Abstract
Titanium (Ti) has been widely used for manufacturing of bone implants because of its mechanical properties, biological compatibility, and favorable corrosion resistance in biological environments. However, Ti implants are prone to infection (peri-implantitis) by bacteria which in extreme cases necessitate painful and costly revision surgeries. An emerging, viable solution for this problem is to use copper (Cu) as an antibacterial agent in the alloying system of Ti. The addition of copper provides excellent antibacterial activities, but the underpinning mechanisms are still obscure. This review sheds light on such mechanisms and reviews how incorporation of Cu can render Ti–Cu implants with antibacterial activity. The review first discusses the fundamentals of interactions between bacteria and implanted surfaces followed by an overview of the most common engineering strategies utilized to endow an implant with antibacterial activity. The underlying mechanisms for antibacterial activity of Ti–Cu implants are then discussed in detail. Special attention is paid to contact killing mechanisms because the misinterpretation of this mechanism is the root of discrepancies in the literature.
Keywords: Antibacterial mechanisms, Ti–Cu implants, Contact killing, Ion releasing, Anti-infection, Biomaterials, Antimicrobial
Graphical abstract
List of abbreviations
- AFM
Atomic Force Microscopy
- AgNP
Silver nanoparticle
- AMP
Antimicrobial Peptide
- at. %
Atomic Percentage
- ATP
Adenosine Triphosphate
- ATPase
Adenosine Triphosphatase
- B. cereus
Bacillus cereus
- D. radiodurans
Deinococcus radiodurans
- DCMS
Direct Current Magnetron Sputtering
- DNA
Deoxyribonucleic Acid
- E. coli
Escherichia coli
- E. hirae
Enterococcus hirae
- EPA
Environmental Protection Agency
- EPS
Extracellular Polymeric Substance
- ETS
Electron Transport System
- HiPIMS
High Power Impulse Magnetron Sputtering
- K. pneumoniae
Klebsiella pneumoniae
- MAPD
Micro-area Potential Difference
- MIC
Minimum Inhibitory Concentration
- MRSA
Methicillin-Resistant Staphylococcus aureus
- MSC
Mesenchymal Stem Cell
- P. aeruginosa
Pseudomonas aeruginosa
- P. gingivalis
Porphyromonas gingivalis
- PEG
Polyethylene Glycol
- PEM
Polyelectrolyte Multilayers
- PIII
Plasma Immersion Ion Implantation
- PMF
Proton Motive Force
- PPO
Propylene Oxide
- RGD
Arginylglycylaspartic Acid
- RNA
Ribonucleic Acid
- ROS
Reactive Oxygen Species
- S. aureus
Staphylococcus aureus
- S. epidermidis
Staphylococcus epidermidis
- S. mutans
Streptococcus mutans
- SBMA
Sulfobetaine Methacrylate
- SEM
Scanning Electron Microscopy
- SLA
Sandblasting and Acid Etching
- SLE
Selective Acid Etching
- SLM
Selective Laser Melting
- TEM
Transmission Electron Microscopy
- TNT
Titanium Nano Tubes
- WHO
World Health Organization
- wt. %
Weight Percentage
1. Introduction
Once an implant is exerted in the human body, the pathogenic bacteria and the human host cells compete for the surface of the implant, as described by the term “race for the surface” [1]. If the bacteria possess the upper hand and adhere to the surface of the implant prior to the tissue cells, biofilm formation ensues, which brings about a precarious state in which the bacteria resist the attack from antibiotics and the host immune system. This process results in bacterial infection around the implant [2,3]. Consequently, the formation of new bone around the surgical implant is hindered, which is conducive to the biomedical implant loosening and several revision surgeries to constrain the infection [4].
The infection associated with implants is the second major cause for postoperative complaints in clinical practice [5]. The prevalence of infection after arthroplasty of the knee, hip, and ankle is estimated to be 0.5–2%, 0.3–1.7%, and 2–9%, respectively [[6], [7], [8]]. In the United States alone, for example, 4.3% of orthopedic implants fail every year due to bacterial infections [9]. In the case of dental implants, infection occurs with a rate of 9.3–12.8% at the implant level and 18.5–19.8% at the patient level [10,11]. In extreme cases, getting rid of such implant-related infections requires revision surgeries which are painful and bring an economic burden to patients and healthcare systems [12,13].
Titanium and its alloys have been extensively used in medical applications because of their high biocompatibility, excellent corrosion resistance, and favorable mechanical properties. Thus, titanium alloys are exploited as the favored materials to fabricate artificial joints (hip, knee, shoulder, ankle, elbow, wrist, knuckle, etc.), bone fixations (intramedullary nail, plate, screw, etc.), braces, and dental implants [[14], [15], [16], [17], [18]]. However, titanium implants are prone to bacterial invasion despite antibiotic administration and strict aseptic techniques, because they are bio-inert and do not resist bacterial adhesion and biofilm formation [19,20]. An effective approach to combat the incidence of bacterial infection on implants is to fabricate an implant with antibacterial activity to inhibit the initial bacterial adherence [19].
Copper has garnered growing interest in this area because of its excellent antibacterial activity and significant role in human metabolism. As such, Ti–Cu alloys with favorable antibacterial properties have been established as highly promising materials for fabricating implantable medical devices [21].
Although extensive efforts have been made to produce antibacterial Ti–Cu alloys, our understanding of why Ti–Cu demonstrates the antibacterial activity is still largely limited. The acquisition of this knowledge is crucial for designing Ti–Cu alloys with engineered microstructures to achieve enhanced antibacterial properties. Since we need to make links between different realms of knowledge, including microbiology, material science, chemistry, and even physics, to understand the multifaceted concepts of antibacterial mechanisms in the Ti–Cu implant, achieving this goal seems to be far-fetched, and also lack of it may be the root of a large body of contradictions found in the published literature [22]. Despite these complexities, deductive and comprehensive analysis of existing data can help us to determine the underlying mechanisms of action.
Here, we first briefly discuss the immediate interactions of bacteria with a surface implanted in the human body. According to these interactions, current stage-of-the-art strategies to prohibit bacterial infections on the implant surface are briefly reviewed. We then discuss the antibacterial behavior of copper and the underpinning mechanisms. Next, the mechanisms of antibacterial activity in Ti–Cu implants are discussed and delineated. Specifically, ion releasing and the contact killing mechanisms and various key factors affecting each are highlighted, because the lack of differentiation between these two mechanisms in the literature has sowed dissension among scholars. The paper finally concludes by providing suggestions for further investigations and possible future directions in the area of Ti–Cu implants. The mechanisms of action elucidated and discussed in this review open up new avenues for fundamental research on understanding the bacteria-surface interactions with important implications for a wide range of practical applications, including the fabrication of next-generation antimicrobial implantable devices.
2. Bacteria and their interactions with implant materials
Bacteria are classified as prokaryotes and can be found practically everywhere on Earth in many shapes and sizes [23]. Each bacterial cell is made of three main parts: cell envelope, cytoplasm, and the nucleoid. In most bacteria, the cell envelope is comprised of a capsule, cell wall, and cytoplasmic membrane, which protect the cytoplasm of the cell and defend the bacteria from external damages. An important component of the cell envelope is the cell wall which is mainly composed of peptidoglycan [24]. Although the cell wall of all bacteria has peptidoglycan, not all cells have the same overall structure of peptidoglycan. In fact, the thickness of peptidoglycan separates two important types of bacteria, namely Gram-negative and Gram-positive bacteria [25].
The Gram-negative cell wall is composed of a single layer of peptidoglycan surrounded by a membranous structure called the outer membrane and a periplasm. The cell wall in Gram-negative is thinner and less compact compared to that of Gram-positive bacteria. Generally, in Gram-positive bacteria, the cell wall is thick and consists of several layers of peptidoglycan. They lack the outer membrane envelope found in Gram-negative bacteria [26] (Scheme.1). Beneath the cell wall, a thin living membrane (cytoplasmic membrane) exists that is made of phospholipids and proteins. This membrane functions as a selective permeability barrier that regulates the passage of substances into and out of the cell and separates vital components of the cell from harmful components of the environment. The bacterial cell membrane has a variety of functions for energy production and biosynthesis in the cell. A membrane is a place where there is an electron transport system (ETS) that produces energy during respiration or photosynthesis, and it contains enzymes called ATP synthetase (ATPase) that convert energy into a useable state in a bacterial cell, so-called adenosine triphosphate (ATP). Other vital components of a bacteria cell are cytoplasm and nucleoid, where the DNA is located [23].
Scheme 1.
(a) General structure of a bacteria cell, and the cell wall structure in (b) Gram-negative bacteria, and (c) Gram-positive bacteria.
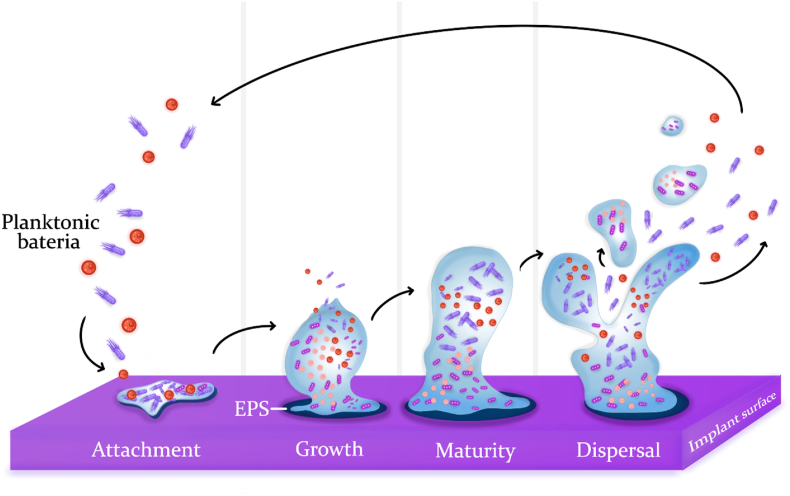
2.1. Biofilm formation
Bacteria that are capable of colonizing the human body are creative and adapt themselves against the defense system of the body [27]. One of the specific methods by which bacteria adapt themselves to environmental conditions is the ability to form biofilm as a strategy to deal with environmental hazards. Biofilm is defined as structured microbial communities embedded in a self-produced extracellular matrix that adheres to a susceptible surface [28]. Biofilm formation is a complex process that consists of several phases: growth, maturity, attachment, and dispersal (Scheme 2). In short, initial colonizers form biofilm by secretion of extracellular polymeric substances (EPS) containing molecules of glycoproteins and polysaccharides. This viscous substance makes thousands of bacteria join together. In the next phases, the secretion of this matrix is multiplied by different species of bacteria until it leads to the formation of a mature mixed-population biofilm [29,30]. Bacteria make up less than 1/3 of the total biofilm volume, and the rest of the volume in biofilm is occupied by the EPS.
Scheme 2.
Different stages of biofilm formation and development on a susceptible surface. Attachment: Initial colonizers adhere to the implant surface due to van der Waals forces and reproduce, followed by secretion of a gel-like substance (extracellular polysaccharides). Growth: The substance tenaciously bounds with the surface and protects bacteria against external threats, providing them with a safe environment to reproduce. Maturity: The population of bacteria in EPS increases until a mature biofilm is formed. Dispersal: The biofilm is dispersed by releasing either a small part of it or planktonic bacteria to colonize other sites of the implant [27].
It has been estimated that about 65% of bacterial infections in the human body are associated with bacterial biofilm formation [31]. After biofilm formation, bacteria can tolerate antibiotics at concentrations of 10–1000 times more than their planktonic mode, making biofilms tremendously difficult to eliminate from living hosts [14,30]. Therefore, antibiotic administration to eliminate biofilm could be dangerous; since, in this state, the probability of the emergence of antibiotic resistance is too high.
3. Strategies to combat biofilm formation in implants
As discussed in the previous section, the bacterial biofilm formed on an implant surface is the main cause of implant-related infections [14]. The current therapy to combat bacterial biofilm on implants mainly relies on antibiotic therapy. However, bacteria in biofilm are more resistant to antibodies, phagocytes, and any antibacterial drugs than planktonic bacteria [32]. Further, the efficiency of antibiotic treatment is gradually reducing due to the abuse of antibiotics and the emergence of deadly superbugs [33,34].
Another noteworthy treatment to tackle biofilm formation, particularly in dental implants, is to mechanically clear the biofilm. The mechanical treatments, however, carry the risk of micromorphological changes in the implant's surface and surface roughness alterations [35]. The roughness of the implant surface is a vital feature for osseointegration [36]. Moreover, with the mechanical clearance procedure, the entire bacteria may not be removed from the implant surface [37,38]. Altogether, it can be concluded that combatting the bacteria only after biofilm formation is not much efficient [39]. Intervening before biofilm formation and preventing it at the early stages is thus a more viable strategy [40].
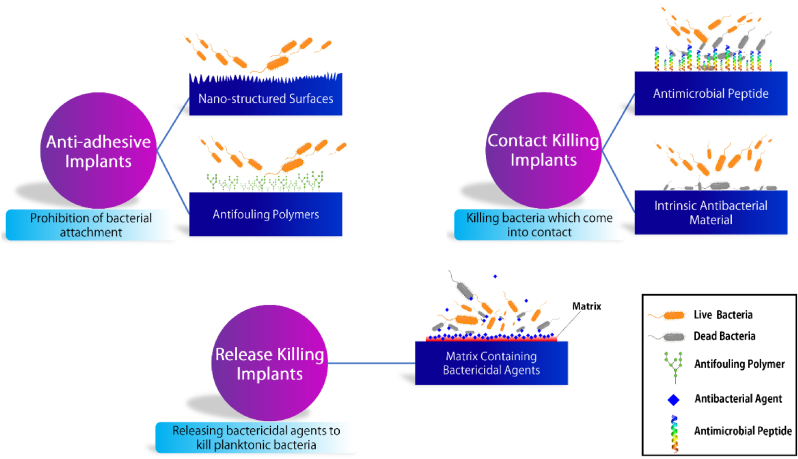
In this regard, various approaches have been developed by equipping the implants with remarkable characteristics by which the initial stages of biofilm formation could be prevented. Such implants, endowed with antibacterial properties, combat bacteria in three different ways: prohibition of bacterial adhesion, killing the bacteria which come in contact with the surface, and releasing antibacterial agents to kill planktonic bacteria [41]. An antibacterial implant may apply one, two, or all three anti-infection strategies against bacteria [42]. In this section, we briefly review these strategies. Scheme 3 illustrates the various strategies currently employed to combat bacterial infection and inhibit biofilm development on the implant surfaces.
Scheme 3.
Various strategies to fabricate an antibacterial implant. Anti-adhesive implants are obtained through either changing the surface chemistry or morphology of the implants. Contact killing implants are fabricated by immobilizing antimicrobial peptides (AMP) on the surface or utilizing antimicrobial chemical compounds. Release-killing implants are acquired by equipping the surface with an antibacterial agent, which could be released from a matrix coated on the surface.
3.1. Anti-adhesion implants
One of the widely applied approaches to prevent biofilm formation on the implants is surface modification with an aim to prevent or reduce bacterial adhesion. Such surfaces possess antifouling or antiadhesive properties and do not allow bacterial adhesion; nonetheless, they may not kill the bacteria. They are classified as passive surfaces which just discourage bacterial adhesion and do not kill bacteria. The antiadhesive implants can be achieved through fabricating micro/nano-structured surfaces or via physically immobilizing antifouling polymers on the surface of implants [41,43]. The immobilization of antifouling polymers can also be applied by chemical grafting that is acquired by UV, plasma, or ozone activation [[44], [45], [46], [47]]. However, the chemistry involved in chemical grafting is often complex, difficult to scale up, and challenging to replicate [48].
In many studies, the antibacterial activity of surfaces with nanotopographic features have been investigated. Hue et al. [49], for example, reported the improvement in antiadhesion properties of selective laser melting (SLM)-modified titanium implants by coating titanium dioxide nanotubes on the SLMed titanium. Yi et al. [50] coated ZnO nanopillars on various surfaces and reported that the surfaces prohibit bacterial adhesion. Inspired by cicada and catkin, Ye and coworkers [51] fabricated a biomimetic nanostructured implant through a hydrothermal chemical reaction technique, and the achieved implants demonstrated remarkable bacterial antiadhesion. Although the exact mechanisms of antiadhesion properties observed in the nano-architecture surfaces have not been completely understood, it seems that the hydrophilicity of high-roughened surfaces combined with the physical electrostatic interactions between the nanoscale topography and the bacteria are conducive to the antiadhesion phenomenon [52,53].
Antifouling polymers have been extensively used for a wide variety of applications such as drug delivery, bioseparation, marine coating, and medical implants to prevent the adsorption of biomolecules and microorganisms on the surface [54]. An antibacterial implant, on which a specific type of antifouling polymer is immobilized, produces a hydrated surface layer that resists protein adhesion [41].
There are a relatively large number of polymers that can confer antifouling properties to an implant surface [54]. Polyethylene glycol (PEG) is the most prominent one among these polymers [[55], [56], [57]]. However, using PEG as an antiadhesion agent on an implant surface is associated with the problem of blocking the adhesion of eukaryotic cells. Such blockage is unwanted because the adhesion and proliferation of eukaryotic cells on the implanted material is an indispensable step for the integration of the implant with the surrounding tissues [58]. This issue can be tackled by immobilizing a cell adhesive sequence such as arginyl glycyl aspartic acid (RGD) to the PEG-containing surfaces to maintain the bioactivity of the implants [59]. In another approach, various surface topographies combined with suitable surface chemistries can be harnessed to achieve anti-adhesion surfaces for the modification of implants [60,61]. Hsiao et al. [48], for example, reported the improvement of resistance to bacterial adhesion on a convex surface compared with a smoother surface when modified with diblock copolymers (containing poly SBMA (poly sulfobetaine methacrylate) and PPO group (poly propylene oxide)).
To turn a passive coating into an active surface that dynamically kills the contaminating bacteria; bactericidal molecules, ions, or other agents can be incorporated with the antiadhesive polymers [62,63]. This incorporation is indeed essential since anti-adhesion surfaces do not necessarily prevent the adhesion of all species of bacteria. It is, thus, required to equip the surface with a bactericidal effect to enhance the antibacterial activity of implants [41].
3.2. Release-killing implants
Local delivery of antibacterial agents from the surfaces of implants is another strategy to actively kill planktonic bacteria around the implant [64]. In fact, the antibacterial agents are confronted with bacteria that surround the wound or those which do not come in contact with the implant and kill them actively. This encounter is of paramount importance because the planktonic bacteria can invade unhealed wounds and bring about infections around the wounds [65]. Importantly, the locally administered antibacterial therapy seems to be more efficient than conventional systemic administration of antibacterial agents because the former faces bacteria in the early stages of infections and before biofilm formation.
In this approach, the surfaces of implants are coated with a matrix film in which antibacterial agents are loaded. The antimicrobial agents are released upon implant insertion into the body, thereby resulting in proficient localized drug delivery. The antibacterial agents that have been typically used in this area include metal ions such as silver (Ag+) [66,67], zinc (Zn2+) [68], and copper (Cu2+) [69], antibiotics like rifampin [70], bactericidal polymers such as polyelectrolyte multilayers [71] and chitosan [72], and antimicrobial peptides (AMP) like GL13K [73] and Mel4 [74].
Besides the type of antibacterial agents, the matrices or carriers on which the bactericides are coated define the releasing behavior of the system. The matrices can be chosen from a wide variety of options such as titanium nanotubes (TNTs) [75,76], mesoporous structures [42], hydroxyapatite [77], hydrogels [78,79], and other appropriate polymers such as chitosan [80], and polyelectrolyte multilayers (PEMs) [81]. Based on the chosen system (type of bactericide and matrix), the functional surface kills the planktonic bacteria through different approaches. For example, the metallic ions enter bacteria, interfere with their respiratory process, and induce reactive oxygen species (ROS) overproduction, which culminates in bacteria death [34].
