Abstract
Objective
To determine if the use of corticosteroids was associated with Intensive Care Unit (ICU) mortality among whole population and pre-specified clinical phenotypes.
Design
A secondary analysis derived from multicenter, observational study.
Setting
Critical Care Units.
Patients
Adult critically ill patients with confirmed COVID-19 disease admitted to 63 ICUs in Spain.
Interventions
Corticosteroids vs. no corticosteroids.
Main variables of interest
Three phenotypes were derived by non-supervised clustering analysis from whole population and classified as (A: severe, B: critical and C: life-threatening). We performed a multivariate analysis after propensity optimal full matching (PS) for whole population and weighted Cox regression (HR) and Fine-Gray analysis (sHR) to assess the impact of corticosteroids on ICU mortality according to the whole population and distinctive patient clinical phenotypes.
Results
A total of 2017 patients were analyzed, 1171 (58%) with corticosteroids. After PS, corticosteroids were shown not to be associated with ICU mortality (OR: 1.0; 95% CI: 0.98–1.15). Corticosteroids were administered in 298/537 (55.5%) patients of “A” phenotype and their use was not associated with ICU mortality (HR = 0.85 [0.55–1.33]). A total of 338/623 (54.2%) patients in “B” phenotype received corticosteroids. No effect of corticosteroids on ICU mortality was observed when HR was performed (0.72 [0.49–1.05]). Finally, 535/857 (62.4%) patients in “C” phenotype received corticosteroids. In this phenotype HR (0.75 [0.58–0.98]) and sHR (0.79 [0.63–0.98]) suggest a protective effect of corticosteroids on ICU mortality.
Conclusion
Our finding warns against the widespread use of corticosteroids in all critically ill patients with COVID-19 at moderate dose. Only patients with the highest inflammatory levels could benefit from steroid treatment.
Keywords: COVID-19, Corticosteroids, Phenotypes, ICU mortality, SARS-CoV2-pneumonia, Unsupervised clustering
Abstract
Objetivo
Evaluar si el uso de corticoesteroides (CC) se asocia con la mortalidad en la unidad de cuidados intensivos (UCI) en la población global y dentro de los fenotipos clínicos predeterminados.
Diseño
Análisis secundario de estudio multicéntrico observacional.
Ámbito
UCI.
Pacientes
Pacientes adultos con COVID-19 confirmado ingresados en 63 UCI de España.
Intervención
Corticoides vs. no corticoides.
Variables de interés principales
A partir del análisis no supervisado de grupos, 3 fenotipos clínicos fueron derivados y clasificados como: A grave, B crítico y C potencialmente mortal. Se efectuó un análisis multivariado después de un propensity optimal full matching (PS) y una regresión ponderada de Cox (HR) y análisis de Fine-Gray (sHR) para evaluar el impacto del tratamiento con CC sobre la mortalidad en la población general y en cada fenotipo clínico.
Resultados
Un total de 2.017 pacientes fueron analizados, 1.171 (58%) con CC. Después del PS, el uso de CC no se relacionó significativamente con la mortalidad en UCI (OR: 1,0; IC 95%: 0,98-1,15). Los CC fueron administrados en 298/537 (55,5%) pacientes del fenotipo A y no se observó asociación significativa con la mortalidad (HR = 0,85; 0,55-1,33). Un total de 338/623 (54,2%) pacientes del fenotipo B recibieron CC sin efecto significativo sobre la mortalidad (HR = 0,72; 0,49-1,05). Por último, 535/857 (62,4%) pacientes del fenotipo C recibieron CC. En este fenotipo, se evidenció un efecto protector de los CC sobre la mortalidad HR (0,75; 0,58-0,98).
Conclusión
Nuestros hallazgos alertan sobre el uso indiscriminado de CC a dosis moderadas en todos los pacientes críticos con COVID-19. Solamente pacientes con elevado estado de inflamación podrían beneficiarse con el tratamiento con CC.
Palabras clave: COVID-19, Corticoides, Fenotipos clínicos, Mortalidad en la unidad de cuidados intensivos, Neumonía por SARS-CoV-2, Agrupamiento no supervisado
Introduction
Patients with COVID-19 are known to develop a major inflammatory response that can lead to acute respiratory distress syndrome (ARDS). As inflammation is thought to contribute to the pathogenesis of ARDS1 it warrants further investigation as to the pharmacokinetic effects of immunomodulatory agents. Further study of the interaction of these drugs with virus/host dynamics is necessary to provide insight into optimal timing of administration, dosing, and association with other interventions.
Corticosteroids are potent anti-inflammatory agents with immunomodulatory properties, which exert inhibitory effects in several stages of the inflammatory cascade, and consequently have been proposed for the treatment of ARDS.2, 3 However, in recent epidemics due to coronavirus infections such as that Middle East respiratory syndrome-related coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome associated coronavirus (SARS-CoV) or influenza viruses the use of corticosteroids was associated with delayed virus clearance and an increase in ICU mortality.4, 5, 6, 7
Several randomized control trials8, 9, 10 found a benefit to the use of corticosteroids in patients with COVID-19, and various clinical guidelines11, 12 recommended its use to all patients requiring oxygen with severe COVID-19 during the second wave. However, there is limited data in relation to ICU admission beyond 28 days that assesses the side effects of medium- and long-term glucocorticoid treatment.13, 14 For example, there are still unanswered questions as which subgroup or rather “phenotype” of patients could have higher response rate to the steroid therapy.15 Therefore, our primary objective is to identify the association of corticosteroids treatment in a whole cohort population and according to three new classified clinical phenotypes identified from 2017 COVID-19 critically ill patients in Spain.16 Our secondary objective is to stratify the competing risk factors associated with use of corticosteroids in each phenotype and clinical outcome.
