir Omar MS, Wagiran A. DNA barcoding and chromatography fingerprints for the authentication of botanicals in herbal medicinal products. Evid Based Complement Altern Med. 2017 doi: 10.1155/2017/1352948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mushtaq S, Abbasi BH, Uzair B, Abbasi R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018;17:420. doi: 10.17179/excli2018-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nahar L, Onder A, Sarker SD. A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010–2019) Phytochem Anal. 2020;31(4):413–457. doi: 10.1002/pca.2906. [DOI] [PubMed] [Google Scholar]
- 61.Nasim N, Behera JK, Sandeep IS, RamaRao VV, Kar B, Mishra A, Nayak S, Mohanty S. Phytochemical analysis of flower from Pandanus odorifer (Forssk.) Kuntze for industrial application. Nat Prod Res. 2018;32(20):2494–7. doi: 10.1080/14786419.2017.1422184. [DOI] [PubMed] [Google Scholar]
- 62.Nasim N, Dey N. Pararetroviruses: plant Infecting dsDNA Viruses. Plant Mol Biol Rep. 2021 doi: 10.1007/s11105-021-01294-7. [DOI] [Google Scholar]
- 63.Nasim N, Ray A, Singh S, Jena S, Sahoo A, Kar B, Sandeep IS, Mohanty S, Nayak S. Characterization of Kewda volatile components by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. Nat Prod Res. 2017;31(7):853–856. doi: 10.1080/14786419.2016.1269099. [DOI] [PubMed] [Google Scholar]
- 64.Nasim N, Sandeep IS, Nayak S, Mohanty S. Cultivation and utilization of Pandanus odorifer for industrial application. In: Ekiert HM. Ramawat KG, Arora J (eds) Medicinal plants. Cham: Springer; 2021. pp. 435–45610.1007/978-3-030-74779-4_15.
- 65.Nasr-Bouzaiene N, Sassi A, Bedoui A, Krifa M, Chekir-Ghedira L, Ghedira K. Immunomodulatory and cellular antioxidant activities of pure compounds from Teucrium ramosissimum Desf. Tumor Biol. 2016;37(6):7703–7712. doi: 10.1007/s13277-015-4635-0. [DOI] [PubMed] [Google Scholar]
- 66.Nooreen Z, Rai VK, Yadav NP. Phytopharmaceuticals: a new class of drug in India. Ann Phytomed. 2018;7(1):27–37. doi: 10.21276/ap.2018.7.1.4. [DOI] [Google Scholar]
- 67.Pasierski M, Szulczyk B. Beneficial effects of capsaicin in disorders of the central nervous system. Molecules. 2022;27(8):2484. doi: 10.3390/molecules27082484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patwardhan B. Rediscovering drug discovery. Comb Chem High Throughput Screen. 2014;17(10):819. doi: 10.2174/138620731710150108230829. [DOI] [PubMed] [Google Scholar]
- 69.Patwardhan B, Vaidya AD, Chorghade M, Joshi SP. Reverse pharmacology and systems approaches for drug discovery and development. Curr Bioact Compd. 2008;4(4):201–212. doi: 10.2174/157340708786847870. [DOI] [Google Scholar]
- 70.Pellá MC, Lima-Tenório MK, Tenório-Neto ET, Guilherme MR, Muniz EC, Rubira AF. Chitosan-based hydrogels: from preparation to biomedical applications. Carbohyd Polym. 2018;196:233–245. doi: 10.1016/j.carbpol.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 71.Pillet S, Couillard J, Trépanier S, Poulin JF, Yassine-Diab B, Guy B, Ward BJ, Landry N. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate—two randomized phase II clinical trials in 18 to 49 and ≥ 50 years old adults. PLoS ONE. 2019;14(6):e0216533. doi: 10.1371/journal.pone.0216533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qnais EY, Elokda AS, Abu Ghalyun YY, Abdulla FA. Antidiarrheal activity of the aqueous extract of Punica granatum. (pomegranate) peels. Pharmaceutical Biology. 2007;45(9):715–20. doi: 10.1080/13880200701575304. [DOI] [Google Scholar]
- 73.Rathod R, Chandak RR. Review on “standardization an imp tool for herbal drug development”. J Drug Deliv Ther. 2019;9(6-s):253–7. doi: 10.22270/jddt.v9i6-s.3784. [DOI] [Google Scholar]
