Highlights
-
•
Food waste blueberry pomace offers a high potential to recover anthocyanins.
-
•
Ultrasonic-assisted extraction of anthocyanins was optimized by Box-Behnken design.
-
•
The optimal parameters of ultrasonic treatment were 40 °C and 400 W for 40 min.
-
•
Highly purified blueberry anthocyanins were obtained after SCX cation exchange.
-
•
Malvidin was the main anthocyanins in the purified fractions from blueberry pomace.
Keywords: Blueberry pomace, Anthocyanins, HPLC, Ultrasound-assisted extraction, Box-Behnken Design
Abstract
Food waste is a potential source to replace fresh materials for obtaining functional ingredients. Blueberry pomace contains considerable amounts of anthocyanins. In this study, we investigated ultrasonic-assisted extraction (UAE) of anthocyanins from blueberry pomace. We used a Box-Behnken design (BBD) to screen and optimize the important factors influencing yield. The optimum extraction conditions were a temperature of 40 °C, an ultrasonic power of 400 W and an extraction time of 40 min. The optimum yield was 108.23 mg/100 g DW. In addition, we used a cation column to separate anthocyanins, and optimized the chromatographic conditions of HPLC to analyze and identify the main anthocyanins. Thirteen anthocyanins were found in blueberry pomace, of which Malvidin-3-Galactoside (22.65 %) was the highest. These findings provide a theoretical basis and optimized process parameters for the recovery of high value-added anthocyanins from blueberry pomace with ultrasound-assisted extraction, thus facilitating the comprehensive utilization of blueberry pomace.
1. Introduction
Blueberries (Vaccinium spp.) are popular throughout the world for their delicious taste and nutritional value (Chu et al., 2017). Furthermore, studies have demonstrated that blueberries have numerous bioactive properties such as antioxidant activity (Morita, Naito, Yoshikawa, & Niki, 2017), anticancer (Luo, Gu, Kennelly, & Long, 2017), anti-inflammatory (Grace et al., 2019) and cardioprotective effects (Eladwy et al., 2018). These pharmacological properties could be related to the presence of bioactive compounds such as anthocyanins and phenolic acids (Li et al., 2017). Therefore, the blueberry has been classified as a “superfood” (Pérez, Laca, Laca, & Díaz, 2022).
Food waste is a global problem that has a negative impact on many aspects of society. This includes the financial cost of disposing of wasted food, the environmental impact of the greenhouse gases produced by decomposing food waste in landfills, and the waste of resources (Jurgilevich et al., 2016). Food wastage occurs at two different stages: pre-purchase waste and post-purchase waste (McCarthy, Kapetanaki, & Wang, 2020). A large number of by-products are produced during the processing and production of blueberry products. For example, the production of blueberry juice produces a large amount of residue (up to 20–30 % of the fresh fruit weight) (Liu et al., 2021). These by-products are valuable sources of various high added-value compounds like polyphenols. Studies have collectively demonstrated that blueberries are rich in multiple types of anthocyanins, and the types of anthocyanins vary among blueberry cultivars (Bunea et al., 2013). The blueberry anthocyanins are mainly derivatives of malvidin and delphinidin, followed by petunidin, cyanidin, and peonidin (Herrera-Balandrano, Chai, Beta, Feng, & Huang, 2021). Blueberry anthocyanins have a variety of proven beneficial functions: they have antioxidant and anti-inflammatory properties, they can improve vision and prevent cardiovascular disease (Yang et al., 2022). Therefore, recovering blueberry anthocyanins is an effective way to convert blueberry waste into a renewable resource that can contribute to the development of the blueberry industry.
The possible methods to extract blueberry anthocyanins primarily include solvent extraction, enzyme-assisted extraction (EAE), ultrasonic-assisted extraction (UAE) and supercritical fluid extraction (Paes et al., 2014, Zheng et al., 2013). Among these, UAE is an effective, economical and environmentally-friendly approach. High-frequency ultrasonic waves can induce cavitation effects and accelerate plant cell disruption, which facilitates solvent penetration and mass transfer (Santos, Aguiar, Barbero, Rezende, & Martínez, 2015). The UAE method has been used extensively to extract anthocyanins from various fruits such as raspberry (Hui, Lee, & Yong, 2013), pear (Belwal et al., 2019), grape (Tan, Li, Xue, & Tang, 2020), blackberry and sweet cherry (Oancea, Ketney, Grosu, & Stoia, 2013). However, this method cannot completely separate out the target anthocyanins; what is obtained is a mixture of anthocyanins, phenolic acids and polymers. For this reason, we used cation-exchange chromatography to separate out the anthocyanins more quickly and easily.
In addition to extracting and separating out anthocyanins, how to accurately identify and quantify the anthocyanins is attracting increasing attention. Thanks to UV/Vis absorption spectroscopy, the pH differential approach can detect anthocyanin concentrations without anthocyanin standards. This method is simple, fast, and accurate for anthocyanin quantification (Wang, 2014). Nevertheless, the pH differential method is not suitable for determining the quantities of individual anthocyanins in the samples. High-performance liquid chromatography (HPLC) is based on the pH difference method. It is easy, economical and effective, and makes it possible to determine individual anthocyanin concentrations. However, it is not easy to optimize chromatographic conditions, particularly when the sample contains multiple anthocyanin monomers.
Therefore, our objectives were i) to develop an HPLC method able to detect all the major anthocyanins in blueberry pomace simultaneously, and ii) to optimize the response surface method (RSM) for UAE of anthocyanins from blueberry pomace with a Box-Behnken (BBD) design.
2. Experimental conditions
2.1. Materials and chemicals
The blueberry pomace samples were obtained from a brewing workshop in Guizhou, China. Food grade lactic acid, fumaric acid, citric acid, malic and acetic acid were purchased from the Hongxin Biotechnology Company. HPLC grade methanol, acetonitrile and formic acid were purchased from the Thermo Fisher company.
