Abstract
The COVID-19 pandemic poses a fundamental challenge to global health. Since the outbreak of SARS-CoV-2, great efforts have been made to identify antiviral strategies and develop therapeutic drugs to combat the disease. There are different strategies for developing small molecular anti-SARS-CoV-2 drugs, including targeting coronavirus structural proteins (e.g. spike protein), non-structural proteins (nsp) (e.g. RdRp, Mpro, PLpro, helicase, nsp14, and nsp16), host proteases (e.g. TMPRSS2, cathepsin, and furin) and the pivotal proteins mediating endocytosis (e.g. PIKfyve), as well as developing endosome acidification agents and immune response modulators. Favipiravir and chloroquine are the anti-SARS-CoV-2 agents that were identified earlier in this epidemic and repurposed for COVID-19 clinical therapy based on these strategies. However, their efficacies are controversial. Currently, three small molecular anti-SARS-CoV-2 agents, remdesivir, molnupiravir, and Paxlovid (PF-07321332 plus ritonavir), have been granted emergency use authorization or approved for COVID-19 therapy in many countries due to their significant curative effects in phase III trials. Meanwhile, a large number of promising anti-SARS-CoV-2 drug candidates have entered clinical evaluation. The development of these drugs brings hope for us to finally conquer COVID-19. In this account, we conducted a comprehensive review of the recent advances in small molecule anti-SARS-CoV-2 agents according to the target classification. Here we present all the approved drugs and most of the important drug candidates for each target, and discuss the challenges and perspectives for the future research and development of anti-SARS-CoV-2 drugs.
Keywords: COVID-19, SARS-CoV-2, RdRp inhibitor, Mpro inhibitor, host-targeted inhibitor, small molecule
Introduction
An infectious respiratory disease with atypical pneumonia emerged in 2019. Its rapid and global spread has been officially declared as a pandemic by the World Health Organization (WHO). A new coronavirus capable of infecting humans was identified as the causing pathogen of the disease.1 This novel virus was named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the disease as coronavirus disease 2019 (COVID-19). Symptoms after infection with SARS-CoV-2 include influenza-like minor or moderate respiratory symptoms, as well as serious lung injury and multiple-organ failure, which eventually lead to death. The high transmission of SARS-CoV-2 and the continuing outbreaks of COVID-19 have posed a significant threat to global public health.
Currently, there are still very few approved effective antiviral drugs for the treatment of patients with COVID-19. Remdesivir, molnupiravir, and Paxlovid (PF-07321332 plus ritonavir) have been approved by the Food and Drug Administration (FDA) for the treatment of COVID-19 patients.2 The drugs promise to reduce the number of COVID-19 hospitalizations and deaths. But as the pandemic has progressed, we have seen the emergence of new viral variants, such as Delta and Omicron. The Delta variant is linked to more serious illness than the original strain, while the Omicron variant is slightly more transmissible. These variants have mutations that could provide SARS-CoV-2 with unusual epidemiological properties and potential immune evasion. The capacity to escape immunity could lead to future infection waves, and exert adverse impacts on diagnostics and therapeutics.3 The variants are mainly characterized by mutations in the viral spike protein. But the possibility of mutations in other viral components still exists. The chance that the variants will develop resistance to the current drugs remains. Therefore, novel and satisfactory therapeutic strategies that could attack the SARS-CoV-2 on multiple fronts are still urgently needed. In this review, we summarize the ongoing efforts and enormous challenges of finding antiviral drugs targeting the crucial components of SARS-CoV-2. The review is divided into the following sections: compounds that inhibit virus entry, compounds that inhibit the polymerase and proteases of SARS-CoV-2, compounds that inhibit host processes required by SARS-CoV-2, and repurposed drugs that inhibit SARS-CoV-2 replication. There are already reviews that describe the small molecules against SARS-CoV-2, especially the drug repurposing strategies.4–7 We here comprehensively summarize the current advances and challenges in small-molecular therapeutics based on almost all potential targets against SARS-CoV-2, including virus-targeted and host-targeted inhibitors, as well as immune response modulators and high-throughput drug repurposing.
Viral structure and potential targets for drug development
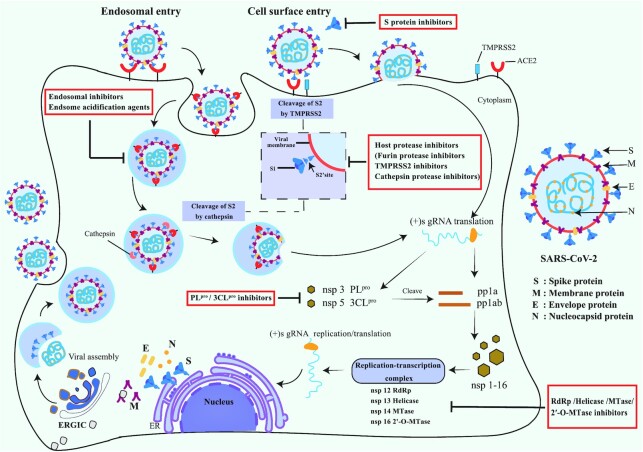
SARS-CoV-2 belongs to the enveloped beta-coronaviruses. It has a positive-sense single-stranded RNA genome. SARS-CoV-2 shares 79% genome sequence similarity to SARS-CoV and 50% identity with Middle East respiratory syndrome coronavirus (MERS-CoV).8 Its genome is organized into six functional open reading frames (ORFs): envelope (E), spike (S), membrane (M), replicase (ORF1a or ORF1b), and nucleocapsid (N).9 The majority of SARS-CoV-2-encoded proteins are similar to the corresponding proteins in SARS-CoV. The two large overlapping replicase genes ORF1a and ORF1b constitute about two-thirds of the SARS-CoV-2 genome. They encode two large replicase polyproteins pp1a and pp1ab. The polyproteins are proteolytically cleaved into 16 non-structural proteins (nsp1–16).10
SARS-CoV-2 infection is initiated by the attachment of its S protein to the human cell entry receptor angiotensin-converting enzyme 2 (ACE2),11,12 followed by membrane fusion (Fig. 1). The receptor binding domain (RBD) in the S1 subunit of the S protein mediates the binding with ACE2. Several residues important for ACE2 binding of the SARS-CoV-2 RBD differ from those of SARS-CoV, which strengthens its ACE2 binding affinity.11 The S2 subunit of the S protein mediates the fusion between the viral and human membrane. SARS-CoV-2 binds to the same host entry receptor ACE2 as SARS-CoV, because their S proteins are highly homologous.13 SARS-CoV-2 broadly recognizes other animals’ ACE2, which implies that SARS-CoV-2 might have a wide host range.
Figure 1.
Schematic representation of the mechanism of action of SARS-CoV-2 infection and pharmacological intervention.
After the entry of viral RNA into the cytoplasm of target host cells, the viral genome is translated. The replicase polyproteins pp1a and pp1ab are then proteolytically cleaved into nsp1–16 by two viral proteases. One is main protease Mpro (also referred to as 3CLpro) and the other one is papain-like protease (PLpro). nsp1–16 form viral enzyme complexes such as the replicase–transcriptase complex, which are involved in SARS-CoV-2 genome replication and transcription. Structural proteins S, E, M, and N are subsequently translated, and are responsible for virion assembly. The N proteins encapsulate the synthesized viral genome RNA and form nucleocapsids. The new virions are assembled in the endoplasmic reticulum, transported by the Golgi apparatus, and eventually secreted to the cell surface (Fig. 1).
Virus-targeted inhibitors
Spike (S) protein inhibitors
Cryo-electron microscopy illustrated that the extracellular domain of the SARS-CoV-2 S protein formed a homotrimeric structure.14,15 The RBD in the SARS-CoV-2 S protein binds to the host cell ACE2 and initiates the interaction between the host cell and the virus. The binding of RBD is regarded as an important process for viral entry. The major structural differences between the SARS-CoV-2 S protein and that of SARS-CoV lies in their RBD. The RBD of SARS-CoV-2 illustrates a stronger binding affinity toward ACE2 than that of SARS-CoV.16 The use of neutralizing antibodies against the S protein of SARS-CoV-2 is believed to be a sound therapeutic strategy for the treatment of patients having COVID-19.
During the fusion process, the heptad repeat 1 (HR1) and 2 (HR2) in the S2 subunit of the S protein interact conjointly and form a six-helix bundle (6-HB) that promotes the viral and host cell membrane fusion.17 Continuous efforts are being devoted to identify potent inhibitors to block the viral 6-HB formation. A series of pan-CoV peptide inhibitors derived from the HR2 domain could block the S protein-mediated membrane fusion of diverse CoVs. Recent studies showed that a designed fusion inhibitor EK1 and a lipopeptide derivative EK1C4 could effectively inhibit SARS-CoV-2 infections with nanomolar activities.18,19 Another SARS-Cov-2 fusion inhibitor mimicking HR2 is the dimeric lipoprotein [SARS-HRC-PEG4]-2-chol.20 Daily intranasal administration of [SARS-HRC-PEG4]-2-chol protected ferrets from direct-contact SARS-CoV-2 infection. Salvianolic acid C, a hydrophilic compound from the traditional Chinese medicine Danshen, exhibited potent antiviral activity against SARS-CoV-2 infection by blocking 6-HB formation and S-mediated viral membrane fusion.21 Despite the potential efficiency of fusion inhibitors, none has been studied in a clinical trial.
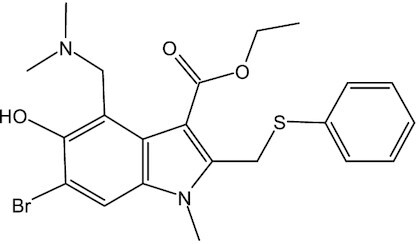
Arbidol, an approved anti-influenza drug in China and Russia, could efficiently inhibit S protein-mediated membrane fusion and impede virus entry into host cells (Table 1). It showed adequate activity against SARS-CoV-2 in vitro with a median effective concentration (EC50) of 4.11 μM.22 An exploratory randomized, placebo-controlled clinical trial showed that monotherapy arbidol presents minor benefit for ameliorating the clinical outcome of hospitalized patients with COVID-19.23 A retrospective cohort study found that arbidol combined with lopinavir/ritonavir (LPV/r) treatment might delay the progression of lung lesions and decrease the viral load of COVID-19 more than LPV/r only.24
Table 1.
Properties of small molecule inhibitors approved or evaluated in clinical trials for COVID-19 therapy: virus-targeted inhibitors.
| Chemical structure | Generic name (trade name) | Code name | Molecular formula | Targets | Effective spectrum | Highest phase | Corporation or trial originator |
|---|---|---|---|---|---|---|---|
|
Umifenovir (Arbidol) | – | C22H25BrN2O3S | Spike (S) protein | SARS-CoV-2, SARS-CoV, Influenza virus | Phase IV | Pharmstandard |