Material and methods
Study design
This study is a pre-planned secondary analysis derived from multicenter, prospective, observational study (NCT04948242) between February 22, 2020 and May 11, 2020, consisting of a large-scale data source of critical ill patients to determine differential clinical response to corticosteroid use in whole populations and in each phenotype group. Recorded variables are shown in e-Table 1. No other superinfections except ventilator-acquired pneumonia were recorded.
The study was approved by the reference institutional review board at Joan XXIII University Hospital (IRB# CEIM/066/2020) and each participating site (63 Spanish ICUs) with a waiver of informed consent.
Clinical phenotypes
The characteristics of the phenotype derivation have been published elsewhere.16 In summary, to determine presence of distinct clinical phenotypes, an unsupervised clustering analysis was applied and three different clinical phenotypes were derived: (1) Cluster A phenotype (severe disease); (2) Cluster B phenotype (critical disease) and (3) Cluster C phenotype (life-threatening disease). The characteristics of each phenotype are shown in Table 1 and more detailed information on the development of phenotypes is available in supplementary material.
Table 1.
Characteristics of 2017 critically ill patients included in machine learning analysis according to overall or cluster (phenotype) population.
| Variable | Overall n = 2017 |
A phenotype n = 537 |
B phenotype n = 623 |
C phenotype n = 857 |
|---|---|---|---|---|
| General characteristics and severity of illness | ||||
| Age, median (p25–75), years | 64 (55–71) | 63 (53–70) | 63 (53.5–71.5) | 66 (58–72)*** |
| Male, n (%) | 1419 (70.3) | 377 (70.2) | 416 (66.8) | 626 (73.0)* |
| APACHE II, median (p25–75), | 13 (10–17) | 12 (9–16) | 13 (10–16) | 17 (14–22)*** |
| SOFA, median (p25–75), | 5 (3.7) | 4 (3–5) | 5 (3–7) | 7 (6–8)*** |
| GAP diagnosis, median (p25–75) | 6.2 (4.0–8.0) | 7.0 (4.0–9.0) | 6.0 (4.0–8.0)* | 6.0 (4.3–8.0)* |
| GAP UCI, median (p25–75) | 2.0 (0.0–4.0) | 2.0 (1.0–4.0) | 2.0 (1.0–4.0) | 1.1 (0.0–3.0)** |
| Laboratory findings | ||||
| d-Lactate dehydrogenase, median (p25–75), U/L | 537 (417–707) | 474 (372–564) | 477 (378–570) | 670 (554–929)*** |
| White blood cell, median (p25–75), ×109 | 8.8 (6.2–12.2) | 7.7 (5.8–10.2) | 8.5 (6–11.7) | 10 (6.9–13.6)*** |
| Serum creatinine, median (p25–75), mg/dL | 0.88 (0.7–1.1) | 0.80 (0.66–1.01) | 0.80 (0.66–1.00) | 0.99 (0.76–1.36)*** |
| C-reactive protein, median (p25–75), mg/mL | 15.5 (9.1–24.3) | 14 (8–2) | 14 (9–22) | 18 (10–26)*** |
| Procalcitonin, median (p25–75), ng/mL | 0.3 (0.1–2.0) | 0.2 (0.1–0.6) | 0.2 (0.1–0.5) | 0.5 (0.2–1.3)*** |
| Serum lactate, median (p25–75), mmol/L | 1.5 (1.1–2.0) | 1.5 (1.1–1.9) | 1.4 (1.0–1.9) | 1.6 (1.2–2.2)*** |
| D dimer, median (p25–75), ng/mL | 1593 (720–3790) | 1090 (580–2100) | 1319 (634–3548) | 2260 (1009–4894)*** |
| Ferritin, median (p25–75), ng/mL | 1600 (1290–2240) | 1538 (1280–1899) | 1554 (1271–1936) | 1800 (1416–2377)*** |
| Coexisting condition and comorbidities | ||||
| Arterial hypertension, n (%) | 932 (46.2) | 211 (39.3) | 173 (27.8) | 548 (63.9)*** |
| Obesity (BMI > 30), n (%) | 653 (32.3) | 159 (29.6) | 200 (32.1) | 294 (34.3) |
| Diabetes, n (%) | 418 (20.7) | 112 (20.9) | 108 (17.3) | 198 (23.1)* |
| Coronary arterial disease, n (%) | 124 (6.1) | 35 (6.5) | 41 (6.6) | 48 (5.6) |
| COPD, n (%) | 148 (7.3) | 37 (6.9) | 38 (6.1) | 73 (8.5) |
| Chronic renal disease, n (%) | 85 (4.2) | 31 (5.8) | 10 (1.6) | 44 (5.1)*** |
| Hematologic disease, n (%) | 72 (3.5) | 20 (3.7) | 22 (3.5) | 30 (3.5) |
| Asthma, n (%) | 120 (5.9) | 41 (7.6) | 45 (7.2) | 34 (4.0)** |
| HIV, n (%) | 5 (0.2) | 2 (0.4) | 1 (0.2) | 2 (0.2) |
| Pregnancy, n (%) | 4 (0.19) | 1 (0.2) | 3 (0.5) | 0 (0.0) |
| Autoimmune disease, n (%) | 74 (3.6) | 20 (3.7) | 18 (2.9) | 36 (4.2) |
| Chronic heart disease, n (%) | 57 (2.8) | 21 (3.9) | 10 (1.6) | 26 (3.0) |
| Neuromuscular disease, n (%) | 16 (0.8) | 3 (0.6) | 5 (0.8) | 8 (0.9) |
| Oxygenation and ventilator support | ||||
| Oxygen mask, n (%) | 325 (16.1) | 124 (23.1) | 105 (16.9)** | 96 (11.2)*** |
| High flow nasal cannula, n (%) | 375 (18.6) | 345 (64.2) | 3 (0.5)*** | 27 (3.2)*** |
| Non-invasive ventilation, n (%) | 140 (6.9) | 64 (11.9) | 26 (4.2)*** | 50 (5.8)*** |
