Abstract
Background
Recovery strategies are often used with the intention of preventing or minimising muscle soreness after exercise. Whole‐body cryotherapy, which involves a single or repeated exposure(s) to extremely cold dry air (below ‐100 °C) in a specialised chamber or cabin for two to four minutes per exposure, is currently being advocated as an effective intervention to reduce muscle soreness after exercise.
Objectives
To assess the effects (benefits and harms) of whole‐body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, CINAHL, the British Nursing Index and the Physiotherapy Evidence Database. We also searched the reference lists of articles, trial registers and conference proceedings, handsearched journals and contacted experts.The searches were run in August 2015.
Selection criteria
We aimed to include randomised and quasi‐randomised trials that compared the use of whole‐body cryotherapy (WBC) versus a passive or control intervention (rest, no treatment or placebo treatment) or active interventions including cold or contrast water immersion, active recovery and infrared therapy for preventing or treating muscle soreness after exercise in adults. We also aimed to include randomised trials that compared different durations or dosages of WBC. Our prespecified primary outcomes were muscle soreness, subjective recovery (e.g. tiredness, well‐being) and adverse effects.
Data collection and analysis
Two review authors independently screened search results, selected studies, assessed risk of bias and extracted and cross‐checked data. Where appropriate, we pooled results of comparable trials. The random‐effects model was used for pooling where there was substantial heterogeneity. We assessed the quality of the evidence using GRADE.
Main results
Four laboratory‐based randomised controlled trials were included. These reported results for 64 physically active predominantly young adults (mean age 23 years). All but four participants were male. Two trials were parallel group trials (44 participants) and two were cross‐over trials (20 participants). The trials were heterogeneous, including the type, temperature, duration and frequency of WBC, and the type of preceding exercise. None of the trials reported active surveillance of predefined adverse events. All four trials had design features that carried a high risk of bias, potentially limiting the reliability of their findings. The evidence for all outcomes was classified as 'very low' quality based on the GRADE criteria.
Two comparisons were tested: WBC versus control (rest or no WBC), tested in four studies; and WBC versus far‐infrared therapy, also tested in one study. No studies compared WBC with other active interventions, such as cold water immersion, or different types and applications of WBC.
All four trials compared WBC with rest or no WBC. There was very low quality evidence for lower self‐reported muscle soreness (pain at rest) scores after WBC at 1 hour (standardised mean difference (SMD) ‐0.77, 95% confidence interval (CI) ‐1.42 to ‐0.12; 20 participants, 2 cross‐over trials); 24 hours (SMD ‐0.57, 95% CI ‐1.48 to 0.33) and 48 hours (SMD ‐0.58, 95% CI ‐1.37 to 0.21), both with 38 participants, 2 cross‐over studies, 1 parallel group study; and 72 hours (SMD ‐0.65, 95% CI ‐2.54 to 1.24; 29 participants, 1 cross‐over study, 1 parallel group study). Of note is that the 95% CIs also included either no between‐group differences or a benefit in favour of the control group. One small cross‐over trial (9 participants) found no difference in tiredness but better well‐being after WBC at 24 hours post exercise. There was no report of adverse events.
One small cross‐over trial involving nine well‐trained runners provided very low quality evidence of lower levels of muscle soreness after WBC, when compared with infrared therapy, at 1 hour follow‐up, but not at 24 or 48 hours. The same trial found no difference in well‐being but less tiredness after WBC at 24 hours post exercise. There was no report of adverse events.
Authors' conclusions
There is insufficient evidence to determine whether whole‐body cryotherapy (WBC) reduces self‐reported muscle soreness, or improves subjective recovery, after exercise compared with passive rest or no WBC in physically active young adult males. There is no evidence on the use of this intervention in females or elite athletes. The lack of evidence on adverse events is important given that the exposure to extreme temperature presents a potential hazard. Further high‐quality, well‐reported research in this area is required and must provide detailed reporting of adverse events.
Keywords: Adult, Female, Humans, Male, Young Adult, Air, Cryotherapy, Cryotherapy/methods, Exercise, Myalgia, Myalgia/etiology, Myalgia/prevention & control, Randomized Controlled Trials as Topic, Rest
Plain language summary
Whole‐body cryotherapy for preventing and treating muscle soreness after exercise
Background and aim of the review
Delayed onset muscle soreness describes the muscular pain, tenderness and stiffness experienced after high‐intensity or unaccustomed exercise. Various therapies are in use to prevent or reduce muscle soreness after exercise and to enhance recovery. One more recent therapy that is growing in use is whole‐body cryotherapy (WBC). This involves single or repeated exposure(s) to extremely cold dry air (below ‐100°C) in a specialised chamber or cabin for two to four minutes per exposure. This review aimed to find out whether WBC reduced muscle soreness, improved recovery and was safe for those people for whom it can be used.
Results of the search
We searched medical databases up to August 2015 for studies that compared WBC with a control intervention such as passive rest or no treatment; or with another active intervention such as cold water immersion. We found four small studies. These reported results for a total of 64 physically active young adults. All but four participants were male. The studies were very varied such as the type, temperature, duration and frequency of the WBC and the exercises used to induce muscle soreness. There were two comparisons: WBC compared with a control intervention; and WBC compared with far‐infrared therapy.
Key results
All four studies compared WBC with either passive rest or no treatment. These provided some evidence that WBC may reduce muscle soreness (pain at rest) at 1, 24, 48 and 72 hours after exercise. However, the evidence also included the possibility that WBC may not make a difference or may make the pain worse. There was some weak evidence that WBC may improve well‐being at 24 hours. There was no report and probably no monitoring of adverse events in these four studies.
One very small study also compared WBC with far‐infrared therapy and reported lower levels of muscle soreness one hour after the treatment.
Quality of the evidence
All four studies had aspects that could undermine the reliability of their results. We decided that the evidence was of very low quality for all outcomes. Thus, the findings remain very uncertain and further research may provide evidence that could change our conclusions.
Conclusions
The currently available evidence is insufficient to support the use of WBC for preventing and treating muscle soreness after exercise in adults. Furthermore, the best prescription of WBC and its safety are not known.
Summary of findings
Summary of findings for the main comparison. Summary of findings: whole‐body cryotherapy (WBC) compared with control (no WBC or passive rest).
| Whole‐body cryotherapy (WBC) compared with control (no WBC or passive rest) for preventing and treating muscle soreness after exercise in adults | ||||||
|
Patient or population: physically‐active adults partaking in exercise designed to produce delayed onset muscle soreness (most trial participants were male) Settings: laboratory‐based Intervention: whole‐body cryotherapy (WBC). The timing, format and modality varied. WBC delivered in either in a specialised cryotherapy chamber (temperature ‐110°C) or partial‐body cryotherapy (head and neck not included) in a cryo‐cabin at temperatures of ‐110°C or between 140 to ‐195°C. Exposure: 3 minutes. Timing after exercise ranged from 10 minutes to 24 hours. Repeat exposures, which were every 24 hours in 2 studies, varied from 0 to 5 additional sessions. Comparison: control: no intervention (in chamber but normal temperatures: 15°C and 21°C) or passive rest | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (rest or no WBC) | WBC | |||||
|
Muscle soreness: pain at rest (VAS) Follow‐up: 1 hour |
The mean difference in muscle soreness in the WBC groups was 0.77 standard deviations lower (1.42 lower to 0.12 lower) | SMD ‐0.77 (‐1.42 to ‐0.12) | 20 participants (2 studies) |
⊕⊝⊝⊝ very low1 | Reported in 2 cross‐over studies only One rule of thumb is that 0.2 represents a small difference, 0.5 a moderate difference and 0.8 a large difference. Based on this 'rule of thumb', this result equates to a moderate to large difference in favour of WBC |
|
|
Muscle soreness: pain at rest (VAS) Follow‐up: 24 hours |
The mean difference in muscle soreness in the WBC groups was 0.57 standard deviations lower (1.48 lower to 0.33 higher) | SMD ‐0.57 ( ‐1.48 to 0.33) | 38 participants (3 studies) | ⊕⊝⊝⊝ very low2 | Reported in one parallel group (which found no difference between the two groups) and two cross‐over studies which found in favour of WBC. Based on the above rule of thumb, this results equates to a moderate difference in favour of WBC but also includes a small to moderate effect in favour of rest or no WBC | |
|
Muscle soreness: pain at rest (VAS) Follow‐up: 48 hours |
The mean difference in muscle soreness in the WBC groups was 0.58 standard deviations lower (1.37 lower to 0.21 higher) | SMD ‐0.58 (‐1.37 to 0.21) | 38 participants (3 studies) | ⊕⊝⊝⊝ very low2 | Reported in one parallel group (which found no difference between the two groups) and two cross‐over studies which found in favour of WBC. Based on the above rule of thumb, this results equates to a moderate difference in favour of WBC but also includes a small effect in favour of rest or no WBC | |
|
Muscle soreness: pain at rest (VAS) Follow‐up: 72 hours |
The mean difference in muscle soreness in the WBC groups was 0.65 standard deviations lower (2.54 lower to 1.24 higher) | SMD ‐0.65 (‐2.54 to 1.24) | 29 participants (2 studies) | ⊕⊝⊝⊝ very low2 | Reported in one parallel group (which found no difference between the two groups) and one cross‐over study which found in favour of WBC. There was substantial heterogeneity between the two trials (Chi² = 7.73, df = 1 (P = 0.005); I² = 87%), which brings both pooling and the validity of this result into question | |
|
Tiredness (0 [no tiredness] to 100 [maximum tiredness]) Follow‐ups: 1, 24 and 48 hours |
The mean tiredness recorded in the study control group at 24 hours was 49.2 | The mean tiredness in the WBC group was 13.30 lower (32.17 lower to 5.57 higher) | 9 participants (1 study) |
⊕⊝⊝⊝ very low1 | Reported in one cross‐over study only. The results at 1 and 48 hours showed a similar lack of differences between the two groups, with all 95% CIs crossed the line of no effect |
|
|
Well‐being (0 [worst well‐being] to 100 [optimal well‐being]) Follow‐ups: 1, 24 and 48 hours |
The mean well‐being recorded in the study control group at 24 hours was 65.4 | The mean well‐being in the WBC group was 21.7 higher (2.20 to 41.20 higher) | 9 participants (1 study) |
⊕⊝⊝⊝ very low1 | It is possible that the difference in well‐being represented a clinically important difference but the minimal clinically important difference is not known. No differences between groups were observed at 1 and 48 hour follow‐ups; the 95% CIs crossed the line of no effect at both time periods |
|
| Adverse events | See comment | See comment | See comment | All studies failed to report active surveillance of predefined adverse events. We found one report of skin burn following WBC in the recent literature. Studies typically exclude people with contradictions to cryotherapy, such as Raynaud’s disease | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The quality of the evidence was downgraded 3 levels for very serious study limitations resulting in a high risk of bias and serious imprecision (very few participants).
2The quality of the evidence was downgraded 3 levels for very serious study limitations resulting in a high risk of bias, serious imprecision (very few participants) and serious inconsistency (substantial heterogeneity).
Background
Elite‐level athletic participation necessitates recovery from many physiological stressors, including fatigue to the musculoskeletal, nervous and metabolic systems (Nédélec 2013). Athletic participation may also result in exercise‐induced muscle damage (EIMD), which may lead to delayed‐onset muscle soreness (DOMS) and decrements in subsequent performance (Howatson 2008). Various therapeutic modalities of recovery are currently used by athletes in an attempt to offset the negative effects of strenuous exercise (Bieuzen 2013;Bleakley 2012; Costello 2014b; Minett 2015; Nédélec 2013).
Description of the condition
DOMS is a broad term used to describe the muscular pain, tenderness and stiffness experienced after high‐intensity, eccentric (when the muscle is forcibly stretched when active) or unaccustomed exercise (Cheung 2003; Ebbeling 1989; Howatson 2008; Newham 1987). Clinically associated with EIMD, DOMS is proposed to result from mechanical disturbances of the muscle membrane that evoke secondary inflammation, swelling and free radical proliferation (Connolly 2003). These events typically peak 24 to 96 hours post exercise (Cheung 2003) and may reduce physical capacity via alterations in muscle length, maximal force and range of motion (Prasartwuth 2006; Saxton 1995). Although damage to the exercised musculature is linked to the biochemical expression of intracellular enzymes, compensatory neuromuscular recruitment patterns may contribute both central and peripheral factors to DOMS aetiology (Byrne 2004).
Symptoms associated with DOMS typically dissipate within five to seven days post exercise with adequate rest (Cheung 2003). Nevertheless, various interventions have been advocated to prevent or treat, or both prevent and treat, EIMD and associated DOMS. Interventions include cool‐down, stretching, nutritional supplements, massage, hydrotherapy, compression, electrotherapy and non‐steroidal anti‐inflammatory medications (Bieuzen 2013; Bleakley 2012; Herbert 2011). Despite their widespread popularity (Nédélec 2013), empirical support for the use of these interventions for DOMS remains tenuous (Bleakley 2012; Herbert 2011).
Description of the intervention
Whole‐body cryotherapy (WBC) is increasingly used in sports medicine as treatment for muscle soreness after exercise. This treatment involves exposing individuals to extremely cold dry air (below ‐100°C) for two to four minutes. To achieve the subzero temperatures required for WBC, two methods are typically used: liquid nitrogen and refrigerated cold air. During these exposures, individuals wear minimal clothing, which usually consists of shorts for males and shorts and a crop top for females. Gloves, a woollen headband covering the ears, and a nose and mouth mask, in addition to dry shoes and socks, are commonly worn to reduce the risk of cold‐related injury.
The first WBC chamber was built in Japan in the late 1970s, but WBC was not introduced to Europe until the 1980s, and has only been used in the USA and Australia in the past decade (Miller 2012). The treatment was initially intended for use in a clinical setting to treat patients with conditions such as multiple sclerosis (Miller 2012) and rheumatoid arthritis (Hirvonen 2006;Metzger 2000); however, elite athletes have recently reported using the treatment to alleviate DOMS after exercise (Bleakley 2014; Costello 2012a; Fonda 2013; Hausswirth 2011; Pournot 2011). WBC is commonly employed shortly (within 0 to 24 hours) after exercise, and the treatment is often repeated on the same day (Costello 2012a) or over several days (Lubkowska 2012).
In the field of athletic training, a new method of exposing people to these extreme temperatures, called partial‐body cryotherapy (PBC), using a portable cryo‐cabin, has recently been developed. This system has an open tank and exposes the body, except the head and neck, to temperatures below ‐100°C. Recently, recreational athletes have started to emulate elite athletes in using these treatments after exercise.
How the intervention might work
Reductions in muscle and skin tissue temperature after WBC exposure (Costello 2012b; Costello 2012c; Costello 2014a) may stimulate cutaneous receptors and excite the sympathetic adrenergic fibres, causing constriction of local arterioles and venules (Costello 2014d; Savic 2013). Consequently, WBC may be effective in relieving soreness through reduced muscle metabolism, skin microcirculation, receptor sensitivity and nerve conduction velocity. In addition, both Bleakley 2012 and Cochrane 2004 describe the potential psychological benefits of using other modalities of cold exposure (e.g. cold water immersion) to reduce the subjective feeling of DOMS following exercise.
Although the research examining WBC is typically limited in terms of quality and statistical power (Costello 2012a; Costello 2012b), some studies have described a reduction in creatine kinase activity after training (Wozniak 2007), increases in parasympathetic activation (Hausswirth 2013; Zalewski 2014) and an increase in anti‐inflammatory cytokines (proteins that serve to regulate the inflammatory response) (Ferreira‐Junior 2014b; Lubkowska 2010; Lubkowska 2011) after WBC exposure. A reduction in the severity of muscle damage after exercise and an increase in anti‐inflammatory cytokines post‐treatment may help to reduce both the initial damage and the secondary inflammatory damage associated with EIMD. However, from a mechanistic perspective, very little is known about the physiological and biochemical rationale for using WBC in the management of DOMS.
Only a few studies have sought to examine the physiological effects (Fonda 2014; Hammond 2014; Selfe 2014) of different WBC protocols on different population. Selfe 2014 studied the effects of a 1, 2 and 3 minutes exposure of WBC at ‐135°C on changes in the inflammatory cytokine interleukin‐six (IL‐6), tissue oxygenation, skin and core temperature, thermal sensation and comfort in professional rugby league players, and concluded that two minutes was the optimum exposure length that should be applied as the basis for future studies. Fonda 2014, employing ‐130 to ‐170°C partial‐body (head out) cryotherapy, supported these findings and demonstrated that longer durations do not substantially affect thermal and cardiovascular response, but do increase thermal discomfort in healthy young male adults.