There are numerous studies investigating the antibacterial properties of metallic ions implanted into various matrices such as titanium. Liu et al. [82], for example, doped Cu into Ti implants using the plasma immersion ion implantation technique (PIII) and reported an enhanced antimicrobial activity for the fabricated Cu-doped Ti. Wan et al. [83] also reported that the antibacterial ability of stainless steel, pure Ti and Ti alloys are enhanced by the implantation of Cu ions into these matrices. In addition, in the research of Hou et al. [84], the antibacterial effect of Ag-doped Ti was confirmed.
The releasing kinetics in release-killing implants is evidently crucial. In the initial period after implantation, the implant releases an inordinate number of bactericide agents known as the burst-release stage, followed by a slow-release stage [41]. At first glance, the burst release may be taken as a virtue because numerous antibacterial agents kill bacteria in a short time. However, it stimulates the cytotoxicity for surrounding tissues and cells and leads to short-term antibacterial activity. Thus, myriad techniques to control the speed and manner of agents’ release are well documented in the literature [80,[85], [86], [87], [88]]. In one of these techniques, the trigger-responsive antibacterial coatings can release the drugs only in the presence of bacteria, where changes in the local microenvironment, aroused by the bacterial infection, can trigger the operation of the drug delivery system [89,90]. As a case in point, Dong et al. [91] designed a pH-dependent silver nanoparticles (AgNPs) releasing titania nanotube arrays implant, in which the nanotube arrays were fabricated on the surface of titanium implant as carriers, and AgNPs were fixed on TNT implant surface via a low pH-sensitive acetal linker. As the pH level around the implant decreases to 5.5, AgNPs are released from the implant surface in high doses. Not only this technique prolongs the lifetime and enhance the efficacy of the antibacterial activity of the implant, but also it diminishes the risk of cytotoxicity of the antibacterial agents.
3.3. Contact-killing implants
Contact-killing implants are made of materials or covered with coatings that kill the bacteria on contact. Contact killing implants show a few advantages over the other two types of implants. First, contact-killing implants, unlike antiadhesion implants, actively kill the sessile bacteria [41]. Second, since the bactericide agents do not detach from the surface in contact with bactericidal implants, there is no risk of cytotoxicity to the surrounding tissues in comparison to the ion-releasing implants. Also, contact-killing implants often have a long-term bactericidal effect that makes them more efficient against bacterial infections [35,41].
Direct contact sterilization implants can be fabricated through various approaches. Implants with surfaces functionalized by AMP have been extensively reported to show antibacterial activity against a broad spectrum of bacteria [[92], [93], [94], [95], [96]]. In this type of implants, AMPs are immobilized on the surfaces through either non-covalent or covalent bonds and kill the adhered bacteria without the delivery of antibacterial agents [97,98]. In one work, as an example, the antibacterial behavior of Mel4, covalently bound to plasma polymer-coated Ti implants, was ascribed to the depolarization of bacterial cytoplasmic membrane by electrostatic interactions occurring between the membrane and Mel4 agents [99].
The other type of direct contact-killing implants are made from ceramics which inherently possess antibacterial activity. For example, zirconia implants prohibit bacteria adhesion and biofilm formation and consequently exhibit lower inflammation than titanium implants [100,101]. Another example is photoinduced titanium oxide (titania) that kills bacteria through photodecomposition and the production of ROS species [102,103].
Another strategy is to utilize the bactericidal activity of metals such as Ag and Cu to make antibacterial implants. Contact killing implants containing metals could be secured either by embedding them to the surface via surface modifications such as plasma activation strategies [104,105] or by intermingling them into the matrix during the fabricating process (alloying) [21,106]. For example, Chen et al. [107] endowed the surface of titanium implants with antibacterial properties via silver and reported that the surface-treated implants exhibited more than 90% antibacterial activity against Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), and Staphylococcus aureus (S. aureus). Alternatively, Lei et al. [108] fabricated Ti–Ag alloys with different silver contents and noted that Ti–Ag with 3 weight percent (wt.%) and 5 wt.% Ag possessed prolonged antibacterial activities and good biocompatibility.
Numerous techniques, including ion implantation [83], oxidation [109], ion-beam assisted deposition [110], dip coating [111], plasma spraying [112,113], electroplating [114], magnetron sputtering [105,[115], [116], [117], [118], [119]], ion-assisted plasma polymerization [[120], [121], [122], [123], [124]] and plasma immersion ion implantation (PIII) [[125], [126], [127], [128], [129]] have been established to fabricate implant coatings and bioactive interfaces. In particular, a large body of works has been devoted to create contact killing surfaces that contain bactericidal agents such as F [130], Cu [83,131], Ag [108,109,132] and Zn [133]. Table 1 lists various techniques utilized to coat copper-containing surfaces and their antibacterial performance. Nevertheless, one important aspect that needs to be taken into consideration is that these types of coatings may lose their antibacterial effectiveness over time because ion-releasing is inevitable, and the antibacterial agents may run out over time. Moreover, surface coatings can be vulnerable to mechanical shear forces and may delaminate from the substrate under physiological environments [134]. Therefore, implant materials alloyed with antibacterial metals can present a more prudent course of action because the bulk of the material exhibits antibacterial activity rather than only the surface and for a long period.
Table 1.
Techniques used to fabricate antibacterial Ti–Cu coating and their antibacterial efficacy.
| Substrate | Antibacterial Agents | Fabricating Method | Description | Antibacterial Rate (%) | Ref. |
|---|---|---|---|---|---|
| Pure Titanium | Copper | Ion Implantation | The concentration of Cu was 4 × 1017 Cu ions/cm2 | 100% in plate count method test | [135] |
| Si wafer | Ti-xCu film | High power impulse magnetron sputtering (HiPIMS) and DC magnetron sputtering (DCMS) | A cuboid Ti target was used for DC magnetron sputtering (DCMS), and a Cu target was sputtered using high power pulsed magnetron sputtering (HPPMS) | The antibacterial activity of Ti-xCuO coatings, x = 3.1–33.9 at.% in plate count method was 99% | [116] |
| Pure Ti | Nitrogen (N) and copper (Cu) dual ions | plasma immersion ion implantation and deposition (PIII&D) | Titanium nitride (TiN) film was embedded with Cu nanoparticles (Cu NPs) | Cu–Ti and N/Cu–Ti samples showed nearly 100% antibacterial efficacy in bacterial counting method | [126] |
| 316L stainless steel and Si | Ti-xCu coating | Hybrid high power impulse magnetron sputtering (HiPIMS) and DC magnetron co-sputtering (DCMS) | Ti-xCu coatings were co-sputtered on silicon wafers and polished 316 L stainless steel substrates using DCMS and HiPIMS techniques | Pure Cu and Ti-xCu coatings with x = 55 and 65 at.% showed an antibacterial efficacy of 99.9% | [105] |
Alloys that are made with antibacterial elements have other merits over ion-releasing and anti-adhesion implants. First, antibacterial alloys are inherently equipped with the ion releasing mechanism, and the antibacterial elements are released in solution and kill bacteria. Secondly, the fabricating process of alloys can be simpler and with lower cost than complex immobilizing procedures to couple antibacterial agents to surfaces.
4. Antibacterial effects of copper
Since the down of civilization, copper and its compounds have been exploited for hygiene in general and as disinfectant agents to sterilize chest wounds and drinking water [22]. In the 19th century, scientists discovered the adverse effect of pathogens on everyday diseases, and since then, many studies have been carried out on antibacterial agents such as copper [136]. The use of copper as an antimicrobial agent proceeded until the advent of antibiotics in the 1930s, but the spread of antibiotic-resistant bacteria at the end of the 1980–1990s raised the need for different approaches to combat pathogens, one of which was to take advantage of copper surfaces in hygiene-sensitive areas. In 2008, the U.S. Environmental Protection Agency (EPA) officially recognized copper and its alloys as the first effective metallic antibacterial agents, and thenceforth, the research community quickly introduced copper into the research cycle of manufacturing disinfectant surfaces and implants [22]. In general, copper has been deployed as an antibacterial agent in various forms, such as thin films, ionic forms, and nanoparticles, depending on its end application in a biomedical device [[137], [138], [139], [140]]. Also, copper can be used either purely or as a component of a wide range of alloys, including those that are based on Ti [141,142].
Vast investigations have addressed the antibacterial activity of metallic copper and copper alloys over a broad spectrum of bacteria. Word Health Organization announced methicillin-resistant Staphylococcus aureus (MRSA) as a high-priority pathogen and a serious threat to public health [143]. However, it has been reported that pure copper kills the whole population (107 cfu/mL) of MRSA in only 45 min [144]. In another study, the results showed that after only 90 min exposure to a copper surface at 20 °C, a high concentration (107 cells) of E. coli vanished [145]. Additionally, it was demonstrated that copper alloys (>70% Cu) completely kill Clostridium difficile (C. difficile) after 24–48 h [146]. Souli et al. [147] investigated the antibacterial activity of copper surfaces on clinical isolates of E. coli, Enterobacter spp., Klebsiella pneumoniae (K. pneumoniae), P. aeruginosa and Acinetobacter baumannii (A. baumannii); and reported that copper surfaces destroyed all tested strains within a few hours.
4.1. The two modes of stress
When bacteria come in contact with a copper surface, a series of events causes the bacteria to be destroyed. In this regard, the main molecular target of copper, the nature of bacterial changes, and the sequence of events that lead to bacterial death have not yet been fully understood. This has been the subject of considerable debate among researchers for many years. In this section, we strived to point out the contradictions around the identification of cellular targets of copper toxicity in order to have a deeper understanding of the antibacterial activity of Ti–Cu alloys. Nonetheless, we emphasize that in works reviewed in this section, copper has been used either purely or with relatively high concentrations (copper coupon with 100 wt.% of copper or alloys with at least 50 wt.% of copper). Bacterial exposure to these forms of antibacterial metal is highly acute and is different from bacterial contact with Ti–Cu alloys with lower content of copper. In the former, cells face more challenging conditions which differ from the latter chronic case [148]. However, we tried to focus on results that can be achieved in both states of copper, namely pure copper or as a component of alloys.
In a controversial work, Airey and Verran [149] reported that the MRSA was not killed after exposure to the surfaces of copper coupons. This finding was based on the Live/Dead staining technique which is used to distinguish damaged cells from those left intact. The authors observed that MRSA cells were undamaged, therefore concluding that the copper surface is unable to kill MRSA. However, Weaver et al. [150] challenged these results by studying the antibacterial effect of copper surfaces against MRSA and analyzing DNA integrity and tracking respiring MRSA cells. Results indicated that although copper exposure does not affect the cell membrane integrity (Fig. 1), copper surfaces are able to kill bacteria by other mechanisms. DNA analysis showed that copper causes MRSA inactivation by inducing maximum damage to the genomic DNA. Also, no activity of respiring cells was found. Thus, the claim of MRSA viability upon copper exposure was refuted and instead, an alternative model was proposed. This model suggests that copper ions enter the cell and rapidly kill MRSA by applying detrimental effects on cellular respiration and DNA integrity without markedly effecting the integrity of the cell membrane.
Fig. 1.
An assessment to detect the cell membrane integrity of MRSA bacteria inoculated with copper surfaces. Bacteria were stained with Backlight™, Systo 9 (a), and propidium iodide (b). Cells with undamaged membranes fluoresce green (a), while bacteria with damaged membranes fluoresce red (b). This assessment shows that copper exposure does not disrupt the cell membrane integrity of MRSA. However, other analyses reported in this work confirmed that bacteria were killed as a result of damages to their DNA and respiratory system [150].
Understanding the damage delivered to the DNA of bacteria upon exposure to a copper-containing surface, or any antibacterial agent in general, is of great importance because otherwise (in the case of intact DNA), there is the possibility of emergence and spread of resistant organisms [151]. Warnes et al. [152] investigated the antibacterial effect of copper surfaces against pathogenic enterococci, a type of bacteria with a high propensity for genetic transfer. The authors reported that copper and copper alloys resulted in the disintegration of Gram-positive enterococci DNA into fairly small fragments and inhibition of respiration, while just little damage to the cell membrane was observed. This finding suggests that the development of copper resistance is unlikely. Warnes et al. confirmed their previous results in other works and added that the membrane depolarization occurs after cell death [153,154].
Nevertheless, Espírito Santo et al. [148] were uncertain about whether DNA damage is causative for or subsequent to cell death and conducted an experiment to investigate the primary target of copper in both Gram-positive [(Bacillus cereus (B. cereus), and Deinococcus radiodurans (D. radiodurans)] and Gram-negative (E. coli) bacteria. Their study showed strong evidence that death in both Gram-negative and Gram-positive cells after contact to copper surface coincided with membrane damage which are combined with the cellular accumulation of copper. Indeed, they suggested that lethality is not caused by DNA damage, and instead, DNA damage is a secondary event ensuing the cell death. Various studies on the different types of bacteria confirm the results reported by Espírito Santo et al. [22,141,[155], [156], [157], [158]]. Grass et al. [22] believed that bacteria-killing proceeds by the following steps: membrane disintegration, copper accumulation in cells, oxidative damage, cell death, and DNA degradation.
Therefore, based on the argument mentioned above, there are generally two competing hypotheses as the two modes of stress about the antibacterial characteristic of copper surfaces:
-
1
The cell membrane is the primary target of copper surface-induced lethality, and DNA damage occurs after cell death.
-
2
The genotoxicity of copper and damage to cellular DNA is the underlying cause of bacteria death, and cell membrane damage occurs after cell death.
There is a possibility that the difference in the cell wall structure, which determines the type of bacteria (Gram-positive and Gram-negative), determines the effective mode of stress. The lack of the outer membrane and periplasmic space in the cell wall of Gram-positive bacteria can make DNA accessible to copper ions and their damage. Thus, the latter hypothesis is the controlling mechanism. In contrast, in Gram-negative bacteria, due to the protection of nucleic acid by the periplasm, the access of copper ions to DNA is restricted until the cytoplasmic membrane becomes disintegrated. As such, the first hypothesis is more likely in this case [153]. Nevertheless, Santo et al. [155] reported that the antibacterial mode of stress for copper is not dependent on the structural difference in the cell wall, and all bacteria are killed as a result of extensive membrane damage. However, the kinetics of killing (time needed to kill bacteria) can be affected by the morphology of the cells [141,155].
Regardless, Vincent et al. [139], in their review paper, have considered this discrepancy inevitable as a result of different protocols and assays, and various types and strands of bacteria used with varying forms of copper (coupons, alloys, and particles) across different works published in the literature. In another review paper, Grass et al. [22] suggested that there is no risk of developing bacterial resistance to copper because copper exposure completely degrades plasmid DNA, which is responsible for the transfer of resistance genes between organisms. Another reason is that copper surfaces kill bacteria rapidly, leaving no time for bacteria to divide and thus preventing the development of resistance genes. The fact that copper has been utilized by humans since the dawn of humanity, yet no bacteria resistant to copper have been detected, supports the claim of no bacterial resistance to copper. Taken together, future studies should be concentrated specifically on this subject by deploying pre-arranged protocols and investigations in a systematic manner to be able to compare determinant factors between studies.
The noteworthy point to bear in mind is that the two modes of stress are inseparably interwoven with the two antibacterial mechanisms discussed above, namely ion releasing and contact killing. It is suggested that during the contact of bacteria with the Cu-containing metal, the metal-bacterial contact damages the cell envelope of the bacteria and, subsequently, makes the cells susceptible to further damage by copper ions [157]. Thus, the contact killing mechanism results in the cell membrane rapture, and the damage to DNA stems from the accumulation of copper ions into the bacteria cells. Therefore, a distinction should be drawn between these two concepts. In a recent publication, for example, the authors incorrectly considered these two concepts identical and used them interchangeably [159].
Using a specific engineering design, Mathews et al. [160] well discriminated the ion releasing mechanism from the contact killing mechanism. They designed a system named contact arrays in which the surface of a copper coupon was coated with an inert polymer so that it prevented contact of the Enterococcus hirae (E. hirae) bacteria with the metal surface (Fig. 2). It was observed that although the quantity of released ion in the sample coated with contact arrays was preserved in comparison to the non-polymeric sample, the antibacterial characteristic of the modified surface was significantly attenuated, indicating the importance of the contact killing mechanism.
Fig. 2.
Scanning electron microscope (SEM) images of the contact array system designed by Mathews et al. [160] This system was used to show the significant role of the contact-killing mechanism in the antibacterial ability of a copper surface. The surface of the copper coupon was coated with an inert polymer to prevent contact of the E. hirae bacteria with the metal surface. The SEM images of the honeycomb-like structure of inert polymer coated on the surface of the copper coupon at relatively low (a) and high (b and c) magnifications. The size of the holes was designed to be smaller than that of bacteria to prevent the contact of bacteria with the copper surface. The red arrows point to E. hirae bacteria.
5. Ti–Cu antibacterial implants
Among the metallic alloys employed for implants, such as stainless steel, cobalt-chromium, niobium, and tantalum, titanium and its alloys have been widely used in the preparation of orthopedic and dental implants because of their superior mechanical properties, high fatigue limit, corrosion resistance, bioactivity, as well as biocompatibility [161]. The mechanical properties of the material and the loading conditions in the host have, conventionally, influenced material selection for different clinical applications. For example, Ti–6Al–4V is commonly used in orthopedics, while commercially pure titanium is utilized in dentistry [162]. However, due to the cytotoxicity and neurotoxic effects of aluminum and vanadium, Ti–6Al–4V alloys should be cautiously and carefully used in biomedical applications [163,164].
Despite the advantages of Ti-based implants, they are susceptible to bacterial adhesion and colonization which leads to biofilm formation and implantation failure in the later stages [40,165]. The bacterial infection caused by biofilm formation upon implantation is one of the most considerable complications in orthopedic surgery and dental implantation. As explained above, the biofilm formation protects the bacteria enmeshed in EPS against environmental dangers such as antibiotic treatment and the immune system [30]. Thus, the most effective approach to prevent biofilm formation on implants is to provide them with an antibacterial surface that discourages the first phase of biofilm formation. This can be achieved through introducing antibacterial elements such as Cu, Ag, and Zn into the Ti-based alloy system [166]. A quick survey of the literature reveals that among these elements, vast investigations have been dedicated to the Ti–Ag implants. However, Ti–Ag implants suffer from a serious drawback: formation of silver salts that may be absorbed into the circulatory system and become deposited in various body tissues, leading to some major medical problems [167]. In addition, the antibacterial activity of Ag is more pH-, humidity-, and temperature-dependent compared to that of Cu, which may restrict its applications [[168], [169], [170]].
These drawbacks encouraged researchers to focus on other alternatives, including titanium-copper alloys [171]. Considering the antimicrobial efficiency, economic cost, and cytotoxicity, the copper element can be a promising agent [164,172]. In an investigation, among 21 metallic elements tested, silver and copper presented similar rates of bacteria-killing, which was 5- to 10-fold higher than that of other elements [173]. In addition, unlike silver element, copper as an essential trace metal, has biological applications in the body and is an important member of several enzymes in the cell and contributes to the formation of red blood cells in the body [174,175]. Copper also endows the alloy with satisfactory strength, ductility and corrosion resistance [165,[176], [177], [178], [179], [180]]. In addition, copper triggers the proliferation and osteogenic differentiation of mesenchymal stem cells (MSCs), and brings about angiogenesis and collagen deposition [181,182].
The appropriate amount of copper added into Ti should be carefully considered since a small amount of copper brings nearly non-antibacterial ability, whilst excess concentrations of copper would cause cytotoxicity. The adequate daily intake of copper for each adult is in the range of 2–3 mg, and the World Health Organization (WHO) recommends the tolerable upper intake level (UL) of approximately 10 mg/day for adults as the excessive amount of Cu is highly toxic for cell, though other ions such as silver are harmful to biological cells even in small quantities [[183], [184], [185]]. Therefore, the development of Ti–Cu alloys with suitable balance among antibacterial properties, cytotoxicity, and formability has important implications for developing dental and orthopedic implants. Considering that excessive copper intake can cause metal toxicity, titanium alloys with a copper content lower than 10 wt.% have been extensively investigated in the literature [172].
