Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a severe life-threatening manifestation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection that often presents with acute cardiac dysfunction and cardiogenic shock. While recovery from acute illness is excellent, the long-term myocardial impact is unknown.
Objective
To compare cardiac MRI findings in children 6–9 months after their hospitalization with MIS-C against MRI findings in healthy controls to assess for residual myocardial disease.
Materials and methods
We prospectively performed cardiac MRI on 13 children 6–9 months following their hospitalization with MIS-C: eight of these children had a history of left ventricle ejection fraction (LVEF) < 50%, persistent symptoms, or electrocardiogram (ECG) abnormalities and underwent clinical MRI; five of these children without cardiac abnormalities during their hospitalization underwent research MRIs. We compared their native T1 and T2 mapping values with those of 20 normal controls.
Results
Cardiac MRI was performed at 13.6 years of age (interquartile range [IQR] 11.9–16.4 years) and 8.2 months (IQR 6.8–9.6 months) following hospitalization. Twelve children displayed normal ejection fraction: left ventricle (LV) 57.2%, IQR 56.1–58.4; right ventricle (RV) 53.1%, IQR 52.0–55.7. One had low–normal LVEF (52%). They had normal extracellular volume (ECV) and normal T2 and native T1 times compared to controls. There was no qualitative evidence of edema. One child had late gadolinium enhancement (LGE) with normal ejection fraction, no edema, and normal T1 and T2 times. When stratifying children who had MIS-C according to history of LVEF <55% on echocardiography, there was no difference in MRI values.
Conclusion
Although many children with MIS-C present acutely with cardiac dysfunction, residual myocardial damage 6–9 months afterward appears minimal. Long-term implications warrant further study.
Keywords: Children, Coronavirus disease 2019, Heart, Magnetic resonance imaging, Modified Look-Locker inversion recovery, Multisystem inflammatory syndrome in children, Severe acute respiratory syndrome coronavirus 2, T1 mapping, T2 mapping
Introduction
While children were relatively spared from severe acute disease secondary to the initial strains of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, some developed a severe hyperinflammatory process associated with SARS-CoV-2 infection, termed multisystem inflammatory syndrome in children (MIS-C) [1–4]. This syndrome was characterized by high fevers, elevated inflammatory markers and, in some, respiratory failure and significant cardiovascular compromise including vasodilatory shock, severe valve regurgitation, ventricular dysfunction and coronary artery dilatation [3]. Fortunately, most children recover rapidly from their acute presentation, with near-resolution of cardiac dysfunction and coronary dilatation by echocardiography by 4–9 months, as previously reported by our group [3].
While mid-term clinical and echocardiographic reports to date have been encouraging, echocardiography cannot characterize the myocardium for the presence of fibrosis or edema that might be associated with long-term cardiac dysfunction or poor outcomes. Considering the similar degrees of systemic inflammation and myocardial disease noted in other viral-induced myocarditis during initial hospitalization, evaluating for the presence of significant long-term myocardial involvement is necessary [5, 6]. Despite significant improvement in symptomatology and echocardiographic markers in children with MIS-C, understanding myocardial involvement and persistent myocardial inflammation could have implications toward exercise restrictions, returning to play, and long-term well-being. A joint American Heart Association and American College of Cardiology statement regarding competitive athletics recommends restricting sports participation until inflammation has subsided or at least 3–6 months following the diagnosis of myocarditis [5], and the American Academy of Pediatrics and others have used this statement to design guidelines for children following MIS-C diagnosis [6]. Limited data exist on the persistence of cardiac inflammation after MIS-C, and expert consensus to date has relied heavily on extrapolation from related conditions. A more nuanced understanding of long-term cardiac inflammation post MIS-C hospital discharge could aid in counseling children and families on return to sports and long-term implications.
Cardiac MRI is the gold-standard noninvasive tool for tissue characterization and is now the mainstay for diagnosing edema and fibrosis in viral-induced myocarditis [7, 8]. More recently, T1 and T2 mapping has been employed to identify and quantify diffuse fibrosis and edema [9–11]. T1 mapping has been shown to correlate with collagen volume fraction and extracellular matrix expansion as measured by histological samples [12–14]. Clinically, this has been correlated with severity of diastolic dysfunction among adults with heart failure with preserved ejection fraction, as well as all-cause mortality, and T1/T2 mapping has been incorporated into updated guidelines in the diagnosis of myocarditis [15, 16].
To date, cardiac MRI publications in children recovering from MIS-C are predominantly within the initial 3 months following hospitalization, and larger studies on mid-term outcomes have not incorporated quantitative markers of fibrosis or edema [17–22]. We performed a prospective cardiac MRI study of pediatric patients hospitalized with MIS-C, 6–9 months following hospitalization, versus healthy controls to assess for residual myocardial disease.
