Abstract
An anatomical understanding of the atrial myocardium is crucial for surgeons and interventionists who treat atrial arrhythmias. We reviewed the anatomy of the inter-nodal and intra-atrial conduction systems. The anterior inter-nodal route (#1) arises from the sinus node and runs through the ventral wall of the atrial chambers. The major branch of route #1 approaches the atrioventricular node from the anterior aspect. Other branches of route #1 are Bachmann’s bundle and a vestibular branch around the tricuspid valve. The middle inter-nodal route (#2) begins with a broad span of fibers at the sinus venarum and extends to the superior limbus of the oval fossa. The major branch of route #2 joins with the branch of route #1 at the anterior part of the atrioventricular node. The posterior inter-nodal route (#3) is at the terminal crest and gives rise to many branches at the pectinate muscles of the right atrium and then approaches the posterior atrioventricular node after joining with the vestibular branch of route #1. The branches of the left part of Bachmann’s bundle and the branches of the second inter-nodal route form a thin myocardial network at the posterior wall of the left atrium. These anatomical structures could be categorized into major routes and side branches. There are 9 or more anatomical circles in the atrial chambers that could be structural sites for macro re-entry. The implications of normal and abnormal structures of the myocardium for the pathogenesis and treatment of atrial arrhythmias are discussed.
Keywords: Cardiac conduction system, Cardiac arrhythmia, Bachmann’s bundle, Atrial flutter, Atrial fibrillation, Maze procedure, Radiofrequency ablation
Introduction
Atrial fibrillation and atrial flutter are common sustained arrhythmias in the adult and pediatric populations, and their diagnosis and treatment have been revolutionized in recent years [1-4]. Surgery (maze operation) or catheter-based interventions are used to treat these conditions, but recurrence is common [1,5-8]. The basic principle of interventional and surgical treatment of atrial arrhythmia is optimizing the intra-atrial conduction routes by physically damaging the relevant anatomical sites. Over-treatment harms cardiac function, whereas under-treatment leads to recurrent arrhythmia. Understanding the anatomy of the atrial conduction system is important for optimal diagnosis and treatment [9,10].
Atrial arrhythmias are also common in patients with structurally abnormal hearts. Examples include mitral stenosis with a dilated left atrium [11,12] and atrial septal defect with increased volume load on the right atrium [13-16]. Dilatation of the atrial chamber and secondary modifications of the myocardial architecture of atrial conduction are the mechanisms that induce and sustain abnormal rhythms in these hearts. Atrial arrhythmias are also practical concerns in older patients with surgically corrected cardiac diseases, particularly those due to congenital heart disease [3,17,18]. Carefully designing incision lines in cardiac surgery may minimize damage to the normal anatomical configuration of intra-atrial myocardium, and understanding the spatial layout of the postoperative scars enables better diagnosis and treatment.
In the classic study by James [19,20] and Sherf and James [21], the inter-nodal conduction routes are very well described, and we would like to express appreciation for his contribution and emphasize the importance of his original description. We also value the interpretation of those routes in this era of current therapeutic modalities. Our review illustrates the morphological anatomy of the conduction pathway in the atrial chambers (intra-atrial conduction) and conduction between the sinus node and the atrioventricular node (inter-nodal conduction). Some practical issues are raised regarding the potential value of surgical procedures or catheter intervention.
Terminology and cardiac specimens
Terminology
The cardiac conduction tissue is morphologically specialized tissue, including the sinus node, atrioventricular node, bundle of His, and their branches. The cardiac conduction system, in contrast, is a composite of the myocardial route of conduction by ordinary myocardial cells and conduction through specialized tissue. The myocardium as a component of the conduction system is of interest for our purposes, although it does not necessarily constitute morphologically specialized tissue.
Inter-nodal conduction in the heart refers to conduction between the sinus node and the atrioventricular node. Inter-atrial conduction is conduction between the right and left atrial chambers, and this term has been used to refer to Bachmann’s bundle and conduction channels at the Waterstone groove [22,23]. The concept of “inter-atrial” conduction is not used in this article because it would mislead readers that the right atrial myocardium and left atrial myocardium are electrically insulated. Instead, the myocardium of the left atrium and the right atrium is a single myocardial mass with a broad connection between the two. Intra-atrial conduction in this paper includes conduction within each of the right or left atrial chambers, as well as inter-nodal or inter-atrial conduction.
We use the term “route” to refer to the major pathway of conduction by ordinary myocardial cells. Other terms, such as “pathway” or “track,” may mean that conduction occurs through an exclusive or insulated road. Three routes are described in this paper; anterior (#1), middle (#2), and posterior (#3). Branches of the route are described by adding sub-numbers to the route, such as #1-1, #1-2, and #1-3. The right and left parts of Bachmann’s bundle are described as L-1, L-2, L-3, and R-1.
Conventional anatomical terms are used for locations in the heart. The sinus venarum is the area with a smooth inner wall between the superior and inferior caval veins and between the atrial septum and the terminal crest. The atrial septum, superior/inferior limbus, and oval fossa are used, as well as the coronary sinus, Eustachian valve, Thebesian valve, and Waterstone groove. The vestibules of the right and left atrial chambers are circular smooth-walled zones at the outlet of the atrial chambers just above the tricuspid and mitral valves.
The walls of the right and left atrial chambers facing the transverse pericardial sinus are described as the ventral wall or medial wall as appropriate.
The walls of the left atrium are described based on the anatomical orientation; the inferior wall is the part facing the diaphragm, the septal wall faces the atrial septum; the posterior wall is a square bordered by four pulmonary veins, and the lateral wall is around the left pulmonary veins.
The pulmonary veins are described as right upper/lower and left upper/lower veins and abbreviated as right upper pulmonary vein, right lower pulmonary vein, left upper pulmonary vein, and left upper pulmonary vein.
Study subjects
We used more than 50 human heart specimens from the heart specimen collection of the Heart Museum [24]. The hearts in this collection are anonymized human hearts from different institutions in Korea. A special collection from the National Yang Ming Chiao Tung University needs special mention, insofar as 9 “old” human heart specimens were used to visualize the muscular anatomy of the atrial wall. More than 30 years had passed after these “old” hearts were harvested for a cadaver anatomy laboratory for medical students; therefore, the epicardial fatty tissue was easily removed and the myocardial bundles were easily exposed [25].
We also analyzed 3-dimensional models of human hearts from computed tomograms. The MEDIP software (Medical IP Co. Ltd., Seoul, Korea) was used to make 3-dimensional models of these hearts. Cardiac cavity models from atrial fibrillation were used to illustrate the in-situ anatomy of atrial chambers and vascular connection. The cavity models were also used to illustrate the atrial myocardial architecture. Cardiac wall models were used to show the inner surface of the atrial chambers in situ. The cavity models were used to analyze the thickness of muscle layers.