Although initially, copper was added to titanium implants in order to improve their mechanical properties and also to increase the casting ability [[186], [187], [188]], Shirai et al. [21] firstly reported the antibacterial properties of a Ti–Cu implant in 2009. In this research, the antibacterial properties of Ti-1 wt.% Cu, and Ti-5 wt.%Cu (hereafter referred to as Ti–1Cu and Ti–5Cu) implants were investigated. All the Cu-containing samples were “antibacterial” according to the GB4789.2–2010 Food Safety Standard, which considered materials with antibacterial efficacy of higher than 90% as “antibacterial” [163]. This research was a distinguished turning point for the development of antibacterial Ti–Cu implants and showed the great potential of the copper element in the fabrication of anti-infection biomaterials. Since then, considerable efforts have been made to further develop this alloy system in order to improve its antibacterial activity, cell biocompatibility, mechanical properties, and anticorrosion behavior.
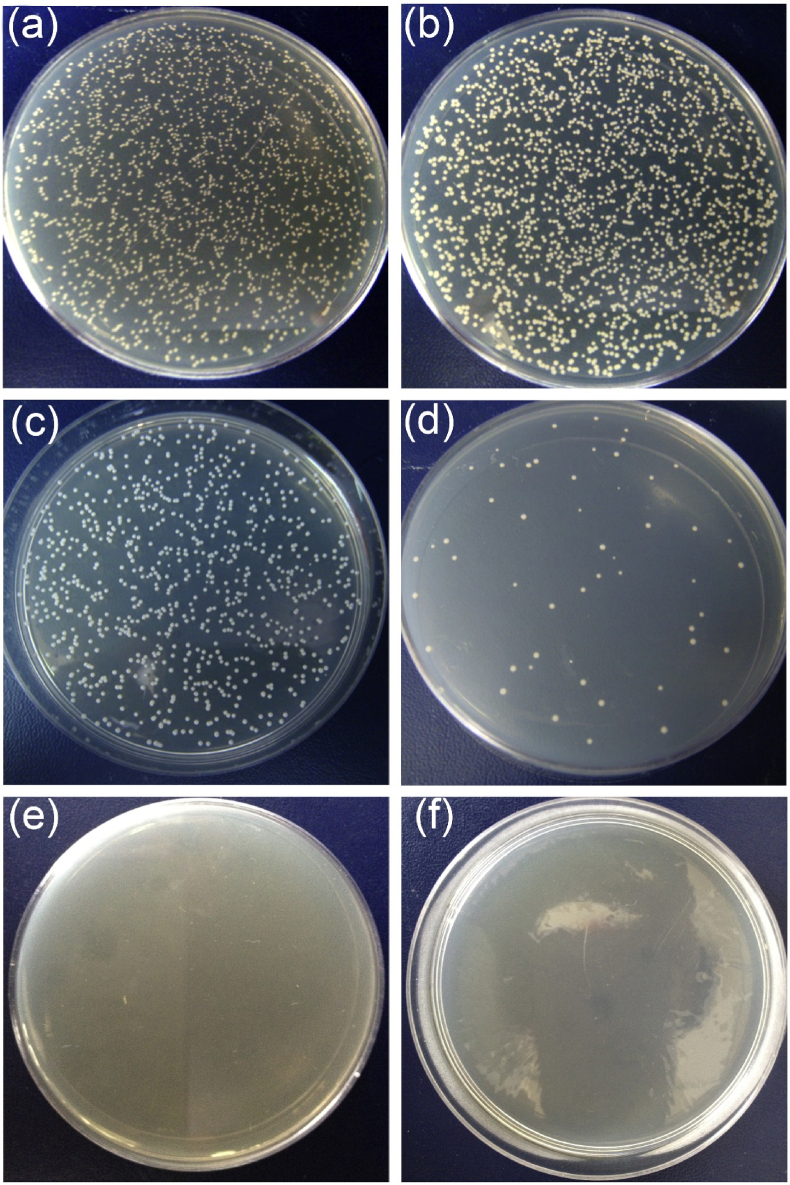
The two most common bacteria that in most cases cause implant-associated infections are S. aureus (Gram-positive) and E. coli (Gram-negative). It has been well documented in the literature that Ti–Cu alloys effectively kill both of these bacteria [176,[189], [190], [191], [192], [193], [194], [195], [196], [197]]. For example, Zhang et al. [19] reported that the Ti–10Cu alloy removed more than 99% of S. aureus and E. coli bacterial colonies in the plate-count method assay even after several times of polishing (Fig. 3). This experiment confirms that antibacterial properties in Ti–Cu alloys exist in the entire materials, including their bulk and surfaces, compared to Cu-coated surfaces, which show antibacterial effect only on the surface. In another study, the antibacterial rates of Ti–3Cu alloy and Ti–4Cu alloy reached 90.33% and 92.57%, respectively [198]. The in vitro results in Lui and co-workers’ study [199] showed that sandblasted and acid etched Ti–5Cu (SLAed alloy) kills at least 90% of Streptococcus mutans (S. mutans) and Porphyromonas gingivalis (P. gingivalis) -two significant oral bacteria-compared to SLAed Ti, and also significantly inhibits biofilm formation. Also, the outstanding antibacterial effects and favorable biocompatibility of the sintered Ti–10Cu alloy have been proved through in vivo rabbit muscle infection model in another study [200].
Fig. 3.
Typical S. aureus and E. coli colonization images of (a)–(b) negative samples, (c)–(d) control samples (cp-Ti), and (e)–(f) Ti–10Cu samples after incubation for 24 h in the plate counting test method. A large number of bacteria were detected on the negative and cp-Ti samples, indicating that cp-Ti does not show antibacterial activity. However, the copper-containing sample (Ti–10Cu) killed nearly all S. aureus and E. coli bacteria, indicating that Ti–10Cu implants exhibit a strong antibacterial activity [19].
Besides the significant antibacterial performance, Ti–Cu alloys can present desirable biocompatibility with bioactivity behavior. Research showed that the Cu-containing antibacterial Ti alloys exert favorable cell spreading behavior with no cytotoxicity to a range of cell types such as rBMSCs, MG63, and MC3T3-E1 [137,178,[201], [202], [203], [204], [205]]. As an example, the study of Zhang et al. [204] demonstrated great cell adhesion and spreading of MG63 cells on Ti–Cu alloys with outstanding cell viability. In another study, it was reported that the Ti–Cu alloys can further encourage cell differentiation in comparison with cp-Ti. Moniri et al. [203] reported that Ti–Cu alloys trigger osteoblast formation due to the release of Cu ions. In vivo investigations also confirmed the favorable potential of Ti–Cu alloys in promoting osteogenesis [206,207].
5.1. Mechanisms of action in Ti–Cu implants
Researchers have not yet reached a general agreement on the mechanisms of copper-induced antibacterial effect, i. e contact-killing or ion-releasing. However, it is clear that copper causes “multiple-hit damage” in bacteria cells by various pathways, including oxidative stress and leakage of essential nutrients; disrupting the structure of enzymes and their functions; damaging the DNA; and oxidation of cell proteins, nucleic acids and lipids [134,136,[208], [209], [210]]. Recently, it was reported that upon the contact of S. mutans with the surface of a Cu-bearing Ti implant, the pathogenicity of bacteria diminished and the biofilm formation was prevented [211]. This activity was explained by down-regulation of the gene expressions related to the bacterial adhesion and acid production. In early articles in the field, the release of copper ions was considered the underlying mechanism of such antimicrobial activity [[212], [213], [214]]. Wang et al. [165] reported that high copper contents in an Ti–Cu alloy result in the release of higher concentrations of Cu ions which yields stronger antibacterial ability. Eriksson [215] believed that continuous release of copper ions from the Ti–Cu alloys surface kills the bacteria by preventing biofilm formation. Liu et al. [202] argued that with the increase in incubation time, the number of ions released from the Ti–Cu alloy is enhanced, and consequently, the antibacterial performance improves. In the research of Cao et al. [216], the antibacterial effect of the surface-oxidized Ti–Cu alloy was attributed to the formation of Cu2O and CuO and release of Cu+ and Cu2+ from the coating.
In other research as well, the antibacterial properties of the Ti-Cu alloys have been attributed to the release of copper ions. Nevertheless, we could trace contradictions in the results. As a case in point, the minimum inhibitory concentration (MIC) of copper ions for the E. coli and S. aureus is determined to be 256 μg/mL and 448 μg/mL, respectively [[217], [218], [219]]. However, the measured amount of released copper ions per day from Ti–5Cu alloy in the research of Lui et al. was 0.003 μg/mL which was considerably lower than the MIC values reported for the two mentioned bacteria [39]. Nevertheless, the antibacterial rate in this alloy was more than 90%. In the research of Zhang et al. [19] the concentration of copper released from the Ti–10Cu was 0.34 mg/L, while zero S. aureus bacterial survival was recorded. Once again, the concentration of released copper ions from the surface was insufficient to generate such a substantial antibacterial effect. Fowler et al. [169] reported that a solution with the copper concentration of 9 × 10−5 g/mL actively killed more than 90% of Staphylococcus epidermidis (S. epidermidis) bacteria. Nonetheless, in their previous study, they showed that the Ti–10Cu with the ion releasing amount of 9 × 10−8 g/mL exhibits a much larger antibacterial effect [171]. From these works, it is clear that the concentration of copper ions released from the surface of Cu-containing materials is far less than the MIC. This implies that considering the release of Cu ions as the main mechanism of antimicrobial activity may not be precisely correct. In summary, for all bacteria to be killed solely through the ion releasing mechanism, the concentration of released copper ions must be equal or greater than the MIC value [220]; otherwise, another mechanism must be playing a role [207].
5.1.1. Contact-killing mechanism
In the previous sections, the two modes of stress exerted by copper on bacteria were described, and it was discussed that membrane damage could occur. However, several researchers believe that this may not be the primary target of copper [150,[152], [153], [154]].
Considering the studies in which transition electron microscopy (TEM) and atomic force microscopy (AFM) analyses were used to monitor the bacteria, it seems that cell envelop rupture plays a significant role and cannot be neglected [39,207,[221], [222], [223]]. Using AFM, Nan et al. [221] demonstrated that after contact of E. coli bacteria with the surface of copper-bearing stainless steel, the cell wall of E. coli is disintegrated. In the research of Liu et al. [39], TEM images of examined bacteria (Fig. 4) indicated that after contact with a copper-bearing alloy, the peptidoglycan layer of bacteria is disappeared (e), the cell membrane is separated from the cell wall (f), and the cellular contents release out of the bacteria (h). In a similar study, E. coli and S. aureus bacteria were exposed to Ti–5Cu alloy, and analogous observations were reported [207]. Furthermore, Li et al. [224] reported S. aureus bacteria were killed when they were exposed to Ti6Al4V5Cu alloy. However, the genomic integrity of bacteria in contact with the copper-containing alloy was not damaged, but the gene replication process was disrupted. The vital point to bear in mind is that it seems the membrane disintegration is not due to the attack of copper ions. Instead, the contact of bacteria with the copper-containing surface may be a key, defining factor [139,160,177,190,225,226].
Fig. 4.
TEM images of S. mutans and P. gingivalis bacteria. (a) S. mutans bacteria exposed to Ti. (b) P. gingivalis bacteria exposed to Ti. (c) S. mutans bacteria exposed to Ti–Cu alloy. (d) P. gingivalis bacteria exposed to Ti–Cu alloy. White and black arrows show the peptidoglycan layer and the cell membrane, respectively. The red arrow determines the separation of the cytoplasmic membrane from the cell wall and expels the contents of the cell. The bacteria exposed to the Cu-containing surfaces exhibit shriveled or cracked morphologies [39].
What is the unique characteristic of a copper-containing surface which is conducive to membrane disintegration? This question cannot yet be answered clearly and confidently because there is no striking information available on the processes involved. It is known that the initial attachment of bacteria on a surface is governed by physical and electrostatic forces such as van der Waals force and hydrophobic interactions [221]. Therefore, the hydrophilicity of the implant surface and the surface charge of bacteria influence the adhesion forces [220]. Nan et al. [221] proved that the adhesion force of “bacteria cell-antibacterial stainless steel” (Cu-containing stainless steel) is more than that of “cell-stainless steel” and even “cell-cell”. These finding provide evidence that bacteria are more strongly absorbed onto the antibacterial surface. To justify the cell envelop rapture due to the contact of bacteria with the copper surface, we suggest that the electrostatic interactions of the Cu-containing phase disrupt the balance in the internal-external osmotic pressure of the cell which is conducive to the disintegration of the cell envelope. It is reported that the concentration of K+ (which plays a significant role in the regulation of cellular osmotic pressure [24]) in the bacterial solution after the contact of bacteria with antibacterial stainless steel tremendously increased, indicative of the disorder in the osmotic pressure of the bacterial cell [221]. In addition, Shi et al. [220] claimed that under the contact killing mechanism, the Cu- or Ag-containing particles cause physiological metabolic disorders of bacteria by disrupting the normal functions of membrane proteins and ion channels on the bacterial cell membrane. This eventually results in the leakage of cytoplasm, as observed in Fig. 4. In another study, it was confirmed that leakage of reducing sugars and proteins from the cells occurs when the S. aureus bacteria come in contact with the Ti6Al4V5Cu alloy [224].
A few recent studies attributed the contact killing mechanism to the galvanic cells generated as a result of the potential difference between the matrix (Ti phase) and the Cu-containing phase (such as Ti2Cu and Cu-rich phase) on the surface [132,[227], [228], [229], [230], [231]]. In the Ti–Cu implants, the intermetallic phase of Ti2Cu owns a nobler standard of electrode potential than the titanium matrix. Therefore, the intermetallic phase appears as an anode, and the matrix acts as a cathode in the Ti–Ti2Cu micro galvanic couple (or micro-electric couple), in the presence of solution [231]. Zhang et al. [227] estimated the potential difference between the Cu-rich phase and stainless-steel matrix is around 40 mV, creating a charge transfer between anode and cathode in the mentioned micro electric couple near the Cu-rich phase.
On the other hand, various respiratory chains embedded in the cell membrane of bacteria generate energy (synthesize adenosine triphosphate (ATP)) for bacterial survival through successive electron transfer reactions. During the release of ATP, the ATP synthetase (ATPase) pushes H+ to the outside of the membrane by dissociation of H2O and leaves OH− within bacteria, which leads to an electrochemical gradient of protons, known as the proton motive force (PMF). Based on the chemiosmotic model, the PMF, the difference in free energy of inside and outside of the bacteria, pumps protons from outside into the cell, facilitating the ATP synthesis. The electrochemical potential also creates a membrane potential (Δψ). Therefore, the PMF is essential for bacteria to grow and remain viable [[232], [233], [234], [235]]. When the bacteria are in contact with the material surface, the potential difference between the Cu-containing phase and the matrix consumes H+ ions. This in turn disrupts the PMF activity of bacteria, resulting in the reduction of ATP production. In addition, electron transfers induced from the potential difference of microdomains (Cu-containing phase and matrix) interfere with the normal respiration of bacteria and hasten the production of intracellular ROS, which in turn trigger the bacteria death.
In the study of Fu et al. [236], this phenomenon was well demonstrated. Knowing that pure Au, Ta, and Zr show no antibacterial activity against S. aureus and E. coli, Fu et al. selected these elements rather than Cu and Ag to prepare Ti–Au, Ti–Ta, and Ti–Zr alloys. The authors found that only the Ti–Au alloy shows significant antibacterial effect due to the presence of intermetallic phase Ti3Au (only Ti–Au had secondary phase). It was reported that the micro-area potential difference (MAPD) between the second phase and the matrix induces electron transfer between them which interferes with the ETS of bacteria and ultimately leads to bacterial death. Moreover, Xie et al. [237] confirmed that the existence of MAPD between Ti2Cu and the matrix results in the antibacterial effect of the Ti–Cu alloy. Thus, the formation of Ti2Cu phase plays a pivotal role in the antibacterial behavior of Ti–Cu alloys [238]. Scheme 4 illustrates how the presence of Ti2Cu in the microstructure of Ti-Cu alloy can actively kill bacteria through the contact killing mechanism.
Scheme 4.
Schematic of contact killing mechanism of Ti–Cu implants containing the intermetallic phase of Ti2Cu in their microstructure. (a) The planktonic bacteria adhere to the surface of the implant. (b) After contact with the implant surface, the cell wall of bacteria is disintegrated and the cellular content leaks out. (c) The potential difference between Ti2Cu and Ti matrix results in the formation of micro-galvanic cell and the generated electron transport between the anode and the cathode of the cell is conducive to the interfering of normal cell function such as ATP synthesis and disturbing the potential membrane. Another suggested mechanism is the disruption of balance in the internal-external osmotic pressure of the cell due to the excessive accumulation of K+ outside the bacteria which is culminated in the disintegration of the cell.
5.1.2. Ion-releasing mechanism
Although our understanding of the contact killing mechanism is still limited, the ion releasing mechanism that encompasses all interactions of copper ions with the internal components of bacteria cells is almost fully known.
Copper ions within the bacteria facilitate the production of ROS [136,239]. Hydrogen peroxide (), superoxide anion (), and hydroxyl radical () are the three common types of ROS [240]. Inside the bacteria, ROS is created as a byproduct of natural metabolism, but it can also be produced in the presence of copper ions, during the Fenton-type reaction and Haber-Weiss cycle [241], and in some cases during the non-Fenton reactions [242]. Oxidative stress is referred to a condition where the balance between environmental ROS production and the ability of a biological system to detoxify them is disturbed. Such condition leads to extreme ROS production and consequently to loss of normal cell function and, ultimately, cell death [160,243,244]. Generally, free radicals attack their nearest molecules to steal their electrons. Thus, the attacked molecule becomes a free radical itself and thereby a chain reaction commences. These radicals spread like an avalanche through the entire bacteria cell, oxidize lipids, amino acids, carbohydrates, and ribonucleic acid (RNA) and also cause DNA mutation [40,158,175,[239], [240], [241],[244], [245], [246], [247], [248]]. Also, free radicals can directly attack the polyunsaturated fatty acid in the cell membrane and initiate lipid peroxidation (cell membrane disruption) [190,224]. Li et al. [224] investigated the effect of copper addition to Ti6Al4V alloy on the ROS production and the killing ability of this alloy in S. aureus. The authors reported that the antibacterial alloy produces a large concentration of ROS in the bacterial cell. Also, Grass et al. [22] discussed that the redox cycling between different copper species, Cu, Cu+, and Cu2+ is the reason for ROS production and showed that the absence of oxygen in this cycle doubles the time required for the complete killing of bacteria. Furthermore, Mathews et al. [160] reported the reduction potential of Cu/Cu+, Cu+/Cu2+, and Ag/Ag+ are in the range of biological reduction potentials which results in the production of ROS species.
In addition to such ROS-regulated damages to critical bacteria components, the copper ion itself also has destructive interactions with components within the bacteria. These interactions have important implications, including a negative impact on the ETS and hence energy production [139,150], an adverse effect on gene expression such as the expression of genes related to the formation of biofilm [245], reducing the amount of protein and polysaccharide in the biofilm [39], effects on Fe–S protein clusters [246,249], damage to the cell membrane by electrostatic reactions [249,250], disturbance of metal/metal ion homeostasis, genotoxicity, photo-cleaning, and protein and enzyme dysfunction [249].
5.2. Aspects affecting the measured antibacterial activity of Ti–Cu implants
Reviewing the literature reveals that a few experimental factors greatly influence the data obtained on the antibacterial performance of Ti–Cu implants. These factors should be carefully considered when evaluating the experimental results, as they can be the underlying reason of the contradictions among different works reported in the literature.