Using the approach described by Anderson 2011, we developed a logic model to capture the wide range of potential effects of WBC exposure (Figure 1). This model is divided into two sections: (1) potential recovery benefits and (2) potential adverse effects. Missing from this model is any appraisal of the logistical, environmental and financial costs associated with WBC.
1.
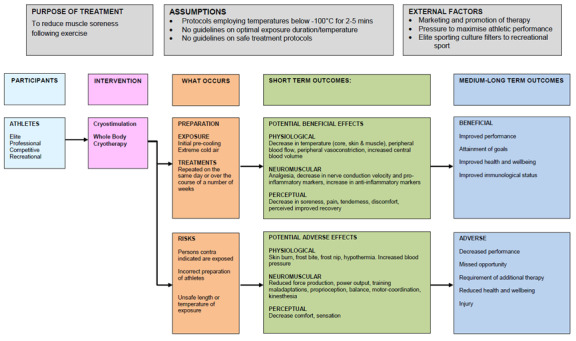
Logic Model describing the potential benefits and adverse effects of whole‐body cryotherapy
Why it is important to do this review
This review aimed to examine the effects, both beneficial and harmful, of WBC used for the purpose of preventing or treating muscle soreness after exercise. Currently, no guidelines for a clinically effective or safe WBC protocol are available. Because of the extreme temperatures employed during WBC, the potential for short‐ and long‐term adverse effects needs to be elucidated. A systematic review of the evidence is also important because of the increasing use of WBC by elite and recreational athletes and the potential for long‐term use throughout a sporting career in an attempt to alleviate DOMS.
Objectives
To assess the effects (benefits and harms) of whole‐body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and quasi‐randomised (method of allocating participants to a treatment that is not strictly random, e.g. by date of birth) controlled clinical trials evaluating whole‐body cryotherapy for prevention and treatment of muscle soreness after exercise in adults. This included randomised cross‐over trials and trials carried out in laboratory or field settings.
Types of participants
No restrictions were placed on gender or on type or level of exercise. All field‐ and laboratory‐based (including eccentric) exercise modalities were included. We excluded studies focusing on children (< 18 years of age) or on injured participants. As anticipated, people with vascular problems, such as Raynaud’s disease, who are contraindicated for cryotherapy, were excluded from trials.
Types of interventions
We included trials in which at least one group in the trial comprised participants treated with whole‐body cryotherapy (WBC) before or after exercise. WBC was defined as exposure of the body (trunk, arms and legs) to extremely cold dry air (below ‐100°C). These exposures are typically administered as a once‐off treatment, or repeated several times on the same days or over several days.
We aimed to include trials that compared the use of WBC versus a passive or control intervention (rest, no treatment or placebo treatment) or active interventions designed to prevent or treat delayed‐onset muscle soreness (DOMS), including, but not limited to, cold water immersion (immersion in water colder than 15°C), warm water immersion (immersion in water warmer than 15°C), contrast water immersion (alternating hot and cold water immersion), cool‐down, stretching, massage and compression garments.
We also aimed to include randomised trials that compared different durations or dosages of WBC. We excluded trials in which the same WBC protocol was used in both arms as a co‐intervention. Comparisons with pharmacological interventions were also excluded.
Types of outcome measures
Trials that did not report any of the primary outcomes were not included in the review.
Primary outcomes
Muscle soreness (e.g. pain measured with the use of visual analogue scales and algometer data)
Subjective recovery (e.g. tiredness, well‐being)
Immediate or long‐term complications or adverse effects (e.g. frost bite, adverse cardiac or vascular events, musculoskeletal injury)
Secondary outcomes
Muscle strength and power (muscle contractile properties)
Objective test of function and performance (e.g. hop test)
Resource use
We also attempted to collect cost and resource data, including cost of the intervention and cost of time off work or professional sports activity.
Timing of outcome assessment
We collected data at the following follow‐up times: up to 1 and 24, 48, 72, 96 and more than 96 hours post intervention. These are typical follow‐up times for studies assessing treatment for DOMS.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (5 August 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2015 Issue 7), MEDLINE (1946 to July Week 4 2015), MEDLINE In‐Process & Other Non‐Indexed Citations (5 August 2015), EMBASE (1974 to 2015 Week 31), the Cumulative Index to Nursing and Allied Health (CINAHL) (1982 to 5 August 2015), the British Nursing Index (BNI) (1985 to 5 August 2015) and the Physiotherapy Evidence Database (PEDro) (1929 to 7 August 2015).
In MEDLINE, the subject‐specific search was combined with the sensitivity‐ and precision‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011). We present the search strategies for CENTRAL, MEDLINE, EMBASE, CINAHL and BNI in Appendix 1.
We also searched Current Controlled Trials and the WHO International Clinical Trials Registry Platform for ongoing and recently completed trials (7 August 2015) (seeAppendix 1).
We applied no language restrictions.
Searching other resources
We searched the reference lists of relevant articles and the table of contents of the following journals not registered as being handsearched by the Cochrane Collaboration:
(Australian) Journal of Science and Medicine in Sport (1998 to September 2014).
British Journal of Sports Medicine (1964 to September 2014).
Clinical Journal of Sport Medicine (1991 to September 2014).
International Journal of Sports Medicine (2005 to September 2014).
Journal of Applied Physiology (1948 to September 2014).
Journal of Sports Medicine and Physical Fitness (1998 to September 2014).
Journal of Sports Sciences (1985 to 1987; 1990 to 1991; 1994; 1996; 2000 to September 2014).
Medicine and Science in Sports and Exercise (1980 to September 2014).
Physical Therapy in Sport (2000 to 2002; 2007 to September 2014).
We also searched the conference proceedings of the following organisations:
American College of Sports Medicine (1986 to September 2014) (in Medicine and Science in Sports and Exercise).
American Physical Therapy Association (1980 to September 2014) (in Physical Therapy).
British Association of Sport and Exercise Medicine (BASEM) (1964 to September 2014) (in British Journal of Sports Medicine).
British Association of Sport and Exercise Sciences (BASES) (1964 to September 2014) (in Journal of Sports Sciences).
World Confederation for Physical Therapy (2003, 2007, 2011) (CD‐ROM).
Experts and colleagues working in the subject area were also asked to notify us on the existence of new or ongoing studies, which we also considered for inclusion.
Data collection and analysis
Selection of studies
Two review authors (JTC, GMM) independently selected trials for inclusion. First, we screened titles and abstracts of publications obtained by the search strategy and removed only those that were obviously outside the scope of the review. We were over‐inclusive at this stage and obtained the full text of any papers that potentially met the review inclusion criteria. We checked for multiple publications and reports of the same study. The same two review authors then independently selected trials using a standardised form to record their choices. We were not blinded during this process with respect to study authors’ names, journal or date of publication. When possible, translation of non–English language studies was undertaken. We contacted primary authors when necessary to ask for clarification of study characteristics. Disagreement between the review authors was resolved by consensus or by third party adjudication (CB, PRAB, IBS).
Data extraction and management
Two review authors (JTC, GMM) used a customised form to independently extract relevant data on methodology, eligibility criteria, interventions (including detailed characteristics of the exercise protocols and the WBC protocol employed), comparisons and outcome measures. Details of the characteristics of trial participants such as training status, age, sex and health status were also recorded. When available, we extracted data on participant subgroups, including any equity considerations such as ethnicity and socioeconomic status. Any included study written by one of the current review authors was reviewed by review authors who did not participate in the original study. Any disagreement was resolved by consensus or by third party adjudication (IBS, PRAB). We contacted primary authors to clarify any omitted data or study characteristics. For intention‐to‐treat analysis, data were extracted according to the original allocation groups, and losses to follow‐up were noted where possible.
Assessment of risk of bias in included studies
Two review authors (JTC, GMM) independently assessed risk of bias using the tool described (and the criteria outlined) in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). To minimise bias in the interpretation of this scale, two review authors (JTC, GMM) initially assessed 10 unrelated studies (not included in the current review); disparities in 'Risk of bias' judgements were reviewed and discussed before any of the included studies were evaluated.
Each study was graded for risk of bias in each of the following domains: sequence generation, allocation concealment, blinding (participants and intervention providers; outcome assessment), incomplete outcome data and selective outcome reporting. Two other sources of bias were also considered on the basis of the following questions: (1) 'Was the exercise protocol clear and consistent between groups?', and (2) 'Were co‐interventions used, and if so, were they standardised across groups?'. For each study, the information pertaining to each of the domains was described as reported in the published study report (or, if appropriate, based on information from related protocols or published comments, or after discussion with the relevant authors) and the associated risk of bias judged by the review authors. Studies were assigned 'high risk', 'low risk', or 'unclear risk' when there was uncertainty or when information was insufficient to allow review authors to make a judgement. Disagreements between review authors regarding the 'Risk of bias' assessment were resolved by consensus.
Measures of treatment effect
For each study, we calculated risk ratios and 95% confidence intervals (CIs) for dichotomous outcomes, and mean differences and 95% CIs for continuous outcomes. For continuous outcomes that were pooled on different scales, we used standardised mean differences (SMDs). Where possible, follow‐up scores were used in preference to change scores. An exception was made for strength outcomes, where recovery to baseline is arguably of most interest to athletes and researchers.
Unit of analysis issues
We extracted data at clinically relevant time points. When available, data were extracted for, and separate analyses conducted at, the following time points: up to 1 hour after the exercise and then at 24‐hour intervals (1 to 24 hours, 25 to 48 hours, 49 to 72 hours, 73 to 96 hours and over 96 hours). In studies using a randomised cross‐over design, and where a carry‐over effect was not thought to be a problem, we aimed to undertake paired analysis when sufficient data were available; otherwise, data were analysed as if these studies used a parallel group design.
Dealing with missing data
In cases where data were missing, we considered why they were missing. We contacted study authors to request missing data or to ask for an explanation as to why data were missing. Unless missing standard deviations were derived from CIs, standard errors or exact P values, we did not assume or impute values for these in order to present results in the analyses.
Assessment of heterogeneity
Assessment of heterogeneity between comparable trials was evaluated visually with the use of forest plots, as well as Chi² tests and I² statistics. The level of significance for the Chi² test was set at P = 0.1 (Deeks 2011): a P value for Chi² < 0.1 was considered to indicate statistically significant heterogeneity between studies. Values of I² were interpreted as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% may represent considerable heterogeneity.
Assessment of reporting biases
We planned to use funnel plots to assess for publication bias; however, there were insufficient studies. Should sufficient trials become available in future, we plan to use funnel plots to assess for publication bias based on the effect estimates (horizontal scale) against standard error (on a reversed scale, vertical) using Review Manager software, with continuous data represented as SMDs, and dichotomous data represented as risk ratios on a logarithmic scale.
Data synthesis
Results of comparable groups of trials were pooled using either fixed‐effect or random‐effects models. The choice of the model to report was guided by careful consideration of the extent of heterogeneity and whether it could be explained, in addition to other factors, such as the number and size of included studies. Ninety‐five per cent CIs were used throughout. We considered not pooling data when considerable heterogeneity (I² > 75%) could not be explained by the diversity of methodological or clinical features observed among trials. When it was inappropriate to pool data, we presented trial data in the analyses or tables for illustrative purposes and reported them in the text.
Subgroup analysis and investigation of heterogeneity
We intended to perform the following subgroup analyses:
Gender (male versus female)
Exposure dose (single versus repeated exposures; short versus long exposure durations)
Exercise type (normal sporting activities and laboratory‐induced delayed‐onset muscle soreness (DOMS))
Training status (elite versus recreational)
We chose these subgroup analyses because gender, type of athletic activity and training status may impact the severity of DOMS experienced after exercise (Howatson 2008; McGinley 2009). In particular, DOMS may be augmented in untrained males after eccentric exercise when compared with trained females performing concentric exercise. Moreover, reductions in tissue temperature may be more pronounced after repeated, or longer, WBC exposures (Costello 2012c).
We conducted an exploratory subgroup analysis based on study design: parallel group versus cross‐over. As well as issues relating to potential carry‐over effects and suboptimal analysis of cross‐over trials, they are likely to be at increased risk of serious bias where there is lack of blinding and subjective assessment of outcome.
We investigated whether the results of subgroups were significantly different by inspecting the overlap of CIs and by performing the test for determining subgroup differences that is available in Review Manager (RevMan 2014).
Sensitivity analysis
If some of the included trials were at high risk of bias for one or more domains, we intended to perform sensitivity analysis to determine whether inclusion of such trials significantly influenced the effect size. We planned to consider trials at high risk of bias in sensitivity analysis if allocation concealment was unclear or at high risk of bias, or if attrition was greater than 20%. We performed sensitivity analysis to explore the effects of using fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity.
'Summary of findings' table
We prepared a 'Summary of findings' table for the main comparison (WBC versus rest, no or a placebo intervention) using the GRADE profiler (Schünemann 2011). We summarised the quality of evidence by applying the principles of the GRADE framework and following the recommendations and worksheets of the Cochrane Effective Practice and Organisation of Care Group for creating 'Summary of findings' tables (EPOC 2011). We assessed the quality of the evidence according to four levels (high, moderate, low and very low). We presented the evidence for primary outcomes only. We selected muscle soreness assessed for pain at rest at 1, 24, 48 and 72 hours; well‐being and tiredness at 24 hours and adverse events as the 7 outcomes for presenting in a 'Summary of findings' table.
Results
Description of studies
Results of the search
We screened a total of 1696 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (2 records); Cochrane Central Register of Controlled Trials (388), MEDLINE (332), EMBASE (463), CINAHL (286), BNI (5), PEDro (87), the WHO International Clinical Trials Registry Platform (63) and Current Controlled Trials (70). We also found 52 potentially‐eligible studies from ongoing searches and through contacting experts and colleagues working in the subject area.
The search identified a total of 29 articles for potential inclusion, for which full reports were obtained where possible. Upon further analysis, 4 trials (6 articles) were included (Costello 2012; Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011) and 17 studies (19 articles) were excluded. Four studies are awaiting classification pending publication (DRKS00006038; NCT02341612) or receipt of further information (Krüger 2015; Ziemann 2014).
A flow diagram summarising the study selection process is shown in Figure 2.
2.
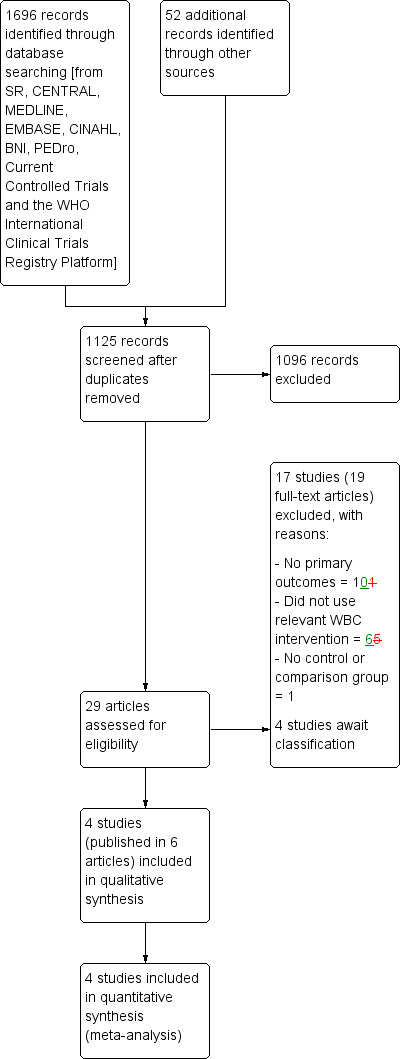
Study flow diagram
Included studies
The four included studies, which were published between 2010 and 2014, were laboratory studies conducted in single centres in France (Hausswirth 2011), Ireland (Costello 2012), Slovenia (Fonda 2013) and Brazil (Ferreira‐Junior 2014). All were published in English and in peer‐reviewed journals. Three were identified via database searches and one was identified from searching conference proceedings (Ferreira‐Junior 2014). A full pre‐publication report was obtained from the authors of this trial. All four studies were randomised controlled trials. Two employed a parallel group design (Costello 2012; Ferreira‐Junior 2014) and two used a cross‐over design (Fonda 2013; Hausswirth 2011). The time between intervention arms in the cross‐over trials was 3 weeks in Hausswirth 2011 and 10 weeks in Fonda 2013.
Further details of individual trials can be found in the Characteristics of included studies.