Surgical and electrical anatomy of atrial chambers
Basic architecture of the atrial muscle
The architecture of the myocardium in the atrial wall could be analyzed in terms of its thickness and the direction of fibers. The atrial wall is thick at the terminal crest, anterior part of the atrial septum, and the left and right atrial wall at the transverse pericardial sinus (the Bachmann’s bundle area). The pectinate muscles are well-defined parallel bundles connecting the terminal crest and the thicker muscle at the vestibule of the tricuspid valve. The muscular layer at the left atrial wall around other pulmonary veins is thin, but thick fibrous endocardium covers these zones. Thicker muscle bundles are found in the left atrial wall at the anatomical extensions from Bachmann’s bundle and the posterior inter-nodal route, particularly at the junction between the left upper pulmonary vein and the left appendage (known as the lateral ridge). There is a thick band-like ring of myocardium at the vestibule of the left and right atrial chambers. The thin muscle of the left atrial wall fades out as the atrial wall continues to the pulmonary veins (i.e., the myocardial sleeve). The atrial wall was thinnest at the floor of the oval fossa and narrow spaces between pectinate muscles at the free wall of the right atrium.
The direction of muscle fibers was recognized by blunt dissection of the atrial wall after the removal of fatty tissue. It was found that the muscles run from the sinus node area to the atrioventricular junction. The terminal crest and Bachmann’s bundle are the 2 most prominent groups of muscles. The atrial septum also has grossly recognizable muscle bundles from the sinus node to the atrioventricular junction. The circular orientation of muscle fibers as mentioned above are recognized around the vestibule at the supra-mitral and supra-tricuspid area. These fibers merge into the atrioventricular node through 2 approaches: from the anterior and posterior aspects.
The thickness and direction of the muscular tissue could be interpreted along with our understanding of the conduction routes as follows. We classified the routes into inter-nodal and inter-atrial routes.
Inter-nodal routes
The first inter-nodal route (the anterior route, #1) is a broad span of myocardial fibers from the sinus node at the right atrial wall facing the aorta (the wall at the transverse pericardial sinus). After an initial short descent, the bundles branch into 3 parts (#1-1, #1-2, #1-3) (Fig. 1). One (#1-1) approaches the atrioventricular node at the shortest distance, and this branch unites with a branch of the second route (#2-1) at the atrial septum to contribute to the short pathway to the atrioventricular node, which is described as the “fast pathway” by electrophysiologists. The second (#1-2) branches to the right and left parts of Bachmann’s bundle. The third (#1-3) has a long course at the vestibule of the tricuspid valve, approaching the posterior end of the atrioventricular node after joining with the major part of the posterior route (#3-1) (Fig. 2). The second branch (#1-2) forms the right and left sub-branches of Bachmann’s bundle to the right and left atrial walls (R-1, L-1, L-2, L-3) (Fig. 1 for details). The right sub-branch (R-1) is a broad span of fibers to the edge of the appendages, attaching to the pectinate muscles. The upper part of the left sub-branch (L-1) supplies the posterior wall of the left atrium through the roof of the left atrium, running between the right and left upper pulmonary veins. The middle part of the left sub-branch (L-2) is at the thick muscular ridge at the lateral ridge between the left upper pulmonary vein and the left appendage. The lower part of the left sub-branch (L-3) runs along the anterior and left wall of the left atrium, only in the supra-mitral part (the vestibule).
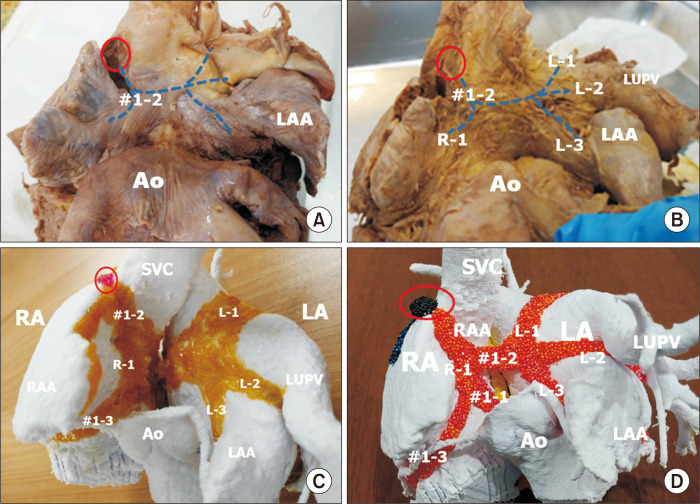
Fig. 1.
The major inter-nodal routes in the atrial wall facing the transverse pericardial sinus. These routes are broad bands or sheets, as shown in (C) and (D), although they are shown as lines in (A) and (B) to demonstrate the shape of the atrial wall. (A) The anterior wall of the right and left atrial chambers at the transverse pericardial sinus. The second branch of the anterior inter-nodal route (#1-2) arises from the sinus node (red circle) to form Bachmann’s bundle. (B) The atrial wall after removal of the epicardium shows Bachmann’s bundle running to the right (R-1) and to the left (L-1, L-2, L-3). Bachmann’s bundle to the left atrium (LA) has 3 sub-branches. Sub-branch L-1 is at the superior wall between the right and left upper pulmonary veins (LUPVs). The second sub-branch (L-2) is at the lateral ridge between the LUPV and the left atrial appendage (LAA). The third sub-branch (L-3) is the myocardium along the vestibule of the LA. (C) Painting the muscular bundles on 3-dimensional–printed atrial chambers at the same view as A and B. The atrial wall in the transverse pericardial sinus is seen from the anterior aspect. The second branch of the first inter-nodal route (#1-2) arises from the sinus node (red circle) and descends along the anterior wall of the right atrium (RA) to form Bachmann’s bundle to the right (R-1) and left atrial chambers. The left Bachmann’s bundle branches to L-1, L-2, and L-3. Figs. 1A–C are modified from the authors’ previous publication [25]. Ao, aorta; RAA, right atrial appendage; SVC, superior caval vein.
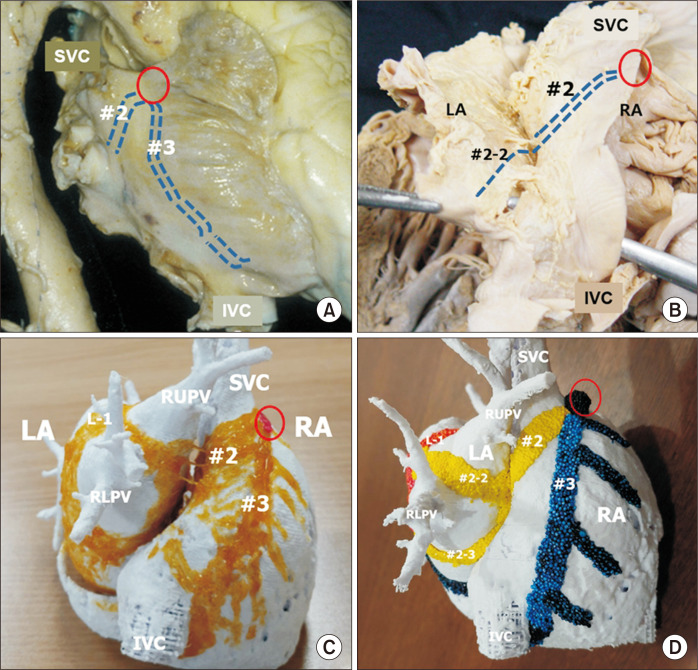
Fig. 2.