|
Remdesivir (Veklury) | GS-5734 | C27H35N6O8P | RdRp | Ebola virus, MERS-CoV, SARS-CoV-2 | Approved in the USA and Japan | Gilead Sciences/Dr Reddy's Laboratories |

|
Favipiravir (Avigan) | T-705 | C5H4FN3O2 | RdRp | Ebola virus, Influenza A, Influenza B, SARS-CoV-2 | Approved in China and EUA in several countries | Fujifilm Toyama Chemical/Hisun Pharmaceutical |

|
Molnupiravir (Lagevrio) | EIDD-2801 MK-4482 | C13H19N3O7 | RdRp | Ebola virus, Chikungunya virus, Influenza viral, MERS-CoV, SARS-CoV-2 | Approved in the UK and EUA in the USA | Merck, Sharp and Dohme (MSD)/ Ridgeback Biotherapeutics |

|
– | VV116 JT001 | C24H31O7N5DBr | RdRp | SARS-CoV-2 | Phase III | Junshi Biosciences/ Vigonvita Life Sciences |

|
Ribavirin/Tribavirin (Rebetol/Copegus) | ICN-1229 RG-964 | C8H12N4O5 | RdRp | Hepatitis C, Hepatitis E, Adenovirus, Papilloma virus, Respiratory syncytial virus, Influenza A, SARS-CoV-2, SARS-CoV | Phase III | Mansoura University (originator) |

|
Bemnifosbuvir hemisulfate | AT-527 RG-6422 | C24H35FN7O11PS | RdRp | Hepatitis C, SARS-CoV-2 | Phase II | Roche/Atea Pharmaceuticals |

|
Azvudine | FNC RO-0622 | C9H11FN6O4 | RdRp | HIV, SARS-CoV-2 | Phase III | Genuine Biotech |

|
Ivermectin | MK-933 | C48H74O14 | 3CLpro | Flavivirus, HIV, Dengue virus, Influenza virus, SARS-CoV-2 | Phase IV | Multiple originators |

|
Lopinavir | ABT-378 | C37H48N4O5 | 3CLpro | HIV, Papilloma virus, HCoV-229E, MERS-CoV, SARS-CoV, SARS-CoV-2 | Phase IV | Beni-Suef University (originator) |

|
Ritonavir | ABT 538 RTV NOR-VIR | C37H48N6O5S2 | 3CLpro | HIV, MERS-CoV, SARS-CoV-2 | Phase IV | Multiple originators |

|
Darunavir | TMC114 UIC-94017 | C27H37N3O7S | 3CLpro | HIV, SARS-CoV-2 | Phase III | Multiple originators |

|
Atazanavir | BMS-232632 | C38H52N6O7 | 3CLpro | HIV, Hepatitis B, Hepatitis C, SARS-CoV-2 | Phase II/III | NeuroActiva |
|
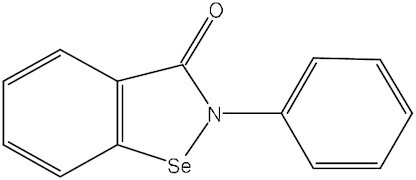
Ebselen (Harmokisane) | DR-3305 PZ-51 SPI-1005 | C13H9NOSe | 3CLpro, PLpro | SARS-CoV-2 | Phase II | Sound Pharmaceuticals |
|
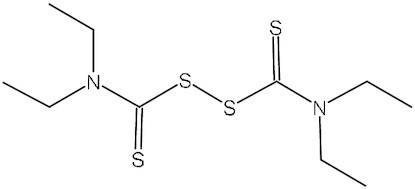
Disulfiram | TETD | C10H20N2S4 | 3CLpro | MERS-CoV, SARS-CoV | Phase II | ETICA |

|
Lufotrelvir | PF-07304814 | C24H33N4O9P | 3CLpro | SARS-CoV-2 | Phase I | Pfizer |

|
Paxlovid (Nirmatrelvir-Ritonavir) | PF-07321332 | C23H32F3N5O4 | 3CLpro | SARS-CoV-2 | Approved in the USA and EUA in multiple countries | Pfizer |

|
Dalcetrapib | JTT-705 RG-1658 | C23H35NO2S | 3CLpro | SARS-CoV-2 | Phase II | DalCor Pharmaceuticals |
| Undisclosed | Tollovir | NLC-V | – | 3CLpro | SARS-CoV-2 | Phase II | Todos Medical |
| Undisclosed | Pentarlandir | SNB-011 | – | 3CLpro, TMPRSS2 | SARS-CoV-2 | Phase II | SyneuRx International (Taiwan) Corp |
| Undisclosed | ASC09F (ASC09- Ritonavir) | ASC09 | – | 3CLpro | HIV, SARS-CoV-2 | Phase III | Ascletis Pharma |

|
– | S-217622 | C22H17O2ClF3N9 | 3CLpro | SARS-CoV-2 | Phase III | Shionogi |
| Undisclosed | – | PBI-0451 | – | 3CLpro | SARS-CoV-2 | Phase I | Pardes Biosciences |

|
– | DC-402234 FB-2001 | C26H36N4O4 | 3CLpro | SARS-CoV-2 | Phase I/II | Frontier Biotechnologies |

|
Isotretinoin/ 13-cis-Retinoic acid | – | C20H28O2 | PLpro | SARS-CoV-2 | Phase IV | Multiple originators |

