Abstract
Our previous study demonstrated that paeoveitol D, a benzofuran compound isolated from Paeonia veitchii, displayed activity on MT1 and MT2 receptors with agonistic ratios of 57.5% and 51.6% at a concentration of 1 mM. To explore the structure–activity relationships, 34 paeoveitol D derivatives were synthesized and evaluated for their MT1 and MT2 agonistic activities using the Fluo-8 calcium assay. Among them, 16 and 18 derivatives increased agonistic activities on the MT1 and MT2 receptors, respectively. Compound 18 indicated EC50 values of 21.0 and 298.9 μM on MT1 and MT2 receptors in agonistic dose response curves with Tango assays and shortened immobility time in the forced swim test. The preliminary mechanism-of-action investigation manifested that the antidepressant activity of compound 18 may be mediated by promoting serotonin (5-HT) and dopamine (DA) levels in the mice brain. Compound 18 also showed favorable pharmacokinetic profiles and low toxicity in vivo. These results suggest that compound 18 could be a potential antidepressant agent.
Paeoveitol D derivatives were synthesized as potent MT1 and MT2 receptors agonists with in vivo antidepressant activity.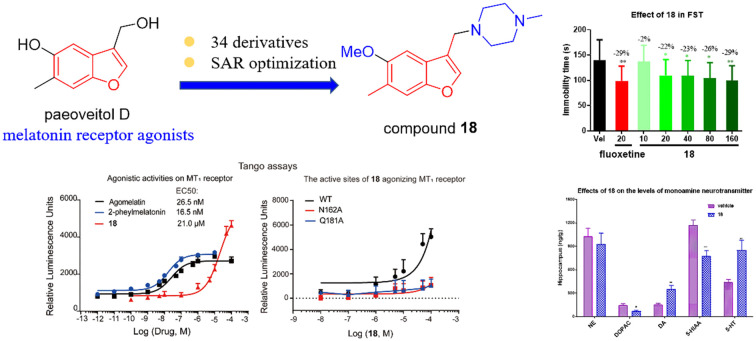
Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is an endogenous neurohormone that is mainly synthesized and secreted by the pineal gland in a light-regulated manner, and regulates a variety of physiological functions by activating two high-affinity G-protein-coupled receptors, known as MT1 and MT2.1 It has been suggested that melatonin and its receptors play an important role in many central nervous system diseases, including rhythm disorders, sleep disorders, depression, Alzheimer's disease, and Parkinson's disease.2 Exogenous melatonin can ameliorate the symptoms of insomnia,3 jetlag4 and mood disorder.5 However, the therapeutic application of melatonin is limited by its poor pharmacokinetic properties.6 Thus, searching for more effective, long-lasting, and orally bioavailable MT1 and MT2 receptor agonists is an interesting target for the development of new therapeutic agents. Accordingly, several MT1 and MT2 receptor ligands with improved pharmacological profiles and effects have been developed during the past three decades. Structurally, these ligands can be divided into the following catalogs:7 melatonin derivatives with larger substituents at C-5 and C-3-side chain; compounds obtained via conformational rigidification of the amide side chain and its incorporation into additional rings; and compounds replacing the indole nucleus with other aromatic moieties, such as benzimidazole, benzoxazole, naphthalene, isoquinoline, and indane.8–11 These attempts resulted in the discovery of nonselective MT1 and MT2 agonists such as ramelteon, agomelatine and tasimelteon as approved drugs for the treatment of insomnia, depression, and non 24 h sleep–wake disorder (Fig. 1).
Fig. 1. Chemical structures of melatonin, ramelteon, agomelatine, tasimelteon and paeoveitols D and E.
The discovery of natural products and their derivatives as melatonin receptor ligands with potential use in depression is a continuous goal of our laboratory.12–14 Our previous phytochemical investigation of Paeonia veitchii (Shao Yao), which is often used in Chinese herbal formulae for the treatment of depression, led to the isolation of a unique norditerpene natural product, paeoveitol,15 and two benzofuran constituents, paeoveitols D (1) and E (2).16 Preliminary biological assays indicated that paeoveitol D showed moderate activity on MT1 and MT2 receptors with agonistic ratios of 57.5% and 51.6% at a concentration of 1 mM. It was reported that benzofuran scaffolds could be regarded as valuable bioisosteres of the indole nucleus in the design of melatonin receptor ligands.17
During our previous total synthesis of paeoveitol, a large scale of paeoveitol D was also synthesized in five steps with 45% overall yield.18 With sufficient amounts of paeoveitol D in hand, paeoveitol D (1) was selected as a lead to explore its structure–activity relationship in search for new melatonin receptor agonists. In this paper, 34 derivatives of paeoveitol D were synthesized and evaluated for their agonistic activities on MT1 and MT2 receptors using the Fluo-8 calcium assay in vitro. The most potent compound 18 was subjected to in vivo experiments to assess its antidepressive activity and influence on the expression of monoamine neurotransmitters in the brain, pharmacokinetic properties and preliminary safety.
Results and discussion
Chemistry
The synthetic routes for paeoveitol D derivatives are outlined in Schemes 1–3. The synthesis started from previously reported compound 3 which can be synthesized on a decagram-scale from commercially available materials in 4 steps. The reduction of 3 with DIBAL afforded 5-O-methy-paeoveitol D (4), which was further transformed to esters 5–6 in good yields via esterification. The primary hydroxyl group of 4 was converted to a –NH2 group using the Mitsunobu protocol followed by treatment with hydrazine hydrate. The reaction of 8 with acetyl chloride and cyclopropanecarbonyl chloride afforded products 9 and 10. Derivatives 12–26 containing amino moieties were prepared from 4via oxidation with Dess–Martin periodinate, followed by reductive amination with various amines (Scheme 1).
Scheme 1. Synthesis of compounds 4–26. Reagents and conditions: (a) DIBAL-H, CH2Cl2, −78 °C to rt, 1 h, 91%. (b) Acetyl chloride or cyclopropanecarbonyl chloride, DMAP, pyridine, CH2Cl2, 0 °C to rt, 5 h, 92% (5), 87% (6). (c) Phthalimide, DIAD, PPh3, THF, rt, 12 h, 79%. (d) H2NNH2·H2O, MeOH, rt, 12 h, 93%. (e) Acetyl chloride or cyclopropanecarbonyl chloride, Et3N, 0 °C to rt, 5 h, 96% (9), 94% (10). (f) DMP, CH2Cl2, rt, 2 h, 89%. (g) Amine, NaBH(OAc)3, AcOH, CH2Cl2, rt, 8–16 h, 40–91%. (h) CF3COOH, rt, 6 h, 98%.
Scheme 3. Synthesis of compounds 32–34. (a) LDA, trimethyl borate, −78 °C, 4 h, 82%. (b) PdCl2(dppf). DCM, iodobenzene, Na2CO3, DME/H2O, 70 °C, 83%. (c) DIBAL-H, CH2Cl2, −78 °C to rt, 1 h, 87%. (d) DMP, CH2Cl2, rt, 2 h, 93%. (e) 1-Methylpiperazine, NaBH(OAc)3, AcOH, CH2Cl2, rt, 8 h, 45%.
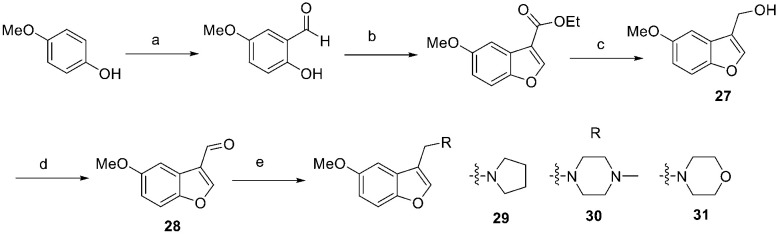
Derivatives 27–31, which lack a methyl substituent at C-6, were prepared from 4-methoxyphenol as described in Scheme 2. A three-step sequence consisting of MgCl2 mediated formylation, condensation with ethyl diazoacetate and reduction delivered compound 27, which was subjected to Dess–Martin oxidation and reductive amination furnishing derivatives 29–31.
Scheme 2. Synthesis of compounds 27–31. (a) (CHO)n, MgCl2, Et3N, CH3CN, reflux, 8 h, 91%. (b) N2CHCO2Et, HBF4·Et2O, 0 °C to rt, 30 min, then H2SO4, rt, 30 min, 84%. (c) DIBAL-H, CH2Cl2, −78 °C to rt, 1 h, 88%. (d) DMP, CH2Cl2, rt, 2 h, 90%. (e) Amine, NaBH(OAc)3, AcOH, CH2Cl2, rt, 8–16 h, 79–90%.
Compounds 32–34 bearing a phenyl substituent at C-2 of the benzofuran core were prepared from ethyl 5-methoxybenzofuran-3-carboxylate via a five-step sequence consisting of (i) LDA-mediated borylation, (ii) Suzuki coupling with iodobenzene,19 (iii) reduction of the esters with DIBAL, (iv) oxidation of the resulting primary alcohol to aldehyde, and (v) reductive amination for the introduction of the amino group (Scheme 3).
Derivatives 35–37 possessing a methyl group at C-2 were synthesized as described in Scheme 4. Palladium-catalyzed annulation of 2-iodo-4-methoxyphenol with methyl-2-butynaote delivered 5-methoxy-2-methylbenzofuran-3-carboxylate,20 which was converted into 37 in three steps through the same synthetic sequences as compound 34.
Scheme 4. Synthesis of compounds 35–37. (a) Methyl but-2-ynoate, Pd2(dba)3, Et3N, DMSO, reflux, 1 h, 48%; (b) DIBAL-H, CH2Cl2, −78 °C to rt, 1 h, 84%; (c) DMP, CH2Cl2, rt, 2 h, 86%; (d) 1-methylpiperazine, NaBH(OAc)3, AcOH, CH2Cl2, rt, 8 h, 57%.
Biology
In vitro agonistic activities on MT1 and MT2 receptors using the Fluo-8 calcium assay
All the synthetic derivatives (4–37) were examined for their agonistic activities at a concentration of 1.0 mM by a Fluo-8 calcium assay on HEK293 cells that stably expressed the human melatonin MT1 or MT2 receptor. As a result, 16 derivatives (4, 8, 12–14, 16–20, 23–26, 29–30) increased agonistic activities on the MT1 receptor, and 18 compounds (4, 8, 12–14, 16–20, 22–26, 29–31) showed enhanced agonistic activity on the MT2 receptor (Table 1). 5-O-Methylated derivative 4 obviously increased activities on MT1 and MT2 receptors with agonistic ratios of 70.4% and 95.2%, indicating that a 5-methoxy substituent is favorable. Compounds 5 and 6 showed no activities, manifesting that esterification was unfavorable. Compound 8 with a primary amine and compound 12 with a tertiary amine significantly improved the activities on both receptors, which were 2–3 fold more potent than paeoveitol D. Compound 8 was further modified via amidation to afford compounds 9 and 10. Unfortunately, the introduction of an acyl group reduced the agonistic activity, as seen with compounds 5–6 in contrast to compound 4. These results drove us to synthesize diverse tertiary amino-containing derivatives, and most of these series of compounds showed good potential on MT1 and MT2 receptors. Compounds 12–15 bearing catenulate tertiary amines with different lengths showed that the agonistic activity on MT1 and MT2 receptors decreased as the chain length increased. Compounds 16, 17, 18 and 24 were obtained by replacing the catenulate tertiary amines with different cyclic tertiary amines, and the general trend in the order of activity was morpholine < pyrrolidine < 1-methylpiperazine ≈ N-methylhomopiperazine. The effect of substituents on the piperazine ring was explored, and the agonistic ratios of derivatives 18–23 ranged from 114.7% to 33.9% on the MT1 receptor and from 132.5% to 44.9% on the MT2 receptor. Compounds 18–19 with an electron-donating group and compound 23 without a substituent at N-1′ showed better activities than compounds 21–22 with an electron-withdrawing group. Among them, N-methylation derivative 18 showed the most promising activity with agonistic ratios of 114.7% and 132.5% on MT1/2 receptors, which were 2 and 3-fold higher than compound 1. Derivatives 25–26 with a bis(benzofuran) structure increased activity on MT1 and MT2 receptors, and compound 25 exhibited 2-fold and 3-fold enhancement of the agonistic effect on MT1 and MT2 receptors.