5.2.1. Methods for in vitro evaluating the antimicrobial activity
In an early work, Zhang et al. [19] investigated the antibacterial performance of Ti–10Cu. The antibacterial activity of this alloy was measured through two approaches: plate-counting method and agar diffusion assay with the presence of S. aureus and E. coli bacteria. According to the obtained results, Ti–10Cu alloy showed no antibacterial characteristics in the agar diffusion test, but this alloy removed more than 99% of bacterial colonies in the plate-count method assay. This research clearly shows that the assay might influence the observed antibacterial activity of the alloy. Generally, the various parameters in the established antibacterial measurement method, such as incubation condition (time [202] and temperature [139]), culture media [251], the size of alloy, the number of bacteria, and the type of bacteria [172] may impact the results. For example, it was reported that the antibacterial activity of a Ti–Cu alloy was enhanced from 90% to 100% by prolonging the incubation time from 7 h to 24 h [202]. In addition, the condition of the implant surface in terms of whether it is dry or wet (inoculation methods) simulates the contact distance between bacteria and the implant. Thus, the applied inoculation method also influences the antibacterial behavior of the implant. It is established that in the dry inoculation, when bacteria are in full contact with the implant, the maximum antibacterial efficacy is observed [227,252]. Table 2 shows the impact of experimental parameters on the observed antibacterial performance of alloys.
Table 2.
The effect of experimental conditions on the antibacterial performance of various Ti–Cu alloys.
| Alloy (Ti- wt.%Cu) | Conducted Method | Bacteria | Experimental Condition | R % (antibacterial efficacy) | Ref. |
|---|---|---|---|---|---|
| Ti–10Cu | Agar diffusion assay | S. aureus | Incubation at 37 °C for 24 h under a humidity of 80% | No inhibition zone | [19] |
| E. coli | No inhibition zone | ||||
| Plate-count method | S. aureus | Incubation at 37 °C for 24 h under a humidity of 90% | >99.6 | ||
| E. coli | >99.2 | ||||
| Ti–5Cu | Biofilm-based gene expression | S. mutans | Incubation for 24 h | Only 43.37% of gene expression | [39] |
| P. gingivalis | Only 26.91% of gene expression | ||||
| Ti–3Cu | Plate-count method | S. aureus | Incubation at 37 °C for 24 h | Not identified but killed all bacteria after 24 h | [177] |
| Ti–5Cu | Quantitative antibacterial test | S. aureus & E. coli | Incubation at 37 °C and 90% humidity for 24 h | ≥99% | [207] |
| Ti–5Cu | Plate-count method | S. aureus & E. coli | Incubation at 37 °C and 90% humidity for 24 h | 92.7% and 96%, respectively | [178] |
| Ti–5Cu | Plate-count method | P. gingivalis | Anaerobic incubation at 37 °C for 18 h | 36% | [142] |
| BHI–S blood agar diffusion assay | No inhibition zone | ||||
| Ti–10Cu | Plate-count method | 68% | |||
| BHI–S blood agar diffusion assay | No inhibition zone | ||||
| Ti–1Cu | Bacteria direct contact test | S. epidermidis | 6 h direct contact, 24 h for growing bacteria followed by direct contact, incubation at 37 °C | 4% | [171] |
| Ti-2.5Cu | 13% | ||||
| Ti–3Cu | 16% | ||||
| Ti–10Cu | 24% |
5.2.2. Concentration of copper in the Ti–Cu alloy
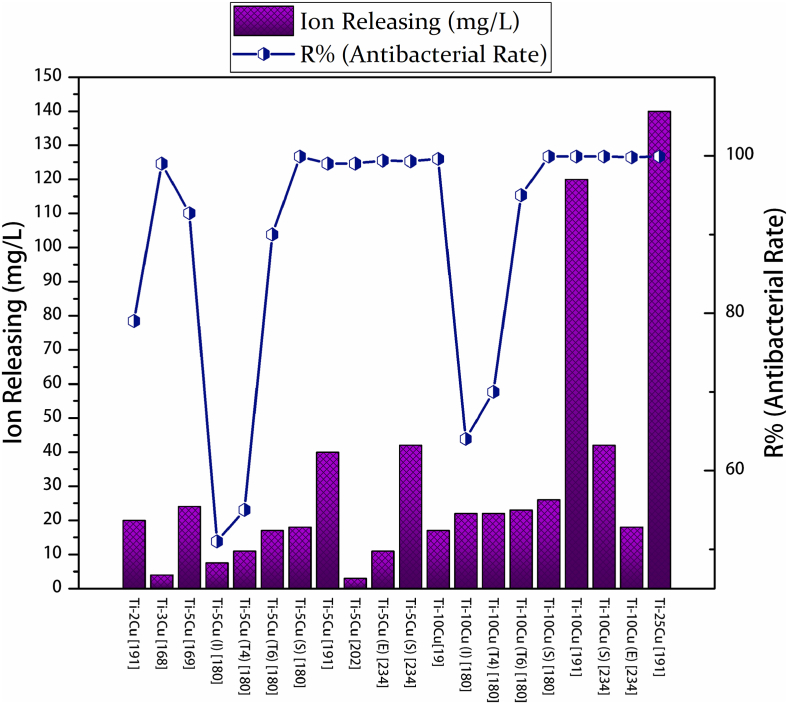
The concentration of copper in the alloy can highly regulates the measured antibacterial properties. Fig. 5 presents the influence of copper content on the antibacterial efficacy of Ti–Cu alloys. Liu et al. [208] proved that the copper concentration in a Ti–Cu alloy improves the intensity of antibacterial activity; and they measured the critical amount of copper for a satisfactory and stable antibacterial performance (more than 90%), to be at least 5 wt.% Cu in a sintered alloy.
Fig. 5.
The effect of copper concentration present in the Ti-x wt.% Cu alloys on the S. aureus bacterial colonies in the plate counting test method. (a) Negative sample, (b) cp-Ti sample (c) Ti–2Cu sample, (d) Ti–5Cu sample, (e) Ti–10Cu sample, and (f) Ti–25Cu sample. While colonies excited in the case of Ti-2.5Cu and Ti–5Cu alloys, by increasing the copper content in the alloys, the bacterial colonies decreased, and alloys that contained more than 10 wt.% copper killed almost all bacteria [208].
There are significant discrepancies in the literature regarding the critical amount of copper needed to achieve effective antibacterial performance. We recognized that these discrepancies are as a result of different experimental conditions used for identifying the antibacterial activity of Ti–Cu alloy (Table 2). For instance, Bao et al. [176] used 36 h incubation time and reported an antibacterial rate of 99.3% for a typical Ti–3Cu alloy against S. aureus. However, in another research, Fowler et al. [171] employed 6 h of direct contact and reported the antibacterial rate of 16% for Ti–3Cu alloys against S. epidermidis. Rui Liu et al. [207] acquired the antibacterial rates of 96% and 80% for the Ti–5Cu alloy against E. coli and S. aureus in 12 h incubation, respectively; and for Ti–10Cu, Jie Liu et al. [202] reported the antibacterial rates of 36% and 43% against E. coli and S. aureus in 3 h incubation, respectively.
5.2.3. Microstructure of Ti–Cu alloy and the role of Ti2Cu phase
The microstructure of Ti–Cu implants, including the existing state of copper (such as Ti2Cu and Cu-rich phase) and its distribution in the alloy, exert a great influence on their antibacterial performance. In regards to the ion-releasing and contact-killing mechanisms, the microstructure of an alloy affects the release behavior of metal ions and determines the quality and the quantity of the contact surface between bacteria and implant surface. Hence, the understanding of Ti–Cu microstructure is essential to predict the antibacterial behavior of a system.
Based on the Ti–Cu binary phase diagram, the solid solubility of copper in α-Ti and β-Ti phase is about 2.1 wt.% at 790 °C and 18 wt.% at 1005 °C, respectively. The eutectoid reaction occurring at Ti-rich side of the diagram is β-Ti → α-Ti + Ti2Cu at the copper concentration of 7 wt.% (Table 3) [253,254]. Therefore, copper can exist in three different states at room temperature in the Ti–Cu alloys, which are ordinarily designed for implants applications (copper concentration less than 10 wt.%): interstitial solid solution in the α-Ti phase or unstable β-Ti phase, precipitated intermetallic Ti2Cu compound [40,226], and unstable copper-rich phases [171,208].
Table 3.
The information related to the Ti–Cu binary system at lower temperature and lower copper content.
| Solid Solubility |
Eutectoid Reaction |
Ti2Cu Phase | ||
|---|---|---|---|---|
| α-Ti | β-Ti | β-Ti → α-Ti + Ti2Cu | ||
| Cu Concentration (wt.%) | 2.1 | 18 | 7 | 40 |
| Temperature (◦C) | 790 | 1005 | 790 | 1005 |
The α-Ti phase is formed during the equilibrium cooling processes, and the microstructures containing β-Ti are fabricated due to the fast cooling of alloys from the eutectoid temperature. The intermetallic phase of Ti2Cu precipitates within the Ti–Cu alloy under equilibrium solidification conditions when the copper content is less than 40 wt.% Cu [226]. In addition, the Cu-rich phase is a result of an incomplete solid-state reaction during the cooling in the sintering process and does not have a specific stoichiometric composition. Therefore, this phase is not an equilibrium phase and occurs under certain sintering conditions [208,255]. As such, the processing methods, heat treatment conditions, and surface modification procedures greatly influence the antibacterial efficacy of Ti–Cu implants. As a case in point, Liu et al. [208] recognized 5 wt.% of copper as the minimum copper concentration for effective antibacterial performance in Ti–Cu made by conventional sintering process, while Ji et al. [256] identified the Ti–3Cu alloy fabricated by selective laser melting process with a stable, highly-effective antibacterial performance of 99%. Table 3 summarizes the critical copper concertation and temperature in the Ti–Cu binary system at low copper contents and temperatures.
As discussed above, the earlier studies proposed that the ions released from the surface of Ti–Cu implants are responsible for killing bacteria. In fact, researchers held two misconceptions in justifying the antibacterial mechanisms of Ti–Cu implants by considering the ion-releasing mechanism as the only mechanism for the antibacterial properties of Ti–Cu alloy. The first group suggested that the ions released from the solid solution phases [165,212,213,215] are responsible for antibacterial activity. It means a microstructure containing a high ratio of solid solution can release more copper ions than a microstructure comprising a high ratio of intermetallic phases, and consequently, it is expected that the former possesses a stronger antibacterial activity than the latter. However, this was not observed in the inspiring research of Zhang et al. [190], and the sample with less ion release showed greater antibacterial activity. In this research, the influence of various forms of copper on the properties of Ti–Cu implant was investigated by fabricating different microstructures (copper existing forms) in the Ti–Cu alloys via various manufacturing processes and several heat-treatment processes (Table 4). Results showed that the Ti–Cu(S) alloy, which holds a higher percentage of Ti2Cu phase and a lower ratio of the solid solution, presented stronger antibacterial activity than the Ti–Cu (T4) alloy, which contains a large amount of solid solution in its microstructure. The data shown in Fig. 6 indicate that ion-releasing cannot be the only factor defining the efficacy of antibacterial properties in Ti–Cu alloys.
Table 4.
The effect of processing methods and the microstructure of Ti–Cu alloys on their antibacterial efficacy.
| Alloy (Ti- wt.% of Cu) | Processing Method | Microstructure | R % (antibacterial efficacy) | Mentioned Mechanism | Ref. |
|---|---|---|---|---|---|
| Ti–1Cu | Ingot melting and heat treatment of 900 °C for 18 h followed by 798 °C for 24 h and finally water quench | Solid solution of Cu in Ti | 4% after 6 h of contact with the alloy | Cu ion releasing | [171] |
| Ti-2.5Cu | Solid solution of Cu in Ti, Cu-rich phase | 13% after 6 h of contact with the alloy | |||
| Ti–3Cu | Solid solution of Cu in Ti, Ti2Cu | 16% after 6 h of contact with the alloy | |||
| Ti–10Cu | Solid solution of Cu in Ti, Ti2Cu | 24% after 6 h of contact with the alloy | |||
| Ti–3Cu | Ti–3Cu wrought bar and solid solution treated (900 °C for 5 h + 400 °C for 16 h) | Solid solution of Cu in Ti, and nano-scale of Ti2Cu | ≥99% | Disrupting the proton motive force and resisting the production of ATP by the nano-scale galvanic cells on the surface of Ti–Cu alloy | [177] |
| Ti–2Cu | Powder metallurgy | Solid solution of Cu in Ti, Ti2Cu | ≤79% | Cu ion releasing | [208] |
| Ti–5Cu | Solid solution of Cu in Ti, Ti2Cu, Cu-rich phase | ≤99.2% | |||
| Ti–10Cu | Solid solution of Cu in Ti, Ti2Cu, Cu-rich phase | 99.99% | |||
| Ti–25Cu | Solid solution of Cu in Ti, Ti2Cu, Cu-rich phase | 99.99% | |||
| Ti–5Cu (S) | Powder metallurgy | α-Ti phase and a small amount of Ti2Cu phases | 99.3% | Cu ion releasing and contact with the Ti2Cu phase | [257] |
| Ti–5Cu(E) | Extrusion process (sintered alloys were extruded at 800 °C at a rate of 10 mm/s) | α-Ti phase and a small amount of Ti2Cu phases, phases are smaller and more homogenized | 99.4% | ||
| Ti–10Cu(S) | Powder metallurgy | α-Ti phase and more Ti2Cu phases | 99.9% | ||
| Ti–10Cu(E) | Extrusion process (sintered alloys were extruded at 800 °C at a rate of 10 mm/s) | α-Ti phase and more Ti2Cu phases with flake shapes, phases are smaller and more homogenized | 99.8% | ||
| Ti–5Cu | Ingot melting followed by heat treatment at 850 °C for 2 h and cooling in the furnace | α -Ti (HCP) matrix and Ti2Cu precipitation | ≥99% | Contacting sterilization of Ti2Cu and Cu ion releasing | [207] |
| Ti–5Cu | Ingot melting followed by heat treatment at 900 °C for 2 h, and air cooling | Ti2Cu phase in the α -Ti matrix | ≤96%, | Cu ion releasing | [178] |
| Ti–5Cu (I) | Alloys were melted and casted in ingot (casting) | Nano-scale Ti2Cu, a relatively high amount of Cu in a solid solution state | 51% | Contact sterilization and Cu ion releasing | [190] |
| Ti–10Cu (I) | More nano-scale Ti2Cu, relatively high amount of Cu in solid solution state | 64% | |||
| Ti–5Cu (T4) | As-cast alloys were heat-treated at 900 °C for 2 h (T4) and quenched in room temperature water | Complete solid solution | <55%a | ||
| Ti–10Cu (T4) | A large amount of solid solution and a small amount of Ti2Cu phase | <70%a | |||
| Ti–5Cu (T6) | As-cast alloys were heat-treated at 900 °C for 2 h and quenched in water, and then at 400 °C for 12 h (T6) | Nano-scale Ti2Cu, a small amount of solid solution | >90%a | ||
| Ti–10Cu (T6) | Nano-scale Ti2Cu phase, a small amount of solid solution | <95%a | |||
| Ti–5Cu (S) | Ti and Cu powders were sintered (sintering) | Micro-scale Ti2Cu, a minimal amount of solid solution | >99%a | ||
| Ti–10Cu (S) | More micro-scale Ti2Cu, very small amount of solid solution | >99%a |
Data were derived from the graphs presented in the referenced paper.
Fig. 6.
The relationship between ion releasing of an alloy and its antibacterial performance estimated in various publications [19,39,177,178,190,207,208,257]. These data well show that the ion-releasing characteristic is not the only factor determining the antibacterial performance. For example, the ion releasing amount of Ti–10Cu(I) alloy [180] is more than that of Ti–5Cu(S) [180], however, the antibacterial rate of the latter is much higher than the former.
Therefore, it has been realized that the intermetallic phase of Ti2Cu has a vital role in antibacterial effectiveness. Accordingly, the second group of researchers, possibly incorrectly, hypothesized that the anti-bacterial property is attributed to the release of Cu2+ ions from the Ti2Cu intermetallic compound [134,159]. It should be mentioned that copper ions can hardly be released from intermetallic compounds such as Ti2Cu due to the stronger binding force of copper in Ti2Cu (ionic or covalent bonding) compared to solid solution copper atoms (metal bonding) [220,222,226]. We thus conclude that the intermetallic phases (here Ti2Cu) exert an extraordinary effect on the antibacterial properties of Ti–Cu alloys through the contact killing mechanism. This effect is illustrated in Fig. 7. Also, the schematic of how the microstructure of Ti–Cu implants is effective in the antibacterial properties is illustrated in Scheme 5 . It was reported that the formation of a high fraction of intermetallic phases of Ti2Cu rather than the solid solution not only increases the antibacterial efficacy of the implants but also decreases the risk of cytotoxicity so that the Ti–57Cu with fully intermetallic microstructure exhibited excellent cell viability to MG-63 osteosarcoma cells [212]
Fig. 7.
SEM images from different microstructures formed by various heat treatments and the corresponding antibacterial activities observed in the plate counting test. (a) Ti–5Cu(I) with a high ratio of solid solution and low content of Ti2Cu phase, and (b) the corresponding bacterial colonization. (c) Ti–5Cu(T4) alloys with fully solid solution microstructure, and (d) the corresponding bacterial colonization. (e) Ti–5Cu (T6) alloy with a high ratio of intermetallic phase and a small amount of solid solution, and (f) the corresponding bacterial colonization. (g) Ti–5Cu(S) alloy with an extremely high ratio of intermetallic phase and an extremely low content of the solid solution, and (h) corresponding bacterial colonization. With the increase in the intermetallic phase, the antibacterial efficacy of alloys enhances [190].
Scheme 5.
Schematic of two existing forms of copper in Ti–Cu alloys and their different roles on the invading bacteria. (a) An alloy with a fully solid solution microstructure that is unable to kill all bacteria coming in contact with the implant surface, resulting in biofilm formation. (b) An alloy containing large concentrations of Ti2Cu and a small fraction of solid solution showing effective antimicrobial performance. In this case, the Ti2Cu particles prevent bacterial adhesion to the surface and thus inhibit the formation of biofilm. The Cu2+ ions kill planktonic bacteria near the surface. The schematic is inspired by Ref. [190].
A careful review of the literature reveals that the antibacterial properties of the Ti–Cu implant are consistent with the volume fraction, the size, and the homogeneity of Ti2Cu phase [165,177,225,226,228,229,258,259]. In this regard, two aspects are significant in increasing the antibacterial activity: the increase in surface area fraction of Ti2Cu to the total surface of the alloy and the improvement in the homogeneity of this phase in the whole microstructure. Therefore, any processes such as heat treatment, surface treatments, and aging procedures that augment the surface area fraction of Ti2Cu exert great influence on the antibacterial properties of the Ti–Cu implant [260,261].
Table 4 presents the effect of processing methods on the microstructure and antibacterial performance of Ti–Cu alloys. To give an example, Lu et al. [231] improved the antibacterial behavior of Ti–Cu alloy by increasing the volume fraction of Ti2Cu phase due to the selective acid etching (SLE) surface treatment, although the surface roughness did not change significantly. In another study, the increase of heat treatment time from 16 to 36 h, resulted in an increase in the fraction of deposited Ti2Cu with enhanced homogeneity, and consequently, the antibacterial character of the alloy increased from 91% to 99% [176,198]. Furthermore, Xu et al. [229] confirmed that the large numbers of smaller Ti2Cu crystals enhance the antibacterial capability of the alloy. In addition, the morphology of Ti2Cu is also influential. In work carried out by Wu et al. [197], it was confirmed that the acicular precipitates of Ti2Cu possess twice contribution in antibacterial efficacy than that of the fine Ti2Cu precipitates. Similar to this result, Xin et al. [262] proved that the Ti–Cu alloy containing lamellar Ti2Cu demonstrated better antibacterial behavior than the Ti–Cu alloy with granular Ti2Cu, due to the fact that the lamellar Ti2Cu formed elongated micro-galvanic cells.
