Abstract
Understanding the historical emergence and growth of long-range fisheries can provide fundamental insights into the timing of ecological impacts and the development of coastal communities during the last millennium. Whole-genome sequencing approaches can improve such understanding by determining the origin of archaeological fish specimens that may have been obtained from historic trade or distant water. Here, we used genome-wide data to individually infer the biological source of 37 ancient Atlantic cod specimens (ca 1050–1950 CE) from England and Spain. Our findings provide novel genetic evidence that eleventh- to twelfth-century specimens from London were predominantly obtained from nearby populations, while thirteenth- to fourteenth-century specimens were derived from distant sources. Our results further suggest that Icelandic cod was indeed exported to London earlier than previously reported. Our observations confirm the chronology and geography of the trans-Atlantic cod trade from Newfoundland to Spain starting by the early sixteenth century. Our findings demonstrate the utility of whole-genome sequencing and ancient DNA approaches to describe the globalization of marine fisheries and increase our understanding regarding the extent of the North Atlantic fish trade and long-range fisheries in medieval and early modern times.
Keywords: cod trade, historical ecology, marine fisheries, zooarchaeology, biological source, genomics
1. Introduction
The expansion of long-range fish trade, not least of Atlantic cod (Gadus morhua), has partly driven the development of urbanized market economies across European societies during the last millennium [1–3]. The importance of this trade is well documented by historical sources from the fourteenth century and can be glimpsed in anecdotal historical records and archaeological evidence from the late eleventh, twelfth and thirteenth centuries [4,5].
Ancient DNA (aDNA) and stable isotopes have previously shown the early transport of air-dried Arctic Norwegian cod (stockfish) to Haithabu in Germany by ca 1066 CE [6,7]. This exchange developed into a major and wide-ranging Atlantic cod trade across medieval northern Europe, linking towns in Scandinavia, Germany, England and the Low Countries (e.g. Bergen, Lübeck, King's Lynn, London and Deventer) [6–8]. In the Iberian Peninsula, the northern ports were developed as strategic trading posts for receiving and distributing luxury and foreign products from both the Mediterranean and northern Europe [9,10]. As a consequence, distant-water fisheries and fish trade along the Atlantic coast, from Sevilla to western Ireland and Flanders, started to receive more interest within the Iberian market [11,12]. Subsequently, post-medieval European expansion to the western Atlantic, especially to Newfoundland, linked the above-mentioned northern and Iberian networks into competing and sometimes complementary long-range fisheries that were sources of both food and wealth [1,13]. For example, seventeenth-century English catches from Newfoundland were often traded to southern Europe, in an economically significant triangular trade that also entailed salt and wine [14].
Tracing the origin of Atlantic cod specimens harvested for these medieval and post-medieval trade networks contributes to our understanding of economic history and historical ecology. Historical and archaeological sources have revealed the extension of distant-water fisheries and trading networks through time and space [8,13]. However, the geographical and biological resolution of text-based and archaeological sources is often limited, and the level of detail in such sources often decreases with time depth [15]. Determining the biological origin of archaeological bone assemblages of species such as Atlantic cod can therefore provide important information about the populations targeted through distant-water fishing and/or trade. Since archaeological cod bones can represent local or long-distance (even intercontinental) fishing, it is important to distinguish between source populations. Thus, there has been an increased interest in the use of aDNA and stable isotope methods to identify the origin of archaeological remains to trace the development of the globalization of marine fisheries [6,16–20]. Here, we use novel whole-genome aDNA approaches to greatly improve the spatial specificity and resolution regarding the inference of source populations of archaeological Atlantic cod bones [21].
We assess the biological origin of 37 Atlantic cod specimens from medieval England (London) and post-medieval Spain (Barcelona, Álava (Castillo de Labastida), Madrid and Sevilla) using low-coverage genome-wide data. We genetically assign such specimens according to patterns of spatial genome-wide differentiation among modern populations of Atlantic cod [22–25]. We specifically investigated significant differentiation in polymorphic chromosomal inversions (i.e. LG1, LG2, LG7 and LG12) [6,26,27] that are associated with migratory behaviour and temperature clines [22,27–30]. Their genetic differentiation can therefore indicate the assignment of specimens towards a particular geographical area [6,31]. Through these methods, we aim to distinguish source populations with improved discriminating power in relation to previous stable isotope and aDNA approaches [6,15,16].
2. Materials and methods
(a) . Sample collection
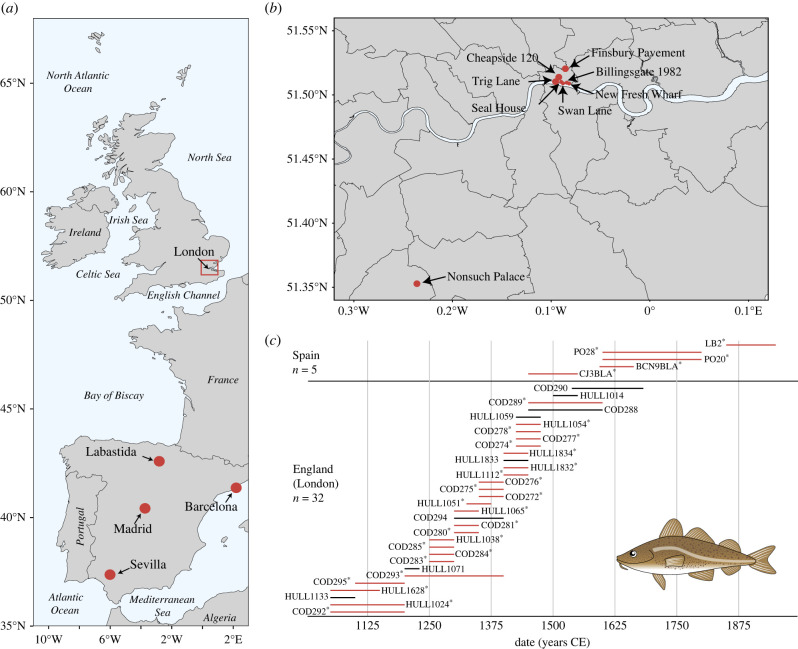
English samples (n = 32) were obtained from eight archaeological locations in London (figure 1b; electronic supplementary material, table S1). Based on archaeological evidence and stable isotope analysis, three of the locations (Finsbury Pavement, Seal House and Trig Lane) have previously been inferred to have imported preserved cod [16]. Specimens from the additional five locations (Billingsgate 1982, Cheapside 120, New Fresh Wharf, Nonsuch Palace and Swan Lane) were included to provide a continuous fishing time series from the eleventh to sixteenth–seventeenth centuries CE (figure 1c). Seven of the archaeological sites in London were urban when occupied. The eighth location (Nonsuch Palace) was a royal residence originally outside London, which was later surrounded by the modern metropolis. Spanish samples (n = 5) were obtained from four different archaeological locations: a monastic-upper class context from La Cartuja (Sevilla, late fifteenth–early sixteenth centuries), an urban context from Plaza de Oriente (Madrid, seventeenth–eighteenth centuries), a context from the fishermen's quarter from Barraques de pescadors (Barcelona; ca seventeenth century) and a rural castle (Álava) that acted as a military centre during the nineteenth century (JA Quirós 2022, personal communication; figure 1a; electronic supplementary material, table S1). Atlantic cod bones are very rare in Iberian archaeological sites [12]; therefore, the present five Spanish specimens represent those available for this study.
