Key Points
Question
Among patients with chronic radicular pain after lumbar spine surgery, does spinal cord burst stimulation affect back pain–related disability?
Findings
In this crossover randomized clinical trial that involved 50 participants who underwent placement of a spinal cord stimulator, there was no significant difference in change from baseline for the self-reported Oswestry Disability Index (range, 0 points [no disability] to 100 points [maximum disability]; minimal clinically important difference, 10 points) during the spinal cord burst stimulation periods vs the placebo stimulation periods (mean change, −10.6 points vs −9.3 points, respectively).
Meaning
Among patients with chronic radicular pain after lumbar spine surgery, spinal cord burst stimulation, compared with placebo stimulation, resulted in no significant difference in back pain–related disability.
Abstract
Importance
The use of spinal cord stimulation for chronic pain after lumbar spine surgery is increasing, yet rigorous evidence of its efficacy is lacking.
Objective
To investigate the efficacy of spinal cord burst stimulation, which involves the placement of an implantable pulse generator connected to electrodes with leads that travel into the epidural space posterior to the spinal cord dorsal columns, in patients with chronic radiculopathy after surgery for degenerative lumbar spine disorders.
Design, Setting, and Participants
This placebo-controlled, crossover, randomized clinical trial in 50 patients was conducted at St Olavs University Hospital in Norway, with study enrollment from September 5, 2018, through April 28, 2021. The date of final follow-up was May 20, 2022.
Interventions
Patients underwent two 3-month periods with spinal cord burst stimulation and two 3-month periods with placebo stimulation in a randomized order. Burst stimulation consisted of closely spaced, high-frequency electrical stimuli delivered to the spinal cord. The stimulus consisted of a 40-Hz burst mode of constant-current stimuli with 4 spikes per burst and an amplitude corresponding to 50% to 70% of the paresthesia perception threshold.
Main Outcomes and Measures
The primary outcome was difference in change from baseline in the self-reported Oswestry Disability Index (ODI; range, 0 points [no disability] to 100 points [maximum disability]; the minimal clinically important difference was 10 points) score between periods with burst stimulation and placebo stimulation. The secondary outcomes were leg and back pain, quality of life, physical activity levels, and adverse events.
Results
Among 50 patients who were randomized (mean age, 52.2 [SD, 9.9] years; 27 [54%] were women), 47 (94%) had at least 1 follow-up ODI score and 42 (84%) completed all stimulation randomization periods and ODI measurements. The mean ODI score at baseline was 44.7 points and the mean changes in ODI score were −10.6 points for the burst stimulation periods and −9.3 points for the placebo stimulation periods, resulting in a mean between-group difference of −1.3 points (95% CI, −3.9 to 1.3 points; P = .32). None of the prespecified secondary outcomes showed a significant difference. Nine patients (18%) experienced adverse events, including 4 (8%) who required surgical revision of the implanted system.
Conclusions and Relevance
Among patients with chronic radicular pain after lumbar spine surgery, spinal cord burst stimulation, compared with placebo stimulation, after placement of a spinal cord stimulator resulted in no significant difference in the change from baseline in self-reported back pain–related disability.
Trial Registration
ClinicalTrials.gov Identifier: NCT03546738
This placebo-controlled, crossover randomized clinical trial compares the effects of spinal cord burst stimulation vs placebo stimulation in patients with chronic radiculopathy after surgery for degenerative lumbar spine disorders.
Introduction
Although the use of spinal cord stimulation for chronic pain has increased, with the global market size expected to grow by more than 8% annually and reach $2.8 billion within 3 years,1 evidence for its efficacy and cost-effectiveness is limited.1,2 It is estimated that up to 50 000 patients currently undergo spinal cord stimulation treatment per year worldwide,1,3 and one of the most common indications is persistent radicular pain following lumbar spine surgery.4 In epidemiological studies from the last decade,5,6,7 surgery for degenerative spine disease did not achieve the desired result for approximately 30% of patients, and residual or worsened pain after spinal surgery is notoriously difficult to treat.
Spinal cord stimulation involves the placement of a subcutaneous implantable pulse generator connected to electrodes with leads that travel into the epidural space posterior to the spinal cord dorsal columns (eFigure 1 in Supplement 1). The mechanisms by which spinal cord stimulation potentially inhibits pain appear to be related to spinal gate control, alteration of neurotransmitter levels, promotion of inhibitory interneuron activation, effects on glial and immune cells, and supraspinal mechanisms.1,8,9 An important element of traditional spinal cord stimulation therapy has been replacement of pain with paresthesia. Recent advances allow paresthesia-free treatment and it has been suggested that these novel modalities, including burst spinal cord stimulation, improve the tolerability of the intervention and offer better relief of chronic pain after spine surgery.9,10,11,12,13
Spinal cord burst stimulation involves delivery of intermittent “trains” of closely spaced, high-frequency electrical stimuli to the spinal cord.9 Spinal cord stimulation devoid of treatment-induced paresthesia allows for placebo-controlled trials, but few have been performed in patients with chronic pain after spine surgery, and the suggested effect remains uncertain due to a high risk of bias.4,13,14 To better inform clinical decision-making for chronic radicular pain after lumbar spine surgery, a crossover randomized clinical trial was conducted to compare spinal cord burst stimulation with placebo stimulation.