| Invasive mechanical ventilation, n (%) | 1172 (58.1) | 3 (0.6) | 475 (76.2)*** | 694 (81.0)*** |
| PaO2/FiO2, median (p25–75) | 132 (96–163) | 111 (82–133) | 165 (144–212)*** | 126 (88–155)*** |
| Complications and outcome | ||||
| Shock, n (%) | 904 (44.8) | 56 (10.4) | 196 (31.5) | 652 (76.1) |
| Acute kidney dysfunction, n (%) | 579 (28.7) | 111 (20.7) | 118 (18.9) | 350 (40.8)*** |
| Myocardial dysfunction, n (%) | 169 (8.3) | 30 (5.6) | 43 (6.9) | 96 (11.2)*** |
| >2 quadrant infiltrates in chest X-ray, n (%) | 1327 (65.7) | 341 (63.5) | 413 (66.3) | 573 (66.8) |
| ICU crude mortality, n (%) | 657 (32.6) | 109 (20.3) | 159 (25.5)* | 389 (45.4)*** |
Abbreviations: p25–27, percentile range; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency viruses; PaO2/FiO2, partial pressure arterial oxygen/fraction of inspired oxygen.
All comparisons were made with respect to phenotype A considered as the reference.
p < .05.
p < .01.
p < .001, others comparison p > .01.
Corticosteroids treatment
Corticosteroid treatment was defined as administration of methylprednisolone or dexamethasone; within 24–48 h prior or first 24 h of ICU admission. Patients receiving corticosteroids outside the established timeframe (i.e., 24–48 h) or when hydrocortisone was administered as rescue therapy due to shock or to treat COPD/asthma exacerbation were excluded. Methylprednisolone (40 mg/day) or dexamethasone (6 mg/day) were administered at the discretion of the attending physician for 7–10 days. High doses bolus of corticosteroids were not administrated at any patients.
Definitions
Ventilator associated pneumonia: the definition was based on current American Thoracic Society and Infectious Disease Society of America guidelines.17
Cardiac dysfunction was defined by the assistant physician. Left ventricular systolic dysfunction was assessed by echocardiographic and EF estimated visually. Left ventricular systolic dysfunction was defined as EF < 50% and was reported in the CRF as present or absent. No specific echocardiography data had been requested at the time of analysis.
Other definitions used in the study are shown in supplemental online content.
Cluster homogeneity
A cluster is intrinsically homogeneous in the basis of the features used to generate the cluster.18 The homogeneity in each cluster allows us to study the impact of a target treatment within clusters and relate that impact among each clusters’ distinctive features. This analysis was possible considering the target treatment under study (corticosteroids) was not used for cluster derivation. Therefore, any further impact can be seen as unbiased and independent from former analyses.
Statistical analysis
Discrete variables were expressed as counts (percentage) and continuous variables as means with standard deviation (SD) or medians and percentile range 25–75% (p25–75). For patient demographics and clinical characteristics, differences between groups were assessed using the chi-squared test and Fisher's exact test for categorical variables, and the Student t test or the Mann–Whitney U test for continuous variables.
Inter-hospital variation in corticosteroids treatment was assessed by multilevel conditional logistic modeling19 with patients nested in each hospital and by to calculate the intraclass correlation coefficient (ICC). The ICC quantifies the degree of homogeneity of the outcome within cluster and represents the proportion of the between-hospital variation in the total variation.
In the first step we assess the impact of corticosteroid treatment among the general population. An optimal full-matching propensity score (PS) analysis was performed. This method performs optimal full matching, which is a form of sub-classification wherein all units, both treatment and control are assigned to a subclass and receive at least one match. Advantage of optimal full matching include is that the number of patients is not reduced.20 We checked model performance with a cross validation and the patients were randomly divided into two subsets: (a) a “training set” with 1613 patients (80%), and (b) a “validation set” with 404 patients (20%). Subsequently, a logistic regression analysis (LRA) for ICU mortality was carried out with the matched population to assess factors independently associated with mortality in the whole population. The results are presented as odds ratios (OR) and 95% CI and forest plots.
