Graphical abstract
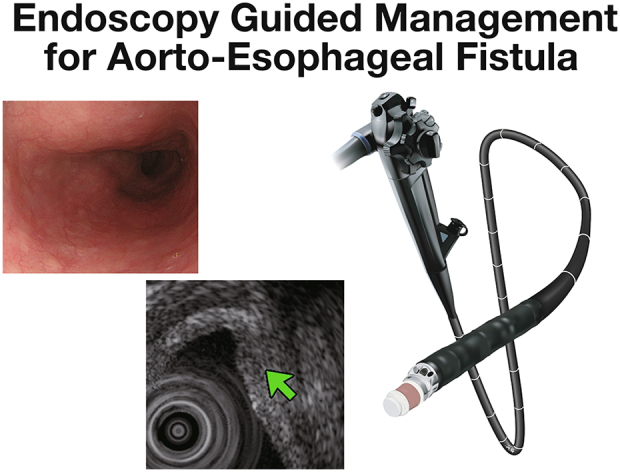
Ultrasonography for aortoesophageal fistula after thoracic endovascular aortic repair.
Central Message.
Repeated endoscopic ultrasound can evaluate transmural changes of the esophageal wall in aortoesophageal fistulae, which may guide patient selection for nonoperative treatment.
Aortoesophageal fistula (AEF) is a rare, life-threatening condition. AEFs usually develop in patients with thoracic aortic disease or those undergoing thoracic aortic surgery. Although there is a lack of consensus regarding the optimal treatment for AEF, definitive surgery involving radical esophagectomy and extensive aortic reconstruction is the only durable approach rather than nonoperative options.1 Thoracic endovascular aortic repair (TEVAR) is considered an initial bridging intervention to stabilize hemodynamics and before subsequent open surgery.2
We report a case of endovascular management and preservation of the esophagus for AEF supported by esophagogastroduodenoscopy (EGD) and endoscopic ultrasonography (EUS). The patient provided informed consent for publication (ethics review board approval #272, March 1, 2022).
Case Report
A 71-year-old man with no significant medical history presented with massive hematemesis that started 10 days before admission. The patient showed hypotension, and his hemoglobin was 7.5 g/dL. The patient's laboratory data showed no signs of infection at arrival (white blood count 6410/μL, C-related protein 0.32 mg/dL, procalcitonin 0.03 ng/dL, and negative blood culture). Computed tomography (CT) revealed a contained rupture of the descending thoracic aneurysm (Figure E1). Imaging could not exclude any mediastinal neoplasms.
Figure E1.
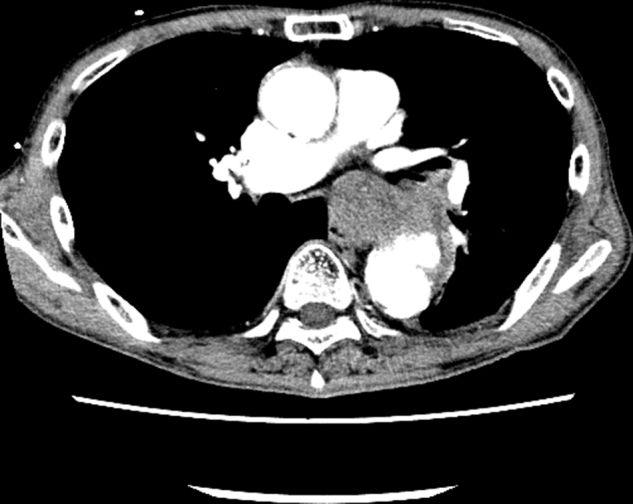
Preoperative computed tomography. Aortoesophageal fistula due to contained rupture of the descending thoracic aortic aneurysm.
Emergent bridging TEVAR was performed to stabilize hemodynamics and achieve hemostasis (Gore C-TAG, 34 mm × 20 cm, 31 mm × 10 cm; W. L. Gore & Associates, Inc) (Figure E2). One day after TEVAR, EGD and EUS were performed to evaluate the esophageal ulcer and underlying conditions. EGD revealed a longitudinal ulcer (Figure 1, A). EUS showed fluid and thrombus outside the esophagus without neoplasm, which confirmed the AEF due to the contained rupture of the aneurysm (Figure 2, A). Intravenous meropenem hydrate was initiated because the longitudinal ulcer was transmural following treatment for esophageal rupture.