Methods
Trial Design and Oversight
The trial was approved by the regional committee for medical research ethics in southeast Norway before starting patient recruitment. All patients provided written informed consent before inclusion in the trial. In this investigator-initiated, single-center, quadruple-blinded, placebo-controlled, crossover randomized clinical trial, we evaluated the effects of spinal cord burst stimulation compared with placebo stimulation in patients with chronic radicular pain after lumbar spine surgery who underwent placement of a spinal cord stimulator. Burst stimulation consisted of closely spaced, high-frequency electrical stimuli delivered to the spinal cord. Details of the trial design, conduct, oversight, and the analyses appear in the trial protocol and statistical analysis plan (Supplement 2).
Assessment of trial eligibility and postoperative follow-up appointments were performed at the multidisciplinary outpatient clinic for back, neck, and shoulder rehabilitation at St Olavs University Hospital in Trondheim, Norway. All surgical procedures were performed at the Department of Neurosurgery, St Olavs University Hospital, which has extensive clinical experience with spinal cord stimulation for more than 40 years. The Unit for Applied Clinical Research at the Norwegian University of Science and Technology was the trial coordinating center and was responsible for the randomization scheme, analyses, and trial coordination.
Patients
Patients aged 18 years or older were assessed for eligibility if (1) they had undergone at least 1 decompressive or fusion procedure for degenerative lumbar spine disease, (2) they experienced postoperative chronic radicular pain refractory to nonsurgical treatment for a minimum of 6 months, (3) they reported average pain intensity with a minimum of 5 on scale of 1 to 10 for leg pain using the Numeric Rating Scale (higher scores indicate more severe pain; 0 meant “no pain” and 10 meant the “worst pain imaginable”), and (4) no additional spine surgery or pharmacological treatment was assumed to be beneficial. A 2-week spinal cord stimulation testing period with an external neurostimulator and epidural leads providing a reduction of at least 2 points for leg pain using the Numeric Rating Scale was mandatory before trial inclusion. Radicular pain was defined as pain arising from 1 or more spinal nerve roots, and the diagnosis was based on pain characteristics, clinical examination, sensorimotor testing, and review of diagnostic imaging.
Patients were ineligible if they had been previously treated with spinal cord stimulation or subcutaneous nerve stimulation. Exclusion criteria also included abnormal pain behavior, unresolved psychiatric illness, unresolved issues of possible secondary gain, and inappropriate medication use (eg, misuse of sedatives or substance use disorders). Race/ethnicity data were collected from participants using fixed categories in the baseline questionnaire to provide a detailed definition of the study population.
Randomization
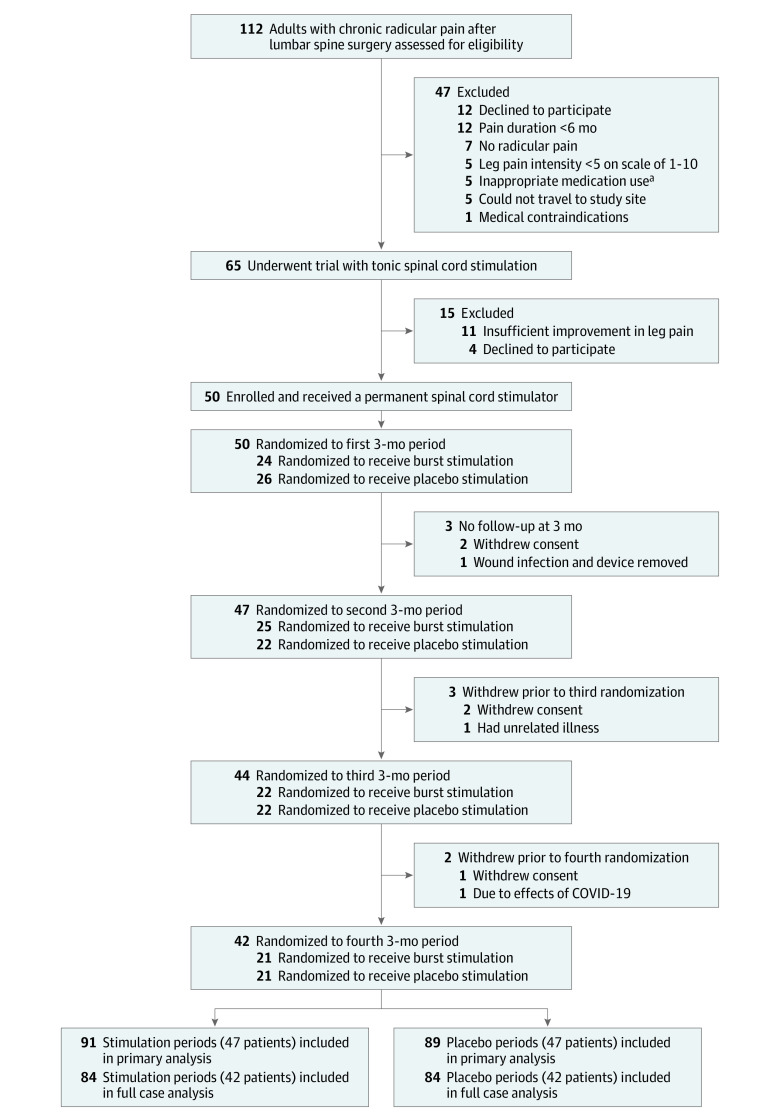
A block randomization sequence was generated manually (block size of 6). Patients underwent 2 periods of burst stimulation and 2 periods of placebo stimulation in a randomized order, meaning 6 treatment allocation sequences were available (Figure 1). The allocation envelopes for the planned number of study participants were made before commencement of the trial. Each allocation envelope contained 4 smaller sealed sequentially numbered envelopes with a paper inside stating the treatment allocation. The allocation envelopes were made in block sizes of 6 that included all possible allocation sequences, and then randomly assigned consecutive participant numbers. With the exception of the clinical trial nurse who performed the actual setting of the stimulators and who collected data from the trial participants, everyone else (ie, patients, surgeons, investigators, and the statistician) was blinded to the actual treatment allocations during the whole duration of the trial. The statistician and all personnel evaluating the trial outcomes were blinded to treatment allocation until the predefined statistical analyses were completed and the data tables were filled in.