|
Clofazimine | NSC-141046 | C27H22Cl2N4 | Helicase, Spike (S) protein | Leprosy, Tuberculosis, MERS-CoV, SARS-CoV-2 | Phase II | The University of Hong Kong (originator) |
Drug-like compound DRI-C23041 targeted the viral attachment stage and blocked the S protein of SARS-CoV-2 from interacting with ACE2 in vitro.25 Protein thermal shift assay indicated that DRI-C23041 selectively binds to the S protein of SARS-CoV-2 but not ACE2. DRI-C23041 inhibited the infection of SARS-CoV-2 pseudo-viruses into cells expressing ACE2 with low micromolar activity.
Clofazimine, an FDA-approved anti-leprosy and anti-tuberculosis drug, has shown antiviral activity against SARS-CoV-2 with a median inhibitory concentration (IC50) of 310 nM in vitro.26 Clofazimine displayed broad-spectrum anti-coronavirus efficacy. It could suppress both SARS-CoV-2 and MERS-CoV replication in ex vivo lung models.27 In a mechanistic study, clofazimine was shown to inhibit membrane fusion mediated by the SARS-CoV-2 S protein. A combination of low-dose remdesivir and clofazimine produced antiviral synergy in vitro and in vivo, and effectively reduced viral load in the lung.27
Coronavirus non-structural protein inhibitors
RNA-dependent RNA polymerase inhibitors
RNA-dependent RNA polymerase (RdRp), also known as non-structural protein 12 (nsp12), is an important enzyme regulating the replication and transcription of viral RNA during infection.28 It catalyzes the synthesis of RNA strands with the assistance of cofactors nsp7 and nsp8. nsp12, nsp7, and nsp8 constitute the minimal core for the replication of virus RNA.29 The structure and function of RdRp is conserved among RNA viruses. Therefore, using RdRp as an anti-RNA virus target is beneficial for the development of wide-spectrum antiviral agents and for the repurposing of existing RdRp inhibitors. Rao's research team reported the structure of the SARS-CoV-2 RdRp complex,30 and SARS-CoV-2 RdRp has 96% sequence identity with that of SARS-CoV, which remarkably promotes structure-based drug design against RdRp and drug repurposing for combating SARS-CoV-2 infection.
There are two types of RdRp inhibitors: non-nucleoside analog and nucleoside analog. The former lacks activity on other types or subtypes of viruses and is prone to drug resistance due to the non-conservation and structural variability of adjacent allosteric sites, which limits their application, particularly in the repurposing of such drugs. The latter is one of the main focuses of anti-SARS-CoV-2 drug research. Currently, three nucleoside RdRp inhibitors have been approved for the treatment of SARS-CoV-2 infection, including remdesivir (approved by the US FDA), favipiravir (approved by the National Medical Products Administration (NMPA) of China), and molnupiravir (approved by the Medicines Regulatory Agency in the UK) (Table 1). Remdesivir, a prodrug of the adenosine analog, was given emergency use authorization (EUA) for COVID-19 therapy by the FDA due to its positive outcomes in a clinical trial conducted in hospitalized adult patients with COVID-19 (NCT04280705). In this double-blind randomized trial, the median recovery time was 10 days in 541 remdesivir-treated subjects, as compared with 15 days in 521 patients treated with placebo.31 However, in the WHO-funded international, randomized trial (NCT04315948), remdesivir had little effect on hospitalized patients with COVID-19, as indicated by ventilation initiation, hospitalization duration, and mortality.32 Therefore, WHO recommends against the use of remdesivir in hospitalized COVID-19 patients. In a recent study conducted in outpatients at high risk of COVID-19 progression (NCT04501952), ramdesivir was reported to reduce the risk of hospitalization or death by 87% compared with placebo.33 Moreover, the combination of remdesivir with baricitinib was more effective in expediting improvement of clinical status and reducing recovery time in hospitalized adults with COVID-19, as compared with remdesivir alone.34 VV116, an orally administered remdesivir derivative, showed excellent oral absorption and efficacy in blocking SARS-CoV-2 replication (Table 1).35 It was reported to have potent inhibitory effects against a panel of SARS-CoV-2 variants, including Alpha, Beta, Delta, and Omicron.36 The results of three phase I studies showed that VV116 had satisfactory safety, tolerability, and pharmacokinetic properties in healthy subjects,36 which pushed it into phase II/III clinical trials (NCT05242042, NCT05279235). Noteworthily, this prodrug has been granted emergency authorization for the treatment of COVID-19 in Uzbekistan. Favipiravir, an FDA-approved anti-influenza guanosine analog prodrug, is also a broad-spectrum anti-RNA virus agent. In a nonrandomized, before–after controlled study, favipiravir-treated COVID-19 patients showed a shorter viral clearance median time and significant improvement in chest computed tomography (CT).37 In a phase III trial conducted in COVID-19 subjects, the median time to clinical cure for favipiravir and supportive care was 3 days and 5 days, respectively, suggesting a potential benefit of favipiravir for patients with mild to moderate COVID-19.38 Nevertheless, the efficacy of favipiravir for COVID-19 therapy has also been challenged by the results of some other clinical trials.39,40 Consequently, large-scale, multicenter, randomized clinical trials are still needed to further verify the effectiveness of favipiravir as a therapeutic agent against COVID-19. Molnupiravir, developed by Merck, Sharp and Dohme (MSD) and Ridgeback Biotherapeutics, is an orally bioavailable prodrug of EIDD-1931 (a cytidine analog).41 It has wide-spectrum activity against influenza viruses and various coronaviruses, including SARS-CoV-2.42 Molnupiravir was well tolerated based on the results of two phase I trials.43,44 The safety, tolerability, and efficacy of molnupiravir in symptomatic adult outpatients with COVID-19 have been evaluated in a phase II study (NCT04405570), but no results have been disclosed. Meanwhile, the interim results of a phase III study showed that molnupiravir decreased the risk of hospitalization or death by ∼50% in mild to moderate COVID-19 patients.45 On 4 November 2021, the UK's Medicines Regulatory Agency issued temporary authorization of molnupiravir for the treatment of adults with mild to moderate COVID-19 and at least one risk factor for severe illness. This is also the first approved oral anti-SARS-CoV-2 agent in the world. Notably, molnupiravir could effectively inhibit the replication of SARS-CoV-2 variants (B.1.1.7 and B.1.351) in hamster infection models, thus it may have therapeutic potential against current or future emerging variants that may influence the efficacy of vaccines and monoclonal antibodies.46 Ribavirin is also a guanosine analog prodrug and has been approved for the treatment of hepatitis C viral or respiratory syncytial virus infection (Table 1).47 Hung et al. evaluated the combination of interferon beta-1b, ribavirin, and LPV/r in the treatment of hospitalized COVID-19 patients. The results indicated that triple antiviral therapy was well tolerated and superior to LPV/r in reducing virus shedding time, alleviating symptoms, and facilitating discharge of COVID-19 patients. However, due to the limitations of this trial, it is still necessary to carry out further research on dual antiviral treatment using interferon beta-1b as a backbone therapy.48 Bemnifosbuvir (AT-527) is a novel optimized guanosine analog prodrug developed as a drug candidate for combating hepatitis C virus infection (Table 1). It is also potent in inhibiting SARS-CoV-2 replication in biological tests.49 Phase III trials are being conducted to evaluate the effects of AT-527 in non-hospitalized adult and adolescent participants with mild or moderate COVID-19 (NCT05126576, NCT04889040). Azvudine, a nucleoside drug that inhibits HIV-1 RdRp, has been approved for HIV infection prevention and treatment (Table 1). It demonstrated potent and broad-spectrum effects against multiple viruses, including SARS-CoV-2 and HCoV-OC43.50 The repurposing use of azvudine in treating COVID-19 was evaluated in animal models and patients, and it could cure COVID-19 through its antiviral activity concentrated in the thymus, which prevents the host immune system from being attacked by SARS-CoV-2 and promotes T-cell immune function.50,51 This mechanism is unique in known RdRp inhibitors. Large-scale, randomized, placebo-controlled phase III trials are being performed to assess the effects of azvudine for COVID-19 therapy (NCT04668235, NCT05033145). In addition, some other RdRp inhibitors, such as galidesivir and penciclovir, have also been reported to be potent in combating SARS-CoV-2 in vitro,52,53 but no related clinical trials have been executed or continued up to now.
3-Chymotrypsin-like protease (Mpro or 3CLpro) inhibitors
nsp5, also referred to as 3-chymotrypsin-like protease (3CLpro) or Mpro, is a 33.8-kDa cysteine protease. It regulates the cleavage of pp1a and pp1ab (overlapping polyproteins) into 16 mature nsps (Fig. 1), which then form replication–transcription complexes for the synthesis of viral RNA.54 The crystal structure of SARS-CoV-2 3CLpro (PDB code: 6LU7) was rapidly solved by Jin et al. after the COVID-19 outbreak.55 It is a dimer structure composed of two monomers and each of them has three domains: domain I (residues 8–101), domain II (residues 102–184), and domain III (201–303). SARS-CoV-2 3CLpro has a Cys-His catalytic dyad, and the substrate-binding site is located in a cleft between domains I and II. This structural feature is similar to those of other coronavirus 3CLpros.56,57 Furthermore, SARS-CoV-2 3CLpro shares 96% sequence identity with SARS-CoV 3CLpro.58 The highly conserved structure of 3CLpro, together with the absence of a closely related human homolog, makes it a promising target for developing anti-coronavirus drugs.