Figure E2.
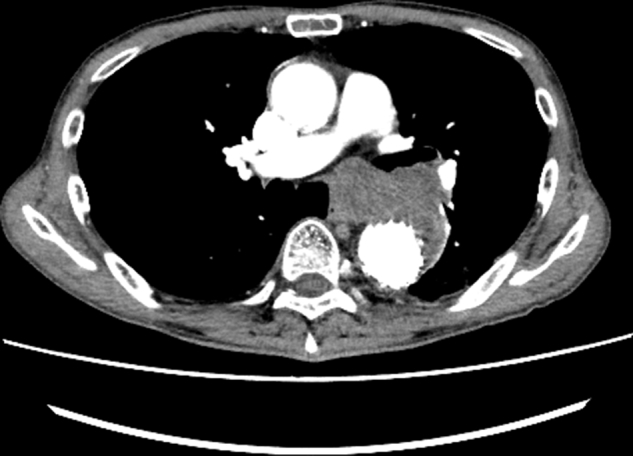
Postoperative computed tomography. The aneurysm was then covered with stent grafts.
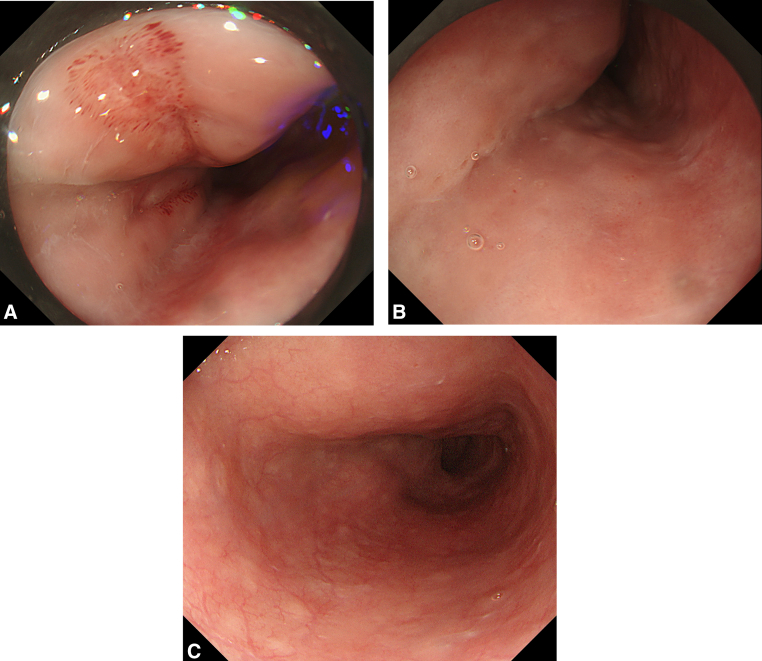
Figure 1.
Esophagogastroduodenoscopy. A, One day after bridging stent-grafting; longitudinal ulcer on the mid-esophagus, massive bleeding was not observed. B, One week after stent-grafting; the ulcer recovered completely. C, At discharge; no exacerbation was observed in the esophageal mucus.
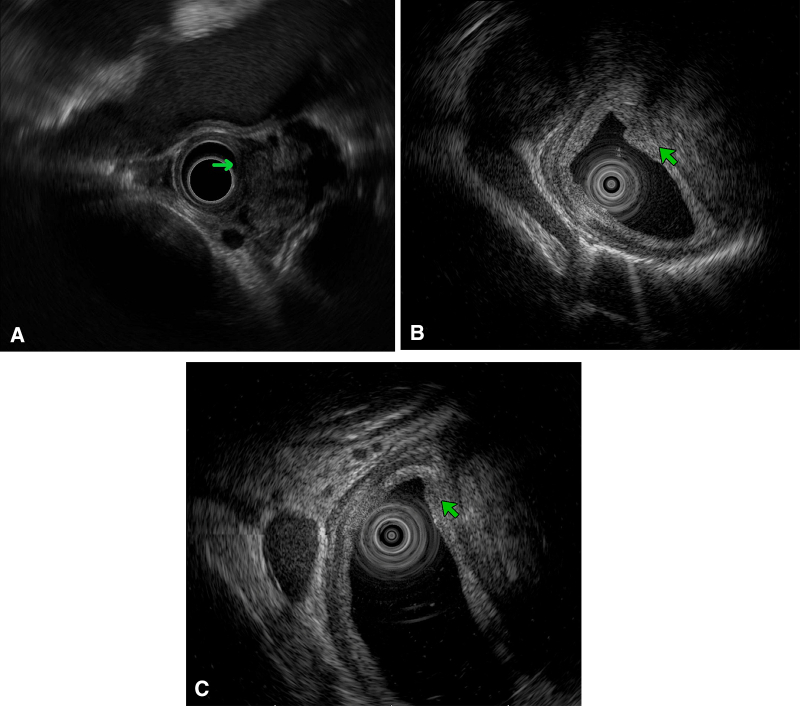
Figure 2.