Figure 1. Screening, Randomization, and Follow-up.
aMisuse of sedatives or substance use disorder.
Interventions
All surgical procedures were performed by the same neurosurgical team specialized in functional neurosurgery. During the testing period, patients received tonic stimulation (ie, they experienced paresthesia in the targeted spinal dermatomes) with an external neurostimulator. Epidural surgical lead insertion was performed while patients were in the prone position using local anesthetics and mild intravenous sedation to enable patient feedback and cooperation. The aim was to optimize lead placement over the dorsal columns of the spinal cord so that paresthesia occurred in the targeted spinal dermatome (ie, tonic conventional stimulation). A 16-contact lead (Infinion CX, Boston Scientific, Inc) was implanted for unilateral leg pain or two 8-contact leads (Linear ST, Boston Scientific, Inc) were implanted for bilateral leg pain through a small skin incision at the L1/L2 or L2/L3 vertebral levels and placed in the epidural space at the T9/T10 level under fluoroscopic guidance.
Intraoperative electrophysiological testing and stimulation were performed during longitudinal lead navigation. The leads were anchored at the optimal localization and their positions were confirmed with x-ray imaging. Leads were then connected to an external neurostimulator using extension cords. Programming software (Illumina 3D, Boston Scientific, Inc) was used to optimize tonic conventional stimulation and determine paresthesia thresholds during the testing period. If there was insufficient improvement in leg pain during the testing period, the leads were removed and the patients were excluded. If there was sufficient improvement in leg pain during the testing period, the patients were included in the trial and their external neurostimulator was replaced with a nonrechargeable implantable pulse generator (Precision Novi, Boston Scientific, Inc) placed subcutaneously on the upper buttock or abdomen under local anesthesia. A nonrechargeable pulse generator was chosen to avoid unblinding of patients.
Immediately after implantation of the stimulator, eligible patients underwent four 3-month periods of treatment. All patients underwent burst stimulation and placebo stimulation in a randomized order for two 3-month periods for each intervention. Burst stimulation consisted of closely spaced, high-frequency stimuli delivered to the spinal cord. The stimulus consisted of a 40-Hz burst mode of constant-current stimuli with 4 spikes per burst and an amplitude corresponding to 50% to 70% of the paresthesia perception threshold. At the end of each treatment allocation period, and after having collected self-reported outcome measures for that period, the trial nurse checked the impedance of the spinal cord stimulation system, used tonic stimulation to ascertain that stimulation could still be provided in the desired spinal dermatomes, and used burst stimulation to ascertain perception threshold measurements. Then patients were allocated to the next treatment period. Patients were only provided with handheld spinal cord stimulation programmers (ie, opportunity to change stimulation patterns) after completing the final randomization period and after collection of all outcome measures. The trial nurse was readily available to the patients in between scheduled follow-up appointments, regardless of treatment allocation.
The outcome measures were collected prior to the testing period and at the end of each of the 4 treatment allocation periods, ensuring a sufficient washout period of potential treatment effects from the preceding treatment allocation. The patients completed questionnaires without assistance from trial personnel.