The major inter-nodal routes at the right lateral aspect of the atrial wall. These routes are broad bands or sheets, as shown in (C) and (D), although they are shown as lines in (A) and (B) to demonstrate the shape of the atrial wall. (A) Right lateral view of the heart showing external indicators of the middle and posterior inter-nodal routes from the sinus node (red circle). The middle route is a broad myocardial bundle at the sinus venarum. (B) After dissection of the Waterstone groove, the inter-atrial muscular connection is noted at the superior limbus of the oval fossa. The main stem of the middle inter-nodal route gives rise to the atrioventricular node at the superior limbus (#2-1) and then to the branches at the left atrial wall (#2-2,3). (C) The atrial chambers from the posterior aspect show the middle (second) inter-nodal route (#2) and the posterior (third) route at the crista terminalis (#3). (D) The middle inter-nodal route is colored yellow, and the posterior inert-nodal tract is colored blue. The middle route branches to form sub-branches extending to the left atrial wall (#2-2, #2-3). The posterior inter-nodal route gives rise to the pectinate muscles and approaches the atrioventricular node and the coronary sinus. Fig. 2C is modified from the authors’ previous publication [25]. SVC, superior caval vein; IVC, inferior caval vein; LA, left atrium; RA, right atrium; RLPV, right lower pulmonary vein.
The second inter-nodal route (the middle route, #2) arises from the sinus node as a broad span of myocardial fibers at the right posterior aspect of the sinus venarum (Figs. 2, 3), in contrast to the left anterior direction of the anterior inter-nodal route (Fig. 3). They form the upper part of the atrial septum and the upper and anterior part of the limbus of the oval fossa. These fibers at the atrial septal wall of the second route (#2-1) unite the first inter-nodal route (#1-1), as described above, to form the short pathway to the atrioventricular node. The other part of the second route covers 2 layers of the atrial wall at the Waterstone groove, and then extends to the left atrial wall around the right pulmonary veins (#2-2 and #2-3) (Figs. 2, 4).
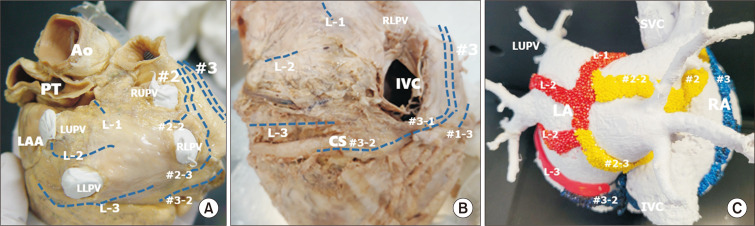
Fig. 3.
The major intra-atrial routes at the posterior aspect of the atrial wall. These routes are broad bands or sheets as shown in (C), although they are shown as lines in (A) and (B) to demonstrate the shape of the atrial wall. (A) Posterior aspect of the left atrium (LA). This part is covered by visceral pericardium at the oblique sinus of the pericardium. The branches of the left part of Bachmann’s bundle merge at the posterior wall along with the branches of the middle inter-nodal routes. (B) The posterior wall of the LA shows approaches from inter-nodal routes #1-3. The distal part of L-3 is close to the coronary sinus (CS). The inter-nodal route from the crista terminalis (#3) approaches the atrioventricular node, and the third branch of the first inter-nodal route (#1-3) also approaches the posterior aspect. (C) Myocardial bundles from 3 different routes of the inter-nodal tracts form complex circles at the left atrial wall. Three branches of Bachmann’s bundle (L-1, L-2, L-3), branches of the middle route (#2-2, #2-3), and a branch of the posterior route along the CS join at the left atrial wall, and pulmonary venous orifices are in the center of each circle. Fig. 3B is modified from the authors’ previous publication [25]. Ao, aorta; PT, pulmonary trunk; LAA, left atrial appendage; LUPV, left upper pulmonary vein; RUPV, right upper pulmonary vein; IVC, inferior caval vein; RLPV, right lower pulmonary vein; SVC, superior caval vein; RA, right atrium.
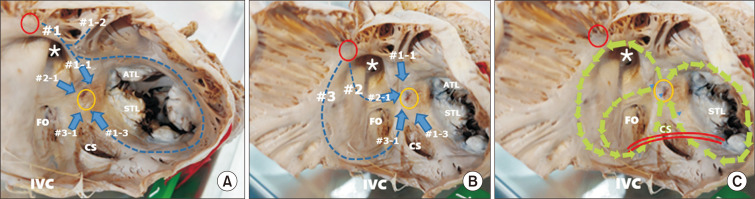
Fig. 4.
Internal views of the right atrium. Three inter-nodal routes arise from the sinus node (red circle) to the atrioventricular (AV) node (yellow circle). (A) The anterior inter-nodal route (#1) is from the sinus node (red circle), running along the medial wall of the right atrium in front of the opening of the superior vena cava (asterisk), and then giving rise to three branches. Branch #1-1 travels anteriorly and descends to the AV node (yellow circle) in a short pathway after joining #2-1. Branch #1-2 spans the anterior wall of the left and right atrial chambers to form Bachmann’s bundle. Branch #1-3 runs around the tricuspid annulus and joins branch #3-1 to approach the posterior part of the AV node in the long pathway. (B) The middle inter-nodal route (#2) runs along the posterior wall of the sinus venarum and descends through the top of the limbus of the oval fossa (FO) (#2-1) to approach in a short pathway to the anterior aspect of the AV node. This route also supplies the left atrial wall between the upper and lower pulmonary veins (#2-2, #2-3) (Fig. 3A, C). The posterior inter-nodal route (#3) is the terminal crest and approaches the AV node through the Eustachian ridge in front of the inferior vena cava (IVC) (#3-1). Route #3-1 joins route #1-3 to approach the posterior part of the AV node in a long pathway. (C) Three anatomical circles are drawn in the same picture as in Fig. 4B. The circle around the FO starts from the anterior part of the AV node and shares #2-1 and #3-1. The large circle uses the root of #1 and #3 and their branches, #1-1 and #3-1. The circle at the tricuspid annulus runs through #1-1 and #1-3. The re-entry circuits in these circles may be in clockwise or counterclockwise directions. The interventional cut at the cavo-tricuspid isthmus (CTI: red lines) is blocking #1-3 and #3-1. Fig. 4A and B are modified from the authors’ previous publication [25]. STL, septal leaflet of the tricuspid valve; ATL, anterior leaflet of the tricuspid valve; CS, coronary sinus.
The third inter-nodal route (the posterior route, #3) is a condensed group of muscle fibers at the terminal crest (Figs. 2, 3). This third route gives rise to the pectinate muscles, which are parallel fibers from the crista terminalis to the right anterior wall of the right appendage at the vestibule of the tricuspid valve. The caudal end of the crista terminalis has 2 terminal branches. The major anterior branch (#3-1) continues to the Eustachian ridge or valve in front of the opening of the inferior vena cava to the right atrium and then approaches the posterior side of the atrioventricular node after joining the branch of the anterior route (#1-3) (Fig. 2). The minor and posterior branch (#3-2) is in the wall of the coronary sinus.
Intra-atrial routes
Contraction of the atrial myocardium occurs through the conduction routes from the original pacer, the sinus node. Therefore, the intra-atrial conduction bundles are peripheral extensions of the inter-nodal routes. They may be terminal dead-end routes, but may also be a form of the complex re-entrant network.