Figure 1.
(a) Distribution of archaeological Atlantic cod specimens in England (London) and Spain. Spanish locations are highlighted (in red) on the map. (b) Detailed distribution (in red) of English archaeological locations in London from which Atlantic cod bones were obtained. (c) Date range of the 37 archaeological specimens as estimated based on archaeological context. Samples in red and with ‘asterisk’ (*) yielded sufficient data to allow more detailed genomic assignments. Other samples could only be assigned to major geographical regions (see Results section for explanation). For details regarding the sample codes, see electronic supplementary material, table S1. Fish illustration was drawn by Lourdes Martínez-García. (Online version in colour.)
Cranial (articular, premaxilla, frontal, dentary and parasphenoid) and postcranial (vertebra and cleithrum) bones were included (electronic supplementary material, table S1 and figure S2). Cranial bones are more likely to represent local fishing because many preserved fish products were decapitated [32], although, complete fish (and/or preserved fish heads) were sometimes traded over long distances [6,33,34]. Cleithra (which support the pectoral fin just behind the cranium) can be found together with cranial remains, or (if fish were decapitated anterior to this element) with postcranial bones [15]. Here, we considered cleithra belonging among the postcranial bones.
After field collection, all samples were stored dry and unfrozen. Dating of the samples was based on archaeological context. Qualitative date ranges were converted into calendar years as per Orton et al. [15] considering an ‘early’ century the first half of that century (e.g. ‘00 to ‘50), ‘mid’ century as ‘25 to ‘75 and ‘late’ century as the second half of that century (e.g. ‘50 to ‘00). The archaeological Atlantic cod samples were morphologically and genetically identified to species.
(b) . aDNA extraction and library preparation
We processed 18 English (London) fish-bone samples in the aDNA laboratory at the University of Oslo [35,36] (electronic supplementary material, table S1). Treatment of samples prior to DNA extraction was according to Ferrari et al. [37] and Martínez-García et al. [38]. In short, fish bones were UV treated for 10 min per side and milled using a stainless-steel mortar [39]. Milled fish-bone powder was divided in two aliquots per specimen (150–200 mg per aliquot) as starting material for DNA extraction. Genomic DNA was extracted from the fish-bone samples using the mild Bleach treatment and Double-Digestion step (BleDD) protocol [40]. In addition, we added to our initial London assemblage aDNA from 14 English fish-bone samples previously processed at the University of Hull following the protocols in Hutchinson et al. [19] (electronic supplementary material, table S1). Three out of the 14 samples were previously inferred to have a southern and central North Sea biological origin (electronic supplementary material, table S1) [19]. Furthermore, we analysed aDNA from five Spanish bone samples previously analysed and processed using a modified protocol of Yang et al. [41] at BioArch, University of York. In short, samples were decontaminated with 6% sodium hypochlorite (bleach) for 5 min and then rinsed three times in distilled water. Samples were further UV treated for 10 to 20 min per side. Samples were powdered prior to the addition of a lysis buffer (EDTA) and Proteinase K. Samples were incubated overnight at 50°C while kept in rotation. After incubation, samples were centrifuged to separate bone powder from buffer solution. The supernatant was transferred to an Amicon Ultra-4, Centrifugal Filter Device, 10 000 NMWL tube to concentrate the solution and the Qiagen QiaQuick MinElute™ kit was used for DNA purification. Contamination controls were taken during every step of the extraction and amplification procedure.
Double-indexed blunt-end sequencing libraries were built from 16 or 20 µl of DNA extract from all samples using the double-stranded Meyer-Kircher protocol [42,43] with the modifications listed in Schroeder et al. [44] or the single-stranded Santa Cruz Reaction (SCR) protocol using tier four adapter dilutions [45] (see electronic supplementary material, table S1 for specifications). Multiple extraction and negative controls during all library sessions were used to detect possible contamination. All samples were assessed for library quality and concentration using a high-sensitivity DNA Assay on the Bioanalyzer 2100 (Agilent) or with a high-sensitivity NGS Fragment Analysis Kit on the Fragment AnalyzerTM (Advanced Analytical). Successful libraries were sequenced using the Illumina HiSeq 4000 with 150 bp paired reads, or on a Novaseq 6000 with 150 bp paired reads at the Norwegian Sequencing Centre. Sequencing reads were demultiplexed allowing zero mismatches in the index tag and they were processed using PALEOMIX v1.2.13 [46]. Trimming of residual adapter contamination, filtering and collapsing of reads was done using AdapterRemoval v.2.1.7 [47]. Mapping of remaining reads was performed against the gadMor2 reference genome [48,49] using BWA v.0.7.12 [50] with the backtrack algorithm, disabled seeding and minimum quality score of 25. aDNA deamination patterns were determined using MapDamage v.2.0.9 [51] and BAM files were indexed with samtools v.1.9 [52].
