Abstract
The versatile structural motif of hydroxypyrone is found in natural products and can be easily converted into hydroxypyridone and hydroxythiopyridone analogues. The favourable toxicity profile and ease of functionalization to access a vast library of compounds make them an ideal structural scaffold for drug design and discovery. This versatile scaffold possesses excellent metal chelating properties that can be exploited for chelation therapy in clinics. Deferiprone [1,2-dimethyl-3-hydroxy-4(1H)-one] was the first orally active chelator to treat iron overload in thalassemia major. Metal complexes of hydroxy-(thio)pyr(id)ones have been investigated as magnetic resonance imaging contrast agents, and anticancer and antidiabetic agents. In recent years, this compound class has demonstrated potential in discovering and developing metalloenzyme inhibitors. This review article summarizes recent literature on hydroxy-(thio)pyr(id)ones as inhibitors for metalloenzymes such as histone deacetylases, tyrosinase and metallo-β-lactamase. Different approaches to the design of hydroxy-(thio)pyr(id)ones and their biological properties against selected metalloenzymes are discussed.
Hydroxypyrone derivatives comprise a versatile class of compounds with massive potential as therapeutic and imaging agents. Their excellent metal chelating properties have been exploited to inhibit metalloenzymes.
1. Hydroxypyr(id)ones in drug discovery
Hydroxypyr(id)one (HOPO) is a versatile scaffold that can be easily structurally modified to optimize pharmacological properties. Several examples such as maltol (3-hydroxy-2-methyl-4(1H)-pyrone) and kojic acid (5-hydroxy-2-hydroxymethyl-4(1H)-pyrone) are commercially available natural products that are used as additives.1,2 They have been investigated for various medicinal applications, including anticancer, antidiabetic, and antimicrobial agents. The procedures for the synthesis of hydroxy-pyrones and their conversion to hydroxy-(thio)pyridones have been outlined in the literature.3–6 Based on the position of the hydroxyl and carbonyl groups on the heterocycle ring, HOPOs are classified into three types including 3-hydroxy-4(1H)-pyr(id)one (3,4-HOPO), 3-hydroxy-2(1H)-pyr(id)one (3,2-HOPO) and 1-hydroxy-2(1H)-pyr(id)one (1,2-HOPO) (Fig. 1).
Fig. 1. Structures of common 3,4-HOPO (maltol 1; deferiprone 2), 3,2-HOPO (3) and 1,2-HOPO (4).
At physiological pH, HOPOs quickly undergo deprotonation and can act as O,O-chelating ligands, forming stable complexes with various metal ions such as iron, zinc, copper, etc.7–10 The chelating ability of HOPOs has been successfully exploited to design and develop compounds for chelation therapy.11,12 Deferiprone 2 was developed by Hider and coworkers as the first orally administered drug to treat iron overload in thalassemia major.13 Since the accumulation of aluminum(iii) in the brain is suggested to be responsible for the development of Alzheimer's disease (AD), the properties of HOPOs as chelators for aluminum(iii) were investigated.14,15 Besides, HOPOs have been explored as therapeutic agents for treating neurodegenerative diseases. For example, high levels of redox-active metal ions such as copper are found in the central nervous system of patients with neurodegenerative diseases.16,17 The increased accumulation of these metal ions causes the production of reactive oxygen species (ROS), leading to the development of oxidative stress in the brain.18 The metal chelator such as those derived from HOPO have shown promise in restoring the balance of metal ions.
HOPOs can compete thermodynamically with the chelating moiety of natural microbial siderophores such as catecholates, hydroxycarboxylates and hydroxamates. In this regard, HOPOs have been investigated as synthetic antimicrobial iron chelators. This relies on the ability of HOPOs to exert bacteriostatic effects by decreasing the amount of iron(iii) available to pathogens.19 Key consideration in the effective growth inhibition of pathogens by synthetic iron(iii) chelators is that they form thermodynamically and kinetically stable iron(iii) complexes, and the resulting iron(iii) complexes cannot be recognized and taken up by the microbes. The HOPO chelators fullfil these criteria. This led to the development of a series of multidentate HOPO-derived macromolecules and investigation of their antimicrobial properties.20–23 For example, hexadentate HOPO chelator 5 and 6 (Fig. 2) showed a strong binding affinity for iron(iii) and were able to inhibit the growth of both Gram +ve and −ve bacteria including clinical isolates of P. aeruginosa and methicillin-resistant S. aureus. The iron(iii) complexes of 5 were not taken up by E. coli or S. aureus. The nature of the linker and/or flexibility of the backbone is essential in the antimicrobial HOPO chelators.
Fig. 2. Structures of hexadentate 3,4-HOPO (5 and 6) chelators investigated as antimicrobial agents.
The coordination chemistry of the HOPO motif has also been exploited for design of imaging agents,24–26 antidiabetic agents27,28 and anticancer agents.29,30 For example, 68Ga-labelled hydroxypyridone–peptide bioconjugates proved as effective radiopharmaceuticals in positron emission tomography (PET) imaging.31 Raymond and coworkers developed a library of GdIII complexes as potential MRI contrast agents based on hexadentate and/or octadentate HOPO ligands.26 The Gd complex of tris[(3-hydroxy-1-methyl-2(1H)-pyridone-4-carboxamido)ethyl]amine 7 (Fig. 3) showed superior properties such as stability and relaxivity compared to contrast agents in clinical use.32 The HOPO based vanadium complexes [bis(maltolato)oxovanadium(iv)] 8 and its ethylmaltol analogue [bis(ethylmaltolato)oxovanadium(iv)] 9 (Fig. 3) have shown insulin enhancing properties and were investigated in clinical phase II trials to treat type-2 diabetes.33,34 Recently, HOPO and their organometallic compounds have demonstrated potent anticancer properties with IC50 values in the low micromolar to nanomolar range in human colorectal cancer (HCT116) and colon cancer (SW480) cell lines.29
Fig. 3. Structures of a Gd complex (7) as MRI contrast agent and vanadium complexes (8 and 9) as insulin enhancing agents for type-2 diabetes mellitus.
2. HOPOs as metalloenzyme inhibitors
More than 30% of all enzymes contain metal ions such as iron, copper, and zinc. The metal ions play essential structural and functional roles in metalloenzymes including histone deacetylases, tyrosinases, matrix metalloproteinases, catechol-O-methyltransferases and metallo-β-lactamases. The metal-containing enzymes are widely distributed from microorganisms to humans and control critical biological processes such as DNA modification, gene expressions, protein-matrix degradation and antibiotic resistance.35 The overexpression, enhanced activation, or misregulation of these metalloenzymes have implicated in many human diseases such as cancer, hypertension, arthritis, malaria, glaucoma and inflammation. For example, a number of metalloenzymes have been identified in the proliferation of pathogenic infections and are crucial for the survival and virulence of both bacteria and viruses.35–38 Considering their involvement in various diseases, these metal-containing enzymes are drug targets for the development of therapeutic agents.39–41
Various strategies and structural motifs have been investigated for the inhibition of disease-associated metalloenzymes.36 The strong binding affinity of HOPOs with various metal ions offers the opportunity to design novel inhibitors for a wide range of metalloenzymes such as HDACs, and MMPs.42 For example, kojic acid has been shown to inhibit the copper-containing enzyme tyrosinase that causes melanization in humans and because of this, it has been used as an additive in cosmetics.43,44 Similarly, antiproliferative properties of deferiprone and its derivatives are suggested due to the inhibition of a subset of iron-dependent histone lysine demethylases.45 Although the exact mechanism of action is not known, depletion of intracellular iron and zinc is attributed as a key contributing factor to antiproliferative properties of these compounds.46–49 In the sections below, we will review recent progress in the design and discovery of hydroxypyr(id)one-derived compounds as metalloenzyme inhibitors.
2.1. Histone deacetylases inhibitors
Histone deacetylases (HDACs) are zinc-containing enzymes responsible for critical biological functions such as gene expression. Over expression of HDACs is associated with poor prognosis in cancer patients.50,51 HDACs have also been involved in the development of neurodegenerative disorders such as AD.52 Therefore, HDACs are a crucial therapeutic target in drug discovery. Metal-binding pharmacophores such as hydroxamic acid have been investigated as HDAC inhibitors. The thionation of the respective HOPOs led to the formation of hydroxy-thiopyr(id)ones (THOPOs)30,53,54 that act as bidentate S,O-chelator and strongly chelate Zn(ii) ions in the active sites of zinc-dependent metalloenzymes such as HDACs. Oyelere and coworkers reported THOPO 10 as a selective inhibitor of HDAC6 and HDAC8 with IC50 values in the low nM range (Table 1).55 The series was further extended to prepare the derivatives 11–25 of HOPO and THOPO for structure–activity relationship (SAR) study. The N-1 benzyl derivative of THOPO 13 proved to be more active against HDAC6 and HDAC8 than other isoforms. 18–20 displayed the highest potency against HDAC6 irrespective of the methyl position, while 20 with a methyl group at ortho position proved to be strongest inhibitor of HDAC6 with IC50 value of 306 nM (Table 1). However, against HDAC8 the para-methyl derivative 18 is more active, with IC50 of 800 nM, than the corresponding meta- and ortho-methyl analogues 19 and 20, respectively (Table 1). The presence of electron donating group N,N-dimethyl at para-position of compound 21 increased selectivity towards HDAC8 while the substitution of pyridine and N,N-dimethyl in 23 resulted in enhance selectivity towards HDAC6. The proximal phenyl ring substituted with 1,2,3-triazole ring in 24 exhibited 2–3 times more activity towards HDAC8 than 14 while displaying marginal inhibitory activity against HDAC6 (Table 1).
Inhibitory activity (IC50 in nM) of compounds 10, 13–24, 27, 28, 32, and 35–40 against HDAC6 and HDAC8.
| Compound | IC50 values (nM) | |
|---|---|---|
| HDAC6 | HDAC8 | |
| 10 | 681 ± 110 | 3675 ± 1201 |
| 13 | 457 ± 27 | 1272 ± 200 |
| 14 | 847 ± 188 | 4283 ± 1548 |
| 15 | 957 ± 159 | 2075 ± 459 |
| 16 | NI | 1701 ± 717 |
| 17 | 372 ± 35 | 1907 ± 771 |
| 18 | 454 ± 42 | 800 ± 304 |
| 19 | 812 ± 286 | 2496 ± 1180 |
| 20 | 306 ± 69 | 3105 ± 1649 |
| 21 | NI | 2858 ± 944 |
| 22 | 2390 ± 458 | NI |
| 23 | 2204 ± 355 | 2780 ± 323 |
| 24 | NI | 1570 ± 1067 |
| 27 | 1023 ± 99 | 1868 ± 723 |
| 28 | 911 ± 173 | 917 ± 139 |
| 32 | 807 ± 207 | 2533 ± 823 |
| 35 | 1100 ± 443 | 1660 ± 416 |
| 36 | 637 ± 160 | 2402 ± 263 |
| 37 | 905 ± 249 | 1465 ± 217 |
| 38 | 356 ± 72 | 2831 ± 520 |
| 39 | 1006 ± 425 | 1482 ± 389 |
| 40 | 661 ± 121 | 2258 ± 1005 |
The promising compounds 17, 20, 21, and 26 as selective inhibitors for HDAC6 and/or HDAC8 were also tested for antiproliferative properties against DU-145 (androgen-independent prostate cancer), LNCaP (androgen-dependent prostate cancer), Jukart (the T-cell leukemia cell line) and Jurkat J.γ1 cancer cell lines. 20 displayed weak activity against DU-145 while the rest of the compounds were inactive (Table 2). The tested compounds 17, 20, 21, and 26 were more active against the LNCaP cancer cell line. 17 and 26 displayed similar cell inhibition activity in this cell line, although 26 is a selective inhibitor of HDAC6 (Table 2). The inhibition of T-cell-derived leukemia cell line Jukart has been attributed to the selectivity of compounds for HDAC8 inhibition. 20 which inhibited both HDAC6 and HDAC8, proved to be a potent cytotoxic agent against Jukart cancer cell line (IC50 =3.19 μM) and LNCaP with IC50 of 3.2 and 7.8 μM, respectively (Table 2). In Jukart J.γ1, the apoptotic pathway is mediated by the activity of phospholipase Cγ1 (PLCγ1) that is initiated by HDAC8.56 However, none of the tested compounds 17, 20, 21, and 26 proved effective in inhibiting the Jukart J.γ1 (Table 2). The inactivity of 21 and 26 attributed to their selectivity in inhibiting the HDAC8 which is important in initiating the antiproliferative effect against Jukart J.γ1. However, the non-cytotoxicity of 17 and 20 (both are HDAC6 and HDAC8 inhibitors)55 on PLCγ1 activity may be due to the attenuation of caspase-dependent apoptosis, which is crucial for the cytotoxicity of HDACi.56
In vitro cytotoxic activity (mean IC50 values ± standard deviations) of lead compounds 17, 20, 21, 26, 31, 33, 36 and 38 against DU-145 (androgen-independent prostate cancer), LNCaP (androgen-dependent prostate cancer), Jukart (the T-cell leukemia cell line) and Jurkat J.γ1 cancer cell lines.