The electrical contribution to right atrial contraction is through the muscle in the terminal crest: the posterior inter-nodal route. The muscular bundles in the terminal crest give rise to the pectinate muscles, and the size of muscle bundle at the terminal crest decreases rapidly as it descends from the sinus node. As the right atrium dilates, the pectinate muscles and terminal crest elongate and become fibrotic. The distance from the sinus node to the atrioventricular node at the terminal crest becomes longer than the first and second inter-nodal routes, and the role of the pectinate muscles for synchronizing the difference diminishes in the elderly. Dilation of the tricuspid annulus also affects the synchronizing role of the muscle at the vestibule of the tricuspid annulus (the third branch of the anterior inter-nodal route: #1-3) (Fig. 3).
The electrical contribution to the left atrial myocardium is through the anterior inter-nodal route (Bachmann’s bundle) (Fig. 1) and the middle inter-nodal route (#2-2 and #2-3) (Figs. 2, 4). Bachmann’s bundle branches to the roof of the left atrium (L-1), to the lateral ridge (L-2) and to the vestibule of the mitral valve (L-3). The second and the third branches of the middle inter-nodal route contribute to the contraction of the left atrium at the right side. Two different routes from the sinus node become asynchronous by dilatation of the left atrium and right atrium. These branches of the 2 routes also make complex circles for re-entry around 4 to 6 openings of the pulmonary veins (Fig. 5).
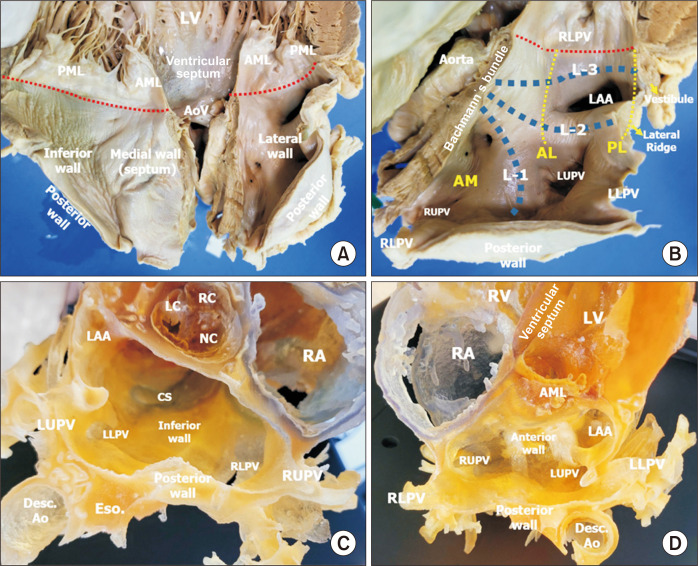
Fig. 5.
(A) Inner wall of the left atrium after sectioning the middle of the anterior (aortic) mitral leaflet. (B) Close-up view of the left lateral wall of the left atrium of the same specimen as in (A). The atrial wall is sectioned at the junction between the septal and left lateral walls. Bachmann’s bundle is cut at its main part to the left. The 3 branches of the left part of Bachmann’s bundle are shown as L-1, L-2, and L-3. L-1 runs upward between the right and left upper pulmonary veins (LUPVs). L-2 runs to the lateral ridge between the LUPV and the left atrial appendage (LAA). L-3 is at the vestibule of the left atrium over the mitral valve. The location of the annulus of the mitral valve from the left atrial aspect is marked in red dotted lines. Three isthmus lines for left atrial ablation are shown by yellow dotted lines. The posterior lateral isthmus line (PL) between the left lower pulmonary vein and the mitral annulus (red dotted lines) is the shortest line to cut L-3 of the mitral vestibule. The antero-lateral isthmus line (AL) is between the LUPV and the mitral annulus, and the procedure blocks L-3 and L-2 at the lateral ridge. The anterior medial isthmus line (AM) is between the right upper pulmonary vein (RUPV) and the mitral annulus, blocking all 3 branches of Bachmann’s bundle (L-1, L-2, and L-3). (C) A 3-dimensional (3D)–printed heart showing the inferior wall of the left atrium. There is a wide space at the inferior wall between the annulus at the posterior mitral leaflet and the inferior pulmonary veins. The coronary sinus runs along the inferior wall of the left atrium. (D) A different view of the 3D-printed heart sectioned on another plane, similar to the view shown in (B). LV, left ventricle; AML, anterior leaflet of the mitral valve; PML, posterior leaflet of the mitral valve; AoV, aortic valve; RLPV, right lower pulmonary vein; LLPV, left lower pulmonary vein; LC, left coronary cusp; RC, right coronary cusp; NC, non-coronary cusp; CS, coronary sinus; Eso., esophagus; Desc. Ao, descending aorta; RA, right atrium; RV, right ventricle.
Discussion
A basic understanding of atrial arrhythmia
Three pathogenetic mechanisms have been introduced as the anatomical substrates of atrial arrhythmias. An abnormal initiation of the rhythm or “trigger” is the first step. Increased automaticity of myocytes enhances propagation of the trigger, and they together make micro and macro re-entry circuits to sustain arrhythmia. The first and the third of these mechanisms are tightly linked with the anatomical characteristics of the atrial wall and the triggering site, and macro re-entry in hearts with atrial fibrillation and flutter are discussed in detail in this article [4,26]. The second mechanism, abnormal automaticity of the myocardium, is a matter of the humoral status of the myocardium, although fibrosis and stretch-activated automaticity of myocardial fibers in heart failure with dilated atrial chambers can be anatomical substrates of these physiological changes [26,27].
The pulmonary venous wall near its opening to the left atrium and the terminal zone of the coronary sinus near the ligament of Marshal are common sources of abnormal triggering in structurally normal hearts [4,28-30]. Pulmonary veins in patients with atrial fibrillation also showed more fibrosis and discontinuities [31]. Myocytes at these foci are reported to have cellular characteristics of nodal cells [32,33]. Exclusion of these sites of the abnormal trigger from the rest of the atrial myocardium is the primary target for surgical and interventional treatment.
The propagation of localized events to sustained atrial fibrillation occurs through re-entry mechanisms at the atrial myocardium. Micro and macro re-entry mechanisms have been suggested, and these abnormal circuits are detected by electrophysiological mapping. Macro re-entry is a grossly recognizable circuit, and anatomical correction is possible either by surgery or intervention. It is crucial for surgeons and interventionists to understand the actual anatomy to localize the circuits involved in these events and to find the best sites for therapeutic intervention in the atrial wall [10].
The atrial myocardium as a part of the conduction system
There is no doubt that these specialized components of the myocardium, or the conduction tissue, have major roles in the conduction system [34,35], but we also know that histologically specialized conduction tissue is not documented in the atrial wall. The myocardial cells, therefore, play roles in the atrial conduction system—that is, conduction between the sinus node and the atrioventricular node and conduction to the atrial myocardium.
The anatomical basis of conduction at the level of atrial myocardium was described long before the current therapeutic strategies were adopted [19,36-38]. The presence of the inter-nodal pathways was illustrated by James [19] and Sherf and James [21] in the human heart [39]. He referred to Wenckebach, Thorel, and Bachmann for their pioneering descriptions of the inter-nodal routes. Wenckebach [40] in 1907 was the first to suggest the presence of the conduction pathway between the nodes. In 1910, Thorel [41] was the first to identify the terminal crest as a route of specialized issue between 2 nodes, but those findings were not fully approved by the German Pathological Society in 1910 [38]. The contribution by Bachmann [42] in 1916 was an incomplete document in the sense that he considered the bundle as being involved in inter-atrial conduction rather than inter-nodal conduction [19].