2.2. Anthocyanins extraction
The blueberry pomace was frozen at −80 °C for 24 h and then freeze dried, crushed in a grinder, filtered through a 60-mesh sieve and stored at room temperature in brown bottles placed in a sealed container. Then the pomace powder was added to the extractant at a certain ratio of material to liquid (1:20, w/w), leached in an ultrasonic cleaner for a certain period of time, then centrifuged at 14,000g for 10 min at room temperature. The supernatant, that is, the crude extract of blueberry anthocyanins, was then concentrated and stored. Finally, to obtain purified anthocyanins, the crude extract was passed through a cationic column to remove phenolic acids and other impurities.
2.3. Single-factor extractions
We carried out single-factor studies to explore the influence of ultrasonic power, acid type, extraction temperature and time on the anthocyanin yield. The UAE process we used to extract the anthocyanins was based on a prior study, with minor adjustments (He et al., 2016). The total anthocyanin concentration, expressed as mg/g of dried blueberry pomace weight, was used to screen the optimal conditions for each of the four above-mentioned factors; these conditions were (i.e. the optimal acid) then applied to all subsequent optimization tests. For the single factor experiments, the ultrasonic power ranged from 250 ∼ 400 W, the extraction temperature from 30 ∼ 45 °C, and extraction time from 20 ∼ 50 min. Four types of acid were present: fumaric acid, lactic acid, malic acid and citric acid. Three parallel experiments were performed for each factor and the mean values were recorded.
2.4. Box-Behnken design (BBD) for optimized UAE
Based on the first experimental results we obtained, a three-level, three-factor Box-Behnken design was employed to investigate the association between extraction conditions (temperature, time and ultrasound power) and total anthocyanin yield (Y) to determine the joint effect of multiple variables on optimization. The three independent variables were coded at three levels (1, 0, +1), as shown below (Table 1): Temperature (°C): 30, 35, 40; Time (min): 20, 30, 40; Ultrasonic power (W): 300, 350, 400.
Table 1.
BBD for independent variables and observed responses.
| Run No.a | Temperature (°C) |
Time (min) |
Power (W) |
Yield (mg/100 g) |
|
|---|---|---|---|---|---|
| Actual value | Predicted value | ||||
| 1 | −1 (30) | −1 (20) | 0 (3 5 0) | 29.19 | 31.61 |
| 2 | 1 (40) | −1 (20) | 0 (3 5 0) | 26.07 | 33.38 |
| 3 | −1 (30) | 1 (40) | 0 (3 5 0) | 50.98 | 43.67 |
| 4 | 1 (40) | 1 (40) | 0 (3 5 0) | 90.98 | 88.56 |
| 5 | −1 (30) | 0 (30) | −1 (3 0 0) | 33.28 | 36.16 |
| 6 | 1 (40) | 0 (30) | −1 (3 0 0) | 47.20 | 45.20 |
| 7 | −1 (30) | 0 (30) | 1 (4 0 0) | 32.57 | 34.58 |
| 8 | 1 (40) | 0 (30) | 1 (4 0 0) | 75.10 | 72.21 |
| 9 | 0 (35) | −1 (20) | −1 (3 0 0) | 29.11 | 23.8 |
| 10 | 0 (35) | 1 (40) | −1 (3 0 0) | 36.72 | 41.15 |
| 11 | 0 (35) | −1 (20) | 1 (4 0 0) | 24.67 | 20.25 |
| 12 | 0 (35) | 1 (40) | 1 (4 0 0) | 64.83 | 70.14 |
| 13 | 0 (35) | 0 (30) | 0 (3 5 0) | 30.28 | 28.45 |
| 14 | 0 (35) | 0 (30) | 0 (3 5 0) | 34.28 | 28.45 |
| 15 | 0 (35) | 0 (30) | 0 (3 5 0) | 24.32 | 28.45 |
| 16 | 0 (35) | 0 (30) | 0 (3 5 0) | 20.27 | 28.45 |
| 17 | 0 (35) | 0 (30) | 0 (3 5 0) | 33.10 | 28.45 |
| Predicted | 114.27 | ||||
| Experimental | 108.23 | ||||
Run number is solely for identification purposes and does not represent the sequence in which the experimental runs were carried out.
2.5. Purifying the anthocyanins
To obtain the anthocyanin fraction, the crude extracts were purified to eliminate impurities following the procedure previously reported by Juadjur and Winterhalter (2012), with some modifications. First, a strong cation exchange (SCX) column was activated in four steps: (i) rinsing with a 1 N NaOH solution; (ii) removing mechanical impurities with water; (iii) rinsing with a 0.01 N HCl solution; (iv) again removing mechanical impurities with water until the filler became neutral. After that, the filler was rinsed with a small amount of methanol/glacial acetic acid at 19:1 (v/v). After adding an appropriate amount of extract solution, the filler was flushed with a large quantity of methanol/glacial acetic acid at 19:1 (v/v) to remove impurities such as flavonoids. During anthocyanin elution, 2 M of an NaCl solution was mixed with acidified methanol at a 1:1 (v/v) ratio, and the eluent was sampled to determine anthocyanin content (Fig. S1). After use, the cationic column was recycled for reuse in the separation and purification processes. It was soaked in methanol and stored at room temperature.