In the second step, we assessed the impact of corticosteroid treatment in each predetermined phenotype. We defined cohort entry hierarchically on the basis of exposure, such as the first prescription for the drug under study. Thus, first, subjects who receive the treatment under study were considered “exposed” and entered the cohort at the time they started exposure. Following with all other subjects that are then considered unexposed, and their cohort entry is defined arbitrarily (ICU admission) by a comparison treatment. When considering patients who received corticosteroids upon admission or 48 h prior ICU admission, we ensure that all patients have received the drug under study at the start of zero follow-up time (defined as ICU admission) and the immortal time bias is reduced. In addition, we performed a competing risks analysis21 to solve immortal time bias and confirm our results.
A Kaplan–Meier survival plot was generated to track ICU mortality over time for corticosteroid-treated and untreated patients in each clinical phenotype. The information provided by each variable regarding ICU mortality was defined using the information value (IV). A IV greater than 0.03 was considered clinically important and this variable was included in the LRA. In addition, a weighted Cox regression (wCox) was performed which yields unbiased estimates of average hazard ratios (HR) in case of non-proportional hazards.22
Finally, to investigate the association between baseline (ICU admission) variables and corticosteroid use; a LRA was performed with variables of clinical interest and all significant covariates in the univariate analysis. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). Data analysis was performed using R software (cran.r-project.org).
Results
A global approach
Corticosteroids response in whole population: a propensity full matching
A total of 2017 critically ill patients were included. The median (p25–75) age was 64 (55–71) years, and 1419 (70.3%) were men with an APACHE II of 13 (10–17) and SOFA of 5 (3–7) scores. Characteristics of whole and phenotypes population are shown in Table 1. An inter-hospital variation effect in the corticosteroids treatment was not observed (ICC = 0.04).
Among 1171 patients with corticosteroid therapy, 825 (70.5%) received methylprednisolone and 346 (29.5%) dexamethasone and 50 (4.2%) patients received hydrocortisone in combination treatment with the other steroids. No patient received hydrocortisone as the only treatment.
Patients received a median (p25–75) daily dose equivalent to 40 (30–60) mg of methylprednisolone and 6 (5–10) mg of dexamethasone, and the median duration of corticosteroid treatment was 7 (5–10) days. Main clinical characteristics of whole population and their distribution in the two groups are shown in e-Table 4.
Patients who received corticosteroid therapy had similar characteristics to those who did not receive them, except for lactate dehydrogenase (LDH), White Blood Cells count (WBC), ferritin and use of invasive mechanical ventilation(IMV). The crude ICU mortality was 32.6% and similar for patients with (33.8%) and without corticosteroids (30.8%).
PS matching was applied, and 846 control and 1171 treated patients were matched. The summaries of balance for unmatched and matched data are shown in e-Figure 1. When LRA for ICU mortality was performed, corticosteroids treatment was not associated with mortality (OR = 1.0; 95% CI 0.98–1.15) (e-Table 5). The discriminatory power of the model (e-Figure 2) was good with an area under ROC (AUC) of 0.78 (95% CI 0.75–0.82, p < 0.01) and an accuracy of 0.75.
A personalized approach
Corticosteroids treatment response among the A phenotype
Therapeutic impact among the A phenotype, was assessed among 298 (55.5%) patients that received corticosteroids as co-adjuvant therapy for viral pneumonia (e-Table 4). The crude ICU mortality was 20.3%. Non-survivors’ (n = 109) were older (70 vs. 60; p = 0.001), with high APACHE II (15 vs. 11, p = 0.001) and SOFA (5 vs. 3, p = 0.001), higher inflammatory status and more incidence of acute kidney injury (AKI: 48.6% vs. 13.6%, p = 0.001) and myocardial dysfunction (15.6% vs. 3.0%, p = 0.001) than survivors (e-Table 6). Conversely, corticosteroid treatment was not associated with mortality. Ventilator-associated pneumonia (VAP) diagnosis was not significantly different between patients with (12.8%) and without (14.6%, p = 0.61) corticosteroids treatment (e-Table 4).
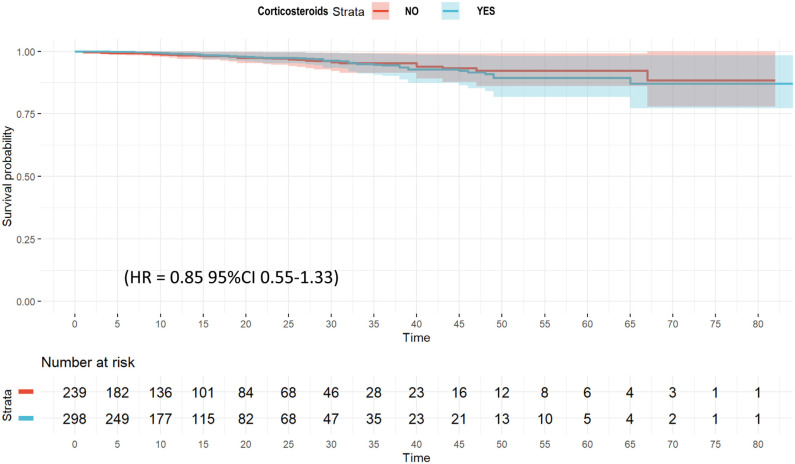
The unadjusted probability of survival (Kaplan–Meier plot) is shown in e-Figure 3. No significant differences were observed (p = 0.58) between groups. Twenty-eight variables were included in the wCox model (e-Table 7) and corticosteroids had no effect on ICU mortality (HR = 0.85; 95% CI 0.55–1.33) (Fig. 1 and e-Table 8). When a regression model for competing risk was performed (e-Figure 4), corticosteroid use remained as a factor not associated with mortality (SHR = 0.85 [95% CI 0.55–1.83).