(c) . Genomic and statistical analysis
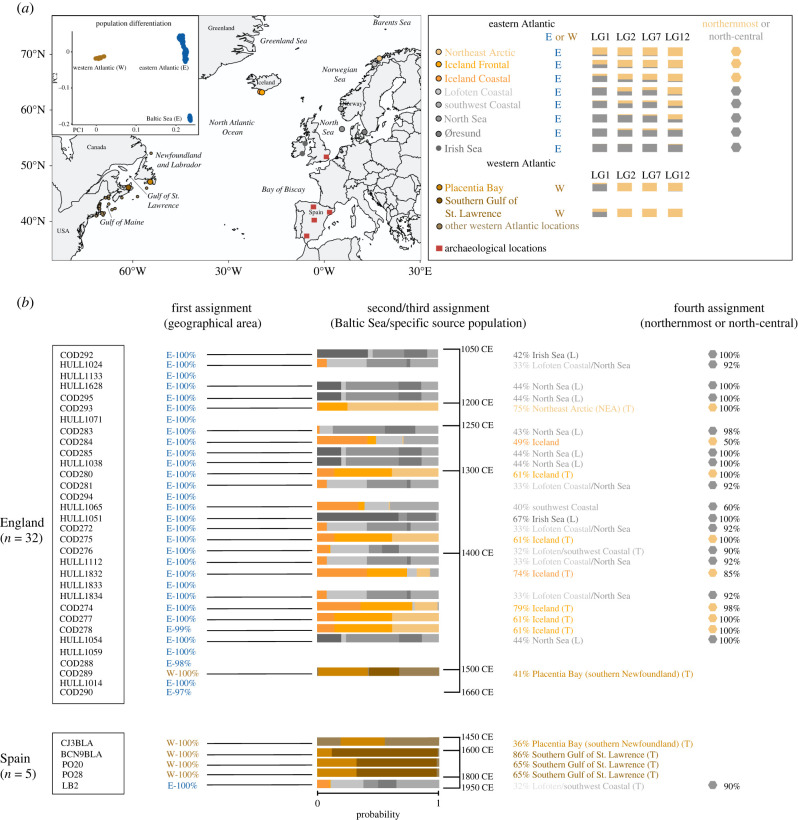
To infer the biological origin (source population) of the archaeological samples of Atlantic cod, we followed a hierarchical procedure (figure 2). First, we used BAMscorer, a software that can assign low-coverage sequences to biological populations following the methodology described in Ferrari et al. [21]. This approach creates databases of spatially divergent genetic data, analysing high-coverage whole-genome data of modern individuals of known provenance. These modern specimens were obtained from Barth et al. [53] and Pinsky et al. [24] and comprise 276 Atlantic cod individuals that represent three broad geographical regions of the species' range: the western Atlantic Ocean, the eastern Atlantic Ocean and the Baltic Sea. All of these regions are genetically differentiated and represent potential sources of distant-water fishing and fish trade over the chronology of our study [24,53]. Three major genetic clusters (here named western Atlantic, eastern Atlantic and Baltic Sea) can be identified and genetically assigned using genome-wide data (excluding the four large chromosomal inversions in Atlantic cod: LG1, LG2, LG7 and LG12, figure 2a). Based on such genome-wide differentiation, the first assignment was used to determine an overall eastern or western Atlantic Ocean origin. Subsequently, using a similar approach, all specimens first assigned to a putative eastern Atlantic region were analysed to determine a possible Baltic Sea origin [21].
Figure 2.
(a) Geographical distribution of inversion frequencies of chromosomal inversions in Atlantic cod (LG1, LG2, LG7 and LG12) across the North Atlantic Ocean. The assignment of specific haplotypes to a geographical area is either eastern (E, in blue) or western (W, in brown) Atlantic. The population PCA plot was modified from Ferrari et al. [21] and shows the differentiation between the eastern and western Atlantic Oceans, and the Baltic Sea (in blue as it is located within the eastern Atlantic Ocean). Specific alleles associated with a northernmost composite genotype distribution are assigned in orange. Alleles associated with a more temperate north-central genotype distribution are assigned in grey. The archaeological locations are indicated with red squares. The modern populations used as possible source populations of our ancient specimens are located in the map. (b) First genomic assignment: overall percentages (%) represent the minimum probability obtained to be from either the eastern or the western Atlantic Ocean. Second/third genomic assignment: source population percentages (%) represent the highest probability to be assigned to a specific modern location (electronic supplementary material, tables S2–S4). Iceland assignment is obtained by adding the probabilities of both frontal and coastal Icelandic ecotypes. Only two locations from the western Atlantic region have divergent inversion genotype frequencies; thus, they have a specific assignment colour (Placentia Bay and Southern Gulf of St. Lawrence). Other western Atlantic locations are represented in light brown colour (electronic supplementary material, tables S3 and S4). The approximate age (CE) of the specimens is indicated on the right side of the bar plot. Specific time periods are found in figure 1, the electronic supplementary material, table S1 and figure S2. A local (L) or traded (T) assignment follows putative source population. Specimens are considered to be of local origin with considerable North Sea or Irish Sea assignments, specimens are considered to have been obtained through trade with significant Northeast Arctic, Norwegian coast, Iceland or western Atlantic assignments. Individuals with ambiguous origin (i.e. HULL1024, COD281, COD272, HULL1112 and HULL1834) or with a northernmost or north-central origin below 75% probability (i.e. COD284 and HULL1065) are not identified as local or traded in this chronology. Individuals COD276 and LB2 are identified as traded specimens as their likely origin is a remote population: Norwegian coast (Lofoten or southwest). Fourth assignment: percentages (%) representing either the northernmost or north-central genotype distribution after adding the scaled probabilities of selected source populations. For details of the sample codes, see electronic supplementary material, table S1. (Online version in colour.)
Then, again using the BAMscorer pipeline [21], we identified specific individual genotypes of the four large chromosomal inversions in our low-coverage ancient samples. These inversions have highly divergent genetic haplotypes [22,27–30] whose spatial distributions show elevated differentiation between different Atlantic cod ecotypes and spawning regions within the western and eastern Atlantic cluster [22–25]. Therefore, these genotypes can be used to further assign each ancient specimen to a more specific source population within these three genome-wide clusters (electronic supplementary material, table S2). For this, we used the binomial sampling method as per Star et al. [6], to infer the overall probability of obtaining a specific composite inversion genotype based on the inversion frequencies of the specific source populations [22–25]. In short, this approach investigates if ancient individuals statistically differ in their affinity towards a particular modern population based on their inversion genotypes. The probability of obtaining an individual inversion genotype follows a binomial distribution given the underlying allele frequencies of a modern population. Since the inversions are located on different chromosomes, we can assume independence between inversion loci. We then calculated the overall probability of obtaining a composite ancient inversion genotype—based on the modern populations’ respective allele frequencies—as a measure of an individual's affinity toward a specific population (electronic supplementary material, table S2).