Ti2Cu phase is capable of killing the bacteria that come in contact with the alloy surface, thereby preventing biofilm maturation as the major cause of infection. However, less ion releasing in such Ti2Cu-rich implants with low content of solid solution phase results in the existence of a high number of planktonic bacteria. In fact, bacteria on the Ti-Cu surface can be killed at a higher rate than those that are not in direct contact with the copper-containing surface [171]. This effect has been demonstrated in a study by Zhang and his coworkers. In this research, it was concluded that the bacteria in the suspension containing Ti-3Cu alloy were so high in number that they were almost equal to the number of bacteria in the suspension containing cp-Ti. However, the bacteria in contact with the surface of the Ti-3Cu implant had completely disappeared [177]. This process is represented in Fig. 8. In addition, Fan et al. [211] reported that a special antibacterial alloy of Ti-Cu possessed a significant antibacterial effect against sessile bacteria, while almost no antibacterial effect was detected for planktonic bacteria in this alloy.
Fig. 8.
Typical S. aureus colonies on agar plate incubated with bacteria obtained from a, b) the suspension with cp-Ti and the suspension with Ti–3Cu; and c, d) the surface of cp-Ti and the surface of Ti–3Cu, respectively [177]. There is no difference in the cell number between the Cu-free and Cu-containing suspensions sample, indicating the Ti–Cu is unable to kill bacteria when they are in planktonic mode. However, bacteria that come in contact with Cu-containing samples are killed at a high rate.
Therefore the contribution of these two mechanisms is conducive to the killing of bacteria, and the absence of either of these mechanisms leads to the reduction in antibacterial activity [263]. According to the study of Mathews et al. [160], it was confirmed that the released ions are not capable of killing bacteria alone. On the other hand, as Lui et al. [39] reported, ion releasing still has its own noteworthy effect.
Several studies indicated that the surface topography and hydrophilicity of Ti–Cu are also effective in the antibacterial performance [199,222,223,256,[264], [265], [266], [267], [268]]. For example, Hu et al. reported that the Ti–Cu implants, which were surface treated by ultrasonic micro-arc oxidation could produce a rough, porous layer, yielding a stronger antibacterial effect [264]. The SLMed Ti–Cu implants in the research of Ji et al. [256] with fine holes on the surface formed as a result of shrinkage during fast solidification of the SLM process showed stronger antibacterial activity compared to the highly-dense Ti–Cu. This phenomenon is due to the fact that fine pores not only increase the effective contact area between the Cu-containing phases and bacteria but also facilitate higher rates of copper ions release compared to the untreated alloy [222]. In fact, pure Ti implants with high surface roughness are in favor of bacterial attachment and biofilm formation compared with smooth surfaces [269,270]; nevertheless, Ti–Cu implants owning high-rough surfaces act as a trap for bacteria and make them more exposed to contact with Cu-containing particles, therefore kill them more efficiently [199]. However, it has been reported that the changes in the antibacterial performance for surface roughness value of smaller than 0.2 μm is negligible [271].
6. Future directions
The information reported on the antibacterial properties of Ti–Cu implants, such as the critical concentration of copper element, varies in the literature from one study to another. Such variation and inconsistencies highly depend on the experimental condition and the type of antimicrobial assessment assay used in each study. Therefore, one indispensable need is that methodologies to test the antimicrobial activity of the Ti–Cu implant follow a consistent protocol. Such protocol should reflect the actual and practical operating conditions of the implant in the human body to compare and evaluate the antimicrobial efficacy of various Ti–Cu alloys. Following a consistent and universal protocol should allow for a better understating of the antibacterial mechanisms underpinning the performance of Ti–Cu implants.
As emphasized in this paper, the exact contact killing mechanism of Ti–Cu antibacterial properties has not yet been fully understood. One approach that can be used to experimentally shed light on this mechanism is to fabricate a Ti–Cu alloy with a microstructure saturated with intermetallic compound phases. A fully-intermetallic phase microstructure simulates the operation of the contact-killing mechanism solely, without the interference of the ion-releasing mechanism. This is because relatively small concentrations of copper ions are released from the intermetallic phases. Therefore, by studying this type of alloy, the role of contact killing mechanism can be investigated in more detail. One important area in this domain that deserves further research is to track the first stages of antibacterial activity and monitor the early interactions of Cu-containing surfaces with the bacteria.
Until now, the biological and antibacterial properties of Ti–Cu implants were primarily evaluated in vitro, and the majority of conclusions reported in the literature have been made based on such experimental data. These studies have limitations in simulating the real in vivo physiological environment where the implant is inserted. As such, comprehensive in vivo studies are needed to obtain reliable evidence on the performance of Ti–Cu implants for clinical applications.
A quick literature survey reveals that the presence of copper in titanium alloys can promote osteogenic differentiation, both in vivo and in vitro, with biocompatibility properties comparable to cp-Ti alloys. However, a critical and in-depth review of the literature on such performance of Ti–Cu is required to gain a comprehensive understanding of how these properties and antibacterial activities can be optimized for Ti–Cu implants.
Although many groups have focused on the applications of Ti–Cu alloys in orthopedic and dental implants, in-depth investigation of specific microstructural features of these materials is still needed. For example, the effect of microstructural changes on the biological behavior of Ti–Cu alloys must be further investigated. As we discussed in Section 5, copper ions released from the implant surface can be associated with mammalian cell toxicity, depending on their concentration. Therefore, the microstructural characteristics of Ti–Cu alloys that regulate the release profile of copper ions are particularly important and should be comprehensively studied. Such understanding will enable the fabrication of Ti–Cu implants with the optimum release of copper ions that are active against bacteria but not cytotoxic. Moreover, the mechanical properties and corrosion resistance of different alloys with various microstructural properties should be taken into account when designing antimicrobial Ti–Cu alloys for optimum, long-term practical performance in-vivo.
7. Conclusions
Bacterial infection caused by biofilm formation has been a long-lasting problem in orthopedic surgery and dental implantation. One of the most viable approaches to prevent biofilm formation on implants is to design biomaterials that discourage the first phase of biofilm formation. Among various anti-infection materials reported in the literature, Ti–Cu implants have been extensively investigated. It has been well documented that the presence of copper in Ti–Cu alloys inhibits biofilm formation and causes strong antibacterial efficacy. However, the mechanisms of action for the antibacterial behavior of such implants are not well-conveyed.
This review paper was designated to compile the existing information around the Ti–Cu antibacterial alloys for understanding the effective factors on antibacterial efficacy, the contradictions in literature, and the underpinning mechanism of antibacterial activity of Ti–Cu, in particular, the contact killing mechanism. It was concluded that through the contact killing mechanism, the cell envelope of bacteria experiences structural damages. When bacteria are in contact with Ti–Cu alloys, the cytoplasmic membrane loses its integrity and provides conditions for the entry of ions into the bacteria. Of particular interest is that the membrane disintegration is not due to the attack of copper ions. Instead, the contact of bacteria with the copper-containing surface may be a key, defining event. It seems the electrostatic interactions of the Cu-containing phase disrupt the balance in the internal-external osmotic pressure of the cell, which is conducive to the disintegration of the cell envelope. We highlighted the pivotal role of the intermetallic phase in the Ti–Cu binary system and the effect of Ti2Cu phase in the disintegration of bacteria cells. It is suggested that the potential difference between the Ti2Cu particles and the Ti matrix is conducive to a micro galvanic cell formation, which in turn creates a charge transfer between anode and cathode in the micro electric couple. The resulting electron transfer interferes with the internal functions of bacteria and culminates in bacterial death. Thus, Ti2Cu, an intermetallic phase, formed in the copper concentration of less than 40 wt.%, plays a key role in the antibacterial efficacy of Ti–Cu alloys.
After cell rapture, released copper ions invade the bacteria and kill cells by damaging the vital components and exerting oxidative stress in the cells (ion releasing mechanism). It was demonstrated that copper ions released from the solid solution state of copper in titanium have a critical function in the antibacterial activity of Ti–Cu alloys. Finally, the overriding importance of fabrication procedures and antimicrobial assessment protocols to measure the activity of Ti–Cu materials were acknowledged. We envision that the mechanisms behind the antibacterial activities of these materials elucidated and discussed in the paper pave the way for the design and fabrication of new titanium and copper-containing alloys, ushering a new dimension to produce anti-infection biomedical devices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank professor Mohammad Maleki Shahraki for his valuable assistance in preparing this paper. The financial support of the Australian Research Council (ARC) through the DECRA program (DE210100662) and Hunter Medical Research Institute (HMRI) through the Precision Medicine Research Program is gratefully acknowledged.
Data availability
No data was used for the research described in the article.
References
- 1.Gristina A.G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 2.Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16(7):397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 3.Stones D.H., Krachler A.M. Against the tide: the role of bacterial adhesion in host colonization. Biochem. Soc. Trans. 2016;44(6):1571–1580. doi: 10.1042/BST20160186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen L.K., Koch J., Aalbaek B., Moodley A., Bjarnsholt T., Kragh K.N., Petersen A., Jensen H.E. Early implant-associated osteomyelitis results in a peri-implanted bacterial reservoir. APMIS. 2017;125(1):38–45. doi: 10.1111/apm.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasouli M.R., Restrepo C., Maltenfort M.G., Purtill J.J., Parvizi J. Risk factors for surgical site infection following total joint arthroplasty. J Bone Joint Surg Am. 2014;96(18):e158. doi: 10.2106/JBJS.M.01363. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014;276(2):111–119. doi: 10.1111/joim.12233. [DOI] [PubMed] [Google Scholar]
- 7.Inzana J.A., Schwarz E.M., Kates S.L., Awad H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials. 2016;81:58–71. doi: 10.1016/j.biomaterials.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laffer R., Graber P., Ochsner P., Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clinical microbiology and infection. 2006;12(5):433–439. doi: 10.1111/j.1469-0691.2006.01378.x. [DOI] [PubMed] [Google Scholar]
- 9.Anguita-Alonso P., Hanssen A.D., Osmon D.R., Trampuz A., Steckelberg J.M., Patel R. High rate of aminoglycoside resistance among staphylococci causing prosthetic joint infection. Clin. Orthop. Relat. Res. 2005;439:43–47. doi: 10.1097/01.blo.0000182394.39601.9d. [DOI] [PubMed] [Google Scholar]
- 10.Lee C.T., Huang Y.W., Zhu L., Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J. Dent. 2017;62:1–12. doi: 10.1016/j.jdent.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Rakic M., Galindo-Moreno P., Monje A., Radovanovic S., Wang H.-L., Cochran D., Sculean A., Canullo L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral Invest. 2018;22(4):1805–1816. doi: 10.1007/s00784-017-2276-y. [DOI] [PubMed] [Google Scholar]
- 12.Yan C.H., Arciola C.R., Soriano A., Levin L.S., Bauer T.W., Parvizi J. Team Approach: the management of infection after total knee replacement. JBJS Rev. 2018;6(4):e9. doi: 10.2106/JBJS.RVW.17.00058. [DOI] [PubMed] [Google Scholar]
- 13.Kapadia B.H., Banerjee S., Cherian J.J., Bozic K.J., Mont M.A. The economic impact of periprosthetic infections after total hip arthroplasty at a specialized tertiary-care center. J. Arthroplasty. 2016;31(7):1422–1426. doi: 10.1016/j.arth.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 14.V. Antoci, A.F. Chen, J. Parvizi, 7.9 Orthopedic Implant Use and Infection ☆, Comprehensive Biomaterials II2017, pp. 133-151.
- 15.Jakubowicz J. Special issue: Ti-based biomaterials: synthesis, properties and applications. Materials. 2020;13(7) doi: 10.3390/ma13071696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams D.F. Springer; 2001. Titanium for Medical Applications, Titanium in Medicine; pp. 13–24. [Google Scholar]
- 17.Oldani C., Dominguez A. Titanium as a biomaterial for implants. Recent advances in arthroplasty. 2012;218:149–162. [Google Scholar]
- 18.Niinomi M., Boehlert C.J. Springer; 2015. Titanium Alloys for Biomedical Applications, Advances in Metallic Biomaterials; pp. 179–213. [Google Scholar]
- 19.Zhang E., Li F., Wang H., Liu J., Wang C., Li M., Yang K. A new antibacterial titanium-copper sintered alloy: preparation and antibacterial property. Mater Sci Eng C Mater Biol Appl. 2013;33(7):4280–4287. doi: 10.1016/j.msec.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Puckett S.D., Taylor E., Raimondo T., Webster T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31(4):706–713. doi: 10.1016/j.biomaterials.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 21.Shirai T., Tsuchiya H., Shimizu T., Ohtani K., Zen Y., Tomita K. Prevention of pin tract infection with titanium-copper alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2009;91(1):373–380. doi: 10.1002/jbm.b.31412. [DOI] [PubMed] [Google Scholar]
- 22.Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011;77(5):1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowy F. Columbia University; 2009. Bacterial Classification, Structure and Function. Lecture notes. Retrieved August 22. [Google Scholar]
- 24.Brooks G., Carroll K., Butel J., Morse S., Mietzner T., Jawetz Melnick. Placebo doo; 2015. Adelberg Medical Microbiology. [Google Scholar]
- 25.Roberts G.C.K. 2013. Encyclopedia of Biophysics. [Google Scholar]
- 26.Cowan S.T., Steel K.J. 1965. Manual for the Identification of Medical Bacteria, Manual for the Identification of Medical Bacteria. [Google Scholar]
- 27.Kostakioti M., Hadjifrangiskou M., Hultgren S.J. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med. 2013;3(4):a010306. doi: 10.1101/cshperspect.a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.S. Maloy, K. Hughes, Brenner's Encyclopedia of Genetics, Academic Press2013.
- 29.Chandki R., Banthia P., Banthia R. Biofilms: a microbial home. J. Indian Soc. Periodontol. 2011;15(2):111–114. doi: 10.4103/0972-124X.84377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jefferson K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004;236(2):163–173. doi: 10.1016/j.femsle.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Foreman A., Boase S., Psaltis A., Wormald P.J. Role of bacterial and fungal biofilms in chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2012;12(2):127–135. doi: 10.1007/s11882-012-0246-7. [DOI] [PubMed] [Google Scholar]
- 32.Del Pozo J.L. Biofilm-related disease. Expert Rev. Anti Infect. Ther. 2018;16(1):51–65. doi: 10.1080/14787210.2018.1417036. [DOI] [PubMed] [Google Scholar]
- 33.Jung W.K., Koo H.C., Kim K.W., Shin S., Kim S.H., Park Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008;74(7):2171–2178. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasuyuki M., Kunihiro K., Kurissery S., Kanavillil N., Sato Y., Kikuchi Y. Antibacterial properties of nine pure metals: a laboratory study using Staphylococcus aureus and Escherichia coli. Biofouling. 2010;26(7):851–858. doi: 10.1080/08927014.2010.527000. [DOI] [PubMed] [Google Scholar]
- 35.Zhang E., Zhao X., Hu J., Wang R., Fu S., Qin G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021;6(8):2569–2612. doi: 10.1016/j.bioactmat.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wennerberg A. The importance of surface roughness for implant incorporation. Int. J. Mach. Tool Manufact. 1998;38(5–6):657–662. [Google Scholar]
- 37.Sahrmann P., Ronay V., Hofer D., Attin T., Jung R.E., Schmidlin P.R. In vitro cleaning potential of three different implant debridement methods. Clin. Oral Implants Res. 2015;26(3):314–319. doi: 10.1111/clr.12322. [DOI] [PubMed] [Google Scholar]
- 38.John G., Becker J., Schwarz F. Rotating titanium brush for plaque removal from rough titanium surfaces--an in vitro study. Clin. Oral Implants Res. 2014;25(7):838–842. doi: 10.1111/clr.12147. [DOI] [PubMed] [Google Scholar]
- 39.Liu R., Memarzadeh K., Chang B., Zhang Y., Ma Z., Allaker R.P., Ren L., Yang K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016;6 doi: 10.1038/srep29985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Z., Liu R., Zhao Y., Ren L., Yang K. Study on the antibacterial mechanism of Cu-bearing titanium alloy in the view of materials science. Mater. Technol. 2019;35(1):11–20. [Google Scholar]