| Compound | IC50 values (μM) | |||
|---|---|---|---|---|
| DU-145 | LNCaP | Jurkat | Jurkat J.γ1 | |
| 17 | >20 | 15 ± 0.8 | 9 ± 0.7 | NI |
| 20 | 13.6 ± 2.9 | 8 ± 0.7 | 3 ± 0.3 | NI |
| 21 | >20 | 16 ± 1.1 | 11 ± 0.7 | NI |
| 26 | >20 | 13 ± 1.5 | 5 ± 0.3 | NI |
| 31 | 9.3 ± 1 | 5 ± 0.5 | 3 ± 0.6 | 2 ± 0.2 |
| 33 | 17.7 ± 3.3 | 11 ± 2 | 9 ± 1.3 | >20 |
| 36 | 11 ± 2.4 | 5 ± 0.4 | 5 ± 1.2 | NT |
| 38 | 5 ± 1.1 | 4 ± 0.3 | 3 ± 0.6 | 0.9 ± 0.1 |
To further expand the scope and establish the SAR, lead compound 26 was modified by changing the linker and the surface recognition group.57 Among the series of compounds 28–33 (Fig. 4) with varying methylene spacer linkers, compound 31 with five methylene linker displayed an increase in HDAC6 and HDAC8 inhibition activity compared to the lead compound 26 (Table 1). An extension in the methylene unit (from n = 5 to 6) resulted in the loss of HDAC inhibition activity. However, on further extension of the methyl linker unit (n = 7), the HDAC6 inhibition activity is restored. This highlights the importance of appropriate linker length for optimum binding in the active site. The most potent compound 31 was further derivatized by introducing different surface recognition groups to obtain 34–40 (Fig. 4). However, the modifications in the surface recognition groups did not yielded compounds with improved activity compared to 31 against HDCA8 (Table 1). Among methyl-substituted derivatives 34–36, only ortho-methyl substituted analogue was more active than 31 against HDAC6. Remarkably, the cyano group substitution at meta position yielded the most effective HDAC6 inhibitor 38, with potency 2.5-folds higher than unsubstituted derivative 31 (Table 1).
Fig. 4. Chemical structures of HOPO and THOPO derivatives 10–40 as HDAC inhibitors.
Molecular docking against HDAC6 showed the phenyl ring of all compounds 28–40 forming π-stacking interaction with Phe680. The cyano group in the lead compound 38 resulted in H-bonding interaction with the hydroxyl side chain and amide backbone of Ser568 that may caused the enhanced activity towards HDAC6. For HDAC8, a comparative docking analysis of all the compounds revealed that the only unsubstituted phenyl ring in 31 best fit in the hydrophobic pocket making it equipotent towards both HDAC6 and HDAC8.
The lead compounds 31, 33, 36 and 38 were evaluated for cytotoxic potential against DU-145, LNCaP, Jukart and Jurkat J.γ1 cancer cell lines (Table 2). The cyano substituted derivative 38 was 2-folds more potent against DU-145 and LNCaP prostate cancer cell lines than unsubstituted analogue 31 (Table 2). The improved sensitivity of 38 towards prostate cancer cell lines, particularly LNCap, may be attributed to its selectivity towards HDAC6 as its inhibition is detrimental to cell viability.58,5931 and 38 displayed similar cytotoxic potential against wild-type Jukart cell line despite their difference (38 being potent HDAC8 inhibitor than 31) in HDAC8 inhibition that favors the cytotoxicity against the Jukart cell line.56 The weak inhibition capacity of 38 towards HDAC8 is compensated by a 3-fold increase in HDAC6 activity relative to 31, resulting in cytotoxicity in the Jukart cell. The compounds 31 and 38 exhibited remarkable anticancer activity in the low micromolar range against the Jukart J.γ1 cancer cell line (Table 2). Although the HDAC6 selective inhibitors SAHA and tubastatin A are inactive against Jukart J.γ1 cell line, the cytotoxicity of the lead compounds 31 and 38 against Jukart J.γ1 might be due to the inhibition of some other cellular targets. Comparatively, the IC50 values of the lead 31 and 38 against healthy mammalian cell line vero were much greater than the highest tested concentration of 20 μM (Table 2). The involvement of HDAC6 inhibition in the mechanism of action of 31 and 38 was confirmed by the upregulation of tubulin acetylation, a common biomarker of intracellular HDAC6 activity.57
2.2. Catechol-O-methyltransferase (COMT) inhibitors
Catechol-O-methyltransferase (COMT) is a magnesium-dependent intracellular enzyme. The enzyme regulates the levels of endogenous neurotransmitters such as dopamine, epinephrine and norepinephrine.60,61 This enzyme exists in soluble form (S-COMT) and membrane-bound form (MB-COMT), while the MB-COMT form exists predominantly in the brain.62,63 The finding that the COMT enzyme is involved in the metabolic degradation of the mainstay antiparkinsonian drug l-DOPA identified it as a critical biological drug target for various neurological disorders such as Parkinson's diseases, depression, schizophrenia and other dopamine deficiency-related diseases.64–67 COMT inhibitors such as tolcapone 41 and entacapone 42 (Fig. 5) were developed68 but their clinical value is limited by adverse effects such as diarrhoea, hepatitis and neurological reactions, particularly for tolcapone.69 The selective inhibition of MB-COMT is critical to minimize the peripheral side effects of S-COMT inhibition. Following this strategy, non-catechol derivatives such as 3-hydroxy-4-pyridones 43 and 5-hydroxy-4-pyrimidinones 44 (Fig. 5) were identified as heterocyclic catechol mimics and exhibited potent inhibition of MB-COMT.70,71 Barrow and his coworkers synthesized derivatives of 43 and 44 comprising a series of 6-hydroxymethyl-4-pyridone 45–57, 2-substituted-5-hydroxy-4-pyridones 58–65 and 5-hydroxy-4-pyrimidinones 66–68, respectively (Fig. 5). The major variation in the structure of 43 and 44 was distant aryl groups at either meta- or para-position to the chelating hydroxy group of the inhibitors.76
Fig. 5. Chemical structures of catechol-O-methyltransferase inhibitors 41–69.
The in vitro experiments revealed 54 and 55 were less potent compared to 43 against h-MB-COMT, while 58 was potent in the low nanomolar to high nanomolar range against both r-MB-COMPT (16–220 nM) and h-MB-COMT (47–13 000 nM). Notably, 57 displayed 10- to 20-folds more selectivity towards human h-MB-COMT and r-MB-COMT, respectively than h-S-COMT. Moreover, N-biphenyl pyridone 57 (Fig. 5) was a selective and potent inhibitor of h MB-COMT (47 nM) over S-COMT (1100 nM). The high potency was attributed to the lipophilicity (clog P 2.53) and the position of attachment of the biphenyl group attached to hydroxypyridone core. To improve the physiochemical and pharmacokinetics properties, N-heteroaryl analogues of 3-hydroxy-4-pyridone 69 (Fig. 5) was prepared.7269 proved an excellent selective inhibitor of MB-COMT (40 nM) than S-COMT (3020 nM). It was proposed that the inhibition of COMT with 69 led to a significant decrease in the concentration of dopamine dihydroxyphenylacetic acid and homovanillic acid at a dosage of 100 mg Kg−1. However, the heterocyclic replacement of biphenyl in 57 with phenylpyridine in 69 resulted in small improvement of intrinsic clearance both in human and rat and accompanied by increase in plasma clearance and suggested that introducing polarity did not have any effect on pyridone glucuronidation. Crystallographic studies revealed the critical interaction of hydroxypyridone pharmacophore and magnesium in the active site of S-COMT.73,74 For example, the X-ray crystallography of novel inhibitor 57 with S-COMT chelation of Mg2+ in the metalloenzyme (Fig. 6).75
Fig. 6. Crystal structure of human SCOMT (PDB 4XUC) with bound HOPO inhibitor 57 that is chelating Mg2+ using the two oxygen atoms of the hydropyridone.75.
Barrow and coworkers prepared a series of bicyclic hydroxypyridones 70–95 (Fig. 7) by modifying the structure of COMT inhibitor 41.70,76 Among the series, only the N-benzyl analogue 73 displayed the potency against h-MB-COMT with an IC50 value of 220 nM. Further modifications to obtain 84 and 85 resulted in an increased potency with IC50 values of 50 and 40 nM, respectively. The most potent inhibitors were disubstituted 2,6-dimethyl 89, 2,4-dichloro 92 and 2-chloro 4-fluoro 91 analogues with IC50 values 6.3, 10 and 16 nM, respectively. The pharmacokinetics studies of lead compounds 73, 85, 88, 91 and 92 were conducted in rats. The investigations revealed the high clearance for these agents and low plasma protein and brain tissue binding, ensuring their availability to interact with the target of interest. In rat biomarker studies, unbound drug concentration of the lead compounds 73, 85, 88, and 91 in the brain exceeded the IC50 values by 2-folds with only compound 92 by 50-folds.
Fig. 7. Chemical structures of bicyclic hydroxypyridones-based catechol-O-methyltransferase inhibitors 70–95.
2.3. Tyrosinase inhibitors
Tyrosinase (also known as polyphenol oxidase) is a copper-containing oxidation enzyme and is widely distributed in microorganisms, animals, and plants.77–79 It is a rate-limiting enzyme that is involved in the biosynthesis of melanin through two distinctive reactions, in particular, the hydroxylation of l-tyrosine to l-3-(3,4-dihydroxyphenyl)-alanine (l-DOPA) in the first step (monophenolase activity) and then subsequent oxidation of DOPA to dopaquinone (diphenolase activity).77–79 Tyrosinase is responsible for the production of neuromelanin in the brain. It has been shown that overexpression of tyrosinase enzyme may lead to neuronal dysfunction and degeneration related to Parkinson's disease and other neurodegenerative diseases.80 In addition, the excessive secretion of tyrosinase is associated with several esthetic problems in humans (e.g., melasma, freckles, solar lentigines and serious hyperpigmentation diseases), antibiotic resistance in bacteria81 and undesirable browning of fruits and vegetables.82 The inhibition of enzymatic activity through chelation of copper in the catalytic site seems to be an important strategy for developing tyrosinase inhibitors.
Starting from commercially available kojic acid as starting material, a series of hydroxypyridone-l-phenylalanine conjugates 96–101 (Fig. 8) bearing hydrophobic alkyl group at N-1 position was reported as potential tyrosinase inhibitors.83 The lead compound 100 with octyl moiety (clog P = 3.697) exhibited the potent inhibitory activity at 12.6 and 4.01 μM for monophenolase and diphenolase, respectively. The kinetic examination showed excellent chelation of copper ion present in the active site of tyrosinase. The inhibitors could bind to both the free enzyme and enzyme–substrate complexes. 100 was found noncytotoxic towards HeLa, A549, and MCF-7 cell lines, demonstrating the potential of compounds as tyrosine inhibitors with low general toxicity and reduced side effects.
Fig. 8. Chemical structures of HOPO derivatives 96–130 as tyrosinase inhibitors.
Zhou and coworkers further extend the library of compounds 102–106 and 107–109 by conjugating hydroxypyridone with l-amino acid and dipeptide, respectively (Fig. 8).84 The compounds 106 and 107 with benzyl moiety were potent tyrosinase inhibitors with IC50 values of 1.95 and 2.79 μM, respectively. 106 and 107 inhibited the diphenolase activity with IC50 values 8.97 and 26.20 μM, respectively. The compounds exhibited reversible and mixed-type inhibition of tyrosinase. Inhibitory constants for 106 to bind with both the free enzyme (KI) and enzyme–substrate complex (KIs) were 17.17 and 22.09 μM, respectively. Molecular docking studies revealed that 106 outcompete histidine residues and strongly chelate copper ions with 3-hydroxyl and 4-carbonyl groups while the two benzyl groups formed hydrophobic interactions with the enzymes.