Given the low-coverage data, we used the negative controls from the library sessions as a baseline to include or exclude specimens for specific genomic assignments. We included those samples with greater than 2000 aligned reads and at least one magnitude more of nuclear coverage and endogenous DNA than our negative controls. Subsequently, we only infer the genotype of samples with at least three chromosomal inversions with greater than 75% probability (see details in electronic supplementary material, tables S1 and S2). Comparative modern inversion frequency data were compiled for a range of different populations [22–25]. From the eastern Atlantic, we included: the Northeast Arctic, the Norwegian Coast (Lofoten and the southwest), the North Sea, the Irish Sea, Øresund and Iceland (both coastal and frontal ecotypes, which differ in their migratory behaviour). Western Atlantic populations included a number of populations south and north of Newfoundland (figure 2a; electronic supplementary material, tables S3 and S4). The highest specific assignment probabilities are reported as percentages (%) in tables and figures, representing the confidence with which one individual is assigned to a specific population (i.e. 75%). In the event of similar assignment probabilities for more than one population (e.g. 50% and 50%), both populations are reported as the putative origin of the individual (i.e. Norwegian Coast or North Sea, see electronic supplementary material, tables S3 and S4 for specific details of assignment percentages). Finally, we recognized that most eastern Atlantic individuals could be further classified with high confidence towards two spatially distinct groups; an overall assignment to a northernmost (Northeast Arctic and Iceland) or north-central (Norwegian Coast, North Sea, Irish Sea and Øresund) distribution (by adding the scaled probabilities of source populations; see details in the electronic supplementary material, tables S3 and S4).
We performed a Fisher's exact test to assess for the existence of an association between bone element and specimen origin (i.e. sourced through trade versus local landings from the North Sea or Irish Sea). The test was implemented in the stats and ggstatsplot libraries in R [54,55] using 22 samples that were assigned to a specific source population (figure 2b; electronic supplementary material, S3). We excluded samples with a northernmost or north-central assignment with less than 75% probability and samples with an indistinguishable origin (see Results section for more details).
3. Results
We sequenced 37 specimens and obtained a total of approximately 342 million paired reads, approximately 9 million aligned reads and endogenous DNA content between less than 0.01% and 34% (electronic supplementary material, table S2). Sequencing reads showed the patterns of DNA fragmentation and deamination rates that are consistent with those of authentic aDNA (electronic supplementary material, figure S1). We successfully assigned these 37 sequenced specimens to one of the two broad geographical areas (eastern or western Atlantic Ocean), finding a total of five samples (England = 1, Spain = 4) from the western Atlantic and 32 specimens (England = 31, Spain = 1) from the eastern Atlantic (figure 2b; electronic supplementary material, tables S1 and S2). Within the eastern Atlantic specimens, we found two that could be assigned to the Baltic Sea at 54% and 98% probability (electronic supplementary material, table S2), however, because of low numbers of obtained sequence reads such assignments were not considered for our final chronology. Subsequently, we assigned a total of 29 out of the 37 samples (England = 24, Spain = 5) to a more specific source population based on the assignment probabilities of their composite inversion genotypes (electronic supplementary material, table S2; figure 2b). For samples assigned to a specific source population, we could identify 102 out of 116 inversion genotypes with more than 95% probability (electronic supplementary material, table S2). Specific assignments are dependent on the source populations provided for comparison, which can result in low probability assignments as several populations can share similar inversion genotype frequencies (e.g. less than 50% probability; figure 2b). In agreement with postcranial bones being commonly assigned to non-local sources, we found a statistically significant association between the bone element (cranial or postcranial) and the origin (local or traded, respectively) of the specimen (p = 0.01; electronic supplementary material, table S1 and figures S2 and S3).
For London, of the 31 specimens assigned to the eastern Atlantic Ocean, 24 passed the initial quality baselines required for specific assignment analysis and had sufficient data for estimating genotypes of inversion loci. We assigned 15 specimens to a north-central haplotype group (with greater than 60% probability). This includes eight specimens with a possible source population like the North Sea or the Irish Sea (42–67% probability), one specimen with a southwest Norwegian Coast origin (40% probability) and six specimens with indistinguishable associations to the Norwegian Coast (both Lofoten and southwest) and the North Sea (32–33% probability). Nonetheless, these within-group assignments are not strongly supported as the inversion frequency differences of the reference populations are limited (figure 2a). Similarly, we assigned seven specimens to a northernmost composite genotype group (with greater than 85% probability), where we found one specimen possibly coming from northern Norway (Northeast Arctic, 75% probability) and six specimens likely coming from Iceland (61–79% probability). We calculated an overall Icelandic origin by adding the probabilities of being Icelandic frontal or coastal ecotypes. The genomic distinction between Icelandic cod and Northeast Arctic cod is predominantly driven by the higher frequency of north-central genotypes (in grey figure 2a) for the chromosomal inversion LG01 in Iceland [22]. However, similar inversion frequencies (for LG01) between deep water Icelandic cod and Northeast Arctic cod have been reported [56]. Considering such similarities, the assignments to Iceland or the Northeast Arctic should be taken with caution. Moreover, we found an unreliable assignment for one specimen to a northernmost or north-central composite genotype group (with 50% probability), resulting in an Icelandic assignment with low confidence (49%; figure 2b; electronic supplementary material, table S3). Our specific genomic assignments further agree with a presumed local origin of two specimens included in our final chronology previously used as ‘local’ (control) samples for aDNA analysis in Hutchinson et al. [19] (electronic supplementary material, table S1). These samples (HULL1038 and HULL1065) have a north-central haplotype group distribution with 60% and 100% probability, respectively. In combination with the significant association between the bone element (cranial or postcranial) and the origin (local or traded) of a specimen, these bones are therefore likely of a local origin. Finally, as noted above, we assigned one London specimen (dated between the late fifteenth and sixteenth centuries) to a western Atlantic origin (with 100% probability) including a possible low confidence assignment to Placentia Bay (41% probability; figure 2b; electronic supplementary material, table S3).
For Spain, we found four specimens assigned to the western Atlantic and one specimen assigned to the eastern Atlantic (figure 2b). The assignments to the western Atlantic (with 100% probability) tentatively included source populations along southwestern Newfoundland (Placentia Bay) or the Gulf of St. Lawrence (with 36–86% probability). We assigned the eastern Atlantic specimen (with 90% probability) to a north-central composite genotype group which includes the Norwegian Coast as a putative origin (indistinguishable association to the Lofoten and southwest coast with 32–33% probability; figure 2b; electronic supplementary material, table S4).
4. Discussion
We have used a novel genomic assignment approach to identify the biological source of individual archaeological Atlantic cod specimens from England and Spain. With high confidence, we assigned fish remains to a large-scale geographical origin (up to 100% assignment probability) and genotype groups within regions (greater than 85% assignment probability). With moderate to low confidence (less than 86% assignment probability), we tentatively identified several of the specimens to more specific spatially constrained populations. Below we describe the resulting spatio-temporal patterns observed in England and Spain, and consider the impact these findings have on our understanding of the globalization of marine fisheries over the last millennium.