Figure 1.
Weighted Cox hazard regression plot for ICU mortality among A phenotype patient's.
No significant differences were observed in laboratory findings or clinical characteristics of patients that received or not corticosteroids (e-Table 4), except for white blood cell counts (WBC), serum ferritin and the number of patients with more than 2 quadrant infiltrates in chest X-ray, more frequent in patients that received corticosteroid. These variables plus APACHE II, SOFA, age, IMV, obesity and CRP were included in LRA. Only presence more than 2 quadrant infiltrates in chest X-ray (OR = 1.5; 95% CI 1.05–2.16) was associated with use of corticosteroids (e-Table 9).
Corticosteroids treatment response among the B Phenotype
Therapeutic impact among the B phenotype, was assessed among 338 (54.2%) patients that received corticosteroids (e-Table 4). The crude ICU mortality was 25.5%. Non-survivors’ (n = 159) patients were older (71 vs. 61; p = 0.001), with high APACHE II (15 vs. 12, p = 0.001) and SOFA (6 vs. 4, p = 0.001), higher inflammatory status and more incidence of AKI (37.7% vs. 12.5%, p = 0.001) and myocardial dysfunction (11.9% vs. 5.2%, p = 0.001) than survivors. VAP was more frequent in patients with (18.6%) than without corticosteroids treatment (11.9%, p = 0.02). Conversely, corticosteroid treatment was not associated with mortality (e-Table 10).
The unadjusted probability of survival (Kaplan–Meier plot) is shown in e-Figure 5. No significant differences were observed between groups (p = 0.58).
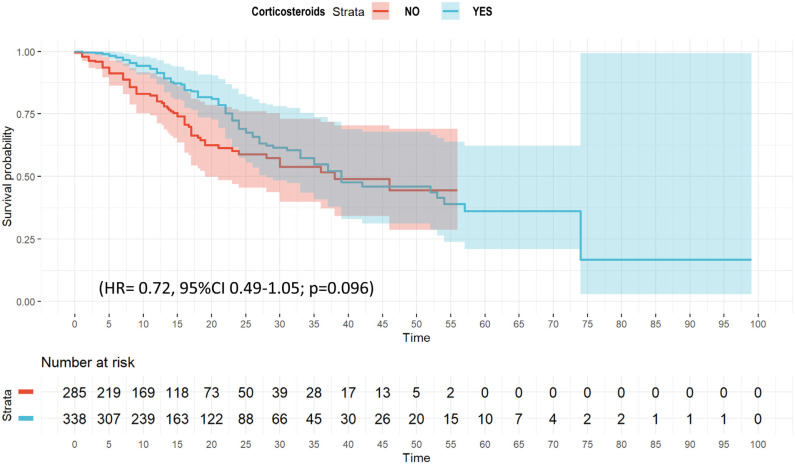
Twenty variables were included in the wCox model (e-Table 7) that confirmed no association between corticosteroid and ICU mortality (HR = 0.72, 95% CI 0.49–1.05; p = 0.096) (Fig. 2 and e-Table 11). The multivariate regression model for competing risk (e-Figure 6), suggest that corticosteroid use was associated with outcome (SHR = 0.65 [95% CI 0.46–0.91]).
Figure 2.
Weighted Cox hazard regression plot for ICU mortality among B phenotype patient's.
No significant differences were observed in laboratory findings or clinical characteristics of patients that received or not corticosteroids (e-Table 4), except for LDH, WBC, serum lactate, and IMV use more frequent in patients with corticosteroid treatment. These variables plus APACHE II, SOFA, age, IMV, obesity and CRP were included in the LRA. Only LDH OR = 1.0 [1.01–1.2], serum lactate (OR = 1.1 [1.03–1.26]) and WBC (OR = 1.04 [1.01–1.08]) were associated with use of corticosteroids (e-Table 12).
Corticosteroids treatment response among the C phenotype
Therapeutic impact among the C phenotype, was assessed among 535 (62.4%) patients that received corticosteroids (e-Table 4). The crude ICU mortality was 45.4%. Non-survivors’ (n = 389) patients were older (68 vs. 63; p = 0.001), with high APACHE II (18 vs. 15, p = 0.001) and SOFA (7.4 vs. 7.0, p = 0.001) than survivors. Corticosteroid treatment was not associated with mortality (e-Table 13).