To discover potential SARS-CoV-2 3CLpro inhibitors, Mody et al. screened FDA-approved drugs for their repurposing use in COVID-19 treatment using computational molecular modeling. In all, 47 drugs with a high score were tested for inhibitory activity against 3CLpro enzyme in vitro. Among them, micafungin, boceprevir, ombitasvir, paritaprevir, and tipranavir showed partial inhibition, while ivermectin (Table 1) could inhibit SARS-CoV-2 3CLpro activity by >85%.59 The efficacy of these potential 3CLpro inhibitors in the treatment of COVID-19 needs further evaluation in clinical trials. Lopinavir and ritonavir are 3CLpro inhibitors earlier evaluated clinically for COVID-19 therapy. Lopinavir combined with ritonavir has been approved for treating HIV infection.60 They also have inhibitory activity against MERS-CoV and SARS-CoV (Table 1).61,62 On 18 January 2020, an open-label, randomized controlled trial (RCT) was carried out to evaluate the efficacy of lopinavir combined with ritonavir in China. A total of 199 adult patients with severe COVID-19 were enrolled in this study, but no benefit was achieved with the combination therapy.63 Further large-scale clinical trials also found no significant differences in efficacy between LPV/r-treated COVID-19 patients and patients receiving usual care or placebo.64,65 Given the similar mechanism with lopinavir in inhibiting HIV replication, darunavir, another 3CLpro inhibitor in combination with a CYP3A inhibitor cobicistat as a pharmaco-enhancer, was also assessed for COVID-19 treatment (Table 1). This combination therapy was well tolerated, but the currently reported efficacy in COVID-19 patients is unsatisfactory.66,67 Atazanavir is another HIV 3CLpro-targeting agent used for COVID‐19 therapy (Table 1). Computer-aided drug screening found that it had the potential to inhibit SARS-CoV-2 3CLpro,68,69 and this result was confirmed in an in vitro study, in which atazanavir suppressed the replication of SARS-CoV-2 alone or in combination with ritonavir in both A549 and Vero cells.70 A clinical study suggested that atazanavir combined with dolutegravir might have an advantage over the LPV/r regimen for COVID-19 treatment, and atazanavir/dolutegravir could be an alternative strategy beyond standard care.71 Moreover, Jin et al. not only resolved the crystal structure of SARS-CoV-2 3CLpro, but also identified seven promising 3CLpro inhibitors, including ebselen, shikonin, carmofur, disulfiram, tideglusib, TDZD-8, and PX-12, using a combination of structure-based virtual and high-throughput screening methods. These compounds inhibited 3CLpro with IC50 values ranging from 0.67 to 21.4 μM. Of these, ebselen has the strongest inhibition of 3CLpro (IC50: 0.67 μM).55 Currently, the efficacy and safety of ebselen and disulfiram in patients with moderate or severe COVID-19 are being evaluated in phase II studies (NCT04484025, NCT04483973, and NCT04485130) (Table 1). Pfizer is developing two 3CLpro inhibitors for COVID-19 treatment: an intravenous drug PF-07304814 and an oral drug PF-07321332 (nirmatrelvir) (Table 1). PF-07304814 is the phosphate prodrug of PF-00835231, a drug candidate against SARS-CoV.72 A phase 1b study has been conducted to evaluate its safety, tolerability, and pharmacokinetics in hospitalized patients with COVID-19 (NCT04535167) (no relevant data was reported), and a phase III trial involving its efficacy in COVID-19 treatment is currently recruiting patients (NCT04501978). PF-07321332 is an orally bioavailable 3CLpro inhibitor with broad activity against all coronaviruses, including alpha-coronaviruses (229E and NL63) and beta-coronaviruses (SARS-CoV, MERS, SARS-CoV-2, OC43, and HKU1).73,74 In an in vivo test conducted in a SARS-CoV-2 MA10 mouse model, PF-07321332 significantly decreased virus levels and protected the lung tissue of mice from damage by virus infection.74 A randomized, double-blind clinical trial has been conducted to assess the safety, tolerability, and pharmacokinetics of PF-07321332 as a single drug or in combination with ritonavir in healthy participants (NCT04756531). PF-07321332 was well tolerated, and showed a prominently increased plasma concentration when combined with ritonavir, which could slow down hepatic metabolism of PF-07321332.74 The lastest phase II/III clinical trial results (NCT04960202) showed that treatment with PF-07321332 plus ritonavir reduced COVID-19-related hospitalization or death by 89%, without significant safety concerns.75 This combination therapy has been developed into a product by Pfizer, named Paxlovid (PF-07321332 tablet and ritonavir tablet). On 22 December 2021, Paxlovid received its first EUA for COVID-19 therapy in the USA. Dalcetrapib, a cholesteryl ester transfer protein inhibitor, used to be a drug candidate for patients with stable coronary heart disease (Table 1). Niesor et al. identified dalcetrapib as a 3CLpro inhibitor, and it could effectively restrain the activity of 3CLpro with an IC50 value of 14.4 ± 3.3 μM and the replication of SARS-CoV-2 with an EC50 value of 17.5 ± 3.5 μM.76 A placebo-controlled phase II study is being performed to assess the safety and efficacy of dalcetrapib in COVID-19 patients (NCT04676867). Hamed et al. performed molecular docking of 20 FDA-approved β-blockers and found carvedilol, nebivolol, and bisoprolol as potential 3CLpro inhibitors against SARS-CoV-2.77 El-Masry et al. have synthesized a series of oxadiazoles carrying an isatin moiety, one of which has shown moderate activity against 3CLpro with an IC50 of 16.6 μM.78 Additionally, several novel 3CLpro inhibitors have been developed and evaluated in clinical studies for their safety, tolerability, and efficacy in COVID-19 patients, such as tollovir (NCT05226767), pentarlandir (NCT04911777), ASC09F (NCT04261270), S-217622 (NCT05305547), PBI-0451 (NCT05011812), and FB2001 (NCT05415241), but few relevant data have been reported so far (Table 1).
Papain-like protease (PLpro) inhibitors
PLpro is the protease domain of nsp3, the largest individual protein encoded by the coronavirus genome. Coronavirus PLpro manages cleavage of nsp1–3 at the N-terminal region of polyproteins, which leads to the release of nsp1–3 from virus polyproteins; this process is necessary for viral replication.79 Moreover, PLpro could also strip ubiquitin and ubiquitin-like protein ISG15 from the host cell to help coronavirus evade the innate immune response of the host.79,80 Hence, targeting coronavirus PLpro could exert antiviral effects through multiple mechanisms. The crystal structure of SARS-CoV-2 PLpro (PDB code: 6W9C) has been resolved for drug design, and there is 83% sequence identity between the PLpro of SARS-CoV-2 and SARS-CoV.81 Based on this structure, researchers revealed the molecular mechanism of PLpro substrate specificity, and then designed and biochemically characterized two selective PLpro inhibitors VIR250 and VIR251.81 Furthermore, they resolved the crystal structures of VIR250 and VIR251 in complex with SARS-CoV-2 PLpro, and revealed their mechanisms of action, providing a structural basis for drug repurposing or the development of more effective agents against SARS-CoV-2 PLpro.81 To discover PLpro inhibitors in a repurposing strategy, Klemm et al. synthesized racemic forms of rac5c, rac3j, and rac3k, three inhibitors of SARS-CoV PLpro. These compounds exhibited sub-micromolar activity against SARS-CoV PLpro, and effectively rescued the cytopathic effect in SARS-CoV-2-infected Vero E6 cells.82,83 Kuo et al. established 3CLpro and PLpro assay platforms to screen SARS-CoV-2 protease inhibitors. They screened thousands of FDA-approved drugs and identified 36 drugs as PLpro inhibitors and 12 drugs as 3CLpro inhibitors. Among them, levothyroxine and manidipine-2HCl were dual inhibitors with activity against both 3CLpro and PLproin vitro.84 In addition, there is still much research concerning SARS-CoV-2 PLpro inhibitors.85,86 However, most of them were tested in silico or in vitro, and few of them have entered clinical trials, except for several multi-target inhibitors, such as ebselen (NCT04483973, NCT04484025) and isotretinoin (NCT04361422, NCT04353180) (Table 1) which were reported to have anti-SARS-CoV-2 PLpro effects.87–89
Helicase inhibitors
Helicase (nsp13) is responsible for the replication and transcription of the SARS-CoV-2 genome. The C-terminal of helicase forms a conserved helicase domain and participates in unraveling double-stranded DNA. nsp13 forms a stable complex with RdRp and is a target for treating COVID-19 disease.90 Clofazimine has been found to inhibit the unwinding activity of helicase on double-stranded DNA and RNA substrates (Table 1).27 However, one of the challenges in targeting helicase is the comparatively low selectivity of helicase inhibitors.91
nsp14 inhibitors
nsp14 of SARS-CoV-2 is a bifunctional enzyme, consisting of N-terminal exoribonuclease (ExoN) and C-terminal guanosine-N7 methyltransferase (MTase). ExoN contributes to the drug resistance of RdRp inhibitors, while the MTase is important for the stability and translation of mRNA.92 nsp14 MTase is a promising target for drug discovery against SARS-CoV-2. It catalyzes the transfer of the methyl group of S-adenosyl-L-methionine (SAM), yielding the methylated capped viral RNA and S-adenosyl-L-homocysteine (SAH). Basu et al. conducted an in vitro high-throughput biochemical assay for screening inhibitors of the MTase of nsp14. Potential nsp14 inhibitors, including PF-03884528, lomeguatrib, and trifluperidol, were identified to effectively block the viral replication of SARS-CoV-2 in cell-based assays at low micromolar concentration.93 When combined with remdesivir, current nsp14 inhibitors had an increased inhibitory effect in reducing the viral load of SARS-CoV-2. Bobileva et al. have developed potential nsp14 MTase inhibitors against SARS-CoV-2 through bioisosteric substitution of the substrate SAM. Of all the compounds synthesized, compound 2a showed the optimum activity towards nsp14 with an IC50 value of 8 nM, lower than that of the known pan-MTase inhibitor sinefungin.94 But the compounds lack selectivity for human glycine N-methyltransferase. Additionally, Otava et al. identified that the modification at the adenine nucleobase of SAH derivatives could produce nsp14 MTase inhibitors.95 Some designed compounds, such as compound 16, have nanomolar activity, along with superior selectivity on human counterpart mRNA cap guanine-N7 methyltransferase. In summary, these results suggested that the MTase activity of nsp14 is indispensable for SARS-CoV-2 replication.
nsp 16 inhibitors
nsp 16, also named 2'-O-methyltransferase (2ʹ-O-MTase), is an important component responsible for SARS-CoV-2 replication. It catalyzes the methylation at the 5'-terminal of viral mRNA molecule which is referred to as mRNA cap structure. This cap formation resembles the host cellular mRNA and prevents the recognition of viral mRNA by host immune systems. Thus, nsp16 is an attractive SARS-CoV-2 target for its viral mRNA protection role. In silico fragment-based screening and subsequent Molecular Mechanics-Poisson Boltzman Surface Area (MM-PBSA) calculations have been used to identify several potential inhibitors of nsp16.96 Krafcikova et al. have shown that the pan-MTase inhibitor sinefungin binds to the SAM binding pocket of nsp16 of SARS-CoV-2 and inhibits MTase activity.97
Host-targeted inhibitors
Host protease inhibitors
Proteolytic cleavage is thought to promote virus–cell membrane fusion and SARS-CoV-2 entry into host target cells. S protein is cleaved at the S1/S2 junction and S2' site by host cell transmembrane serine protease 2 (TMPRSS2) and furin protease. The inhibitors of TMPRSS2, such as camostat and nafamostat, can be used as possible antiviral agents against SARS-CoV-2 (Table 2). The clinically proven serine protease inhibitor camostat, which inhibits TMPRSS2 activity, significantly reduced S-driven cell entry of SARS-2-CoV.11 Camostat is then converted into the active metabolite 4-(4-guanidinobenzoyloxy) phenylacetic acid (GBPA, FOY-251) in animal models, which inhibits SARS-CoV-2 entry with almost similar efficacy to camostat.98 In a phase 2 double-blind, randomized, placebo-controlled trial, administration of camostat orally did not significantly improve many clinical outcomes of hospitalized COVID-19 patients.99 In the camostat arm, the proportion of patients requiring mechanical ventilation or dying is 10%, but is 18% in the placebo arm. Nafamostat, another broad-spectrum serine protease inhibitor, could inhibit TMPRSS2 activity. Nafamostat potently prevented S protein-mediated membrane fusion and blocked SARS-CoV-2 infection with EC50 22.50 μM.100 In another in vitro study, nafamostat has been shown to inhibit SARS-CoV-2 infection in a cell-type dependent manner.101 Nafamostat exerted augmented antiviral activity in human lung tissue ex vivo compared with camostat and GBPA.102
Table 2.
Properties of small molecule inhibitors approved or evaluated in clinical trials for COVID-19 therapy: host-targeted inhibitors and immune response modulators.
| Chemical structure | Generic name (trade name) | Code name | Molecular formula | Targets | Effective spectrum | Highest phase | Corporation or trial originator |
|---|---|---|---|---|---|---|---|