(a) . London: an increasingly North Atlantic trade
Previous zooarchaeological evidence and stable isotope data [15,16] implied that Atlantic cod trade stemmed predominantly from local fisheries during the eleventh to twelfth centuries, after which longer distance imports appeared during the thirteenth to fourteenth centuries. Our individual assignments provide confident aDNA evidence that supports this chronology, with fish assigned to northern Atlantic regions appearing in increasing frequency over time. Assignments to specific source populations are often associated with lower individual probabilities (approx. 32% probability); therefore, these should be considered as indicative only. Interestingly, our assignment analysis indicates that imported Atlantic cod dated between the thirteenth and the fourteenth centuries derived not only from northern Norway but possibly from Iceland (figure 2b). According to historical evidence, Iceland first became a major supplier of dried cod to England during the fifteenth century, when fishermen and merchants from England and Germany first defied the Norwegian royal monopoly on trade with Iceland [57,58]. However, exports of stockfish from Iceland via Norway commenced ca 1300 CE or earlier [59,60]. Our results are consistent with this chronology. Icelandic cod in medieval London would probably have reached England via Bergen, on Norwegian, English, and/or Hanseatic ships that are known to have traded between Norway and ports of the English east coast [61,62].
Furthermore, England's participation in the western Atlantic cod fisheries expanded in south-eastern Newfoundland during the late sixteenth century (figure 2b; electronic supplementary material, table S4) [63]. Therefore, the observation of a specimen from the western Atlantic dated to the late fifteenth to the sixteenth centuries currently represents one of the earliest known genetic examples of this trans-Atlantic expansion. This chronology is consistent with existing knowledge regarding the emergence of trans-Atlantic cod trade, although, as discussed further below, English catches in North America were often destined for southern Europe rather than home markets like London [14,64].
(b) . Spain: an increasingly trans-Atlantic trade
Similar to the single late fifteenth- to sixteenth-century London cod bone, our findings in Spain are consistent with known western Atlantic fishing expansion of the early modern period [65]. In a historical context, the Basques and Galicians provided Atlantic cod for Spain throughout the sixteenth century [64–66]. The fifteenth- to sixteenth-century sample from Spain assigned to waters of the western Atlantic is consistent with historical sources, which indicate that Basque fishermen (from Spain and France) and Galicians acquired fish from southern to western Newfoundland (i.e. Placentia Bay, the Gulf of St. Lawrence, St. Pierre and Miquelon) to fulfil the demand for Atlantic cod in Spain [65,67–70]. The three later specimens (seventeenth century and seventeenth to eighteenth centuries) could have derived from Spanish fishermen operating in Newfoundland [66,70] or as trade items with English or French fishermen that had been operating in these grounds since the sixteenth century. In fact, the English engaged in unofficial trade even when political hostilities disrupted relations with Spain [64]. These specimens may thus relate to the triangular trade involving an exchange of Atlantic cod from the western North Atlantic for wine and salt from southern Europe [14]. By the late nineteenth to early twentieth century, dried Atlantic cod from across the Norwegian waters could have been used to provision the military centre in Álava (JA Quirós 2022, personal communication). Our most recent Spanish sample originating from the Norwegian Coast (figure 2b; electronic supplementary material, table S4) is therefore consistent with the supply of northern European air-dried Atlantic cod since the eighteenth century [71,72].
5. Conclusion
Altogether, our results provide genetic evidence for an expanding trade and increasing demand for marine fish leading to the exploitation of a great diversity of distant-water sources already in the Middle Ages. Our evidence also tracks the culmination of the marine fisheries extension with European exploitation of the western Atlantic fishing grounds around Newfoundland, starting in the sixteenth century. Our findings emphasize the utility of whole-genome sequencing and aDNA methods to describe the increasing demand for Atlantic cod for European societies during medieval and post-medieval periods. We expect that the inclusion of more archaeological sites and larger sample sizes through space and time will reveal additional patterns about those populations targeted for long-distance trade in the past and possibly provide greater insight about which populations have experienced long-term impacts of fisheries. Overall, our results corroborate and significantly increase existing knowledge about the globalization of marine fisheries and fish trade in medieval and early modern times.
Acknowledgements
We thank Lori Lawson Handley for providing ancient DNA resources for this research. We thank Martin Biddle (Oxford University), Francis Grew (Museum of London), Cath Maloney (Museum of London), Natasha Powers (MOLA) and Roy Stephenson (Museum of London) for assistance with sampling and sampling permissions. We thank M. Skage, S. Kollias, A. Tooming-Klunderud and the Ullevål Sequencing team at the Norwegian Sequencing Centre for sequencing and processing of the genomic samples. Analyses were performed on resources provided by UNINETT Sigma2 – the National Infrastructure for High Performance Computing and Data Storage in Norway.
Contributor Information
Lourdes Martínez-García, Email: l.m.garcia@ibv.uio.no.
Bastiaan Star, Email: bastiaan.star@ibv.uio.no.
James H. Barrett, Email: james.barrett@ntnu.no.
Data accessibility
The raw reads for the ancient specimens are released under the ENA accession number PRJEB52865. This manuscript is available as a pre-print in BioRxiv at https://www.biorxiv.org/content/10.1101/2022.06.03.494519v1 [73].
The data are provided in the electronic supplementary material [74].
Authors' contributions
L.M.-G.: conceptualization, data curation, formal analysis, methodology, visualization, writing—original draft and writing—review and editing; G.F.: data curation, methodology and writing—review and editing; A.C.: methodology and writing—review and editing; L.M.A.: methodology and writing—review and editing; B.L.-A.: methodology and writing—review and editing; M.C.: methodology and writing—review and editing; L.L.-R.: methodology, resources and writing—review and editing; A.M.-M.: resources and writing—review and editing; E.R.-I.: resources and writing—review and editing; J.A.Q.: resources and writing—review and editing; R.M.-M.: resources and writing—review and editing; B.H.: resources and writing—review and editing; W.H.: funding acquisition, resources and writing—review and editing; K.S.J.: funding acquisition and writing—review and editing; S.J.: funding acquisition and writing—review and editing; D.O.: funding acquisition, resources and writing—review and editing; B.S.: conceptualization, funding acquisition, project administration, supervision, visualization and writing—review and editing; J.H.B.: conceptualization, funding acquisition, project administration, resources, supervision and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by the Research Council of Norway project ‘Catching the Past’ (grant no. 262777), the Leverhulme Trust (grant nos. F/00 181/R and MRF-2013-065), the European Union's Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement No. 813383 (SeaChanges), the 4-OCEANS Synergy grant agreement no. 951649, the FISHARC-IF 658022 Marie-Curie-Sklodowska-IF fellowship for Career development and the European Molecular Biology Organization (ASTF 354-2016). The European Research Agency is not responsible for any use that may be made of the information this work contains. This research is also under the framework of the PID-118662GB-100 (FISHCIIS - Fishing Isotopes) project from the Spanish Ministry of Science and Innovation.