- 41.Chen Z., Wang Z., Qiu W., Fang F. Overview of antibacterial strategies of dental implant materials for the prevention of peri-implantitis. Bioconjugate Chem. 2021;32(4):627–638. doi: 10.1021/acs.bioconjchem.1c00129. [DOI] [PubMed] [Google Scholar]
- 42.Atefyekta S., Ercan B., Karlsson J., Taylor E., Chung S., Webster T.J., Andersson M. Antimicrobial performance of mesoporous titania thin films: role of pore size, hydrophobicity, and antibiotic release. Int. J. Nanomed. 2016;11:977–990. doi: 10.2147/IJN.S95375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mas-Moruno C., Su B., Dalby M.J. Multifunctional coatings and nanotopographies: toward cell instructive and antibacterial implants. Adv Healthc Mater. 2019;8(1) doi: 10.1002/adhm.201801103. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Deng Q., Xiao J., Nie H., Wu L., Zhou W., Huang B. Controlled grafting from poly (vinylidene fluoride) microfiltration membranes via reverse atom transfer radical polymerization and antifouling properties. Polymer. 2007;48(26):7604–7613. [Google Scholar]
- 45.Chang Y., Ko C.-Y., Shih Y.-J., Quémener D., Deratani A., Wei T.-C., Wang D.-M., Lai J.-Y. Surface grafting control of PEGylated poly (vinylidene fluoride) antifouling membrane via surface-initiated radical graft copolymerization. J. Membr. Sci. 2009;345(1–2):160–169. [Google Scholar]
- 46.Susanto H., Ulbricht M. Photografted thin polymer hydrogel layers on PES ultrafiltration membranes: characterization, stability, and influence on separation performance. Langmuir. 2007;23(14):7818–7830. doi: 10.1021/la700579x. [DOI] [PubMed] [Google Scholar]
- 47.Chiang Y.-C., Chang Y., Higuchi A., Chen W.-Y., Ruaan R.-C. Sulfobetaine-grafted poly (vinylidene fluoride) ultrafiltration membranes exhibit excellent antifouling property. J. Membr. Sci. 2009;339(1–2):151–159. [Google Scholar]
- 48.Hsiao S.-W., Venault A., Yang H.-S., Chang Y. Bacterial resistance of self-assembled surfaces using PPOm-b-PSBMAn zwitterionic copolymer–concomitant effects of surface topography and surface chemistry on attachment of live bacteria. Colloids Surf. B Biointerfaces. 2014;118:254–260. doi: 10.1016/j.colsurfb.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 49.Hu X., Xu R., Yu X., Chen J., Wan S., Ouyang J., Deng F. Enhanced antibacterial efficacy of selective laser melting titanium surface with nanophase calcium phosphate embedded to TiO2 nanotubes. Biomed. Mater. 2018;13(4) doi: 10.1088/1748-605X/aac1a3. [DOI] [PubMed] [Google Scholar]
- 50.Yi G., Yuan Y., Li X., Zhang Y. ZnO nanopillar coated surfaces with substrate-dependent superbactericidal property. Small. 2018;14(14) doi: 10.1002/smll.201703159. [DOI] [PubMed] [Google Scholar]
- 51.Ye J., Deng J., Chen Y., Yang T., Zhu Y., Wu C., Wu T., Jia J., Cheng X., Wang X. Cicada and catkin inspired dual biomimetic antibacterial structure for the surface modification of implant material. Biomater. Sci. 2019;7(7):2826–2832. doi: 10.1039/c9bm00082h. [DOI] [PubMed] [Google Scholar]
- 52.Elbourne A., Crawford R.J., Ivanova E.P. Nano-structured antimicrobial surfaces: from nature to synthetic analogues. J. Colloid Interface Sci. 2017;508:603–616. doi: 10.1016/j.jcis.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 53.Li Y., Yang Y., Li R., Tang X., Guo D., Qing Y., Qin Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: a review of current techniques. Int. J. Nanomed. 2019;14:7217–7236. doi: 10.2147/IJN.S216175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L., Li W., Liu Q. Recent development of antifouling polymers: structure, evaluation, and biomedical applications in nano/micro-structures. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(6):599–614. doi: 10.1002/wnan.1278. [DOI] [PubMed] [Google Scholar]
- 55.Pranjali P., Meher M.K., Raj R., Prasad N., Poluri K.M., Kumar D., Guleria A. Physicochemical and antibacterial properties of PEGylated zinc oxide nanoparticles dispersed in peritoneal dialysis fluid. ACS Omega. 2019;4(21):19255–19264. doi: 10.1021/acsomega.9b02615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buwalda S., Rotman S., Eglin D., Moriarty F., Bethry A., Garric X., Guillaume O., Nottelet B. Synergistic anti-fouling and bactericidal poly(ether ether ketone) surfaces via a one-step photomodification. Mater Sci Eng C Mater Biol Appl. 2020;111 doi: 10.1016/j.msec.2020.110811. [DOI] [PubMed] [Google Scholar]
- 57.Neoh K.G., Kang E.T. Combating bacterial colonization on metals via polymer coatings: relevance to marine and medical applications. ACS Appl. Mater. Interfaces. 2011;3(8):2808–2819. doi: 10.1021/am200646t. [DOI] [PubMed] [Google Scholar]
- 58.Skovdal S.M., Jorgensen N.P., Petersen E., Jensen-Fangel S., Ogaki R., Zeng G., Johansen M.I., Wang M., Rohde H., Meyer R.L. Ultra-dense polymer brush coating reduces Staphylococcus epidermidis biofilms on medical implants and improves antibiotic treatment outcome. Acta Biomater. 2018;76:46–55. doi: 10.1016/j.actbio.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Harris L.G., Tosatti S., Wieland M., Textor M., Richards R.G. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(L-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials. 2004;25(18):4135–4148. doi: 10.1016/j.biomaterials.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 60.Fortunati E., Mattioli S., Visai L., Imbriani M., Fierro J.L., Kenny J.M., Armentano I. Combined effects of Ag nanoparticles and oxygen plasma treatment on PLGA morphological, chemical, and antibacterial properties. Biomacromolecules. 2013;14(3):626–636. doi: 10.1021/bm301524e. [DOI] [PubMed] [Google Scholar]
- 61.Armentano I., Arciola C.R., Fortunati E., Ferrari D., Mattioli S., Amoroso C.F., Rizzo J., Kenny J.M., Imbriani M., Visai L. The interaction of bacteria with engineered nanostructured polymeric materials: a review. Sci. World J. 2014;2014 doi: 10.1155/2014/410423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L., Ning C., Zhou T., Liu X., Yeung K.W., Zhang T., Xu Z., Wang X., Wu S., Chu P.K. Polymeric nanoarchitectures on Ti-based implants for antibacterial applications. ACS Appl. Mater. Interfaces. 2014;6(20):17323–17345. doi: 10.1021/am5045604. [DOI] [PubMed] [Google Scholar]
- 63.Hetrick E.M., Schoenfisch M.H. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 2006;35(9):780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 64.Norden C.W. Antibiotic prophylaxis in orthopedic surgery. Rev. Infect. Dis. 1991;13(Suppl 10):S842–S846. doi: 10.1093/clinids/13.supplement_10.s842. (Supplement_10) [DOI] [PubMed] [Google Scholar]
- 65.Duran L.W. Preventing medical device related infections. Med. Device Technol. 2000;11(6):14–17. [PubMed] [Google Scholar]
- 66.Arkusz K., Paradowska E., Nycz M., Mazurek-Popczyk J., Baldy-Chudzik K. Evaluation of the antibacterial activity of Ag-and Au-nanoparticles loaded TiO2 nanotubes. J. Biomed. Nanotechnol. 2020;16(9):1416–1425. doi: 10.1166/jbn.2020.2979. [DOI] [PubMed] [Google Scholar]
- 67.Balamurugan A., Balossier G., Laurent-Maquin D., Pina S., Rebelo A.H., Faure J., Ferreira J.M. An in vitro biological and anti-bacterial study on a sol-gel derived silver-incorporated bioglass system. Dent. Mater. 2008;24(10):1343–1351. doi: 10.1016/j.dental.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 68.Vu A.A., Robertson S.F., Ke D., Bandyopadhyay A., Bose S. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019;92:325–335. doi: 10.1016/j.actbio.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 69.Ding Z., Wang Y., Zhou Q., Ding Z., Wu Y., Zhu Y., Shi W., He Q. The preparation and properties of multilayer Cu-MTa2O5 composite coatings on Ti6Al4V for biomedical applications. Nanomaterials. 2019;9(10):1498. doi: 10.3390/nano9101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bigham A., Saudi A., Rafienia M., Rahmati S., Bakhtiyari H., Salahshouri F., Sattary M., Hassanzadeh-Tabrizi S. Electrophoretically deposited mesoporous magnesium silicate with ordered nanopores as an antibiotic-loaded coating on surface-modified titanium. Mater. Sci. Eng. C. 2019;96:765–775. doi: 10.1016/j.msec.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 71.Chua P.H., Neoh K.G., Kang E.T., Wang W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials. 2008;29(10):1412–1421. doi: 10.1016/j.biomaterials.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 72.Hashemi A., Ezati M., Mohammadnejad J., Houshmand B., Faghihi S. Chitosan coating of TiO2 nanotube Arrays for improved metformin release and osteoblast differentiation. Int. J. Nanomed. 2020;15:4471–4481. doi: 10.2147/IJN.S248927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li T., Wang N., Chen S., Lu R., Li H., Zhang Z. Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide. Int. J. Nanomed. 2017;12:2995–3007. doi: 10.2147/IJN.S128775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dutta D., Kumar N., M D.P.W. Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling. 2016;32(4):429–438. doi: 10.1080/08927014.2015.1129533. [DOI] [PubMed] [Google Scholar]
- 75.Esmaeilnejad A., Mahmoudi P., Zamanian A., Mozafari M. Synthesis of titanium oxide nanotubes and their decoration by MnO nanoparticles for biomedical applications. Ceram. Int. 2019;45(15):19275–19282. [Google Scholar]
- 76.Gunputh U.F., Le H., Handy R.D., Tredwin C. Anodised TiO2 nanotubes as a scaffold for antibacterial silver nanoparticles on titanium implants. Mater. Sci. Eng. C. 2018;91:638–644. doi: 10.1016/j.msec.2018.05.074. [DOI] [PubMed] [Google Scholar]
- 77.Li B., Xia X., Guo M., Jiang Y., Li Y., Zhang Z., Liu S., Li H., Liang C., Wang H. Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-49941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madzovska-Malagurski I., Vukasinovic-Sekulic M., Kostic D., Levic S. Towards antimicrobial yet bioactive Cu-alginate hydrogels. Biomed. Mater. 2016;11(3) doi: 10.1088/1748-6041/11/3/035015. [DOI] [PubMed] [Google Scholar]
- 79.Walia R., Akhavan B., Kosobrodova E., Kondyurin A., Oveissi F., Naficy S., Yeo G.C., Hawker M., Kaplan D.L., Dehghani F. Hydrogel− solid hybrid materials for biomedical applications enabled by surface-embedded radicals. Adv. Funct. Mater. 2020;30(38) [Google Scholar]
- 80.Akhtar M.A., Ilyas K., Dlouhy I., Siska F., Boccaccini A.R. Electrophoretic deposition of copper(II)-Chitosan complexes for antibacterial coatings. Int. J. Mol. Sci. 2020;21(7):2637. doi: 10.3390/ijms21072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malcher M., Volodkin D., Heurtault B., Andre P., Schaaf P., Mohwald H., Voegel J.C., Sokolowski A., Ball V., Boulmedais F., Frisch B. Embedded silver ions-containing liposomes in polyelectrolyte multilayers: cargos films for antibacterial agents. Langmuir. 2008;24(18):10209–10215. doi: 10.1021/la8014755. [DOI] [PubMed] [Google Scholar]
- 82.Kun-Qiang L., Yu-Qin Q., Xuan-Yong L. Titanium modified by copper ion implantation: anti-bacterial and cellular behaviors. J. Inorg. Mater. 2020;35(2):158–164. [Google Scholar]
- 83.Wan Y., Xiong G., Liang H., Raman S., He F., Huang Y. Modification of medical metals by ion implantation of copper. Appl. Surf. Sci. 2007;253(24):9426–9429. [Google Scholar]
- 84.Hou X., Ma H., Liu F., Deng J., Ai Y., Zhao X., Mao D., Li D., Liao B. Synthesis of Ag ion-implanted TiO2 thin films for antibacterial application and photocatalytic performance. J. Hazard Mater. 2015;299:59–66. doi: 10.1016/j.jhazmat.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Li B., Zhang L., Wang D., Peng F., Zhao X., Liang C., Li H., Wang H. Thermosensitive-hydrogel-coated titania nanotubes with controlled drug release and immunoregulatory characteristics for orthopedic applications. Mater. Sci. Eng. C. 2021;122 doi: 10.1016/j.msec.2021.111878. [DOI] [PubMed] [Google Scholar]
- 86.Xie K., Zhou Z., Guo Y., Wang L., Li G., Zhao S., Liu X., Li J., Jiang W., Wu S., Hao Y. Long-term prevention of bacterial infection and enhanced osteoinductivity of a hybrid coating with selective silver toxicity. Adv Healthc Mater. 2019;8(5) doi: 10.1002/adhm.201801465. [DOI] [PubMed] [Google Scholar]
- 87.Escobar A., Muzzio N.E., Andreozzi P., Libertone S., Tasca E., Azzaroni O., Grzelczak M., Moya S.E. Antibacterial layer-by-layer films of poly (acrylic acid)–gentamicin complexes with a combined burst and sustainable release of gentamicin. Adv. Mater. Interfac. 2019;6(22) [Google Scholar]
- 88.Niu X., Sun L., Zhang X., Sun Y., Wang J. Fabrication and antibacterial properties of cefuroxime-loaded TiO 2 nanotubes. Appl. Microbiol. Biotechnol. 2020;104(7):2947–2955. doi: 10.1007/s00253-020-10446-w. [DOI] [PubMed] [Google Scholar]
- 89.Yuan Z., Huang S., Lan S., Xiong H., Tao B., Ding Y., Liu Y., Liu P., Cai K. Surface engineering of titanium implants with enzyme-triggered antibacterial properties and enhanced osseointegration in vivo. J. Mater. Chem. B. 2018;6(48):8090–8104. doi: 10.1039/c8tb01918e. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y., Zhang L., Li B., Han Y. Enhancement in sustained release of antimicrobial peptide from dual-diameter-structured TiO2 nanotubes for long-lasting antibacterial activity and cytocompatibility. ACS Appl. Mater. Interfaces. 2017;9(11):9449–9461. doi: 10.1021/acsami.7b00322. [DOI] [PubMed] [Google Scholar]
- 91.Dong Y., Ye H., Liu Y., Xu L., Wu Z., Hu X., Ma J., Pathak J.L., Liu J., Wu G. pH dependent silver nanoparticles releasing titanium implant: a novel therapeutic approach to control peri-implant infection. Colloids Surf. B Biointerfaces. 2017;158:127–136. doi: 10.1016/j.colsurfb.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 92.Geng H., Yuan Y., Adayi A., Zhang X., Song X., Gong L., Zhang X., Gao P. Engineered chimeric peptides with antimicrobial and titanium-binding functions to inhibit biofilm formation on Ti implants. Mater Sci Eng C Mater Biol Appl. 2018;82:141–154. doi: 10.1016/j.msec.2017.08.062. [DOI] [PubMed] [Google Scholar]
- 93.Boix-Lemonche G., Guillem-Marti J., D'Este F., Manero J.M., Skerlavaj B. Covalent grafting of titanium with a cathelicidin peptide produces an osteoblast compatible surface with antistaphylococcal activity. Colloids Surf. B Biointerfaces. 2020;185 doi: 10.1016/j.colsurfb.2019.110586. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X., Geng H., Gong L., Zhang Q., Li H., Zhang X., Wang Y., Gao P. Modification of the surface of titanium with multifunctional chimeric peptides to prevent biofilm formation via inhibition of initial colonizers. Int. J. Nanomed. 2018;13:5361–5375. doi: 10.2147/IJN.S170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silva R.R., Avelino K.Y., Ribeiro K.L., Franco O.L., Oliveira M.D., Andrade C.A. Chemical immobilization of antimicrobial peptides on biomaterial surfaces. Front. Biosci. 2016;8(1):129–142. doi: 10.2741/s453. [DOI] [PubMed] [Google Scholar]
- 96.Long T., Li H., Wang X., Yue B. A comparative analysis of antibacterial properties and inflammatory responses for the KR-12 peptide on titanium and PEGylated titanium surfaces. RSC Adv. 2017;7(55):34321–34330. [Google Scholar]
- 97.Stewart C., Akhavan B., Wise S.G., Bilek M.M. A review of biomimetic surface functionalization for bone-integrating orthopedic implants: mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019;106 [Google Scholar]
- 98.Godoy-Gallardo M., Mas-Moruno C., Fernández-Calderón M.C., Pérez-Giraldo C., Manero J.M., Albericio F., Gil F.J., Rodríguez D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014;10(8):3522–3534. doi: 10.1016/j.actbio.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 99.Akhavan B., Michl T.D., Giles C., Ho K., Martin L., Sharifahmadian O., Wise S.G., Coad B.R., Kumar N., Griesser H.J., Bilek M.M. Plasma activated coatings with dual action against fungi and bacteria. Appl. Mater. Today. 2018;12:72–84. [Google Scholar]
- 100.Roehling S., Astasov-Frauenhoffer M., Hauser-Gerspach I., Braissant O., Woelfler H., Waltimo T., Kniha H., Gahlert M. In vitro biofilm formation on titanium and zirconia implant surfaces. J. Periodontol. 2017;88(3):298–307. doi: 10.1902/jop.2016.160245. [DOI] [PubMed] [Google Scholar]
- 101.Hanawa T. Zirconia versus titanium in dentistry: a review. Dent. Mater. J. 2020;39(1):24–36. doi: 10.4012/dmj.2019-172. [DOI] [PubMed] [Google Scholar]
- 102.Suketa N., Sawase T., Kitaura H., Naito M., Baba K., Nakayama K., Wennerberg A., Atsuta M. An antibacterial surface on dental implants, based on the photocatalytic bactericidal effect. Clin. Implant Dent. Relat. Res. 2005;7(2):105–111. doi: 10.1111/j.1708-8208.2005.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 103.Iwatsu M., Kanetaka H., Mokudai T., Ogawa T., Kawashita M., Sasaki K. Visible light-induced photocatalytic and antibacterial activity of N-doped TiO2. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108(2):451–459. doi: 10.1002/jbm.b.34401. [DOI] [PubMed] [Google Scholar]
- 104.Akhavan B., Bakhshandeh S., Najafi-Ashtiani H., Fluit A.C., Boel E., Vogely C., van der Wal B.C.H., Zadpoor A.A., Weinans H., Hennink W.E., Bilek M.M., Amin Yavari S. Direct covalent attachment of silver nanoparticles on radical-rich plasma polymer films for antibacterial applications. J. Mater. Chem. B. 2018;6(37):5845–5853. doi: 10.1039/c8tb01363b. [DOI] [PubMed] [Google Scholar]
- 105.Huang B., Jing F., Akhavan B., Ji L., Leng Y., Xie D., Bilek M., Huang N. Multifunctional Ti-xCu coatings for cardiovascular interfaces: control of microstructure and surface chemistry. Mater Sci Eng C Mater Biol Appl. 2019;104 doi: 10.1016/j.msec.2019.109969. [DOI] [PubMed] [Google Scholar]
- 106.Nakajo K., Takahashi M., Kikuchi M., Takada Y., Okuno O., Sasaki K., Takahashi N. Inhibitory effect of Ti-Ag alloy on artificial biofilm formation. Dent. Mater. J. 2014;33(3):389–393. doi: 10.4012/dmj.2013-334. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y., Zheng X., Xie Y., Ji H., Ding C. Antibacterial properties of vacuum plasma sprayed titanium coatings after chemical treatment. Surf. Coating. Technol. 2009;204(5):685–690. [Google Scholar]