- 74.Rosales-Mendoza S. Will plant-made biopharmaceuticals play a role in the fight against COVID-19? Expert Opin Biol Ther. 2020;20(6):545–548. doi: 10.1080/14712598.2020.1752177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shakeri A, Sahebkar A. Nanotechnology: a successful approach to improve oral bioavailability of phytochemicals. Recent Pat Drug Deliv Formul. 2016;10(1):4–6. doi: 10.2174/1872211309666150611120724. [DOI] [PubMed] [Google Scholar]
- 76.Sharma G, Anabousi S, Ehrhardt C, Ravi Kumar MN. Liposomes as targeted drug delivery systems in the treatment of breast cancer. J Drug Target. 2006;14(5):301–310. doi: 10.1080/10611860600809112. [DOI] [PubMed] [Google Scholar]
- 77.Simion IM, Casoni D, Sârbu C. Classification of Romanian medicinal plant extracts according to the therapeutic effects using thin layer chromatography and robust chemometrics. J Pharm Biomed Anal. 2019;163:137–143. doi: 10.1016/j.jpba.2018.09.047. [DOI] [PubMed] [Google Scholar]
- 78.Singh RJ, Lebeda A, Tucker O. Chapter 2. Medicinal plants—nature’s pharmacy. In: Singh RJ, editor. Genetic resources, chromosome engineering, and crop improvement. Medicinal plants. Boca Raton: CRC Press; 2012. pp. 13–51. [Google Scholar]
- 79.Singh S, Pathak N, Fatima E, Negi AS. Plant isoquinoline alkaloids: advances in the chemistry and biology of berberine. Eur J Med Chem. 2021;226:113839. doi: 10.1016/j.ejmech.2021.113839. [DOI] [PubMed] [Google Scholar]
- 80.Srinivasan K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr. 2016;56(9):1488–1500. doi: 10.1080/10408398.2013.772090. [DOI] [PubMed] [Google Scholar]
- 81.Süntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev. 2020;19(5):1199–1209. doi: 10.1007/s11101-019-09629-9. [DOI] [Google Scholar]
- 82.Surh YJ. Reverse pharmacology applicable for botanical drug development–inspiration from the legacy of traditional wisdom. J Tradit Complement Med. 2011;1(1):5. doi: 10.1016/s2225-4110(16)30051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeyama N, Kiyono H, Yuki Y. Plant-based vaccines for animals and humans: recent advances in technology and clinical trials. Ther Adv Vaccines. 2015;3(5–6):139–154. doi: 10.1177/205101361561327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tembe-Fokunang EA, Charles F, Kaba N, Donatien G, Michael A, Bonaventure N. The potential pharmacological and medicinal properties of neem (Azadirachta indica A. Juss) in the drug development of phytomedicine. J Complement Altern Med Res. 2019;7(1):1–8. doi: 10.9734/JOCAMR/2019/v7i130093. [DOI] [Google Scholar]
- 85.Tistaert C, Dejaegher B, Vander Heyden Y. Chromatographic separation techniques and data handling methods for herbal fingerprints: a review. Anal Chim Acta. 2011;690(2):148–161. doi: 10.1016/j.aca.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 86.Tuli HS, Tuorkey MJ, Thakral F, Sak K, Kumar M, Sharma AK, Sharma U, Jain A, Aggarwal V, Bishayee A. Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol. 2019;10:1336. doi: 10.3389/fphar.2019.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R. Molecular farming in plants: host systems and expression technology. Trends Biotechnol. 2003;21(12):570–578. doi: 10.1016/j.tibtech.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Vasir JK, Tambwekar K, Garg S. Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm. 2003;255(1–2):13–32. doi: 10.1016/S0378-5173(03)00087-5. [DOI] [PubMed] [Google Scholar]
- 89.Wagh VD, Patil PN, Surana SJ, Wagh KV. Forskolin: upcoming antiglaucoma molecule. J Postgrad Med. 2012;58(3):199. doi: 10.4103/0022-3859.101396. [DOI] [PubMed] [Google Scholar]