Materials and methods
Subject acquisition and consent
The institutional review board at Columbia University Irving Medical Center approved this study. We recruited children and adolescents < 18 years of age with a history of hospitalization for MIS-C at our institution, April 2020 and February 2021, and with no known prior cardiac disease, for participation in this study. In the spring of 2020, as the coronavirus disease 2019 (COVID-19) pandemic soared in New York City, our institution developed the Columbia University Interdisciplinary MIS-C Team and offered longitudinal follow-up by the Columbia University Interdisciplinary MIS-C Follow-up Program. All children meeting the Centers for Disease Control and Prevention definition of MIS-C were evaluated by the Interdisciplinary MIS-C Team and all suspected cases were independently adjudicated to confirm accurate diagnosis, as described [3]. Diagnosis, management and follow-up of MIS-C at our institution follow our interdisciplinary team protocols and have been described [1, 3].
Our institutional protocol recommended frequent clinical visits, electrocardiograms, echocardiograms and laboratory immunological evaluations. As these children all presented in the earliest months of the United States experience with COVID-19, limited data existed regarding the appropriateness or timing of cardiac MRIs. In consultation with others around the country, we determined that an MRI was clinically indicated for all children with history of ventricular dysfunction (LVEF <50%); residual cardiac symptoms including chest pain, palpitations or exercise intolerance; persistently elevated inflammatory markers; or electrocardiogram (ECG) changes/persistent dysrhythmia 3–9 months after presentation. Given concerns about the safety of staff and patients of semi-elective procedures in the earliest months of the pandemic and a necessary triaging of urgent MRIs, our patients were referred for cardiac MRI 6–9 months following hospitalization for MIS-C. For children undergoing clinical studies, clinicians discussed with subjects the existing literature on non-COVID myocarditis and their beliefs as to why the study was likely clinically indicated, and then the child, family and clinician jointly decided upon appropriateness for MRI referral. Families of all other children 10 years and older in the Interdisciplinary MIS-C Follow-up Clinic were approached for research MRI. This age cutoff was chosen to maximize the likelihood of a successful completion of the MRI exam without the need for sedation. We obtained informed consent and assent for all children. For children referred for a clinically indicated MRI, consent focused on privacy and use of medical record data. For children undergoing MRIs without a presumed clinical indication, consent included the risks of intravenous lines, gadolinium administration and privacy.
Cardiac magnetic resonance imaging acquisition
All studies were performed on a 1.5-tesla (T) Explorer MRI scanner (GE Healthcare Systems, Waukesha, WI). The cardiac MRI protocol included conventional balanced steady-state free precession (bSSFP) cine imaging in long- and short-axis planes using end-expiratory breath-holding or apnea based on anesthesia requirement. Cine imaging slice thickness was modified to obtain 10–12 images per short-axis stack. Pre-contrast T2 mapping was performed in basal, mid and apical short-axis slices using a breath-held or apnea motion-corrected turbo spin-echo sequence with a T2 preparatory pulse and bSSFP readout with the following typical parameters: echo times (TEs) of 11 ms, 41 ms, 71 ms, 100 ms; slice thickness, 8 mm; bandwidth, 780 Hz/pixel; pixel size, 2.2 × 2.2 mm; field of view (FOV), 350 mm; phase FOV, 70%; temporal resolution, 127 ms; repetition time (TR), 1×R-R interval; phase resolution, 160×160; acceleration factor of 2.
T2-weighted turbo spin-echo (TSE) with fat suppression in basal, mid and apical short-axis slices was acquired. Native T1 mapping was obtained using ECG-triggered modified Look-Locker inversion recovery (MOLLI) sequences in basal, mid and apical short-axis slices during diastole using a 5(3)3 sequence. Typical sequence parameters were: slice thickness, 8 mm; flip angle, 35°; echo time, 1.6 ms; repetition time (TR), 3.8 ms; bandwidth 1,250 Hz/pixel; pixel size, 2.2×2.2 mm; FOV, 350 mm; phase FOV, 70%; temporal resolution, 261 ms; phase resolution, 160×160; acceleration factor of 2.
Post-contrast MOLLI sequences were performed 15 ± 5 min following administration of 0.2 mmol/kg of gadobutrol. First-pass perfusion imaging was performed immediately following the administration of 0.2 mmol/kg of gadobutrol. Phase-sensitive inversion recovery imaging was performed in long- and short-axis planes to assess for late gadolinium enhancement (LGE). All static sequences except for first-pass perfusion were performed using breath-holding or apnea (based on anesthesia needs) at end-diastole. Hematocrit was determined by lab sample during intravenous (IV) line placement for calculation of extracellular volume (ECV).