Some histological and ultrastructural studies showed different kinds of cells in the atrial myocardium [21,43]. However, further studies denied the presence of histologically specialized conduction tissue [38]. According to the classical definition, conduction tissue must be histologically different from the surrounding myocardium and covered or insulated by a sheath from the surrounding myocardium [38].
Any morphological changes in individual myocytes may produce abnormal conduction at the cellular level. Death of myocytes or discontinuity of myocytic connections would be examples of abnormal conduction. Increased length of individual myocytes due to a distended atrial cavity cause delays in conduction. Fibrosis and fatty changes of interstitial tissue modify cellular conduction. The micro-environment and the autonomic nervous system modulate the cellular characteristics of myocytes [26]. The abnormal conduction of individual myocytes might present with an abnormal trigger or abnormal automaticity in terms of the mechanism that produces arrhythmia.
The macroscopic architecture of myocytes as fascicles or bundles explains synchronized conduction through different routes in normal conduction, but these routes may also produce abnormal non-synchronized conduction to produce a re-entry mechanism. Cardiac dilatation and damage to the myocardium produce changes in myocardial integrity in some of these routes, affecting the myocardial conduction speed to produce asynchrony in conduction through different routes.
We described the atrial myocardial architecture based on our hypothesis that there are 2 types of atrial myocardial bundles; major preferential routes and side branches interconnecting those routes. We used the term “route” to refer to the major inter-nodal and intra-atrial bundles that would be targets for intervention or surgery. The length of the major routes and the lag time of conduction through different routes are similar in normal hearts. Side branches are used to synchronize the conduction speed of different routes.
Inter-nodal, inter-atrial, and intra-atrial conduction
There are 2 types of atrial conduction: intra-atrial conduction, which produces atrial contraction, and inter-nodal conduction, which delivers the pacing message from the sinus node to the atrioventricular node.
There are 3 inter-nodal routes from the sinus node: anterior, middle, and posterior routes. There are also 2 possible approaches to the atrioventricular node. The anterior approach to the atrioventricular nodes is a fused muscle group consisting of the anterior and middle inter-nodal routes, which are in front of the oval fossa. The posterior approach to the atrioventricular node is also a fused muscle group, mainly consisting of the terminal branch of the posterior route through the terminal crest and Eustachian ridge (the posterior route) and the minority from the anterior route through the supra-tricuspid vestibular muscle (#1-3) (Figs. 2, 3).
Intra-atrial conduction occurs within and to the right and left atrial chambers. Conduction to the free wall of the right atrium involves conduction at the pectinate muscle from the terminal crest. Conduction to the right atrium at its septum is from the anterior and middle routes. Bachmann’s bundle is at the anterior wall or the lesser curvature of the right and left atrial chambers, corresponding to the right and left branches of the anterior route. The major conduction to the left atrial wall is through Bachmann’s bundles and their branches from the anterior route. A minor contribution is through the middle inter-nodal route. The coronary sinus receives conduction from the posterior route as a terminal posterior branch.
Bachmann’s bundle was the first classical concept of inter-atrial conduction, and this bundle is still accepted as the most important pathway between atrial chambers [44]. Inter-atrial conduction at the posterior aspect of the interatrial septum, known as the Waterstone groove, was illustrated in human heart specimens [22]. The pathways in their diagram were tiny threads crossing over the groove, which would not likely have more value than the folded atrial wall connected at the limbus of the oval fossa (personal observation).
The anatomical re-entry circuits
Interventional block at the cavo-tricuspid isthmus in hearts with atrial flutter is performed to cut anatomical circuits at the posterior approaches to the atrioventricular node. The particular isthmus is located at the critical zone as described below (Fig. 3C). Electrical signals from the sinus node are delivered to the atrioventricular node through 3 inter-nodal routes. When they arrive at the atrioventricular node, the branches of 3 routes merge together into 2 approaches: the anterior short and posterior long tracts. The atrioventricular node is like a fortress to which only 2 approaches are open. The electrical approach to the node is blocked superiorly by the oval fossa and inferiorly by the electrical insulation at the atrioventricular junction. The distance from the sinus node through the anterior approach is shorter than the posterior approach. The 2 components of the anterior approach are through the anterior route (#1-1) and the middle route (#2-1), but they join early to form a common route. Two components (#1-3 and #3-1) of the posterior approach, however, join just before they are in contact with the node. The 2 components are separated by intervening structures (the orifice of the coronary sinus and the sub-Eustachian sinus).
The 3 circuits at the atrioventricular node area include the atrioventricular node, such that the time difference between the anterior and posterior approaches makes re-entry circles that produce atrial flutter. The first is around the oval fossa. The second is around the tricuspid annulus, and the third is the larger circle of the anterior inter-nodal route and the third route (the terminal crest). Time differences are normally produced between the anterior and posterior approaches, but the energy is compensated by minor interconnecting branches such as the pectinate muscles. The time difference is then at critical value, as they produce re-entry through a mechanism like resonance. The cavo-tricuspid isthmus has 3 components: the vestibular muscle (#1-3) at the tricuspid end; the sub-Eustachian sinus, which is a thin-walled pouch with little electrical signaling; and the terminal branch of the terminal crest (#3-1). The main target of intervention on cavo-tricuspid isthmus is to cut 2 major bundles (#1-3 and #3-1).
Re-entry circuits are formed around pulmonary venous openings. The muscle around the pulmonary veins is not thick, but these circuits are located immediately next to the trigger points at the myocardial sleeve of the pulmonary veins.
Circuits are also formed at the orifice of the superior and inferior caval veins, but re-entry at these sites is disregarded due to the stronger electrical current of the nearby conduction routes.
The vestibular muscle at the mitral annulus (L-3) also has an anatomical circuit, but this circle is fed back to Bachmann’s bundle and hardly produces flutter.
Atrial fibrillation arising from the left atrium is mostly controlled through the exclusion of trigger sites by isolating the myocardial sleeve around the pulmonary vein orifice. If abnormal fibrillation persists, additional blocks are performed. Three isthmus lines are applied at the left atrium [45]. The posterior lateral isthmus line between the left lower pulmonary vein and the mitral annulus is the shortest line to cut the L-3 of the mitral vestibule. The antero-lateral isthmus line is between the left upper pulmonary vein and the mitral annulus, and the procedure blocks L-3 and L-2 at the lateral ridge. The anterior medial isthmus line is between the right upper pulmonary vein and the mitral annulus to block all 3 branches of Bachmann’s bundle (L-1, L-2, and L-3).
Dilatation and fibrosis of the atrial wall
The small number of heart specimens is a major limitation of pathological studies compared to clinical reviews. Previous studies based on a number of heart specimens have shown details of the anatomy of the atrial chambers in regard to the conduction system [38,46,47]. Here we describe our observational review rather than a scientific analysis. The 3 major routes and their side branches to atrial myocardium are not fixed permanent structures, but we describe them in terms of expedient groups in the myocardial tissue as observed by blunt dissection. The significance of the 3 routes changes depending on the cardiac size and electrical conditions. When the heart is young and it is not large, these routes will be active and one route will synchronize with others. In a heart that is old and dilated, some of the muscular bundles in these routes will attenuate.