Primary Outcome
The primary outcome was difference in change from baseline in the self-reported Oswestry Disability Index (ODI), version 2.0, score between periods with active burst stimulation and periods with placebo stimulation.15 The ODI has been translated into Norwegian and tested for psychometric properties.16 The ODI questionnaire is used to quantify back pain–related disability and covers 10 activities of daily living. For each item, there are 6 response alternatives (0-5) that are converted to a percentage index score; the score range is from 0 points (no disability) to 100 points (maximum disability). The minimal clinically important difference (MCID) for the ODI score is 10 points.17
Secondary Outcomes
The secondary outcomes were (1) changes from baseline in leg and back pain using the Numeric Rating Scale (score range, 0 to 10; higher scores indicate more severe pain; 0 indicates “no pain” and 10 indicates “worst pain imaginable”; MCID, 1.0 points),18 (2) changes from baseline in generic health-related quality of life measured with the 5-dimension EuroQol 3L index score (range, −0.594 to 1.000; 0 indicates a health status equivalent to death and 1 indicates perfect health; MCID, 0.03),19 (3) changes from baseline in the number of steps per day and the amount of time spent standing or walking measured using a body-worn accelerometer (ActivPAL, PAL Technologies, Ltd),20 (4) adverse events, (5) surgical revisions of the implanted spinal cord stimulator systems, and (6) a cost-effectiveness analyses if superiority of burst stimulation vs placebo stimulation was claimed.
Adverse events were self-reported by patients at 3 months using fixed categories, surgeons reported perioperative adverse events using fixed categories, and surgical revisions were registered until trial closure. Treatment allocation guesses were collected from participants using fixed categories in the follow-up questionnaires.
The Brief Pain Inventory and use of analgesics were specified as secondary outcomes in the trial protocol (§3.2 in Supplement 2), but were omitted before trial registration and commencement of the trial. The reason for omitting the Brief Pain Inventory was that pain is extensively covered by the other self-reported outcomes. The reason for omitting use of analgesics was that we did not want to overburden the study participants with data registration; several analgesics (ie, acetaminophen [paracetamol], ibuprofen) are available over-the-counter without a prescription and inappropriate medication use was an exclusion criterion.
Sample Size Calculation
For the sample size calculation, the outcome variable was defined as the difference between each participant’s mean ODI scores after undergoing burst stimulation and placebo stimulation. The trial was designed to detect a between-group difference of 10 points, corresponding to the MCID, in change in the mean ODI score between periods with burst stimulation and periods with placebo stimulation.17 Assuming that the population mean was 10 and the SD was 18 for the differences, a 1-sample t test of the differences at the .05 significance level needed 34 patients to achieve 90% power. Due to expected rates of 10% to 20% for patients lost to follow-up and potential breakthrough of paresthesia with risk of unblinding during burst stimulation in 20% to 30% of patients, we aimed at including 50 trial participants.
Statistical Analysis
The null hypothesis was that there was no between-group difference in mean change of ODI from baseline to the end of each intervention period. The primary efficacy analyses were performed in the full analysis population, which included all the patients who underwent randomization, received treatment, and had at least 1 complete follow-up questionnaire for the ODI.
Sensitivity analyses were performed in the complete case set, which included the subset of patients in the full analysis set that had ODI measurements at all follow-up visits. The 2 interventions were compared using a linear mixed model, accounting for repeated measurements in each patient. The fixed effect was the combination of time (baseline vs follow-up) and treatment, yielding 3 levels representing baseline, burst stimulation, and placebo stimulation. Due to variance heterogeneity over time, the covariance structure for the repeated measurements for each patient was handled as unstructured.
Statistical tests for the primary and secondary outcomes were performed at the 2-sided significance level of .05. The absolute between-intervention differences and 95% CIs were determined for the self-reported outcomes and daily physical activity. Missingness of data was handled with the use of mixed modeling and no imputations were performed.21 Period and sequence effects were not assessed in the statistical analyses.
A between-group difference in the intervention effect would be claimed if the null hypothesis was rejected. Superiority of burst stimulation would be claimed if the 2-sided P value was less than .05 from the test comparing the ODI change from baseline and the effect was in favor of burst stimulation. Because of the potential for type I error due to multiple comparisons, the findings from the analyses of the secondary outcomes should be interpreted as exploratory. The statistical analyses were performed using SPSS version 25.0 (IBM) and R version 3.6.3 (R Foundation for Statistical Computing).
Results
Patients
Patients were enrolled from September 5, 2018, through April 28, 2021. The 12-month follow-up finished on May 20, 2022. A total of 112 patients were screened for trial inclusion. Of the 65 patients who underwent a testing period with tonic spinal cord stimulation, 50 fulfilled all inclusion criteria and were randomized (Figure 1). Of these 50 patients, 47 (94%) had at least 1 follow-up ODI score and constituted the full analysis population. Of 180 treatment allocations (91 burst stimulation periods and 89 placebo stimulation periods) in the full analysis population, corresponding ODI measurements were available for 178. The full case analysis population consisted of 42 patients (84%) who completed all randomization periods and had ODI measurements at all follow-up visits.
Delay of follow-up appointments by more than 2 weeks due to COVID-19–related issues resulted in protocol deviations for 3 patients. The baseline characteristics of the patients appear in Table 1. The mean age was 52.2 years (SD, 9.9 years) and 27 of 50 patients (54%) were women. All were White patients and 47 (94%) were native Norwegian speakers.