|
Camostat mesylate (Foipan) | FOY305 FOY-S980 | C20H22N4O5. CH4O3S | TMPRSS2 | SARS-CoV, MERS-CoV, HCoV-229E, SARS-CoV-2 | Phase III | Ono Pharmaceutical |

|
Nafamostat mesylate (Futhan) | DS-2319 FUT-175 | C19H17N5O2. 2CH4O3S | TMPRSS2 | SARS-CoV-2, MERS-CoV | Phase III | Multiple originators |

|
Proxalutamide | GT0918 | C24H19F4N5O2S | Host protease inhibitor (androgen receptor antagonist) | SARS-CoV-2 | Phase III | Kintor Pharmaceutical |

|
Chloroquine | – | C18H26ClN3 | Endosome acidification agent | HCoV-229E, HCoV-OC43, HIV, Ebola virus, SARS-CoV, MERS-CoV, SARS-CoV-2 | EUA by FDA at early outbreak | Multiple originators |
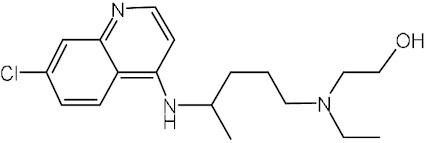
|
Hydroxychloroquine | – | C18H26ClN3O | Endosome acidification agent | SARS-CoV, MERS-CoV, SARS-CoV-2 | Phase IV | Multiple originators |

|
Mefloquine | – | C17H16F6N2O | Endosome acidification agent | MERS-CoV, SARS-CoV | Phase II/III | Multiple originators |

|
Apilimod | STA-5326 | C23H26N6O2 | Host endosomal inhibitor (PIKfyve kinase inhibitor) | Ebola virus, Lassa virus, Marburg virus, SARS-CoV-2 | Phase II | AI Therapeutics |

|
Dexamethasone (Hexadecadrol, Prednisolone F) | AR-1105 BDI-1 ISV-305 | C22H29FO5 | Immune response modulator | SARS-CoV-2 | Phase IV | Multiple originators |

|
Baricitinib (Olumiant) | LY3009104 INCB028050 | C16H17N7O2S | Immune response modulator (JAK inhibitor) | SARS-CoV-2 | EUA in the USA | Eli Lilly and Company/Incyte |

|
Ruxolitinib (Jakfi) | INCB18424 | C17H18N6 | Immune response modulator (JAK inhibitor) | SARS-CoV-2 | Phase III | Novartis/Incyte |

|
Tofacitinib | CP-690550 | C16H20N6O | Immune response modulator (JAK inhibitor) | SARS-CoV-2 | Phase III | Pfizer |