Endoscopic ultrasonography. A, One day after bridging stent-grafting, there was a transmural fistula (green arrow). Fluid and thrombus outside of the esophagus were also confirmed. B, One week after stent-grafting, transmural scar and thickness were found in the esophageal wall. C, At discharge, the thickness of the esophageal wall decreased significantly.
EGD and EUS were performed before proceeding with the radical surgical approach. The longitudinal ulcer resolved (Figure 1, B), and there was a transmural scar and thickness in the esophageal wall (Figure 2, B). No other findings suggested infection (biomarker, blood culture, CT, and magnetic resonance imaging) (Figure E3) at this time point. Therefore, we decided not to perform operative management to preserve the esophagus after the bridging TEVAR.
Figure E3.
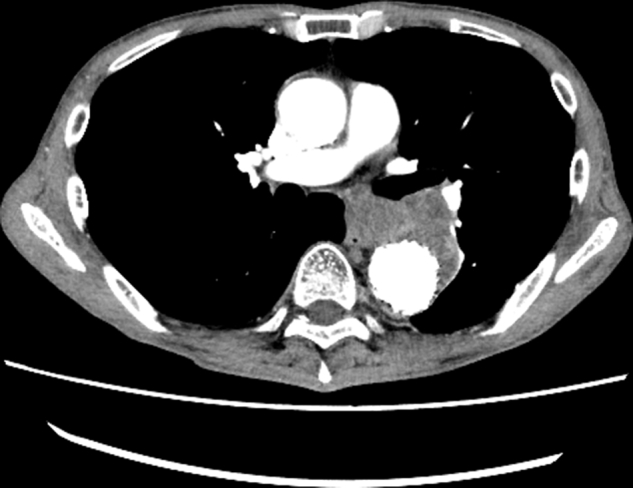
Computed tomography at discharge. The diameter of the aneurysm decreased without fluid or gas retention.
The patient's subsequent clinical course was uneventful. Repeated CT imaging revealed decreased aneurysm diameter without any signs of infection (air or fluid retention). After 4 weeks of treatment, the antibiotics were converted from intravenous meropenem hydrate to lifelong oral potassium clavulanate. The patient was discharged 50 days after confirmation of further recovery of the esophageal wall by the third EGD and EUS (Figure 1, B, and Figure 2, C). No recurrence of infection was observed in 6 months after discharge.