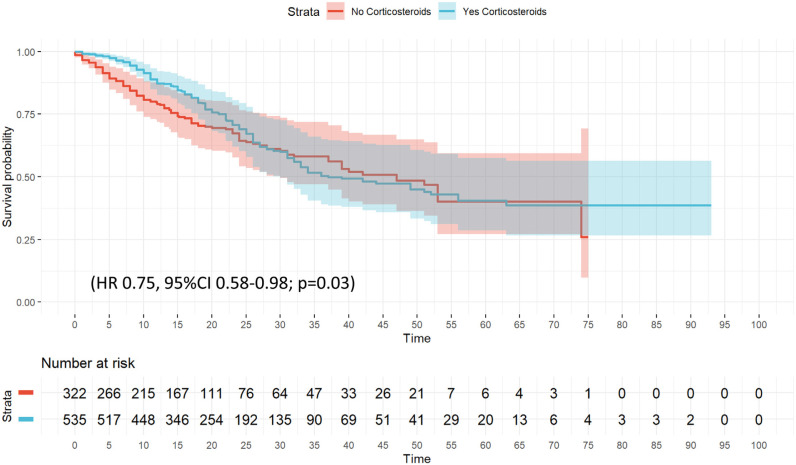
The unadjusted probability of survival (Kaplan–Meier plot) is shown in e-Figure 7. No significant differences were observed (p = 0.06). Twenty variables were included in the wCox model (e-Table 7) and corticosteroid treatment was associated with a protected effect (HR 0.75, 95% CI 0.58–0.98; p = 0.03) for ICU mortality (Fig. 3 and e-Table 14). The competing risk regression model (e-Figure 8) confirmed corticosteroid treatment as a protective factor for ICU mortality (SHR = 0.79 [95% CI 0.63–0.98]).
Figure 3.
Weighted Cox hazard regression plot for ICU mortality among C phenotype patient's.
No significant differences were observed in laboratory findings or clinical characteristics of patients that received or not corticosteroids (e-Table 4), except for LDH higher in patients with corticosteroid treatment. Development of VAP was higher in patients with corticosteroid treatment (20.4% vs. 14.6%, p = 0.04) (e-Table 4). These variables plus clinically relevant variables as APACHE II, SOFA, age, IMV, obesity and CRP were included in LRA. Only LDH (OR = 1.0, 95% CI 1.01–1.02) was associated with use of corticosteroids (e-Table 15).
Discussion
This represents the first built machine learning model used to assess the effect of corticosteroids therapy according to pre-defined clinical phenotypes among a large cohort of critically ill patients with severe COVID-19 disease. The main finding of our study is that the use of corticosteroids was not associated with improved outcomes in all critically ill patients with COVID-19 at moderate dose.
The challenge in developing optimal treatment strategies is the extreme heterogeneity of presentation in COVID-19 patients who are critically ill.15, 16 Consequently, our study suggests that only clinical phenotypes with a high degree of systemic inflammation, such as the defined phenotype C, may have an early benefit from steroid treatment. Benefits offered by corticosteroids in attenuating immune dysregulation must be balanced with their inhibitory effect on the immune response needed to control viral replication, as well as risk of opportunistic infections and associated side-effects.13, 14 Specifically, our results show a higher incidence of VAP in patients who have received corticosteroids in B and C phenotypes.
Data from the RECOVERY Trial8 and WHO meta-analysis,11 supported the administration of 6 mg dexamethasone for all patients with COVID-19 who required oxygen supplementation or IMV. However, the role of corticosteroids in the treatment of COVID-19 remains controversial.23, 24, 25, 26, 27, 28, 29 A recent study in France30 comparing first vs. second wave reported that, despite of the systematic and early administration of glucocorticoids in the second wave, the ICU mortality (50% vs. 52%, p = 0.96) and duration of ICU stay did not differ between the two waves. In contrast, Wu et al.31 observed in 380 patients that, low-dose corticosteroid treatment was associated with reduced risk of in-hospital death within 60 days in COVID-19 patients who developed ARDS. However, it should be noted that this study only included patients with ARDS, and corticosteroids were initiated 13 days after symptom and this is not the usual clinical practice.
Chen et al.15 observed presence of two phenotypes (hypo and hyper-inflammatory) among COVID-19 patients. Interestingly, after applying a marginal structural modeling, the association between corticosteroid therapy and 28-day mortality was only observed in patients with the hyper-inflammatory phenotype. These findings are consistent with our results, where only the phenotype C (with a higher inflammatory status), seem to have benefit from corticosteroid treatment. This observation is contrary to the current recommendation of dexamethasone treatment according to the RECOVERY trial,8 that showed that the mortality from COVID-19 was lower among patients who were randomized to receive dexamethasone than among those who received the standard of care. Several limitations have been reported since its publication.2, 3, 32, 33 Possibly the most important limitations are the lack of an adjustment according to severity of illness to minimize potential bias and that mortality has been censored at 28 days, and no data have been published from the mortality at ICU or hospital discharge.
Survival benefit of corticosteroids8 appeared greatest among patients who required IMV. These findings are consistent with our results, as between 70 and 80% of patients in phenotypes B and C required ventilation and then could benefit from steroids. In the other hand, in the RECOVERY trial8 a favorable effect on survival was evident with the use of steroids treatment among patients who only required supplemental oxygen. This sub-group of patients can be said to represent a similar profile to that of the A phenotype from our study, where more than 80% of patients received only supplemental oxygen at ICU admission. However, we do not observe the impact of corticosteroid treatment on survival in this phenotype and our results strongly suggest that corticosteroid treatment should not be administered to patients who do not require mechanical ventilation independently of their hypoxemia level. Differences in results could be due to our patient adjustment modeling according to severity in illness and the absence of stratification and incomplete information about some factors associated with outcome in the RECOVERY trial may have resulted in imbalance between the treated and control.26 In addition, a recent prospective study34 with more than 3000 elderly critically ill COVID-19 patients, observed an independent association of steroid use with increased 30-days mortality after multivariable adjustment (aOR 1.60; 95% CI 1.26–2.04).