Cardiac magnetic resonance imaging post-processing
Image post-processing was performed using Circle CVI42 (Circle Cardiovascular Imaging Inc., Alberta, CA). Left and right ventricular (LV and RV, respectively) end-diastole and end-systole endocardial and epicardial borders were traced to determine end-diastolic and end-systolic volumes (EDV and ESV, respectively) and myocardial mass. Ejection fraction (EF) was calculated by the equation ([EDV–ESV]/EDV)*100 and reported as percentages. Indexed EDV, ESV and masses were calculated by dividing by the body surface area (BSA). Volume and LV mass z-scores were generated using published data [23]. Z-scores of 2.0 to 3.5 were considered mildly enlarged, 3.5 to 5.0 were considered moderately enlarged, and >5.0 were considered severely enlarged.
We analyzed T2 and T1 mapping in the respective CVI42 modules. Endocardial and epicardial borders were traced with 10% offset to ensure that only myocardium was included in tracings. T2 and native T1 values (ms) are reported as the mean value for the given basal, mid-ventricular or apical slice.
We reviewed T2-W turbo spin-echo (TSE), first-pass perfusion and inversion recovery imaging to determine the presence of edema, perfusion defects and LGE, respectively. All imaging analyses were performed by a single pediatric cardiologist with extensive training and 5 years of experience in pediatric cardiac MRI (M.P.D.).
Controls
We retrospectively identified controls for native T1 and T2 mapping by reviewing the MRI database for children with structurally normal hearts undergoing cardiac MRI to evaluate coronary anatomy with normal coronary origins, ECG abnormalities or isolated premature ventricular contractions (PVCs)/premature atrial contractions (PACs) to rule out arrhythmogenic RV dysplasia with normal biventricular size and systolic function and no evidence of LGE. We included controls with cardiac MR imaging performed solely to assess normal scanner T2 and native T1 times per Society of Cardiovascular Magnetic Resonance (SCMR) recommendation with qualitatively normal biventricular size and function.
Statistical analysis
Descriptive statistics are presented as frequency counts and percentages for categorical variables, and mean ± standard deviation (SD) or median (interquartile range; IQR) for continuous variables, as appropriate. Based on the distribution of the data, we used Student’s t-test or Wilcoxon rank sum to compare T1 and T2 values between subjects and controls. We used Spearman rho or Pearson correlation coefficient to assess associations between T1, T2 and LVEF as compared to lowest LVEF by echocardiography during hospitalization. The two-sided statistical significance level was set at 0.05. Our study was powered to detect a 50-ms difference in T1 values and a 3.6-ms difference in T2 values. All data analyses were performed using STATA 14.1 (StataCorp, College Station, TX).
Results
Baseline data
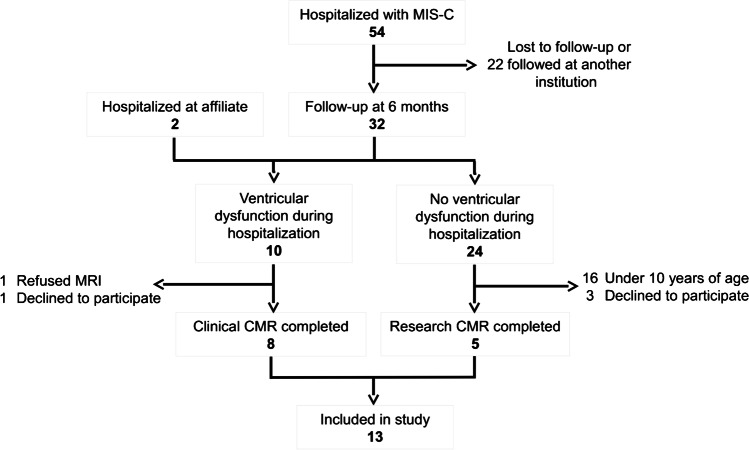
In total, 54 children were admitted to Morgan Stanley Children’s Hospital with MIS-C between April 2020 and February 2021 and were eligible for inclusion. Thirty-two were followed for at least 6 months. Eight of these had cardiac dysfunction during initial hospitalization and, therefore, met clinical criteria for cardiac MRI. Of these, seven underwent cardiac MRI at our institution. Of these seven, one family declined to participate in this research. Two additional children hospitalized at an affiliated institution were referred to our center for cardiac MRI by their primary cardiologist. One of these children had significant ventricular dysfunction at the time of hospitalization, and the other had moderate mitral regurgitation and high-degree heart block with an abnormal ECG on follow-up. Of the eight children with normal cardiac function on admission who were followed for more than 6 months in our MIS-C Longitudinal Follow-up Program and were eligible for enrollment (10 years or older), five agreed to participate (Fig. 1).
Fig. 1.