Dilatation of atrial chambers is a major modifying factor for the proper functioning of these routes. Dilatation of a chamber refers to the elongation of its constituent muscle fibers. Every part of the chamber is not the same in terms of the degree of these changes. The right atrium, for example, has fixed parts, such as the areas around the connected veins, and mobile parts, such as the appendage. The mobile parts are more strongly affected by dilatation of the chamber and cellular elongation. The terminal crest is usually more elongated than the atrial septum in atrial dilatation. In hearts with increased shunt from the left atrium, changes at the atrial septum will ensue. The left atrium also has fixed parts at the junctions with the pulmonary veins, but the atrial free wall between these pulmonary venous openings and the left appendage is freely expanded in the cardiac cycle.
In hearts with right atrial dilatation, the lengths of the 3 inter-nodal routes have different impacts. The first branch of the anterior inter-nodal route remains constant, but the third branch of the anterior inter-nodal route is hampered by an enlarged tricuspid annulus. The second or middle inter-nodal route is less damaged, but this route is the most variable structure from the beginning. The third route at the terminal crest is slightly damaged at its proximal end, but the distal part of the crista is damaged by the dilatation of the lower part of the right atrium.
Dilatation of the left atrium does not cause much damage to the inter-nodal route, but the attenuation of intra-atrial conduction will produce micro re-entry circuits in the atrial wall.
Dilatation of the mitral annulus in left-side heart failure and the tricuspid annulus in right-side heart failure makes vestibular conduction bundles longer, in addition to dilatation of the atrial chambers. Furthermore, aging and fibrosis of the atrial myocardium, as well as dilatation, modify the inter-nodal and intra-atrial routes and their branches.
Cardiac development and remodeling
In the initial developmental period, cardiac cells in the primitive cardiac tube have characteristics of sinus nodal cells based on morphological and immunohistochemical data [48,49]. Myocardial cells in later developmental periods are derived from those nodal cells to form a lineage of predecessor nodal cells. In other words, every cardiac cell has its own pedigree, but they form lateral junctions with neighboring cells to harmonize their contraction.
Through the growth of the chambers, 3 or more major conduction routes are secondarily produced. Other branches to the left atrium are dead ends and disappear after they produce myocardial contractions in the left atrium without further conduction to the atrioventricular node. The 3 major routes are common pathways that remain inter-nodal routes. The lengths of these routes are similar when the heart is small, but they become different due to the growth of the chambers. If the length of the routes or the time of conduction through these routes is significantly different, it is possible for 1 impulse to disappear or for 2 impulses to interfere with each other. Interference does not necessarily produce arrhythmia, but may be a temporary subclinical modification of the impulse.
Maze operation and radiofrequency ablation lines
Several previous surgical techniques were designed before the current maze operation [9]. The left atrial isolation procedure described in 1980 involved total exclusion of the left atrial wall by incising the right side of the atrial septum and cryo-damaging the wall of the coronary sinus [50]. The “corridor” procedure involved making an incision on the atrial septum to isolate the narrow direct pathway from the sinus node to the atrioventricular node from the rest of atrial wall [9]. The atrial transection procedure cut the postero-superior and lateral walls of both atria and the septum, which separates the sinus node and superior vena cava and anterior wall from the pulmonary venous chamber and inferior vena cava [51]. A common feature of these 3 procedures was that the short pathway from the sinus node to the atrioventricular node was kept intact and a variable amount of atrial wall was excluded.
The incision lines for current maze procedures or the ablation lines for interventional procedures include isolation of the orifices of pulmonary veins and additional lines to cut macro re-entry circuits [9]. Two principles are to exclude sources of triggering (pulmonary vein isolation) and to cut the macro re-entry circuits (additional lines). Additional incision or ablation lines reduce the chance of recurrence of arrhythmia, but also decrease the functional outcomes of atrial contraction. Electrophysiological mapping is of great value for detecting remaining abnormal circuits during the procedures, but understanding the baseline anatomical character of the atrial wall is of critical importance.
Potential value of this observation on incision lines for cardiac surgery
Any incision lines at the atrial wall produce fibrous scars blocking the original conduction routes. In young hearts, these scars do not produce significant abnormalities in inter-nodal and intra-atrial conduction. In aged and diseased hearts, surgical scars will hamper harmony in conduction through different routes. Although damaging the myocardial integrity is inevitable in cardiac surgery, significant damage to the major inter-nodal pathways may be avoided by some minor changes in current incision procedures for cardiac surgery.
Right atriotomy for the surgical closure of ventricular septal defects does not cut any major routes, but a vertical incision in parallel to the crista cuts many pectinate muscle fibers, which are interconnecting channels between the proximal part of the posterior inter-nodal route (terminal crest) and the second or third branches of the anterior inter-nodal route. This damage can be avoided by making an incision in parallel to the pectinate muscle when performing right atriotomy. Surgeons can minimize damage to the pectinate muscle to keep interconnecting channels intact.
A left atriotomy incision for mitral valve surgery also has some possibilities for minimizing injuries. An incision made parallel to the Waterstone groove may cut the posterior branch of the middle inter-nodal route between the upper and lower right pulmonary veins. Modifying the incision starting from the level of the right lower pulmonary vein can save the branch to the left atrial myocardium.
Working hypothesis
We described the atrial myocardial bundles in 2 groups: the first group comprises the major routes between the nodes and between the 2 atrial chambers, while the other group contains the side branches interconnecting major routes. Three major routes arise from the sinus node and then approach the atrioventricular node through anterior (short) and posterior (long) approaches. Branches of these routes are found on their way to the atrioventricular node and to the left atrial myocardium. These branches form several circuits: around the tricuspid valve, around the oval fossa, around pulmonary venous channels, and around the mitral valve. The conduction channels through different circuits result in synchronized conduction when the chambers are of normal size and myocardial tissue is not damaged but they produce macro re-entry circuits when they have different lengths and speeds of conduction.
The anatomy can be basically understood as the sum of histological and ultrastructural cellular elements, and individual cellular details characterize the types of electrical conduction between cardiac cells. However, a more important factor may be the macroscopic alignment of these cells, since the conduction capacity of individual cells is influenced by neighboring cells and they together form macroscopic conduction in the heart. The macroscopic anatomy of cells in the atrial chambers is never static; instead, it changes in response to cardiac contraction cycles, cardiac dilatation, stretching and fibrosis of the wall, post-inflammatory or post-traumatic scars, and aging of the myocardium. Knowledge of the basic anatomical features is an important step not only for understanding complicated arrhythmia, but also for therapeutic planning of intervention and surgery.