References
- 1.Holm P, Nicholls J, Hayes PW, Ivinson J, Allaire B. 2021. Accelerated extractions of North Atlantic cod and herring, 1520–1790. Fish Fish. 23, 54-72. ( 10.1111/faf.12598) [DOI] [Google Scholar]
- 2.Barrett JH, Locker AM, Roberts CM. 2004. ‘Dark Age Economics’ revisited: the English fish bone evidence AD 600–1600. Antiquity 78, 618-636. ( 10.1017/S0003598X00113262) [DOI] [Google Scholar]
- 3.Barrett JH. 2016. Medieval sea fishing AD 500–1550: chronology, causes and consequences. In Cod and herring: the archaeology and history of medieval sea fishing (eds Barret JH, Orton DC), pp. 250-271. Oxford, UK: OxBow books. [Google Scholar]
- 4.Orton DC, Locker A, Morris J, Barrett JH. 2016. Chapter 16: Fish for London. In Cod and herring: the archaeology and history of medieval sea fishing (eds Barrett JH, Orton DC). Oxford, UK: Oxbow Books. [Google Scholar]
- 5.Barrett JH, Locker AM, Roberts CM. 2004. The origins of intensive marine fishing in medieval Europe: the English evidence. Proc. R. Soc. Lond. Ser. B 271, 2417-2421. ( 10.1098/rspb.2004.2885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Star B, et al. 2017. Ancient DNA reveals the Arctic origin of Viking Age cod from Haithabu, Germany. Proc. Natl Acad. Sci. USA 114, 9152-9157. ( 10.1073/pnas.1710186114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett J, et al. 2008. Detecting the medieval cod trade: a new method and first results. J. Archaeol. Sci. 35, 850-861. ( 10.1016/j.jas.2007.06.004) [DOI] [Google Scholar]
- 8.Nedkvitne A. 2016. The development of the Norwegian long-distance stockfish trade. In (eds JH Barrett, DC Orton), Cod and herring: The archaeology and history of medieval sea fishing, pp. 50–59. Oxford, England: Oxbow Books. [Google Scholar]
- 9.Priegue EMF. 1982. Las rutas marí timas y comerciales del flanco ibé rico, desde Galicia hasta Flandes”. El Fuero de San Sebastiá n y su é poca (Congreso de Estudios Histó ricos) San Sebastiá n, Sociedad de Estudios Vascos, pp. 217–234. [Google Scholar]
- 10.Hinojosa Montalvo J. 1982. De Valencia a Portugal y Flandes: relaciones durante la Edad Media. Anales de la Universidad de Alicante. Historia Medieval 1, 149-168. ( 10.14198/medieval.1982.1.08) [DOI] [Google Scholar]
- 11.Breen C. 2016. Marine fisheries and society in medieval Ireland. In Cod and herring. The archaeology and history of medieval sea fishing (eds Barrett JH, Orton DC), pp. 91-98. Oxford, UK: Oxbow Books. [Google Scholar]
- 12.Morales A, Morales DC, Roselló E. 1991. Sobre la presencia del bacalao (Gadus morhua) en la Cartuja Sevillana de Santa Maria de las Cuevas (siglos xv-xvi). Arquivo Hispalense 226, 17-24. [Google Scholar]
- 13.Pope PE. 2003. Early European occupations in southeast Newfoundland: Archaeological perspectives on competition for fishing rooms, 1530–1680. In (eds C Roy, J Bélisle, M-A Bernier, B Loewen). Mer et monde: Questions d'archéologie maritime. Archéologiques, Collection hors série 1, Quebec, QC, Canada, pp. 122–133. [Google Scholar]
- 14.Pope PE. 2004. Fish into Wine: The Newfoundland Plantation in the 17th century. Chapel Hill, NC, USA: University of North Carolina Press and Omohundro Institute of Early American History and Culture. [Google Scholar]
- 15.Orton DC, Morris J, Locker A, Barrett JH. 2014. Fish for the city: meta-analysis of archaeological cod remains and the growth of London's northern trade. Antiquity 88, 516-530. ( 10.1017/S0003598X00101152) [DOI] [Google Scholar]
- 16.Barrett JH, et al. 2011. Interpreting the expansion of sea fishing in medieval Europe using stable isotope analysis of archaeological cod bones. J. Archaeol. Sci. 38, 1516-1524. ( 10.1016/j.jas.2011.02.017) [DOI] [Google Scholar]
- 17.Orton DC, et al. 2011. Stable isotope evidence for Late Medieval (14th–15th C) origins of the eastern Baltic Cod (Gadus morhua) fishery. PLoS ONE 6, e27568. ( 10.1371/journal.pone.0027568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glykou A, Ritchie K, Hargrave MS, Visch W, Lidén K. 2021. Strontium isotope analysis in prehistoric cod otoliths by laser ablation multi-collector inductively coupled plasma mass spectrometry. J. Archaeol. Sci. 37, 102976. ( 10.1016/j.jasrep.2021.102976) [DOI] [Google Scholar]
- 19.Hutchinson WF, Culling M, Orton DC, Hanfling B, Lawson Handley L, Hamilton-Dyer S, O'Connell TC, Richards MP, Barrett JH. 2015. The globalization of naval provisioning: ancient DNA and stable isotope analyses of stored cod from the wreck of the Mary Rose, AD 1545. R. Soc. Open Sci. 2, 150199. ( 10.1098/rsos.150199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conrad C, DeSilva U, Bingham B, Kemp BM, Gobalet KW, Bruner K, Pastron AG. 2021. Finny Merchandise: the Atlantic Cod (Gadus morhua) trade in Gold Rush-Era San Francisco, California. J. Anthropol. Res. 77, 520-549. ( 10.1086/716744) [DOI] [Google Scholar]
- 21.Ferrari G, Atmore LM, Jentoft S, Jakobsen KS, Makowiecki D, Barrett JH, Star B. 2021. An accurate assignment test for extremely low-coverage whole-genome sequence data. Mol. Ecol. Resour. 1, 15. ( 10.1111/1755-0998.13551) [DOI] [PubMed] [Google Scholar]