Agonistic activities of paeovetiol D and its derivatives on melatonin receptorsa.
| Compounds | Agonistic activitiesb | Compounds | Agonistic activitiesb | ||
|---|---|---|---|---|---|
| MT1 | MT2 | MT1 | MT2 | ||
| Paeovetiol D | 57.5 ± 2.9 | 51.7 ± 2.1 | 21 | 55.2 ± 1.6 | 44.9 ± 2.5 |
| 4 | 70.4 ± 4.4 | 95.2 ± 3.6 | 22 | 33.9 ± 0.7 | 60.2 ± 3.6 |
| 5 | −4.4 ± 0.4 | −4.7 ± 0.5 | 23 | 98.3 ± 3.1 | 111.8 ± 4.6 |
| 6 | −2.3 ± 0.1 | −7.4 ± 0.2 | 24 | 113.8 ± 5.2 | 101.8 ± 5.8 |
| 7 | 10.2 ± 0.9 | 5.3 ± 0.6 | 25 | 121.2 ± 5.5 | 145.3 ± 8.3 |
| 8 | 107.8 ± 4.9 | 177.8 ± 7.8 | 26 | 87.8 ± 5.7 | 76.3 ± 9.6 |
| 9 | 1.9 ± 0.2 | −11.2 ± 0.8 | 27 | 23.6 ± 1.2 | 16.9 ± 0.9 |
| 10 | −3.4 ± 0.1 | −11.6 ± 2.5 | 28 | 19.3 ± 0.6 | 21.1 ± 1.3 |
| 11 | 35.9 ± 3.8 | 40.9 ± 2.9 | 29 | 83.6 ± 2.8 | 122.6 ± 6.6 |
| 12 | 109.7 ± 5.5 | 160.4 ± 5.9 | 30 | 105.8 ± 8.9 | 85.5 ± 9.5 |
| 13 | 142.5 ± 10.1 | 175.0 ± 8.7 | 31 | 56.2 ± 4.4 | 60.1 ± 6.3 |
| 14 | 98.4 ± 7.2 | 65.9 ± 3.4 | 32 | 17.9 ± 0.2 | 15.3 ± 0.3 |
| 15 | 53.9 ± 6.4 | 28.9 ± 2.3 | 33 | 9.6 ± 0.4 | 20.3 ± 0.3 |
| 16 | 102.5 ± 9.1 | 101.9 ± 6.7 | 34 | 35.1 ± 5.1 | 56.9 ± 4.0 |
| 17 | 67.9 ± 3.4 | 77.2 ± 4.8 | 35 | 13.7 ±0.3 | 11.0 ± 0.8 |
| 18 | 114.7 ± 8.2 | 132.5 ± 4.6 | 36 | 8.6 ± 0.7 | 5.9 ± 0.6 |
| 19 | 101.0 ± 3.6 | 97.5 ± 8.3 | 37 | 8.9 ± 1.2 | 18.2 ± 0.7 |
| 20 | 79.8 ± 1.9 | 85.4 ± 3.0 | |||
Compounds were tested at 1.0 mM.
Agomelatine was used as a positive control with EC50 values of 5.8 ± 1.1 nM (MT1) and 17.3 ± 2.1 nM (MT2). The highest agonistic activity achieved by agomelatine at the highest concentration was set as 100%, the agonistic activities of the tested compounds were obtained by comparison to the highest agonistic activity of agomelatine.
Compound 27 without a methyl substitution at C-6 is less active than paeoveitol D. Similarly, the agonistic effects of compounds 29–31 are weaker than those of compounds 16–18. These results suggested that a methyl substitution at C-6 contributed positively to agonistic activity. Introduction of a benzyl or methyl group at the C-2 position caused an obvious decrease on MT1 and MT2 receptors (compounds 32 and 35vs.4, compounds 34 and 37vs.18).
From the above results, the preliminary SARs can be concluded: (i) methylation of the 5-hydroxy group of paeoveitol D was favorable for agonistic activity, (ii) the presence of a methyl unit at C-6 enhanced the activity; (iii) a substituent at C-2 was unfavorable for the activity; (iv) the introduction of tertiary and primary amines at the C-3 side chain significantly improved the agonistic activity (Fig. 2).
Fig. 2. The summarized SARs of paeoveitol D derivatives.
The agonistic ratios of compounds 8, 12, 13, 16, 18, 24 and 25 were higher than 100% on both MT1 and MT2 receptors at a concentration of 1.00 mM, which prompted us to determine their EC50 values from dose–response curves. Compounds 4, 8, 12, 13, 16, 18, and 23–25 exhibited significant activities on MT1 and MT2 receptors with EC50 values ranging from 0.13 to 0.70 mM (Table 2).
The EC50 (mM) values of derivatives 4, 8, 12, 13, 16, 18, and 23–25 on MT1 and MT2 receptorsa.
| Compounds | EC50 (mM) | |
|---|---|---|
| MT1 | MT2 | |
| 4 | 0.70 ± 0.09 | 0.52 ± 0.03 |
| 8 | 0.25 ± 0.03 | 0.35 ± 0.04 |
| 12 | 0.44 ± 0.08 | 0.13 ± 0.02 |
| 13 | 0.36 ± 0.02 | 0.36 ± 0.09 |
| 16 | 0.31 ± 0.05 | 0.35 ± 0.04 |
| 18 | 0.33 ± 0.05 | 0.26 ± 0.07 |
| 23 | 0.46 ± 0.06 | 0.38 ± 0.05 |
| 24 | 0.38 ± 0.03 | 0.27 ± 0.07 |
| 25 | 0.42 ± 0.04 | 0.43 ± 0.03 |
EC50 values for derivatives 4, 8, 12, 13, 16, 18, and 23–25 were determined from the dose–response curves obtained with seven concentrations in the range of 0.02–2 mM, and calculated by GraphPad Prism 6.0 software.
In vitro agonistic activities on MT1 and MT2 receptors via the Tango GPCR assay
Considering that the piperazine framework was a valuable pharmacophore for many drugs for the treatment of central nervous system diseases,21 the most active compound 18 with a piperazine fragment was selected for further research. To confirm the agonistic activity of compound 18 on MT1 and MT2 receptors, we performed a Tango GPCR assay.22 Agomelatine and 2-pheylmelatonin were used as positive controls. For the MT1 receptor, the EC50 values of agomelatine, 2-pheylmelatonin, and compound 18 were 26.5 nM, 16.5 nM, and 21.0 μM, respectively. Furthermore, the highest mean values of the relative luminescence units of agomelatine, 2-pheylmelatonin, and compound 18 were 2952, 3168, and 4624, respectively. These results showed that compound 18 increased the maximum agonistic activity by 57% and 46% by comparison with the maximum agonist activity of agomelatine and 2-phenylmelatonin. For the MT2 receptor, the EC50 values of agomelatine, 2-pheylmelatonin, and compound 18 were 88.9 nM, 7.0 μM, and 298.9 μM, respectively (Fig. 3). Our results demonstrated that compound 18 was an agonist on MT1 and MT2 receptors, and the agonistic activity of compound 18 at the MT1 receptor was better than its agonist activity at the MT2 receptor.
Fig. 3. Concentration–response curves for the response of agomelatine, 2-pheylmelatonin, and compound 18 at MT1 and MT2 receptors. Data are shown as mean ± SD, and curves were fitted with GraphPad Prism.
The active sites of compound 18 agonizing MT1 and MT2 receptors
Key amino acids are essential to the structure and function of the receptors. Mutation of key sites will have a huge effect on the activity of the active compounds. By detecting the activity of active compounds on site-directed amino acid mutants, we evaluated whether selected amino acid sites were the key active sites for compound 18 to play a role, and the N162 or Q181 MT1 mutant receptor and the N175 or Q194 MT2 mutant receptor were structured. As shown in Fig. 4, regardless of whether 2-phenylmelatonin or compound 18 was used, the concentration–response curves did significantly shift at the N162 or Q181 MT1 mutant receptor, which suggested that N162 and Q181 were two key important active sites for 2-pheylmelatonin and compound 18. In addition, agomelatine indicated a good mutant-function relationship for MT2 mutant receptors, the mutant receptors significantly decreased the dose response of agomelatine, and the curve shifted. However, compound 18 showed a nontypical mutant-function relationship, the Q194 mutant did not shift the dose curve of compound 18, while N175 shifted the dose curve of compound 18 a little. These results suggested that compound 18 did not activate MT2 through Q194 and N175.
Fig. 4. Concentration–response curves of agomelatine, 2-pheylmelatonin, and compound 18 at MT1 and MT2 wide-type and mutant receptors. (A) Concentration–response curves of 2-pheylmelatonin or compound 18 to MT1 wide-type or mutants N162A and Q181A. (B) Concentration–response curves of agomelatine or compound 18 to MT2 wide-type or mutants N175A and Q194A. Data are shown as the mean ± SD, and curves were fitted with GraphPad Prism.
Rat forced swimming test (FST)
In view of the fact that melatonin receptor agonists such as agomelatine exhibited well-documented effects for the treatment of depression, compound 18 was further evaluated for its antidepressive potential in the forced swim test (FST) which is a classic method that can quickly assess the effects of antidepressants. The clinically used antidepressant fluoxetine was selected as a positive control at a dose of 20 mg kg−1. Initially, compound 18 was administered orally two times (24 h, 1 h) before testing at doses of 10, 20, 40, 80 and 160 mg kg−1. The results are shown in Fig. 5; compound 18 decreased the immobility time in the FST gradually by increasing the dose compared with the negative control group. Compound 18 at doses of 80 and 160 mg kg−1 significantly reduced the immobility time by 26 and 29%, which were comparable to fluoxetine (29% reduction in immobility time at 20 mg kg−1). The immobility time of the mice in the FST after 14 days of administration of compound 18 was also recorded with agomelatine as an additional positive control. Pretreatment of mice with 20 mg kg−1 agomelatine for 14 days reduced the immobility time compared with that of the control group. Administration of compound 18 at different doses for 14 days dose-dependently decreased the immobility time in the FST. Compound 18 at doses of 40, 80 and 160 mg kg−1 significantly decreased the immobility time of mice similar to that of fluoxetine and the percent decrease in immobility time was 26%, 26% and 28%, respectively, which were more potent than agomelatine. These results indicated that compound 18 possesses potential antidepressant-like effects.