Conclusion
Modification of the myocardial architecture of the atrial chambers is the basic principle of surgery and catheter-based interventions for arrhythmia. We reviewed the anatomy of 3 inter-nodal conduction routes and their intra-atrial branches. The 3-dimensional architecture of the routes is described and terminology is proposed. The anterior inter-nodal route (#1) arises from the sinus node to the ventral wall of the atrial chambers at the transverse pericardial sinus near the atrial septum. The major branch of route #1 approaches the anterior end of the atrioventricular node (#1-1), which is the shortest inter-nodal pathway (short tract). The second branch (#1-2) is Bachmann’s bundle, branching to the right and left. The 3 branches of the left part of Bachmann’s bundle extend to the roof (L-1), to the lateral ridge (L-2), and to the vestibule of the left atrium (L-3). The right part of Bachmann’s bundle supplies the ventral wall of the right atrium and connects to the pectinate muscles. The third branch (#1-3) runs to the vestibule of the right atrium and approaches the posterior end of the atrioventricular node. The middle inter-nodal route (#2), previously described as the Wenckebach pathway, begins with a broad span of fibers at the lateral and proximal part of the sinus venarum and merges into the superior limbus of the oval fossa. The major branch of route #2 joins with #1-1 and approaches the anterior end of the atrioventricular node (#2-1). Route #2 at the superior limbus makes a U-loop to supply the left atrial wall around the right upper and lower pulmonary veins (#2-2, #2-3). The posterior inter-nodal route (#3), which was previously described as the Thorel pathway, is the muscle ridge at the terminal crest. Route #3 gives rise to many pectinate muscles in the anterior lateral wall of the right atrium. The lowest part of route #3 branches to #3-1 and #3-2. Branch #3-1 is the muscle approaching the posterior end of the atrioventricular node to form the long tract after joining with branch #1-3. The branch #3-2 is at the coronary sinus. Anatomical circles could be described as preferred structural sites for macro re-entry in atrial flutter: around the oval fossa, tricuspid annulus, the large circle around the terminal crest, and the middle inter-nodal route. The anatomical components of the cavo-tricuspid isthmus are illustrated. Re-entry circles in the left atrial chamber are circles around pulmonary venous orifices and the mitral annulus. The anatomical components of mitral isthmus lines are illustrated. Different disease processes could produce a spectrum of dilatation and fibrosis of the atrial wall at different locations.
Article information
Author contributions
Conceptualization: JWS, JSK, HMT, CHL, SO. Data curation: JWS, MJK, HMT, CHL. Formal analysis: JWS, JSK, MJC, HMT, CHL. Methodology: JWS, JSK, HMT, CHL. Project administration: JWS, JSK, MJC, HMT. Visualization: JWS, JSK, HMT. Writing–original draft: JWS, MJC, HMT. Writing–review & editing: JWS, JSK, MJC, JKY, MJK, HMT, CHL, SO.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Baman JR, Passman RS. The future of long-term monitoring after catheter and surgical ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2022 Jan 20; doi: 10.1111/jce.15375. [Epub]. https://doi.org/10.1111/jce.15375 . [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Villarreal OA. Standardization in maze procedure: a step towards a better future. J Thorac Dis. 2018;10(Suppl 33):S3887–9. doi: 10.21037/jtd.2018.08.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labombarda F, Hamilton R, Shohoudi A, et al. Increasing prevalence of atrial fibrillation and permanent atrial arrhythmias in congenital heart disease. J Am Coll Cardiol. 2017;70:857–65. doi: 10.1016/j.jacc.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Park HS, Jeong DS, Yu HT, et al. 2018 Korean Guidelines for Catheter Ablation of Atrial Fibrillation: part I. Int J Arrhythm. 2018;19:186–234. doi: 10.18501/arrhythmia.2018.011. [DOI] [Google Scholar]
- 5.Lee JM, Jeong DS, Yu HT, et al. 2018 Korean Guidelines for Catheter Ablation of Atrial Fibrillation: part III. Int J Arrhythm. 2018;19:285–339. doi: 10.18501/arrhythmia.2018.013. [DOI] [Google Scholar]
- 6.Shim H, Yang JH, Park PW, Jeong DS, Jun TG. Efficacy of the maze procedure for atrial fibrillation associated with atrial septal defect. Korean J Thorac Cardiovasc Surg. 2013;46:98–103. doi: 10.5090/kjtcs.2013.46.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–74. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan K, Xie A, La Meir M, Black D, Yan TD. Surgical ablation for treatment of atrial fibrillation in cardiac surgery: a cumulative meta-analysis of randomised controlled trials. Heart. 2014;100:722–30. doi: 10.1136/heartjnl-2013-305351. [DOI] [PubMed] [Google Scholar]
- 9.Cox JL, Schuessler RB, Boineau JP. The development of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12:2–14. doi: 10.1016/S1043-0679(00)70010-4. [DOI] [PubMed] [Google Scholar]
- 10.Ho SY, McCarthy KP, Faletra FF. Anatomy of the left atrium for interventional echocardiography. Eur J Echocardiogr. 2011;12:i11–5. doi: 10.1093/ejechocard/jer093. [DOI] [PubMed] [Google Scholar]
- 11.Pawar P, Mumtaz Z, Phadke M, Bharati A, Mahajan A. Is left atrial fibrosis an independent determinant of atrial fibrillation in mitral stenosis? Indian Heart J. 2021;73:503–5. doi: 10.1016/j.ihj.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westermann D, Schrage B. Mitral stenosis and atrial fibrillation. Heart. 2020;106:713. doi: 10.1136/heartjnl-2019-316282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwak JG, Seo JW, Oh SS, et al. Histopathologic analysis of atrial tissue in patients with atrial fibrillation: comparison between patients with atrial septal defect and patients with mitral valvular heart disease. Cardiovasc Pathol. 2014;23:185–92. doi: 10.1016/j.carpath.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Karunanithi Z, Nyboe C, Hjortdal VE. Long-term risk of atrial fibrillation and stroke in patients with atrial septal defect diagnosed in childhood. Am J Cardiol. 2017;119:461–5. doi: 10.1016/j.amjcard.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Wu SJ, Fan YF, Chien CY. Surgical or interventional treatment for adult patients with atrial septal defect and atrial fibrillation: a systemic review and meta-analysis. Asian J Surg. 2022;45:62–7. doi: 10.1016/j.asjsur.2021.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Ogiso M, Ejima K, Shoda M, et al. Efficacy of catheter ablation for patients with atrial fibrillation and atrial septal defect. J Cardiovasc Electrophysiol. 2021;32:279–86. doi: 10.1111/jce.14862. [DOI] [PubMed] [Google Scholar]
- 17.Moak JP. Ablation of atrial arrhythmias in postoperative congenital heart disease patients: have we reached the upper limit of success or is it time for a paradigm shift in strategy? Circ Arrhythm Electrophysiol. 2017;10:e006021. doi: 10.1161/CIRCEP.117.006021. [DOI] [PubMed] [Google Scholar]
- 18.Houck CA, de Groot NM, Kardys I, Niehot CD, Bogers AJ, Mouws EM. Outcomes of atrial arrhythmia surgery in patients with congenital heart disease: a systematic review. J Am Heart Assoc. 2020;9:e016921. doi: 10.1161/JAHA.120.016921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James TN. The connecting pathways between the sinus node and A-V node and between the right and the left atrium in the human heart. Am Heart J. 1963;66:498–508. doi: 10.1016/0002-8703(63)90382-X. [DOI] [PubMed] [Google Scholar]
- 20.James TN. The internodal pathways of the human heart. Prog Cardiovasc Dis. 2001;43:495–535. doi: 10.1053/pcad.2001.24598. [DOI] [PubMed] [Google Scholar]
- 21.Sherf L, James TN. Fine structure of cells and their histologic organization within internodal pathways of the heart: clinical and electrocardiographic implications. Am J Cardiol. 1979;44:345–69. doi: 10.1016/0002-9149(79)90327-8. [DOI] [PubMed] [Google Scholar]
- 22.Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:1525–33. doi: 10.1111/j.1540-8167.1999.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 23.Ho SY, Anderson RH, Sanchez-Quintana D. Gross structure of the atriums: more than an anatomic curiosity? Pacing Clin Electrophysiol. 2002;25:342–50. doi: 10.1046/j.1460-9592.2002.00342.x. [DOI] [PubMed] [Google Scholar]
- 24.Heart Museum, author. The heart museum 2019 [Internet] Heart Museum; Incheon: 2019. [cited 2022 Jun 20]. Available from: http://heartmuseum.kr/ [Google Scholar]
- 25.Seo JW, Yoon JK, Kim J, Tsao HM, Oh S. Intra-atrial inter-nodal conduction at the heart specimen. APHRS Newsl [Internet] 2020. [cited 2022 Jun 20]. ;(51):3-4. Available from: https://www.aphrs.org/attachments/category/16/APHRS%20News%20No.51.pdf .