Zhou and coworkers introduced an oxime ether moiety in the hydroxypyridone scaffold to prepare derivatives 110–115 (Fig. 8). These were investigated as potential tyrosinase inhibitors and for their use in retarding the browning of freshly-cut apple slices.85 The increase in clog P or lipophilicity proved to be an important parameter for increasing the enzyme inhibiting potential. It also helped them bind with the hydrophobic pocket of the tyrosinase active site. The lead compounds 114 and 115 exhibited potent inhibition of monophenolase (IC50 = 2.04 and 1.60 μM, respectively) and diphenolase (IC50 = 13.89 and 7.99 μM, respectively). 114 and 115 demonstrated strong inhibitory constants both for the free enzyme (KI = 24.84 and 32.54 μM, respectively) and enzyme–substrate complex (KIs = 18.07 and 21.34 μM, respectively). Molecular docking revealed the 4,5-dihydroxy moiety of 115 strongly chelated the copper ions, while the aliphatic chain at 1-position and side-chain at 2-position formed hydrophobic interaction with the active site of tyrosinase.
A series of chalcone–hydroxypyridone hybrids 116–130 were reported as potential tyrosinase inhibitors (Fig. 8).86 The promising compounds 116, 119 and 129 had shown relatively strong inhibition of monophenolase (IC50 = 3.07, 2.25 and 2.75 μM, respectively) and diphenolase (IC50 = 17.05, 11.70 and 19.3 μM, respectively) while sharing almost same clog P values (1.60, 1.78 and 1.81, respectively) and the same electron-withdrawing effect of F, OH and OMe group on the benzene ring. The inhibition kinetics of diphenolase of 116 and 119 induced reversible inhibition mechanism on tyrosinase. In molecular docking, 119 formed strong chelation to copper of tyrosinase enzyme along with several hydrophobic interactions to form a stable conformation.
2.4. Monoamine oxidase inhibitors
Monoamine oxidases (MAOs) are flavin adenine dinucleotide containing enzymes, which exist as two different isoforms, MAO-A and MAO-B87 and are involved in catalytic oxidative deamination of biogenic amines, xenobiotic amines and hormones in the brains and peripheral tissues and hence modulating their concentrations.88,89 MAOs are biochemically important in metabolising amine neurotransmitters, and termination of their physiological functions depends upon the nature of their substrate.90 The substrates includes several biogenic amines such as indoleamines (serotonin and tryptamine),91 catecholamines (dopamine, norepinephrine and epinephrine)92 and trace amines (β-phenylethylamine, tyramine and octopamine) effectively metabolized by MAOs.93 Therefore, inhibition of MAOs is of significant therapeutic importance. For example, inhibitors of MAO-B have been employed to treat neurodegenerative diseases such as AD94,95 and Parkinson's disease.96–98
Xie and coworkers combined the iron-chelating properties of hydroxypyridones with MAO-inhibitory coumarin derivatives in one scaffold 131–141 as multimodal agents for the treatment of Alzheimer's disease (Fig. 9).16 Most of the compounds proved excellent iron chelators. At a concentration of 1 μM, most of the compounds exhibited weak inhibition of MAO-B, but at 10 μM concentration, the inhibition capacity of most of the compounds was over 90%. Compounds 131 and 140 were the most potent inhibitors of MAO-B with IC50 values of 0.68 and 0.86 μM, respectively.
Fig. 9. Chemical structures of HOPO derivatives 131–195 as MAO-B inhibitors.
Direct incorporation of coumarin into hydroxypyridone scaffold led to a series of hybrid compounds 142–162 (Fig. 9).99 The compounds proved as effective iron chelators and displayed moderate to good inhibition of MOA-B activity at 10 μM concentration. 154 exhibited the most potent MAO-B inhibitory activity with an IC50 value 14.7 nM. Additionally, 154 did not show any effect on healthy human glioma cells (U251) and displayed a cytoprotective effect on oxidative stress. In animal studies, 154 significantly ameliorated the cognitive dysfunction of scopolamine-induced AD mice. In molecular docking, 154 showed binding to the entrance and substrate cavity and formed several hydrophobic interactions.99
The SAR studies by modifying the substituents at the coumarin core and linker between coumarin and hydroxypyridone led to 163–195 (Fig. 9).100 These hybrid compounds exhibited remarkable iron-chelating effect with pFe3+ values in the range of 16.91 to 20.16 similar to reference drug deferiprone (pFe3+ = 17.50). The most potent 166 showed the highest MAO-B inhibition activity IC50 = 87.9 nM in comparison with reference drug pargyline IC50 = 107.3 nM. 166 significantly increased the cell viability of P12 cell line up to 88% by reversing the amyloid β1–42 (Aβ1–42) induced cellular damage and also proved cytoprotectant against antagonizing oxidative stress. In vivo examination revealed that 166 significantly improved the cognitive dysfunction in a scopolamine-induced mice AD model. In molecular modelling, hydroxypyridones moiety of 166 acts as a key fragment in binding with MAO-B, while coumarin moiety forms several hydrophobic interactions with the substrate cavity.
2.5. Metallo-β-lactamase (MBL) inhibitors
Since the discovery of penicillin, β-lactam antibiotics remain the most important drugs for treating infectious diseases.101,102 However, the practice of excessive use, and misuse of β-lactam drugs against minor infections have led to the development of β-lactam resistance.103 The mechanism of action of β-lactam antibiotics involves inhibiting the penciling-binding proteins responsible for making bacterial cell wall.104 The most resistant Gram-negative bacteria express the β-lactamases that hydrolyse the β-lactam ring of antibiotics and hence halt their mechanism of action.105 VIM-2 is one of the most common carbapenem-hydrolysing MBL found in many drug-resistant Gram-negative bacteria utilizing the Zn cofactor necessary for catalysis.106 The carbapenem-resistant Enterobacteriaceae, along with carbapenem-resistant Acinetobacter baumannii and carbapenem-resistant Pseudomonas aeruginosa have emerged as urgent threats107 that can inactivate all β-lactam antibiotics.108 With this immense increase in antibiotic resistance, immediate interventions are required to develop MBL inhibitors.
The Zn complex of 1-hydroxypyridine-2-thione (THOPO) 196 (Fig. 10) was reported to have a broad range of antibacterial activities.109 In contrast, sodium salt of THOPO 197 (Fig. 10) as specific zinc chelating inhibitors was capable of suppressing the activity of vanX expressing bacteria.110 The promising results of 197 against zinc enzymes led to an increasing interest in developing THOPO as MBL inhibitors. Sham and coworkers synthesized 1-hydroxypyridone-2(1H)-thione-6-carboxylic acid 198 (Fig. 10) that produced remarkable biochemical inhibition (98%) of VIM-2 producing bacteria with an inhibition constant Ki of 13 nM that corresponds to potent ligand efficiency (LE) of 0.99.111 It is important to note that 197 exhibited IC50 value of 0.908 μM with an inhibition constant Ki of 0.217 μM and LE of 1.15 indicating its promising potential as an antibacterial agent. Incorporating amino acids with phenyl side chain into THOPO 199 (Fig. 10) resulted in a significant loss in inhibition potency against VIM-2. Further examination of 198, restored the efficacy of amoxicillin against E. coli strains expressing VIM-2 and displayed a therapeutic index of 8800 with a low cytotoxicity of 97 μM making it an ideal candidate for further development as MBL inhibitor. The molecular modeling analysis of 198 against VIM-2 revealed the presence of sulfur as bridging ligand between the zinc ions while the charged carboxylate ions formed chelation to the zinc ions.
Fig. 10. Chemical structures of THOPO derivatives 196–199 as MBL inhibitors.
2.6. Cholinesterase inhibitors
Alzheimer's disease is mainly associated with the deposition of amyloid-β (Aβ) plaques in the form of neurofibrillary tangles of tau protein and a decrease in the cholinergic system.112,113 In the cholinergic system, acetylcholine neurotransmitters play an important role in maintaining the intracellular cascade of events that maintains the cognitive system.114 In clinical trials, the efforts to diminish the Aβ plaques have not been very fruitful, resulting in interest in other targets such as targeting the cholinesterases enzymes, namely acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Both AChE and BChE play a vital role in the aggregation of Aβ plaques in the form of senile plaques/neurofibrillary tangles in the brain.115–118 In the healthy human brain, AChE is mainly responsible for the hydrolysis of acetylcholine.119 Therefore, inhibition of AChE and BuChE has been reported as a critical therapeutic strategy for effective AD treatment.
The functional group carbamate is found in some of the active AD drugs available in the market. Considering this, Orvig and coworkers reported that the carbamate functionalization of multifunctional drug candidate deferiprone for AD increases its pharmacokinetics by reducing metabolism and secretion.120 For this 3-hydroxy-2-methyl-1-phenyl-4(1H)-pyridone 200 (Fig. 11) derived from deferiprone scaffold converted into carbamate conjugate 201 (Fig. 11) and its AChE inhibitory activity (IC50 = 48.8 μM) is increased four times as compared 200 (IC50 = 210 μM). In molecular docking analysis, 201 is well placed in the active site of the enzyme but did not modify the enzyme covalently and hence would act in a reversible manner that was also confirmed by the kinetic study analysis. In addition, 201 displayed non-toxicity at high concentrations (450–650 μM) against neuronal cell line.
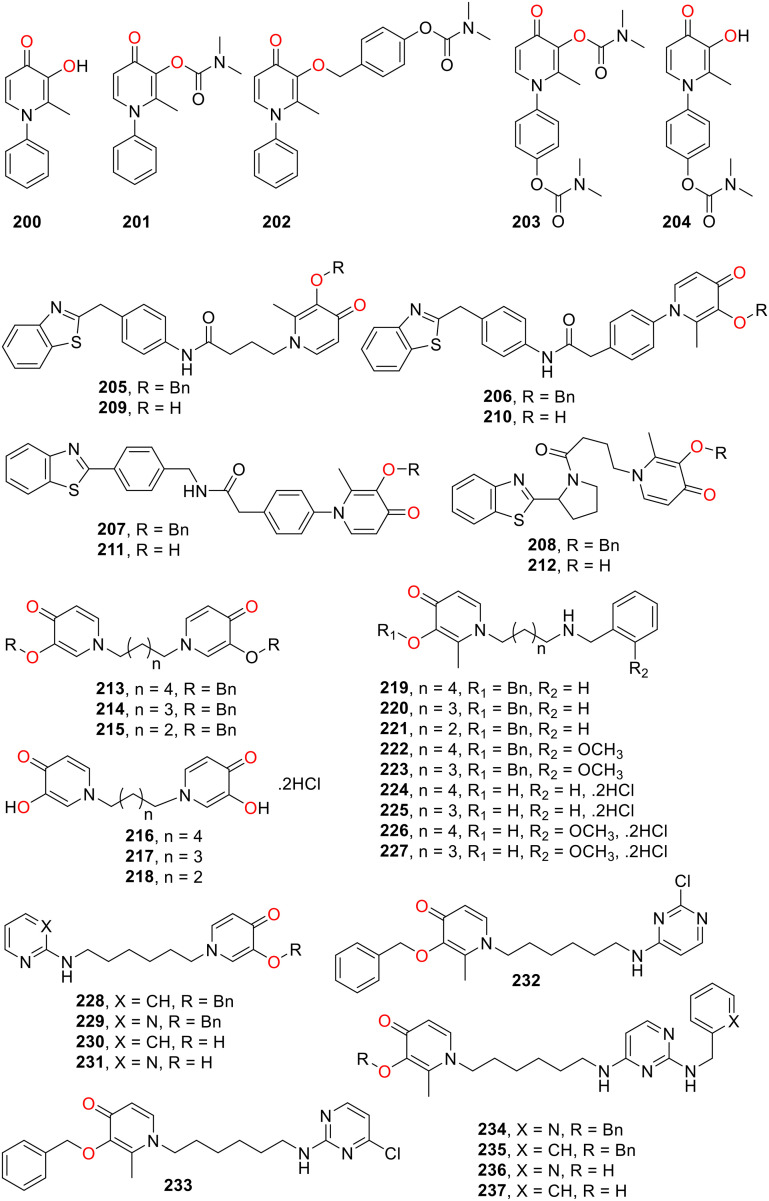
Fig. 11. Chemical structures of HOPO derivatives 200–237 as cholinesterase inhibitors.
The multifunctional HOPO scaffold as a cholinesterase inhibitor was further explored by making a virtual library of second-generation HOPO-carbamate using two strategies: one benzyl ether linker is attached between the carbamate and HOPO ring, and a second carbamate moiety is positioned on the N-substituent of the HOPO.121 After preliminary screening, 15 compounds were selected for molecular docking studies against AChE to understand the bioactivity. Within the series, 202 exhibited the best binding affinity (−5.59 kcal mol−1), followed by 203 (−5.31 kcal mol−1) and 204 (−4.96 kcal mol−1) (Fig. 11). In molecular docking, 204–206 did not enter the active site of Torpedo californica TcAChE and exhibited the reversible inhibition mechanism with inhibitory activity in the range of 216.5 to 347.6 μM. It is important to note that 201 bearing carbamate directly attached to the hydroxyl group of the pyridone ring is a more potent inhibitor of AChE than the second-generation carbamate functionalised HOPO.