|
Nitazoxanide (Alinia/Colufase/Cryptaz) | NT-300 NTZ PH-5776 | C12H9N3O5S | Immune response modulator (IFN inducer) | Influenza virus, Hepatitis B, Hepatitis C, HIV, SARS-CoV, MERS-CoV, SARS-CoV-2 | Phase IV | Multiple originators |
Besides, androgen is the only known regulator of TMPRSS2, and targeting TMPRSS2 transcription with antiandrogens can affect the entry of viruses into host cells.103 Notably, androgen was reported to be highly expressed in elderly males and smokers (>70 years old), and elevated androgen level was closely related to higher disease severity in men.104,105 Therefore, older men are more likely than women to be infected with SARS-CoV-2 and develop severe COVID-19. Despite multifactorial mechanisms involved in the gender disparities observed in COVID-19 outcomes, androgen signaling plays a pivotal role in increasing COVID-19 susceptibility and severity in men. This is not only due to the regulatory effects of androgen on TMPRSS2, but also because of its suppressive role in the immune response.106 Proxalutamide (GT0918), a second-generation androgen receptor inhibitor, was originally developed as an anti-prostate cancer agent.107 The therapeutic effects of proxalutamide on COVID-19 have been evaluated in multiple clinical trials (Table 2). Compared to usual care, it could reduce by 91% the hospitalization rate in male outpatients with COVID-19.108 In a placebo-controlled, two-arm, randomized clinical study, proxalutamide significantly increased recovery rate, shortened hospital stay, and reduced mortality rate in hospitalized COVID-19 patients in both arms.109
Human cathepsin activity is typically required for proper proteolytic processing of the S protein of SARS-CoV-2 during infection. Riva et al. screened the FDA-approved or clinical-stage small molecular library. They identified four cathepsin protease inhibitors VBY-825, Z LVG CHN2, MDL-28170, and ONO 5334 that inhibited viral replication of SARS-CoV-2 with EC50 < 500 nM in Vero cells in a dose–response relationship.26 Rodon et al. screened existing approved drugs and found that cathepsin inhibitor MDL-28170 had antiviral efficacy to halt SARS-CoV-2 replication in vitro.110 Calpain inhibitors II and XII inhibit the activities of both cathepsins and SARS-CoV-2 Mpro with low micromolar IC50 values.111 One of the advantages of the dual inhibitors is that they can target both the human and viral proteases.
E64-d, a broad endosomal cysteine proteases cathepsin inhibitor, showed inhibitory activity against SARS-CoV-2 infection.110 However, directed expression of TMPRSS2 rescued the S-driven entry of SARS-CoV-2 from inhibition by E-64d. When combined with the TMPRSS2 inhibitor camostat, E-64d could interfere with SARS-CoV-2 entry into host cells more effectively,11,112 indicating that SARS-CoV-2 employs both TMPRSS2 and cathepsins for S protein priming. In pulmonary cells, the endosomal viral entry route is absent,113 thus camostat should be counted as the primary inhibitor to limit the entry of SARS-CoV-2 into pulmonary cells expressing TMPRSS2.11
Furin cleavage of S protein is essential for the viral replication of SARS-CoV-2. Furin inhibitors block the virus entry and are potential antiviral agents for SARS-CoV-2 infection. Two furin inhibitors, naphthofluorescein and decanoyl-RVKR-chloromethylketone (CMK), were shown to inhibit S protein processing and block SARS-CoV-2 infection in vitro.114 Although both are potential antiviral agents to control SARS-CoV-2 pathogenesis, they have shown distinct antiviral effects. The reason might be that the two inhibitors have different target specificity. Furin-mediated cleavage at the S1/S2 junction might promote subsequent TMPRSS2-dependent viral entry into target cells.11 Thus, inhibition of both TMPRSS2 and furin proteases might be required for tight blockade of SARS-CoV-2 entry.
Endosome acidification agents
Endosome and lysosome are potential host cell targets to combat SARS-CoV-2 infections. The inhibitors of lysosomal or endosomal function could become a notable therapy for improving COVID-19 treatment. Two interesting candidates are chloroquine and its derivative hydroxychloroquine, which are normally indicated for malaria or autoimmune diseases (Table 2). They are widely recommended for the potential treatment of COVID-19. Chloroquine and hydroxychloroquine prevent viral infection by increasing the endosomal pH. The elevated pH inhibits the hydrolytic activity of cathepsins. They have shown inhibitory activity against SARS-CoV-2 in vitro.100 However, although hydroxychloroquine and chloroquine could inhibit SARS-CoV-2 infection in monkey kidney cells, they did not hamper SARS-CoV-2 infection in lung cells expressing TMPRSS2.115 Neither hydroxychloroquine nor its combination with azithromycin showed a pronounced effect on viral load in a macaque model.113 Chloroquine and hydroxychloroquine might target a pathway for SARS-CoV-2 cellular entry that is not active in lung cells. This could explain why hydroxychloroquine in randomized clinical trials has failed to show a profound effect against SARS-CoV-2 infection.116 Additionally, concerns about their adverse cardiac effects including QT-interval prolongation have been raised and may limit their widespread use.117
In addition, four lysosomotropic compounds have been evaluated and found to block the cytopathic effect of SARS-CoV-2 in vitro, including hycanthone, clomipramine, ROC-325, and mefloquine (Table 2).118 These compounds increased lysosomal pH, prevented pseudotyped particle entry, and reduced viral titers in a tissue model. Ammonium chloride has been employed to elevate endosomal pH and thereby block endosomal cathepsins' activity, which strongly inhibited the S-driven cellular entry of SARS-CoV-2.11
Host endosomal inhibitors
Apilimod is a small molecular inhibitor of endosomal phosphatidylinositol-3-phosphate/phosphatidylinositol 5-kinase (PIKfyve kinase). It has demonstrated broad-spectrum antiviral activity and could efficiently inhibit the Marburg virus, Lassa virus, and Ebola virus in human cell lines. Consistent with these findings, apilimod was found to antagonize SARS-CoV-2 replication in human cells (Table 2).26,119,120 PIKfyve kinase resides principally in early endosomes and has a substantial role in the maintenance of the homeostasis of endomembrane. PIKfyve kinase phosphorylates phosphatidylinositol-3-phosphate (PI3P) to produce phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2). The inhibition of PIKfyve kinase potently blocks the intracellular dynamic trafficking of pathogens among the subcompartments of the endosomal system. Apilimod exhibited a desirable safety profile in humans, indicating that therapeutic dosing may be achievable in patients at concentrations with antiviral efficacy.119
High-throughput drug repurposing
High-throughput screenings have identified several drugs that could be repurposed to inhibit SARS-CoV-2 in vitro. Many inhibitory compounds have favorable safety profiles and may act by influencing the host cellular signaling or metabolism pathways. However, the detailed molecular mechanism of action for the antiviral activity remains unknown (Supplementary Table 1, see online supplementary material). Bojkova et al. have identified that SARS-CoV-2 reshaped and depended on several host cellular pathways, such as translation, spliceosome, nucleic acid metabolism, protein homeostasis, and carbon metabolism.121 The replication of SARS-CoV-2 is assumed to be sensitive to the perturbation of these pathways. Pladienolide B, a spliceosome inhibitor that targets splicing factor SF3B1, inhibited SARS-CoV-2 replication with an IC50 of 7 nM in Caco-2 cells. A small-molecule inhibitor of p97 ATPase, NMS-873, could effectively perturb host cell proteostasis and inhibit SARS-CoV-2 replication with an IC50 of 25 nM. Two translation inhibitors emetine (a 40S ribosomal protein S14 inhibitor) and cycloheximide (a translation elongation inhibitor) dramatically inhibited the replication of SARS-CoV-2 in human Caco-2 cells with IC50 values of 0.17 and 0.47 μM, respectively. Ribavirin, which interferes with nucleic acid metabolism, could inhibit SARS-CoV-2 replication at low micromolar concentrations. The above results revealed essential host cellular pathways for SARS-CoV-2 amplification that might be potential therapeutic targets.
Two independent studies have reported a marine-derived cyclic peptide plitidepsin against SARS-CoV-2 in vitro.110,122 The antiviral activity of plitidepsin against SARS-CoV-2 is mainly due to the inhibition of eukaryotic translation elongation factor 1A (eEF1A). The in vivo antiviral activity in SARS-CoV-2 infected mouse models of plitidepsin has been demonstrated with reduced viral replication in the lungs.122 In addition, Gordon et al. observed that a class of protein biogenesis inhibitors ternatin-4 and zotatifin could effectively reduce the viral infectivity of SARS-CoV-2.10 Zotatifin is a eukaryotic initiation factor 4A (eIF4A) inhibitor, and ternatin-4 is an eEF1A inhibitor. These data demonstrated that the rate of translation elongation is crucial for the proper production of viral proteins.
The inhibitors of sigma-1 and sigma-2 receptors (also known as TMEM97) have been found to exert antiviral activity against SARS-CoV-2.10 The inhibitors of sigma-1 and sigma-2 receptors include progesterone, PD-144418, siramesine, cloperastine, PB28, clemastine, and haloperidol. Their antiviral effect is probably related to the inhibition of Sigma receptors, not other aminergic receptors; however the detailed mechanism responsible is uncertain.
High-content screenings have found the antiparasitic compound niclosamide and the antibiotic salinomycin to be effective against SARS-CoV-2 with nanomolar potency.123 Niclosamide exerts its effects by suppressing the activity of transmembrane protein 16F (TMEM16F), which is a calcium-activated ion channel and regulates phospholipid scrambling. Treatment with either niclosamide or salinomycin inhibited SARS-CoV-2 spike-mediated syncytia formation and cell–cell fusion. A small phase 1 trial of inhalation and intranasal administration of niclosamide has found an acceptable safety and well-tolerated profile.124 It is a promising candidate for further clinical trials in the treatment of COVID-19.
Another antiparasitic compound, ivermectin, inhibited SARS-CoV-2 in Vero cells with an EC50 of 2 μM.125 Ivermectin was previously shown to have broad-spectrum in vitro antiviral activity. The activity might relate to its ability to inhibit importin α/β1 nuclear transport proteins in the host, which have been hijacked by viruses to import viral proteins and enhance infection.126 A retrospective study supported that ivermectin treatment was related to decreased mortality of hospitalized COVID-19 patients.127 However, a placebo-controlled randomized clinical trial revealed no significant virological and clinical improvement related to the receiving of ivermectin for treatment of COVID-19 patients, compared with placebo.128