Patient flowchart. Fifty-four children were hospitalized at Morgan Stanley Children’s Hospital with multisystem inflammatory syndrome in children (MIS-C), with 12 ultimately undergoing cardiac MRI and 11 consenting for inclusion. Two additional children hospitalized at an affiliate institution underwent cardiac MRI and were included in the analysis. CMR cardiac magnetic resonance imaging
In total, 13 children were included in this analysis (eight clinical and five research) and underwent cardiac MRI. Two children (15%) required anesthesia with endotracheal intubation for MRI. Of the children who underwent MRI, 9 (69%) were boys. The median age at hospitalization was 13.1 years (IQR 11.3–15.0), with a median length of hospital stay of 5 days (IQR 4–6 days). Four children (31%) were polymerase chain reaction (PCR)-positive for COVID-19 during hospitalization and all children had positive immunoglobulin G (IgG) antibodies for COVID-19. Nine (69%) children required intensive care unit admission. Five (41%) children required inotropic support and 5 (41%) required respiratory support, including intubation in one child. All children received intravenous immunoglobulin, aspirin and pulse steroids during the acute presentation, with a steroid taper following discharge. LV dysfunction and coronary artery dilation or prominence was present in the majority (69% and 77%, respectively). Two children had residual ventricular dysfunction on discharge ECG, which resolved by 3 months following discharge. Demographics and hospitalization data are described in Table 1.
Table 1.
Baseline characteristics and demographics for children with multisystem inflammatory syndrome in children (MIS-C) who underwent cardiac MRI (n = 13)
| Median (IQR) or frequency (%)a | Range | |
|---|---|---|
| Gender, male | 9 (69) | |
| Race | ||
|
White Black/African American Other Declined to say |
2 (15) 5 (38) 3 (23) 3 (23) |
|
| Ethnicity | ||
|
Hispanic/Latino Not Hispanic/Latino Declined to say |
6 (46) 5 (38) 2 (15) |
|
| Age at hospitalization, years | 13.1 (11.3–15.0) | 5.2–16.2 |
| Cardiac MRI indicationsb | ||
|
Diminished LVEF Heart block Research |
7 (54) 1 (8) 5 (38) |
|
| Time from hospitalization to MRI, months | 8.2 (6.8–9.6) | |
| Length of hospitalization, days | 5 (4–6) | 3–32 |
| Symptoms at presentation (n = 12)c | ||
|
Fever Shortness of breath Cough Nausea/vomiting Diarrhea Rash Malaise Conjunctivitis Headache |
12 (100) 6 (50) 2 (17) 11 (92) 10 (83) 8 (67) 4 (33) 4 (33) 5 (42) |
|
| Intensive care unit stay | 9 (69) | |
| Inotropic support | 5 (38) | |
| Respiratory support | ||
|
Intubation CPAP/BiPAP or HFNC |
1 (8) 4 (31) |
|
| LV function (n = 12) | ||
|
Normal Mildly diminished Moderately diminished |
5 (42) 5 (42) 2 (17) |
|
| Coronary dilation/prominence | 10 (77) | |
| Mitral regurgitation | ||
|
Trivial Mild Mild to moderate Moderate Severe |
7 (54) 2 (15) 1 (8) 2 (15) 1 (8) |
|
| Tricuspid regurgitation | ||
|
Trivial Mild Mild to moderate Moderate Severe |
5 (38) 4 (31) 2 (15) 1 (8) 1 (8) |
|
BiPAP bilevel positive airway pressure, CPAP continuous positive airway pressure, HFNC high-flow nasal cannula, IQR interquartile range, LV left ventricle, LVEF left ventricular ejection fraction, MRI magnetic resonance imaging
a Data presented as median (IQR) or frequency (%), as appropriate
b Echo parameters are qualitative based on echo report
c Symptoms based on available report
Cardiac magnetic resonance imaging data
Cardiac MRIs were performed at a median of 8.2 months (IQR 6.8–9.6) months post hospital discharge and at a median age of 15.3 years (IQR 11.9–16.4). Twelve children (93%) had follow-up ECG around the time of cardiac MRI at a median of 6.4 (IQR 5.9–7.4) months following hospitalization. By ECG, 11 children had normal biventricular function, and 1 child had mildly diminished biventricular function. All children were asymptomatic and had normal ventricular size with no evidence of significant mitral or tricuspid regurgitation.
On cardiac MRI, children overall had normal LVEF (57.2%, IQR 56.1–58.4%) and right ventricular ejection fraction (RVEF) (53.1%, IQR 52.0–55.7%). Children overall had normal biventricular size and LV mass based on indexed ventricular size and mass and z-score (Table 2). One clinical case with ventricular dysfunction during hospitalization had low normal LVEF (52%) with qualitatively diminished apical wall motion relative to mid-ventricular and basal motion, but otherwise normal ventricular size and mass, and no evidence of perfusion defects, LGE or edema. Two children had mild LV dilation (z-score + 2.2 and + 2.8) with overall normal LV and RVEF and no evidence of edema or fibrosis. T1 and T2 times in these children were normal.