Participants
In total, there were data for 64 trial participants of which only 4 (6.3%) were female; all 4 were recruited in 1 trial of 18 participants (Costello 2012). The mean age of participants in individual trials was 21 years in Costello 2012, 20 years in Ferreira‐Junior 2014, 27 years in Fonda 2013 and 32 years in Hausswirth 2011. The largest trial included 26 participants (Ferreira‐Junior 2014).
Participants were described as physically active (Costello 2012; Ferreira‐Junior 2014; Fonda 2013) or well‐trained runners (Hausswirth 2011).
Details of exercise
The type, duration and intensity of exercise performed varied across studies. In three studies, the exercise was designed to produce delayed onset muscle soreness (DOMS) under laboratory‐controlled conditions (Costello 2012; Ferreira‐Junior 2014; Fonda 2013). The exercise comprised multiple repetitions (100 repetitions) of resistance to lengthening (eccentric exercise) in Costello 2012; drop jumps (100 jumps) in Ferreira‐Junior 2014; and a combination of drop jumps (50 jumps), bilateral leg curls (50 repetitions) and eccentric leg curls (10 repetitions) in Fonda 2013. The methodology employed by Costello 2012 targeted a single muscle group (quadriceps). Athough there was a focus on the hamstring and quadriceps muscles respectively, Fonda 2013 and Ferreira‐Junior 2014 targeted a number of related muscle groups using a jumping protocol. Hausswirth 2011 employed a 48 minute simulated trail run (incorporating five 3‐minute downhill blocks at a ‐15% gradient) on a treadmill.
Details of whole‐body cryotherapy
All studies employed some type of whole‐body cryotherapy (WBC) after exercise.
Two studies used WBC and exposed participants to a controlled temperature of ‐110°C in a specialised cryotherapy chamber (Costello 2012; Hausswirth 2011). Two studies used partial‐body cryotherapy in a cryo‐cabin at temperatures of ‐110°C (Ferreira‐Junior 2014) and between ‐140 to ‐195°C (Fonda 2013). It was noted by Fonda 2013 that the temperature was measured on the inner wall of the cabin and not next to the skin. It is likely that the temperature around the skin surface was lower (Fonda 2013). Although similar, there are some differences between partial‐ and whole‐body cryotherapy including:
the head is not exposed during partial‐body cryotherapy,
partial‐body cryotherapy uses liquid nitrogen and WBC uses refrigerated cold air,
participants typically walk slowly around a small chamber during WBC and stand during partial‐body cryotherapy, and
the temperature is uniformly distributed in the WBC chamber and not in the cabin (i.e. it is cooler at the bottom of the cabin compared with the top).
All four studies exposed participants to the cryotherapy for three minutes. However, before entering the ‐110°C chamber, Costello 2012 included an additional 20 seconds standing in a ‐60°C chamber and Hausswirth 2011 stated that participants traversed through two separate chambers (at ‐10°C and ‐60°C respectively). Fonda 2013 also stated that the participants were instructed to turn around continuously in the cabin during exposure. The timing of initiating cryotherapy after exercise was not consistent across the studies. Ferreira‐Junior 2014 and Hausswirth 2011 initiated WBC approximately 10 to 15 minutes after exercise, while Fonda 2013 and Costello 2012 waited until 1 and 24 hours respectively after exercise. Three studies undertook additional WBC interventions after completing the exercise session: Costello 2012 (26 hours); Hausswirth 2011 (at 24, 48, 72 and 96 hours); and Fonda 2013 (at 24, 48, 72, 96 and 120 hours).
Details of comparisons
All four included trials compared WBC versus control (no WBC or passive rest). One three‐group trial also compared WBC versus far‐infrared therapy (Hausswirth 2011). This study therefore appears in both comparisons.
We found no trials evaluating WBC versus other interventions: cold water immersion (immersion in water colder than 15°C), warm water immersion (immersion in water warmer than 15°C), contrast water immersion (alternating hot and cold water immersion), cool‐down, stretching, massage or compression garments. Nor were there trials evaluating the effectiveness of different durations or dosages of WBC.
Whole‐body cryotherapy versus control (no intervention, passive rest or sham treatment)
The participants in the control groups of Costello 2012 and Ferreira‐Junior 2014 followed the same procedures as the intervention groups but the chamber or cryo‐cabin temperature was set to a temperature of 15°C and 21°C respectively. The control group comprised 'passive rest' in Fonda 2013 and seated rest for 30 minutes in a temperate room in Hausswirth 2011.
Whole‐body cryotherapy versus far‐infrared therapy
One study compared WBC with far‐infrared therapy (Hausswirth 2011). During the far‐infrared therapy, participants lay supine on the table and the whole body, except the head, was exposed to the treatment for 30 minutes (4 to 14 µm, 45°C). The number of treatments was the same for both interventions (15 minutes, 24, 48, 72 and 96 hours post exercise).
Details of outcome measurement
Primary outcomes
All four trials reported on muscle soreness, which was assessed using a visual analogue scale (VAS). Three studies measured muscle soreness at rest (Costello 2012; Fonda 2013; Hausswirth 2011); one during a squat (Fonda 2013) and one while performing an isometric knee extension (Ferreira‐Junior 2014). Fonda 2013 assessed the soreness using a 10 cm (0 “no pain” to 10 “severe pain”) VAS during the resting and the exercise assessment. Both Hausswirth 2011 (0 "no pain" to 100 "maximum possible" on a web interface) and Ferreira‐Junior 2014 (0 mm "no soreness" to 100mm "severe soreness") used a 100‐point visual analogue scale. Costello 2012 employed a 10‐point (1 “normal, no pain” to 10 "very, very sore") VAS to assess muscle soreness across eight different sites in the lower limb, with the results combined together.
Hausswirth 2011 also measured 'tiredness' and 'well‐being' using the same web interface 100‐point VAS.
The included studies did not monitor adverse events or complications relating to the interventions. One study measured tympanic temperature changes associated with WBC and reported that the lowest mean temperature (36.6 ± 0.4°C) was observed eight minutes after WBC (Costello 2012).
Secondary outcomes
A range of secondary outcomes was reported. Strength was assessed by all four studies. Three studies used an isokinetic dynamometer to assess either maximal voluntary torque production knee flexion (Fonda 2013) or maximal voluntary isometric knee extensor force (Ferreira‐Junior 2014; Hausswirth 2011). Costello 2012 assessed maximal voluntary isometric knee extensor force on a modified Tornvall chair.
Two studies reported on power: Costello 2012 assessed peak power output (% of baseline) during repeated‐sprint cycling on a cycle ergometer and Fonda 2013 examined height (m), max force (N/kg), max power (W/kg), work (J), and push off duration (s) during a squat and a counter movement jump on a force plate.
None of the included studies included objective tests of function or performance (e.g. hop test).
Missing data
In order to calculate effect sizes, raw data from four included studies were requested from study authors (Costello 2012; Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011). All were successfully contacted, with each providing the requested information.
Follow‐up
All trials undertook multiple follow‐up observations for each outcome. These included immediately after exercise (Costello 2012; Ferreira‐Junior 2014; Hausswirth 2011), 1 hour (Fonda 2013; Hausswirth 2011), 24 hours (Costello 2012; Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011), 48 hours (Costello 2012; Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011), 72 hours (Costello 2012; Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011), 96 hours (Costello 2012; Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011) and 120 hours (Fonda 2013) hours post exercise.
This equated to follow‐up observations at 10 to 15 minutes (Fonda 2013; Costello 2012), 30 to 45 minutes (Hausswirth 2011) and 24 hours (Ferreira‐Junior 2014) after the cryotherapy treatment.
Excluded studies
After appraisal of the full study reports, we excluded 17 studies. Ten studies were excluded because they did not include a primary outcome measure of the review (rating of perceived exertion and rating of thermal discomfort/sensation were not considered to be measures of subjective recovery) and six studies because they did not use a relevant WBC intervention. Banfi 2008 was a cohort study only. Reasons for exclusion for individual studies can be found in the Characteristics of excluded studies.
Studies awaiting classification
Details of the four studies in this category are shown in the Characteristics of studies awaiting classification. We have been unable to locate a full report of DRKS00006038, which recruited 24 female football players, or of NCT02341612, which recruited 65 male college students. Additional information is being sought from the authors of Krüger 2015 and Ziemann 2014; these recruited 11 and 18 physically‐active young men respectively.
Risk of bias in included studies
All corresponding authors of the included trials responded to our requests for any unclear or missing methodological details. Our requests for information were open‐ended to avoid any bias resulting from leading questions. Unless an author specifically stated that he or she did not understand our question, we avoided making multiple requests for information. 'Risk of bias' judgements were made by two independent authors, based on information from trial reports and author correspondence; the results are summarised in Figure 3 and Figure 4. Full details of our 'Risk of bias' assessments for individual trials are given in the Characteristics of included studies.
3.
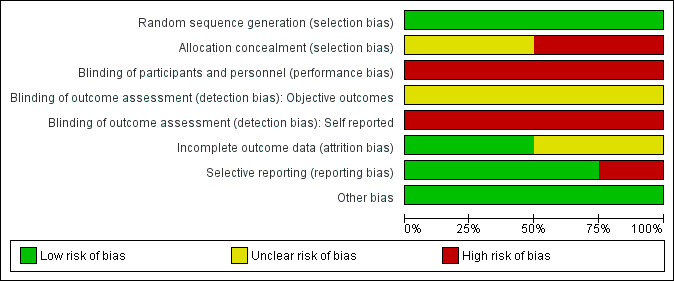
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation procedure was described in all four studies. Two studies used a random numbers generator (Costello 2012; Fonda 2013); Ferreira‐Junior 2014 used a random numbers table and Hausswirth 2011 used a hat draw (confirmed following personal communication).
Allocation concealment was not adequately described in any of the included studies. However, in two studies, there was no clear indication that the investigators would be unable to predict the prospective group (Fonda 2013; Hausswirth 2011), or in the case of cross‐over trials, the order of treatments to which participants would be allocated. However, the allocations of the final treatment in the cross‐over studies were predictable following knowledge of the first treatment. After personal communication, both Costello 2012 and Ferreira‐Junior 2014 confirmed that an open random allocation schedule was employed. Thus both these trials were rated at high risk of bias for this item.
Blinding
Given the type of intervention, it is impractical to blind participants or personnel and thus all four trials were inevitably at high risk of performance bias. We assessed detection bias separately for objective (e.g. strength) and self‐reported outcome measures (e.g. DOMS). The risk of detection bias was classified as high for self‐reported outcome measures. As it is unclear if the lack of assessor blinding would influence the objective measures (e.g. strength), the risk of bias for the objective measures was classified as unclear.
Incomplete outcome data
In general, the losses to follow‐up and missing data were poorly described in the published reports of the included studies. After correspondence, the authors of two trials confirmed no losses to follow‐up or violation from the study protocol (Costello 2012; Fonda 2013), whereas the authors of the two other studies confirmed there were missing data (Ferreira‐Junior 2014; Hausswirth 2011). Two (of 11) participants were dismissed because of incomplete outcomes in Hausswirth 2011. Ferreira‐Junior 2014 stated that there were missing data from one individual at two follow‐up points. In personal correspondence, Ferreira‐Junior 2014 indicated that these participants were included in the analysis and that the statistical software, SigmaPlot 11.0, calculated the missing data and automatically decreased the degree of freedom accordingly. The risk of attrition bias for both Hausswirth 2011 and Ferreira‐Junior 2014 was classified as unclear.
Selective reporting
None of the studies made any reference to a published protocol or trial registration. Therefore, bias from selective reporting of results was difficult to ascertain fully. Hausswirth 2011 did not report on measured outcomes (biomarkers of inflammation) within the trial report; however, these data were available from a secondary publication (Pournot 2011) based on communication with the authors. Additionally, Hausswirth 2011 only reported data up to 48 hours post exercise but upon contact, the authors provided unpublished data on muscle strength for follow‐up at 72 and 96 hours following exercise. We thus rated this study at high risk of reporting bias. All studies described outcomes and follow‐up times with corresponding results presented by intervention group. In all four studies, additional raw data were provided by corresponding authors in order to calculate percentage change from baseline scores and effect sizes. There was an absence of reporting of adverse events.
Other potential sources of bias
All studies provided in‐depth descriptions of the exercise protocols based on exercise type, duration, and intensity. Three studies stated that participants were asked to refrain from using the following specified co‐interventions for the duration of the study; medications/supplements (Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011), electrostimulation (Hausswirth 2011), cold water immersion (Hausswirth 2011), and stretching (Hausswirth 2011). Diet was controlled over the course of two trials (Fonda 2013; Hausswirth 2011) and exercise using a heart rate monitor in another (Hausswirth 2011). Ferreira‐Junior 2014 also stated that participants were not allowed to perform any vigorous physical activity or unaccustomed exercise during the experiment. Costello 2012 did not provide any details on co‐interventions in the published trial but the authors confirmed that participants were instructed to refrain from exercise and using co‐interventions over the course of the experiment. All four studies were rated as low risk of other bias.
Effects of interventions
See: Table 1
The four included studies were divided into two different groups based on comparison of the primary outcome. Within each comparison, results are presented in subcategories based on follow‐up time (1, 24, 48, 72, 96 and 120 hours).
Whole‐body cryotherapy (WBC) versus control (no intervention/rest)
Four studies made this comparison (Costello 2012; Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011).
Primary outcomes
Pain at rest (muscle soreness: VAS, various scales or scores; highest values = worst pain)
Three studies presented data on muscle soreness at rest based on various visual analogue scores. Results are presented at six follow‐up times (seeAnalysis 1.1). The pooled results using the fixed‐effect model showed significantly lower levels of soreness in the WBC group at 1 hour (SMD ‐0.77, 95% CI ‐1.42 to ‐0.12; 2 trials); 24 hours (SMD ‐0.57, 95% CI ‐1.12 to ‐ 0.03; 3 trials) and 48 hours (SMD ‐0.58, 95% CI ‐1.12 to ‐0.04; 3 trials). However, there was significant heterogeneity in the analyses at 24 and 72 hours and when applying the random‐effects model, the significant findings in favour of WBC were not upheld: 24 hours (SMD ‐0.57, 95% CI ‐1.48 to 0.33); 48 hours (SMD ‐0.58, 95% CI ‐1.37 to 0.21); seeAnalysis 1.2, Figure 5). The results for remaining follow‐up times also showed no significant differences between groups: 72 hours (SMD ‐0.65, 95% CI ‐2.54 to 1.24; 2 trials); 96 hours (SMD ‐0.33, 95% CI ‐0.95 to 0.30; 2 trials) and 120 hours (SMD ‐0.32, 95 CI 1.16 to 0.52; 1 trial).
1.1. Analysis.
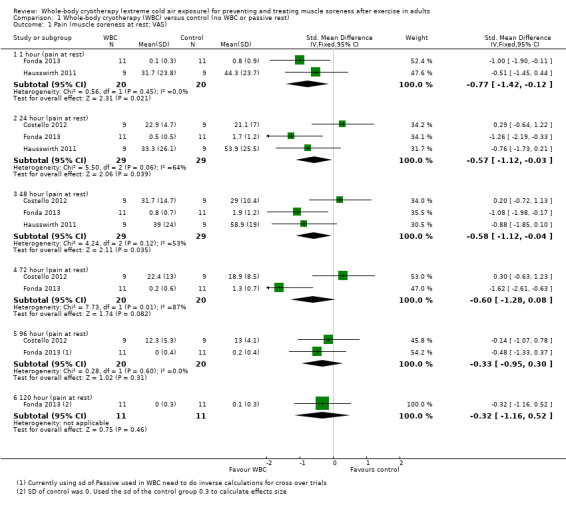
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 1 Pain (muscle soreness at rest: VAS).
1.2. Analysis.
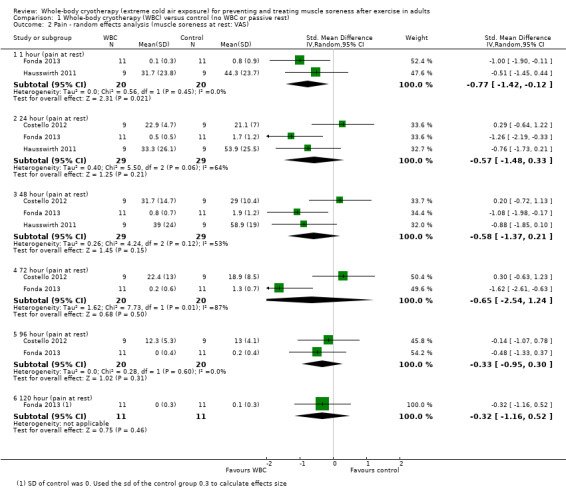
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 2 Pain ‐ random effects analysis (muscle soreness at rest: VAS).