However, some study limitations should be noted. First, although phenotypes were found to be generalizable in our population (after validation), risk factors and characteristics that pre-defined these clinical phenotypes were derived initially from data at ICU admission of a multicenter observational study in Spain. However, at the same time these risk factors are similar to those that have been reported by other investigators31, 35, 36 which suggests its applicability to other populations.
Second, only routinely available clinical data at ICU admission was used to identify risk factors and clinical phenotypes, and the inclusion of other data related to clinical evolution of patients in the ICU could change risk factors or phenotype assignments. However, our objective was to study early risk factors and phenotypes at ICU admission that may allow for early treatment implementation and as a result improve patient outcome.
Third, this is a sub-analysis conducted following the author's primary observational study in order to consider only segmental measured confounders. The authors are aware of the limitations presented by the exclusion of other residual measured confounders and unmeasured confounders that could not be included fully.
Fourth, we cannot affirm that an echocardiographic assessment has been carried out in all patients, so the incidence of cardiac dysfunction may be higher than that observed. This incidence should be considered with caution.
Finally, this study did not collect data that could assess the impact of ethnicity, socioeconomic factors o long-term complications. These factors may play a role in the prevalence of pre-existing comorbidities and mortality due to COVID-19.
Conclusion
Our findings warn against the widespread use of corticosteroids in all critically ill patients with COVID-19 according to the moderate dose and suggest the need to determine within each phenotype what subset of patients may really benefit from treatment.
Authors’ contributions
Alejandro Rodríguez, Gerard Moreno, Manuel Ruiz-Botella, Ignacio Martín-Loeches, María Jiménez Herrera, Jordi Sole-Violán, Josep Gómez, María Bodí, Sandra Trefler, Fernándo Armestar, Asunción Marques Parra, Angel Estella, Ruth Jorge García, Pablo Vidal-Cortes, Emili Díaz, Ricard Ferrer, Antonio Albaya-Moreno, Ana Loza, Laura Sánchez Montori, María deAlba-Aparicio, Mercedes Nieto, Judith Marín-Corral, Lorena Forcelledo Espina and Immaculada Vallverdú had substantial contributions to conception and design of the work.
Alejandro Rodríguez, Sandra Trefler, Manuel Ruiz-Botella, Ana Casamitjana Ortega, Fátima Martín Serano, Josep Gómez, Josep María Bonell Goytisolo, Susana Sancho Chinesta, Virgina Fraile Gutierrez, Angel Estella and Lorenzo Socias Crespi had substantial contribution for data acquisition.
Alejandro Rodríguez, Gerard Moreno, Manuel Ruiz-Botella, Ignacion Martín-Loeches, Josep Gomez, María Bodí, Judith Marín-Corral, Antonio Alabaya Moreno and Angel Estella had substantial contribution for data analysis and interpretation of data for the work.
Alejandro Rodríguez, Gerard Moreno, Ignacio Martín-Loeches, Josep Gómez and Emili Díaz drafting of the manuscript.
Ricard Ferrer, María Bodí, Judith Marín-Corral, Juan Carlos Pozo Laderas, Antonio Albaya Moreno and Jordi Solé-Violán critically reviewed the draft manuscript.
The corresponding author (Alejandro Rodríguez) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript. The views expressed in this article are those of the authors and not necessarily those of the SEMICYUC.
Funding
This study was supported by the Spanish Intensive Care Society (SEMICYUC), Ricardo Barri Casanovas Foundation Grant (A. Rodríguez) and Grant of Pla Estratègic de Recerca i Innovació en Salut-Generalitat de Catalunya PERIS SLT017/20/000030 (M. Bodi, M. Ruíz-Botella, J. Gómez, A. Rodríguez). The study sponsors have no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Acknowledgements
Alexis Garduno, MSc provided a factual review and helped edit the manuscript and the COVID-19/SEMICYUC Working Group (complete list of investigators in Appendix 2).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.medine.2021.10.016.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Thompson B.T., Chambers R.C.L.K. Acute respiratory distress syndrome. N Engl J Med. 2017;10:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Arabi Y.M., Chrousos G.P., Meduri G.U. The ten reasons why corticosteroid therapy reduces mortality in severe COVID-19. Intensive Care Med. 2020;46:2067–2070. doi: 10.1007/s00134-020-06223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Pascale G., Bello G., Dell’Anna A.M., Montini L., Antonelli M., Moreno G., et al. Steroids and severe pneumonia. Ready for the winter? Discussion on “Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study”. Intensive Care Med. 2018;44:2319–2320. doi: 10.1007/s00134-018-5414-3. [DOI] [PubMed] [Google Scholar]
- 4.Hui D.S. Systemic corticosteroid therapy may delay viral clearance in patients with middle east respiratory syndrome coronavirus infection. Am J Respir Crit Care Med. 2018;197:700–701. doi: 10.1164/rccm.201712-2371ED. [DOI] [PubMed] [Google Scholar]