By utilizing human pluripotent stem cells (hPSC-LOs), Han et al. developed a lung organoid drug screening model. They identified some SARS-CoV-2 entry inhibitors, including quinacrine dihydrochloride (QNHC) (EC50 = 2.83 μM), mycophenolic acid (MPA) (EC50 = 0.15 μM), and imatinib (EC50 = 4.86 μM).129 Surface plasmon resonance binding analysis suggested that both QNHC and imatinib interfere with ACE2. Treatment with MPA or QNHC lowers the expression levels of furin. This suggested that the organoid models developed can serve as disease models to study SARS-CoV-2 infection and provide valuable in vitro platforms for high-throughput drug screening.
Immune response modulators
At the late stage of SARS-CoV-2 infection, patients might develop life-threatening acute respiratory distress syndrome (ARDS). The associated severe clinical symptoms or death are mostly due to the uncontrolled antiviral inflammatory cell infiltration and cytokine storm.130 Immune response modulators such as cytokine inhibitors could help in managing the lung inflammation that causes severe disorders in cases of SARS-CoV-2 infection. Multiple anti-cytokine therapies have been evaluated for COVID-19 therapy in clinic and even approved in some countries, such as anakinra, tocilizumab, and sarilumab. Anakinra is a recombinant IL-1α/β receptor antagonist. Clinical benefit from anakinra treatment was found in the phase III SAVE-MORE randomized controlled trial, with reduced mortality and shorter hospital stay for COVID-19 patients.131 Sarilumab and tocilizumab alike are monoclonal antibodies against interleukin-6 (IL-6) receptor. Both of them were reported to improve COVID-19 outcomes, including survival,132–134 and were recommended by the WHO as a class of agent that is lifesaving in severely or critically ill patients with COVID-19. Moreover, high doses of corticosteroids have been traditionally considered effective in decreasing ARDS-induced multiple organ failure and mortality. Corticosteroids have been widely used to treat patients during outbreaks of SARS-CoV and MERS-CoV. However, using corticosteroids to treat COVID-19 patients remains debatable due to the adverse effects, including increased risk of secondary infection and delayed viral clearance.135 A recent large-scale randomized clinical trial named the RECOVERY project indicated that dexamethasone therapy reduces the mortality rate in COVID-19 patients who require ventilation or supplemental oxygen treatment (Table 2).136
Various cytokines bind type I and type II cytokine receptors and employ the Janus kinase (JAK) pathway to exert their effect. Inhibitors of the JAK family have attracted great attention to manage the cytokine storm in patients with severe COVID-19.137 JAK inhibitors, including baricitinib, ruxolitinib, and tofacitinib, could hinder the signaling of several cytokines involved in the pathogenesis of SARS-CoV-2 infections and the emergence of the cytokine storm (Table 2). Several clinical trials were initiated to examine the use of JAK inhibitors in patients with COVID-19. A non-randomization clinical trial provided preliminary evidence that baricitinib treatment achieved significant improvements in clinical characteristics and respiratory function parameters compared with controls.138 None of the patients in the baricitinib arm required intensive care unit support. One concern for using JAK inhibitors in COVID-19 patients is that the interference with JAK signaling might delay the virus clearance efficacy.137
Interferon (IFN) response represents the canonical host innate immune response to viral infections.47 IFN serves to promote antiviral restriction factors and inhibit the replication of viruses in vivo. Although the IFN response might be suppressed and antagonized by the accessory proteins of CoVs, the administration of recombinant IFN can enhance the antiviral response. Both SARS-CoV and MERS-CoV remain susceptible to the treatment of recombinant IFN.139 In an uncontrolled study of patients with SARS-CoV, combination therapy using corticosteroid and IFN was associated with better oxygen saturation and quite fast resolving of radiographic lung abnormalities rather than using corticosteroid alone.140 IFN has been proposed to be used to stimulate innate antiviral responses in SARS-CoV-2 infection patients. In an uncontrolled exploratory study, treatment with IFN-α2b significantly decreased the detectable virus’ duration time in the upper respiratory tract, whether or not in conjunction with arbidol.141 A retrospective study of COVID-19 patients treated with IFN-α alone or combined with LPV/r found similar results.142 In a prospective open-label randomized phase 2 trial, the triple combination of IFN-β1b with LPV/r and ribavirin significantly suppressed viral load and completely alleviated symptoms in a shorter time in COVID-19 patients.48
Nitazoxanide is another potential IFN inducer that has been applied in humans for the treatment of parasitic infections and has exhibited broad-spectrum antiviral activities (Table 2).143 Clinical trials for the treatment of viral infections have shown the good safety profile of nitazoxanide.144 A recent study revealed that nitazoxanide could inhibit SARS-CoV-2 at a micromolar concentration in vitro.100 The antiviral efficacy of nitazoxanide has been assessed in a placebo-controlled double-blind randomized clinical trial. The symptoms of COVID-19 patients did not differ between placebo and nitazoxanide groups, but early nitazoxanide therapy was safe and reduced viral load significantly.145 The results of the studies concerning IFN signaling suggested that the stage of the COVID-19 disease is a critical parameter underlying the treatment success or failure of the immune response modulators for SARS-CoV-2 infections.146
Concluding remarks: challenges and future perspectives
With the continuous development of vaccines against SARS-CoV-2, there is a realistic expectation that the pandemic caused by the virus will be under control. However, immunocompromised persons might respond poorly to vaccine immunization.147 Uncertainties about the emergence of immune escape variants mean that vaccine failure may occur even in immunocompetent individuals.148 Antiviral drugs are indispensable against future pandemic coronaviruses. At present, anti-COVID-19 drugs mainly include neutralizing antibodies and small molecules. Compared with macromolecule antibody agents, small molecule antiviral drugs have advantages in many aspects, including conquering the immune escape variants, pharmacokinetic properties, administration, cost, mass production, storage, and transportation. Several small molecule anti-SARS-CoV-2 agents have been approved recently (Table 1), particularly Paxlovid and molnupiravir, which effectively abate the risk of hospitalization or death of patients with COVID-19. Meanwhile, many small molecular anti-SARS-CoV-2 drugs are undergoing preclinical tests or clinical trials (Tables 1 and 2, and supplementary Table 1).
Despite the significant progress achieved, there are still some challenges that small molecular anti-SARS-CoV-2 agents face. The first and foremost is the efficacy issue. The early drug discovery strategy for combating SARS-CoV-2 was the massive screening of available drugs for repurposing. Researchers quickly identified potential anti-SARS-CoV-2 agents according to this strategy and advanced some of them to the clinic, such as remdesivir, favipiravir, and chloroquine. However, their efficacies were controversial. Although molnupiravir and Paxlovid bring hope to COVID-19 treatment and infection prevention, they cannot fully meet the clinical needs. For example, molnupiravir needs to be given as quickly as possible after infection with SARS-CoV-2, as late administration is less beneficial to patients. This requires frequent and effective diagnostic tests. Specifically, when to start dosing for asymptomatic patients is an issue.149 Meanwhile, the potency of momupiravir in individuals with breakthrough infection after vaccination remains to be assessed.150 Therefore, there is still a pressing need to develop novel anti-SARS-CoV-2 agents with structural diversity and different mechanisms to improve efficacy and meet distinct treatment needs.
Second, previous lessons remind us that RNA viruses have high mutation rates and can easily develop resistance under selection pressure via mutation of the pivotal targets. For SARS-CoV-2, resistance to small molecular antiviral drugs caused by target mutations is well known in the in vitro studies.151–153 Currently, very low levels of escape mutation were observed in patients treated with anti-SARS-CoV-2 small molecular drugs.154,155 This situation may be attributed to inadequate drug selectivity pressure due to short-term administration, the high genetic barrier these drugs construct to prevent viruses escaping, the broad-spectrum inhibitory effects of these agents on different SARS-CoV-2 variants, or the self-clearing ability of immunocompetent individuals against resistant virus.46,156,157 Nevertheless, with the extensive application of anti-SARS-CoV-2 agents and extension of monitoring time, resistance remains a non-negligible problem and will appear predictably. An effective strategy for conquering resistance is combination therapy, particularly the combination of agents with distinct molecular mechanisms. Numerous combination regimens have been studied but still need to be verified in clinical trials.158–160 Meanwhile, the possible side effects caused by combination therapy are another critical concern.
Third, the side effects of drugs need continuous attention, especially in some special populations. Molnupiravir is not recommended for use in patients under 18 years of age as it may influence the growth of bone and cartilage, in gravidas as it may cause fetal harm, and in those during breastfeeding as there is no sufficient data to support it.161 Similarly, the safety and efficacy of Paxlovid in a subset of patients are not yet known, including children, adolescents, pregnant and breastfeeding women, and patients with renal impairment .162 More clinical evidence is still needed to prove whether it is available to these patients. Notably, Paxlovid (nirmatelvir/ritonavir) is a compound preparation, which contains ritonavir, a cytochrome P450 (CYP450) inhibitor. Potential drug interactions may be very challenging to its clinical application. In a kidney transplant patient with SARS-CoV-2 infection, the drug interaction between tacrolimus and Paxlovid has been observed, which significantly increased the levels of tacrolimus and its metabolites, resulting in acute kidney injury. Tacrolimus can be metabolized by CYP3A4 enzyme, which has been inhibited by ritonavir.163 Moreover, Zhou et al. warned of the possible risk of host mutagenesis after treatment with molnupiravir because of the homogeneity of RNA and DNA precursors utilized by both virus and host.164 Mutagenic effects have occurred in mammalian cells, but its impact on the whole organism is still unknown.164 All of the above underscores the necessity of continuous monitoring of side effects of anti-SARS-2-CoV drugs.