Table 1. Baseline Characteristics, Coexisting Illnesses, and Measures of Health Status.
| Patients with chronic radicular pain (N = 50)a | |
|---|---|
| Age, median (IQR), y | 50 (45-59) |
| Sex | |
| Male | 23 (46) |
| Female | 27 (54) |
| Any college | 19 (38) |
| Current smoker | 14 (28) |
| Body mass index, median (IQR)b | 27.2 (24.3-29.8) |
| American Society of Anesthesiologists physical status class >IIc | 5 (10) |
| Had relevant comorbidityd | 32 (64) |
| Hypertension | 9 (18) |
| Chronic musculoskeletal pain | 7 (14) |
| Chronic lung disease | 6 (12) |
| Gastrointestinal disease | 4 (8) |
| Cardiovascular disease | 3 (6) |
| Osteoarthritis in knee, hip, or both | 3 (6) |
| Other endocrine disorders | 2 (4) |
| Cerebrovascular disease | 2 (4) |
| Diabetes | 1 (2) |
| Osteoporosis | 1 (2) |
| Chronic kidney disease | 1 (2) |
| Chronic neurological disease | 1 (2) |
| Vascular claudication | 1 (2) |
| Depression or anxiety | 1 (2) |
| Ankylosing spondylitis | 1 (2) |
| Cancer | 0 |
| Rheumatoid arthritis | 0 |
| Other rheumatic diseases | 0 |
| Daily pain medication usee | 32 (64) |
| Opioid analgesics | 18 (36) |
| Gabapentinoids | 17 (34) |
| Acetaminophen (paracetamol) | 17 (34) |
| Nonsteroidal anti-inflammatory drugs | 5 (10) |
| Antidepressants | 3 (6) |
| Lumbar spine surgeries | |
| No. of prior procedures, median (IQR) | 2 (1-3) |
| Diskectomy | 38 (76) |
| Fusion | 13 (26) |
| Decompressive surgery | 11 (22) |
Data are expressed as No. (%) unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
Used to assess a patient’s physical health and comorbidities to predict perioperative risk prior to surgery. Patients with class III or higher have significant systemic disease.
Recorded by the surgeon using a standard registration form and a predefined set of comorbidities.
Recorded by the surgeon using a standard registration form.
Primary Outcome
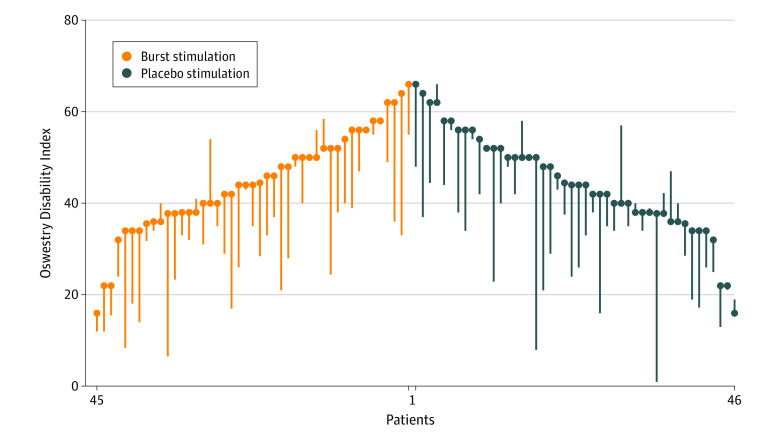
At baseline, the mean ODI score was 44.7 points (SD, 11.3 points). The mean changes in ODI were −10.6 points (95% CI, −14.1 to −7.2 points) for burst stimulation and −9.3 points (95% CI, −12.7 to −5.9 points) for placebo stimulation, resulting in a mean between-group difference of −1.3 points (95% CI, −3.9 to 1.3 points; P = .32) (Figure 2).
Figure 2. Comparative Outcomes Assessment for the Primary Outcome.
Oswestry Disability Index scores range from 0 points (no disability) to 100 points (maximum disability). Scores greater than 40 indicate severe disability. The minimal clinically important difference was 10 points. Each bar extends from a patient’s baseline score to their mean score at the end of the treatment allocation periods. Among the 50 patients who were randomized, 2 patients allocated to burst stimulation and 1 allocated to placebo stimulation did not have follow-up scores starting at 3 months. Among the 47 patients who underwent randomization and had at least 1 follow-up score, there were 91 burst stimulation periods and 89 placebo stimulation periods. Of these 180 treatment periods, follow-up scores were available for 178. The mean score at baseline was 44.7 points and the mean score changes were −10.6 points (95% CI, −14.1 to −7.2 points) for burst stimulation and −9.3 points (95% CI, −12.7 to −5.9 points) for placebo stimulation. For patients with 2 placebo stimulation or burst stimulation follow-up periods, the outcomes are presented as the mean of the 2 burst stimulation periods and the mean of the 2 placebo stimulation periods.