5.
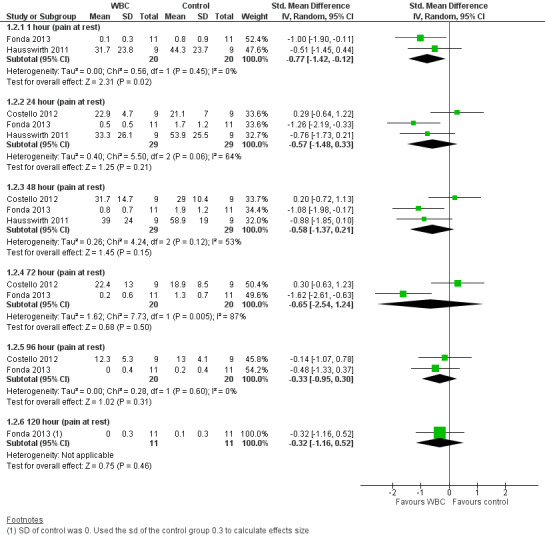
Forest plot of comparison: Whole‐body cryotherapy (WBC) versus passive (no WBC intervention/rest), outcome: 1.2 Pain ‐ random effects analysis (muscle soreness at rest: VAS).
In the 24, 48, 72 and 96 hours analyses, the cross‐over trials have been combined with Costello 2012, a parallel group trial. Of note, is that Costello 2012 found no difference between the two groups, whereas the two cross‐over trials found in favour of WBC. An exploratory subgroup analysis by study design at 24 hours follow‐up illustrates this observation, with a highly statistically significant test for subgroup differences (Chi² = 4.98, df = 1 (P = 0.03), I² = 79.9%; seeAnalysis 1.3).
1.3. Analysis.
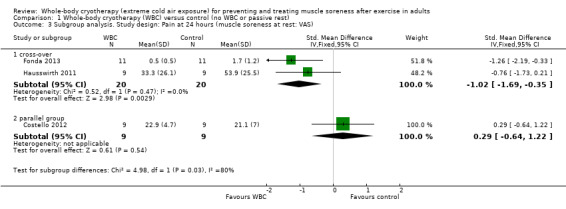
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 3 Subgroup analysis. Study design: Pain at 24 hours (muscle soreness at rest: VAS).
Pain on movement (muscle soreness: VAS, different scales; highest values = worst pain)
Two studies presented data on muscle soreness during subsequent exercise based on various visual analogue scores (Ferreira‐Junior 2014; Fonda 2013). Pooled SMD results are presented for six follow‐up times (seeAnalysis 1.4). Though the pooled results for follow‐ups at 1, 24, 48, 72 and 96 hours were in favour of WBC, the 95% CIs clipped the line of no effect except at 24 hours (SMD ‐0.66, 95% CI ‐1.25 to ‐0.07; 2 trials). Moreover, the results were moderately to substantially heterogenous for this follow‐up. The parallel group trial (Ferreira‐Junior 2014) showed no difference between the two groups at all five follow‐up times, whereas the cross‐over trial (Fonda 2013) found significant differences at the first four follow‐up times but not at 96 hours or 120 hours.
1.4. Analysis.
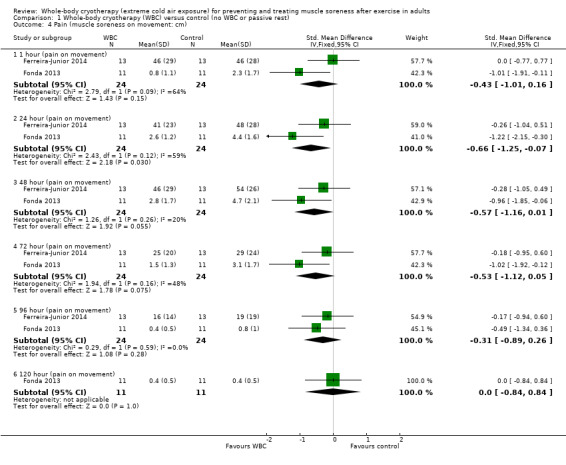
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 4 Pain (muscle soreness on movement: cm).
Subjective recovery
Tiredness (VAS; highest values = worst tiredness)
Hausswirth 2011, the only study to report subjective recovery, found no differences between groups in levels of tiredness at 1, 24 and 48 hour follow‐ups (seeAnalysis 1.5).
1.5. Analysis.
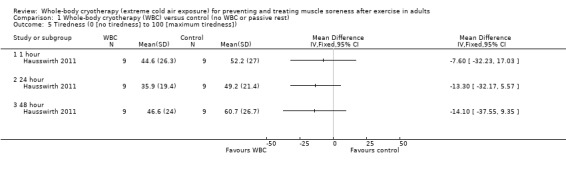
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 5 Tiredness (0 [no tiredness] to 100 [maximum tiredness]).
Well‐being (VAS; highest values = best well‐being)
Hausswirth 2011 reported that well‐being was increased at 24 hours (MD 21.70, 95% CI 2.20 to 41.20) following WBC but found no differences between groups at 1 and 48 hour follow‐ups (seeAnalysis 1.6).
1.6. Analysis.
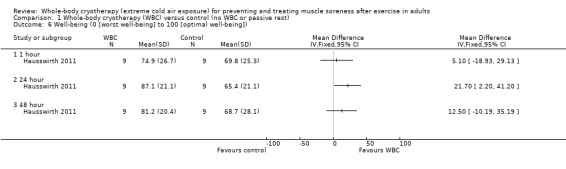
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 6 Well‐being (0 [worst well‐being] to 100 [optimal well‐being]).
Immediate or long‐term complications or adverse effects
None of the included studies recorded or reported adverse events or complications relating to the interventions. Moreover, no study examined the effects of the chronic use of WBC. It was unclear whether any study actively monitored specific adverse effects as part of its outcomes.
Secondary outcomes
Strength (% of baseline; highest values = increased strength)
Maximal strength, which was reported in all four studies, was assessed over a range of time points post intervention. In order to complete a meta‐analysis on strength as a percentage of baseline, raw data were sought from all four studies. Despite not being included in the report of the original study, Hausswirth 2011 provided additional raw data for follow‐ups at 72 and 96 hour post intervention and these data are subsequently included in the analysis of this review.
Although there was some variation in the measurement device and contraction type, all four studies tested lower limb strength. Pooled results over six follow‐ups are displayed in Analysis 1.7. At all follow‐up times the best estimates favoured the WBC group but the mean differences were very small and the 95% CI included the line of no effect at 1 hour (MD 1.76%, 95% CI ‐4.50 to 8.01%; 3 trials) and at 48 hours (MD 2.69%, 95% CI ‐2.21 to 7.60%; 4 trials) post exercise. The pooled results showed modest strength increases in the WBC group at 24 hours (MD 5.16%, 95% CI 0.66 to 9.65%; 3 trials), 72 hours (MD 6.92%, 95% CI 2.32 to 11.52%; 4 trials) and 96 hours (MD 6.73%, 95% CI 1.87 to 10.87%; 4 trials). Only Fonda 2013 reported results at 120 hours; also finding a significant difference in favour of WBC (MD 12.60%, 95% CI 0.48 to 24.72%).
1.7. Analysis.
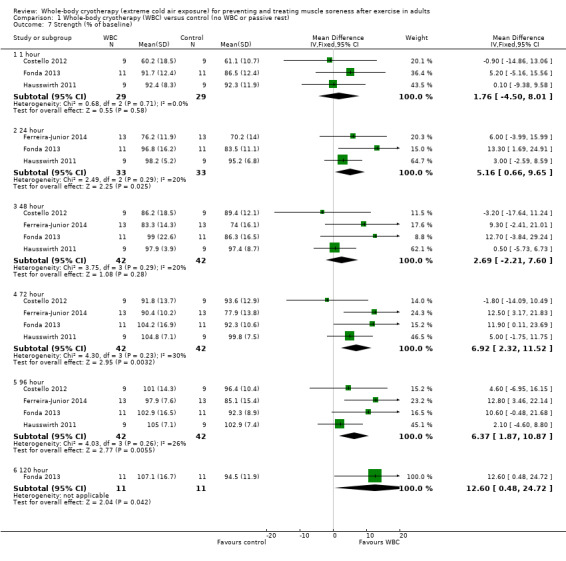
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 7 Strength (% of baseline).
The results of statistical tests for heterogeneity for the pooled data together with the visual inspection of the CIs showed little important heterogeneity in the results. An exploratory subgroup analysis by study design was conducted for the 72 hour follow‐up as this had the greatest heterogeneity (Chi² = 4.30, df = 3 (P = 0.23); I² = 30%). This found no statistically significant differences between the pooled results of cross‐over trials and parallel group trials (test for subgroup differences: Chi² = 0.01, df = 1 (P = 0.91); I² = 0%; seeAnalysis 1.8).
1.8. Analysis.
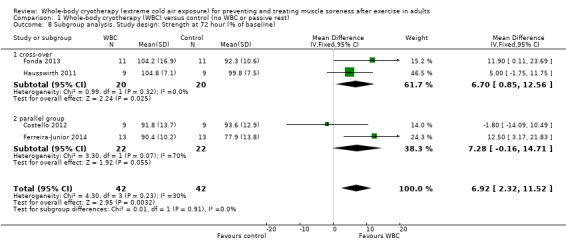
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 8 Subgroup analysis. Study design: Strength at 72 hour (% of baseline).
Objective tests of function and performance
Power (jumping performance: final outcome data in centimetres)
Fonda 2013 found no significant difference between interventions in squat and counter movement jump (CMJ) height at 1, 24, 48, 72, 96 and 120 hour follow‐ups (seeAnalysis 1.9).
1.9. Analysis.
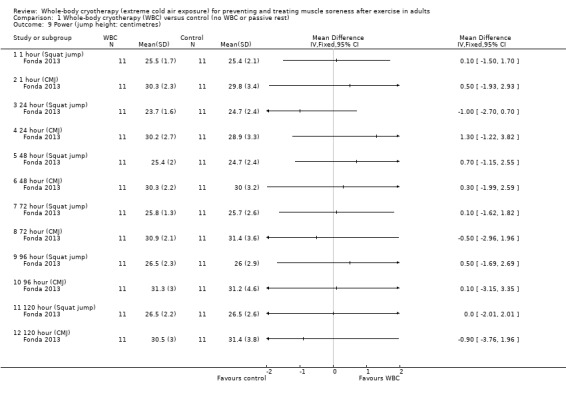
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 9 Power (jump height: centimetres).
Power (peak power output: % of baseline)
Costello 2012 found no significant difference between interventions in a 5x6 maximal cycle repeated‐sprint test at 48, 72 and 96 hour follow‐ups (seeAnalysis 1.10).
1.10. Analysis.
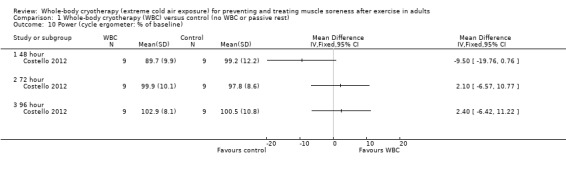
Comparison 1 Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest), Outcome 10 Power (cycle ergometer: % of baseline).
No other secondary outcomes were reported in these studies.
Subgroup and sensitivity analyses
Aside from exploratory subgroup analysis based on study design, there were insufficient data to carry out our planned subgroup analyses (seeSubgroup analysis and investigation of heterogeneity). Notably, only one study included a sample of female participants (Costello 2012), and there was significant heterogeneity in the dosage of WBC and the type of exercise performed in the studies. Additionally, we performed very limited sensitivity analysis; this being limited to the inspection of the results of using fixed‐effect versus random‐effects models for pooling.
Whole‐body cryotherapy versus far‐infrared therapy
One cross‐over trial involving nine participants compared WBC with far‐infrared therapy after a 48 minute simulated trial run (Hausswirth 2011).
Primary outcomes
Pain at rest (muscle soreness: VAS: 100 = worst pain)
Although lower levels of muscle soreness were reported after the WBC intervention at 1 hour follow‐up (MD ‐26.60 units, 95% CI ‐46.25 to ‐6.95; 100‐point VAS, 0 ‘no pain’ to 100 ‘maximum pain’), no differences between the interventions were observed at 24 and 48 hours (seeAnalysis 2.1).
2.1. Analysis.
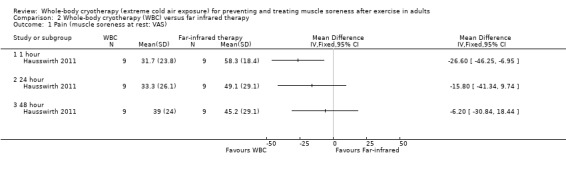
Comparison 2 Whole‐body cryotherapy (WBC) versus far infrared therapy, Outcome 1 Pain (muscle soreness at rest: VAS).
Subjective recovery (tiredness: VAS; 100 = worst tiredness)
Participants reported lower levels of tiredness following WBC at 1 and 24 hour follow‐ups (seeAnalysis 2.2). The results at 48 hours follow‐up still favoured WBC but to a lesser extent and the 95% CI crossed the line of no effect.
2.2. Analysis.
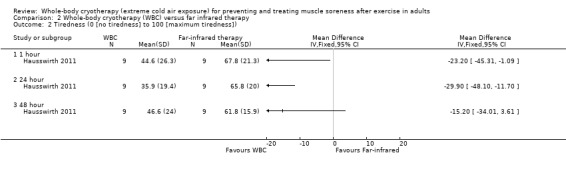
Comparison 2 Whole‐body cryotherapy (WBC) versus far infrared therapy, Outcome 2 Tiredness (0 [no tiredness] to 100 [maximum tiredness]).
Subjective recovery (well‐being: VAS; highest values = best well‐being)
No significant differences between WBC and far‐infrared therapy interventions were observed in well‐being at 1, 24 and 48 hour follow‐ups (see Analysis 2.3).
2.3. Analysis.
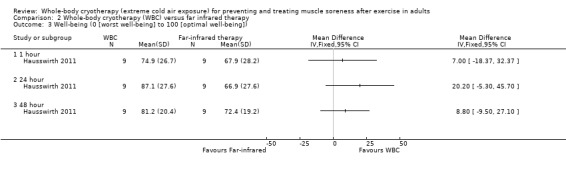
Comparison 2 Whole‐body cryotherapy (WBC) versus far infrared therapy, Outcome 3 Well‐being (0 [worst well‐being] to 100 [optimal well‐being]).
Immediate or long‐term complications or adverse effects
Hausswirth 2011 did not record or report adverse events or complications relating to the interventions.
Secondary outcomes
Strength (% of baseline)
Knee extensors’ isometric strength was slightly lower after the WBC intervention at 48 hours (MD ‐4.50%, 95% CI ‐8.34 to ‐0.66; seeAnalysis 2.4). No other significant differences were observed at the 4 other follow‐up points (1, 24, 72, and 96 hours). Notably, strength was slightly greater (> 100%) than baseline in 6 of the 10 results presented in Analysis 2.4.
2.4. Analysis.
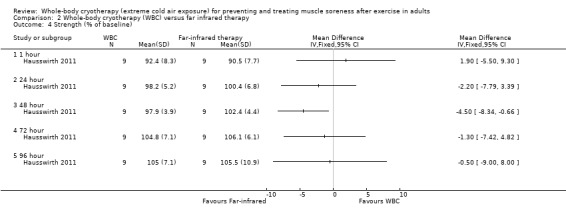
Comparison 2 Whole‐body cryotherapy (WBC) versus far infrared therapy, Outcome 4 Strength (% of baseline).
Objective tests of function and performance
Hausswirth 2011 did not record or report any other secondary outcome measures.
Discussion
Summary of main results
This review examined the effectiveness of whole‐body cryotherapy (WBC) for preventing and treating muscle soreness after exercise. Four small laboratory‐based randomised controlled trials, reporting on a total of 64 physically‐active adults (60 male; 4 female; mean age 23 years), were included. The trials were clinically and methodologically heterogeneous with considerable variation such as in: study design (two were parallel group studies and two were cross‐over studies); the type and application of WBC, including modality (whole chamber versus cryo‐cabin), timing (WBC undertaken immediately after exercise or 24 hours after exercise), and the temperature and frequency of WBC; and the type of exercise (simulation trial run/drop jumps versus 100 eccentric contractions).
The included studies made two comparisons: WBC versus control (rest or no WBC), tested in four studies; and WBC versus far‐infrared therapy, also tested in one study.The results for the primary outcomes of muscle soreness (pain at rest at 1, 24, 48 and 72 hours), subjective recovery (tiredness at 24 hours; well‐being at 24 hours) and adverse events are summarised here. The very poor quality of the available evidence for both comparisons means that we are very uncertain about these results.