Benzothiazole is known for its high affinity for Aβ aggregation. Therefore, it was incorporated into the HOPO pharmacophore to prepare a series of hybrid compounds 205–212 (Fig. 11). The optimization of linkers between two pharmacophores has been decided through virtual screening against AChE. The compounds were tested for their potential as multifunctional prodrug candidates for the treatment of AD.122212 exhibited the highest iron-chelating ability (pFe = 19.2–19.4) and best antioxidant activity (147 μM) along with the highest druglikeness.123205–207 and 212 showed remarkable AChE inhibitory activities (IC50 = 14.7–29.2 μM) which was attributed to a strong interaction between the lipophilic active sites of AChE and hydrophobic residues of these compounds as suggested by the docking studies. 207 and 212 proved to be potent inhibitors of Aβ42 self-aggregation with inhibition capacity 68% and 59% at 100 μM but only 207 significantly restored the cell viability to 100% and efficiently prevented Aβ42-induced toxicity.
Deferiprone derivatives 213–237 (Fig. 11) containing 3-hydroxy-4-pyridone fragment connected through flexible alkyl linker were prepared to investigate their potential as multifunctional molecule for AD therapy.124 In enzymatic inhibition studies 219, 222, 224 and 226 proved to be potent inhibitors of Electrophorus electricus AChE (EeAChE) while 219, 221, 222, 228, 233, 235 and 237 exhibited potent inhibition of equine BChE (eqBChE). Selected compounds showed uncompetitive mechanism (219 and 224) or mixed (222 and 226) inhibition against EeAChE (Ki = 0.788–2.787 μM) and mixed inhibition against eqBChE for compounds 219, 221, 222, 228, 233, 235 and 237 (Ki = 0.182–2.364 μM). 219 exhibited the highest inhibition ability against EeAChE with a Ki of 788 nM, while 228 and 235 proved potent inhibitors of eqBChE with Ki values of 182 and 258 nM, respectively. Molecular docking towards AChE demonstrated that 219, 222, 224 and 226 formed π–π-interactions with both the catalytic active site and peripheral anionic site of hAChE that supported their binding mode as suggested by the enzymatic inhibition. Metal chelation properties of the most potent compounds 224, 228, 233 and 235 were investigated. Moreover, the most active inhibitors also displayed very low toxicity against U-87 MG cell line from the human brain (glioblastoma astrocytoma).
Turel and coworkers synthesized novel thiopyridone derivatives 238a–238h and their ruthenium–cymene complexes 239a–239h (Fig. 12) to evaluate their cholinesterase inhibition potential for the treatment of AD.125 The compounds exhibited stability in aqueous solutions determined by 1H NMR spectroscopy and supported by ESI-MS. In enzymatic inhibition assay, the ligands 238a–238h did not show any activity towards cholinesterase enzymes but their metal complexes 239a–239h showed excellent inhibition activity against electric eel acetylcholinesterase (eeAChE) and horse serum butyrylcholinesterase (hsBChE) in low micromolar range with IC50 values in the range of 4.3–14.3 μM and 0.2–2.7 μM, respectively. The complex 239g demonstrated the best inhibition against eeAChE and hsBuChE with IC50 values of 4.3 and 0.2 μM, respectively. The molecular docking of the most potent compound 239g with TcAChE confirmed the formation of H-bonding, π-stacking, and several hydrophobic interactions. These biological results demonstrated that the inhibition capacity of these ruthenium cymene complexes towards cholinesterase can be fine-tuned by increasing the lipophilicity and modifications in the thiopyridone scaffold.
Fig. 12. Chemical structures of compounds 238a–238h and 239a–239h.125.
2.7. 5-Lipoxygenase (5-LOX) inhibitors
5-Lipoxygenase (5-LOX) is a nonheme iron-dependent metalloenzyme that employs Fe3+ for catalysis. Lipid hydroperoxides activate the enzyme that oxidizes the inactive Fe2+ into the active Fe3+, leading to the enzyme's entrance into its catalytic cycle.
5-LOXs are inflammatory mediators playing a pathophysiological role in several diseases such as asthma,126 allergic rhinitis, inflammatory disorders such as rheumatoid arthritis, psoriasis,127,128 and cardiovascular diseases129 and certain types of cancer.130 Therefore, 5-LOX inhibition is an important therapeutic strategy for treating inflammation-related disease pathways. Up to now, there is only one clinically approved 5-LOX inhibitor zileuton, for the treatment of asthma.131
Hider and coworkers synthesized 2-substituted HOPO derivatives 2 (Fig. 1) and 240–254 (Fig. 13) by introducing a hydrophilic substituent at the 1-position and a bulky substituent at the 2-position. The compounds were investigated to inhibit 5-LOX and ribonucleotide reductase (RR).132 The SAR indicated that the introduction of large substituents at the 2-position of HOPO interferes with the chelation of the iron centre. In this regard, compound 240 with large bulky substituents caused only 10% inhibition of 5-LOX, while simpler HOPOs such as deferiprone 2 (Fig. 1) caused 70% of inhibition. Similarly, 240 inhibited the RR at a much slower rate than 2. However, 240 immobilizes the hepatocellular iron much more rapidly. These findings suggested the quick accessibility towards intracellular iron pools while inhibiting nonheme iron-containing enzymes at a much slower rate are the ideal parameters to design the effective HOPOs for inhibition of 5-LOX and RR. The molecular dimensions and lipophilic character of these ligands played an important role in the inhibition of 5-LOX. Ligand 254 with bulky R1 substituent with hydrophilic nature caused 2% inhibition of 5-LOX as compared to a small molecule of hydrophobic character i.e., deferiprone was found to have 40% inhibition of enzyme activity.133
Fig. 13. Chemical structures of compounds 240–254.132,133.
A series of N-hydroxypyridones 255–257 (Fig. 14) functionalized with benzenesulfonamide through a linear acetylene spacer were synthesised as 5-LOX inhibitors.134 The position of SO2NH2 at phenyl ring of acetylene revealed the inhibition potency order against 5-LOX as follows ortho-SO2NH2255 (IC50 = 10 μM) > meta-SO2NH2256 (IC50 = 15 μM) > para-SO2NH2257 (IC50 = 68 μM). The ortho-SO2NH2255 regioisomers (ED50 = 86.0 mg kg−1 po) showed more potent anti-inflammatory activity than aspirin (ED50 = 128.9 mg kg−1), demonstrating the great potential of these compounds to develop as orally active 5-LOX inhibitory anti-inflammatory drug.
Fig. 14. Chemical structures of 255–257,134258a–b, 259a–b and 260a–b.135.
A novel series of celecoxib analogues having a 2-chloropyridyl 258a–b (Fig. 14), 1-difluoromethyl-1,2-dihydropyrid-2-one 259a–b (Fig. 14), or N-hydroxy-1,2-dihydropyrid-2-one 260a–b (Fig. 14) moiety has led to the development of dual inhibitors of COX-2/5-LOX.135 Celecoxib derivative 259b displayed an IC50 value of 5.0 μM against 5-LOX, 13.1 μM against COX-1 and 0.69 μM against COX-2, with excellent oral anti-inflammatory activity (ED50 = 27.7 mg kg−1 po) comparable to that of reference drugs celecoxib (ED50 = 10.8 mg kg−1 po) and ibuprofen (ED50 = 67.4 mg kg−1 po). Thus N-difluoromethyl-1,2-dihydropyridin-2-one moiety can be utilized as novel 5-LOX inhibitor pharmacophore in cyclic hydroxamic mimetics for the development of COX-2/5-LOX inhibitory anti-inflammatory drugs.
2.8. Matrix metalloproteinases (MMPs)
Matrix metalloproteinases (MMPs) are hydrolytic Zn2+ dependent endopeptidases that are involved in the breakdown of the extracellular matrix (ECM) and basement membrane components such as aggrecan, collagen, elastin, fibronectin, gelatin, and laminin.136,137 In normal conditions, MMPs are required in several metabolic processes such as reproduction, body maintenance, nerve, and bone growth, wound healing, angiogenesis, and apoptosis.138 Under pathologic conditions, overexpression of MMPs is associated with various diseases such as rheumatoid arthritis,139 cancer invasion, and metastasis,139,140 periodontal disease,141 neuroinflammatory dysfunctions,142 liver cirrhosis,143 multiple sclerosis,144 and cardiomyopathy.145 Therefore, MMPs have been identified as an important therapeutic target in drug development.
The molecular scaffold of MMPs inhibitors consists of the chelating moiety to bind the catalytic Zn and thus inhibit the proteolytic action of MMP. Mostly hydroxamic acid (HA) derivatives have been explored as zinc-binding groups (ZBGs) because of their Zn-chelation and hydrogen bonding interaction with neighbouring amino acids in the active site of enzyme.146 However, HA offers unfavourable pharmacokinetics, and clinical trials have not been successful. This has led to investigations of other ZBGs.147 Hydroxypyridones and their derivatives have been shown to have strong Zn2+ chelation accompanied by less toxicity and more easily extra-functionalization.148,149
To understand the Zn-binding properties of HOPO, Cohen and coworkers synthesized model zinc complexes using HOPO/THOPO derivatives 1–4 (Fig. 1), 261, and 262 (Fig. 15) to generate the complexes of the type [(TpPh,Me)Zn(ZBG)], (TpPh,Me = hydrotris(3,5-phenylmethylpyrazolyl)borate).150 The single crystal X-ray diffraction analysis, IR and 1HNMR data confirmed the bidentate chelation of these ZBGs to Zn2+ ion. Molecular modelling demonstrated that ZBGs easily fit in the active site of MMP-3 and exhibited one of the conformations favorable for developing inhibitors with a peptidomimetic substrate backbone.150
Fig. 15. Chemical structures of compounds 261–276.150,152,153.
HOPO 1–4 (Fig. 1) and THOPO derivatives 261–267 (Fig. 15) were tested for inhibitory activity against MMPs.150 In both fluorescence and colorimetric assay, most of the compounds exhibited moderate to very high potency than the acetohydroxamic acid (AHA; known best inhibitor of MMPs). 262 displayed very low micromolar activity against MMP3 with IC50 value 35 and 20 μM in fluorescence and colorimetric assay, respectively. The poor solubility of 2 precluded its evaluation in MMP assays. It was shown that O,O-chelating HOPO ligands showed at least 2 fold increase in affinity over AHA, while O,S-chelating HOPO inhibitors exhibited a binding constant with 14-fold greater affinity than AHA. Both the IC50 values and binding constant revealed that HOPO-based compounds inhibit the MMP-3 due to direct chelation with the Zn2+ ion in the active catalytic site. In a cell culture assay using neonatal rat cardiac fibroblast cells revealed that HOPO-based ZBGs were typically 10–100 folds more effective inhibitors than AHA. Moreover, most of these HOPO-based ZBGs proved to be non-toxic to neonatal rat fibroblast cardiac cells at 100 μM.151
The compound library was further expanded to 268–276 (Fig. 15), containing biphenyl as peptidomimetic backbone and was tested for selective inhibition of MMPs.152268–272 proved to be low to moderate inhibitors of MMP-1, MMP-7, and MMP-9 with IC50 values between ∼50 μM and 25 μM but greater inhibition was observed for MMP-13 with IC50 values ∼4–20 μM. 268 demonstrated excellent inhibiting potential against MMP-2 with low nanomolar IC50, but 269–272 showed low micromolar potential against MMP-2. 268–270 strongly inhibited the MMP-8 and MMP-12 with IC50 values ranging from 0.018–0.248 μM, while a similar trend was observed against MMP-3 with IC50 values of 0.56, 0.077, and 0.24 μM, respectively. However, MMP-8 and MMP-1 were inhibited with lesser potency by 271 and 272 (1.2–5 μM). In comparison, 273 and 274 that lack HOPO chelating moiety demonstrated very weak or no activity against MMPs. This indicated that the biphenyl backbone alone is not sufficient for efficacy, the presence of ZBG is crucial for MMP inhibition. The biphenyl peptidomimetic backbone was proved as an essential feature,152 as seen from two effective MMP inhibitors 275 and 276. 275 showed low inhibition capacity (21–34%) against MMP-1, MMP-2, MMP-3, and MMP-9 at 100 μM concentration. However, 276 showed 94% inhibition of MMP-2 and moderately inhibited the percent activity of MMP-1, MMP-3, and MMP-9 by 52%, 45%, and 54%.153
Molecular modelling helped to explain the reasons for the low potency of 271 and 272 towards MMP-3 than their pyrone analogues 269 and 270. The presence of methyl substituent causes a steric clash with the active site of MMP-3. In contrast, methyl substituents in the 6-position of pyrone 270 did not lead to steric conflict towards MMP-3; hence, this methyl imparts isoform selectivity for MMP-3. It is also observed that 268 and 270 containing hydrophobic substituents proved to be more potent than 269 in the hydrophobic binding pocket of MMP-8 and MMP-12 versus the more polar binding pocket of MMP-3. Together these results showed that ZBGs and the nature of substituents (polar vs. hydrophobic) are important in dictating the isoform selectivity of MMP inhibitors. Moreover, the importance of ZBG was evaluated in an isolated perfused rat heart system which is subjected to ischemia–reperfusion injury. 269 yielded no recovery, but 268 exhibited significant (∼80%) and sustained heart recovery, demonstrating that the choice of ZBG can have a significant impact.