Fig. 5. Effects of different doses of compound 18 (10, 20, 40, 80, 160 mg kg−1, O.P.) after administration two (A) and fourteen (B) times in the FST. *p < 0.05, **p < 0.01.
Neurochemical tests
Dysfunction and deficiency of monoamine neurotransmitters such as dopamine (DA), 5-hydroxytrylamine (5-HT) and norepinephrine (NE) in the brain play an important role in the pathomechanisms of depression. After the behavioral test, the anti-depressive mechanism of compound 18 was explored by quantitatively measuring the levels of monoamine neurotransmitters, including dopamine (DA) and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), 5-hydroxytrylamine (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA), and norepinephrine (NE). The effects of derivative 18 on the levels of monoamine neurotransmitters in the frontal cortex, hippocampus, striatum, hypothalamus and thalamus are shown in Fig. 6. Compared with the control group, mice pretreated with 160 mg kg−1 compound 18 for 14 days showed significantly elevated DA and 5-HT levels in multiple brain regions, while the DOPAC and 5-HIAA levels were reduced compared with the control group, indicating that compound 18 could inhibit the metabolism of 5-HT and DA to increase their content. It was noted that no obvious difference in NE levels was observed in the mice brain. These results indicated that the antidepressant effect of compound 18 might be mediated by the central serotonergic and dopaminergic systems.
Fig. 6. Effects of compound 18 on monoamine neurotransmitters and their metabolite levels in different regions of mice brain. (A) Frontal cortex; (B) hippocampus; (C) striatum; (D) thalamus; (E) hypothalamus; data are expressed as means ± SEM with units of ng g−1. (n = 12) *P < 0.05, **P < 0.01, ***P < 0.005.
Pharmacokinetic study
The pharmacokinetic (PK) properties of compound 18 were evaluated after a single oral administration of 80 mg kg−1, and the key PK parameters were determined. Compound 18 displayed a half-life of 5.47 h. The drug concentration rapidly reached a maximum in plasma after 0.083 h of administration and Cmax and AUC0–24 were 1336.87 ng mL−1 and 5395.55 ng h−1 mL−1, indicating that compound 18 possessed high Cmax and exposures (Table 3).
Pharmacokinetic characteristics of compound 18 in mice.
| Parameters | Unit | 18 (80 mg kg−1) |
|---|---|---|
| t 1/2 | h | 5.47 |
| C max | ng mL−1 | 1336.87 |
| T max | h | 0.083 |
| AUC0–24 | ng h−1 mL−1 | 5395.55 |
| AUC0-inf | ng h −1 mL−1 | 5624.05 |
| CL | L h−1 | 14.22 |
In vivo subacute toxicity test
To preliminarily evaluate the safety of compound 18, we performed an in vivo oral subacute toxicity test. Treatment with compound 18 at a dosage of 240 mg kg−1 for 14 days did not cause any animal deaths. No significant difference in the structures of the heart, liver, spleen, lung, and kidney was observed compared with the vehicle group, suggesting that compound 18 appears to have a good safety profile.
Conclusions
In summary, 34 paeovetiol D derivatives were synthesized and examined for their agonistic activities on MT1 and MT2 receptors using the Fluo-8 calcium assay. Of these derivatives, 16 displayed higher agonistic activities on the MT1 receptor, and 18 derivatives showed higher activity on the MT2 receptor. In particular, derivatives 8, 12, 13, 16, 18, 24 and 25 exhibited significant agonistic activities on both MT1 and MT2 receptors with EC50 values ranging from 0.13 to 0.44 mM. The SAR analyses indicated that 5-methoxyl, methyl substitution at C-6 and the introduction of tertiary and primary amines at the C-3 side chain played significant roles in agonistic activities, while substituents at C-2 were unfavorable. The agonistic activity of piperazine-containing compound 18 on MT1 and MT2 receptors was further confirmed by the Tango assay. Compound 18 was found to be orally effective with a significant reduction of immobility in the forced swim test. It was also found that compound 18 significantly increased the concentrations of 5-HT and DA by inhibiting their metabolism. In vivo, compound 18 also exhibited a good pharmacokinetic profile and low toxicity. These results suggest that derivative 18 is a promising lead compound for antidepressant drugs.
Experimental
Chemical analysis
General methods
The reagents and solvents for the reaction were commercially available and used without further purification. 1H NMR and 13C NMR spectra were obtained on an Avance III HD 400 (Bruker, Germany) and Avance III 500 (Bruker, Germany) with TMS as the internal standard. All synthetic compounds were purified by column chromatography on silica gel (200–300 mesh, Qingdao Makall Group Co., Ltd., Qingdao, China). High resolution mass spectra were acquired on a Shimadzu LC/MS-IT-TOF mass spectrometer (Shimadzu, Kyoto, Japan). Reactions were monitored by thin layer chromatography (TLC) (250 μm thickness, F-254 indicator) and visualized by UV irradiation and heating silica gel plates sprayed with 10% H2SO4 in EtOH.
Synthesis of (5-methoxy-6-methylbenzofuran-3-yl)methanol (4)
To a solution of ethyl 5-methoxy-6-methylbenzofuran-3-carboxylate (2.34 g, 10 mmol) in 25 mL CH2Cl2 was added DIBAL-H (25 mL, 1.2 M in toluene, 30 mmol) dropwise over 10 min at −78 °C. The reaction mixture was slowly warmed to 0 °C and stirred at that temperature for 1 h quenching with saturated NH4Cl (50 mL). The reaction mixture was extracted with EtOAc (3 × 50 mL), and the combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was subjected to flash column chromatography on silica gel (acetone–petroleum ether, 15 : 85) to provide compound 4 (1.75 g, 91% yield) as a white solid. 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 1H, H-2), 7.25 (s, 1H, H-7), 7.03 (s, 1H, H-4), 4.79 (s, 3H, –OMe), 3.87 (s, 2H, H-1′), 2.31 (s, 3H, H-2′); 13C NMR (100 MHz, CDCl3) δ 154.6 (C-5), 150.4 (C-8), 142.1 (C-2), 125.1 (C-9), 124.8 (C-6), 120.6 (C-3), 113.1 (C-7), 99.9 (C-4), 56.2 (C-1′), 56.0 (–OMe), 17.2 (C-2′).
General synthetic procedures for compounds 5–6
To a solution of (5-methoxy-6-methylbenzofuran-3-yl)methanol (76.9 mg, 0.4 mmol) in 3 mL CH2Cl2 were added DMAP (2.4 mg, 0.02 mmol) and pyridine (48.2 μL, 0.6 mmol). The mixture was cooled to 0 °C and acetyl chloride (42.7 μL, 0.6 mmol) or cyclopropanecarbonyl chloride (54.4 μL, 0.6 mmol) was added. The reaction mixture was slowly warmed to room temperature and stirred for 5 h before quenching with saturated NaHCO3 (5 mL), and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL). The combined organic phases were washed with brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was subjected to flash column chromatography on silica gel (ethyl acetate–petroleum ether, 10 : 90) to provide compound 5 or 6.
(5-Methoxy-6-methylbenzofuran-3-yl)methyl acetate (5)
White solid (86.1 mg, 92% yield). 1H NMR (500 MHz, CDCl3) δ 7.59 (s, 1H, H-2), 7.26 (s, 1H, H-7), 7.00 (s, 1H, H-4), 5.23 (s, 2H, H-1′), 3.89 (s, 3H, –OMe), 2.32 (s, 3H, H-2′), 2.10 (s, 3H, H-4′); 13C NMR (125 MHz, CDCl3) δ 171.1 (C-3′), 154.7 (C-5), 150.1 (C-8), 144.0 (C-2), 125.1 (C-9), 124.7 (C-6), 116.0 (C-3), 113.1 (C-7), 99.7 (C-4), 56.8 (–OMe), 55.9 (C-1′), 21.0 (C-4′), 17.1 (C-2′).
(5-Methoxy-6-methylbenzofuran-3-yl)methyl cyclopropane carboxylate (6)
White solid (90.5 mg, 87% yield); 1H NMR (500 MHz, CDCl3) δ 7.59 (s, 1H, H-2), 7.27 (s, 1H, H-7), 7.00 (s, 1H, H-4), 5.25 (s, 2H, H-1′), 3.89 (s, 3H, –OMe), 2.33 (s, 3H, H-2′), 1.68–1.63 (m, 1H, H-4′), 1.06–1.03 (m, 2H, H-5′), 0.90–0.86 (m, 2H, H-5′); 13C NMR (125 MHz, CDCl3) δ 175.0 (C-3′), 154.7 (C-5), 150.2 (C-8), 143.8 (C-2), 125.1 (C-9), 124.8 (C-6), 116.2 (C-2), 113.0 (C-7), 99.7 (C-4), 56.8 (–OMe), 55.8 (C-1′), 17.1 (C-2′), 13.0 (C-4′), 8.7 (C-5′).
Synthesis of 2-((5-methoxy-6-methylbenzofuran-3-yl)methyl)isoindoline-1,3-dione (7)
DIAD (1.02 mL, 5.2 mmol) was added dropwise to a solution of (5-methoxy-6-methylbenzofuran-3-yl)methanol (0.77 g, 4 mmol), phthalimide (0.77 g, 5.2 mmol) and PPh3 (1.36 g, 5.2 mmol) in anhydrous THF (30 mL) at room temperature. After stirring the mixture for 12 h, water (50 mL) was added and the resultant mixture was extracted with EtOAc (3 × 50 mL), and the combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was subjected to flash column chromatography on silica gel (ethyl acetate–petroleum ether, 10 : 90) to provide compound 7 (1.01 g, 79% yield) as a white solid. 1H NMR (500 MHz, CDCl3) δ 7.83–7.80 (m, 2H, H-5′), 7.70 (s, 1H, H-2), 7.69–7.65 (m, 2H, H-6′), 7.27 (s, 1H, H-7), 7.20 (s, 1H, H-4), 4.91 (s, 3H, –OMe), 3.92 (s, 2H, H-1′), 2.28 (s, 3H, H-2′); 13C NMR (125 MHz, CDCl3) δ 168.1 (C-3′), 154.6 (C-5), 150.0 (C-8), 144.5 (C-2), 134.1 (C-6′), 132.2 (C-4′), 124.9 (C-9), 124.7 (C-6), 123.4 (C-5′), 115.9 (C-3), 112.9 (C-7), 100.2 (C-4), 55.9 (–OMe), 31.1 (C-1′), 17.1 (C-2′).