Whole‐body cryotherapy (WBC) versus control (passive rest or no WBC intervention)
A summary of the evidence available for the primary outcomes for this comparison is presented in Table 1. Pooled standardised mean difference (SMD) results for muscle soreness (pain at rest) favoured WBC after delayed‐onset muscle soreness (DOMS)‐inducing exercise at all four follow‐ups: 1 hour (20 participants, 2 cross‐over studies); 24 and 48 hours (38 participants, 2 cross‐over studies, 1 parallel group study); 72 hours (29 participants, 1 cross‐over study, 1 parallel group study). However, the 95% confidence intervals (CIs) also included either no between‐group differences or a benefit in favour of the control group. The trials were heterogeneous, including in terms of the 'control group' where that of the two cross‐over studies was passive rest and that of the parallel group study was standing in the chamber with the temperature set at 15°C. There was statistical heterogeneity, also shown by the statistically significant results of the subgroup analysis by study design, where the data from the cross‐over studies were in favour of WBC but those from the parallel group study showed no difference between the two groups. One small cross‐over trial found no difference in tiredness but better well‐being after WBC at 24 hours post exercise. There was no report and probably no monitoring of adverse events.
WBC versus far‐infrared therapy
One small cross‐over trial involving 9 well‐trained runners provided very low quality evidence of lower levels of muscle soreness after WBC at 1 hour follow‐up, but not at 24 hours or 48 hours. The same trial found no difference in well‐being but less tiredness after WBC at 24 hours post exercise. There was no report and probably no monitoring of adverse events.
Overall completeness and applicability of evidence
We included four laboratory‐based studies with a total of 64 participants; but substantially fewer participants were available for pooling in several primary and secondary outcomes. The majority of participants were young (mean ages between 20.2 and 31.8 years); we suspect that this probably reflects the current WBC user population. However, only four (6.25%) were female. This finding tallies with our findings of low participation by females in research relating to other recovery interventions (Costello 2014c). It is possible that the results may not be applicable to females. For example, a recent study demonstrated that skin temperature following WBC depends upon anthropometric variables and sex, with females and individuals with a higher adiposity cooling more (Hammond 2014). It has also been demonstrated that the severity of muscle damage, and subsequent levels of inflammation and muscle soreness, is related to the gender of the participants (Costello 2014c; Enns 2010) as well as to the exercise performed (Paulsen 2012).
Only two comparisons were tested by the included studies. Notably, these studies did not allow a comprehensive review of the relative effectiveness of different methods of WBC in comparison with other interventions (e.g. cold water immersion) in treating DOMS post exercise or in elite athletes.
There was also no evidence on different types and timings of WBC. Of particular note on timing is that three studies (Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011) both attempted to ‘prevent’ DOMS by treating participants immediately after WBC, while Costello 2012 attempted to ‘treat’ soreness by utilising WBC at 24‐hour post exercise when inflammation and DOMS are suggested to peak.
The clinical relevance of any differences in DOMS may be dependent on several factors such as the time of the outcome, the capacity for natural recovery after exercise, and the time and costs associated with treatment (Bennett 2005; Bleakley 2012; Herbert 2011). Bleakley 2012 has suggested that a 13% to 22% difference in muscle soreness would be important for athletes, particularly those in an elite sporting environment. In the current review, a reduction in muscle soreness of between ˜7% to ˜20% was observed in three studies (Ferreira‐Junior 2014; Fonda 2013; Hausswirth 2011), demonstrating a potential positive effect after WBC at 1, 24 and 48 hour follow‐ups. This suggests that if WBC is utilised immediately after exercise, reductions in DOMS may be clinically relevant. Due to the limitations of the current evidence base, further research is required to support these findings.
Increases in muscle strength that exceed baseline (> 100%) occurred at several follow‐up times in Fonda 2013 and Hausswirth 2011. Such increases are unlikely to be observed in a highly‐trained group of athletes and, aside from statistical variation, may reflect the training status (‘physically active’) of the participants.
Although we could not find any reports of adverse events, given the absence of active surveillance these findings do not preclude their existence (Figure 1). Recently, Selfe 2014, examining the optimal duration of WBC exposure in 14 professional rugby league players, reported a case of mild superficial skin burn bilaterally on a mid‐portion of the anterior thigh in one athlete. The investigators described the skin damage as consisting of “erythema and minor blistering which appeared in a horizontal strip approximately 2 cm high and 10 cm wide the day following WBC”. The athlete was a Samoan player with an intolerance to ice packs who did not disclose his cold intolerance to the study team or personnel. It is well established that cold injuries are more prevalent in individuals of African descent compared with their Caucasian counterparts (Golden 2014; Imray 2009). This greater sensitivity to cold may be explained by a more intense protracted vasoconstriction in the peripheries during cold exposure (Iampietro 1959; Maley 2014). However, as the studies included in this review did not undertake active surveillance for predefined adverse events, the short‐ and long‐term safety of WBC remains unknown.
As with most recovery interventions, trained or elite athletes are most likely to use WBC on a regular basis. Although DOMS is most prevalent in novice athletes, we acknowledge that it may also occur in elite sport: e.g. at the beginning of the season (when returning to training following a period of reduced activity), after injury or after the introduction of a new movement or training method, especially if eccentric in nature. WBC has also been advocated in a clinical and rehabilitative setting as a way to reduce pain and inflammation (symptoms also associated with DOMS); however, our findings are less applicable to these settings.
Quality of the evidence
On the basis of GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria, the quality of evidence for the primary outcomes, muscle soreness and subjective recovery, was classified as ‘very low’ across all follow‐up times. The reasons for downgrading the evidence were study limitations (especially lack of blinding) resulting in a high risk of bias, imprecision reflecting the very small sample sizes, and, where pooling was possible, inconsistency reflecting substantial heterogeneity. The reasons for downgrading for individual outcomes for the WBC versus control comparison are detailed in Table 1. Although there were no complications or side effects reported within any of the individual studies, it was unclear whether any study actively monitored specific adverse effects. For similar reasons, the quality of evidence was also judged as 'very low' for all other outcomes including strength. Reflecting serious study limitations and serious imprecision, the evidence for the second comparison, WBC versus far‐infrared therapy, was classified as 'very low'.
There is a high degree of inter‐individual differences in the level and duration of muscle soreness experienced after exercise. Cross‐over designs are therefore popular in this area of research, with two such studies included in this review (Fonda 2013; Hausswirth 2011). The two other studies in the review employed a parallel group design (Costello 2012; Ferreira‐Junior 2014). Bleakley 2012 and Bleakley 2014 have previously described how cross‐over designs can risk certain carry‐over effects between exercise and treatment periods, which are not present in parallel group designs. Insufficient recovery from the first exercise bout is a likely carry‐over effect, particularly when studies induce DOMS on an untrained population. Secondly, during subsequent exercise bouts patients may experience reduced levels of muscle soreness or muscle damage. This repeated bout effect has been demonstrated to last for several months in humans (Howatson 2008; McHugh 1999; McHugh 2003; Nosaka 2005; Nosaka 1995). The two cross‐over studies in this review used a time frame of 3 weeks (Hausswirth 2011) and 10 weeks (Fonda 2013) between treatment periods. However, it is still possible that the timeframe used by Hausswirth 2011 may be appropriate as the trialists used well‐trained individuals completing familiar running exercises, who were likely to recover faster.
Potential biases in the review process
In this review, we undertook an extensive search of electronic databases and grey literature sources. Although we think this is likely to have identified all relevant published studies, it is possible that some unpublished studies have been missed and we cannot, in addition, rule out publication bias. To the best of our knowledge, the first randomised controlled trial conducted in this area was accepted for publication in December 2010 (Costello 2012). This appears to be the first study which sought to examine the effectiveness of WBC as a post exercise recovery intervention to reduce DOMS. We anticipate that the effectiveness of this intervention on treating or preventing DOMS post exercise is likely to be the focus of more research in the near future. We have not, however, identified any ongoing trials.
One potential source of bias is the post‐hoc exclusion of trials not reporting our primary outcomes (seeTypes of outcome measures). However, none of the excluded studies were aimed at the treatment of DOMS.
As none of the included studies had a registered protocol, bias from selective reporting of results was difficult to fully ascertain. However, we made a concerted attempt to retrieve missing summary and raw data, and were able to contact all of the authors.
Two of the included studies used a randomised cross‐over design. However, we were unable to perform any paired analysis and data were analysed as if these studies used a parallel group design. Cross‐over studies were also combined with parallel group trials in the same meta‐analysis. Although this approach gives rise to bias through unit of analysis error, this is expected to be conservative as cross‐over studies analysed in this way tend to be under‐weighted (Deeks 2011). Other Cochrane reviews (Bleakley 2012; Herbert 2011) have used a similar approach when assessing interventions to ameliorate DOMS. Inspection of the findings for muscle soreness of the two cross‐over studies, and our exploratory subgroup analysis based on study design, showed that cross‐over designs had a more positive effect on the primary outcome (muscle soreness at rest), compared with the parallel group trial reporting these data (Costello 2012). The reasons for this are not clear and the sample size is inadequate to speculate. Of note, however, is that a similar finding applied in Bleakley 2012. We suggest that parallel studies represent the better methodological design for future research in this area.
Agreements and disagreements with other studies or reviews
A recent review, conducted by several authors of the current review, that includes a more general perspective has also noted the limited evidence base supporting the use of WBC in an athletic or rehabilitative setting (Bleakley 2014). Bleakley 2014 included 10 controlled trials, of which 3 randomised controlled trials (RCTs) appear in this review (Costello 2012; Fonda 2013; Hausswirth 2011). It did not include data from the latest published RCT (Ferreira‐Junior 2014) included in the current review. Bleakley 2014 demonstrated that WBC induces tissue‐temperature reductions that are comparable to, or less significant than, traditional forms of cryotherapy such as cold water immersion and ice pack application. Although WBC was reported to have a positive influence on inflammatory mediators, antioxidant capacity, and autonomic function during sporting recovery, the findings were based on weak evidence from controlled studies (Bleakley 2014). Bleakley 2014 concluded that there is some weak evidence that WBC improves the perception of recovery and soreness after exercise but that this does not translate into enhanced functional recovery or performance. None of the 10 trials reported adverse events associated with WBC; but as found in our review, none of the studies appeared to undertake active surveillance of predefined adverse events.
Of the three other Cochrane reviews examining the effectiveness of cold water immersion, stretching, and hyperbaric oxygen therapy for treating muscle soreness after exercise, only that for cold water immersion found some evidence of benefit (Bleakley 2012). Bleakley 2012 found some evidence that cold water immersion reduces delayed onset muscle soreness after exercise compared with passive interventions involving rest or no intervention; however, there was insufficient evidence to draw conclusions on other outcomes or for other comparisons in this review. Herbert 2011 concluded that available evidence suggests that muscle stretching does not produce clinically important reductions in DOMS in healthy adults. Bennett 2005 also found little evidence to support the use of hyperbaric oxygen therapy to reduce DOMS after exercise, but also noted some evidence that this intervention may increase interim pain. The finding of benefit from cold water immersion, which is a cheaper intervention than WBC, provides an important context in which to view the results of our review.
Authors' conclusions
Implications for practice.
There is insufficient evidence from randomised controlled trials to determine whether whole‐body cryotherapy (WBC) reduces self‐reported muscle soreness, or improves subjective recovery, after exercise compared with passive rest or no WBC in physically‐active young adult males. There is no evidence on adverse events nor or on the use of this intervention in females or elite athletes. There is insufficient evidence to draw any conclusions on the relative effects of WBC versus far‐infrared therapy, and no evidence to inform on the relative effects of WBC versus other active interventions such as cold water immersion, or on the best protocol for WBC.
Given the limitations of the evidence, the safety concerns relating to any or repeated exposure to extreme temperatures, and the self‐limiting nature of most forms of muscle soreness resulting from exercise, there is currently insufficient evidence to support the use of WBC after exercise.
Implications for research.
Based on the apparent increasing use of whole‐body cryotherapy (WBC) in elite and recreational sport, and the costs associated with the WBC, there is an urgent need for high‐quality, well‐reported research in the area. It is recommended that future studies should: incorporate a randomised controlled parallel group design with adequate sequence generation and allocation concealment; use appropriate sample sizes with power to detect expected differences; and undertake active surveillance of predefined adverse events. Future studies comparing the effects of WBC with other commonly used recovery interventions for which there is evidence of effectiveness, such as for cold water immersion, is also warranted. Of note is the identification of an unpublished trial comparing the effects of three different formats of cold water immersion (single exposure at 5°C; single exposure at 15°C and multiple exposures at 10°C), WBC at 110°C and passive recovery (control group) on DOMS that has recently been completed, and is likely to be included in subsequent updates of this review. Trials including females and elite athletes in this area are warranted. Should WBC be found to be effective, research on the best protocols for WBC is needed; this might aim to establish, for instance, whether there is a specific time frame after exercise when this intervention might be most effective. Also for consideration is research, in particular evaluating safety issues, assessing the effects of long‐term or chronic use of WBC.