268 and 269 (Fig. 15) were further investigated as MMP inhibitors for the protection of myocardial structure–function in ischemia–reperfusion injury.154268 showed slightly stronger inhibitory capacities than 269 against MMP-2, -8, and -12. These inhibitors showed a non-toxic environment in cocultures of cardiac cells. In the ex vivo model of isolated stunned rat hearts, 268 displayed improved cardiac performance (∼23% higher rate-pressure product) and coronary flow rates (∼22%), reducing muscle (∼25%) and fibrillar collagen damage (∼60%). Evidence also suggested that MMP-2 activation played an important role in these activities. Overall, the promising results of this study indicated further investigation of small molecule inhibitors such as 268 in in vivo ischemia–reperfusion injury models.
Cohen and coworkers adopted an approach of protecting the metal chelation site of (T)HOPO to synthesize the glycoconjugate prodrugs 277–280 (Fig. 16), possessing a glucose protecting group on ZBGs.155 Upon activation by β-glucosidase, the percent inhibition of MMP-9 was close to 50% in most derivatives. The glycoconjugate prodrug 278 was inactive towards MMP-8 and MMP-9, but upon activation, percent inhibition increased by 33% and 73%, respectively. Hence, this strategy clearly reflects that the development of MMP pro-inhibitor then its activation by the enzyme can be useful in enhancing the water solubility, targeting properties, and minimizing the side effects associated with the delivery of drugs.
Fig. 16. Chemical structures of compounds 277–283.
The involvement of MMP-9 has been found in the breakdown of the blood–brain barrier during cerebral ischemia. Hence, inhibition of MMP-9 comes out to be an important therapeutic invention in the treatment of stroke.156 A novel series of 1-hydroxy-2-pyridones 281–283 (Fig. 16) containing sulfonamide scaffold was prepared as MMPs inhibitors.149In vitro MMPs inhibition assay revealed inhibitory activities in the subnanomolar range.152281–283 demonstrated excellent inhibition activity against MMP-2, MMP-3, MMP-9 and MMP-13 with IC50 values 5.0, 56.5, 2.4 and 2.5 nM, respectively. The most potent compound 282, significantly reduced the early brain edema after transient ischemia in mice and also showed good bioavailability.149
In another approach, a novel series of α- or β-arylsulfone-based MMP-2/-9 inhibitors 284–300 (Fig. 17) containing 6- to 8-membered heterocycles (i–iv) as ZBGs was reported.157285 and 288 proved to be potent nanomolar inhibitors of MMP9 with IC50 values of 7.3 and 4 nM, respectively. In general, the 7- and 8-membered heterocycles 295, 296 and 300 proved to be effective inhibitors of both MMP2 (IC50 = 37–96 nM) and MMP9 (IC50 = 21–31 nM). However, 288 demonstrated very low inhibitory activity against both MMP2 (IC50 = 13 nM) and MMP9 (IC50 = 4 nM). The most active compounds 285, 296, and 300 screened against other MMPs and displayed excellent selectivity for MMP1 and MMP13. The homology model indicated that the size of N-hydroxy-lactam, size and nature of R group was responsible for dictating the binding mode and inhibitory activity of these molecules.
Fig. 17. Chemical structures of compounds 284–300.157.
A library of 22 THOPO isosteres 10 (Fig. 4), 238a, 238f, 238g, 238h (Fig. 12), 264, 265, 266 (Fig. 15) and 301–314 (Fig. 18) have been screened against MMP-12.158238f, 303, 304, 312, 314, and 266 all displayed 93% or better inhibition against MMP-12.158
Fig. 18. Chemical structures of compounds 301–314.158.
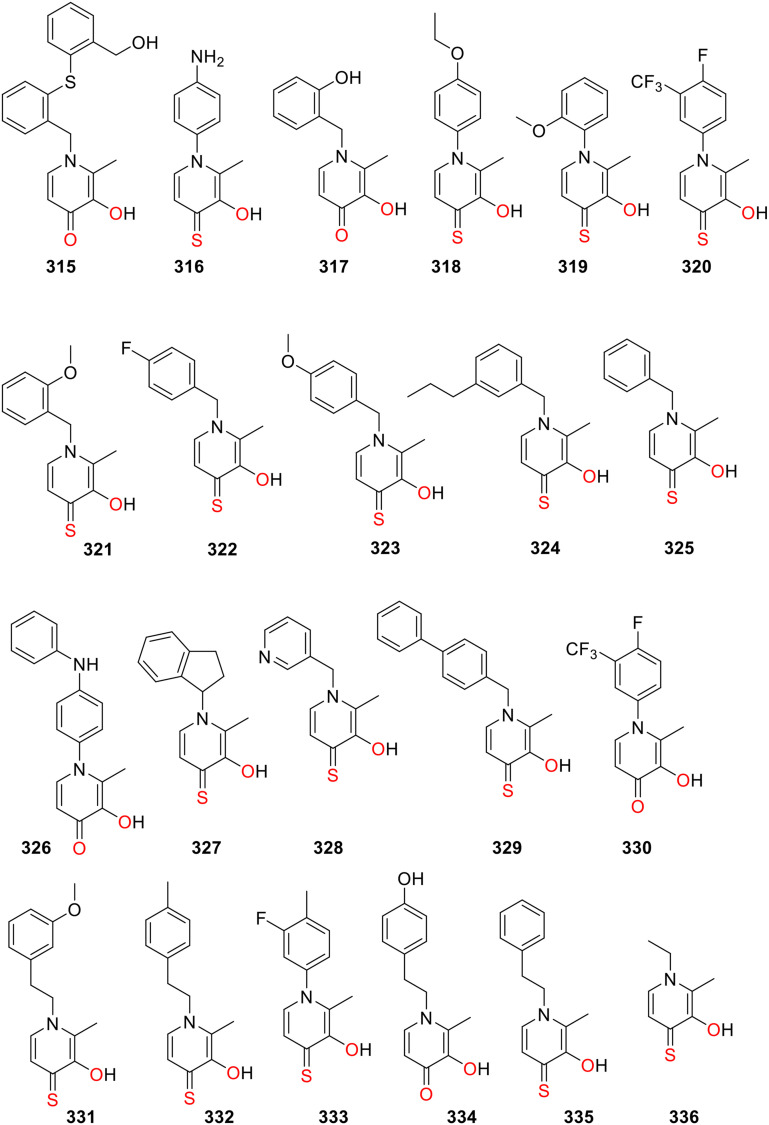
Cohen and coworkers employed a fragment-based drug discovery approach to screen a metal chelator fragment library of 96 structural cores against MMP-2 that produced several hits. HOPO and THOPO were identified as lead chelators. Then an expanded fragment library of HOPO and THOPO fragments 265 (Fig. 15) and 315–336 (Fig. 19) was synthesized and screened against MMP-2, MMP-9, and MMP-3.53 This screening revealed that the HOPO fragment (O,O chelator) produced only 1 hit against MMP-2 265 and 2 hits against MMP-9 330, 265 while the THOPO fragment (O,S chelator) with the highest activity generated 16 hits against MMP-2, 16 hits against MMP-9, and 5 hits against MMP-3. 329 proved to be the most potent inhibitor with IC50 values of 1 μM against MMP-2, 4 μM against MMP-3 and 2 μM against MMP-9. Interestingly, 329 features O,S chelation, and biphenyl moiety as the backbone, which is also potent inhibitors for other metalloenzymes.3,159 These results showed that a chelator fragment library is an excellent approach to developing metalloenzyme inhibitors. In comparing the inhibitory properties of HOPOs and THOPOs against MMPs, the THOPOs were identified as the most potent inhibitors. The high inhibitory activity of THOPOs is attributed to the thiophilicity of the metal ions such as zinc(ii) present in the active site of metalloenzymes.53,160
Fig. 19. Chemical structures of compounds 315–336.
2.9. Anthrax lethal factor
Anthrax is one of the oldest diseases known to infect animals and humans but still pose a serious threat to the human population.161 Anthrax is caused by the virulent spore-forming bacterium Bacillus anthracis which secretes anthrax toxin162,163 known as a lethal factor (LF).159,160 It is a Zn-dependent metalloprotease that is lethal to the host and disrupts the key signaling molecules, such as mitogen-activated protein kinase kinases, ultimately causing cellular destruction and circulatory shocks.164–166 Currently, the FDA-approved ciprofloxacin as an antibiotic is used to target Bacillus anthracis bacteria, but it is ineffective towards toxin secreted by the bacterium.167 Hence inhibition of toxic proteolytic LF enzymes reflects an important therapeutic strategy for treating lethal anthrax infection.
Cohen and coworkers investigated HOPO/THOPO derivatives 1–4 (Fig. 1) and 261–267 (Fig. 15) as lethal factor inhibitors (LFi).3 It has been shown that a ZBG and a biphenyl backbone incorporated at position 2 of the HOPO scaffold are essential for obtaining the potent, selective metalloprotein inhibitors. In the fluorescence-based assay, the THOPO (O,S donor ligands) proved to be more potent inhibitors of LF than HOPO (O,O donor ligands). It is in accordance with the SHAB theory that soft Zn2+ prefers to bind with the soft sulfur donor than the hard oxygen.168 The novel THOPO chelator 337 (Fig. 20) displayed excellent inhibition potential of the LF with an IC50 value of 13.9 μM, verifying the importance of biphenyl backbone.3 The screening of HOPO and THOPO derivatives 265 (Fig. 15) and 315–336 (Fig. 20) generated 10 hits (three HOPO and seven THOPO fragments) against LF among the 87-fragments of the expanded library. 329 proved to be the most potent inhibitor with IC50 values of 3 μM.53
Fig. 20. Chemical structures of compounds 337–363.159.
Further studies identified 268, 269 (Fig. 15) and 338–363 (Fig. 20) as effective LFi.159In vitro fluorescence-based assay revealed that compounds consisting of a THOPO as ZBG and thioamide linkers (356 and 360) are the most potent LF inhibitors with IC50 values in the low micromolar range (5–11 μM). In contrast, 345–352, 361 and 363 bearing THOPO as ZBG and amide linker (O,S,O) showed moderate potency (IC50 = 13 to >100 μM) against LF. However, the HOPO-based ZBGs 353–360, 268 and 361 coupled via an amide linker (O,O,O) were ineffective against LF. This again demonstrates the thiophilic effect of thione moiety, making strong interaction between S donor and Zn ion in the active site of the metalloenzyme. A similar trend has been observed for other metalloenzymes.3
2.10. Miscellaneous metalloenzyme inhibition
The HOPO and THOPO are relatively less explored for inhibiting other disease-associated metalloenzymes such as carbonic anhydrase II and influenza endonuclease. Cohen and coworkers screened the THOPO derivatives 10 (Fig. 4), 238a, 238f, 238g, 238h (Fig. 12), 264, 265, 266 (Fig. 15) and 301–314 (Fig. 18) against three metalloenzymes including human carbonic anhydrase II (hCAII), New Delhi metallo-β-lactamase-1 (NDM-1), and influenza endonuclease (PAN).158 hCAII is mononuclear Zn2+-dependent metalloenzymes and established as cancer-associated enzymes,169,170 while NDM-1 is a dinuclear Zn2+-dependent metalloenzyme famous for its development as bacterial resistance antibiotics,171 and PAN is a dinuclear Mn2+ dependent metalloenzyme play a role for influenza virus replication.172 The compounds 238f, 265, 266, 303, 304, 305 and 307 all displayed 90% or better inhibition against the NMD-1, while only 238f turned out to be an effective inhibitor of hCAII.158 The compounds 238h, 266, 304 and 314 had the best activity against PAN with greater than 90% inhibition.158 These results proved that THOPO scaffold is an ideal pharmacophore for developing inhibitors for various metalloenzymes.