- 26.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 27.Ferrier GR, Moffat MP, Lukas A. Possible mechanisms of ventricular arrhythmias elicited by ischemia followed by reperfusion: studies on isolated canine ventricular tissues. Circ Res. 1985;56:184–94. doi: 10.1161/01.RES.56.2.184. [DOI] [PubMed] [Google Scholar]
- 28.Cabrera JA, Ho SY, Climent V, Sanchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–62. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 29.Ho SY. Anatomy and myoarchitecture of the left ventricular wall in normal and in disease. Eur J Echocardiogr. 2009;10:iii3–7. doi: 10.1093/ejechocard/jep159. [DOI] [PubMed] [Google Scholar]
- 30.Saito T, Waki K, Becker AE. Left atrial myocardial extension onto pulmonary veins in humans: anatomic observations relevant for atrial arrhythmias. J Cardiovasc Electrophysiol. 2000;11:888–94. doi: 10.1111/j.1540-8167.2000.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 31.Hassink RJ, Aretz HT, Ruskin J, Keane D. Morphology of atrial myocardium in human pulmonary veins: a postmortem analysis in patients with and without atrial fibrillation. J Am Coll Cardiol. 2003;42:1108–14. doi: 10.1016/S0735-1097(03)00918-5. [DOI] [PubMed] [Google Scholar]
- 32.Weiss C, Gocht A, Willems S, Hoffmann M, Risius T, Meinertz T. Impact of the distribution and structure of myocardium in the pulmonary veins for radiofrequency ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2002;25:1352–6. doi: 10.1046/j.1460-9592.2002.01352.x. [DOI] [PubMed] [Google Scholar]
- 33.Kugler S, Nagy N, Racz G, Tokes AM, Dorogi B, Nemeskeri A. Presence of cardiomyocytes exhibiting Purkinje-type morphology and prominent connexin45 immunoreactivity in the myocardial sleeves of cardiac veins. Heart Rhythm. 2018;15:258–64. doi: 10.1016/j.hrthm.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 34.Padala SK, Cabrera JA, Ellenbogen KA. Anatomy of the cardiac conduction system. Pacing Clin Electrophysiol. 2021;44:15–25. doi: 10.1111/pace.14107. [DOI] [PubMed] [Google Scholar]
- 35.Sternick EB, Sanchez-Quintana D. Critical assessment of the concepts and misconceptions of the cardiac conduction system over the last 100 years: the personal quest of Robert H. Anderson. J Cardiovasc Dev Dis. 2021;8:5. doi: 10.3390/jcdd8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies MJ. Pathology of conducting tissue of the heart. Butterworths; London: 1971. [Google Scholar]
- 37.Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J. 1972;34:520–5. doi: 10.1136/hrt.34.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson RH, Ho SY, Smith A, Becker AE. The internodal atrial myocardium. Anat Rec. 1981;201:75–82. doi: 10.1002/ar.1092010110. [DOI] [PubMed] [Google Scholar]
- 39.Holsinger JW, Jr, Wallace AG, Sealy WC. The identification and surgical significance of the atrial internodal conduction tracts. Ann Surg. 1968;167:447–53. doi: 10.1097/00000658-196804000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenckebach KF. Beitrage zur Kenntnis der menschlichen Herztatigkeit [Contributions to the knowledge of the human heart activity] Arch Anat Physiol. 1907;1:1–2. [Google Scholar]
- 41.Thorel C. Uber den Aufbau des Sinusknotens und seine Verbindung mit der Cava superior und den Wenckebachschen Bundeln [On the structure of the sinus node and its connection with the superior cava and Wenckebach's bundles] Munch Med Wschr. 1910;57:183–6. [Google Scholar]
- 42.Bachmann G. The inter-auricular time interval. Am J Physiol. 1916;41:309–20. doi: 10.1152/ajplegacy.1916.41.3.309. [DOI] [Google Scholar]
- 43.Hoffman BF. Fine structure of internodal pathways. Am J Cardiol. 1979;44:385–6. doi: 10.1016/0002-9149(79)90332-1. [DOI] [PubMed] [Google Scholar]
- 44.Ho SY, Anderson RH, Sanchez-Quintana D. Atrial structure and fibres: morphologic bases of atrial conduction. Cardiovasc Res. 2002;54:325–36. doi: 10.1016/S0008-6363(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 45.Cho Y, Lee W, Park EA, et al. The anatomical characteristics of three different endocardial lines in the left atrium: evaluation by computed tomography prior to mitral isthmus block attempt. Europace. 2012;14:1104–11. doi: 10.1093/europace/eus051. [DOI] [PubMed] [Google Scholar]
- 46.James TN. The development of ideas concerning the conduction system of the heart. Ulster Med J. 1982;51:81–97. [PMC free article] [PubMed] [Google Scholar]
- 47.DeSimone CV, Noheria A, Lachman N, et al. Myocardium of the superior vena cava, coronary sinus, vein of Marshall, and the pulmonary vein ostia: gross anatomic studies in 620 hearts. J Cardiovasc Electrophysiol. 2012;23:1304–9. doi: 10.1111/j.1540-8167.2012.02403.x. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharyya S, Munshi NV. Development of the cardiac conduction system. Cold Spring Harb Perspect Biol. 2020;12:a037408. doi: 10.1101/cshperspect.a037408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson RH, Moorman AF. Recent developmental findings relevant to the clinical significance of the myocardial venous sleeves. J Cardiovasc Electrophysiol. 2012;23:1310–2. doi: 10.1111/j.1540-8167.2012.02424.x. [DOI] [PubMed] [Google Scholar]
- 50.Williams JM, Ungerleider RM, Lofland GK, Cox JL. Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg. 1980;80:373–80. doi: 10.1016/S0022-5223(19)37762-1. [DOI] [PubMed] [Google Scholar]
- 51.Cox JL, Schuessler RB, D'Agostino HJ, Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–83. doi: 10.1016/S0022-5223(19)36684-X. [DOI] [PubMed] [Google Scholar]