Secondary Outcomes
There were no statistically significant differences between burst stimulation and placebo stimulation in the changes of the 5-dimension EuroQol 3L index score, the Numeric Rating Scale for leg pain and back pain, steps per day, or hours in upright position (Table 2 and eFigure 2 in Supplement 1). Because there was no significant between-group difference for the primary outcome, the cost-effectiveness analyses were not performed. The sensitivity analyses that were performed in the complete case set showed no significant between-group differences for any of the outcomes (eTable in Supplement 1). Patients’ treatment allocation guesses at the end of each randomization period were correct in 100 of 171 instances (58%).
Table 2. Effect of Spinal Cord Burst Stimulation on Primary and Secondary Outcomes.
| Mean score (95% CI) | P value | ||||
|---|---|---|---|---|---|
| At baseline | Spinal cord burst stimulation | Placebo stimulation | Between-group difference | ||
| No. of stimulation periods | 91 | 89 | |||
| Primary outcome | |||||
| Oswestry Disability Index, pointsa | 44.7 (41.4 to 47.9) | 34.0 (30.0 to 38.1) | 35.4 (31.3 to 39.4) | ||
| Change from baseline | −10.6 (−14.1 to −7.2) | −9.3 (−12.7 to −5.9) | −1.3 (−3.9 to 1.3) | .32 | |
| Secondary outcomes | |||||
| Numerical Rating Scaleb | |||||
| Leg pain | 7.3 (6.8 to 7.7) | 5.9 (5.3 to 6.4) | 6.1 (5.6 to 6.6) | −0.2 (−0.7 to 0.2) | .32 |
| Back pain | 6.8 (6.4 to 7.3) | 5.7 (5.2 to 6.2) | 6.1 (5.6 to 6.6) | −0.4 (−0.8 to 0.04) | .07 |
| 5-Dimension EuroQol indexc | 0.21 (0.13 to 0.28) | 0.48 (0.39 to 0.56) | 0.44 (0.35 to 0.53) | 0.04 (−0.03 to 0.11) | .32 |
| Physical activity leveld | |||||
| No. of steps per day | 6775 (5651 to 7899) | 7561 (6411 to 8710) | 7155 (6006 to 8305) | 405 (−422 to 1233) | .34 |
| Time spent standing or walking, h/d | 3.8 (3.3 to 4.3) | 4.0 (3.5 to 4.4) | 4.0 (3.6 to 4.4) | −0.02 (−0.4 to 0.3) | .89 |
Scores range from 0 points (no disability) to 100 points (maximum disability). The minimal clinically important difference (MCID) was 10 points. A typical patient with moderate back pain and disability would have a score between 20 and 40. Scores greater than 40 indicate severe disability.
Scores range from 0 (no pain) to 10 (worst pain imaginable). The MCID was 1.0 points.
A score of 0 indicates death and a score of 1 indicates a perfect health state. The MCID was 0.03. Scores between 0.2 and 0.5 represented a severe to moderate reduction in overall health-related quality of life.
Measured using a body-worn accelerometer (ActivPAL, PAL Technologies, Ltd).
Adverse Events
Nine patients (18%) experienced adverse events, including 4 (8%) who required surgical revision of the implanted system (Table 3). There were no life-threatening adverse events.
Table 3. Adverse Events That Occurred Within 3 Months of Spinal Cord Stimulator Implantationa.
| No. (%) | |
|---|---|
| Any adverse eventb | 9 (18) |
| Unintentional durotomy during lead placement | 3 (6) |
| Revision of leads | 2 (4) |
| Deep surgical site infection requiring removal of the implanted system | 1 (2) |
| Superficial surgical site infection treated with antibiotics | 1 (2) |
| Pulse generator replacement | 1 (2) |
| Micturition problems | 1 (2) |
Patients self-reported events at 3 months using fixed categories. Surgeons reported intra- and perioperative events using fixed categories. Surgical revisions were registered until trial closure. In total, 3 patients withdrew consent before 3 months, including 1 patient with a deep surgical site infection that required removal of the implanted system.
No patient had more than 1 adverse event. There were no recorded events of postoperative hematoma, pneumonia, thromboembolism, cardiovascular complication, anaphylactic reaction, or urinary tract infection.
Discussion
In this placebo-controlled, crossover, randomized clinical trial involving patients with chronic radicular pain after surgery for degenerative lumbar spine disease who subsequently underwent placement of a spinal cord stimulator, there were no significant between-group differences for the change from baseline in ODI score. There were also no significant between-group differences in leg pain, back pain, health-related quality of life, and physical activity levels.