Conclusions
HOPOs and THOPOs comprise a versatile class of compounds that have shown massive potential in wide-ranging drug design and discovery areas. They strongly chelate a wide range of metal ions with hydroxyl and carbonyl (or thione) groups, acting as O,O- or O,S-chelators. They have been successfully used in clinics for chelation therapy. They have shown promise as receptors or chemosensors for pharmacologically interesting ions such as Li+, magnetic resonance imaging contrast agents, anticancer agents, antidiabetic agents, and antibacterial. Recently, excellent metal chelation properties of HOPOs and THOPOs have been exploited to design novel inhibitors of metalloenzymes, which are involved in diseases from cancer to neurodegenerative diseases to antibiotic resistance. Crystallographic studies of representative examples have demonstrated that hydroxypyridone pharmacophore can strongly chelate metal ions in the active site of metalloenzymes.73–75
In vivo studies revealed that these compounds were well tolerated in animals, along with high clearance and low binding with plasma protein and brain tissue.72,99 These investigations demonstrate their favourable toxicology and pharmacokinetic profile for further development. The use HOPO/THOPO as a chelating moiety has opened new potential in developing metalloenzyme inhibitors. The new derivatives of this class will continue to be developed and assessed for clinical translation in the future.
Conflicts of interest
No conflict of interest to declare.
Supplementary Material
Acknowledgments
We would like to thank the University of Auckland for a Faculty Research Development Fund (M. H.), the Health Research Council of New Zealand for a Sir Charles Hercus Fellowship (M. H.) and the Higher Education Commission of Pakistan for IRSIP and Start-up Research Grant (J. A.). We are grateful to Matthew Sullivan for help with figures.
Biographies
Biography
Jahan Zaib Arshad.

Dr. Jahanzaib Arshad earned his PhD in chemistry from Quaid-I-Azam University (Pakistan). He has completed most of his PhD research work at The University of Auckland (New Zealand) under the kind supervision of Christian Hartinger and Muhammad Hanif on the International Research Support Initiative Program (IRSIP) Scholarship funded by the Higher Education Commission (Pakistan). Currently, he is working as Assistant Professor at Government College Women University Sialkot (Pakistan). His research interests are in bioinorganic and medicinal chemistry, where he uses an interdisciplinary approach to drug discovery. He is especially interested in the development of metal-based anticancer agents.
Biography
Muhammad Hanif.

Dr. Muhammad Hanif completed his PhD (Dr. rer. Nat) in Chemistry with Professor Bernhard Keppler from the University of Vienna, Austria. After a short stint at the COMSATS University Islamabad (Pakistan), he moved to New Zealand as an Erwin Schrödinger Fellow with Professor Christian Hartinger at the University of Auckland. He started his independent research funded through a Sir Charles Hercus Fellowship (Health Research Council of New Zealand) at the University of Auckland, where he is a Senior Research Fellow (above the bar). His research focuses on synthetic inorganic chemistry, and bioinorganic chemistry, utilising an interdisciplinary approach to develop new compounds and materials for biomedical applications.
References
- Bentley R. Nat. Prod. Rep. 2006;23:1046–1062. doi: 10.1039/B603758P. [DOI] [PubMed] [Google Scholar]
- Gralla E. J. Stebbins R. B. Coleman G. L. Delahunt C. S. Toxicol. Appl. Pharmacol. 1969;15:604–613. doi: 10.1016/0041-008X(69)90062-3. [DOI] [PubMed] [Google Scholar]
- Lewis J. A. Mongan J. McCammon J. A. Cohen S. M. ChemMedChem. 2006;1:694–697. doi: 10.1002/cmdc.200600102. [DOI] [PubMed] [Google Scholar]
- Yan Y.-L. Miller M. T. Cao Y. Cohen S. M. Bioorg. Med. Chem. Lett. 2009;19:1970–1976. doi: 10.1016/j.bmcl.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif M. Meier S. M. Adhireksan Z. Henke H. Martic S. Movassaghi S. Labib M. Kandioller W. Jamieson S. M. F. Hejl M. Jakupec M. A. Kraatz H.-B. Davey C. A. Keppler B. K. Hartinger C. G. ChemPlusChem. 2017;82:841–847. doi: 10.1002/cplu.201700050. [DOI] [PubMed] [Google Scholar]
- Kandioller W. Kurzwernhart A. Hanif M. Meier S. M. Henke H. Keppler B. K. Hartinger C. G. J. Organomet. Chem. 2011;696:999–1010. doi: 10.1016/j.jorganchem.2010.11.010. [DOI] [Google Scholar]
- Hancock R. D. Martell A. E. Chem. Rev. 1989;89:1875. doi: 10.1021/cr00098a011. [DOI] [Google Scholar]
- Dobbin P. S. Hider R. C. Hall A. D. Taylor P. D. Sarpong P. Porter J. B. Xiao G. van der Helm D. J. Med. Chem. 1993;36:2448–2458. doi: 10.1021/jm00069a002. [DOI] [PubMed] [Google Scholar]
- Scarrow R. E. Riley P. E. Abu-Dari K. White D. L. Raymond K. N. Inorg. Chem. 1985;24:954–967. doi: 10.1021/ic00200a030. [DOI] [Google Scholar]
- Xu J. Jr. Whisenhunt D. W. Veeck A. C. Uhlir L. C. Raymond K. N. Inorg. Chem. 2003;42:2665–2674. doi: 10.1021/ic0259888. [DOI] [PubMed] [Google Scholar]
- Jiang X. Zhou T. Bai R. Xie Y. J. Med. Chem. 2020;63:14470–14501. doi: 10.1021/acs.jmedchem.0c01480. [DOI] [PubMed] [Google Scholar]
- Cilibrizzi A. Abbate V. Chen Y.-L. Ma Y. Zhou T. Hider R. C. Chem. Rev. 2018;118:7657–7701. doi: 10.1021/acs.chemrev.8b00254. [DOI] [PubMed] [Google Scholar]
- Liu Z. D. Hider R. C. Coord. Chem. Rev. 2002;232:151–171. doi: 10.1016/S0010-8545(02)00050-4. [DOI] [Google Scholar]
- Finnegan M. M. Lutz T. G. Nelson W. O. Smith A. Orvig C. Inorg. Chem. 1987;26:2171–2176. doi: 10.1021/ic00260a033. [DOI] [Google Scholar]
- Santos M. A. Gil M. Marques S. Gano L. Cantinho G. Chaves S. J. Inorg. Biochem. 2002;92:43–54. doi: 10.1016/S0162-0134(02)00483-X. [DOI] [PubMed] [Google Scholar]
- Mi Z. Gan B. Yu S. Guo J. Zhang C. Jiang X. Zhou T. Su J. Bai R. Xie Y. J. Enzyme Inhib. Med. Chem. 2019;34:1489–1497. doi: 10.1080/14756366.2019.1634703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell M. Robertson J. Teesdale W. Campbell J. Markesbery W. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/S0022-510X(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Schrag M. Mueller C. Zabel M. Crofton A. Kirsch W. Ghribi O. Squitti R. Perry G. Neurobiol. Dis. 2013;59:100–110. doi: 10.1016/j.nbd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Bilitewski U. Blodgett J. A. V. Duhme-Klair A.-K. Dallavalle S. Laschat S. Routledge A. Schobert R. Angew. Chem., Int. Ed. 2017;56:14360–14382. doi: 10.1002/anie.201701586. [DOI] [PubMed] [Google Scholar]
- Qiu D.-H. Huang Z.-L. Zhou T. Shen C. Hider R. C. FEMS Microbiol. Lett. 2011;314:107–111. doi: 10.1111/j.1574-6968.2010.02153.x. [DOI] [PubMed] [Google Scholar]
- Zhou T. Ma Y. Kong X. Hider R. C. Dalton Trans. 2012;41:6371–6389. doi: 10.1039/C2DT12159J. [DOI] [PubMed] [Google Scholar]
- Zhou T. Winkelmann G. Dai Z.-Y. Hider R. C. J. Pharm. Pharmacol. 2011;63:893–903. doi: 10.1111/j.2042-7158.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- Workman D. G. Hunter M. Dover L. G. Tétard D. J. Inorg. Biochem. 2016;160:49–58. doi: 10.1016/j.jinorgbio.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Datta A. Hooker J. M. Botta M. Francis M. B. Aime S. Raymond K. N. J. Am. Chem. Soc. 2008;130:2546–2552. doi: 10.1021/ja0765363. [DOI] [PubMed] [Google Scholar]
- Moore E. G. Seitz M. Raymond K. N. Inorg. Chem. 2008;47:8571–8573. doi: 10.1021/ic801060x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. Raymond K. N. Acc. Chem. Res. 2009;42:938–947. doi: 10.1021/ar800250h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakusch T. Hollender D. Enyedy E. A. Gonzalez C. S. Montes-Bayon M. Sanz-Medel A. Pessoa J. C. Tomaz I. Kiss T. Dalton Trans. 2009:2428–2437. doi: 10.1039/B817748A. [DOI] [PubMed] [Google Scholar]
- Katoh A. Matsumura Y. Yoshikawa Y. Yasui H. Sakurai H. J. Inorg. Biochem. 2009;103:567–574. doi: 10.1016/j.jinorgbio.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Shakil M. S. Parveen S. Rana Z. Walsh F. Movassaghi S. Söhnel T. Azam M. Shaheen M. A. Jamieson S. M. F. Hanif M. Rosengren R. J. Hartinger C. G. Biomedicines. 2021;9:123. doi: 10.3390/biomedicines9020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanif M. Schaaf P. Kandioller W. Hejl M. Jakupec M. A. Roller A. Keppler B. K. Hartinger C. G. Aust. J. Chem. 2010;63:1521–1528. doi: 10.1071/CH10232. [DOI] [Google Scholar]
- Young J. D. Abbate V. Imberti C. Meszaros L. K. Ma M. T. Terry S. Y. A. Hider R. C. Mullen G. E. Blower P. J. J. Nucl. Med. 2017;58:1270–1277. doi: 10.2967/jnumed.117.191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. Franklin S. J. Whisenhunt D. W. Raymond K. N. J. Am. Chem. Soc. 1995;117:7245–7246. doi: 10.1021/ja00132a025. [DOI] [Google Scholar]
- Sheela A. Roopan S. M. Vijayaraghavan R. Eur. J. Med. Chem. 2008;43:2206–2210. doi: 10.1016/j.ejmech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Thompson K. H. Lichter J. LeBel C. Scaife M. C. McNeill J. H. Orvig C. J. Inorg. Biochem. 2009;103:554–558. doi: 10.1016/j.jinorgbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Rouffet M. Cohen S. M. Dalton Trans. 2011;40:3445–3454. doi: 10.1039/C0DT01743D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. Y. Adamek R. N. Dick B. L. Credille C. V. Morrison C. N. Cohen S. M. Chem. Rev. 2018;119:1323–1455. doi: 10.1021/acs.chemrev.8b00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzellotti A. Farrell N. Chem. Soc. Rev. 2008;37:1629–1651. doi: 10.1039/B617121B. [DOI] [PubMed] [Google Scholar]
- Blanquart C. Linot C. Cartron P.-F. Tomaselli D. Mai A. Bertrand P. Curr. Med. Chem. 2019;26:2748–2785. doi: 10.2174/0929867325666180706105903. [DOI] [PubMed] [Google Scholar]
- Martin D. P. Blachly P. G. McCammon J. A. Cohen S. M. J. Med. Chem. 2014;57:7126–7135. doi: 10.1021/jm500984b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran C. T. Carta F. Scozzafava A. Expert Opin. Ther. Pat. 2013;23:777–788. doi: 10.1517/13543776.2013.777042. [DOI] [PubMed] [Google Scholar]
- White R. J. Margolis P. S. Trias J. Yuan Z. Curr. Opin. Pharmacol. 2003;3:502–507. doi: 10.1016/S1471-4892(03)00115-2. [DOI] [PubMed] [Google Scholar]