- 108.Lei Z., Zhang H., Zhang E., You J., Ma X., Bai X. Antibacterial activities and biocompatibilities of Ti-Ag alloys prepared by spark plasma sintering and acid etching. Mater Sci Eng C Mater Biol Appl. 2018;92:121–131. doi: 10.1016/j.msec.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 109.Necula B.S., Fratila-Apachitei L.E., Zaat S.A., Apachitei I., Duszczyk J. In vitro antibacterial activity of porous TiO2-Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomater. 2009;5(9):3573–3580. doi: 10.1016/j.actbio.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 110.Bai X., More K., Rouleau C.M., Rabiei A. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 2010;6(6):2264–2273. doi: 10.1016/j.actbio.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Yang M., Wang Y., Yue F., Jing F., Qin L., Xie D., Zhao Y., Huang N., Akhavan B., Leng Y. Shellac: a bioactive coating for surface engineering of cardiovascular devices. Adv. Mater. Interfac. 2022 [Google Scholar]
- 112.Zheng X., Chen Y., Xie Y., Ji H., Huang L., Ding C. Antibacterial property and biocompatibility of plasma sprayed hydroxyapatite/silver composite coatings. J. Therm. Spray Technol. 2009;18(3):463. 463. [Google Scholar]
- 113.Li B., Liu X., Meng F., Chang J., Ding C. Preparation and antibacterial properties of plasma sprayed nano-titania/silver coatings. Mater. Chem. Phys. 2009;118(1):99–104. [Google Scholar]
- 114.Secinti K.D., Ayten M., Kahilogullari G., Kaygusuz G., Ugur H.C., Attar A. Antibacterial effects of electrically activated vertebral implants. J. Clin. Neurosci. 2008;15(4):434–439. doi: 10.1016/j.jocn.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 115.Tian X., Wang Z., Yang S., Luo Z., Fu R.K., Chu P.K. Antibacterial copper-containing titanium nitride films produced by dual magnetron sputtering. Surf. Coating. Technol. 2007;201(19–20):8606–8609. [Google Scholar]
- 116.Ren Q., Qin L., Jing F., Cheng D., Wang Y., Yang M., Xie D., Leng Y., Akhavan B., Huang N. Reactive magnetron co-sputtering of Ti-xCuO coatings: multifunctional interfaces for blood-contacting devices. Mater Sci Eng C Mater Biol Appl. 2020;116 doi: 10.1016/j.msec.2020.111198. [DOI] [PubMed] [Google Scholar]
- 117.Wang Q., Akhavan B., Jing F., Cheng D., Sun H., Xie D., Leng Y., Bilek M.M., Huang N. Catalytic formation of nitric oxide mediated by Ti–Cu coatings provides multifunctional interfaces for cardiovascular applications. Adv. Mater. Interfac. 2018;5(6) [Google Scholar]
- 118.Qin L., Ma D., Li Y., Jing P., Huang B., Jing F., Xie D., Leng Y., Akhavan B., Huang N. Ti–Cu coatings deposited by a combination of HiPIMS and DC magnetron sputtering: the role of vacuum annealing on Cu diffusion, microstructure, and corrosion resistance. Coatings. 2020;10(11):1064. [Google Scholar]
- 119.Ganesan R., Akhavan B., Hiob M.A., McKenzie D.R., Weiss A.S., Bilek M.M. HiPIMS carbon coatings show covalent protein binding that imparts enhanced hemocompatibility. Carbon. 2018;139:118–128. [Google Scholar]
- 120.Croes M., Akhavan B., Sharifahmadian O., Fan H., Mertens R., Tan R.P., Chunara A., Fadzil A.A., Wise S.G., Kruyt M.C., Wijdicks S., Hennink W.E., Bilek M.M.M., Amin Yavari S. A multifaceted biomimetic interface to improve the longevity of orthopedic implants. Acta Biomater. 2020;110:266–279. doi: 10.1016/j.actbio.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 121.Stewart C.A.C., Akhavan B., Hung J., Bao S., Jang J.H., Wise S.G., Bilek M.M.M. Multifunctional protein-immobilized plasma polymer films for orthopedic applications. ACS Biomater. Sci. Eng. 2018;4(12):4084–4094. doi: 10.1021/acsbiomaterials.8b00954. [DOI] [PubMed] [Google Scholar]
- 122.Akhavan B., Croes M., Wise S.G., Zhai C., Hung J., Stewart C., Ionescu M., Weinans H., Gan Y., Yavari S.A. Radical-functionalized plasma polymers: stable biomimetic interfaces for bone implant applications. Appl. Mater. Today. 2019;16:456–473. [Google Scholar]
- 123.Stewart C.A., Akhavan B., Santos M., Hung J., Hawkins C.L., Bao S., Wise S.G., Bilek M.M. Cellular responses to radical propagation from ion-implanted plasma polymer surfaces. Appl. Surf. Sci. 2018;456:701–710. [Google Scholar]
- 124.Sharifahmadian O., Zhai C., Hung J., Shineh G., Stewart C.A., Fadzil A.A., Ionescu M., Gan Y., Wise S.G., Akhavan B. Mechanically robust nitrogen-rich plasma polymers: biofunctional interfaces for surface engineering of biomedical implants. Materials Today Advances. 2021;12 [Google Scholar]
- 125.Huang N., Yang P., Leng Y., Wang J., Sun H., Chen J., Wan G. Surface modification of biomaterials by plasma immersion ion implantation. Surf. Coating. Technol. 2004;186(1–2):218–226. [Google Scholar]
- 126.Xia C., Cai D., Tan J., Li K., Qiao Y., Liu X. Synergistic effects of N/Cu dual ions implantation on stimulating antibacterial ability and angiogenic activity of titanium. ACS Biomater. Sci. Eng. 2018;4(9):3185–3193. doi: 10.1021/acsbiomaterials.8b00501. [DOI] [PubMed] [Google Scholar]
- 127.Kondyurin A., Lau K., Tang F., Akhavan B., Chrzanowski W., Lord M.S., Rnjak-Kovacina J., Bilek M.M. Plasma ion implantation of silk biomaterials enabling direct covalent immobilization of bioactive agents for enhanced cellular responses. ACS Appl. Mater. Interfaces. 2018;10(21):17605–17616. doi: 10.1021/acsami.8b03182. [DOI] [PubMed] [Google Scholar]
- 128.Lau K., Akhavan B., Lord M.S., Bilek M.M., Rnjak-Kovacina J. ACS Biomaterials Science & Engineering; 2020. Dry Surface Treatments of Silk Biomaterials and Their Utility in Biomedical Applications. [DOI] [PubMed] [Google Scholar]
- 129.Tan R.P., Chan A.H., Wei S., Santos M., Lee B.S., Filipe E.C., Akhavan B., Bilek M.M., Ng M.K., Xiao Y. Bioactive materials facilitating targeted local modulation of inflammation. JACC (J. Am. Coll. Cardiol.): Basic to Translational Science. 2019;4(1):56–71. doi: 10.1016/j.jacbts.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoshinari M., Oda Y., Kato T., Okuda K. Influence of surface modifications to titanium on antibacterial activity in vitro. Biomaterials. 2001;22(14):2043–2048. doi: 10.1016/s0142-9612(00)00392-6. [DOI] [PubMed] [Google Scholar]
- 131.Heidenau F., Mittelmeier W., Detsch R., Haenle M., Stenzel F., Ziegler G., Gollwitzer H. A novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005;16(10):883–888. doi: 10.1007/s10856-005-4422-3. [DOI] [PubMed] [Google Scholar]
- 132.Fu S., Zhang Y., Qin G., Zhang E. Antibacterial effect of TiAg alloy motivated by Ag-containing phases. Mater Sci Eng C Mater Biol Appl. 2021;128 doi: 10.1016/j.msec.2021.112266. [DOI] [PubMed] [Google Scholar]
- 133.Jeyachandran Y., Venkatachalam S., Karunagaran B., Narayandass S.K., Mangalaraj D., Bao C., Zhang C. Bacterial adhesion studies on titanium, titanium nitride and modified hydroxyapatite thin films. Mater. Sci. Eng. C. 2007;27(1):35–41. [Google Scholar]
- 134.Zhuang Y., Ren L., Zhang S., Wei X., Yang K., Dai K. Antibacterial effect of a copper-containing titanium alloy against implant-associated infection induced by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2021;119:472–484. doi: 10.1016/j.actbio.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 135.Wan Y.Z., Xiong G.Y., Liang H., Raman S., He F., Huang Y. Modification of medical metals by ion implantation of copper. Appl. Surf. Sci. 2007;253(24):9426–9429. [Google Scholar]
- 136.Vincent M., Duval R.E., Hartemann P., Engels-Deutsch M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018;124(5):1032–1046. doi: 10.1111/jam.13681. [DOI] [PubMed] [Google Scholar]
- 137.Ren L., Ma Z., Li M., Zhang Y., Liu W., Liao Z., Yang K. Antibacterial properties of Ti–6Al–4V–xCu alloys. J. Mater. Sci. Technol. 2014;30(7):699–705. [Google Scholar]
- 138.Peng C., Zhao Y., Jin S., Wang J., Liu R., Liu H., Shi W., Kolawole S.K., Ren L., Yu B., Yang K. Antibacterial TiCu/TiCuN multilayer films with good corrosion resistance deposited by axial magnetic field-enhanced arc ion plating. ACS Appl. Mater. Interfaces. 2019;11(1):125–136. doi: 10.1021/acsami.8b14038. [DOI] [PubMed] [Google Scholar]
- 139.Vincent M., Hartemann P., Engels-Deutsch M. Antimicrobial applications of copper. Int. J. Hyg Environ. Health. 2016;219(7 Pt A):585–591. doi: 10.1016/j.ijheh.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 140.Dalecki A.G., Crawford C.L., Wolschendorf F. Copper and antibiotics: discovery, modes of action, and opportunities for medicinal applications. Adv. Microb. Physiol. 2017;70:193–260. doi: 10.1016/bs.ampbs.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 141.San K., Long J., Michels C.A., Gadura N. Antimicrobial copper alloy surfaces are effective against vegetative but not sporulated cells of gram-positive Bacillus subtilis. Microbiol. 2015;4(5):753–763. doi: 10.1002/mbo3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bai B., Zhang E., Liu J., Zhu J. The anti-bacterial activity of titanium-copper sintered alloy against Porphyromonas gingivalis in vitro. Dent. Mater. J. 2016;35(4):659–667. doi: 10.4012/dmj.2016-001. [DOI] [PubMed] [Google Scholar]
- 143.Organization W.H. 2017. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. [Google Scholar]
- 144.Noyce J., Michels H., Keevil C. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006;63(3):289–297. doi: 10.1016/j.jhin.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 145.Wilks S.A., Michels H., Keevil C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005;105(3):445–454. doi: 10.1016/j.ijfoodmicro.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 146.Weaver L., Michels H.T., Keevil C.W. Survival of Clostridium difficile on copper and steel: futuristic options for hospital hygiene. J. Hosp. Infect. 2008;68(2):145–151. doi: 10.1016/j.jhin.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 147.Souli M., Galani I., Plachouras D., Panagea T., Armaganidis A., Petrikkos G., Giamarellou H. Antimicrobial activity of copper surfaces against carbapenemase-producing contemporary Gram-negative clinical isolates. J. Antimicrob. Chemother. 2013;68(4):852–857. doi: 10.1093/jac/dks473. [DOI] [PubMed] [Google Scholar]
- 148.Espirito Santo C., Lam E.W., Elowsky C.G., Quaranta D., Domaille D.W., Chang C.J., Grass G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011;77(3):794–802. doi: 10.1128/AEM.01599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Airey P., Verran J. Potential use of copper as a hygienic surface; problems associated with cumulative soiling and cleaning. J. Hosp. Infect. 2007;67(3):271–277. doi: 10.1016/j.jhin.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 150.Weaver L., Noyce J.O., Michels H.T., Keevil C.W. Potential action of copper surfaces on meticillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2010;109(6):2200–2205. doi: 10.1111/j.1365-2672.2010.04852.x. [DOI] [PubMed] [Google Scholar]
- 151.Paulsen I.T., Banerjei L., Myers G.S., Nelson K.E., Seshadri R., Read T.D., Fouts D.E., Eisen J.A., Gill S.R., Heidelberg J.F., Tettelin H., Dodson R.J., Umayam L., Brinkac L., Beanan M., Daugherty S., DeBoy R.T., Durkin S., Kolonay J., Madupu R., Nelson W., Vamathevan J., Tran B., Upton J., Hansen T., Shetty J., Khouri H., Utterback T., Radune D., Ketchum K.A., Dougherty B.A., Fraser C.M. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299(5615):2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- 152.Warnes S.L., Green S.M., Michels H.T., Keevil C.W. Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNAs. Appl. Environ. Microbiol. 2010;76(16):5390–5401. doi: 10.1128/AEM.03050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Warnes S.L., Caves V., Keevil C.W. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ. Microbiol. 2012;14(7):1730–1743. doi: 10.1111/j.1462-2920.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- 154.Warnes S.L., Keevil C.W. Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact. Appl. Environ. Microbiol. 2011;77(17):6049–6059. doi: 10.1128/AEM.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Santo C.E., Quaranta D., Grass G. Antimicrobial metallic copper surfaces kill Staphylococcus haemolyticus via membrane damage. Microbiol. 2012;1(1):46–52. doi: 10.1002/mbo3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tian W.X., Yu S., Ibrahim M., Almonaofy A.W., He L., Hui Q., Bo Z., Li B., Xie G.L. Copper as an antimicrobial agent against opportunistic pathogenic and multidrug resistant Enterobacter bacteria. J. Microbiol. 2012;50(4):586–593. doi: 10.1007/s12275-012-2067-8. [DOI] [PubMed] [Google Scholar]
- 157.Mathews S., Kumar R., Solioz M. Copper reduction and contact killing of bacteria by iron surfaces. Appl. Environ. Microbiol. 2015;81(18):6399–6403. doi: 10.1128/AEM.01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hong R., Kang T.Y., Michels C.A., Gadura N. Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl. Environ. Microbiol. 2012;78(6):1776–1784. doi: 10.1128/AEM.07068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Machio C., Mathabathe M.N., Bolokang A.S. A comparison of the microstructures, thermal and mechanical properties of pressed and sintered Ti–Cu, Ti–Ni and Ti–Cu–Ni alloys intended for dental applications. J. Alloys Compd. 2020;848 [Google Scholar]
- 160.Mathews S., Hans M., Mucklich F., Solioz M. Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl. Environ. Microbiol. 2013;79(8):2605–2611. doi: 10.1128/AEM.03608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Niespodziana K., Jurczyk K., Jurczyk M. The synthesis of titanium alloys for biomedical applications. Rev. Adv. Mater. Sci. 2008;18:236–240. [Google Scholar]
- 162.Barão V.A., Mathew M.T., Assunção W.G., Yuan J.C.C., Wimmer M.A., Sukotjo C. Stability of cp-Ti and Ti-6 Al-4 V alloy for dental implants as a function of saliva pH–an electrochemical study. Clin. Oral Implants Res. 2012;23(9):1055–1062. doi: 10.1111/j.1600-0501.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- 163.Alqattan M., Peters L., Alshammari Y., Yang F., Bolzoni L. Antibacterial Ti-Mn-Cu alloys for biomedical applications. Regen Biomater. 2021;8(1) doi: 10.1093/rb/rbaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alqattan M., Alshammari Y., Yang F., Peters L., Bolzoni L. Biomedical Ti–Cu–Mn alloys with antibacterial capability. J. Mater. Res. Technol. 2021;10:1020–1028. [Google Scholar]
- 165.Wang J., Zhang S., Sun Z., Wang H., Ren L., Yang K. Optimization of mechanical property, antibacterial property and corrosion resistance of Ti-Cu alloy for dental implant. J. Mater. Sci. Technol. 2019;35(10):2336–2344. [Google Scholar]
- 166.Orapiriyakul W., Young P.S., Damiati L., Tsimbouri P.M. Antibacterial surface modification of titanium implants in orthopaedics. J. Tissue Eng. 2018;9 doi: 10.1177/2041731418789838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Russell A.D., Hugo W.B. 1994. Antimicrobial Activity and Action of Silver; pp. 351–370. [DOI] [PubMed] [Google Scholar]
- 168.Qing Y., Cheng L., Li R., Liu G., Zhang Y., Tang X., Wang J., Liu H., Qin Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018;13:3311–3327. doi: 10.2147/IJN.S165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fowler L., Engqvist H., Ohman-Magi C. Effect of copper ion concentration on bacteria and cells. Materials. 2019;12(22) doi: 10.3390/ma12223798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Russell A.D., Hugo W.B. Antimicrobial activity and action of silver. Prog. Med. Chem. 1994;31:351–370. doi: 10.1016/s0079-6468(08)70024-9. [DOI] [PubMed] [Google Scholar]
- 171.Fowler L., Janson O., Engqvist H., Norgren S., Ohman-Magi C. Antibacterial investigation of titanium-copper alloys using luminescent Staphylococcus epidermidis in a direct contact test. Mater Sci Eng C Mater Biol Appl. 2019;97:707–714. doi: 10.1016/j.msec.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 172.Tao S.C., Xu J.L., Yuan L., Luo J.M., Zheng Y.F. Microstructure, mechanical properties and antibacterial properties of the microwave sintered porous Ti–3Cu alloys. J. Alloys Compd. 2020;812 [Google Scholar]
- 173.Kawakami H., Yoshida K., Nishida Y., Kikuchi Y., Sato Y. Antibacterial properties of metallic elements for alloying evaluated with application of JIS Z 2801: 2000. ISIJ Int. 2008;48(9):1299–1304. [Google Scholar]
- 174.Chinn R.Y., Sehulster L. HICPAC); 2003. Guidelines for Environmental Infection Control in Health-Care Facilities; Recommendations of CDC and Healthcare Infection Control Practices Advisory Committee. [PubMed] [Google Scholar]
- 175.Zhang E.-L., Fu S., Wang R.-X., Li H.-X., Liu Y., Ma Z.-Q., Liu G.-K., Zhu C.-S., Qin G.-W., Chen D.-F. Role of Cu element in biomedical metal alloy design. Rare Met. 2019;38(6):476–494. [Google Scholar]
- 176.Bao M., Liu Y., Wang X., Yang L., Li S., Ren J., Qin G., Zhang E. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of wrought Ti-3Cu alloy by heat treatment. Bioact. Mater. 2018;3(1):28–38. doi: 10.1016/j.bioactmat.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang Z., Zheng G., Li H., Yang L., Wang X., Qin G., Zhang E. Anti-bacterium influenced corrosion effect of antibacterial Ti-3Cu alloy in Staphylococcus aureus suspension for biomedical application. Mater Sci Eng C Mater Biol Appl. 2019;94:376–384. doi: 10.1016/j.msec.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 178.Ma Z., Li M., Liu R., Ren L., Zhang Y., Pan H., Zhao Y., Yang K. In vitro study on an antibacterial Ti-5Cu alloy for medical application. J. Mater. Sci. Mater. Med. 2016;27(5):91. doi: 10.1007/s10856-016-5698-1. [DOI] [PubMed] [Google Scholar]
- 179.Qin P., Liu Y., Sercombe T.B., Li Y., Zhang C., Cao C., Sun H., Zhang L.C. Improved corrosion resistance on selective laser melting produced Ti-5Cu alloy after heat treatment. ACS Biomater. Sci. Eng. 2018;4(7):2633–2642. doi: 10.1021/acsbiomaterials.8b00319. [DOI] [PubMed] [Google Scholar]
- 180.Zhang J.-Q., Cao S., Liu Y., Bao M.-M., Ren J., Li S.-Y., Zhang E.-L., Wang J.-J. Tribocorrosion behavior of antibacterial Ti–Cu sintered alloys in simulated biological environments. Rare Met. 2022;41(6):1921–1932. [Google Scholar]
- 181.Gerard C., Bordeleau L.J., Barralet J., Doillon C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;31(5):824–831. doi: 10.1016/j.biomaterials.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 182.Burghardt I., Luthen F., Prinz C., Kreikemeyer B., Zietz C., Neumann H.G., Rychly J. A dual function of copper in designing regenerative implants. Biomaterials. 2015;44:36–44. doi: 10.1016/j.biomaterials.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 183.Trumbo P., Yates A.A., Schlicker S., Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet Assoc. 2001;101(3):294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 184.W.H. Organization, Trace Elements in Human Nutrition and Health, World Health Organization1996.