2.6. Determining total anthocyanins (TA)
As previously mentioned, results were expressed in terms of total anthocyanin content, which was obtained by differentiating pH levels (Borges, Vieira, Copetti, Gonzaga, & Fett, 2011). The blueberry pomace extracts were diluted with a 25-mM KCl-HCl solution (pH 1.0) and a 0.4-M NaAc-HAc solution (pH 4.5), and then stored at room temperature away from the light for 30 min to reach equilibrium. Absorbance was measured in a spectrophotometer at 520 nm and 700 nm, respectively. Total anthocyanin concentrations in the extract were determined and expressed as cyanidin-3-O-glucoside (C3G) according to the following equation (Eq. (1)):
| (1) |
where A = (A520 nm-A700 nm) pH 1.0-(A520 nm-A700 nm) pH 4.5; MW = the molecular weight of C3G (449.2 g/mol); DF = the dilution factor; V = the total volume of the diluted sample (mL); ε = the molar extinction coefficient of C3G (26,900 mol/L × cm); L = the path length (1 cm); m = the weight of dried blueberry pomace (g). All results are presented as mg C3G equivalents per 1 g dry weight of blueberry pomace (mg C3G/g).
2.7. Optimizing HPLC conditions
The spectrophotometric method described in this article is quick and inexpensive, but it is not targeted, it is susceptible to various interferences and can result in overestimated anthocyanin content. By contrast, chromatographic techniques such as HPLC can efficiently and precisely separate, characterize and quantify individual anthocyanins (Cañadas, Díaz, Rodríguez, González, & González-Miquel, 2022). We therefore performed chromatographic analyses on a Thermo Scientific™ UltiMate™ 3000 series HPLC equipped with an autosampler injector and a diode array detector (DAD). We used a C18 reverse column (4.6 × 250 mm, 5 μm, Thermo Fisher Scientific, USA) to separate the anthocyanins. To separate the main anthocyanins in blueberries simultaneously, we used blueberry anthocyanin extracts as samples to improve the chromatographic conditions that influence peak separation.
To obtain better results, we tested different mobile phases. Mobile phase A was either 2 % or 5 % formic acid and mobile phase B was 100 % acetonitrile, methanol acetonitrile (6:4, v/v) or 5 % formic acid acetonitrile. Classically, the change of flow has little influence on the time and shape of the peak; we therefore chose to compare two flow rates of 0.5 mL/min and 1.0 mL/min. After this preliminary comparison, we chose 5 % formic acid (mobile phase A) and methanol acetonitrile (6:4, v/v) (mobile phase B) for further optimization. There are many anthocyanin monomers in blueberry. Their similarity in structure and polarity makes them difficult to separate well by isocratic elution. Therefore, we used gradient elution to separate the individual anthocyanins present in the blueberry extracts. We then adjusted the elution gradient based on our preliminary results, to ensure that the baseline of the elution chromatogram remained steady and the peaks were well separated.
2.8. HPLC-MS analysis of anthocyanins
We used an HPLC system (Shimadzu Corporation, Kyoto, Japan) equipped with a Shimadzu triple quadrupole HPLC/MS-8045 mass spectrometer (Shimadzu Corp., Kyoto, Japan) and a column oven (30 °C) to analyze the samples. Volumes of 3.0 μL were injected into a reversed-phase C18 column (2.1 × 100 mm, 1.8 μm, Phenomenex, Tianjin, China). The mobile phase consisted of 1 % formic acid (A) and acetonitrile (B). The elution gradient for solvent B was as follows: 0–3 min, 6 %–6%; 3–6 min, 6 %–13 %; 6–26 min, 13 %–30 %; 26–28 min, 30 %-60 %; 28–30 min, 60 %-60 %; 30–32 min, 60 %–6%; 32–38 min, 6 %–6%, with a constant flow rate of 0.2 mL/min. Mass data were obtained in positive ion and full scan mode at a range of 100–1000 m/z. The curtain and collision gas were both nitrogen. For the MS analysis, we applied a source temperature of 300 °C, a desolvation temperature of 250 °C, a capillary voltage of 3 kV; a cone voltage of 30 V, and an elution solvent (nitrogen gas) flow rate of 650 L/h.
2.9. Statistical analysis
We used analysis of variance (ANOVA) to calculate the linear, quadratic and interaction regression coefficients on the Design Expert 13 software, and then analyzed the significance of the polynomial equation. We employed a regression coefficient for the statistical calculations and calculated R2 to determine the applicability of the statistical calculations. For some interactions, we created response surface plots. All experiments were repeated three times.
3. Results and discussion
3.1. Single-factor analysis
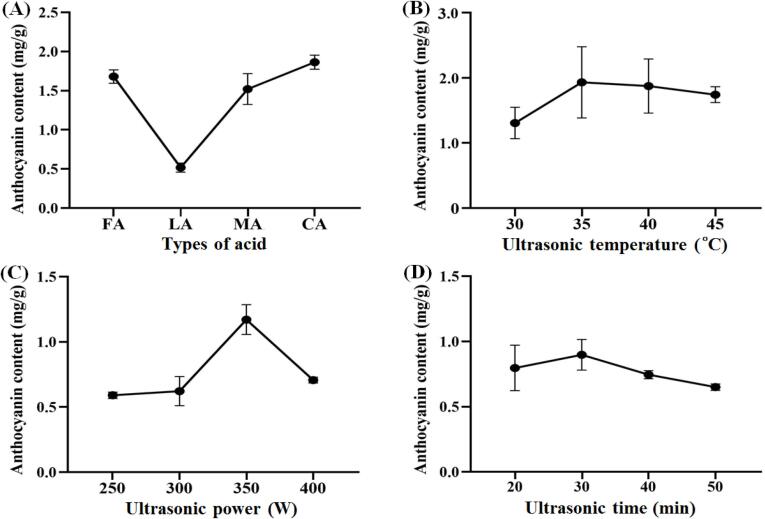
Several variables such as solvent composition, temperature, ultrasonic time and power can influence the efficiency of UAE (Carrera et al., 2012, Setyaningsih et al., 2016). As shown in Fig. 1A, among the four acids used to prepare the acidified ethanol, citric acid extracted the highest anthocyanin content from the blueberry wine pomace, while lactic acid was the lowest. Therefore, we used citric acid to prepare the acidified ethanol solution in subsequent experiments. When the extraction temperature was 35 °C, anthocyanin yield was relatively high (Fig. 1B). Furthermore, with increasing temperatures, anthocyanin content decreased, probably due to the degradation of anthocyanins in this process. Fig. 1C shows that anthocyanin content reached a maximum at 350 W and then decreased dramatically, which may have been caused by excessive ultrasonic cavitation that destroyed the structure of the anthocyanins (He et al., 2016). Finally, different ultrasound times (Fig. 1D) made a small difference in the anthocyanin quantities extracted.