- 90.Wagner AH, Coffman AC, Ainscough BJ, Spies NC, Skidmore ZL, Campbell KM, Krysiak K, Pan D, McMichael JF, Eldred JM, Walker JR. DGIdb 2.0: mining clinically relevant drug–gene interactions. Nucleic Acids Res. 2016;44(D1):D1036–44. doi: 10.1093/nar/gkv1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wan Y, Xu L, Liu Z, Yang M, Jiang X, Zhang Q, Huang J. Utilising network pharmacology to explore the underlying mechanism of Wumei Pill in treating pancreatic neoplasms. BMC Complement Altern Med. 2019;19(1):1–2. doi: 10.1186/s12906-019-2580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z, Li J, Dang R, Liang L, Lin J. PhIN: a protein pharmacology interaction network database. CPT Pharmacomet Syst Pharmacol. 2015;4(3):160–6. doi: 10.1002/psp4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wangchuk P. Therapeutic applications of natural products in herbal medicines, biodiscovery programs, and biomedicine. J Biol Act Prod Nat. 2018;8(1):1–20. doi: 10.1080/22311866.2018.1426495. [DOI] [Google Scholar]
- 94.Wee JJ, Park KM, Chung AS. Biological activities of ginseng and its application to human health. In: Herbal medicine: biomolecular and clinical aspects. 2011 https://www.ncbi.nlm.nih.gov/books/NBK92776/. [PubMed]
- 95.Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J, Xi ST, Mu DZ, Du JB, Li GH, Wallenstein S, Sampson H. Efficacy and tolerability of antiasthma herbal medicine intervention in adult patients with moderate-severe allergic asthma. J Allergy Clin Immunol. 2005;116(3):517–524. doi: 10.1016/j.jaci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 96.Wu RM, Jiang B, Li H, Dang WZ, Bao WL, Li HD, Ye G, Shen X. A network pharmacology approach to discover action mechanisms of Yangxinshi Tablet for improving energy metabolism in chronic ischemic heart failure. J Ethnopharmacol. 2020;246:112227. doi: 10.1016/j.jep.2019.112227. [DOI] [PubMed] [Google Scholar]
- 97.Xuan XY, Wang YJ, Tian H, Pi JX, Sun SZ, Zhang WL. Study on prescription of self-microemulsifying drug delivery system of Mangiferin phospholipid complex. Zhong yao cai = Zhongyaocai = J Chin Med Mat. 2012;35(9):1508–11. [PubMed] [Google Scholar]
- 98.Yang H, Qin C, Li YH, Tao L, Zhou J, Yu CY, Xu F, Chen Z, Zhu F, Chen YZ. Therapeutic target database update 2016: enriched resource for bench to clinical drug target and targeted pathway information. Nucleic Acids Res. 2016;44(D1):D1069–D1074. doi: 10.1093/nar/gkv1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang M, Chen JL, Xu LW, Ji G. Navigating traditional Chinese medicine network pharmacology and computational tools. Evid Based Complement Altern Med. 2013 doi: 10.1155/2013/731969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yin SY, Efferth T, Jian FY, Chen YH, Liu CI, Wang AH, Chen YR, Hsiao PW, Yang NS. Immunogenicity of mammary tumor cells can be induced by shikonin via direct binding-interference with hnRNPA1. Oncotarget. 2016;7(28):43629. doi: 10.18632/oncotarget.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.You X, Wang R, Tang W, Li Y, He Z, Hu H, Wu C. Self-microemulsifying drug delivery system of patchoulic alcohol to improve oral bioavailability in rats. Zhongguo Zhong yao za zhi = Zhongguo Zhongyao Zazhi = China J Chin Mater Med. 2010;35(6):694–8. doi: 10.4268/cjcmm20100607. [DOI] [PubMed] [Google Scholar]
- 102.Zhang R, Zhu X, Bai H, Ning K. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol. 2019;10:123. doi: 10.3389/fphar.2019.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang RZ, Yu SJ, Bai H, Ning K. TCM-Mesh: the database and analytical system for network pharmacology analysis for TCM preparations. Sci Rep. 2017;7(1):1–4. doi: 10.1038/s41598-017-03039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng XS, Chan TF, Zhou HH. Genetic and genomic approaches to identify and study the targets of bioactive small molecules. Chem Biol. 2004;11(5):609–618. doi: 10.1016/j.chembiol.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 105.Zhou X, Liu H, Zhang M, Li C, Li G. Spectrum-effect relationship between UPLC fingerprints and anti-lung cancer effect of Panax ginseng. Phytochem Anal. 2021;32(3):339–346. doi: 10.1002/pca.2980de. [DOI] [PMC free article] [PubMed] [Google Scholar]