- 185.Copper I. World Health Organisation; 1998. Environmental Health Criteria 200, Geneva, International Programme on Chemical Safety. [Google Scholar]
- 186.Sun Q.Y., Yu Z.T., Zhu R.H., Gu H.C. Mechanical behavior and deformation mechanisms of Ti–2.5Cu alloy reinforced by nano-scale precipitates at 293 and 77 K. Mater. Sci. Eng. 2004;364(1–2):159–165. [Google Scholar]
- 187.Kikuchi M., Takada Y., Kiyosue S., Yoda M., Woldu M., Cai Z., Okuno O., Okabe T. Mechanical properties and microstructures of cast Ti-Cu alloys. Dent. Mater. 2003;19(3):174–181. doi: 10.1016/s0109-5641(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 188.Lütjering G., Weissmann S. Mechanical properties and structure of age-hardened Ti-Cu alloys. Metall. Trans. A. 1970;1(6):1641–1649. [Google Scholar]
- 189.Zhang E., Li F., Wang H., Liu J., Wang C., Li M., Yang K. A new antibacterial titanium–copper sintered alloy: preparation and antibacterial property. Mater. Sci. Eng. C. 2013;33(7):4280–4287. doi: 10.1016/j.msec.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 190.Zhang E., Wang X., Chen M., Hou B. Effect of the existing form of Cu element on the mechanical properties, bio-corrosion and antibacterial properties of Ti-Cu alloys for biomedical application. Mater Sci Eng C Mater Biol Appl. 2016;69:1210–1221. doi: 10.1016/j.msec.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 191.Ke Z., Yi C., Zhang L., He Z., Tan J., Jiang Y. Characterization of a new Ti-13Nb-13Zr-10Cu alloy with enhanced antibacterial activity for biomedical applications. Mater. Lett. 2019;253:335–338. [Google Scholar]
- 192.Zhang D., Qiu D., Gibson M.A., Zheng Y., Fraser H.L., StJohn D.H., Easton M.A. Additive manufacturing of ultrafine-grained high-strength titanium alloys. Nature. 2019;576(7785):91–95. doi: 10.1038/s41586-019-1783-1. [DOI] [PubMed] [Google Scholar]
- 193.Tao S., Xu J., Yuan L., Luo J., Zheng Y. Microstructure, mechanical properties and antibacterial properties of the microwave sintered porous Ti–3Cu alloys. J. Alloys Compd. 2020;812 [Google Scholar]
- 194.Wang X., Qiao J., Yuan F., Hang R., Huang X., Tang B. In situ growth of self-organized Cu-containing nano-tubes and nano-pores on Ti90− xCu10Alx (x= 0, 45) alloys by one-pot anodization and evaluation of their antimicrobial activity and cytotoxicity. Surf. Coating. Technol. 2014;240:167–178. [Google Scholar]
- 195.Liu J., Zhang X., Wang H., Li F., Li M., Yang K., Zhang E. The antibacterial properties and biocompatibility of a Ti–Cu sintered alloy for biomedical application. Biomed. Mater. 2014;9(2) doi: 10.1088/1748-6041/9/2/025013. [DOI] [PubMed] [Google Scholar]
- 196.Javadhesari S.M., Alipour S., Akbarpour M. Biocompatibility, osseointegration, antibacterial and mechanical properties of nanocrystalline Ti-Cu alloy as a new orthopedic material. Colloids Surf. B Biointerfaces. 2020;189 doi: 10.1016/j.colsurfb.2020.110889. [DOI] [PubMed] [Google Scholar]
- 197.Wu J.-H., Chen K.-K., Chao C.-Y., Chang Y.-H., Du J.-K. Effect of Ti2Cu precipitation on antibacterial property of Ti-5Cu alloy. Mater. Sci. Eng. C. 2020;108 doi: 10.1016/j.msec.2019.110433. [DOI] [PubMed] [Google Scholar]
- 198.Zhang E., Ren J., Li S., Yang L., Qin G. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of as-cast Ti-Cu alloys. Biomed. Mater. 2016;11(6) doi: 10.1088/1748-6041/11/6/065001. [DOI] [PubMed] [Google Scholar]
- 199.Liu R., Tang Y., Liu H., Zeng L., Ma Z., Li J., Zhao Y., Ren L., Yang K. Effects of combined chemical design (Cu addition) and topographical modification (SLA) of Ti-Cu/SLA for promoting osteogenic, angiogenic and antibacterial activities. J. Mater. Sci. Technol. 2020;47:202–215. [Google Scholar]
- 200.Wang X., Dong H., Liu J., Qin G., Chen D., Zhang E. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater Sci Eng C Mater Biol Appl. 2019;100:38–47. doi: 10.1016/j.msec.2019.02.084. [DOI] [PubMed] [Google Scholar]
- 201.Guo S., Lu Y., Wu S., Liu L., He M., Zhao C., Gan Y., Lin J., Luo J., Xu X. Preliminary study on the corrosion resistance, antibacterial activity and cytotoxicity of selective-laser-melted Ti6Al4V-xCu alloys. Mater. Sci. Eng. C. 2017;72:631–640. doi: 10.1016/j.msec.2016.11.126. [DOI] [PubMed] [Google Scholar]
- 202.Liu J., Zhang X., Wang H., Li F., Li M., Yang K., Zhang E. The antibacterial properties and biocompatibility of a Ti-Cu sintered alloy for biomedical application. Biomed. Mater. 2014;9(2) doi: 10.1088/1748-6041/9/2/025013. [DOI] [PubMed] [Google Scholar]
- 203.Moniri Javadhesari S., Alipour S., Akbarpour M.R. Biocompatibility, osseointegration, antibacterial and mechanical properties of nanocrystalline Ti-Cu alloy as a new orthopedic material. Colloids Surf. B Biointerfaces. 2020;189 doi: 10.1016/j.colsurfb.2020.110889. [DOI] [PubMed] [Google Scholar]
- 204.Zhang E., Zheng L., Liu J., Bai B., Liu C. Influence of Cu content on the cell biocompatibility of Ti–Cu sintered alloys. Mater. Sci. Eng. C. 2015;46:148–157. doi: 10.1016/j.msec.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 205.Zhang D., Ren L., Zhang Y., Xue N., Yang K., Zhong M. Antibacterial activity against Porphyromonas gingivalis and biological characteristics of antibacterial stainless steel. Colloids Surf. B Biointerfaces. 2013;105:51–57. doi: 10.1016/j.colsurfb.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 206.Bai B., Zhang E., Dong H., Liu J. Biocompatibility of antibacterial Ti-Cu sintered alloy: in vivo bone response. J. Mater. Sci. Mater. Med. 2015;26(12):265. doi: 10.1007/s10856-015-5600-6. [DOI] [PubMed] [Google Scholar]
- 207.Liu R., Tang Y., Zeng L., Zhao Y., Ma Z., Sun Z., Xiang L., Ren L., Yang K. In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application. Dent. Mater. 2018;34(8):1112–1126. doi: 10.1016/j.dental.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 208.Liu J., Li F., Liu C., Wang H., Ren B., Yang K., Zhang E. Effect of Cu content on the antibacterial activity of titanium-copper sintered alloys. Mater Sci Eng C Mater Biol Appl. 2014;35:392–400. doi: 10.1016/j.msec.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 209.Chatterjee A.K., Chakraborty R., Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 2014;25(13) doi: 10.1088/0957-4484/25/13/135101. [DOI] [PubMed] [Google Scholar]
- 210.Peng C., Zhang S., Sun Z., Ren L., Yang K. Effect of annealing temperature on mechanical and antibacterial properties of Cu-bearing titanium alloy and its preliminary study of antibacterial mechanism. Mater Sci Eng C Mater Biol Appl. 2018;93:495–504. doi: 10.1016/j.msec.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 211.Fan D.-Y., Yi Z., Feng X., Tian W.-Z., Xu D.-K., Cristino Valentino A., Wang Q., Sun H.-C. Antibacterial property of a gradient Cu-bearing titanium alloy by laser additive manufacturing. Rare Met. 2022;41(2):580–593. [Google Scholar]
- 212.Akbarpour M.R., Moniri Javadhesari S. Densification and mechanical and antibacterial properties of low-cost powder metallurgy TiCu intermetallic alloy as a potential biomedical. Material, Jom. 2020;72(9):3262–3268. [Google Scholar]
- 213.Li Y.-H., Chen N., Cui H.-T., Wang F. Fabrication and characterization of porous Ti–10Cu alloy for biomedical application. J. Alloys Compd. 2017;723:967–973. [Google Scholar]
- 214.Korda A.A., Munawaroh S., Basuki E.A. The antimicrobial activity and characterization of the cast titanium copper alloys with variations of copper content. IOP Conf. Ser. Mater. Sci. Eng. 2019;547(1) [Google Scholar]
- 215.Eriksson A. 2019. Ti-Cu Alloys for Medical Applications. [Google Scholar]
- 216.Cao S., Zhang Z.-M., Zhang J.-Q., Wang R.-X., Wang X.-Y., Yang L., Chen D.-F., Qin G.-W., Zhang E.-L. Improvement in antibacterial ability and cell cytotoxicity of Ti–Cu alloy by anodic oxidation. Rare Met. 2022;41(2):594–609. [Google Scholar]
- 217.Du W.-L., Niu S.-S., Xu Y.-L., Xu Z.-R., Fan C.-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009;75(3):385–389. [Google Scholar]
- 218.Ren G., Hu D., Cheng E.W., Vargas-Reus M.A., Reip P., Allaker R.P. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents. 2009;33(6):587–590. doi: 10.1016/j.ijantimicag.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 219.Ning C., Wang X., Li L., Zhu Y., Li M., Yu P., Zhou L., Zhou Z., Chen J., Tan G., Zhang Y., Wang Y., Mao C. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: implications for a new antibacterial mechanism. Chem. Res. Toxicol. 2015;28(9):1815–1822. doi: 10.1021/acs.chemrestox.5b00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shi A., Zhu C., Fu S., Wang R., Qin G., Chen D., Zhang E. What controls the antibacterial activity of Ti-Ag alloy, Ag ion or Ti2Ag particles? Mater Sci Eng C Mater Biol Appl. 2020;109 doi: 10.1016/j.msec.2019.110548. [DOI] [PubMed] [Google Scholar]
- 221.Nan L., Liu Y., Lu M., Yang K. Study on antibacterial mechanism of copper-bearing austenitic antibacterial stainless steel by atomic force microscopy. J. Mater. Sci. Mater. Med. 2008;19(9):3057–3062. doi: 10.1007/s10856-008-3444-z. [DOI] [PubMed] [Google Scholar]
- 222.Liu S., Zhang Z., Zhang J., Qin G., Zhang E. Construction of a TiO2/Cu2O multifunctional coating on Ti-Cu alloy and its influence on the cell compatibility and antibacterial properties. Surf. Coating. Technol. 2021;421 [Google Scholar]
- 223.Zhang W., Zhang S., Liu H., Ren L., Wang Q., Zhang Y. Effects of surface roughening on antibacterial and osteogenic properties of Ti-Cu alloys with different Cu contents. J. Mater. Sci. Technol. 2021;88:158–167. [Google Scholar]
- 224.Li M., Ma Z., Zhu Y., Xia H., Yao M., Chu X., Wang X., Yang K., Yang M., Zhang Y., Mao C. Toward a molecular understanding of the antibacterial mechanism of copper-bearing titanium alloys against Staphylococcus aureus. Adv Healthc Mater. 2016;5(5):557–566. doi: 10.1002/adhm.201500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang E., Liu C. Effect of surface treatments on the surface morphology, corrosion property, and antibacterial property of Ti-10Cu sintered alloy. Biomed. Mater. 2015;10(4) doi: 10.1088/1748-6041/10/4/045009. [DOI] [PubMed] [Google Scholar]
- 226.Wu J.H., Chen K.K., Chao C.Y., Chang Y.H., Du J.K. Effect of Ti2Cu precipitation on antibacterial property of Ti-5Cu alloy. Mater Sci Eng C Mater Biol Appl. 2020;108 doi: 10.1016/j.msec.2019.110433. [DOI] [PubMed] [Google Scholar]
- 227.Zhang X., Yang C., Yang K. Contact killing of Cu-bearing stainless steel based on charge transfer caused by the microdomain potential difference. ACS Appl. Mater. Interfaces. 2020;12(1):361–372. doi: 10.1021/acsami.9b19596. [DOI] [PubMed] [Google Scholar]
- 228.Xu D., Wang T., Lu Z., Wang Y., Sun B., Wang S., Fu Q., Bi Z., Geng S. Ti-6Al-4V-5Cu synthesized for antibacterial effect in vitro and in vivo via contact sterilization. J. Mater. Sci. Technol. 2021;90:133–142. [Google Scholar]
- 229.Xu D., Lu Z., Wang T., Wang S., Jiang Y., Xu Z., Bi Z., Geng S. Novel Ti-based alloys prepared with different heat treatment strategies as antibacterial biomedical implants. Mater. Des. 2021;205 [Google Scholar]
- 230.Jiao J., Zhang S., Qu X., Yue B. Recent advances in research on antibacterial metals and alloys as implant materials. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.693939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lu M., Zhang Z., Zhang J., Wang X., Qin G., Zhang E. Enhanced antibacterial activity of Ti-Cu alloy by selective acid etching. Surf. Coating. Technol. 2021;421 [Google Scholar]
- 232.Cook G.M., Greening C., Hards K., Berney M. Energetics of pathogenic bacteria and opportunities for drug development. Adv. Microb. Physiol. 2014;65:1–62. doi: 10.1016/bs.ampbs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 233.C.W. Pratt, K. Cornely, Essential Biochemistry, John Wiley & Sons2021.
- 234.Farha M.A., Verschoor C.P., Bowdish D., Brown E.D. Collapsing the proton motive force to identify synergistic combinations against Staphylococcus aureus. Chem. Biol. 2013;20(9):1168–1178. doi: 10.1016/j.chembiol.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 235.Maloney P.C., Kashket E.R., Wilson T.H. A protonmotive force drives ATP synthesis in bacteria. Proc. Natl. Acad. Sci. U. S. A. 1974;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fu S., Zhang Y., Yang Y., Liu X., Zhang X., Yang L., Xu D., Wang F., Qin G., Zhang E. An antibacterial mechanism of titanium alloy based on micro-area potential difference induced reactive oxygen species. J. Mater. Sci. Technol. 2022;119:75–86. [Google Scholar]
- 237.Xie Y., Lu M., Cui S., Yu H., Wang L., Ke H., Zhang E. Construction of a rough surface with submicron Ti2Cu particle on Ti-Cu alloy and its effect on the antibacterial properties and cell biocompatibility. Metals. 2022;12(6):1008. [Google Scholar]
- 238.Sim J.W., Kim J.H., Park C.H., Hong J.-K., Yeom J.-T., Lee S.W. Effect of phase conditions on tensile and antibacterial properties of Ti-Cu alloys with Ti2Cu intermetallic compound. J. Alloys Compd. 2022 [Google Scholar]
- 239.Slauch J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011;80(3):580–583. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kashmiri Z., Mankar S. Free radicals and oxidative stress in bacteria. Int J Curr Microbiol App Sci. 2014;3(9):34–40. [Google Scholar]
- 241.Liochev S.I., Fridovich I. The Haber-Weiss cycle -- 70 years later: an alternative view. Redox Rep. 2002;7(1):55–57. doi: 10.1179/135100002125000190. author reply 59-60. [DOI] [PubMed] [Google Scholar]
- 242.Warnes S.L., Keevil C.W. Lack of involvement of Fenton chemistry in death of methicillin-resistant and methicillin-sensitive strains of Staphylococcus aureus and destruction of their genomes on wet or dry copper alloy surfaces. Appl. Environ. Microbiol. 2016;82(7):2132–2136. doi: 10.1128/AEM.03861-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen M., Yang L., Zhang L., Han Y., Lu Z., Qin G., Zhang E. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater Sci Eng C Mater Biol Appl. 2017;75:906–917. doi: 10.1016/j.msec.2017.02.142. [DOI] [PubMed] [Google Scholar]
- 244.Cabiscol Català E., Tamarit Sumalla J., Ros Salvador J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000;3(1):3–8. (2000) [PubMed] [Google Scholar]
- 245.Li Y., Liu L., Wan P., Zhai Z., Mao Z., Ouyang Z., Yu D., Sun Q., Tan L., Ren L., Zhu Z., Hao Y., Qu X., Yang K., Dai K. Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: in vitro and in vivo evaluations. Biomaterials. 2016;106:250–263. doi: 10.1016/j.biomaterials.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 246.Macomber L., Imlay J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(20):8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dutta R.K., Nenavathu B.P., Gangishetty M.K., Reddy A.V. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces. 2012;94:143–150. doi: 10.1016/j.colsurfb.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 248.Chen S., Guo Y., Zhong H., Chen S., Li J., Ge Z., Tang J. Synergistic antibacterial mechanism and coating application of copper/titanium dioxide nanoparticles. Chem. Eng. J. 2014;256:238–246. [Google Scholar]
- 249.Raghunath A., Perumal E. Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int. J. Antimicrob. Agents. 2017;49(2):137–152. doi: 10.1016/j.ijantimicag.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 250.Stoimenov P.K., Klinger R.L., Marchin G.L., Klabunde K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18(17):6679–6686. [Google Scholar]
- 251.Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang Z., Zhang X.R., Jin T., Yang C.G., Sun Y.P., Li Q., Yang K. Antibacterial mechanism of Cu-bearing 430 ferritic stainless steel. Rare Met. 2022;41(2):559–569. doi: 10.1007/s12598-021-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dyal Ukabhai K., Curle U., Masia N., Smit M., Mwamba I., Norgren S., Öhman-Mägi C., Hashe N., Cornish L. formation of Ti2Cu in Ti-Cu alloys. J. Phase Equilibria Diffus. 2022;43(3):332–344. [Google Scholar]
- 254.Akbarpour M.R., Mirabad H.M., Hemmati A., Kim H.S. Processing and microstructure of Ti-Cu binary alloys: a comprehensive review. Prog. Mater. Sci. 2022 [Google Scholar]
- 255.Chen M., Zhang E., Zhang L. Microstructure, mechanical properties, bio-corrosion properties and antibacterial properties of Ti-Ag sintered alloys. Mater Sci Eng C Mater Biol Appl. 2016;62:350–360. doi: 10.1016/j.msec.2016.01.081. [DOI] [PubMed] [Google Scholar]
- 256.Ji H., Zhao M.-C., Xie B., Zhao Y.-C., Yin D., Gao C., Shuai C., Atrens A. Corrosion and antibacterial performance of novel selective-laser-melted (SLMed) Ti-xCu biomedical alloys. J. Alloys Compd. 2021;864 [Google Scholar]
- 257.Zhang E., Li S., Ren J., Zhang L., Han Y. Effect of extrusion processing on the microstructure, mechanical properties, biocorrosion properties and antibacterial properties of Ti-Cu sintered alloys. Mater Sci Eng C Mater Biol Appl. 2016;69:760–768. doi: 10.1016/j.msec.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 258.Fowler L., Masia N., Cornish L.A., Chown L.H., Engqvist H., Norgren S., Ohman-Magi C. Development of antibacterial Ti-cux alloys for dental applications: effects of ageing for alloys with up to 10 wt% Cu. Materials. 2019;12(23) doi: 10.3390/ma12234017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shi L., Xiang C., Yu F., Li Z. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2020. The hot deformation behaviourof antibacterial Ti-10wt.% CuSintered alloy. [Google Scholar]
- 260.Ma C., Zhu M., Wang J., Zhou X., Xing H., Ji S., Yang H. Mechanisms of improving the mechanical and antibacterial properties of Ti-3wt.% Cu alloys. Mater. Lett. 2022;319 [Google Scholar]
- 261.Yang H.-L., Zhu M.-Z., Wang J.-Y., Ma C.-X., Zhou X.-W., Xing H.-X., Zhang E.-L., Ji S.-X. Optimization of mechanical and antibacterial properties of Ti-3wt% Cu alloy through cold rolling and annealing. Rare Met. 2022;41(2):610–620. [Google Scholar]
- 262.Xin C., Wang N., Chen Y., He B., Zhao Q., Chen L., Tang Y., Luo B., Zhao Y., Yang X. Biological corrosion behaviour and antibacterial properties of Ti-Cu alloy with different Ti2Cu morphologies for dental applications. Mater. Des. 2022;215 [Google Scholar]
- 263.Bolzoni L., Alqattan M., Yang F., Peters L. Design of β-eutectoid bearing Ti alloys with antibacterial functionality. Mater. Lett. 2020;278 [Google Scholar]
- 264.Hu J., Li H., Wang X., Yang L., Chen M., Wang R., Qin G., Chen D.F., Zhang E. Effect of ultrasonic micro-arc oxidation on the antibacterial properties and cell biocompatibility of Ti-Cu alloy for biomedical application. Mater Sci Eng C Mater Biol Appl. 2020;115 doi: 10.1016/j.msec.2020.110921. [DOI] [PubMed] [Google Scholar]
- 265.Zhang Y., Fu S., Yang L., Qin G., Zhang E. A nano-structured TiO2/CuO/Cu2O coating on Ti-Cu alloy with dual function of antibacterial ability and osteogenic activity. J. Mater. Sci. Technol. 2022;97:201–212. [Google Scholar]
- 266.Zietz C., Fritsche A., Finke B., Stranak V., Haenle M., Hippler R., Mittelmeier W., Bader R. Analysis of the release characteristics of cu-treated antimicrobial implant surfaces using atomic absorption spectrometry. Bioinorgan. Chem. Appl. 2012;2012 doi: 10.1155/2012/850390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Liu H., Liu R., Ullah I., Zhang S., Sun Z., Ren L., Yang K. Rough surface of copper-bearing titanium alloy with multifunctions of osteogenic ability and antibacterial activity. J. Mater. Sci. Technol. 2020;48:130–139. [Google Scholar]
- 268.Cordeiro J.M., Nagay B.E., Dini C., Souza J.G., Rangel E.C., da Cruz N.C., Yang F., van den Beucken J.J., Barão V.A. Copper source determines chemistry and topography of implant coatings to optimally couple cellular responses and antibacterial activity. Mater. Sci. Eng. C. 2021 doi: 10.1016/j.msec.2021.112550. [DOI] [PubMed] [Google Scholar]
- 269.Han A., Tsoi J.K., Rodrigues F.P., Leprince J.G., Palin W.M. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhesion Adhes. 2016;69:58–71. [Google Scholar]
- 270.Han A., Li X., Huang B., Tsoi J.K.-H., Matinlinna J.P., Chen Z., Deng D.M. The effect of titanium implant surface modification on the dynamic process of initial microbial adhesion and biofilm formation. Int. J. Adhesion Adhes. 2016;69:125–132. [Google Scholar]
- 271.Bollenl C.M., Lambrechts P., Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent. Mater. 1997;13(4):258–269. doi: 10.1016/s0109-5641(97)80038-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.