Fig. 1.
Single-factor experiments on solubilization of total anthocyanin from dried blueberry pomace. Factors: types of acid (A), FA, Fumaric acid, LA, Lactic acid, MA, Malic acid, CA, Citric acid; ultrasonic temperature (B); ultrasonic power (C); ultrasonic time (D).
3.2. Optimizing HPLC conditions
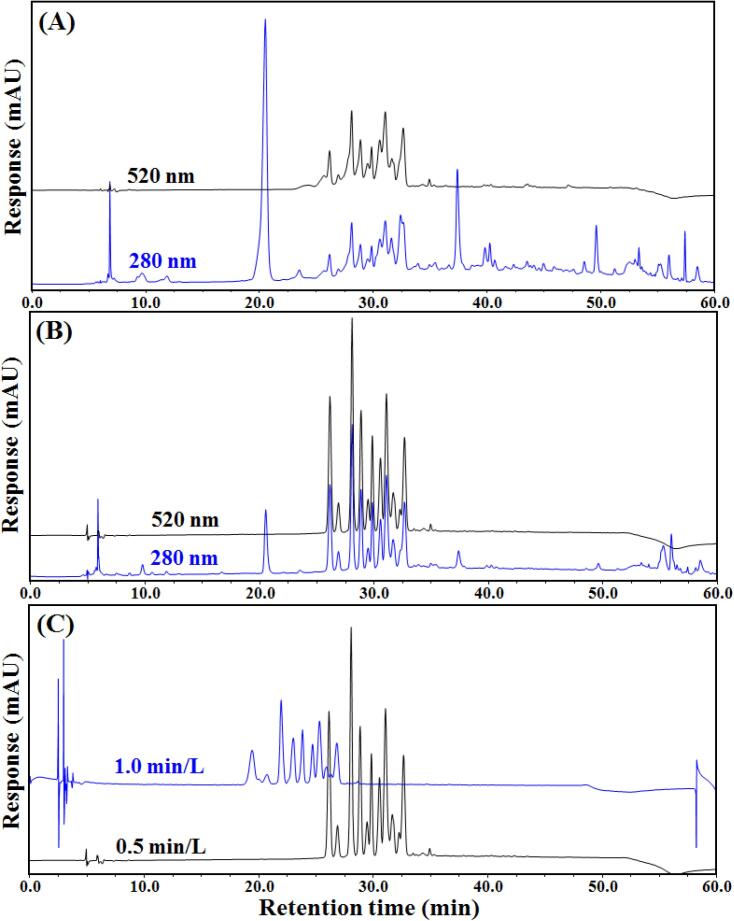
Blueberry contains a variety of bioactive substances including anthocyanins, procyanidins, phenolic acids, stilbenes and organic acids. Fig. 2 shows the HPLC chromatograms of anthocyanins isolated from the original blueberry pomace extract (A), from the extract after purification through an SCX column (B), and at different elution flow rates (C). With a chromatograph, anthocyanins can be distinguished from other flavonoids because they have different absorption limits at different absorbance values. Flavonoids only absorb at 280 nm, whereas anthocyanins also absorb at 520 nm. Therefore, chromatogram (Fig. 2A) shows the peaks of both anthocyanins and other flavonoids in the extract. In contrast, we obtained purified anthocyanin fraction using an SCX column to remove most of the other flavonoids, the chromatograms at 280 nm and 520 nm being almost identical (Fig. 2B). The characteristic peaks of anthocyanins were clearer and the separation had improved. These chromatograms show that it is possible to separate the anthocyanins from most of the other flavonoids in the blueberry pomace extracts with an SCX column.
Fig. 2.
HPLC elution patterns of anthocyanin extracts obtained from blueberry pomace. Sample before (A) and after (B) purification using the cation column, and flow rate for HPLC was 0.5 mL/min; Different flow rates (0.5 mL/min vs 1.0 mL/min) for HPLC was compared at 520 nm (C). The elution gradient was the same for all samples. Solvent A = 2 % formic acid; Solvent B = acetonitrile. The elution conditions for solvent B were: 0–15 min, 5–10 %; 15–45 min, 10–40 %; 45–50 min, 40–90 %; 50–55 min, 90 %; 50–51 min, 90 %-5%, 55–65 min, 5 %. Column temperature was 30 °C.
We then tested the effects of flow rate on the separation of peaks. We found that better separation of peaks was achieved at a flow rate 0.5 mL/min than that at a flow rate 1.0 mL/min (Fig. 2C). This is because the inter-column conversion reaction is slower at higher flow rates, and the resolution between critical peak pairs decreases with increasing flow rates (Yildirim, Kadioglu, Saglam, Yasar, & Sellitepe, 2016). We therefore conducted our subsequent experiments at a flow rate of 0.5 mL/min.