Acknowledgements
We are grateful to Nigel Hanchard, Helen Handoll, Cathie Sherrington and Matthew Wright for helpful feedback on drafts of the review and protocol. We would also like to thank Joanne Elliott for her help with the search strategy and Lindsey Elstub for her help during the editorial process. We would also like to acknowledge Daniel Francis for his assistance with the logic model.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (Wiley Online Library)
#1 MeSH descriptor: [Cryotherapy] this term only (475) #2 MeSH descriptor: [Hypothermia, Induced] this term only (753) #3 cryotherapy:ti,ab,kw (Word variations have been searched) (1027) #4 cryostimulation:ti,ab,kw (Word variations have been searched) (2) #5 cooling:ti,ab,kw (Word variations have been searched) (1720) #6 ((cold or cool*) near/3 (air or treat* or chamber*)):ti,ab,kw (Word variations have been searched) (726) #7 "liquid nitrogen":ti,ab,kw (Word variations have been searched) (142) #8 low near/2 temp*:ti,ab,kw (Word variations have been searched) (427) #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 (4060) #10 MeSH descriptor: [Exercise] explode all trees (14383) #11 MeSH descriptor: [Sports] this term only (669) #12 MeSH descriptor: [Muscle, Skeletal] this term only (5028) #13 MeSH descriptor: [Athletic Injuries] this term only (521) #14 MeSH descriptor: [Soft Tissue Injuries] this term only (71) #15 MeSH descriptor: [Creatine Kinase] explode all trees (1254) #16 MeSH descriptor: [Physical Exertion] this term only (3338) #17 MeSH descriptor: [Muscle Fatigue] this term only (596) #18 MeSH descriptor: [Muscle Cramp] this term only (138) #19 MeSH descriptor: [Spasm] this term only (181) #20 MeSH descriptor: [Muscle Rigidity] this term only (59) #21 MeSH descriptor: [Sprains and Strains] this term only (295) #22 MeSH descriptor: [Muscle Weakness] this term only (272) #23 sore* near/3 musc*:ti,ab,kw (Word variations have been searched) (472) #24 DOMS:ti,ab,kw (Word variations have been searched) (135) #25 (exercise induced and (muscle* near/2 (damage* or injur*))):ti,ab,kw (Word variations have been searched) (250) #26 MeSH descriptor: [Lactic Acid] this term only (1730) #27 lactate* or lactic:ti,ab,kw (Word variations have been searched) (7071) #28# 10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 (28127) #29 #9 and #28 Publication Year from 2013 to 2015 (388) [trials]
MEDLINE (Ovid Online)
1 Cryotherapy/ (4011) 2 Hypothermia, Induced/ (17249) 3 cryotherapy.tw. (5716) 4 cryostimulation.tw. (45) 5 cooling.tw. (28287) 6 ((cold or cool*) adj3 (air or treat* or chamber*)).tw. (5673) 7 "liquid nitrogen".tw. (6876) 8 (low adj2 temp*).tw. (42977) 9 or/1‐8 (101089) 10 Exercise/ (71646) 11 Sports/ (24259) 12 Muscle, Skeletal/ (110110) 13 Athletic Injuries/ (21484) 14 Soft Tissue Injuries/ (3943) 15 exp Creatine Kinase/ (24183) 16 Physical Exertion/ (53739) 17 Muscle Fatigue/ (6144) 18 Muscle Cramp/ or Spasm/ or Muscle Rigidity/ or "Sprains and Strains"/ or Muscle Weakness/ (19715) 19 (sore$ adj3 musc$).tw. (1339) 20 DOMS.tw. (452) 21 (exercise induced and (muscle$ adj2 (damage$ or injur$))).tw. (722) 22 Lactic Acid/ (33460) 23 (lactate$ or lactic).tw. (115241) 24 or/10‐23 (416548) 25 9 and 24 (3169) 26 Randomized controlled trial.pt. (406278) 27 Controlled clinical trial.pt. (91297) 28 randomized.ab. (329655) 29 placebo.ab. (166774) 30 Clinical trials as topic/ (177484) 31 randomly.ab. (237727) 32 trial.ti. (145097) 33 26 or 27 or 28 or 29 or 30 or 31 or 32 (987830) 34 exp Animals/ not Humans/ (4084521) 35 33 not 34 (911579) 36 25 and 35 (332)
EMBASE (Ovid Online)
1 Cryotherapy/ (13557) 2 Induced Hypothermia/ (11090) 3 cryotherapy.tw. (7578) 4 cryostimulation.tw. (67) 5 cooling.tw. (31796) 6 ((cold or cool*) adj3 (air or treat* or chamber*)).tw. (6950) 7 "liquid nitrogen".tw. (8759) 8 (low adj2 temp*).tw. (39013) 9 or/1‐8 (105759) 10 Exercise/ (202759) 11 exp Sport/ (113046) 12 Skeletal Muscle/ (88670) 13 Sport Injury/ (25404) 14 Sport Injury/ (25404) 15 Creatine Kinase/ (37855) 16 Muscle Fatigue/ or Muscle Spasm/ or Muscle Cramp/ or Muscle Weakness/ or Muscle Rigidity/ (66767) 17 (sore* adj3 musc*).tw. (1530) 18 DOMS.tw. (515) 19 (exercise induced and (muscle* adj2 (damage* or injur*))).tw. (777) 20 Lactic Acid/ (51949) 21 (lactate* or lactic).tw. (136672) 22 or/10‐21 (613290) 23 9 and 22 (4416) 24 exp Randomized Controlled Trial/ or exp Single Blind Procedure/ or exp Double Blind Procedure/ or Crossover Procedure/ (431724) 25 (random* or RCT or placebo or allocat* or crossover* or 'cross over' or trial or (doubl* adj1 blind*) or (singl* adj1 blind*)).ti,ab. (1434026) 26 24 or 25 (1511947) 27 (exp Animal/ or animal.hw. or Nonhuman/) not (exp Human/ or Human Cell/ or (human or humans).ti.) (5673507) 28 26 not 27 (1331832) 29 23 and 28 (463)
CINAHL (Ebsco)
S1 (MH "Cryotherapy") (1,636) S2 (MH "Hypothermia, Induced") (2,854) S3 TX cryotherapy (2,028) S4 TX cryostimulation (15) S5 TX cooling (1,823) S6 TX ((cold or cool*) n3 (air or treat* or chamber*)) (615) S7 TX "liquid nitrogen" (147) S8 TX low n2 temp* (507) S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 (6,985) S10 (MH "Exercise") (31,154) S11 (MH "Recovery, Exercise") (1,708) S12 (MH "Muscle, Skeletal") (16,524) S13 (MH "Athletic Injuries+") (15,373) S14 (MH "Creatine Kinase+") (2,481) S15 (MH "Muscle Fatigue") (2,041) S16 (MH "Muscle Cramp") (458) S17 (MH "Muscle Weakness") (2,705) S18 (MH "Sprains and Strains") (1,851) S19 sore* n3 musc* (658) S20 DOMS (175) S21 TX (exercise induced and (muscle* n2 (damage* or injur*))) (293) S22 (MH "Lactic Acid") (1,769) S23 TX lactate* or lactic (9,377) S24 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 (78,164) S25 S9 AND S24 (505) S26 (MH "Clinical Trials+") (190,046) S27 (MH "Evaluation Research+") (21,423) S28 (MH "Comparative Studies") (81,240) S29 (MH "Crossover Design") (13,149) S30 PT Clinical Trial (78,277) S31 (MH "Random Assignment") (39,485) S32 S26 or S27 or S28 or S29 or S30 or S31 (298,918) S33 TX ((clinical or controlled or comparative or placebo or prospective or randomi?ed) and (trial or study)) (547,042) S34 TX (random* and (allocat* or allot* or assign* or basis* or divid* or order*)) (71,634) S35 TX ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)) (798,149) S36 TX ( crossover* or 'cross over' ) or TX cross n1 over (16,303) S37 TX ((allocat* or allot* or assign* or divid*) and (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)) (89,578) S38 S33 or S34 or S35 or S36 or S37 (1,239,776) S39 S32 or S38 (1,311,825) S40 S25 AND S39 (286)
British Nursing Index (ProQuest)
((ti(cryotherapy OR cryostimulation OR cooling) OR ab(cryotherapy OR cryostimulation OR cooling)) OR ((cold OR cool*) NEAR/3 (air OR treat* OR chamber*)) OR "liquid nitrogen" OR (low NEAR/2 tempo*)) AND (SU.EXACT.EXPLODE("Physical Fitness") OR SU.EXACT.EXPLODE("Sports Medicine") OR (exercise OR sport* OR muscle* OR "creatine kinase" OR cramp OR spasm OR rigidity OR sprain OR strain OR domes OR "lactic acid")) (5)
PEDro
1. Abstract and title: cryotherapy Method: clinical trial (70)
2. Abstract and title: cold air Method: clinical trial (17)
WHO International Clinical Trials Registry Platform
1. cryotherapy AND sore* OR cryotherapy AND pain OR cryotherapy AND recovery OR cryotherapy AND fatigue OR cryotherapy AND damage* OR cryotherapy AND injur* (38)
2. cold AND muscle (25)
Current Controlled Trials
(cryotherapy OR cryostimulation OR cold OR cool*) AND (soreness OR pain OR recovery OR fatigue OR damage OR injury) AND muscle (32)
(cryotherapy OR cryostimulation OR cold OR cool*) AND (soreness OR pain OR recovery OR fatigue OR damage OR injury) AND exercise (38)
Data and analyses
Comparison 1. Whole‐body cryotherapy (WBC) versus control (no WBC or passive rest).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (muscle soreness at rest: VAS) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 1 hour (pain at rest) | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.42, ‐0.12] |
| 1.2 24 hour (pain at rest) | 3 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.12, ‐0.03] |
| 1.3 48 hour (pain at rest) | 3 | 58 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.12, ‐0.04] |
| 1.4 72 hour (pain at rest) | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.28, 0.08] |
| 1.5 96 hour (pain at rest) | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.95, 0.30] |
| 1.6 120 hour (pain at rest) | 1 | 22 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐1.16, 0.52] |
| 2 Pain ‐ random effects analysis (muscle soreness at rest: VAS) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 1 hour (pain at rest) | 2 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.42, ‐0.12] |
| 2.2 24 hour (pain at rest) | 3 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.48, 0.33] |
| 2.3 48 hour (pain at rest) | 3 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.37, 0.21] |
| 2.4 72 hour (pain at rest) | 2 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.54, 1.24] |
| 2.5 96 hour (pain at rest) | 2 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.95, 0.30] |
| 2.6 120 hour (pain at rest) | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐1.16, 0.52] |
| 3 Subgroup analysis. Study design: Pain at 24 hours (muscle soreness at rest: VAS) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 cross‐over | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.69, ‐0.35] |
| 3.2 parallel group | 1 | 18 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.64, 1.22] |
| 4 Pain (muscle soreness on movement: cm) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 1 hour (pain on movement) | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.01, 0.16] |
| 4.2 24 hour (pain on movement) | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.25, ‐0.07] |
| 4.3 48 hour (pain on movement) | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.16, 0.01] |
| 4.4 72 hour (pain on movement) | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.12, 0.05] |
| 4.5 96 hour (pain on movement) | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.89, 0.26] |
| 4.6 120 hour (pain on movement) | 1 | 22 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.84, 0.84] |
| 5 Tiredness (0 [no tiredness] to 100 [maximum tiredness]) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 1 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 24 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 48 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Well‐being (0 [worst well‐being] to 100 [optimal well‐being]) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 1 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 24 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 48 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Strength (% of baseline) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 1 hour | 3 | 58 | Mean Difference (IV, Fixed, 95% CI) | 1.76 [‐4.50, 8.01] |
| 7.2 24 hour | 3 | 66 | Mean Difference (IV, Fixed, 95% CI) | 5.16 [0.66, 9.65] |
| 7.3 48 hour | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | 2.69 [‐2.21, 7.60] |
| 7.4 72 hour | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | 6.92 [2.32, 11.52] |
| 7.5 96 hour | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | 6.37 [1.87, 10.87] |
| 7.6 120 hour | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 12.60 [0.48, 24.72] |
| 8 Subgroup analysis. Study design: Strength at 72 hour (% of baseline) | 4 | 84 | Mean Difference (IV, Fixed, 95% CI) | 6.92 [2.32, 11.52] |
| 8.1 cross‐over | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [0.85, 12.56] |
| 8.2 parallel group | 2 | 44 | Mean Difference (IV, Fixed, 95% CI) | 7.28 [‐0.16, 14.71] |
| 9 Power (jump height: centimetres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 1 hour (Squat jump) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 1 hour (CMJ) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 24 hour (Squat jump) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 24 hour (CMJ) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 48 hour (Squat jump) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 48 hour (CMJ) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.7 72 hour (Squat jump) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.8 72 hour (CMJ) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.9 96 hour (Squat jump) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.10 96 hour (CMJ) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.11 120 hour (Squat jump) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.12 120 hour (CMJ) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Power (cycle ergometer: % of baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 48 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 72 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 96 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Whole‐body cryotherapy (WBC) versus far infrared therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (muscle soreness at rest: VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 1 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 24 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 48 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Tiredness (0 [no tiredness] to 100 [maximum tiredness]) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 1 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 24 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 48 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Well‐being (0 [worst well‐being] to 100 [optimal well‐being]) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 1 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 24 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 48 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Strength (% of baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 1 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 24 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 48 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 72 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 96 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Costello 2012.
| Methods | Randomised controlled trial (parallel group) | |
| Participants | Setting: laboratory, Ireland n = 18 (2 groups of 9) healthy physically‐active university students (14 males), mean age 21.2 (SD 2.1). Inclusion/exclusion criteria Participants were excluded if they had a history of lower limb injuries in the past 12 months, ear or vestibular conditions or if they had any contradiction to cryotherapy including Raynaud’s disease. They were also excluded if they were not between the ages of 18 and 40, not physically active for an average of 3 days a week or not comfortable with being blind‐folded during testing. |
|
| Interventions |
Exercise 100 maximal eccentric contractions (5 reps, 20 sets) of the left knee extensors completed on an isokinetic dynamometer set at an angular velocity of 1.57 rad/s. There was a minimum rest period of 1 minute between sets. Intervention Treatment occurred 24 hours post‐exercise and again 2 hours later (26 hours post‐exercise).
|
|
| Outcomes |
Outcomes included in this review Primary outcomes Muscle soreness Self‐palpation at 8 sites corresponding to the proximal and distal regions of the knee extensor and flexors (10‐point visual analogue scale: 1 "normal, no pain" to 10 "very, very sore"). Secondary outcomes Force Maximal voluntary isometric (MVIC) knee extensor force (modified Tornvall chair, 90° flexion, % of baseline) Power Peak power output (PPO) during repeated‐sprint cycling (cycle ergometer, % of baseline) Outcomes not reported in this trial Subjective recovery (return to previous activity) Immediate or long‐term implications Objective test of function or performance Outcomes included in the trial but excluded from this review Tympanic temperature (°C) Joint position sense (absolute, relative and variable error, °) (Follow‐up: MVIC 1, 24, 48, 72 and 96 hours post‐exercise; soreness 24, 48, 72 and 96 hours post‐exercise; PPO 24, 48 and 72 hours post‐exercise) |
|
| Source of Funding | Manuscript states "The authors would like to acknowledge ... Shannon Cryotherapy Clinic, Ennis, Co. Clare, Ireland, for the use of their cryotherapy chamber.'"(p. 197) | |
| Notes | Raw data on muscle soreness, power and force provided by Costello J in March 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were randomly assigned, using a random number generator" (p. 191) |
| Allocation concealment (selection bias) | High risk | An open random allocation schedule was employed (personal correspondence) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Incomplete blinding. Treatment concealed until after exercise. Difficult to blind participant or personnel to treatment. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No details in manuscript. |
| Blinding of outcome assessment (detection bias) Self reported | High risk | Participant was aware of the treatment intervention, thus muscle soreness is likely to be biased. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs reported (confirmed with author). |
| Selective reporting (reporting bias) | Low risk | No published protocol available. Outcomes and follow‐ups stated in methods. Means and standard deviations (SD) presented by intervention group for all outcomes, at all follow‐ups. |
| Other bias | Low risk | Exercise protocol clearly described. "Subjects were also instructed to refrain from consuming alcohol or caffeine 24 h before testing commenced." (p. 191) Manuscript states "The authors would like to acknowledge ... Shannon Cryotherapy Clinic, Ennis, Co. Clare, Ireland, for the use of their cryotherapy chamber." (p. 197) |
Ferreira‐Junior 2014.
| Methods | Randomised controlled trial (parallel group) | |
| Participants | Setting: laboratory, Brazil n = 26 (2 groups of 13) healthy physically‐active male university students, mean age 20.2 (SD 2.5). Inclusion/exclusion criteria Participants were excluded if they had untreated arterial hypertension, cardiovascular and respiratory diseases, angina, peripheral artery occlusive disease, venous thrombosis, urinary tract diseases, severe anaemia, allergy to cold, tumour diseases, viral and bacterial infections, Raynaud’s syndrome, claustrophobia or convulsions. |
|
| Interventions |
Exercise 100 drop jumps (20 reps, 5 sets) from a 0.6 m box with 2 minute rest intervals between sets Intervention
|
|
| Outcomes |
Outcomes included in this review Primary outcomes Muscle soreness Knee extensor muscle strength soreness measured during a maximal isometric contraction of the right extensors (100 mm visual analogue scale: 0 "no soreness" to 100 "severe soreness"). Secondary outcomes Force Maximal voluntary isometric knee extensor peak torque (dynamometer, 60° flexion, % of baseline) Outcomes not reported in this trial Subjective recovery (return to previous activity) Immediate or long‐term implications Objective test of function or performance Outcomes included in the trial but excluded from this review Muscle thickness (Follow‐up: 1, 24, 48, 72 and 96 hours post exercise) |
|
| Source of Funding | None. The study was partially funded by CAPES ‐ Brazil. | |
| Notes | Raw data on muscle soreness and force provided by Ferreira‐Junior J in March 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were randomly assigned, using a random number table" (manuscript via personal correspondence) |
| Allocation concealment (selection bias) | High risk | "we didn't conceal the allocation of the individuals" (personal correspondence) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details in manuscript. Difficult to blind participant or personnel to treatment. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No details in manuscript. |
| Blinding of outcome assessment (detection bias) Self reported | High risk | Participant was aware of the treatment intervention, thus muscle soreness is likely to be biased. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "one missing data in the control group (one subject at 48 h) and another in the WBC group (one subject at 96 h). The statistical software that we used, SigmaPlot 11.0, calculates the missing data and automatically decreases the degree of freedom accordingly the number of missing data" (personal correspondence). |
| Selective reporting (reporting bias) | Low risk | No published protocol available. Outcomes and follow‐ups stated in methods. Means and SD presented by intervention group for all outcomes, at all follow‐ups. |
| Other bias | Low risk | Exercise protocol clearly described. Manuscript states "Volunteers were not allowed to perform any vigorous physical activities or unaccustomed exercise during the experiment period. They were also instructed not to take medications or supplements during the study." (p. 3) |
Fonda 2013.
| Methods | Randomised cross‐over (10 weeks between interventions) | |
| Participants | Setting: laboratory, Slovenia n = 11 healthy young male adults, mean age 26.9 years (SD 3.8) Inclusion/exclusion criteria Inclusion criteria were that the participants were familiar with plyometric exercise, but they did not perform this type of exercise for at least 3 months prior to the study, were not injured or receiving any medications in the last 9 months, were omnivore, and were within normal baseline levels for biochemical markers. |
|
| Interventions |
Exercise 15 min warm‐up. Five sets of 10 drop jumps from a 0.6 m high box. Followed by 5 x 10 bilateral leg curls (75% of concentric 1RM in a prone position. Finally, participants completed 10 eccentric leg curls. The series of drop jump and leg curl exercises were separated by 1 minute breaks. Intervention Treatment occurred ˜1 hour after exercise and at the same time of the day for the next 6 days.