Synthesis of (5-methoxy-6-methylbenzofuran-3-yl)methanamine (8)
To a solution of compound 7 (0.64 g, 2 mmol) in MeOH (20 mL) was added N2H4·H2O (290 μL, 6 mmol). After stirring the mixture for 30 min at room temperature, 5% HCl (20 mL) was added and the resultant mixture was stirred for an additional 12 h. The suspension was filtered and the filtrate was diluted with 40 mL of water, acidified with 10% hydrochloric acid (pH < 2) and washed with Et2O and the organic phase was discarded. Then the aqueous phase was basified with solid NaOH (pH > 10) and the resultant mixture was extracted with EtOAc (3 × 50 mL), and the combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was subjected to flash column chromatography on silica gel (MeOH–CHCl3, 10 : 90) to provide compound 8 (356 mg, 93% yield) as a light yellow powder. 1H NMR (500 MHz, CDCl3) δ 7.46 (s, 1H, H-2), 7.23 (s, 1H, H-7), 6.95 (s, 1H, H-4), 3.96 (s, 2H, H-1′), 3.87 (s, 3H, –OMe), 2.53 (brs, 2H, –NH2), 2.30 (s, 3H, H-2′); 13C NMR (125 MHz, CDCl3) δ 154.5 (C-5), 150.3 (C-8), 141.4 (C-2), 124.9 (C-9), 124.8 (C-6), 121.6 (C-3), 113.1 (C-7), 99.6 (C-4), 56.0 (–OMe), 36.1(C-1′), 17.2 (C-2′).
General synthetic procedures for compounds 9–10
To a solution of the amine (28.7 mg, 0.15 mmol) and Et3N (31 μL, 0.225 mmol) in anhydrous CH2Cl2 (4 mL) was added acetyl chloride (11.7 μL, 0.165 mmol) or cyclopropanecarbonyl chloride (15.0 μL, 0.165 mmol) at 0 °C. The reaction mixture was slowly warmed to room temperature and stirred for 5 h before quenching with saturated NaHCO3 (5 mL), and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL). The combined organic phases were washed with brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was subjected to flash column chromatography on silica gel (ethyl acetate–petroleum ether, 50 : 50) to give compound 9 or 10.
N-((5-Methoxy-6-methylbenzofuran-3-yl)methyl)acetamide (9)
White solid (34 mg, 96% yield); 1H NMR (500 MHz, CDCl3) δ 7.45 (s, 1H, H-2), 7.23 (s, 1H, H-7), 6.96 (s, 1H, H-4), 5.81 (brs, 1H, NH), 4.49 (d, J = 5.5 Hz, 2H, H-1′), 3.85 (s, 3H, –OMe), 2.30 (s, 3H, H-2′), 1.99 (s, 3H, H-4′); 13C NMR (125 MHz, CDCl3) δ 170.2 (C-3′), 154.6 (C-5), 150.2 (C-8), 142.4 (C-2), 125.2 (C-9), 124.7 (C-6), 117.8 (C-3), 113.1 (C-7), 99.8 (C-4), 55.9 (–OMe), 33.5 (C-1′), 23.3 (C-4′), 17.2(C-2′).
N-((5-Methoxy-6-methylbenzofuran-3-yl)methyl) cyclopropane carboxamide (10)
White solid (36 mg, 94%); 1H NMR (500 MHz, CDCl3) δ 7.47 (s, 1H, H-2), 7.25 (s, 1H, H-7), 6.97 (s, 1H, H-4), 4.55 (d, J = 5.5 Hz, 2H, H-1′), 3.86 (s, 3H, –OMe), 2.31 (s, 3H, H-2′), 1.35–1.30 (m, 1H, H-4′), 1.04–1.01 (m, 2H, H-5′), 0.76–0.74 (m, 2H, H-5′); 13C NMR (125 MHz, CDCl3) δ 173.7 (C-3′), 154.6 (C-5), 150.3 (C-8), 142.3 (C-2), 125.1 (C-9), 124.8 (C-6), 118.1 (C-3), 113.1 (C-7), 100.0 (C-4), 55.9 (–OMe), 33.7 (C-1′), 17.2 (C-2′), 14.7 (C-4′), 7.4 (C-5′).
Synthesis of 5-methoxy-6-methylbenzofuran-3-carbaldehyde (11)
To a solution of compound 4 (0.96 g, 5 mmol) in CH2Cl2 (300 mL), Dess–Martin periodinane (3.18 g, 7.5 mmol) was added slowly. After stirring at room temperature for 2 h, the mixture was diluted with CH2Cl2 (20 mL) and quenched with saturated Na2S2O3 (20 mL), and the aqueous layer was extracted with CH2Cl2 (3 × 20 mL). The combined organic phases were washed with saturated NaHCO3 (20 mL) and brine (20 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was subjected to flash column chromatography on silica gel (ethyl acetate–petroleum ether, 10 : 90) to provide compound 11 (0.85 g, 89% yield) as a pale yellow solid. 1H NMR (500 MHz, CDCl3) δ 10.11 (s, 1H, –CHO), 8.16 (s, 1H, H-2), 7.53 (s, 1H, H-7), 7.30 (s, 1H, H-4), 3.90 (s, 3H, –OMe), 2.32 (s, 3H, H-2′); 13C NMR (125 MHz, CDCl3) δ 185.2 (C-1′), 156.0 (C-5), 155.3 (C-6), 150.7 (C-8), 126.9 (C-1), 124.1 (C-8), 121.1 (C-6), 113.0 (C-7), 102.0 (C-4), 56.0 (OMe), 17.3 (C-2′).
General procedure for the synthesis of compounds 12–22, and 24–26
A mixture of aldehyde (57.1 mg, 0.3 mmol), amine (0.45 mmol) and NaBH(OAc)3 (95.4 mg, 0.45 mmol) and AcOH (3.4 μL, 0.06 mmol) in CH2Cl2 (4 mL) was stirred at room temperature for 12 h under an Ar atmosphere. The reaction was quenched with 1 M NaOH solution (5 mL) and extracted with EtOAc (3 × 10 mL). The combined extracts were washed with brine, dried over Na2SO4, and concentrated. The crude product was purified by column chromatography on silica gel to afford products 12–22, 24–26.
1-(5-Methoxy-6-methylbenzofuran-3-yl)-N,N-dimethyl methanamine (12)
White powder (48 mg, 73% yield); 1H NMR (500 MHz, CDCl3) δ 7.47 (s, 1H, H-2), 7.24 (s, 1H, H-7), 7.04 (s, 1H, H-4), 3.89 (s, 3H, –OMe), 3.56 (s, 2H, H-1′), 2.31 (overlap, 9H, H-2′, –NMe2); 13C NMR (125 MHz, CDCl3) δ 154.5 (C-5), 150.2 (C-8), 143.1 (C-2), 126.0 (C-9), 124.7 (C-6), 117.4 (C-1), 112.9 (C-7), 100.3 (C-4), 56.0 (OMe), 53.2 (C-1′), 45.4 (–NMe2), 17.2 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C13H18NO2]+ 220.1332, found 220.1324.
N-Ethyl-N-((5-methoxy-6-methylbenzofuran-3-yl)methyl)ethanamine (13)
White powder (60 mg, 81% yield); 1H NMR (500 MHz, CDCl3) δ 7.44 (s, 1H, H-2), 7.24 (s, 1H, H-7), 7.10 (s, 1H, H-4), 3.88 (s, 3H, –OMe), 3.68 (s, 2H, H-1′), 2.56 (q, J = 7.0 Hz, 4H, H-3′), 2.32 (s, 3H, H-2′), 1.09 (t, J = 7.0 Hz, 6H, H-4′); 13C NMR (125 MHz, CDCl3): δ 154.2 (C-5), 150.2 (C-8), 142.6 (C-2), 126.4 (C-9), 124.4 (C-6), 118.4 (C-1), 112.8 (C-7), 100.7 (C-4), 55. 9 (OMe), 47.0 (C-1′), 46.9 (C-3′), 17.1 (C-2′), 12.2 (C-4′); HRMS (ESI, m/z): [M + H]+ calcd for [C15H22NO2]+ 248.1645, found 248.1640.
N-((5-Methoxy-6-methylbenzofuran-3-yl)methyl)-N-propylpropan-1-amine (14)
White powder (63 mg, 77% yield); 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 1H, H-2), 7.23 (s, 1H, H-7), 7.13 (s, 1H, H-4), 3.87 (s, 3H, OMe), 3.64 (s, 2H, H-1′), 2.41 (t, J = 7.5 Hz, 4H, H-4′), 2.32 (s, 3H, H-2′), 1.56–1.49 (m, 4H, H-4′), 0.88 (t, J = 7.5 Hz, 6H, H-5′); 13C NMR (125 MHz, CDCl3) δ 154.2 (C-5), 150.3 (C-8), 142.5 (C-2), 126.4 (C-9), 124.3 (C-6), 118.8 (C-1), 112.7 (C-7), 101.0 (C-4), 56.0 (C-3′), 55.9 (OMe), 48.5 (C-1′), 20.5 (C-4′), 17.2 (C-2′), 12.1 (C-5′); HRMS (ESI, m/z): [M + H]+ calcd for [C17H26NO2]+ 276.1958, found 276.1953.
N-Butyl-N-((5-methoxy-6-methylbenzofuran-3-yl)methyl)butan-1-amine (15)
White powder (64 mg, 70% yield); 1H NMR (500 MHz, CDCl3) δ 7.41 (s, 1H, H-2), 7.22 (s, 1H, H-7), 7.11 (s, 1H, H-4), 3.86 (s, 3H, OMe), 3.64 (s, 2H, H-1′), 2.44 (t, J = 7.5 Hz, 4H, H-3′), 2.32 (s, 3H, H-2′), 1.51–1.46 (m, 4H, H-4′), 1.35–1.28 (m, 4H, H-5′), 0.88 (t, J = 7.5 Hz, 6H, H-6′); 13C NMR (125 MHz, CDCl3) δ 154.2 (C-5), 150.3 (C-8), 142.5 (C-2), 126.4 (C-9), 124.3 (C-2), 118.4 (C-1), 112.7 (C-7), 101.0 (C-4), 55.8 (OMe), 53.7 (C-3′), 48.4 (C-1′), 29.5 (C-4′), 20.8 (C-5′), 17.2 (C-2′), 14.2 (C-6′); HRMS (ESI, m/z): [M + H]+ calcd for [C19H31NO2]+ 304.2271, found 304.2262.
1-((5-Methoxy-6-methylbenzofuran-3-yl)methyl)pyrrolidine (16)
Pale yellow oil (55 mg, 75% yield); 1H NMR (500 MHz, CDCl3) δ 7.49 (s, 1H, H-2), 7.24 (s, 1H, H-7), 7.05 (s, 1H, H-4), 3.88 (s, 3H, OMe), 3.77 (s, 2H, H-1′), 2.65–2.62 (m, 4H, H-3′), 2.31 (s, 3H, H-2′), 1.83–1.80 (m, 4H, H-4′); 13C NMR (125 MHz, CDCl3) δ 154.5 (C-5), 150.1 (C-8), 142.9 (C-2), 126.0 (C-9), 124.6 (C-2), 117.7 (C-1), 112. 9 (C-7), 100.3 (C-4), 56.0 (OMe), 54.2 (C-3′), 49.1 (C-1′), 23.7 (C-4′), 17.1 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C15H20NO2]+ 246.1489, found 246.1480.