- 22.Berg PR, Star B, Pampoulie C, Bradbury IR, Bentzen P, Hutchings JA, Jentoft S, Jakobsen KS. 2017. Trans-oceanic genomic divergence of Atlantic cod ecotypes is associated with large inversions. Heredity 119, 418-428. ( 10.1038/hdy.2017.54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clucas GV, Lou RN, Therkildsen NO, Kovach AI. 2019. Novel signals of adaptive genetic variation in northwestern Atlantic cod revealed by whole-genome sequencing. Evol. Appl. 12, 1971-1987. ( 10.1111/eva.12861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinsky ML, et al. 2021. Genomic stability through time despite decades of exploitation in cod on both sides of the Atlantic. Proc. Natl Acad. Sci. USA 118, e2025453118. ( 10.1073/pnas.2025453118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen T, Besnier F, Quintela M, Jorde PE, Glover KA, Westgaard JI, Dahle G, Lien S, Kent MP. 2020. Genomic analysis reveals neutral and adaptive patterns that challenge the current management regime for East Atlantic cod Gadus morhua L. Evol. Appl. 13, 2673-2688. ( 10.1111/eva.13070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matschiner M, et al. 2022. Supergene origin and maintenance in Atlantic cod. Nat. Ecol. Evol. 6, 469-481. ( 10.1038/s41559-022-01661-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg PR, Star B, Pampoulie C, Sodeland M, Barth JMI, Knutsen H, Jakobsen KS, Jentoft S. 2016. Three chromosomal rearrangements promote genomic divergence between migratory and stationary ecotypes of Atlantic cod. Sci. Rep. 6, 23246. ( 10.1038/srep23246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barney BT, Munkholm C, Walt DR, Palumbi SR. 2017. Highly localized divergence within supergenes in Atlantic cod (Gadus morhua) within the Gulf of Maine. BMC Genomics 18, 1-14. ( 10.1186/s12864-017-3660-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sodeland M, et al. 2016. ‘Islands of Divergence’ in the Atlantic cod genome represent polymorphic chromosomal rearrangements. Genome Biol. Evol. 8, 1012-1022. ( 10.1093/gbe/evw057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breistein B, et al. 2022. Geographic variation in gene-flow from a genetically distinct migratory ecotype drives population genetic structure of coastal Atlantic cod (Gadus morhua L.). Evol. Appl. 15, 1162-1176. ( 10.1111/eva.13422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-García L, Ferrari G, Hufthammer AK, Jakobsen KS, Jentoft S, Barrett JH, Star B. 2022. Ancient DNA reveals a southern presence of the Northeast Arctic cod during the Holocene. Biol. Lett. 18, 20220021. ( 10.1098/rsbl.2022.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrett JH. 2016. Studying medieval sea fishing and fish trade: how and why. In Cod and herring: the archaeology and history of medieval sea fishing (eds Barrett JH, Orton DC), pp. 1-10. Oxford, UK: Oxbow Books. [Google Scholar]
- 33.Maltin E, Jonsson L. 2018. Cod heads, stockfish, and dried spurdog: unexpected commodities in Nya Lödöse (1473–1624), Sweden. Int. J. Hist. Archaeol. 22, 343-363. ( 10.1007/s10761-017-0405-6) [DOI] [Google Scholar]
- 34.Orton DC, Rannamäe E, Lõugas L, Makowiecki D, Hamilton-Dyer S, Pluskowski A, O'Connell T, Barrett JH. 2019. The Teutonic order's role in the development of a medieval eastern Baltic cod fishery: Evidence from Fish Bone Isotopes. In (ed. AG Pluskowski), The Ecology of Crusading, Colonisation and Religious Conversion in the Medieval Eastern Baltic: Terra Sacra II. Turnhout: Brepols. [Google Scholar]
- 35.Cooper A, Poinar HN. 2000. Ancient DNA: do it right or not at all. Science 289, 1139-1139. ( 10.1126/science.289.5482.1139b) [DOI] [PubMed] [Google Scholar]
- 36.Gilbert MTP, Bandelt HJ, Hofreiter M, Barnes I. 2005. Assessing ancient DNA studies. Trends Ecol. Evol. 20, 541-544. ( 10.1016/j.tree.2005.07.005) [DOI] [PubMed] [Google Scholar]
- 37.Ferrari G, et al. 2021. The preservation of ancient DNA in archaeological fish bone. J. Archaeol. Sci. 126, 105317. ( 10.1016/j.jas.2020.105317) [DOI] [Google Scholar]
- 38.Martínez-García L, et al. 2021. Historical demographic processes dominate genetic variation in ancient Atlantic cod mitogenomes. Front. Ecol. Evol. 9, 342. ( 10.3389/fevo.2021.671281) [DOI] [Google Scholar]
- 39.Gondek AT, Boessenkool S, Star B. 2018. A stainless-steel mortar, pestle and sleeve design for the efficient fragmentation of ancient bone. Biotechniques 64, 266-269. ( 10.2144/btn-2018-0008) [DOI] [PubMed] [Google Scholar]
- 40.Boessenkool S, Hanghøj K, Nistelberger HM, Der Sarkissian C, Gondek AT, Orlando L, Barrett JH, Star B. 2017. Combining bleach and mild predigestion improves ancient DNA recovery from bones. Mol. Ecol. Resour. 17, 742-751. ( 10.1111/1755-0998.12623) [DOI] [PubMed] [Google Scholar]
- 41.Yang DY, Cannon A, Saunders SR. 2004. DNA species identification of archaeological salmon bone from the Pacific Northwest Coast of North America. J. Archaeol. Sci. 31, 619-631. ( 10.1016/j.jas.2003.10.008) [DOI] [Google Scholar]
- 42.Meyer M, Kircher M. 2010. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protocols 2010, pdb. prot5448. ( 10.1101/pdb.prot5448) [DOI] [PubMed] [Google Scholar]
- 43.Kircher M, Sawyer S, Meyer M. 2012. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3-e3. ( 10.1093/nar/gkr771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroeder H, et al. 2015. Genome-wide ancestry of 17th-century enslaved Africans from the Caribbean. Proc. Natl Acad. Sci. USA 112, 3669-3673. ( 10.1073/pnas.1421784112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapp JD, Green RE, Shapiro B. 2021. A fast and efficient single-stranded genomic library preparation method optimized for ancient DNA. J. Hered. 112, 241-249. ( 10.1093/jhered/esab012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schubert M, et al. 2014. Characterization of ancient and modern genomes by SNP detection and phylogenomic and metagenomic analysis using PALEOMIX. Nat. Protoc. 9, 1056. ( 10.1038/nprot.2014.063) [DOI] [PubMed] [Google Scholar]