- Gaeta A. Molina-Holgado F. Kong X. L. Salvage S. Fakih S. Francis P. T. Williams R. J. Hider R. C. Bioorg. Med. Chem. 2011;19:1285–1297. doi: 10.1016/j.bmc.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Santos M. A. Coord. Chem. Rev. 2008;252:1213–1224. doi: 10.1016/j.ccr.2008.01.033. [DOI] [Google Scholar]
- Cabanes J. Chazarra S. Garcia-Carmona F. J. Pharm. Pharmacol. 1994;46:982–985. doi: 10.1111/j.2042-7158.1994.tb03253.x. [DOI] [PubMed] [Google Scholar]
- Khodaverdian V. Tapadar S. MacDonald I. A. Xu Y. Ho P.-Y. Bridges A. Rajpurohit P. Sanghani B. A. Fan Y. Thangaraju M. Hathaway N. A. Oyelere A. K. Sci. Rep. 2019;9:4802. doi: 10.1038/s41598-019-39214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto E. Nakano K. Nakayachi T. Morshed S. R. M. Hashimoto K. Kikuchi H. Nishikawa H. Kawase M. Sakagami H. Anticancer Res. 2004;24:755–762. [PubMed] [Google Scholar]
- Hoyes K. P. Hider R. C. Porter J. B. Cancer Res. 1992;52:4591–4599. [PubMed] [Google Scholar]
- Cooper C. E. Lynagh G. R. Hoyes K. P. Hider R. C. Cammack R. Porter J. B. J. Biol. Chem. 1996;271:20291–20299. doi: 10.1074/jbc.271.34.20291. [DOI] [PubMed] [Google Scholar]
- Maclean K. H. Cleveland J. L. Porter J. B. Blood. 2001;98:3831–3839. doi: 10.1182/blood.V98.13.3831. [DOI] [PubMed] [Google Scholar]
- Yoo C. B. Jones P. A. Nat. Rev. Drug Discovery. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- Falkenberg K. J. Johnstone R. W. Nat. Rev. Drug Discovery. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- De Simone A. Milelli A. ChemMedChem. 2019;14:1067–1073. doi: 10.1002/cmdc.201900174. [DOI] [PubMed] [Google Scholar]
- Agrawal A. Johnson S. L. Jacobsen J. A. Miller M. T. Chen L. H. Pellecchia M. Cohen S. M. ChemMedChem. 2010;5:195–199. doi: 10.1002/cmdc.200900516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harringer S. Happl B. Ozenil M. Kast C. Hejl M. Wernitznig D. Legin A. A. Schweikert A. Gajic N. Roller A. Koellensperger G. Jakupec M. A. Kandioller W. Keppler B. K. Chem. – Eur. J. 2020;26:5419–5433. doi: 10.1002/chem.201905546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil V. Sodji Q. H. Kornacki J. R. Mrksich M. Oyelere A. K. J. Med. Chem. 2013;56:3492–3506. doi: 10.1021/jm301769u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S. Ramos J. Luo W. Sirisawad M. Verner E. Buggy J. Leukemia. 2008;22:1026–1034. doi: 10.1038/leu.2008.9. [DOI] [PubMed] [Google Scholar]
- Sodji Q. H. Patil V. Kornacki J. R. Mrksich M. Oyelere A. K. J. Med. Chem. 2013;56:9969–9981. doi: 10.1021/jm401225q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldana-Masangkay G. I. Sakamoto K. M. J. Biomed. Biotechnol. 2010;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdar M. Perez G. Ngo L. Marks P. A. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20003–20008. doi: 10.1073/pnas.1013754107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J. Senoh S. Witkop B. J. Biol. Chem. 1958;233:697–701. doi: 10.1016/S0021-9258(18)64730-1. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Tomchick R. J. Biol. Chem. 1958;233:702–705. doi: 10.1016/S0021-9258(18)64731-3. [DOI] [PubMed] [Google Scholar]
- Magarkar A. Parkkila P. Viitala T. Lajunen T. Mobarak E. Licari G. Cramariuc O. Vauthey E. Róg T. Bunker A. Chem. Commun. 2018;54:3440–3443. doi: 10.1039/C8CC00221E. [DOI] [PubMed] [Google Scholar]
- Abdolmaleky H. M. Cheng K.-H. Faraone S. V. Wilcox M. Glatt S. J. Gao F. Smith C. L. Shafa R. Aeali B. Carnevale J. Hum. Mol. Genet. 2006;15:3132–3145. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L. S. E. Soares-da-Silva P. J. Med. Chem. 2014;57:8692–8717. doi: 10.1021/jm500572b. [DOI] [PubMed] [Google Scholar]
- Jatana N. Apoorva N. Malik S. Sharma A. Latha N. Cent. Nerv. Syst. Agents Med. Chem. 2013;13:166–194. doi: 10.2174/1871524913666140109113341. [DOI] [PubMed] [Google Scholar]
- Bonifácio M. J. Palma P. N. Almeida L. Soares-da-Silva P. CNS Drug Rev. 2007;13:352–379. doi: 10.1111/j.1527-3458.2007.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. F. Goldberg T. E. Kolachana B. S. Callicott J. H. Mazzanti C. M. Straub R. E. Goldman D. Weinberger D. R. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees A. J. CNS Neurosci. Ther. 2008;14:83–93. doi: 10.1111/j.1755-5949.2007.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaakkola S. Drugs. 2000;59:1233–1250. doi: 10.2165/00003495-200059060-00004. [DOI] [PubMed] [Google Scholar]
- Borchardt R. T. J. Med. Chem. 1973;16:581–583. doi: 10.1021/jm00263a047. [DOI] [PubMed] [Google Scholar]
- Harrison S. T. Poslusney M. S. Mulhearn J. J. Zhao Z. Kett N. R. Schubert J. W. Melamed J. Y. Allison T. J. Patel S. B. Sanders J. M. ACS Med. Chem. Lett. 2015;6:318–323. doi: 10.1021/ml500502d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z. Harrison S. T. Schubert J. W. Sanders J. M. Polsky-Fisher S. Zhang N. R. McLoughlin D. Gibson C. R. Robinson R. G. Sachs N. A. Bioorg. Med. Chem. Lett. 2016;26:2952–2956. doi: 10.1016/j.bmcl.2016.03.095. [DOI] [PubMed] [Google Scholar]
- Vidgren J. Svensson L. A. Liljas A. Nature. 1994;368:354–358. doi: 10.1038/368354a0. [DOI] [PubMed] [Google Scholar]
- Bai H.-W. Shim J.-Y. Yu J. Zhu B. T. Chem. Res. Toxicol. 2007;20:1409–1425. doi: 10.1021/tx700174w. [DOI] [PubMed] [Google Scholar]
- Harrison S. T. Poslusney M. S. Mulhearn J. J. Zhao Z. Kett N. R. Schubert J. W. Melamed J. Y. Allison T. J. Patel S. B. Sanders J. M. ACS Med. Chem. Lett. 2015;6:318–323. doi: 10.1021/ml500502d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst G. Akuma D. Au V. Buchler I. P. Byers S. Carr G. V. Defays S. de León P. Demaude T. DePasquale M. Durieu V. Huang Y. Jigorel E. Kimos M. Kolobova A. Montel F. Moureau F. Poslusney M. Swinnen D. Vandergeten M. C. Van Houtvin N. Wei H. White N. Wood M. Barrow J. C. ACS Med. Chem. Lett. 2019;10:1573–1578. doi: 10.1021/acsmedchemlett.9b00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolghadri S. Bahrami A. Hassan Khan M. T. Munoz-Munoz J. Garcia-Molina F. Garcia-Canovas F. Saboury A. A. J. Enzyme Inhib. Med. Chem. 2019;34:279–309. doi: 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.-S. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z. Wang G. Zeng Q.-H. Li Y. Liu H. Wang J. J. Zhao Y. Crit. Rev. Food Sci. Nutr. 2021:1–42. doi: 10.1080/10408398.2021.1871724. [DOI] [PubMed] [Google Scholar]
- Carballo-Carbajal I. Laguna A. Romero-Giménez J. Cuadros T. Bové J. Martinez-Vicente M. Parent A. Gonzalez-Sepulveda M. Peñuelas N. Torra A. Nat. Commun. 2019;10:1–19. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rivera J. Casadevall A. Med. Mycol. 2001;39:353–357. doi: 10.1080/mmy.39.4.353.357. [DOI] [PubMed] [Google Scholar]
- Tinello F. Lante A. Innovative Food Sci. Emerging Technol. 2018;50:73–83. doi: 10.1016/j.ifset.2018.10.008. [DOI] [Google Scholar]
- Li D.-F. Hu P.-P. Liu M.-S. Kong X.-L. Zhang J.-C. Hider R. C. Zhou T. J. Agric. Food Chem. 2013;61:6597–6603. doi: 10.1021/jf401585f. [DOI] [PubMed] [Google Scholar]
- Zhao D.-Y. Zhang M.-X. Dong X.-W. Hu Y.-Z. Dai X.-Y. Wei X. Hider R. C. Zhang J.-C. Zhou T. Bioorg. Med. Chem. Lett. 2016;26:3103–3108. doi: 10.1016/j.bmcl.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Shao L.-L. Wang X.-L. Chen K. Dong X.-W. Kong L.-M. Zhao D.-Y. Hider R. C. Zhou T. Food Chem. 2018;242:174–181. doi: 10.1016/j.foodchem.2017.09.054. [DOI] [PubMed] [Google Scholar]
- Singh L. R. Chen Y.-L. Xie Y.-Y. Xia W. Gong X.-W. Hider R. C. Zhou T. J. Enzyme Inhib. Med. Chem. 2020;35:1562–1567. doi: 10.1080/14756366.2020.1801669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J. Chen K. Ridd M. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim M. B. Edmondson D. Tipton K. F. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- Westlund K. Denney R. Kochersperger L. Rose R. Abell C. Science. 1985;230:181–183. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- Singh R. Sharma N. Chem. Biol. Lett. 2014;1:33–39. [Google Scholar]
- Johnston J. Biochem. Pharmacol. 1968;17:1285–1297. doi: 10.1016/0006-2952(68)90066-X. [DOI] [PubMed] [Google Scholar]
- Neff N. H. Costa E. Life Sci. 1966;5:951–959. doi: 10.1016/0024-3205(66)90204-9. [DOI] [PubMed] [Google Scholar]
- Youdim M. Riederer P. J. Neural Transm.: Gen. Sect. 1993;91:181–195. doi: 10.1007/BF01245231. [DOI] [PubMed] [Google Scholar]
- Thomas T. Neurobiol. Aging. 2000;21:343–348. doi: 10.1016/S0197-4580(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Riederer P. Danielczyk W. Grünblatt E. NeuroToxicology. 2004;25:271–277. doi: 10.1016/S0161-813X(03)00106-2. [DOI] [PubMed] [Google Scholar]
- Dezsi L. Vecsei L. CNS Neurol. Disord.: Drug Targets. 2017;16:425–439. doi: 10.2174/1871527316666170124165222. [DOI] [PubMed] [Google Scholar]
- Fernandez H. H. Chen J. J. Pharmacotherapy. 2007;27:174S–185S. doi: 10.1592/phco.27.12part2.174S. [DOI] [PubMed] [Google Scholar]
- Schapira A. H. CNS Drugs. 2011;25:1061–1071. doi: 10.2165/11596310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Zhang C. Yang K. Yu S. Su J. Yuan S. Han J. Chen Y. Gu J. Zhou T. Bai R. Eur. J. Med. Chem. 2019;180:367–382. doi: 10.1016/j.ejmech.2019.07.031. [DOI] [PubMed] [Google Scholar]
- Jiang X. Guo J. Lv Y. Yao C. Zhang C. Mi Z. Shi Y. Gu J. Zhou T. Bai R. Bioorg. Med. Chem. 2020;28:115550. doi: 10.1016/j.bmc.2020.115550. [DOI] [PubMed] [Google Scholar]
- Versporten A. Bolokhovets G. Ghazaryan L. Abilova V. Pyshnik G. Spasojevic T. Korinteli I. Raka L. Kambaralieva B. Cizmovic L. Lancet Infect. Dis. 2014;14:381–387. doi: 10.1016/S1473-3099(14)70071-4. [DOI] [PubMed] [Google Scholar]
- Bush K. Bradford P. A. Cold Spring Harbor Perspect. Med. 2016;6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Courvalin P. Dantas G. Davies J. Eisenstein B. Huovinen P. Jacoby G. A. Kishony R. Kreiswirth B. N. Kutter E. Nat. Rev. Microbiol. 2011;9:894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J. Strominger J. L. Annu. Rev. Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Bush K. Antimicrob. Agents Chemother. 2018;62:e01076-18. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopeit T. Carlsen T. J. O. Helland R. Leiros H.-K. S. J. Med. Chem. 2015;58:8671–8682. doi: 10.1021/acs.jmedchem.5b01289. [DOI] [PubMed] [Google Scholar]