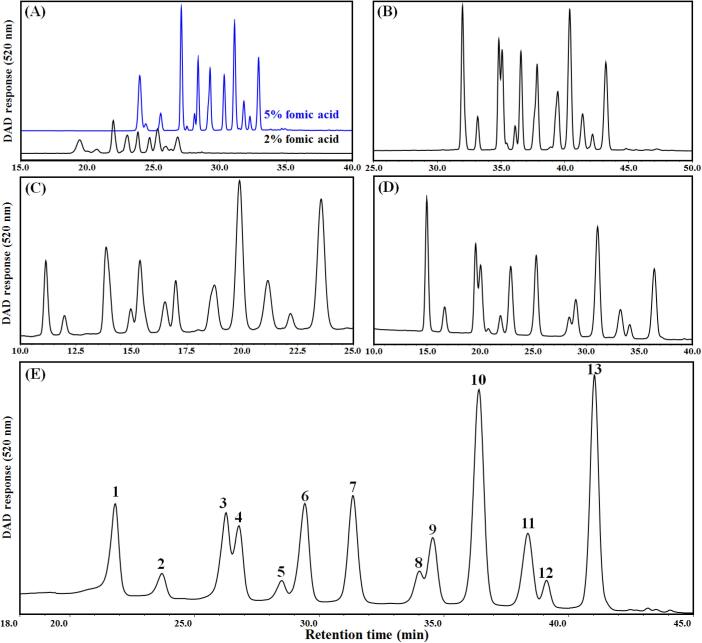
We also compared the different mobile phase for optimal separation of anthocyanins. Anthocyanins are a class of flavonoids that are readily soluble in water and several other organic solvents. To efficiently detect anthocyanins through HPLC, it is critical to select a suitable elution solvent. Methanol and acetonitrile, as well as mildly acidified solvents like formic acid and acetic acid, are the most commonly-used elution solvents in HPLC. Taking into account both safety considerations and the need for maximum anthocyanin release, we tested various solvents to elute anthocyanins from the HPLC stationary phase. We first increased the content of formic acid in mobile phase A and found that a 5 % formic acid solution worked the best (Fig. 3A). We then tested different mobile phase B. A 5 % formic acid acetonitrile (B, v/v) together with 5 % formic acid (A, v/v) improved the peak separation (Fig. 3B). To further separate the anthocyanins in blueberry extracts, we tried a mobile phase B of methanol acetonitrile (6:4, v/v) together with a mobile phase A of 5 % formic acid used in a previous study for anthocyanin separation (Yang et al., 2019). By using this, a better separation of blueberry anthocyanins was achieved with a longer retention time of the components and better peak shape and symmetry (Fig. 3C and 3D) comparing to the mobile phase used for the pattern in Fig. 3B.
Fig. 3.
HPLC patterns of purified-anthocyanin elution with different solvents and elution gradients. Mobile phase A: 2 % or 5 % formic acid and mobile phase B: Acetonitrile (A); Mobile phase A: 5 % formic acid and mobile phase B: 5 % formic acid acetonitrile (v/v) (B); Mobile phase A: 5 % formic acid and mobile phase B: methanol acetonitrile (6:4, v/v) (C, D, E). The elution conditions for mobile phase B were: 0–15 min, 5–10 %; 15–45 min, 10–40 %; 45–50 min, 40–90 % (A, B). 0–35 min, 18–40 % (C). 0–20 min, 15–22 %; 20–50 min, 22–40 % (D). 0–30 min, 10–25 %; 30–40 min, 25–35 %; 40–45 min, 35–90 %; 45–50 min, 90 % (E). Column temperature was 30 °C.
We then studied different elution gradients to obtain better anthocyanin monomers from the blueberry anthocyanin extracts, based on the above-mentioned optimization experiments. These elution conditions included a 10-uL injection volume, a 0.5-mL/min flow rate, a 30-°C column temperature, and a 520-nm monitoring wavelength. As shown in Fig. 3D, almost all the peaks occurred within 40 mins. In order to shorten anthocyanin separation time and increase the baseline separation of some peaks, we successively adjusted the elution gradient. Eventually, we reached an optimal initial composition of 90 % solvent A and 10 % solvent B. The elution conditions for solvent B (0–30 min, 10–25 %; 30–40 min, 25–35 %; 40–45 min, 35–90 %; 45–50 min, 90 %) proved to have a good separation effect and suitable time intervals. Under these conditions, we successfully isolated 13 anthocyanin monomers from the blueberry anthocyanin extracts (Fig. 3E).
3.3. Identification of anthocyanins
In this study, a total of 13 anthocyanins were identified (see Table 2, which includes other relevant information like proportion). It is known from the literature that galactose, glucose or arabinose can be used for glycosylation of anthocyanins; elution occurs in this order. Thus, the first peak of each anthocyanin is a galactoside, the second a glucoside, and the third an arabinoside (Gavrilova et al., 2011, Muller et al., 2012). We identified glucoside, galactoside or arabinoside derivatives of five anthocyanidins: delphinidin, cyanidin, petunidin, peonidin and malvidin. Moreover, the malvidin (48.05 %) glycosides constituted most of the anthocyanins, accounting for nearly half of their total, and malvidin-3-galactoside (peak 11; 24.34 %) was the most abundant individual anthocyanin. We identified fewer anthocyanin monomers than Yousef et al. (2013), who identified 18 monomers, but more than the number reported by Bunea et al. (2013). There are many reasons for this result, including differences in the blueberry varieties used, in the mobile phases and in flow gradients. Furthermore, because blueberry contains a variety of anthocyanins and not all standards are accessible or affordable, we based peak identification in this study on retention times, elution sequences, etc. expected from previous publications (Gapski et al., 2019, Hu et al., 2019, Juadjur and Winterhalter, 2012, Muller et al., 2012, Oh et al., 2018, Si et al., 2020).
Table 2.