Table 2.
Cardiac MRI data
| Subjects (n = 13)a | Controls (n = 20)a | P-valueb | |
|---|---|---|---|
| Age at cardiac MRI, years | 15.3 (11.9–16.4) | 17.5 (16–32.5) | 0.01 |
| LVEF, % | 57 (56–58) | ||
| RVEF, % | 53 (52–56) | ||
| LVEDV, mL | 139 (128.1–191.3) | ||
| Indexed LVEDV, mL/m2 | 84.2 (76.5–97.7) | ||
| LVEDV z-score | –0.4 (–1.0–0.9) | ||
| LV mass, g | 64.7 (51.9–78) | ||
| Indexed LV mass, g/m2 | 41.9 (36.7–43.5) | ||
| RVEDV, mL | 147.4 (130.1–188.7) | ||
| Indexed RVEDV, mL/m2 | 91 (77.6–103.6) | ||
| RVEDV z-score | –1.0 (–2.0–0.4) | ||
| Native T1, ms | |||
| Base | 1,032 (984–1062) | 997 (972–1042) | 0.61 |
| Mid | 1,000 (984–1035) | 981 (954–1014) | 0.22 |
| Apex | 1,024 (983–1102) | 1,013 (996–1074) | 0.92 |
| Global | 1,010 (991–1051) | 997 (963–1048) | 0.42 |
| T2 mapping, ms | |||
| Base | 49.6 (47.4–51.7) | 51.0 (48.0–54.2) | 0.53 |
| Mid | 49.2 (45.9–51.1) | 50.2 (47.0–52.7) | 0.63 |
| Apex | 52.7 (51.0–55.8) | 54.0 (51.8–57) | 0.32 |
| ECV, % | |||
| Base (n = 8) | 24 (23–25) | ||
| Mid (n = 10) | 24 (21–28) | ||
| Apex (n = 7) | 24 (22–32) | ||
| Global (n = 8) | 22.5 (21.5–26.5) | ||
ECV extracellular volume, LV left ventricle, LVEDV left ventricular end-diastolic volume, LVEF left ventricular ejection fraction, MRI magnetic resonance imaging, RVEDV right ventricular end-diastolic volume, RVEF right ventricular ejection fraction
a Data presented as median (IQR)
b P < 0.05 is significant (bold)
There was no evidence of edema based on T2-W TSE imaging, and no perfusion defects were noted on first-pass perfusion. One clinical case — different from the aforementioned — had mid-myocardial LGE at the inferior insertion point and mid-ventricular inferolateral region, with normal ventricular function, no evidence of edema or perfusion defects, and normal T1 and T2 times. This child had a history of moderate LV dysfunction by echocardiography, with an LVEF of 44%. Characteristics of the two children with diminished function and LGE are presented in Table 3.
Table 3.
Characteristics of children with left ventricular dysfunction or late gadolinium enhancement (LGE) on cardiac MRI
| Subject | Age in years (gender) at CMR |
Time from admission to CMR (mo) | Admission LV function | Time to EF recovery | Length of hospitalization | ICU? | CMR LVEF | CMR RVEF | LGE location | Edema | ECV | Global native T1 | Perfusion defects? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15.4 (M) | 9.7 | Moderately diminished | 8 days | 9 days | No | 52% | 53% | None | None | 22% | 1,047 | None |
| 2 | 6.1 (M) | 11.5 | Moderately diminished | 4 days | 5 days | Yes | 57% | 50% | Inferior insertion, mid-ventricular inferolateral wall | None | 23% | 981 | None |
CMR cardiac magnetic resonance imaging, ECV extracellular volume, EF ejection fraction, ICU intensive care unit, LV left ventricle, LVEF left ventricular ejection fraction, M male, mo months, RVEF right ventricular ejection fraction
Mid-ventricular ECV was calculable in 10 children, basal and global ECV in 8, and apical ECV in 7. The remaining children had significant motion artifact or motion between the native and post-contrast images, precluding accurate ECV polar map generation. T2, native T1 and ECV are presented in Table 2. Subjects were younger than controls at time of cardiac MRI: 15.3 (IQR 11.9–16.4) years vs. 17.5 (16–32.5) years; P = 0.01. There was no difference in T2 or native T1 time between subjects and controls (Table 2; Figs. 2 and 3).
Fig. 2.

Native T1 values in subjects vs. controls. Violin plot demonstrates native T1 values in subjects as compared to controls in basal, mid-ventricle, apical and global short-axis slices. Subjects (n = 13) are in black, controls (n = 20) in white. The median, interquartile ranges and 1.5 times the interquartile ranges are represented by the dots, thick lines and thin lines, respectively. Relative thicknesses of the individual plots represent the relative frequencies of each value. Separate plots represent outliers. All subject-to-control comparisons are statistically insignificant
Fig. 3.