This trial underlines the powerful placebo effect of invasive neuromodulation therapies and inherent weaknesses of open-label studies. The magnitude of the placebo effect on change in ODI score was comparable with the suggested MCID in an observational study on spinal cord stimulation for chronic pain after lumbar spine surgery.22 The clinically significant placebo effect should therefore be considered when interpreting the results from open-label studies. The placebo effect in surgery may be augmented by patient expectations of highly specialized, expensive technological and surgical interventions; repeat visits; attentive patient and care provider interactions; and lack of other treatment options and the placebo effect also could be enhanced by obvious treatment effects such as paresthesia.23,24,25
The evidence supporting spinal cord stimulation has received increased scrutiny because of concerns regarding equipoise, bias, lack of a placebo control, and blinding.1,26,27,28 In a systematic review of spinal cord stimulation for any comparison across 15 randomized clinical trials, including a total of 908 patients, the evidence for benefit of spinal cord stimulation was of low certainty.4 Many trials have close ties with the spinal cord stimulation industry, which might explain favorable reports, the absence of replication studies, and the emphasis on testing novel stimulation parameters instead of confirming efficacy.28,29 Although industry-sponsored randomized trials have almost uniformly reported positive results, an industry-independent observational study in workers’ compensation recipients with chronic pain after spine surgery30 found no evidence for greater effectiveness of spinal cord stimulation vs alternative treatments.
Considering the limited high-quality evidence for benefits and the increasing costs associated with its use,1,2,4 spinal cord stimulation for chronic radicular pain outside well-designed clinical trials is of questionable benefit and must be weighed against the common incidence of device-related complications such as infection, lead failure and migration, and surgical revisions. Data on adverse events are often not provided in trials on spinal cord stimulation,11 but complication and revision rates in the current trial are in line with what has previously been reported.31
Limitations
This study has several limitations. First, the blinding of this treatment prohibits the fine-tuning of stimulation parameters in a completely open dialogue with patients. A thorough review of the spinal cord stimulation system prior to each new treatment allocation was therefore conducted and stimulation programming was performed according to the manufacturer’s recommendations.
Second, the risk of unblinding patients precluded comparisons of different stimulation patterns and waveforms. Third, there are inherent limitations in the crossover design, including that the disorders must be stable throughout the observation period, a sufficient washout period is required until the effect of the preceding treatment allocation subsides, and the burden on patients with multiple and repeated treatment allocations.32,33 Potential carryover effects from the preceding treatment allocation were limited by the relatively long duration of randomization periods and the collection of trial outcomes at the end of each randomization period.
Fourth, an effect of surgery on outcomes was not accounted for separate from burst stimulation and placebo stimulation. Fifth, other burst stimulation systems are available and any differences in the mechanisms of action might have an influence on the outcomes.11,34 Additional placebo-controlled trials are needed to clarify the potential effect of other stimulation modalities and patterns as well as spinal cord stimulation for other indications.
Conclusions
Among patients with chronic radicular pain after lumbar spine surgery, spinal cord burst stimulation, compared with placebo stimulation, after placement of a spinal cord stimulator resulted in no significant difference in the change from baseline in self-reported back pain–related disability.
eFigure 1. The concept of spinal cord burst stimulation
eFigure 2. Patient reported outcomes at baseline and following burst spinal cord stimulation for chronic radicular pain after lumbar spine surgery
eTable. Effect of spinal cord burst stimulation on outcomes in the complete case set
Trial protocol and statistical analysis plan
Data sharing statement
References
- 1.Knotkova H, Hamani C, Sivanesan E, et al. Neuromodulation for chronic pain. Lancet. 2021;397(10289):2111-2124. doi: 10.1016/S0140-6736(21)00794-7 [DOI] [PubMed] [Google Scholar]
- 2.Lad SP, Kalanithi PS, Arrigo RT, et al. A socioeconomic survey of spinal cord stimulation (SCS) surgery. Neuromodulation. 2010;13(4):265-268. doi: 10.1111/j.1525-1403.2010.00292.x [DOI] [PubMed] [Google Scholar]
- 3.Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 2018;18(8):1048-1067. doi: 10.1111/papr.12692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connell NE, Ferraro MC, Gibson W, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev. 2021;12(12):CD013756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsbu MA, Solberg TK, Salvesen Ø, Nygaard ØP, Gulati S. Surgery for herniated lumbar disk in individuals 65 years of age or older: a multicenter observational study. JAMA Surg. 2017;152(5):503-506. doi: 10.1001/jamasurg.2016.5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nerland US, Jakola AS, Solheim O, et al. Minimally invasive decompression versus open laminectomy for central stenosis of the lumbar spine: pragmatic comparative effectiveness study. BMJ. 2015;350:h1603. doi: 10.1136/bmj.h1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austevoll IM, Gjestad R, Solberg T, et al. Comparative effectiveness of microdecompression alone vs decompression plus instrumented fusion in lumbar degenerative spondylolisthesis. JAMA Netw Open. 2020;3(9):e2015015. doi: 10.1001/jamanetworkopen.2020.15015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain: still in the dark after 50 years. Eur J Pain. 2019;23(4):652-659. doi: 10.1002/ejp.1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013;80(5):642-649.e1. doi: 10.1016/j.wneu.2013.01.040 [DOI] [PubMed] [Google Scholar]