4-((5-Methoxy-6-methylbenzofuran-3-yl)methyl)morpholine (17)
Pale yellow oil (69 mg, 88% yield); 1H NMR (500 MHz, CDCl3) δ 7.45 (s, 1H, H-2), 7.25 (s, 1H, H-7), 7.12 (s, 1H, H-4), 3.89 (s, 3H, –OMe), 3.72 (t, J = 5.0 Hz, 4H, H-4′), 3.60 (s, 2H, H-1′), 2.50 (t, J = 4.5 Hz, 4H, H-3′), 2.34 (s, 3H, H-2′); 13C NMR (125 MHz, CDCl3) δ 154.3 (C-5), 150.2 (C-8), 142.8 (C-2), 126.0 (C-9), 124.7 (C-6), 116.9 (C-1), 112.8 (C-7), 100.6 (C-4), 67.2 (C-4′), 55.9 (OMe), 53.7 (C-3′), 52.7 (C-1′), 17.1 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C15H20NO3]+ 262.1438, found 262.1436.
1-((5-Methoxy-6-methylbenzofuran-3-yl)methyl)-4-methylpiperazine (18)
Pale yellow powder (66 mg, 81% yield); 1H NMR (500 MHz, CDCl3) δ 7.43 (s, 1H, H-2), 7.22 (s, 1H, H-7), 7.08 (s, 1H, H-4), 3.87 (s, 3H, OMe), 3.60–3.59 (m, 2H, H-1′), 2.57–2.40 (m, 8H, H-3′, H-4′), 2.30 (s, 3H, H-2′), 2.30 (s, 3H, H-2′), 2.27 (s, 3H, H-5′); 13C NMR (125 MHz, CDCl3) δ 154.3 (C-5), 150.1 (C-8), 142.8 (C-1), 126.1 (C-9), 124.5 (C-6), 117.2 (C-1), 112.8 (C-7), 100.6 (C-4), 55.9 (OMe), 55.3 (C-4′), 53.1 (C-3′), 52.2 (C-1′), 46.1 (C-5′), 17.1 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C16H23N2O2]+ 275.1754, found 275.1745.
1-Ethyl-4-((5-methoxy-6-methylbenzofuran-3-yl)methyl) piperazine (19)
Pale yellow powder (63 mg, 73% yield); 1H NMR (500 MHz, CDCl3) δ 7.44 (s, 1H, H-2), 7.22 (s, 1H, H-7), 7.09 (s, 1H, H-4), 3.87 (s, 3H, OMe), 3.61 (s, 2H, H-1′), 2.59–2.26 (m, 8H, H-3′, H-4′), 2.39 (q, J = 7.2 Hz, 2H, H-5′), 2.30 (s, 3H, H-2′), 1.07 (t, J = 7.0 Hz, 1H, H-6′); 13C NMR (125 MHz, CDCl3) δ 154.3 (C-5), 150.2 (C-8), 142.8 (C-2), 126.2 (C-9), 124.5 (C-6), 117.3 (C-1), 112.8 (C-7), 100.7 (C-4), 55.9 (OMe), 53.2 (C-4′), 53.1 (C-3′), 52.4 (C-1′), 52.2 (C-5′), 17.1 (C-2′), 12.1 (C-6′); HRMS (ESI, m/z): [M + H]+ calcd for [C17H26N2O2]+ 289.1911, found 289.1901.
2-(4-((5-Methoxy-6-methylbenzofuran-3-yl)methyl)piperazin-1-yl)pyrazine (20)
Pale yellow powder (68 mg, 67% yield); 1H NMR (500 MHz, CDCl3) δ 8.11 (d, J = 1.5 Hz, 1H, H-6′), 8.05–8.04 (m, 1H, H-7′), 7.83 (d, J = 2.5 Hz, 1H, H-8′), 7.46 (s, 1H, H-2), 7.25 (s, 1H, H-7), 7.12 (s, 1H, H-4), 3.87(s, 3H, OMe), 3.66 (s, 2H, H-1′), 3.60 (t, J = 5.5 Hz, 4H, H-4′), 2.61 (t, J = 5.0 Hz, 4H, H-3′), 2.32 (s, 3H, H-2′); 13C NMR (125 MHz, CDCl3) δ 155.1 (C-5′), 154.4 (C-5), 150.3 (C-8), 142.9 (C-2), 141.8 (C-8′), 133.0 (C-7′), 131.1(C-6′), 125.9 (C-9), 124.7 (C-6), 116.9 (C-3), 112.9 (C-7), 100.6 (C-4), 55.9 (OMe), 52.7 (C-3′), 52.4 (C-1′), 44.7 (C-4′), 17.1 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C19H23N4O2]+ 339.1816, found 339.1793.
1-(4-((5-Methoxy-6-methylbenzofuran-3-yl)methyl)piperazin-1-yl)ethan-1-one (21)
Pale yellow powder (67 mg, 74% yield); 1H NMR (500 MHz, CDCl3) δ 7.41 (s, 1H, H-3), 7.22 (s, 1H, H-7), 7.06 (s, 1H, H-4), 3.85 (s, 3H, OMe), 3.61 (t, J = 5.0 Hz, 2H, H-4′), 3.59 (s, 2H, H-1′), 3.42 (t, J = 5.0 Hz, 2H, H-6′), 2.46–2.43 (m, 4H, H-3′, H-5′) 2.29 (s, 3H, H-2′), 2.05 (s, 3H, H-8′); 13C NMR (125 MHz, CDCl3) δ 168.9 (C-8′), 154.3 (C-5), 150.1 (C-8), 142.8 (C-2), 125.8 (C-9), 124.7 (C-6), 116.8 (C-3), 112.8 (C-7), 100.4 (C-4), 55.8 (OMe), 52.9 (C-3′), 52.8 (C-5′), 52.1 (C-1′), 46.4 (C-4′), 41.5 (C-6′), 21.3 (C-8′), 17.0 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C17H23N2O3]+ 303.1703, found 303.1689.
Tert-butyl 4-((5-methoxy-6-methylbenzofuran-3-yl)methyl)piperazine-1-carboxylate (22)
Pale yellow powder (63 mg, 58% yield); 1H NMR (500 MHz, CDCl3) δ 7.43 (s, 1H, H-2), 7.24 (s, 1H, H-7), 7.09 (s, 1H, H-4), 3.88 (s, 3H, OMe), 3.61 (s, 2H, H-1′), 3.43 (d, J = 5.0 Hz, 4H, H-4′), 2.44 (d, J = 5.0 Hz, 4H, H-3′), 2.31 (s, 3H, H-2′), 1.45 (s, 9H, H-7′); 13C NMR (125 MHz, CDCl3) δ 154.9 (C-5′), 154.4 (C-5), 150.3 (C-8), 142.9 (C-2), 126.0 (C-9), 124.7 (C-6), 117.1 (C-1), 112.9 (C-7), 100. 7 (C-4), 79.7 (C-6′), 56.0 (OMe), 53.0 (C-3′), 53.0 (C-4′), 52.4 (C-1′), 28. 6 (C-7′), 17.2 (C-2′).
1-((5-Methoxy-6-methylbenzofuran-3-yl)methyl)-4-methyl-1,4-diazepane (24)
Pale yellow oil (73 mg, 85% yield); 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 1H, H-2), 7.22 (s, 1H, H-7), 7.13 (s, 1H, H-4), 3.88 (s, 3H, OMe), 3.70 (s, 2H, H-1′), 2.76 (t, J = 6.0 Hz, 2H, H-3′), 2.75–2.73 (m, 2H, H-3′, H-7′), 2.69 (t, J = 6.0 Hz, 2H, H-5′), 2.63–2.61 (m, 2H, H-6′), 2.36 (s, 3H, H-8′), 2.31 (s, 3H, H-2′), 1.86–1.82 (m, 2H, H-4′); 13C NMR (125 MHz, CDCl3) δ 154.2 (C-5), 150.3 (C-8), 142.5 (C-2), 126.1 (C-9), 124.5 (C-6), 118.6 (C-1), 112.8 (C-7), 100.9 (C-4), 58.6 (C-6′), 56.5 (C-7′), 55.9 (OMe), 54.4 (C-5′), 54.2 (C-3′), 52.6 (C-1′), 47.0 (C-8′), 27.4 (C-4′), 17.1 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C17H25N2O2]+ 289.1911, found 289.1909.
1,4-Bis((5-methoxy-6-methylbenzofuran-3-yl)methyl) piperazine (25)
Pale yellow powder (11 mg, 63% yield); 1H NMR (500 MHz, CDCl3) δ 7.44 (s, 2H, H-2), 7.23 (s, 2H, H-7), 7.08 (s, 2H, H-4), 3.87 (s, 6H, OMe), 3.62 (s, 4H, H-1′), 2.58 (brs, 8H, H-3′), 2.31 (s, 6H, H-2′); 13C NMR (125 MHz, CDCl3) δ 154.3 (C-5), 150.2 (C-8), 142.8 (C-2), 126.2 (C-9), 124.6 (C-2), 117.3 (C-1), 112.8 (C-7), 100.7 (C-4), 56.0 (OMe), 53.3 (C-1′), 52.2 (C-3′), 17.1 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C26H30N2O4]+ 435.2278, found 435.2238.
1,4-Bis((5-methoxy-6-methylbenzofuran-3-yl)methyl)-1,4-diazepane (26)
Pale yellow powder (27 mg, 40% yield); 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 2H, H-2), 7.23 (s, 2H, H-7), 7.10 (s, 2H, H-4), 3.83(s, 6H, OMe), 3.74 (s, 4H, H-1′), 2.80 (t, J = 6.0 Hz, 4H, H-3′), 2.74 (s, 4H, H-5′), 2.32 (s, 6H, H-2′), 1.88–1.84 (m, 2H, H-4′); 13C NMR (125 MHz, CDCl3) δ 154.3 (C-5), 150.3 (C-8), 142.6 (C-2), 126.1 (C-9), 124.6 (C-6), 118.2 (C-1), 112.8 (C-7), 100.9 (C-4), 55.9 (OMe), 55.4 (C-5′), 54.1 (C-3′), 52.4 (C-1′), 27.8 (C-4′), 17.2 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C27H33N2O4]+ 449.2435, found 449.2411.
Synthesis of 1-((5-methoxy-6-methylbenzofuran-3-yl)methyl)piperazine (23)
To a solution of compound 22 (36 mg, 0.1 mmol) in CH2Cl2 (3 mL), trifluoroacetic acid (0.2 mL) was added slowly. After stirring at room temperature for 6 h, the mixture was quenched with saturated NaHCO3 (5 mL), and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL). The combined organic phases were washed with saturated NaHCO3 (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was subjected to flash column chromatography on silica gel (methanol–chloroform, 5 : 95) to provide compound 23 (25 mg, 98% yield) as a pale-yellow solid. 1H NMR (500 MHz, MeOD) δ 7.55 (s, 1H, H-2), 7.19 (s, 1H, H-7), 7.13 (s, 1H, H-4), 3.85 (s, 3H, OMe), 3.61 (s, 2H, H-1′), 2.88 (t, J = 5.0 Hz, 4H, H-4′) 2.52 (brs, 4H, H-3′), 2.25 (s, 3H, H-2′); 13C NMR (125 MHz, MeOD) δ 155.8 (C-5), 151.5 (C-9), 144.9 (C-2), 127.3 (C-8), 125.6 (C-6), 117.4 (C-3), 113.5 (C-7), 101.3 (C-4), 56.2 (OMe), 53.9 (C-3′), 52.8 (C-1′), 46.0 (C-4′), 17.1 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C15H21N2O2]+ 261.1598, found 261.1605.