- 5.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Lim W.S. Corticosteroids as adjunctive therapy in the treatment of influenza (Review) Cochrane Database Syst Rev. 2016:1–51. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 7.Moreno G., Rodríguez A., Reyes L.F., Gomez J., Sole-Violan J., Díaz E., et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 2018;44:1470–1482. doi: 10.1007/s00134-018-5332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Meng Q., Rao X., Wang B., Zhang X., Donget F., et al. Corticosteroid therapy in critically ill patients with COVID-19: a multicenter, retrospective study. Crit Care. 2020;24:1–10. doi: 10.1186/s13054-020-03429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., Zhang S., Dong X., Li Z., Xu Q., Feng H., et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130:6417–6428. doi: 10.1172/jci140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . 2020. Corticosteroids for COVID-19. Living Guidance – 2 September 2020; pp. 1–25. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1%0Ahttps://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d?question_domain=5b1dcd8ae611de7ae84e8f14&population=5e7fce7e3d05156b5f5e032a&intervention=5d2b2b62daeedf1d3af33331. [Google Scholar]
- 12.NIH. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Nih. 2020;2019:130. Available from: https://covid19treatmentguidelines.nih.gov/. [PubMed]
- 13.Rodriguez-Morales A.J., Sah R., Millan-Oñate J., Gonzalez A., Montenegro-Idrogo J.J., Scherger S., et al. COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs. Ther Adv Infectious Dis. 2021;8:1–5. doi: 10.1177/20499361211027065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song G., Liang G., Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185:599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H., Xie J., Su N., Wang J., Sun Q., Li S., et al. Corticosteroid therapy is associated with improved outcome in critically ill COVID-19 patients with hyperinflammatory phenotype. Chest. 2020 doi: 10.1016/j.chest.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez A., Ruiz-Botella M., Martín-Loeches I., Jimenez Herrera M., Solé-Violan J., Gómez J., et al. Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit Care. 2021;25:63. doi: 10.1186/s13054-021-03487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalil A.C., Metersky M.L., Klompas M., Muscedere J., Sweeney D.A., Palmer L.B., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato-Ilic M. Homogeneous cluster analysis. Proc Comput Sci. 2018;140:269–275. doi: 10.1016/j.procs.2018.10.320. [DOI] [Google Scholar]
- 19.Sommet N., Morselli D. Keep calm and learn multilevel logistic modeling: a simplified three-step procedure using Stata, R, Mplus, and SPSS. Int Rev Soc Psychol. 2017;30:203–218. doi: 10.5334/irsp.90. [DOI] [Google Scholar]
- 20.Austin P.C., Stuart A.E. Optimal full matching for survival outcomes: a method that merits more widespread use. Stat Med. 2015;34:3949–3967. doi: 10.1002/sim.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 22.Dunkler D., Ploner M., Schemper M., Heinze G. Weighted Cox regression using the R package coxphw. J Stat Software. 2018;84:1–26. doi: 10.18637/jss.v084.i02. [DOI] [Google Scholar]
- 23.Peter J.V., John P., Graham P.L., Moran J.L., George I.A., Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336:1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auyeung T.W., Lee J.S.W., Lai W.K., Choi C.H., Lee H.K., Lee J.S., et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. 2005;51:98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cano E.J., Fonseca Fuentes X., Corsini Campioli C., O’Horo J.C., Saleh O.A., Odeyemi Y., et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest. 2020 doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Backer D., Azoulay E., Vincent J.L. Corticosteroids in severe COVID-19: a critical view of the evidence. Crit Care. 2020;24:1–3. doi: 10.1186/s13054-020-03360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasin L., Navalesi P., Zangrillo A., Kuzovlev A., Fresilli S. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2021;35:578–584. doi: 10.1053/j.jvca.2020.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zangrillo A., Landoni G., Monti G., Yavorovskiy A.G., Baiardo Redaelli M. Dexamethasone in COVID-19: does one drug fits all? Med Intensiva (Engl Ed) 2021 doi: 10.1016/j.medin.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estella Á., Garcia Garmendia J.L., de la Fuente C., Machado Casas J.F., Yuste M.E., Amaya Villar R., et al. Predictive factors of six-week mortality in critically ill patients with SARS-CoV-2: a multicenter prospective study. Med Intensiva (Engl Ed) 2021 doi: 10.1016/j.medin.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contou D., Fraissé M., Pajot O., Tirolien J.A., Mentec H., Plantefève G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care. 2021;25:3. doi: 10.1186/s13054-020-03449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C., Hou D., Du C., Cai Y., Zheng J., Xu J., et al. Corticosteroid therapy for coronavirus disease 2019-related acute respiratory distress syndrome: a cohort study with propensity score analysis. Crit Care. 2020;24:1–10. doi: 10.1186/s13054-020-03340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershengorn H.B. Early adoption of critical care interventions is unjustifiable without concomitant effectiveness study. Crit Care. 2020;24:10–12. doi: 10.1186/s13054-020-03382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattos-silva P., Felix N.S., Silva P.L., Robba C., Battaglini D., Pelosi P., et al. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir Physiol Neurobiol. 2020;280:103492. doi: 10.1016/j.resp.2020.103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung C., Wernly B., Fjølner J., Romano Bruno R., Dudzinski D., Artigas A., et al. Steroid use in elderly critically ill COVID-19 patients. Eur Respir J. 2021 doi: 10.1183/13993003.00979-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;02115:1–11. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.