Identification of anthocyanins in blueberry pomace.
| Peak | Compound | Ret.Time (min) | +ESIMS (m/z) | Formula | Proportion (%) |
|---|---|---|---|---|---|
| 1 | Delphinidin-3-galactoside | 21.827 | 465 | C21H21O12 | 7.74 |
| 2 | Delphinidin-3-glucoside | 23.703 | 465 | C21H21O12 | 1.99 |
| 3 | Cyanidin-3-galactoside | 26.273 | 449 | C21H21O11 | 3.87 |
| 4 | Delphinidin-3-arabinoside | 26.787 | 435 | C20H19O11 | 3.22 |
| 5 | Cyanidin-3-glucoside | 28.503 | 449 | C21H21O11 | 1.22 |
| 6 | Petunidin-3-galactoside | 29.437 | 479 | C22H23O12 | 9.73 |
| 7 | Cyanidin-3-arabinoside | 31.363 | 419 | C20H19O10 | 11.33 |
| 8 | Petunidin-3-glucoside | 34.030 | 479 | C22H23O12 | 1.02 |
| 9 | Peonidin-3-glucoside | 34.567 | 463 | C22H23O11 | 4.41 |
| 10 | Petunidin-3-arabinoside | 38.377 | 449 | C21H21O11 | 7.33 |
| 11 | Malvidin-3-galactoside | 36.413 | 493 | C23H25O12 | 24.34 |
| 12 | Malvidin-3-glucoside | 39.120 | 493 | C23H25O12 | 1.52 |
| 13 | Malvidin-3-arabinoside | 41.047 | 463 | C22H23O11 | 22.19 |
3.4. Analysis of variance and optimization of the interactions among factors
To determine the coefficients of the equation and to analyze the variance data, we subjected the total anthocyanin model to ANOVA, with anthocyanin yield as the response value (Y). The regression equation (model) among the three variables and Y was as follows (Eq. (2)):
| (2) |
where Y is total anthocyanin content (mg/100 g), A is temperature (°C), B is extraction time (Min) and C is ultrasonic power (W).
As shown in Table 3, the model was highly significant for the experimental results with a 95 % confidence level (p-value = 0.0018). R2 (0.94), R2adj (0.86) and Adeq Precision (12.0620, >4.0) values clearly proved that the model fitted the experimental data well. As the lack of fit was not significant (p-value = 0.2273), the model we propose is robust for predicting anthocyanin content based on the factors studied.
Table 3.
Results of ANOVA analysis for the total anthocyanin model.
| Variable | Sum of Squares | Df | Mean Square | F-value | P-value | Significant |
|---|---|---|---|---|---|---|
| Model | 5823.27 | 9 | 647.03 | 11.87 | 0.0018 | ** |
| A-Temperature | 1088.98 | 1 | 1088.98 | 19.97 | 0.0029 | ** |
| B-Time | 2260.10 | 1 | 2260.10 | 41.45 | 0.0004 | ** |
| C-Power | 323.38 | 1 | 323.38 | 5.93 | 0.0451 | ** |
| AB | 464.85 | 1 | 464.85 | 8.53 | 0.0223 | ** |
| AC | 204.60 | 1 | 204.60 | 3.75 | 0.0939 | * |
| BC | 264.72 | 1 | 264.72 | 4.85 | 0.0643 | * |
| A2 | 888.99 | 1 | 888.99 | 16.30 | 0.0049 | ** |
| B2 | 168.53 | 1 | 168.53 | 3.09 | 0.1221 | |
| C2 | 69.39 | 1 | 69.39 | 1.27 | 0.2964 | |
| Residuals | 381.68 | 7 | 54.53 | |||
| Lack of Fit | 238.77 | 3 | 79.59 | 2.23 | 0.2273 | Not significant |
| Pure Error | 142.91 | 4 | 35.73 | |||
| Cor Total | 6204.95 | 16 | ||||
| Adeq Precision | 12.0620 | |||||
| R2 | 0.9385 | |||||
| R2adj | 0.8594 |
Level of significance: **, p < 0.05; *, 0.05 < p < 0.1. Df, Degree of freedom.
Furthermore, three linear (A, B and C) and quadratic coefficients (A2) were significant for anthocyanin extraction while the other quadratic coefficients (B2, C2) had an insignificant effect. The interaction between extraction power, temperature and time showed a less significant effect. Among the three independent factors, time was the most important in anthocyanin extraction, followed by extraction temperature and power.
To predict the associations between the independent and dependent variables, we used the full model resulting from the regression equation to generate 3D response surface plots and contour plots. Fig. S2A and B show a steep slope in the response surface for the interaction between temperature and time, indicating that the interaction is significant, which is also supported by the coefficients in Table 3. The extraction rate of anthocyanin in blueberry increases significantly with time at the same sonication temperature. In contrast, when time was kept constant and the ultrasonic temperature was gradually increased, the increase in extraction rate was slower. Fig. S2C and D show the relationship between time and power on anthocyanin content, which was similar to the relationship between temperature and power. As seen in Fig. S2E and F, the response surface of the interaction between temperature and power has a gentle slope and sparse contour lines, indicating that the significance of the interaction between the two factors is relatively low. This conclusion is consistent with the three independent variables (F) in the analysis of variance. The trend reflected by the value ordering is the same. The maximum predicted total anthocyanin content (114.27 mg/100 g, DW) was therefore obtained under the estimated optimum conditions (temperature = 40 °C, time = 60 min and power = 400 W) (Table 1).
On this basis, we observed the changes in contents for each anthocyanin in the blueberry pomace under 17 different ultrasonic treatments. The anthocyanins were quantified by HPLC as C3G equivalents at 520 nm using commercially available C3G as a reference standard. Results shown in Supplementary Fig. S3 and significant differences are shown in Supplementary Table S1. The graph shows a consistent trend for each anthocyanin, with Group 6 having the highest anthocyanin content under ultrasound conditions, followed by Groups 8 and 11. This phenomenon shows that, under suitable temperature conditions, prolonging the time and power of ultrasonic treatment increases the extraction of blueberry anthocyanins. In addition, one study has shown that the relative levels of individual anthocyanins vary greatly among different genotypes. For example, quantities for delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidin-3-O-glucoside, and malvidin-3-O-glucoside were respectively up to 77, 52, 75 and 33 fold higher in high-yielding genotypes compared to other genotypes (Yousef et al., 2013). This may account for the low anthocyanin content in our study, in addition to the low anthocyanin levels in the blueberry residue itself.