|
|
| Outcomes |
Outcomes included in this review Primary outcomes Pain Perceived pain at rest and during a squat (10 cm visual analogue scale, 0 “no pain” to 10 “severe pain”) Secondary outcomes Force Maximal voluntary torque production knee flexion (dynamometer, 60° flexion, % of baseline). Peak average torque in a 1 second time interval. Rate of torque development in the first 200 ms. Power Squat jump (force plate, height {m}, max force {N/kg}, max power {W/kg}, work {J}, push off duration {s}) Counter movement jump (force plate, height {m}, max force {N/kg}, max power {W/kg}, work {J}, push off duration {s}, counter movement duration {s}) Outcomes not reported in this trial Subjective recovery (return to previous activity) Immediate or long‐term implications Outcomes included in the trial but excluded from this review Aspartate aminotransferase (IU/L) Creatine kinase (IU/L) Lactate dehydrogenase (IU/L) (Follow‐up: 1, 24, 48, 72, 96 and 120 hours post exercise) |
|
| Source of Funding | Manuscript states "The authors would like to thank Bostjan Benedetti (Krios Ltd, Slovenia) and Butan Plin Ltd for providing the cryo‐cabin and the liquid nitrogen, respectively." (p. 8) | |
| Notes | Raw data on muscle soreness, torque, rate of torque development and jump height provided by Fonda B in March 2014. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned into two groups and exposed to a crossover study design. Randomization has been carried out with a custom‐written algorithm in LabVIEW (National Instruments, Austin, Texas, USA)." (p. 2) |
| Allocation concealment (selection bias) | Unclear risk | No details in manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details in manuscript. Difficult to blind participant or personnel to treatment. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No details in manuscript. |
| Blinding of outcome assessment (detection bias) Self reported | High risk | Participant was aware of the treatment intervention, thus muscle soreness is likely to be biased. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed all trials (personal correspondence). |
| Selective reporting (reporting bias) | Low risk | No published protocol available. Outcomes and follow‐ups stated in methods. Means and SD presented by intervention group for all outcomes, at all follow‐ups. |
| Other bias | Low risk | Exercise protocol clearly described. Manuscript states “Between the sessions, the subjects were not allowed to participate in any kind of vigorous physical activity.” (p. 3) Manuscript states "they were not permitted to drink alcohol or take any medications or dietary supplements." (p. 2) Manuscript states “The influence of repeated bout effect was evident in pain measures, squat jump start power, and squat jump work. However, due to small sample size, it is difficult to draw conclusions that in other parameters the repeated bout effect was not present." (p. 7) Manuscript states "The authors would like to thank Bostjan Benedetti (Krios Ltd, Slovenia) and Butan Plin Ltd for providing the cryo‐cabin and the liquid nitrogen, respectively." (p. 8) |
Hausswirth 2011.
| Methods | Randomised cross‐over (3 weeks between interventions) | |
| Participants | Setting: laboratory, France n = 9 well‐trained [male: (personal correspondence)] runners, mean age 31.8 (SD 6.5) Note: 11 participants were recruited but 2 were excluded because of incomplete outcomes (personal correspondence). Inclusion/exclusion criteria Minimal performance of 38 minutes in a 10 km running race and completing a minimum of 4 training sessions per week. Presented no contradictions to WBC or far‐infrared radiation therapy. |
|
| Interventions |
Exercise Simulated trial run lasting 48 minutes. The protocol was divided into 5 blocks; 6 minutes flat (0% gradient), 3 minutes uphill (10% gradient) and 3 minutes downhill (‐15% gradient). Intervention Treatment occurred following the trial run (within the first hour) and again at 24 and 48 hours [participants were also treated at 72 and 96 hours; personal correspondence]
|
|
| Outcomes |
Outcomes included in this review Primary outcomes Pain Perceived pain (web interface 100 point visual analogue scale, 0 “no pain” to 100 “maximum possible”) Secondary outcomes Force Maximal voluntary isometric knee extensor force (isokinetic ergometer, 70° flexion, % of baseline) Outcomes not reported in this trial Subjective recovery (return to previous activity) Immediate or long term implications Objective test of function or performance Outcomes included in the trial but excluded from this review Plasma creatine kinase (% of baseline) Perceived tiredness (web interface 100 point visual analogue scale, 0 “no tiredness” to 100 “maximum possible”) Perceived well‐being (web interface 100 point visual analogue scale) (Follow‐up: post, 1, 24 and 48 hours post exercise) [Follow‐up data at 72 and 96 hours for knee extensor force were also provided following personal correspondence] |
|
| Source of Funding | The authors had no funding to report. | |
| Notes | Raw data on muscle soreness and force provided by Bieuzen F in March 2014. The authors confirmed that 11 participants were recruited but 2 were excluded in the final analysis due to missing outcomes at various time points (personal correspondence with Bieuzen F in March 2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned…" (p. 3). [drawn from a hat: (personal correspondence)] |
| Allocation concealment (selection bias) | Unclear risk | No details in manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details in manuscript. Difficult to blind participant or personnel to treatment. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No details in manuscript. |
| Blinding of outcome assessment (detection bias) Self reported | High risk | Participant was aware of the treatment intervention, thus muscle soreness is likely to be biased. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "There are missing data. Two subjects were excluded because they did not complete all the tests (these two participants did not complete the strength tests even if they have completed all of the other tests). But all presented data were complete." (personal correspondence) |
| Selective reporting (reporting bias) | High risk | No published protocol available. Outcomes and follow‐ups stated in methods. Means and SD presented by intervention group for all outcomes, at all follow‐ups except at 72 and 96 hours. |
| Other bias | Low risk | Exercise protocol clearly described. Training loads between treatments were monitored and controlled. Manuscript states “to control the influence of other recovery modalities, nutritional recommendations were sent to runners during all the experiment and they were asked to respect identical menus during the three days preceding and succeeding the running sessions” (p. 3) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Banfi 2008 | This was a cohort study involving 10 male athletes |
| Brand 2010 | No WBC intervention |
| Chen 1996 | Unclear if intervention is WBC (from abstract). Although unable to source full text or to contact the authors, it is unlikely to be WBC as the intervention lasted for 15 minutes |
| Ferreira‐Junior 2014c | No primary outcomes |
| Gailiera 2013 | No primary outcomes |
| Guilhem 2013 | Treatment not WBC (‐30°C air pulse cryotherapy) |
| Hausswirth 2013 | No primary outcomes |
| Lapier 1995 | No WBC intervention |
| Schaal 2013 | No outcomes relating to DOMS. This study incorporated active recovery in a swimming pool with WBC |
| Schaal 2015a | No outcomes relating to DOMS |
| Selfe 2014 | No outcomes relating to DOMS |
| Smolander 2004 | No primary outcomes |
| Szpotowicz‐Czech 2014 | No primary outcomes |
| Utsunomiya 2010 | No WBC intervention |
| Venter 2014 | No WBC intervention |
| Wechsler 2014 | No primary outcomes (conference abstract only) |
| Ziemann 2012 | No primary outcomes |
Characteristics of studies awaiting assessment [ordered by study ID]
DRKS00006038.
| Methods | Randomised controlled trial. Masking: open (not blinded) |
| Participants | 24 female football players Inclusion criteria required the participants to be in good overall health, possess health insurance, provide written consent for participation in the study, and not miss any of the training sessions held during the study's duration. Age 19 to 26 years Exclusion criteria included contraindication to being subjected to extremely low temperatures and presence of any form of injury to the lower limbs |
| Interventions | WBC: 10 cryo‐stimulation treatments held everyday for a period of 10 days; 1 minute in an atrium of the cryo‐chamber at the temperature –60°C and then, it was continued for 3 minutes in the main cryogenic chamber at the temperature –130°C Control: no treatment |
| Outcomes | Quadriceps muscles strength was tested using the Biodex Multi‐Joint Testing and Rehabilitation System 3 PRO. Static, dynamic and endurance tests were performed. Examinations of quadriceps peak torque were conducted on the first day (Pre 1) before the experimental group had their first cryotherapy treatment and on the tenth day (Post 2) after the last cryotherapy treatment was completed. The isokinetic strength tests were performed by the experimental group both before (A) and after (B) the first cryotherapy treatment and before (C) and after (D) the tenth cryotherapy treatment. The same measurements at the same time intervals were performed in the control group |
| Notes | This completed trial, conducted in Poland, was retrospectively registered on 1 April 2014. It is not clear, but seems unlikely, that muscle soreness was assessed. We have not found a full report of this trial. |
Krüger 2015.
| Methods | Randomised controlled trial |
| Participants | 11 healthy, non‐smoking, endurance‐trained male athletes |
| Interventions | Running ramp test followed by high intensity intermittent running, followed by WBC/control and another ramp test. WBC: 3 minutes of WBC at ‐110°C Control: slow walking for 3 minutes at 22°C |
| Outcomes | Perceived physical fitness
Perceived physical energy
Perceived physical flexibility
Perceived physical health
Perceived sensation of recovery
Readiness to train [Follow‐up: post treatment and post second ramp test] |
| Notes | Currently awaiting more information regarding methodology and outcome measures. |
NCT02341612.
| Methods | Randomised controlled trial |
| Participants | 65, 18 to 30 year old male college students |
| Interventions | Exercise protocol for exercise‐induced muscle damage is unclear WBC: 3 minutes of WBC at ‐110°C Cold water immersion (CWI): 5°C single exposure for 20 minutes CWI: 15°C single exposure for 20 minutes CWI: 10°C multiple exposures [immediately, 24, 48 and 72 hours post exercise] for 20 minutes Control: passive rest |
| Outcomes | Muscle soreness: knee extensors (visual analogue scale) Power: counter‐movement vertical jump [Follow‐up: 0, 24, 48, 72, 96 and 168 post exercise] |
| Notes | This trial, conducted in Brazil, has a registered protocol. The trial registration document indicates that the trial was completed by January 2015. We have not found a full report of this trial. |
Ziemann 2014.
| Methods | Randomised controlled trial |
| Participants | 18 healthy and physically‐active, college‐aged men |
| Interventions | 30‐minute step up/down work‐out. WBC: 3 minutes of WBC at ‐110°C twice a day for 5 days (n = 9) Control: passive rest (n = 9) |
| Outcomes | Muscle soreness Ratings of perceived soreness (DOMS) [Follow‐up: 24 hours post exercise] |
| Notes | Currently awaiting more information regarding methodology |
Differences between protocol and review
Since publication of the protocol (Costello 2013) the following changes were made:
We now state explicitly that cross‐over trials are included.
We now state explicitly that trials that did not report any of our prespecified primary outcomes are excluded.
As the studies differed in the outcome measures used, the standard mean difference was calculated.
For objective outcome measures related to strength, we report percentage change from baseline, instead of our planned analysis which was to use final scores in preference.
We have included study design (parallel versus cross‐over) as a subgroup analysis.
Contributions of authors
JTC, GMM, IBS, PRAB, and CMB developed the research idea, and wrote the original protocol. Study selection was carried out by JTC and GMM. Data extraction and interpretation were carried out by JTC, GMM, IBS, PRAB, and CMB. Risk of bias assessment and data analysis were carried by JTC, FB, GMM and PRAB. All authors commented on drafts and approved the final version. JTC wrote the final review and is the guarantor.
Declarations of interest
Joseph Costello (Costello 2012a) and François Bieuzen (Hausswirth 2011; Pournot 2011) co‐authored studies that are included in this review. Decisions on inclusion of these studies, the risk of bias assessment and data extraction of these studies were undertaken by other review authors (IBS, PRAB, CB and GMM), who had no involvement in the studies.
New
References
References to studies included in this review
Costello 2012 {published data only}
- Costello J. Personal communication March 2014.
- Costello JT, Algar LA, Donnelly AE. Effects of whole‐body cryotherapy (−110°C) on proprioception and indices of muscle damage. Scandinavian Journal of Medicine and Science in Sports 2012;22(2):190‐8. [DOI: 10.1111/j.1600-0838.2011.01292.x] [DOI] [PubMed] [Google Scholar]
Ferreira‐Junior 2014 {published data only}
- Ferreira‐Junior J. Personal communication March 2014.
- Ferreira‐Junior J, Bottaro M, Vieira A, Sigueira A, Vieira C, Quaglioyyi D, et al. One session of whole body cryotherapy (‐110°C) improves muscle damage recovery. Scandinavian Journal of Medicine and Science in Sports 2014 Dec 30 [Epub ahead of print]. [DOI: 10.1111/sms.12353] [DOI] [PubMed] [Google Scholar]
- Vieira A, Ferreira‐Junior J, Vieira C, Sigueira A, Cleto V, Durigan J, et al. One session of whole‐body cryotherapy (‐110 °C) Improves recovery from exercise‐induced muscle damage. ECSS 19th Annual Congress of the European College of Sport Science; 2014 Jul 2‐5; Amsterdam. 2014.
Fonda 2013 {published data only}
- Fonda B. Personal communication March 2014.
- Fonda B, Sarabon N. Effects of whole‐body cryotherapy on recovery after hamstring damaging exercise: a crossover study. Scandinavian Journal of Medicine and Science in Sports 2013;23(5):e270‐8. [DOI: 10.1111/sms.12074] [DOI] [PubMed] [Google Scholar]
Hausswirth 2011 {published data only}
- Bieuzen F. Personal communication March 2014.
- Hausswirth C, Louis J, Bieuzen F, Pournot H, Fournier J, Filliard JR. Effects of whole‐body cryotherapy vs. far‐infrared vs. passive modalities on recovery from exercise‐induced muscle damage in highly‐trained runners. PLoS ONE 2011;6(12):e27749. [DOI: 10.1371/journal.pone.0027749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournot H, Bieuzen F, Louis J, Fillard JR, Barbiche E, Hausswirth C. Time‐course of changes in inflammatory response after whole‐body cryotherapy multi exposures following severe exercise. PLoS ONE 2011;7(7):e22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Banfi 2008 {published data only}
- Banffi G, Krajewska M, Melegati G, Patacchini M. Effects of whole‐body cryotherapy on haematological values in athletes. British Journal of Sports Medicine 2008;42(10):858. [PubMed] [Google Scholar]
- Banfi G, Melegati G, Barassi A, Dogliotti G, Melzi d’Eril G, Dugue B, et al. Effects of whole‐body cryotherapy on serum mediators of inflammation serum muscle enzymes in athletes. Journal of Thermal Biology 2009;34:55‐9. [Google Scholar]
- Banfi G, Melegati G, Barassi A, d’Eril GM. Effects of the whole‐body cryotherapy on NTproBNP, hsCRP and troponin I in athletes. Journal of Science and Medicine in Sport 2009;12(6):609‐10. [DOI] [PubMed] [Google Scholar]
Brand 2010 {published data only}
- Brand ME, Ortega C. Efficacy of a cooling technique in athletes with SCI. American Spinal Injury Association 36th Annual Scientific Meeting; 2010 May 26‐28; Nashville (TN). 2010.
Chen 1996 {published data only}
- Chen TC, Hsieh SS. The effects of stretching and cryotherapy on delayed onset muscle soreness. Medicine and Science in Sports and Exercise 1996;28(5 Suppl):S181. [Google Scholar]
Ferreira‐Junior 2014c {published data only}
- Ferreira‐Junior J, Bottaro M, Vieira C, Soares S, Vieira A, Cleto, et al. Effects of partial‐body cryotherapy (‐ 110°C) on muscle recovery between high‐intensity exercise bouts. International Journal of Sports Medicine 2014;35(14):1155‐60. [DOI] [PubMed] [Google Scholar]
Gailiera 2013 {published data only}
- Galliera E, Dogliotti G, Melegati G, Corsi Romanelli MM, Cabitza P, Banfi G. Bone remodelling biomarkers after whole body cryotherapy (WBC) in elite rugby players. Injury 2013;44(8):1117‐21. [DOI: 10.1016/j.injury.2012.08.057] [DOI] [PubMed] [Google Scholar]
Guilhem 2013 {published data only}
- Guilhem G, Hug F, Couturier A, Regnault S, Bournat L, Filliard JR, et al. Effects of air‐pulsed cryotherapy on neuromuscular recovery subsequent to exercise‐induced muscle damage. American Journal of Sports Medicine 2013;41(8):1942‐51. [DOI] [PubMed] [Google Scholar]
Hausswirth 2013 {published data only}
- Hausswirth C, Schaal K, Meur Y, Bieuzen F, Filliard J‐R, Volondat M, et al. Parasympathetic activity and blood catecholamine responses following a single partial‐body cryostimulation and a whole‐body cryostimulation. PLoS ONE 2013;8(8):e72658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lapier 1995 {published data only}
- Lapier TK, Sheely SL, Moore R, Hubert CL, Brower C, Barnhard ML, et al. The effects of massage or cryotherapy on isometric torque and perceived pain in muscles with contraction‐induced delayed onset muscle soreness. 12th International Congress World Confederation of Physical Therapy; 1995 Jun 25; Washington DC. 1995.