T2 values in subjects vs. controls. Violin plot demonstrates T2 values in subjects as compared to controls in basal, mid-ventricle and apical short-axis slices. Subjects (n = 13) are in black, controls (n = 15) in white. The median, interquartile ranges and 1.5 times the interquartile ranges are represented by the dots, thick lines and thin lines, respectively. Relative thicknesses of the individual plots represent the relative frequencies of each value. Separate plots represent outliers. All subject-to-control comparisons are statistically insignificant
When stratifying subjects by ventricular function during initial admission, LVEF and left ventricular end-diastolic volume (LVEDV) medians on cardiac MRI were marginally lower but not statistically different among those with and without a history of ventricular dysfunction (56% vs. 59%, P = 0.06; 129 mL vs. 199 mL, P = 0.05, respectively). There was no difference in indexed ventricular volumes, RVEF, or T1 and T2 mapping values (Table 4). Children with a history of low LVEF on admission ECG tended to be younger (P = 0.03). Among the 11 children with available LVEF on ECG during admission, there was no association between the lowest available EF and LVEF by cardiac MRI (Spearman rho 0.42, P = 0.2).
Table 4.
Cardiac MRI data among children with a history of left ventricle (LV) dysfunction vs. normal LV function during hospitalization
| Parameter | Low LVEF (n = 7)a | Normal LVEF (n = 6)a | P-valueb |
|---|---|---|---|
| Age at cardiac MRI, years | 11.9 (6.1–15.3) | 16.2 (13.1–16.7) | 0.03 |
| LVEF, % | 56 (55–58) | 59 (57–61) | 0.06 |
| RVEF, % | 53 (52–54) | 55 (51–59) | 0.39 |
| LVEDV, mL | 128.9 (81–153) | 199.2 (139–212.8) | 0.05 |
| Indexed LVEDV, mL/m2 | 84.2 (72.3–93.5) | 90.8 (80.1–116.4) | 0.39 |
| LV mass, g | 61.0 (44.4–68.6) | 76.2 (51.9–96.5) | 0.20 |
| Indexed LV mass, g/m2 | 41.3 (36.7–42.4) | 42.9 (31.4–45.2) | 0.78 |
| RVEDV, mL | 140.1 (86.3–149.4) | 207.0 (130.1–235.7) | 0.12 |
| Indexed RVEDV, mL/m2 | 88.2 (77.6–92.3) | 99.8 (74.9–114.8) | 0.32 |
| Native T1, ms | |||
| Base | 1,032 (975–1,036) | 1,028.5 (993–1,090) | 0.63 |
| Mid | 988 (977–1,019) | 1,020 (996–1,043) | 0.20 |
| Global | 1,002 (990–1,047) | 1,022 (993–1,083) | 0.52 |
| T2 mapping, ms | |||
| Base | 49.6 (46.2–53.7) | 49.6 (48.4–51.6) | 0.89 |
| Mid | 48.1 (45.6–52.5) | 49.9 (48.1–51.1) | 0.62 |
| Apex | 52.7 (51–55.7) | 53.7 (51–56.6) | 0.75 |
| ECV, % | |||
| Base (n = 8) | 25 (20.5–26.0) | 23.5 (23–24) | 0.37 |
| Mid (n = 10) | 21 (21–27.4) | 26 (22–28) | 0.60 |
| Global (n = 8) | 22.5 (21–26) | 23.5 (21.5–26.5) | 0.88 |
ECV extracellular volume, LV left ventricle, LVEDV left ventricular end-diastolic volume, LVEF left ventricular ejection fraction, MRI magnetic resonance imaging, RVEDV right ventricular end-diastolic volume, RVEF right ventricular ejection fraction
a Data presented as median (IQR)
b P < 0.05 is significant (bold)
Discussion
The emergence of SARS-CoV-2 has resulted in significant morbidity and mortality across the globe. We are only just starting to ascertain the long-term impact that this new virus will have on human health. Acute cardiac dysfunction is a prominent and critical manifestation for children who develop MIS-C following infection with SARS-CoV-2. Whether acute cardiac dysfunction during MIS-C ultimately results in permanent cardiac dysfunction or persistent inflammation as seen in other viral-induced myocarditis syndromes remains undefined. Determining the presence of cardiac inflammation and myocardial damage has significant implications toward counseling because residual inflammation can necessitate restrictions from sports participation until such inflammation has resolved. Improving our understanding of the potential lifelong impact that MIS-C has on children’s cardiac health is therefore essential to our ability to guide families and determine treatment plans.
We report on the mid-term cardiac MRI findings in children 6–9 months following hospitalization for MIS-C. Subjects overall had normal ventricular size and systolic function, with LV dysfunction present in only one child. We found no evidence of edema, and T1 and T2 mapping values were normal as compared to controls. LGE was present in only one child, with no evidence of perfusion defects and overall normal T1 times in this child. Importantly, we found normal ejection fraction among the seven children with a history of ventricular dysfunction.