4. Conclusion
This study optimized ultrasonic-assisted extraction (UAE) conditions with a Box-Behnken design (BBD) to produce maximum anthocyanin extracts from blueberry residue. The crude anthocyanin extracts were then purified in a cation column. Finally, the anthocyanins were quantified and identified through optimized HPLC and HPLC-MS. The HPLC-MS results showed that a total of 13 anthocyanins were isolated, including mainly malvidin, petunidin, peonidin, delphinidin and cyanidin. The optimum extraction conditions were a temperature of 40 °C, an ultrasonic power of 400 W and an extraction time of 40 min. The optimum yield was 108.23 mg/100 g DW, which was close to the predicted value. In conclusion, the mathematical models described in this paper can provide practical information on the recovery of anthocyanins from blueberry pomace, improve the efficiency and productivity of the blueberry processing industry, while potentially reducing the environmental impact of blueberry waste products.
Funding
This work was supported by the International Science and Technology Cooperation Project of Guangdong (2021A0505030043), the Foundation of Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences (AB202110), the Pearl River Talent Plan (2017GC010387), and the Youth Science and Technology Innovation Talent of Guangdong TeZhi Plan (2019TQ05N770).
CRediT authorship contribution statement
Xuan Zhang: Methodology, Investigation, Validation, Writing – original draft. Songen Wang: Methodology, Validation, Visualization. Qixia Wu: Methodology, Validation. Maurizio Battino: Writing – review & editing. Francesca Giampieri: Writing – review & editing. Weibin Bai: Supervision, Writing – review & editing. Lingmin Tian: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100476.
Contributor Information
Weibin Bai, Email: baiweibin@163.com.
Lingmin Tian, Email: tianlinmin@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Belwal T., Huang H., Li L., Duan Z., Zhang X., Aalim H., Luo Z. Optimization model for ultrasonic-assisted and scale-up extraction of anthocyanins from Pyrus communis ‘Starkrimson’ fruit peel. Food Chemistry. 2019;297 doi: 10.1016/j.foodchem.2019.124993. [DOI] [PubMed] [Google Scholar]
- Borges G.D.S.C., Vieira F.G.K., Copetti C., Gonzaga L.V., Fett R. Optimization of the extraction of flavanols and anthocyanins from the fruit pulp of Euterpe edulis using the response surface methodology. Food Research International. 2011;44(3):708–715. [Google Scholar]
- Bunea A., Rugina D., Sconta Z., Pop R., Pintea A., Socaciu C.…VanCamp J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16–F10 metastatic murine melanoma cells. Phytochemistry. 2013;95:436–444. doi: 10.1016/j.phytochem.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Cañadas R., Díaz I., Rodríguez M., González E.J., González-Miquel M. An integrated approach for sustainable valorization of winery wastewater using bio-based solvents for recovery of natural antioxidants. Journal of Cleaner Production. 2022;334 [Google Scholar]
- Carrera C., Ruiz-Rodríguez A., Palma M., Barroso C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Analytica chimica acta. 2012;732:100–104. doi: 10.1016/j.aca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Chu W., Gao H., Cao S., Fang X., Chen H., Xiao S. Composition and morphology of cuticular wax in blueberry (Vaccinium spp.) fruits. Food Chemistry. 2017;219:436–442. doi: 10.1016/j.foodchem.2016.09.186. [DOI] [PubMed] [Google Scholar]
- Eladwy R.A., Mantawy E.M., El-Bakly W.M., Fares M., Ramadan L.A., Azab S.S. Mechanistic insights to the cardioprotective effect of Blueberry Nutraceutical Extract in Isoprenaline-Induced Cardiac Hypertrophy. Phytomedicine. 2018;51:84–93. doi: 10.1016/j.phymed.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Gapski A., Gomes T.M., Bredun M.A., Ferreira-Lima N.E., Ludka F., Bordignon-Luiz M.T., Burin V. Digestion behavior and antidepressant-like effect promoted by acute administration of blueberry extract on mice. Food Research International. 2019;125 doi: 10.1016/j.foodres.2019.108618. [DOI] [PubMed] [Google Scholar]
- Gavrilova V., Kajdzanoska M., Gjamovski V., Stefova M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. Journal of Agricultural and Food Chemistry. 2011;59(8):4009–4018. doi: 10.1021/jf104565y. [DOI] [PubMed] [Google Scholar]
- He B., Zhang L., Yue X., Liang J., Jiang J., Gao X., Yue P. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chemistry. 2016;204:70–76. doi: 10.1016/j.foodchem.2016.02.094. [DOI] [PubMed] [Google Scholar]
- Herrera-Balandrano D.D., Chai Z., Beta T., Feng J., Huang W. Blueberry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends in Food Science & Technology. 2021;118:808–821. [Google Scholar]
- Hu W., Gong H., Li L., Chen S., Ye X. Ultrasound treatment on stability of total and individual anthocyanin extraction from blueberry pomace: Optimization and comparison. Molecules. 2019;24(14):2621. doi: 10.3390/molecules24142621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui T., Lee W.Y., Yong H.C. Optimization of microwave-assisted extraction for anthocyanins, polyphenols, and antioxidants from raspberry (Rubus Coreanus Miq.) using response surface methodology. Journal of Separation Science. 2013;36(18):3107–3114. doi: 10.1002/jssc.201300303. [DOI] [PubMed] [Google Scholar]
- Jurgilevich A., Birge T., Kentala-Lehtonen J., Korhonen-Kurki K., Pietikäinen J., Saikku L., Schösler H. Transition towards Circular Economy in the Food System. Sustainability. 2016;8(1):69. [Google Scholar]