Synthesis of 2-hydroxy-5-methoxybenzaldehyde
To a solution of 4-methoxyphenol (1.24 g, 10 mmol), anhydrous magnesium dichloride (1.43 g, 15 mmol) and dry triethylamine (5.6 mL, 40 mmol) in dry CH3CN was added paraformaldehyde (1.5 g, 50 mmol), and the reaction mixture was heated under reflux for 8 h. After cooling to room temperature, the mixture was quenched with aqueous HCl (50 mL of a 2 N solution). The resultant mixture was extracted with EtOAc (3 × 50 mL), washed with brine (50 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (EtOAc–petroleum ether, 10 : 90) to provide 2-hydroxy-5-methoxybenzaldehyde (1.38 g, 91% yield) as a pale yellow oil. 1H NMR (400 MHz, CDCl3) δ 10.60 (s, 1H, CHO), 9.77 (s, 1H, OH), 7.09–7.05 (m, 1H, H-6), 6.93–6.92 (m, 1H, H-3), 6.85 (d, J = 9.2 Hz, 1H, H-5), 3.75 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 196.1 (CHO), 155.9 (C-1), 152.7 (C-4), 125.1 (C-5), 120.0 (C-2), 118.6 (C-6), 115.1 (C-3), 55. 8 (OMe).
Synthesis of ethyl 5-methoxy-benzofuran-3-carboxylate
To a solution of 2-hydroxy-5-methoxybenzaldehyde (767 mg, 5 mmol) in CH2Cl2 (10 mL) was added a tetrafluoroboric acid–diethyl ether complex (67 μL, 0.5 mmol) at 0 °C. The resulting mixture was stirred at that temperature for 10 min before ethyl diazoacetate (0.9 mL, 8.5 mmol) was added dropwise. The mixture was stirred until nitrogen no longer evolved and for 20–30 min more at room temperature. Concentrated sulfuric acid (0.5 mL) was added and stirred for 30 min at room temperature. The reaction mixture was quenched with saturated NaHCO3 (15 mL), and the aqueous layer was extracted with CH2Cl2 (2 × 15 mL). The combined organic phases were washed with brine (30 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was subjected to flash column chromatography on silica gel (EtOAc–petroleum ether, 4 : 96) to provide ethyl 5-methoxy-benzofuran-3-carboxylate (924 mg, 84% yield) as a pale yellow oil. 1H NMR (400 MHz, CDCl3) δ 8.03–8.01 (m, 1H, H-2), 7.35–7.34 (m, 1H, H-7), 7.22–7.16 (m, 1H, H-4), 6.79–6.76 (m, 1H, H-6), 4.23 (q, J = 6.8 Hz, 2H, H-2′), 3.70 (s, 3H, OMe), 1.25 (t, J = 6.8 Hz, 3H, H-3′); 13C NMR (100 MHz, CDCl3) δ 163.3 (C-1′), 156.8 (C-5), 151.3 (C-1), 150.3 (C-8), 125.3 (C-9), 114.4 (C-3), 114.3 (C-6), 110.0 (C-7), 103.6 (C-4), 60.3 (C-1′), 55.6 (OMe), 14.2 (C-2′).
Synthesis of (5-methoxybenzofuran-3-yl)methanol (27)
To a solution of the ethyl 5-methoxy-benzofuran-3-carboxylate (661 mg, 3 mmol) in CH2Cl2 (10 mL) was added DIBAL-H (1.0 M, 9 mL, 9 mmol) dropwise over 10 min at −78 °C. The reaction mixture was stirred at that temperature for 20 min before it was quenched with saturated NH4Cl (20 mL). The resultant mixture was extracted with EtOAc (3 × 20 mL), and the combined organic phases were washed with brine (40 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was subjected to flash column chromatography on silica gel (acetone–petroleum ether, 20 : 80) to provide 27 (496 mg, 88% yield) as a pale-yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.38 (s, 1H, H-2), 7.22 (d, J = 8.8 Hz, 1H, H-7), 6.96 (s, 1H, H-4), 6.77 (d, J = 8.8 Hz, 1H, H-6), 4.58 (s, 2H, C-1′), 3.68 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 155.9 (C-5), 150.5 (C-8), 143.2 (C-2), 127.3 (C-9), 120.5 (C-1), 113.4 (C-6), 112.0 (C-7), 102.3 (C-4), 55.9 (OMe), 55.6 (C-1′).
Synthesis of 5-methoxybenzofuran-3-carbaldehyde (28)
Compound 28 was prepared from compound 27 following a similar procedure to that for 11. Pale-yellow powder (464 mg, 88% yield); 1H NMR (400 MHz, CDCl3) δ 10.14 (s, 1H, CHO), 8.23 (s, 1H, H-2), 7.63 (d, J = 2.8 Hz, 1H, H-7), 7.43 (d, J = 9.2 Hz, 1H, H-4), 7.00 (dd, J = 9.2, 2.8 Hz, 1H, H-6), 3.88 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 185.0 (C O), 157.6 (C-5), 156.1 (C-2), 151.0 (C-8), 123.9 (C-8), 123.7 (C-3), 115.9 (C-6), 112.4 (C-7), 104.1 (C-4), 56.1 (OMe).
Synthesis of compounds 29–31
Compounds 29–31 were prepared from compound 28 following a similar procedure to that for compounds 16–18.
1-((5-Methoxybenzofuran-3-yl)methyl)pyrrolidine (29)
Pale-yellow oil (54 mg, 79% yield); 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 1H, H-2),7.28 (d, J = 8.8 Hz, 1H, H-7), 7.05 (d, J = 2.4 Hz, 1H, H-4), 6.82 (dd, J = 8.8, 2.4 Hz, 1H, H-6), 3.78 (s, 3H, OMe), 3.72 (s, 2H, H-1′), 2.59 (t, J = 6.0 Hz, 4H, H-2′), 1.76–1.73 (m, 4H, H-3′); 13C NMR (100 MHz, CDCl3) δ 156.0 (C-5), 150.4 (C-8), 144.3 (C-2), 128. 6 (C-9), 117.3 (C-3), 113.1 (C-6), 112.0 (C-7), 102.7 (C-4), 56.1 (OMe), 53.9 (C-2′), 48.7 (C-1′), 23.6 (C-3′); HRMS (ESI, m/z): [M + H]+ calcd for [C14H18NO2]+ 232.1332, found 232.1316.
1-((5-Methoxybenzofuran-3-yl)methyl)-4-methylpiperazine (30)
Pale-yellow powder (67 mg, 86% yield); 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 1H, H-2), 7.32 (d, J = 8.8 Hz, 1H, H-7), 7.09 (d, J = 2.4 Hz, 1H, H-4), 6.86 (dd, J = 8.8, 2.4 Hz, 1H, H-5), 3.82 (s, 3H, OMe), 3.64 (s, 2H, H-1′), 2.85–2.73 (m, 8H, H-2′, H-3′), 2.51 (s, 3H, H-4′); 13C NMR (100 MHz, CDCl3) 155.9 (C-5), 150.5 (C-8), 144.5 (C-2), 128.3 (C-9), 116.2 (C-1), 113.0 (C-6), 112.0 (C-7), 103.1 (C-4), 56.1 (OMe), 54.0 (C-3′), 51.4 (C-1′), 50.7 (C-2′), 44.2 (C-4′); HRMS (ESI, m/z): [M + H]+ calcd for [C15H21N2O2]+ 261.1598, found 261.1598.
4-((5-Methoxybenzofuran-3-yl)methyl)morpholine (31)
Pale-yellow oil (67 mg, 90% yield); 1H NMR (400 MHz, CDCl3) δ 7.40 (s, 1H, H-2), 7.24 (d, J = 8.8 Hz, 1H, H-7), 7.09 (d, J = 2.4 Hz, 1H, H-4), 6.80 (dd, J = 8.8, 2.4 Hz, 1H, H-6), 3.75 (s, 3H, OMe), 3.60 (t, J = 4.4 Hz, 4H, H-3′), 3.48 (s, 2H, H-1′), 2.38 (t, J = 4.4 Hz, 4H, H-3′); 13C NMR (100 MHz, CDCl3) δ 155.8 (C-5), 150.5 (C-8), 144.0 (C-2), 128.5 (C-9), 116.9 (C-3), 112.9 (C-6), 111.8 (C-7), 103.2 (C-4), 67.1 (C-3′), 55.9 (OMe), 53.6 (C-2′), 52.5 (C-1′); HRMS (ESI, m/z): [M + H]+ calcd for [C14H18NO3]+ 248.1281, found 248.1277.
Synthesis of (3-(ethoxycarbonyl)-5-methoxybenzofuran-2-yl)boronic acid
To a solution of the ethyl 5-methoxy-benzofuran-3-carboxylate (3.3 g, 15 mmol) and trimethyl borate (3.7 mL, 33 mmol) in THF (75 mL) was added LDA (2.0 M, 16.5 mL, 33 mmol) dropwise at −78 °C. The reaction mixture was stirred at that temperature for 20 min before it was quenched with 5% aqueous HCl (50 mL). The resultant mixture was extracted with EtOAc (3 × 50 mL), and the combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was subjected to flash column chromatography on silica gel (acetone–petroleum ether, 20 : 80) to provide (3-(ethoxycarbonyl)-5-methoxybenzofuran-2-yl)boronic acid (3.26 g, 82% yield) as a brown solid. 1H NMR (400 MHz, CDCl3) δ 7.57 (Brs, 2H, OH), 7.51 (d, J = 8.8 Hz, 1H, H-7), 7.46 (d, J = 2.8 Hz, 1H, H-4), 7.06 (dd, J = 8.8, 2.8 Hz, 1H, H-6), 4.54 (q, J = 7.2 Hz, 2H, H-2′), 3.88 (s, 3H, OMe), 1.53 (t, J = 7.2 Hz, 3H, H-3′); 13C NMR (100 MHz, CDCl3) δ 166.7 (C-1′), 157.8 (C-5), 153.4 (C-8), 126.6 (C-9), 123.5 (C-2), 117.1 (C-6), 112.8 (C-7), 105.6 (C-4), 62.4 (C-2′), 56.0 (OMe), 14.3 (C-3′).