Our research group previously reported on 6-month echocardiographic findings in this population and demonstrated excellent recovery by ECG in almost all children [3]. While ECG is a valuable tool to assess global ventricular function, cardiac MRI remains the gold standard for assessing myocardial tissue characterization [9]. Furthermore, LVEF by ECG requires numerous assumptions on LV geometry, and assessment of RV function is predominantly qualitative; cardiac MRI allows for a more granular calculation of EF.
Early cardiac MRI data among children hospitalized with MIS-C has been reported, with variable degrees of edema and LGE, as well as diminished LV strain in those with LV dysfunction [17, 20, 22]. These findings are consistent with our prior data demonstrating significant functional impairment in many children with MIS-C [1, 3]. Our cardiac MRI cohort demonstrated a similar degree of dysfunction during hospitalization, with almost half of our subjects having had LV dysfunction by echocardiography.
Webster et al. [18] reported on early term outcomes among children with a history of symptomatic COVID-19 at 1–3 months after presentation, including six subjects with MIS-C. They demonstrated globally normal ventricular function and T1 and T2 mapping values by cardiac MRI among the six children with MIS-C, though prior cardiac dysfunction in their cohort was isolated to two subjects with a history of MIS-C. Similarly, Capone et al. [19] demonstrated normal LV systolic function and no evidence of edema or fibrosis among 11 subjects up to 8 weeks following initial hospitalization for MIS-C. Our findings are consistent with theirs and further reinforce the narrative that mid-term outcomes are overall excellent with minimal residual myocardial disease by cardiac MRI.
While we have supported evidence that residual myocardial disease and systolic dysfunction are minimal, evaluation of diastolic dysfunction was beyond the scope of this manuscript. It is therefore plausible that diastolic dysfunction is present, particularly in children with borderline LV systolic dysfunction and scarring. The lack of symptoms in these subjects would suggest that, even if present, diastolic dysfunction is likely minimal. However, incorporation of diastolic function parameters in clinical ECGs for these children, and monitoring for the development of diastolic dysfunction in the long term, might be warranted.
Beyond presenting prospective data further from hospitalization, our study is novel in that we incorporated the use of T1 and T2 mapping for myocardial tissue characterization in a cohort exclusively composed of an MIS-C population. Parametric mapping is quickly becoming a standard of care in the evaluation of myocardial edema and diffuse fibrosis, and it has recently been incorporated as part of revised Lake Louise criteria in the evaluation of myocarditis [7]. Webster et al. [18] evaluated native T1 and T2 values in pediatric patients with symptomatic COVID-19; however, they did not administer contrast agent in their cohort and therefore were unable to assess for LGE and could not calculate ECV, which is less susceptible to age, gender and temperature variation than native T1. While Barris et al. [21] reported on mid-term cardiac MRI findings in children hospitalized with MIS-C, they did not perform T1 and T2 mapping; evaluation of edema and fibrosis was therefore limited to a qualitative analysis. Furthermore, while LGE can identify focal or replacement fibrosis, it is unable to identify diffuse fibrosis [11, 18, 21].
This study has several limitations. Our sample size was limited by an a priori decision to only obtain clinical cardiac MRI in children who had evidence of cardiac dysfunction during initial presentation and also by few subjects being willing to consent to a research cardiac MRI with contrast administration. Therefore, we are likely underpowered to detect differences in functional and myocardial tissue characterization parameters. It is therefore plausible that differences in myocardial tissue characterization between subjects and controls exist, so our conclusions must be taken in context of this small subject pool. However, our conclusions remain in line with the available literature to conclude that children with MIS-C do not have persistent myocardial tissue abnormalities. Furthermore, even if statistically significant, any differences in values between controls and subjects would likely not be clinically relevant. Furthermore, for ethical concerns, we excluded children younger than 10 from research cardiac MRIs and, as a result, among the MIS-C cohort, children with initial cardiac dysfunction, residual cardiac symptoms (i.e. chest pain, palpitations or exercise intolerance), persistently elevated inflammatory markers, or ECG changes/persistent dysrhythmia (clinical indication for cardiac MRI) were by default younger than those recruited as research subjects and controls. Therefore, we would not recommend inferring information about the effects of age from this study. That said, our sample is biased to those with initial cardiac dysfunction, and therefore would be biased to those most at risk. Because of concern for the safety of MRI staff and the limited resources at the time of this study, cardiac MRI was not performed during initial hospitalization. We therefore are unable to compare function in our cohort with baseline LVEF by MRI. Furthermore, our evaluation was limited to systolic function because assessment of diastolic function by cardiac MRI is not validated or readily available. Finally, control data were available for native T1 and T2 values as recommended by SCMR to obtain local references [11]; because control data were based on subjects referred for clinical MRIs, it is possible that myocardial tissue abnormalities were present. However, we ensured that all subjects used as controls had normal biventricular size and systolic function with no evidence of identifiable congenital heart disease or systemic diseases. Therefore, the likelihood of myocardial abnormalities is minimal. Given that the availability of normal control myocardial T1 data were limited, we could not age-match in this study, and therefore it is possible that lack of differences between controls and subjects was a result of an age-dependent phenomenon. Furthermore, normal controls were not available for ECV because of the need for contrast administration. While comparisons to published reference values have been deemed acceptable, the availability of published data in children is limited to small single-center cohorts [1, 10].