- Juadjur A., Winterhalter P. Development of a novel adsorptive membrane chromatographic method for the fractionation of polyphenols from bilberry. Journal of Agricultural and Food Chemistry. 2012;60(10):2427–2433. doi: 10.1021/jf2047724. [DOI] [PubMed] [Google Scholar]
- Li D., Li B., Ma Y., Sun X., Lin Y., Meng X. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. Journal of Food Composition and Analysis. 2017;62:84–93. [Google Scholar]
- Liu H., Qin S., Sirohi R., Ahluwalia V., Zhou Y., Sindhu R.…Binod P. Sustainable blueberry waste recycling towards biorefinery strategy and circular bioeconomy: A review. Bioresour Technol. 2021;332 doi: 10.1016/j.biortech.2021.125181. [DOI] [PubMed] [Google Scholar]
- Luo B., Gu R., Kennelly E.J., Long C. Gaultheria ethnobotany and bioactivity: Blueberry relatives with anti-inflammatory, antioxidant, and anticancer constituents. Current Medicinal Chemistry. 2017;25(38):5168–5176. doi: 10.2174/0929867324666171003122502. [DOI] [PubMed] [Google Scholar]
- Grace M.H., Xiong J., Esposito D., Ehlenfeldt M., Lila M. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chemistry. 2019;277:336–346. doi: 10.1016/j.foodchem.2018.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B., Kapetanaki A.B., Wang P. Completing the food waste management loop: Is there market potential for value-added surplus products (VASP)? Journal of Cleaner Production. 2020;256 [Google Scholar]
- Morita M., Naito Y., Yoshikawa T., Niki E. Antioxidant capacity of blueberry extracts: Peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by multiple oxidants. Journal of Berry Research. 2017;7(1):1–9. [Google Scholar]
- Muller D., Schantz M., Richling E. High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. Journal of Food Science. 2012;77(4):C340–C345. doi: 10.1111/j.1750-3841.2011.02605.x. [DOI] [PubMed] [Google Scholar]
- Oancea S., Ketney O., Grosu C., Stoia M. Conventional and ultrasound-assisted extraction of anthocyanins from blackberry and sweet cherry cultivars. Acta Chimica Slovenica. 2013;60(2):383–389. [PubMed] [Google Scholar]
- Oh H.D., Yu D.J., Chung S.W., Chea S., Lee H.J. Abscisic acid stimulates anthocyanin accumulation in 'Jersey' highbush blueberry fruits during ripening. Food Chemistry. 2018;244:403–407. doi: 10.1016/j.foodchem.2017.10.051. [DOI] [PubMed] [Google Scholar]
- Paes J., Dotta R., Barbero G.F., Martínez J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. Journal of Supercritical Fluids. 2014;95:8–16. [Google Scholar]
- Pérez R., Laca A., Laca A., Díaz M. Environmental behaviour of blueberry production at small-scale in Northern Spain and improvement opportunities. Journal of Cleaner Production. 2022;339 [Google Scholar]
- Santos P., Aguiar A.C., Barbero G.F., Rezende C.A., Martínez J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrasonics Sonochemistry. 2015;22:78–88. doi: 10.1016/j.ultsonch.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Setyaningsih W., Duros E., Palma M., Barroso C.G. Optimization of the ultrasound-assisted extraction of melatonin from red rice (Oryza sativa) grains through a response surface methodology. Applied Acoustics. 2016;103:129–135. [Google Scholar]
- Si X., Tian J., Shu C., Wang Y., Gong E., Zhang Y.…Li B. Serum ceramide reduction by blueberry anthocyanin-rich extract alleviates insulin resistance in hyperlipidemia mice. Journal of Agricultural and Food Chemistry. 2020;68(31):8185–8194. doi: 10.1021/acs.jafc.0c01931. [DOI] [PubMed] [Google Scholar]
- Tan J., Li Q., Xue H., Tang J. Ultrasound-assisted enzymatic extraction of anthocyanins from grape skins: Optimization, identification, and antitumor activity. Journal of Food Science. 2020;85(11):3731–3744. doi: 10.1111/1750-3841.15497. [DOI] [PubMed] [Google Scholar]
- Wang H. Rapid quantitative analysis of individual anthocyanin content based on high-performance liquid chromatography with diode array detection with the pH differential method. Journal of Separation Science. 2014;37(18):2535–2544. doi: 10.1002/jssc.201400364. [DOI] [PubMed] [Google Scholar]
- Yang N., Qiu R., Yang S., Zhou K., Wang C., Ou S., Zheng J. Influences of stir-frying and baking on flavonoid profile, antioxidant property, and hydroxymethylfurfural formation during preparation of blueberry-filled pastries. Food Chemistry. 2019;287:167–175. doi: 10.1016/j.foodchem.2019.02.053. [DOI] [PubMed] [Google Scholar]
- Yang W., Guo Y., Liu M., Chen X., Xiao X., Wang S., Gong P. Structure and function of blueberry anthocyanins: A review of recent advances. Journal of Functional Foods. 2022;88 [Google Scholar]
- Yildirim S., Kadioglu A., Saglam A., Yasar A., Sellitepe H.E. Fast determination of anthocyanins and free pelargonidin in fruits, fruit juices, and fruit wines by high-performance liquid chromatography using a core-shell column. Journal of Separation Science. 2016;39(20):3927–3935. doi: 10.1002/jssc.201600661. [DOI] [PubMed] [Google Scholar]
- Yousef G.G., Brown A.F., Funakoshi Y., Mbeunkui F., Grace M.H., Ballington J.R.…Lila M.A. Efficient quantification of the health-relevant anthocyanin and phenolic acid profiles in commercial cultivars and breeding selections of blueberries (Vaccinium spp.) Journal of Agricultural and Food Chemistry. 2013;61(20):4806–4815. doi: 10.1021/jf400823s. [DOI] [PubMed] [Google Scholar]
- Zheng X., Xu X., Liu C., Sun Y., Lin Z., Liu H. Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Separation and Purification Technology. 2013;104:17–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.