- 47.Lindgreen S. 2012. AdapterRemoval: easy cleaning of next-generation sequencing reads. BMC Res. Notes 5, 337. ( 10.1186/1756-0500-5-337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Star B, et al. 2011. The genome sequence of Atlantic cod reveals a unique immune system. Nature 477, 207-210. ( 10.1038/nature10342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tørresen OK, et al. 2017. An improved genome assembly uncovers prolific tandem repeats in Atlantic cod. BMC Genomics 18, 95. ( 10.1186/s12864-016-3448-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754-1760. ( 10.1093/bioinformatics/btp324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jónsson H, Ginolhac A, Schubert M, Johnson PL, Orlando L. 2013. mapDamage2. 0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29, 1682-1684. ( 10.1093/bioinformatics/btt193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078-2079. ( 10.1093/bioinformatics/btp352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barth JMI, et al. 2019. Disentangling structural genomic and behavioural barriers in a sea of connectivity. Mol. Ecol. 28, 1394-1411. ( 10.1111/mec.15010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Team RC. 2022. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 55.Patil I. 2021. Visualizations with statistical details: the ‘ggstatsplot’ approach. J. Open Source Softw. 6, 3167. ( 10.21105/joss.03167) [DOI] [Google Scholar]
- 56.Pampoulie C, Ruzzante DE, Chosson V, Jörundsdóttir TD, Taylor L, Thorsteinsson V, Daníelsdóttir AK, Marteinsdóttir G. 2006. The genetic structure of Atlantic cod (Gadus morhua) around Iceland: insight from microsatellites, the Pan I locus, and tagging experiments. Can. J. Fish. Aquat. Sci. 63, 2660-2674. ( 10.1139/f06-150) [DOI] [Google Scholar]
- 57.McGhee R. 2003. Epilogue: was there continuity from Norse to post-medieval exploration of the New World? In Contact, continuity and collapse: the Norse colonization of the North Atlantic (ed. Barrett JH), pp. 239-248. [Google Scholar]
- 58.Hoffmann RC. 2001. Frontier foods for late medieval consumers: culture, economy, ecology. Environ. Hist. 7, 131-167. ( 10.3197/096734001129342432) [DOI] [Google Scholar]
- 59.Perdikaris S, McGovern TH. 2008. Codfish and kings, seals and subsistence: Norse marine resource use in the North Atlantic. 2008 In (eds TC Rick, JM Erlandson), Human Impacts on Ancient Marine Ecosystems: A Global Perspective, University of California Press, pp. 187–214. [Google Scholar]
- 60.Edvardsson R. 2010. The role of marine resources in the medieval economy of Vestfirðir, Iceland. Doctoral thesis, City University of New York, New York, NY, USA. [Google Scholar]
- 61.Helle K. 2019. Bergen's role in the medieval North Atlantic trade. In (eds N Mehler, M Gardiner, E Elvestad), German Trade in the North Atlantic c. 1400–1700. Interdisciplinary Perspectives, Museum of Archaeology, University of Stavanger, Stavanger, pp. 43–51. [Google Scholar]
- 62.Nedkvitne A. 2014. The German Hansa and Bergen 1100–1600. Köln, Germany: Böhlau Verlag. [Google Scholar]
- 63.Janzen OU. 2013. The Logic of English Saltcod: an historiographical revision. The Northern Mariner/Le Marin du nord 23, 123-134. ( 10.25071/2561-5467.284) [DOI] [Google Scholar]
- 64.Janzen OU. 1996. The illicit trade in English cod into Spain, 1739–1748. Int. J. Maritime Hist. 8, 1-22. ( 10.1177/084387149600800103) [DOI] [Google Scholar]
- 65.Candow JE. 2009. Migrants and residents: the interplay between European and domestic fisheries in northeast North America, 1502–1854. In A history of the North Atlantic fisheries: volume 1, from early times to the mid-nineteenth century (eds Starkey DJ, Thór JT, Heidbrink I), pp. 416-452. Bremen, Germany: Deutsches Schiffahrtsmuseum. [Google Scholar]
- 66.Ménard C. 2008. La pesca gallega en Terranova, siglos XVI–XVIII. Doctoral thesis, Universidade de Santiago de Compostela, pp. 324. [Google Scholar]
- 67.Barkham M. 2000. La industria pesquera en el País Vasco peninsular al principio de la Edad Moderna: ¿una edad de oro?. Itsas Memoria. Revista de estudios marítmos del País Vasco 3, 29-75. [Google Scholar]
- 68.Pope PE. 2008. The archaeology of France’s migratory fishery on Newfoundland’s Petit Nord. In Rêves d'Amériques: regard sur l'archéologie de la Nouvelle-France (eds H Côté, C Roy) Archéologiques, Collection Hors Série 2, pp. 38-54. [Google Scholar]
- 69.Fitzhugh WW, Herzog A, Perdikaris S, McLeod B. 2011. Ship to shore: Inuit, early Europeans, and maritime landscapes in the Northern Gulf of St. Lawrence. In The archaeology of maritime landscapes. When the land meets the sea (eds Ford B), pp. 99-128. New York, NY: Springer. [Google Scholar]
- 70.Pereira-Fernández XM. 2005. Los mareantes pontevedreses y la pesca de altura en el siglo XVI. Cuadernos de Estudios Gallegos 118, 289-301. ( 10.3989/ceg.2005.v52.i118.97) [DOI] [Google Scholar]
- 71.Lindkvist KB, Gallart-Jornet L, Stabell MC. 2008. The restructuring of the Spanish salted fish market. Can. Geographer/Le Géographe Canadien 52, 105-120. ( 10.1111/j.1541-0064.2008.00203.x) [DOI] [Google Scholar]
- 72.García Orellán R. 2006. Rumbo al Gran Banco: una etnohistoria de la pesca industrial del bacalao en los bancos de Terranova. Revista Internacional de Estudios Vascos 51, 577-592. [Google Scholar]
- 73.Martínez-García L, et al. 2022. Ancient DNA evidence for the ecological globalization of cod fishing in medieval and post-medieval Europe. bioRxiv. ( 10.1101/2022.06.03.494519). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martínez-García L2022. Ancient DNA evidence for the ecological globalization of cod fishing in medieval and post-medieval Europe. Figshare. ( ) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw reads for the ancient specimens are released under the ENA accession number PRJEB52865. This manuscript is available as a pre-print in BioRxiv at https://www.biorxiv.org/content/10.1101/2022.06.03.494519v1 [73].
The data are provided in the electronic supplementary material [74].