Schaal 2013 {published data only}
- Schaal K, Meur Y, Bieuzen F, Petit O, Hellard P, Toussaint JF, et al. Effect of recovery mode on postexercise vagal reactivation in elite synchronized swimmers. Applied Physiology, Nutrition, and Metabolism 2013;38(2):126‐33. [DOI: 10.1139/apnm-2012-0155] [DOI] [PubMed] [Google Scholar]
Schaal 2015a {published data only}
- Schaal K, LE MY, Louis J, Filliard JR, Hellard P, Casazza G, et al. Whole‐body cryostimulation limits overreaching in elite synchronized swimmers. Medicine and Science in Sports and Exercise 2015;47(7):1416‐25. [DOI] [PubMed] [Google Scholar]
Selfe 2014 {published data only}
- Selfe J, Alexander J, Costello JT, May K, Garratt N, Atkins S, et al. The effect of three different (‐135°C) whole body cryotherapy exposure durations on elite rugby league players. PLoS ONE 2014;9(1):e86420. [DOI: 10.1371/journal.pone.0086420] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smolander 2004 {published data only}
- Smolander J, Mikkelsson M, Oksa J, Westerlund T, Leppäluoto J, Huttunen P. Thermal sensation and comfort in women exposed repeatedly to whole‐body cryotherapy and winter swimming in ice‐cold water. Physiology and Behavior 2004;82:691‐5. [DOI] [PubMed] [Google Scholar]
Szpotowicz‐Czech 2014 {published data only}
- Szpotowicz‐Czech B, Szygula Z, Więcek M, Szymura J, Maciejczyk M, Czech P, et al. Effect of whole‐body cryostimulation on serum mediators of inflammation and serum muscle enzyme in healthy men. Medicine and Science in Sport and Exercise 2014;E42:2583. [Google Scholar]
Utsunomiya 2010 {published data only}
- Utsunomiya M, Nitta K, Sawagichi H, Yoshikawa A, Karasuno H, Morozumi K, et al. Changes in blood flow, temperature and muscle endurance in association with cryotherapy. Journal of Physical Therapy Science 2010;22:43‐9. [Google Scholar]
Venter 2014 {published data only}
- Venter R. Perceptions of team athletes on the importance of recovery modalities. European Journal of Sport Science 2014;14(1):S69‐S6. [DOI: 10.1080/17461391.2011.643924] [DOI] [PubMed] [Google Scholar]
Wechsler 2014 {published data only}
- Wechsler K, Jedlicka D, Marees M, Mester J. The effect of whole body cryotherapy on the regenerative capacity following an eccentric jump protocol on biochemical markers. ECSS 19th Annual Congress of the European College of Sport Science; 2014 Jul 2‐5; Amsterdam. 2014.
Ziemann 2012 {published data only}
- Ziemann E, Olek RA, Kujach S, Grzywacz T, Antosiewicz J, Garsztka T, et al. Five‐day whole‐body cryostimulation, blood inflammatory markers, and performance in high‐ranking professional tennis players. Journal of Athletic Training 2012;47(6):664‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
DRKS00006038 {published data only}
- Skrzek A, Podbielska H. The impact of whole body cryotherapy on quadriceps muscle strength in the training process of female football players. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00006038 (accessed 27 October 2014).
Krüger 2015 {published data only}
- Krüger M, Dittmar KH, Sperlich B, Mester J. Whole‐body cryotherapy's enhancement of acute recovery of running performance in well‐trained athletes. International Journal of Sports Physiology and Performance 2015;10(5):605‐12. [DOI] [PubMed] [Google Scholar]
NCT02341612 {published data only}
- NCT02341612. Cold therapy in the treatment of exercise‐induced muscle damage. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02341612 (accessed 7 August 2015).
Ziemann 2014 {published data only}
- Ziemann E, Olek RA, Grzywacz T, Kaczor JJ, Antosiewicz J, Skrobot W, et al. Whole‐body cryostimulation as an effective way of reducing exercise‐induced inflammation and blood cholesterol in young men. European Cytokine Network 2014;25(1):14‐23. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 2011
- Anderson LM, Petticrew M, Rehfuess E, Armstrong R, Ueffing E, Baker P, et al. Using logic models to capture complexity in systematic reviews. Research Synthesis 2011;2(1):33‐42. [DOI] [PubMed] [Google Scholar]
Bennett 2005
- Bennett MH, Best TM, Babul‐Wellar S, Taunton JE. Hyperbaric oxygen therapy for delayed onset muscle soreness and closed soft tissue injury. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004713.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bieuzen 2013
- Bieuzen F, Bleakley CM, Costello JT. Contrast water therapy and exercise induced muscle damage: a systematic review and meta‐analysis. PLoS ONE 2013;8(4):e62356. [DOI: 10.1371/journal.pone.0062356] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bleakley 2012
- Bleakley C, McDonough S, Gardner E, Baxter GD, Hopkins JT, Davison GW. Cold‐water immersion (cryotherapy) for preventing and treating muscle soreness after exercise. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008262.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bleakley 2014
- Bleakley CM, Bieuzen F, Davison GW, Costello JT. Whole‐body cryotherapy: empirical evidence and theoretical perspectives. Open Access Journal of Sports Medicine 2014;10(5):25‐36. [DOI: 10.2147/OAJSM.S41655] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byrne 2004
- Byrne C, Twist C, Eston R. Neuromuscular function after exercise‐induced muscle damage. Sports Medicine 2004;34(1):49‐69. [DOI] [PubMed] [Google Scholar]
Cheung 2003
- Cheung K, Hume P, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Medicine 2003;33(2):145‐64. [DOI] [PubMed] [Google Scholar]
Cochrane 2004
- Cochrane DJ. Alternating hot and cold water immersion for athlete recovery: a review. Physical Therapy in Sport 2004;5(1):26‐32. [Google Scholar]
Connolly 2003
- Connolly DA, Sayers SE, McHugh MP. Treatment and prevention of delayed onset muscle soreness. Journal of Strength and Conditioning Research 2003;17(1):197‐208. [DOI] [PubMed] [Google Scholar]
Costello 2012a
- Costello JT, Algar LA, Donnelly AE. Effects of whole body cryotherapy (−110°C) on proprioception and indices of muscle damage. Scandinavian Journal of Medicine and Science in Sports 2012;22(2):190‐8. [DOI] [PubMed] [Google Scholar]
Costello 2012b
- Costello JT, Culligan K, Selfe J, Donnelly AE. Muscle, skin and core temperature after −110°C cold air and 8°C water treatment. PLoS ONE 2012;7(11):e48190. [DOI: 10.1371/journal.pone.0048190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costello 2012c
- Costello JT, McInerney CD, Bleakley CM, Selfe J, Donnelly AE. The use of thermal imaging in assessing skin temperature following cryotherapy: a review. Journal of Thermal Biology 2012;37(2):103‐10. [Google Scholar]
Costello 2014a
- Costello JT, Donnelly AE, Karki A, Selfe J. Effects of whole body cryotherapy and cold water immersion on knee skin temperature. International Journal of Sports Medicine 2014;35(1):35‐40. [DOI: 10.1055/s-0033-1343410] [DOI] [PubMed] [Google Scholar]
Costello 2014b
- Costello JT. What do we know about recovery interventions used in the management of delayed‐onset muscle soreness?. OA Sports Medicine 2013;1(2):17. [Google Scholar]
Costello 2014c
Costello 2014d
- Costello JT, McNamara PM, O’Connell ML, Algar LA, Leahy MJ, Donnelly AE. Tissue viability imaging of skin microcirculation following exposure to whole body cryotherapy (‐110°C) and cold water immersion (8°C). Archives of Exercise in Health and Disease 2014;4(1):243‐50. [DOI: 10.5628/aehd.v4i1.152] [DOI] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Ebbeling 1989
- Ebbeling C, Clarkson P. Exercise‐induced muscle damage and adaptation. Sports Medicine 1989;7(4):207‐34. [DOI] [PubMed] [Google Scholar]
Enns 2010
- Enns D, Tiidus P. The influence of estrogen on skeletal muscle: sex matters. Sports Medicine 2010;40(1):41‐58. [DOI] [PubMed] [Google Scholar]
EPOC 2011
- EPOC. Worksheets for preparing a summary of findings using GRADE, 2011. http://epocoslo.cochrane.org/sites/epocoslo.cochrane.org/files/uploads/EPOC%20worksheets%20for%20preparing%20a%20summary%20of%20findings%20using%20GRADE_0.doc Vol. (accessed 23 May 2013).
Ferreira‐Junior 2014b
- Ferreira‐Junior J, Bottaro M, Loenneke J, Vieira A, Vieira C, Bemben M. Could whole‐body cryotherapy (below −100°C) improve muscle recovery from muscle damage?. Frontiers in Physiology 2014;5:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fonda 2014
- Fonda B, Nardi M, Sarabon N. Effects of whole‐body cryotherapy duration on thermal and cardio‐vascular response. Journal of Thermal Biology 2014;42:52‐5. [DOI] [PubMed] [Google Scholar]
Golden 2014
- Golden FS, Francis TJ, Gallimore D, Pethybridge R. Lessons from history: morbidity of cold injury in the Royal Marines during the Falklands Conflict of 1982. Extreme Physiology and Medicine 2013;2(1):23. [DOI: 10.1186/2046-7648-2-23] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammond 2014
- Hammond LE, Cuttell S, Nunley P, Meyler J. Anthropometric characteristics and sex influence magnitude of skin cooling following exposure to whole body cryotherapy. BioMed Research International 2014 Jun 29 [Epub ahead of print]. [DOI: 10.1155/2014/628724] [DOI] [PMC free article] [PubMed]
Herbert 2011
- Herbert RD, Noronha M, Kamper SJ. Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD004577.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirvonen 2006
- Hirvonen H, Mikkelsson M, Kautiainen H, Pohjolainen TH, Leirisalo‐Repo M. Effectiveness of different cryotherapies on pain and disease activity in active rheumatoid arthritis: a randomised single blinded controlled trial. Clinical and Experimental Rheumatology 2006;24(3):295‐301. [PubMed] [Google Scholar]
Howatson 2008
- Howatson G, Someren KA. The prevention and treatment of exercise induced muscle damage. Sports Medicine 2008;38(6):483‐503. [DOI] [PubMed] [Google Scholar]
Iampietro 1959
- Iampietro PF, Goldman RF, Buskirk ER, Bass DE. Responses of negro and white males to cold. Journal of Applied Physiology 1959;14:798‐800. [DOI] [PubMed] [Google Scholar]
Imray 2009
- Imray C, Grieve A, Dhillon S, Caudwell Xtreme Everest Research Group. Cold damage to the extremities: frostbite and non‐freezing cold injuries. Postgraduate Medical Journal 2009;85(1007):481‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lubkowska 2010
- Lubkowska A, Szygula Z, Klimek A, Torii M. Do sessions of cryostimulation have influence on white blood cell count, level of IL6 and total oxidative and antioxidative status in healthy men?. European Journal of Applied Physiology 2010;109(2):67‐72. [DOI] [PubMed] [Google Scholar]
Lubkowska 2011
- Lubkowska A, Szyguła Z, Chlubek D, Banfi G. The effect of prolonged whole‐body cryostimulation treatment with different amounts of sessions on chosen pro‐ and anti‐inflammatory cytokine levels in healthy men. Scandinavian Journal of Clinical and Laboratory Investigation 2011;71(5):419‐25. [DOI] [PubMed] [Google Scholar]
Lubkowska 2012
- Lubkowska A, Dołęgowska B, Szyguła Z. Whole‐body cryostimulation—potential beneficial treatment for improving antioxidant capacity in healthy men—significance of the number of sessions. PLoS ONE 2012;7(10):e46352. [DOI: 10.1371/journal.pone.0046352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maley 2014
McGinley 2009
- McGinley C, Shafat A, Donnelly AE. Does antioxidant vitamin supplementation protect against muscle damage?. Sports Medicine 2009;39(12):1011‐32. [DOI] [PubMed] [Google Scholar]
McHugh 1999
- McHugh MP, Connolly DAJ, Eston RG, Gleim GW. Exercise‐induced muscle damage and potential mechanisms for the repeated bout effect. Sports Medicine 1999;27:157‐70. [DOI] [PubMed] [Google Scholar]
McHugh 2003
- McHugh MP. Recent advances in the understanding of the repeated bout effect against muscle damage from a single bout of eccentric exercise. Scandinavian Journal of Medicine and Science in Sports 2003;13:88‐97. [DOI] [PubMed] [Google Scholar]
Metzger 2000
- Metzger D, Zwingmann C, Protz W, Jackel WH. Whole‐body cryotherapy in rehabilitation of patients with rheumatoid diseases—pilot study. Rehabilitation 2000;39(2):93‐100. [DOI] [PubMed] [Google Scholar]
Miller 2012
- Miller E, Markiewicz L, Saluk J, Majsterek I. Effect of short‐term cryostimulation on antioxidative status and its clinical applications in humans. European Journal of Applied Physiology 2012;112(5):1645‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minett 2015
- Minett GM, Costello JT. Specificity and context in post‐exercise recovery: it is not a one‐size‐fits‐all approach. Frontiers in Physiology 2015;6:130. [DOI: 10.3389/fphys.2015.00130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Newham 1987
- Newham DJ, Jones DA, Clarkson PM. Repeated high‐force eccentric exercise: effects on muscle pain and damage. Journal of Applied Physiology 1987;63(4):1381‐6. [DOI] [PubMed] [Google Scholar]
Nosaka 1995
- Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Medicine and Science in Sports an Exercise 1995;27:1263‐9. [PubMed] [Google Scholar]
Nosaka 2005
- Nosaka K, Newton MJ, Sacco P. Attenuation of protective effect against eccentric exercise‐induced muscle damage. Canadian Journal of Applied Physiology 2005;30(5):529‐42. [DOI] [PubMed] [Google Scholar]
Nédélec 2013
- Nédélec M, McCall A, Carling C, Legall F, Berthoin S, Dupont G. Recovery in soccer: part II—recovery strategies. Sports Medicine 2013;43(1):9‐22. [DOI] [PubMed] [Google Scholar]
Paulsen 2012
- Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise?. Exercise Immunology Review 2012;18:42‐97. [PubMed] [Google Scholar]
Pournot 2011
- Pournot H, Bieuzen F, Louis J, Fillard JR, Barbiche E, Hausswirth C. Time‐course of changes in inflammatory response after whole‐body cryotherapy multi exposures following severe exercise. PLoS ONE 2011;6(7):e22748. [DOI: 10.1371/journal.pone.0022748] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasartwuth 2006
- Prasartwuth O, Allen TJ, Butler JE, Gandevia SC, Taylor JL. Length‐dependent changes in voluntary activation, maximum voluntary torque and twitch responses after eccentric damage in humans. Journal of Physiology 2006;571(1):243‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Savic 2013
- Savic M, Fonda B, Sarabona N. Actual temperature during and thermal response after whole‐body cryotherapy in cryo‐cabin. Journal of Thermal Biology 2013;38(4):186‐91. [Google Scholar]
Saxton 1995
- Saxton JM, Clarkson PM, James R, Miles M, Westerfer M, Clark S, et al. Neuromuscular dysfunction following eccentric exercise. Medicine and Science in Sports and Exercise 1995;27(8):1185‐93. [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Wozniak 2007
- Wozniak A, Wozniak B, Drewa G, Mila‐Kierzenkowska C, Rakowski A. The effect of whole‐body cryostimulation on lysosomal enzyme activity in kayakers during training. European Journal of Applied Physiology 2007;100(2):137‐42. [DOI] [PubMed] [Google Scholar]
Zalewski 2014
- Zalewski P, Bitner A, Slomko J, Szrajda J, Klawe J, Tafil‐Klaweb M, et al. Whole‐body cryostimulation increases parasympathetic outflow and decreases core body temperature. Journal of Thermal Biology 2014;45:75‐80. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Costello 2013
- Costello JT, Baker PRA, Minett GM, Bieuzen F, Stewart IB, Bleakley C. Whole‐body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010789] [DOI] [PMC free article] [PubMed] [Google Scholar]