Synthesis of ethyl 5-methoxy-2-phenylbenzofuran-3-carboxylate
A mixture of boronic acid (1.32 g, 5 mmol), iodobenzene (613 μL, 5.5 mmol), PdCl2(dppf), DCM (250 mg, 0.25 mmol), and Na2CO3(2.11 g, 20 mmol) in 60 mL DME–H2O(1 : 1) were heated at 70 °C for 24 h. The cooled mixture was eluted with H2O (30 mL) and ethyl acetate (60 mL), and the aqueous layer was extracted with ethyl acetate (2 × 50 mL), and the combined organic phases were washed with brine (100 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was subjected to flash column chromatography on silica gel (acetone–petroleum ether, 5 : 95) to give ethyl 5-methoxy-2-phenylbenzofuran-3-carboxylate (1.238 g, 83% yield). 1H NMR (400 MHz, CDCl3) δ 8.00–7.97 (m, 2H, H-5′), 7.56 (d, J = 2.8 Hz, 1H, H-4), 7.49–7.45 (m, 3H, H-7, H-6′), 7.41 (d, J = 9.2 Hz, 1H, H-7′), 6.95 (dd, J = 8.8, 2.8 Hz, 1H, H-6), 4.40 (q, J = 7.2 Hz, 2H, H-2′), 3.89 (s, 3H, OMe), 1.39 (t, J = 7.2 Hz, 3H, H-3′); 13C NMR (100 MHz, CDCl3) δ 164.2 (C-2), 161.5 (C-1′), 157.0 (C-8), 149.0 (C-5), 130.3 (C-7′), 129.9 (C-8), 129.6 (C-6′), 128.1 (C-5′), 114.3 (C-1), 111.8 (C-6), 109.1 (C-7), 105.0 (C-4), 60.7 (C-2′), 56.0 (OMe), 14.3 (C-3′).
Synthesis of (5-methoxy-2-phenylbenzofuran-3-yl)methanol (32)
Compound 32 was prepared from ethyl 5-methoxy-2-phenylbenzofuran-3-carboxylate following a similar procedure to that for compound 4. Pale-yellow solid (87% yield); 1H NMR (400 MHz, CDCl3) δ 7.79 (d, J = 7.2 Hz, 2H, H-3′), 7.45 (dd, J = 7.6, 7.6 Hz, 2H, H-4′), 7.39–7.34 (m, 2H, H-7, H-5′), 7.10 (d, J = 2.8 Hz, 1H, H-4), 6.87 (dd, J = 8.8, 2.8 Hz,1H, H-6), 4.88 (s, 2H, H-1′), 3.81 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 156.5 (C-2), 154.3 (C-5), 149.0 (C-8), 130.4 (C-2′), 129.9 (C-9), 128.9 (C-5′), 128.8 (C-4′), 127.3 (C-3′), 115.8 (C-1), 113.0 (C-6), 111.8 (C-7), 103.4 (C-4), 56.0 (OMe), 55.6 (C-1′).
Synthesis of 5-methoxy-2-phenylbenzofuran-3-carbaldehyde (33)
Compound 33 was prepared from compound 32 following a similar procedure to that for compound 11. Pale-yellow solid (93% yield); 1H NMR (400 MHz, CDCl3) δ 10.32 (s, 1H, CHO), 7.84–7.82 (m, 2H, H-3′), 7.74 (d, J = 3.5 Hz, 1H, H-4), 7.56–7.55 (m, 2H, H-4′), 7.43 (d, J = 9.2 Hz, 1H), 6.98 (dd, J = 9.2, 3.5 Hz, 1H, H-6), 3.89 (s, 3H, H-7); 13C NMR (100 MHz, CDCl3) δ 186.9 (C-1′), 166.0 (C-2), 157.6 (C-5), 149.0 (C-8), 131.1 (C-5′), 129.2 (C-4′), 129.2 (C-3′), 128.8 (C-2′), 126.2 (C-9), 117.8 (C-1), 115.5 (C-6), 111.9 (C-7), 104.3 (C-4), 56.1 (OMe); HRMS (ESI, m/z): [M + H]+ calcd for [C16H13O3]+ 253.0859, found 253.0877.
Synthesis of 1-((5-methoxy-2-phenylbenzofuran-3-yl)methyl)-4-methylpiperazine (34)
Compound 34 was prepared from compound 33 and 1-methylpiperazine following a similar procedure to that for compound 18. Pale-yellow powder (45 mg, 45% yield); 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 7.2 Hz, 2H, H-6′), 7.41–7.37 (m, 2H, H-7′), 7.32–7.28 (m, 2H, H-7, H-8′), 7.14 (d, J = 2.8 Hz, 1H, H-4), 6.83 (dd, J = 8.8, 2.8 Hz, 1H, H-6), 3.80 (s, 3H, OMe), 3.63 (s, 2H, H-1′), 2.55–2.38 (m, 8H, H-2′, H-3′), 2.22 (s, 3H, H-4′); 13C NMR (100 MHz, CDCl3) δ 155.9 (C-2), 154.3 (C-5), 148.9 (C-8), 131.5 (C-5′), 131.1 (C-8), 128.7 (C-7′), 128.5 (C-8′), 127.7 (C-5′), 113.0 (C-3), 112.9 (C-6), 111.5 (C-7), 103.0 (C-4), 56.0 (OMe), 55.4 (C-3′), 53.1 (C-2′), 52.3 (C-4′), 46.1 (C-1′); HRMS (ESI, m/z): [M + H]+ calcd for [C21H25N2O2]+ 337.1911, found 337.1896.
Synthesis of methyl 5-methoxy-2-methylbenzofuran-3-carboxylate
In a round-bottom flask equipped with a reflux condenser, 2-iodo-4-methoxyphenol (1.035 g, 4 mmol), DMSO (18 mL), Et3N (1080 μL, 8 mmol, 2 equiv.), methyl propiolate (600 μL, 6 mmol), and Pd2(dba)3 (368 mg, 0.2 mmol) were added sequentially, and the reaction mixture was heated under reflux for 20 h. After cooling to room temperature, the mixture was poured into water (50 mL), extracted with EtOAc (3 × 50 mL), and the combined organic phases were washed with brine (50 mL), dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The residue was subjected to flash column chromatography on silica gel (acetone–petroleum ether, 10 : 90) to provide methyl 5-methoxy-2-methylbenzofuran-3-carboxylate as a white powder (422 mg, 48% yield). 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 2.8 Hz, 1H, H-4), 7.30 (d, J = 8.8 Hz, 1H, H-7), 6.87 (dd, J = 8.8, 2.8 Hz, 1H, H-6), 3.94 (s, 3H, OMe), 3.87 (s, 3H, OMe), 2.74 (s, 3H, H-2′); 13C NMR (100 MHz, CDCl3) δ 165.1 (C-1′), 164.5 (C-5), 156.8 (C-2), 148.6 (C-8), 127.0 (C-9), 113.0 (C-3), 111.3 (C-6), 109.1 (C-7), 104.4 (C-4), 56.0 (OMe), 51.5 (C-3′), 14.8 (C-2′).
Synthesis of (5-methoxy-2-methylbenzofuran-3-yl)methanol (35)
Compound 35 was prepared from 5-methoxy-2-methylbenzofuran-3-carboxylate following a similar procedure to that for compound 4. Pale-yellow solid (84% yield); 1H NMR (400 MHz, CDCl3) δ 7.27 (d, J = 8.8 Hz, 1H, H-7), 7.05(d, J = 2.8 Hz, 1H, H-4), 6.82 (dd, J = 8.8, 2.8 Hz, 1H, H-6), 4.72 (s, 2H, H-1′), 3.83 (s, 3H, OMe), 2.42 (s, 3H, H-2′); 13C NMR (100 MHz, CDCl3) δ 156.0 (C-5), 153.7 (C-6), 149.0 (C-8), 129.1 (C-9), 114.6 (C-3), 112.1 (C-6), 111.2 (C-7), 102.0 (C-2), 56.1 (OMe), 55.6 (C-1′), 12.2 (C-2′).
Synthesis of 5-methoxy-2-methylbenzofuran-3-carbaldehyde (36)
Compound 36 was prepared from compound 35 following a similar procedure to that for compound 11. White solid (84% yield); 1H NMR (400 MHz, CDCl3) δ 10.19 (s, 1H, CHO), 7.60 (d, J = 2.4 Hz, 1H, H-4), 7.32 (d, J = 8.8 Hz, 1H, H-7), 6.90 (dd, J = 8.8, 2.4 Hz, 1H, H-6), 3.87 (s, 3H, OMe), 2.74 (s, 3H, H-2′); 13C NMR (100 MHz, CDCl3) δ 185.1 (C-1′), 167.5 (C-2), 157.4 (C-5), 148.8 (C-8), 125.4 (C-9), 118.2 (C-1), 114.3 (C-6), 111.5 (C-7), 103.8 (C-4), 56.1 (OMe), 13.2 (C-2′).
Synthesis of 1-((5-methoxy-2-methylbenzofuran-3-yl)methyl)-4-methylpiperazine (37)
Compound 37 was prepared from compound 36 and 1-methylpiperazine following a similar procedure to that for compound 18. Pale-yellow powder (47 mg, 57% yield); 1H NMR (400 MHz, CDCl3) δ 7.26–7.24 (m, 1H, H-7), 7.12–7.11 (m, 1H, H-4), 6.81–6.78 (m, 1H, H-6), 3.84 (s, 3H, OMe), 3.53 (s, 2H, H-1′), 2.55–2.45 (m, 8H, H-3′, H-4′), 2.40 (s, 3H, H-5′), 2.28 (s, 3H, H-2′); 13C NMR (100 MHz, CDCl3) δ 155.7 (C-5), 153.8 (C-2), 149.0 (C-8), 130.5 (C-9), 111.5 (C-1), 111.2 (C-6), 110.8 (C-7), 103.2 (C-4), 56.1 (OMe), 55.4 (C-4′), 53.1 (C-3′), 52.2 (C-1′), 46.1 (C-5′), 12.5 (C-2′); HRMS (ESI, m/z): [M + H]+ calcd for [C16H23N2O2]+ 275.1754, found 275.1750.
Biological evaluation
In vitro MT agonistic activity evaluation using the Fluo-8 calcium assay
Following our previous paper,12 HEK293 cells that stably expressed the human MT1 or MT2 receptor were cultivated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, and cultured with 95% O2/5% CO2 at 37 °C. The cells were seeded in a Matrigel coated 96-well black plate with a plating volume of 100 μL per well at a density of 4 × 104 per well, and incubated in a CO2 incubator (Thermo Forma 3310, Gaithersburg, U.S.) overnight. Then the cells were dyed with the HDB wash free calcium assay kit, and placed in the CO2 incubator for 1 h. The tested compounds and the positive drug were dissolved in 10 μL dimethyl sulfoxide and 990 μL HBSS buffer, respectively, and extracted at a plating volume of 100 μL per well in a Matrigel coated 96-well clear bottom plate. Two 96-well plates were put into the Flexstation 3 Benchtop Multi-Mode Microplate Reader. The absorption values were read by the Flexstation 3 Benchtop Multi-Mode Microplate Reader at room temperature with wavelength (excitation: 485 nm; emission:525 nm; emission cut-off: 515 nm). EC50 values for derivatives 4, 8, 12, 13, 16, 18 and 23–25 were determined from the dose–response curves obtained with seven concentrations in the range of 0.02 to 2 mM. The results were calculated by the software GraphPad Prism 6.0.
Tango assays
Plasmids and reagents
The plasmids used in this study were Tet-inducible luciferase reporter (#64127), pCDNA3.1(+)-CMV-bArrestin2-tev (#107245), MTNR1A-Tango (https://www.addgene.org/66443/) (#66443) and MTNR1B-Tango (https://www.addgene.org/66444/) (#66444). These plasmids were obtained from Addgene. Endotoxin-free plasmid DNA isolation kits (TianGen, DP117) were used in this study. Bright-Glo™ Luciferase Assay System (E2620) was purchased from Promega. Lipofectamine 3000 transfection reagent and SYBR® Green Master Mix (150603) were purchased from Invitrogen.