- Tacconelli E. Carrara E. Savoldi A. Harbarth S. Mendelson M. Monnet D. L. Pulcini C. Kahlmeter G. Kluytmans J. Carmeli Y. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- Tehrani K. H. Martin N. I. MedChemComm. 2018;9:1439–1456. doi: 10.1039/C8MD00342D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheselli M. Conzo F. Mauri M. Simonini R. Aquat. Toxicol. 2010;98:204–210. doi: 10.1016/j.aquatox.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Muthyala R. Rastogi N. Shin W. S. Peterson M. L. Sham Y. Y. Bioorg. Med. Chem. Lett. 2014;24:2535–2538. doi: 10.1016/j.bmcl.2014.03.097. [DOI] [PubMed] [Google Scholar]
- Shin W. S. Bergstrom A. Bonomo R. A. Crowder M. W. Muthyala R. Sham Y. Y. ChemMedChem. 2017;12:845–849. doi: 10.1002/cmdc.201700182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria-Castro A. Alvarado-Echeverría I. Monge-Bonilla C. Ann. Neurosci. 2017;24:46–54. doi: 10.1159/000464422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero C. E. Mostile G. Vasta R. Rapisarda V. Santo Signorelli S. Ferrante M. Zappia M. Nicoletti A. Environ. Res. 2017;159:82–94. doi: 10.1016/j.envres.2017.07.048. [DOI] [PubMed] [Google Scholar]
- Fabiani C. Antollini S. S. Front. Cell. Neurosci. 2019;13:309. doi: 10.3389/fncel.2019.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talesa V. N. Mech. Ageing Dev. 2001;122:1961–1969. doi: 10.1016/S0047-6374(01)00309-8. [DOI] [PubMed] [Google Scholar]
- Castro A. Martinez A. Mini-Rev. Med. Chem. 2001;1:267–272. doi: 10.2174/1389557013406864. [DOI] [PubMed] [Google Scholar]
- Bartolini M. Bertucci C. Cavrini V. Andrisano V. Biochem. Pharmacol. 2003;65:407–416. doi: 10.1016/S0006-2952(02)01514-9. [DOI] [PubMed] [Google Scholar]
- Rees T. Hammond P. Soreq H. Younkin S. Brimijoin S. Neurobiol. Aging. 2003;24:777–787. doi: 10.1016/S0197-4580(02)00230-0. [DOI] [PubMed] [Google Scholar]
- Froede H. C. Wilson I. B. J. Biol. Chem. 1984;259:11010–11013. doi: 10.1016/S0021-9258(18)90614-9. [DOI] [PubMed] [Google Scholar]
- Telpoukhovskaia M. A. Patrick B. O. Rodríguez-Rodríguez C. Orvig C. Mol. BioSyst. 2013;9:792–805. doi: 10.1039/C3MB25600F. [DOI] [PubMed] [Google Scholar]
- Telpoukhovskaia M. A. Patrick B. O. Rodríguez-Rodríguez C. Orvig C. Bioorg. Med. Chem. Lett. 2016;26:1624–1628. doi: 10.1016/j.bmcl.2016.01.080. [DOI] [PubMed] [Google Scholar]
- Nunes A. Marques S. M. Quintanova C. Silva D. F. Cardoso S. M. Chaves S. Santos M. A. Dalton Trans. 2013;42:6058–6073. doi: 10.1039/C3DT50406A. [DOI] [PubMed] [Google Scholar]
- Bickerton G. R. Paolini G. V. Besnard J. Muresan S. Hopkins A. L. Nat. Chem. 2012;4:90–98. doi: 10.1038/nchem.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolami M. Pandolfi F. De Vita D. Carafa C. Messore A. Di Santo R. Feroci M. Costi R. Chiarotto I. Bagetta D. Eur. J. Med. Chem. 2020;198:112350. doi: 10.1016/j.ejmech.2020.112350. [DOI] [PubMed] [Google Scholar]
- Kladnik J. Ristovski S. Kljun J. Defant A. Mancini I. Sepčić K. Turel I. Int. J. Mol. Sci. 2020;21:5628. doi: 10.3390/ijms21165628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montuschi P. Mini-Rev. Med. Chem. 2008;8:647–656. doi: 10.2174/138955708784567395. [DOI] [PubMed] [Google Scholar]
- Werz O. Steinhilber D. Pharmacol. Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Zaman K. Hanigan M. H. Smith A. Vaughan J. Macdonald T. Jones D. R. Hunt J. F. Gaston B. Am. J. Respir. Cell Mol. Biol. 2006;34:387–393. doi: 10.1165/rcmb.2005-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C. D. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Chen Y. Hu Y. Zhang H. Peng C. Li S. Nat. Genet. 2009;41:783–792. doi: 10.1038/ng.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno F. Spaziano G. Liparulo A. Roviezzo F. Nabavi S. M. Sureda A. Filosa R. D'Agostino B. Eur. J. Med. Chem. 2018;153:65–72. doi: 10.1016/j.ejmech.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Kayyali R. Porter J. B. Davies N. A. Nugent J. H. Cooper C. E. Hider R. C. J. Biol. Chem. 2001;276:48814–48822. doi: 10.1074/jbc.M109551200. [DOI] [PubMed] [Google Scholar]
- Liu Z. D. Kayyali R. Hider R. C. Porter J. B. Theobald A. E. J. Med. Chem. 2002;45:631–639. doi: 10.1021/jm010817i. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. A. Chen H. Abdellatif K. R. Dong Y. Petruk K. C. Knaus E. E. Bioorg. Med. Chem. Lett. 2008;18:4195–4198. doi: 10.1016/j.bmcl.2008.05.071. [DOI] [PubMed] [Google Scholar]
- Chowdhury M. A. Abdellatif K. R. Dong Y. Das D. Suresh M. R. Knaus E. E. J. Med. Chem. 2009;52:1525–1529. doi: 10.1021/jm8015188. [DOI] [PubMed] [Google Scholar]
- Sternlicht M. D. Werb Z. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. R. Fingleton B. Rothenberg M. L. Matrisian L. M. J. Clin. Oncol. 2000;18:1135. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- Kupai K. Szucs G. Cseh S. Hajdu I. Csonka C. Csont T. Ferdinandy P. J. Pharmacol. Toxicol. Methods. 2010;61:205–209. doi: 10.1016/j.vascn.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Hu J. Van den Steen P. E. Sang Q.-X. A. Opdenakker G. Nat. Rev. Drug Discovery. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- Seiki M. Yana I. Cancer Sci. 2003;94:569–574. doi: 10.1111/j.1349-7006.2003.tb01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapna G. Gokul S. Bagri-Manjrekar K. Oral Dis. 2014;20:538–550. doi: 10.1111/odi.12159. [DOI] [PubMed] [Google Scholar]
- Kaplan A. Spiller K. J. Towne C. Kanning K. C. Choe G. T. Geber A. Akay T. Aebischer P. Henderson C. E. Neuron. 2014;81:333–348. doi: 10.1016/j.neuron.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele A. Abbate I. Oakley C. Casamassima P. Savino E. Casamassima A. Sciortino G. Fazio V. Gadaleta-Caldarola G. Catino A. Int. J. Biochem. Cell Biol. 2016;77:91–101. doi: 10.1016/j.biocel.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Anthony D. Ferguson B. Matyzak M. Miller K. Esiri M. Perry V. Neuropathol. Appl. Neurobiol. 1997;23:406–415. doi: 10.1111/j.1365-2990.1997.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Creemers E. E. Cleutjens J. P. Smits J. F. Daemen M. J. Circ. Res. 2001;89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- Whittaker M. Floyd C. D. Brown P. Gearing A. Chem. Rev. 1999:2735–2776. doi: 10.1021/cr9804543. [DOI] [PubMed] [Google Scholar]
- Coussens L. M. Fingleton B. Matrisian L. M. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Marques S. R. M. Tuccinardi T. Nuti E. Santamaria S. André V. N. Rossello A. Martinelli A. Santos M. A. L. J. Med. Chem. 2011;54:8289–8298. doi: 10.1021/jm200593b. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-M. Fan X. Chakaravarty D. Xiang B. Scannevin R. H. Huang Z. Ma J. Burke S. L. Karnachi P. Rhodes K. J. Bioorg. Med. Chem. Lett. 2008;18:409–413. doi: 10.1016/j.bmcl.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Puerta D. T. Cohen S. M. Inorg. Chem. 2003;42:3423–3430. doi: 10.1021/ic026029g. [DOI] [PubMed] [Google Scholar]
- Puerta D. T. Griffin M. O. Lewis J. A. Romero-Perez D. Garcia R. Villarreal F. J. Cohen S. M. J. Biol. Inorg. Chem. 2006;11:131–138. doi: 10.1007/s00775-005-0053-x. [DOI] [PubMed] [Google Scholar]
- Agrawal A. Romero-Perez D. Jacobsen J. A. Villarreal F. J. Cohen S. M. ChemMedChem. 2008;3:812. doi: 10.1002/cmdc.200700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.-L. Cohen S. M. Org. Lett. 2007;9:2517–2520. doi: 10.1021/ol0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Perez D. Agrawal A. Jacobsen J. Yan Y. Thomas R. Cohen S. Villarreal F. J. Cardiovasc. Pharmacol. 2009;53:452. doi: 10.1097/FJC.0b013e3181a6aa83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourden J. L. M. Cohen S. M. Chem. Commun. 2010;46:1241–1243. doi: 10.1039/B923302D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. Estrada E. Dencoff J. Stroke. 1998;29:2189–2194. doi: 10.1161/01.STR.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-M. Fan X. Yang S.-M. Scannevin R. H. Burke S. L. Rhodes K. J. Jackson P. F. Bioorg. Med. Chem. Lett. 2008;18:405–408. doi: 10.1016/j.bmcl.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Adamek R. N. Credille C. V. Dick B. L. Cohen S. M. JBIC, J. Biol. Inorg. Chem. 2018;23:1129–1138. doi: 10.1007/s00775-018-1593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A. de Oliveira C. A. F. Cheng Y. Jacobsen J. A. McCammon J. A. Cohen S. M. J. Med. Chem. 2009;52:1063–1074. doi: 10.1021/jm8013212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen F. E. Lewis J. A. Cohen S. M. ChemMedChem. 2007;2:152–171. doi: 10.1002/cmdc.200600204. [DOI] [PubMed] [Google Scholar]
- Pile J. C. Malone J. D. Eitzen E. M. Friedlander A. M. Arch. Intern. Med. 1998;158:429–434. doi: 10.1001/archinte.158.5.429. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Young J. A. Annu. Rev. Cell Dev. Biol. 2003;19:45–70. doi: 10.1146/annurev.cellbio.19.111301.140655. [DOI] [PubMed] [Google Scholar]
- Bradley K. A. Mogridge J. Mourez M. Collier R. J. Young J. A. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- Agrawal A. Pulendran B. Cell. Mol. Life Sci. 2004;61:2859–2865. doi: 10.1007/s00018-004-4251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A. P. Boone S. A. Liang X. Duesbery N. S. J. Biol. Chem. 2003;278:9402–9406. doi: 10.1074/jbc.M211262200. [DOI] [PubMed] [Google Scholar]
- Tonello F. Ascenzi P. Montecucco C. J. Biol. Chem. 2003;278:40075–40078. doi: 10.1074/jbc.M306466200. [DOI] [PubMed] [Google Scholar]
- Forino M. Johnson S. Wong T. Y. Rozanov D. V. Savinov A. Y. Li W. Fattorusso R. Becattini B. Orry A. J. Jung D. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9499–9504. doi: 10.1073/pnas.0502733102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. G. J. Am. Chem. Soc. 1963;85:3533–3539. doi: 10.1021/ja00905a001. [DOI] [Google Scholar]
- Supuran C. T. Scozzafava A. Casini A. Med. Res. Rev. 2003;23:146–189. doi: 10.1002/med.10025. [DOI] [PubMed] [Google Scholar]
- Impola U. Uitto V.-J. Hietanen J. Hakkinen L. Zhang L. Larjava H. Isaka K. Saarialho-Kere U. J. Pathol. 2004;202:14–22. doi: 10.1002/path.1479. [DOI] [PubMed] [Google Scholar]
- Watkins K. Curr. Emerg. Hosp. Med. Rep. 2018;6:86–93. doi: 10.1007/s40138-018-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.-S. Kumar G. Shadrick W. R. Zhou W. Jeevan T. Li Z. Slavish P. J. Fabrizio T. P. Yoon S.-W. Webb T. R. Proc. Natl. Acad. Sci. U. S. A. 2016;113:3669–3674. doi: 10.1073/pnas.1519772113. [DOI] [PMC free article] [PubMed] [Google Scholar]