Discussion
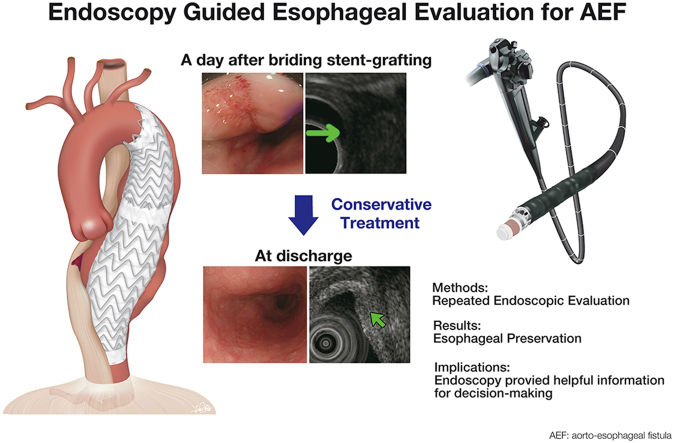
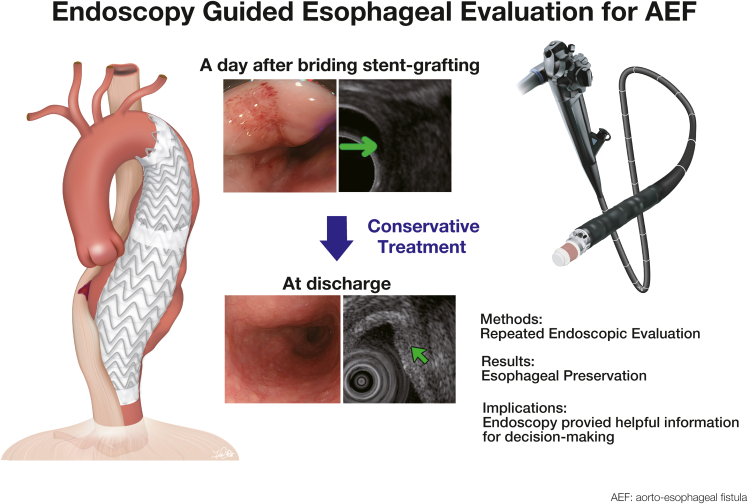
Since the first report by Dubrueil3 in 1818, primary AEF has been an infrequent but lethal clinical entity. Although the first survivor after a surgical repair was reported by Snyder and Crawford4 in 1983, no large series have been published. To control or prevent fatal bleeding, definitive aortic treatment should be accompanied by methods to stop continuous contamination through the fistula to the aortic wall and mediastinum.2 Typically, nonoperative medical management usually does not result in long-term survival. Chiesa and colleagues5 reported the utility of endovascular management for AEF, particularly in emergent and urgent settings. However, the additional esophageal repair is necessary in most cases. In the present case, we initially considered TEVAR as a bridging treatment for open repair. The patient did not exhibit any signs of infection upon arrival. Therefore, we performed repeated endoscopic evaluation and imaging studies to meticulously evaluate esophageal condition, including appearance by EGD and transmural changes by EUS. Finally, esophageal preservation was selected after deliberate discussion. Adding to imaging, in conjunction with favorable clinical signs and laboratory studies, we believe that endovascular repair alone was a viable option. As AEF has a spectrum of severity, radical treatment may not be always mandatory in mild cases. Although the lack of long-term follow-up limits our ability to make any solid conclusions, endoscopy involving EGD and EUS provided helpful information for decision-making, which led to short-term success (Figure 3). The protocol may be applicable for the patient with secondary AEF.
Figure 3.
Endoscopy-guided esophageal evaluation for AEF. AEF, Aortoesophageal fistula.
Conclusions
Endoscopy (EGD/EUS)-guided esophageal evaluation after bridging TEVAR can be useful in determining if esophageal preservation is valid treatment option.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
References
- 1.Czerny M., Eggebrecht H., Sodeck G., Weigang E., Livi U., Verzini F., et al. New insights regarding the incidence, presentation and treatment options of aorto-oesophageal fistulation after thoracic endovascular aortic repair: the European Registry of Endovascular Aortic Repair Complications. Eur J Cardiothorac Surg. 2014;45:452–457. doi: 10.1093/ejcts/ezt393. [DOI] [PubMed] [Google Scholar]
- 2.Yamazato T., Nakamura T., Abe N., Yokawa K., Ikeno Y., Koda Y., et al. Surgical strategy for the treatment of aortoesophageal fistula. J Thorac Cardiovasc Surg. 2018;155:32–40. doi: 10.1016/j.jtcvs.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 3.Dubrueil O. Observation sur la perforation de l'esophague et de l'aorte thoracique par une portion d'os oval: avec des réflexions. J Univ Sci Med. 1818;9:357–363. [Google Scholar]
- 4.Snyder D.M., Crawford E.S. Successful treatment of primary aorta-esophageal fistula resulting from aortic aneurysm. J Thorac Cardiovasc Surg. 1983;85:457–463. [PubMed] [Google Scholar]
- 5.Chiesa R., Melissano G., Marone E.M., Kahlberg A., Marrocco-Trischitta M.M., Tshomba Y. Endovascular treatment of aortoesophageal and aortobronchial fistulae. J Vas Surg. 2010;51:1195–1202. doi: 10.1016/j.jvs.2009.10.130. [DOI] [PubMed] [Google Scholar]