- 10.De Andres J, Monsalve-Dolz V, Fabregat-Cid G, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med. 2017;18(12):2401-2421. doi: 10.1093/pm/pnx241 [DOI] [PubMed] [Google Scholar]
- 11.Chakravarthy K, Malayil R, Kirketeig T, Deer T. Burst spinal cord stimulation: a systematic review and pooled analysis of real-world evidence and outcomes data. Pain Med. 2019;20(suppl 1):S47-S57. doi: 10.1093/pm/pnz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deer T, Slavin KV, Amirdelfan K, et al. Success Using Neuromodulation With BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56-66. doi: 10.1111/ner.12698 [DOI] [PubMed] [Google Scholar]
- 13.Schu S, Slotty PJ, Bara G, von Knop M, Edgar D, Vesper J. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation. 2014;17(5):443-450. doi: 10.1111/ner.12197 [DOI] [PubMed] [Google Scholar]
- 14.Al-Kaisy A, Palmisani S, Pang D, et al. Prospective, randomized, sham-control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS frequency study). Neuromodulation. 2018;21(5):457-465. doi: 10.1111/ner.12771 [DOI] [PubMed] [Google Scholar]
- 15.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273. [PubMed] [Google Scholar]
- 16.Grotle M, Brox JI, Vøllestad NK. Cross-cultural adaptation of the Norwegian versions of the Roland-Morris Disability Questionnaire and the Oswestry Disability Index. J Rehabil Med. 2003;35(5):241-247. doi: 10.1080/16501970306094 [DOI] [PubMed] [Google Scholar]
- 17.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90-94. doi: 10.1097/BRS.0b013e31815e3a10 [DOI] [PubMed] [Google Scholar]
- 18.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121. doi: 10.1016/j.jpain.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Solberg TK, Olsen JA, Ingebrigtsen T, Hofoss D, Nygaard OP. Health-related quality of life assessment by the EuroQol-5D can provide cost-utility data in the field of low-back surgery. Eur Spine J. 2005;14(10):1000-1007. doi: 10.1007/s00586-005-0898-2 [DOI] [PubMed] [Google Scholar]
- 20.O’Brien MW, Wu Y, Petterson JL, Bray NW, Kimmerly DS. Validity of the ActivPAL monitor to distinguish postures: a systematic review. Gait Posture. 2022;94:107-113. doi: 10.1016/j.gaitpost.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 21.Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol. 2013;66(9):1022-1028. doi: 10.1016/j.jclinepi.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 22.Paul AR, Kumar V, Roth S, Gooch MR, Pilitsis JG. Establishing minimal clinically important difference of spinal cord stimulation therapy in post-laminectomy syndrome. Neurosurgery. 2017;81(6):1011-1015. doi: 10.1093/neuros/nyx153 [DOI] [PubMed] [Google Scholar]
- 23.Turner JA, Deyo RA, Loeser JD, Von Korff M, Fordyce WE. The importance of placebo effects in pain treatment and research. JAMA. 1994;271(20):1609-1614. doi: 10.1001/jama.1994.03510440069036 [DOI] [PubMed] [Google Scholar]
- 24.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710-719. doi: 10.1056/NEJM200103083441002 [DOI] [PubMed] [Google Scholar]
- 25.Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347(2):81-88. doi: 10.1056/NEJMoa013259 [DOI] [PubMed] [Google Scholar]
- 26.Duarte RV, Nevitt S, McNicol E, et al. Systematic review and meta-analysis of placebo/sham controlled randomised trials of spinal cord stimulation for neuropathic pain. Pain. 2020;161(1):24-35. doi: 10.1097/j.pain.0000000000001689 [DOI] [PubMed] [Google Scholar]
- 27.Duarte RV, McNicol E, Colloca L, Taylor RS, North RB, Eldabe S. Randomized placebo-/sham-controlled trials of spinal cord stimulation: a systematic review and methodological appraisal. Neuromodulation. 2020;23(1):10-18. doi: 10.1111/ner.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferraro MC, Gibson W, Rice ASC, Vase L, Coyle D, O’Connell NE. Spinal cord stimulation for chronic pain. Lancet Neurol. 2022;21(5):405. doi: 10.1016/S1474-4422(22)00096-5 [DOI] [PubMed] [Google Scholar]
- 29.Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2(2):MR000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner JA, Hollingworth W, Comstock BA, Deyo RA. Spinal cord stimulation for failed back surgery syndrome: outcomes in a workers’ compensation setting. Pain. 2010;148(1):14-25. doi: 10.1016/j.pain.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 31.Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603-608. doi: 10.1111/ner.12312 [DOI] [PubMed] [Google Scholar]
- 32.Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109(15):276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim CY, In J. Considerations for crossover design in clinical study. Korean J Anesthesiol. 2021;74(4):293-299. doi: 10.4097/kja.21165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Ridder D, Vancamp T, Falowski SM, Vanneste S. All bursts are equal, but some are more equal (to burst firing): burstDR stimulation versus Boston burst stimulation. Expert Rev Med Devices. 2020;17(4):289-295. doi: 10.1080/17434440.2020.1736560 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. The concept of spinal cord burst stimulation
eFigure 2. Patient reported outcomes at baseline and following burst spinal cord stimulation for chronic radicular pain after lumbar spine surgery
eTable. Effect of spinal cord burst stimulation on outcomes in the complete case set
Trial protocol and statistical analysis plan
Data sharing statement