Site-directed mutation
We used site-directed mutation to construct MT1 and MT2 mutants. Wide MT1 and MT2 plasmids were used as PCR templates, and PrimeSTAR® Max DNA polymerase (Takara, R045A) was used in the PCR reaction. The primers for site-directed mutation were the following:
MT1N162A: F-tgccgtcctgccggctctgagggcaggc, R-gcctgccctcagagccggcaggacggca; MT1Q181A: F-ttattcatgtactttcgctgcgagcgtgtctagcgcctac, R-gtaggcgctagacacgctcgcagcgaaagtacatgaataa; MT2N175A: F-agttgtggccctgttgcctgcctttttcgttggatccttg, R-caaggatccaacgaaaaaggcaggcaacagggccacaact; MT2Q194A: F-cccgaatttactcatgcacctttatagcaaccgccagcaccc, R-gggtgctggcggttgctataaaggtgcatgagtaaattcggg.
Method
Tango arrestin recruitment assays were carried out according to the previously published procedure.22 Briefly, 293T cells (ATCC, CRL-3216) were transiently transfected with tet-inducible luciferase reporter, pCDNA3.1(+)-CMV-bArrestin2-tev, and TANGO plasmids. The following day (day2), transfected cells were transferred at 20 000 cells per well into poly-l-lysine coated 96-well white clear-bottom cell culture plates (Corning, 3610). On day 3, drug simulation solutions were added to each well. On day 4, Bright-Glo reagent (40 μL per well) was added and after incubation for 20 minutes, luminescence was counted in a luminescence plate reader (Molecular Devices, Flexstation® 3). Results were analyzed using GraphPad Prism 6.
Forced swim test (FST)
Male Kunming mice (weighing 15–18 g) were bought from Beijing HFK Bioscience CO. Ltd. (License: SCXK (JING) 2019-0008, Beijing, China). All mice were housed in a group of six animals per cage at an ambient temperature of 22 ± 1 °C and relative humidity of 55–65% under a normal 12 h light/dark cycle (lights on at 7:00 a.m.), with free access to food and water.
The forced swim test was performed according to a literature method.23 Mice were individually placed into glass cylinders (height: 25 cm, diameter: 15 cm) filled with water to a height of 10 cm and the temperature was maintained at 25 ± 1 °C. Mice were gently placed onto the water and forced to swim for 6 min, and the total duration of immobility during the last 4 min was automatically measured by the ANY-maze video tracking system (Anymaze, Stoelting Co., Wood Dale, USA).
Neurochemical tests
After administration of the vehicle or compound 18 (160 mg kg−1) for 14 days, male Kunming mice were executed by the cervical dislocation method, brains were collected and rapidly dissected on an ice-chilled glass plate, and the frontal cortex, hippocampus, striatum, hypothalamus, and thalamus were separated. The tissue samples were weighed and homogenized in an ice-cold solution of 0.2 M perchloric acid (10 μ mg−1) containing 0.1 mM EDTA, and then centrifuged at 4 °C for 20 min at 15 000 rpm. The supernatant was collected and the contents of NE, 5-HT, 5-HIAA, DA, DOPAC and HVA were determined by HPLC-ECD. An Agilent 1200 pump (Agilent, California,U.S.A) was set to a flow rate of 0.6 mL min−1. The detector (Coulochem III-ECD, Thermo Fisher Scientific) was set at +0.50 V. The mobile phase consisted of 69 mM sodium dihydrogen phosphate, 0.01% (v/v) Et3N, 0.025 mM EDTA, 1.7 mM sodium octanesulfonate (pH = 3.0) and 12% methanol. The injection volume is 20 mL.
Pharmacokinetic study
After oral administration of compound 18 (80 mg kg−1), about 100 μL of blood was collected from the eyes of rats at 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, 8 h and 24 h. Whole blood specimens were placed in 1.5 mL tubes containing heparin anticoagulant. The blood samples were centrifuged at 2000 rpm for 10 min to separate the plasma, and the upper plasma specimen was taken into a sample tube and stored at −20 °C for testing.
Subacute toxicity test
A subacute oral toxicity study was conducted according to the guidelines of the Organization for Economic Co-operation and Development (OECD, 425). The animals were randomly allocated into two groups of six animals each. Group I was set as the blank control: animals were administered orally for 14 consecutive days with vehicle normal 0.5% CMC-Na. Group II was administered orally for 14 consecutive days with 240 mg kg−1 body weight of compound 18.
Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Kunming Institute of Botany (CAS) and approved by the Animal Ethics Committee of Kunming Institute of Botany (CAS).
Author contributions
Tian-Ze Li: investigation, visualization, formal analysis, writing – original draft, funding acquisition. Jing Hu: investigation, formal analysis. Jin Jin Sun: investigation. Huang-Xiao Yan: investigation. Chang-An Geng: writing – review & editing. Shu-Bai Liu: resources, methodology. Xue-Mei Zhang: resources. Ji-Jun Chen: conceptualization, writing – review & editing, supervision, funding acquisition.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81803405), the Xingdian Yingcai Project (YNWR – KJLJ – 2019-002), the Youth Innovation Promotion Association, CAS (2020386), the Reserve Talents of Young and Middle-aged Academic and Technical Leaders in Yunnan Province (202105AC160021), the Science and Technology Plan Projects in Yunan Province (202101AT070200), and State Key Laboratory of Phytochemistry and Plant Resources in West China.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2md00156j
Notes and references
- Barrenetxe J. Delagrange P. Martinez J. A. J. Physiol. Biochem. 2004;60:61–72. doi: 10.1007/BF03168221. [DOI] [PubMed] [Google Scholar]
- Liu J. B. Clough S. J. Hutchinson A. J. Adamah-Biassi E. B. Popovska-Gorevski M. Dubocovich M. L. Annu. Rev. Pharmacol. Toxicol. 2016;56:361–383. doi: 10.1146/annurev-pharmtox-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attenburrow M. E. J. Cowen P. J. Sharpley A. L. Psychopharmacology. 1996;126:179–181. doi: 10.1007/BF02246354. [DOI] [PubMed] [Google Scholar]
- Brown G. M. Pandi-Perumal S. R. Trakht I. Cardinali D. P. Travel Med. Infect. Dis. 2009;7:69–81. doi: 10.1016/j.tmaid.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Lanfumey L. Mongeau R. Hamon M. Pharmacol. Ther. 2013;138:176–184. doi: 10.1016/j.pharmthera.2013.01.005. [DOI] [PubMed] [Google Scholar]
- DeMuro R. L. Nafziger A. N. Blask D. E. Menhinick A. M. Bertino J. S. J. Clin. Pharmacol. 2000;40:781–784. doi: 10.1177/00912700022009422. [DOI] [PubMed] [Google Scholar]
- Ferreira M. A. Azevedo H. Mascarello A. Segretti N. D. Russo E. Russo V. Guimarães C. R. W. J. Med. Chem. 2021;64:1904–1929. doi: 10.1021/acs.jmedchem.0c00627. [DOI] [PubMed] [Google Scholar]
- Zlotos D. P. Arch. Pharm. 2005;338:229–247. doi: 10.1002/ardp.200400996. [DOI] [PubMed] [Google Scholar]
- Zlotos D. P. Curr. Med. Chem. 2012;19:3532–3549. doi: 10.2174/092986712801323153. [DOI] [PubMed] [Google Scholar]
- Jockers R. Delagrange P. Dubocovich M. L. Markus R. P. Renault N. Tosini G. Cecon E. Zlotos D. P. Br. J. Pharmacol. 2016;173:2702–2725. doi: 10.1111/bph.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin J. A. Witt-Enderly P. A. Sotriffer C. Zlotos D. P. J. Pineal Res. 2020;69:12672. doi: 10.1111/jpi.12672. [DOI] [PubMed] [Google Scholar]
- Yang T. H. Ma Y. B. Geng C. A. Yan D. X. Huang X. Y. Li T. Z. Zhang X. M. Chen J. J. Eur. J. Med. Chem. 2018;156:381–393. doi: 10.1016/j.ejmech.2018.07.027. [DOI] [PubMed] [Google Scholar]
- Yin X. J. Geng C. A. Huang X. Y. Chen H. Ma Y. B. Chen X. L. Sun C. L. Yang T. H. Zhou J. Zhang X. M. Chen J. J. RSC Adv. 2016;6:45059–45063. doi: 10.1039/C6RA06748D. [DOI] [Google Scholar]
- Chen S. Y. Geng C. A. Ma Y. B. Huang X. Y. Yang X. T. Su L. H. He X. F. Li T. Z. Deng Z. T. Gao Z. Zhang X. M. Chen J. J. Bioorg. Med. Chem. 2019;27:3299–3306. doi: 10.1016/j.bmc.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Liang W. J. Geng C. A. Zhang X. M. Chen H. Yang C. Y. Rong G. Q. Zhao Y. Xu H. B. Wang H. Zhou N. J. Ma Y. B. Huang X. Y. Chen J. J. Org. Lett. 2014;16:424–427. doi: 10.1021/ol403315d. [DOI] [PubMed] [Google Scholar]
- Liang W. J. Ma Y. B. Geng C. A. Huang X. Y. Xu H. B. Zhang X. M. Chen J. J. Fitoterapia. 2015;106:36–40. doi: 10.1016/j.fitote.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Wallez V. Durieux-Poissonnier S. Chavatte P. Boutin J. A. Audinot V. Nicolas J. P. Bennejean C. Delagrange P. Renard P. Lesieur D. J. Med. Chem. 2002;45:2788–2800. doi: 10.1021/jm0005252. [DOI] [PubMed] [Google Scholar]
- Li T. Z. Geng C. A. Yin X. J. Yang T. H. Chen X. L. Huang X. Y. Ma Y. B. Zhang X. M. Chen J. J. Org. Lett. 2017;19:429–431. doi: 10.1021/acs.orglett.6b03801. [DOI] [PubMed] [Google Scholar]
- Qin L. Vo D.-D. Nakhai A. Andersson C. D. Elofsson M. ACS Comb. Sci. 2017;19:370–376. doi: 10.1021/acscombsci.7b00014. [DOI] [PubMed] [Google Scholar]
- Zhou L. X. Shi Y. X. Zhu X. Y. Zhang P. Tetrahedron Lett. 2019;60:2005–2008. doi: 10.1016/j.tetlet.2019.06.054. [DOI] [Google Scholar]
- Sharma A. Wakode S. Fayaz F. Khasimbi S. Pottoo F. H. Kaur A. Curr. Pharm. Des. 2020;26:4373–4385. doi: 10.2174/1381612826666200417154810. [DOI] [PubMed] [Google Scholar]
- Kroeze W. K. Sassano M. F. Huang X. P. Lansu K. McCorvy J. D. Giguere P. M. Sciaky N. Roth B. L. Nat. Struct. Mol. Biol. 2015;22:362–369. doi: 10.1038/nsmb.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A. Dao D. T. Arad M. Terrillion C. E. Piantadosi S. C. Gould T. D. J. Visualized Exp. 2012;59:e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.