Conclusion
Our study demonstrates overall excellent midterm cardiac outcomes among this small cohort of children with MIS-C. Normal ventricular function was nearly universal with minimal evidence of residual myocardial disease in our cohort. Long-term, larger-scale studies are needed to ensure complete recovery in those with residual disease and to identify whether any children remain at risk for long-term myocardial disease and dysfunction after MIS-C.
Acknowledgments
Research reported in this publication was supported, in part, by the National Institutes of Health National Heart, Lung and Blood Institute grant numbers K23AI141686 (J.D.M.) and K23HL133454 (B.R.A.), U01 AI100119-09S1 (D.L.F.) and by Genentech ML42866 (M.P.D., B.R.A., K.M.F.).
Declarations
Conflicts of interest
None
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 3.Farooqi KM, Chan A, Weller RJ, et al. Longitudinal outcomes for multisystem inflammatory syndrome in children. Pediatrics. 2021;148:e2021051155. doi: 10.1542/peds.2021-051155. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Health Alert Network (2020) Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). CDC. https://emergency.cdc.gov/han/2020/han00432.asp. Accessed 13 Oct 2021
- 5.Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis. Circulation. 2015;132:e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 6.Dean PN, Jackson LB, Paridon SM(2020) Returning to play after coronavirus infection: pediatric cardiologists’ perspective. American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2020/07/13/13/37/returning-to-play-after-coronavirus-infection. Accessed 7 Jan 2022
- 7.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 8.Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. 2021;144:e123–e135. doi: 10.1161/CIR.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 mapping. JACC Cardiovasc Imaging. 2016;9:67–81. doi: 10.1016/j.jcmg.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Riesenkampff E, Messroghli DR, Redington AN, Grosse-Wortmann L. Myocardial T1 mapping in pediatric and congenital heart disease. Circ Cardiovasc Imaging. 2015;8:e002504. doi: 10.1161/CIRCIMAGING.114.002504. [DOI] [PubMed] [Google Scholar]
- 11.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (Scardiac MRI) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 13.Kehr E, Sono M, Chugh SS, Jerosch-Herold M. Gadolinium-enhanced magnetic resonance imaging for detection and quantification of fibrosis in human myocardium in vitro. Int J Cardiovasc Imaging. 2007;24:61–68. doi: 10.1007/s10554-007-9223-y. [DOI] [PubMed] [Google Scholar]
- 14.Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (Scardiac MRI) and cardiac MRI working group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller CA, Naish JH, Bishop P, et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–383. doi: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 16.Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirico D, Basso A, Reffo E, et al. Early echocardiographic and cardiac MRI findings in multisystem inflammatory syndrome in children. J Clin Med. 2021;10:3360. doi: 10.3390/jcm10153360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster G, Patel AB, Carr MR, et al. Cardiovascular magnetic resonance imaging in children after recovery from symptomatic COVID-19 or MIS-C: a prospective study. J Cardiovasc Magn Reson. 2021;23:86. doi: 10.1186/s12968-021-00786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capone CA, Misra N, Ganigara M, et al. Six month follow-up of patients with multi-system inflammatory syndrome in children. Pediatrics. 2021;148:e2021050973. doi: 10.1542/peds.2021-050973. [DOI] [PubMed] [Google Scholar]
- 20.Theocharis P, Wong J, Pushparajah K, et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2021;22:896–903. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barris DM, Keelan J, Ahluwalia N, et al. Midterm outcomes and cardiac magnetic resonance imaging following multisystem inflammatory syndrome in children. J Pediatr. 2022;241:237–241. doi: 10.1016/j.jpeds.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Aeschlimann FA, Misra N, Hussein T, et al. Myocardial involvement in children with post-COVID multisystem inflammatory syndrome: a cardiovascular magnetic resonance based multicenter international study — the CARDOVID registry. J Cardiovasc Magn Reson. 2021;23:140. doi: 10.1186/s12968-021-00841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buechel E, Kaiser T, Jackson C, et al. Normal right- and left ventricular volumes and myocardial mass in children measured by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:19. doi: 10.1186/1532-429X-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]