In summary, fighting against COVID-19 requires a multi-pronged approach, including comprehensive utilization of diagnostic screening, vaccines, small molecules, and neutralizing antibodies. Vaccination is the best measure to prevent SARS-CoV-2 infection, while small molecules and neutralizing antibodies play important roles in managing treatment for vulnerable individuals. Antiviral small molecules, especially oral drugs, have irreplaceable advantages in patient compliance, which can greatly relieve the pressure on public medical resources. At present, the research and development of small molecule antiviral drugs are still in full swing. We believe we will eventually defeat COVID-19 with the availability of more drugs in the clinic and the application of comprehensive anti-COVID-19 strategies.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Research Fund of Science & Technology Department of Sichuan Province(Grants No. 2021YJ0134 and 2019YFS0514), the Fundamental Research Funds for the Central Universities (Grant No. ZYGX2019J106), and the National Natural Science Foundation of China (Grants No. 61876034 and 81472859).
Contributor Information
Lei Zhong, Department of Pharmacy, Personalized Drug Therapy Key Laboratory of Sichuan Province, Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu 610072, China.
Zhipeng Zhao, Department of Pharmacy, Personalized Drug Therapy Key Laboratory of Sichuan Province, Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu 610072, China.
Xuerun Peng, Department of Pharmacy, Personalized Drug Therapy Key Laboratory of Sichuan Province, Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu 610072, China.
Jun Zou, Center for Clinical Translational Innovations, Molecular Medicine Research Center, West China Biomedical Big Data Center, State Key Laboratory of Biotherapy, West China Hospital, West China School of Medicine, Sichuan University, Chengdu 610041, China.
Shengyong Yang, State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, West China School of Medicine, Sichuan University, Chengdu 610041, China.
Conflict of interest
None declared.
References
- 1. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–9.. 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ledford H. Hundreds of COVID trials could provide a deluge of new drugs. Nature. 2022;603:25–7.. 10.1038/d41586-022-00562-0. [DOI] [PubMed] [Google Scholar]
- 3. Callaway E. Are COVID surges becoming more predictable? New Omicron variants offer a hint. Nature. 2022;605:204–6.. 10.1038/d41586-022-01240-x. [DOI] [PubMed] [Google Scholar]
- 4. Ashour NA, Abo Elmaaty A, Sarhan AA, et al. A systematic review of the global intervention for SARS-CoV-2 combating: from drugs repurposing to molnupiravir approval. Drug Des Dev Ther. 2022;16:685–715.. 10.2147/DDDT.S354841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li G, De Clercq E.. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discovery. 2020;19:149–50.. 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 6. Liu C, Zhou Q, Li Y, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Central Science. 2020;6:315–31.. 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duan Y, Yao Y, Kumar SA, et al. Current and future therapeutical approaches for COVID-19. Drug Discovery Today. 2020;25:1545–52.. 10.1016/j.drudis.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet North Am Ed. 2020;395:565–74.. 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–54.. 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–68.. 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020:181;271. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–4.. 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–20.. 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 14. Walls AC, Park Y-J, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281. 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3.. 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894. 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai Y, Zhang J, Xiao T, et al. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369:1586–92.. 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xia S, Liu M, Wang C, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–55.. 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xia S, Zhu Y, Liu M, et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cellular & Molecular Immunology. 2020;17:765–7.. 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Vries RD, Schmitz KS, Bovier FT, et al. Intranasal fusion inhibitory lipopeptide prevents direct-contact SARS-CoV-2 transmission in ferrets. Science. 2021;371:1379–82.. 10.1126/science.abf4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang C, Pan X, Xu X, et al. Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein. Signal Transduction and Targeted Therapy. 2020;5:220. 10.1038/s41392-020-00325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Cao R, Zhang H, et al. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discovery. 2020;6:28. 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Xie Z, Lin W, et al. Efficacy and safety of Lopinavir/Ritonavir or Arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med. 2020;1: 105–13. 10.1016/j.medj.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: A retrospective cohort study. J Infect. 2020;81:e1–5.. 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bojadzic D, Alcazar O, Chen J, et al. Small-molecule inhibitors of the coronavirus spike: ACE2 protein-protein interaction as blockers of viral attachment and entry for SARS-CoV-2. ACS Infectious Diseases. 2021;7:1519–34.. 10.1021/acsinfecdis.1c00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riva L, Yuan S, Yin X, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586:113–9.. 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan S, Yin X, Meng X, et al. Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nature. 2021;593:418–23.. 10.1038/s41586-021-03431-4. [DOI] [PubMed] [Google Scholar]
- 28. Koonin EV, Gorbalenya AE, Chumakov KM.. Tentative identification of RNA-dependent RNA polymerases of dsRNA viruses and their relationship to positive strand RNA viral polymerases. FEBS Lett. 1989;252:42–6.. 10.1016/0014-5793(89)80886-5. [DOI] [PubMed] [Google Scholar]
- 29. Kirchdoerfer RN, Ward AB.. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat Commun. 2019;10:2342. 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao Y, Yan L, Huang Y, et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–82.. 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383:1813–26.. 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan H, Peto R, Henao-Restrepo A-M, et al. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511.. 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386:305–15.. 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2021;384:795–807.. 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xie Y, Yin W, Zhang Y, et al. Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2. Cell Res. 2021;31:1212–4.. 10.1038/s41422-021-00570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qian H-J, Wang Y, Zhang M-Q, et al. Safety, tolerability, and pharmacokinetics of VV116, an oral nucleoside analog against SARS-CoV-2, in Chinese healthy subjects. Acta Pharmacol Sin. 2022. 10.1038/s41401-022-00895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering. 2020;6:1192–8.. 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Udwadia ZF, Singh P, Barkate H, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71.. 10.1016/j.ijid.2020.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Solaymani-Dodaran M, Ghanei M, Bagheri M, et al. Safety and efficacy of favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol. 2021;95:107522. 10.1016/j.intimp.2021.107522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lou Y, Liu L, Yao H, et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 2021;157:105631. 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toots M, Yoon J-J, Cox RM, et al. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019;11. 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wahl A, Gralinski LE, Johnson CE, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–7.. 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Painter WP, Holman W, Bush JA, et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother. 2021;65. 10.1128/AAC.02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khoo SH, Fitzgerald R, Fletcher T, et al. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study. J Antimicrob Chemother. 2021;76:3286–95.. 10.1093/jac/dkab318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahase E. Covid-19: Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ. 2021;375:n2422. 10.1136/bmj.n2422. [DOI] [PubMed] [Google Scholar]
- 46. Abdelnabi R, Foo CS, De Jonghe S, et al. Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model. J Infect Dis. 2021;224:749–53.. 10.1093/infdis/jiab361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zumla A, Chan JFW, Azhar EI, et al. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discovery. 2016;15:327–47.. 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hung IFN, Lung KC, Tso EYK, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet North Am Ed. 2020;395:1695–704.. 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Good SS, Westover J, Jung KH, et al. AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 and a promising oral antiviral for treatment of COVID-19. Antimicrob Agents Chemother. 2021;65. 10.1128/AAC.02479-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ren Z, Luo H, Yu Z, et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Advanced Science. 2020;7:2001435. 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang J-L, Li Y-H, Wang L-L, et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduction and Targeted Therapy. 2021;6:414. 10.1038/s41392-021-00835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253:117592. 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dey SK, Saini M, Dhembla C, et al. Suramin, penciclovir, and anidulafungin exhibit potential in the treatment of COVID-19 via binding to nsp12 of SARS-CoV-2. J Biomol Struct Dyn. 1–17., 2021. 10.1080/07391102.2021.2000498. [DOI] [PubMed] [Google Scholar]
- 54. Wang H, Xue S, Yang H, et al. Recent progress in the discovery of inhibitors targeting coronavirus proteases. Virologica Sinica. 2016;31:24–30.. 10.1007/s12250-015-3711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jin Z, Du X, Xu Y, et al. Structure of M from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–93.. 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 56. Yang H, Yang M, Ding Y, et al. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci. 2003;100:13190–5.. 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ren Z, Yan L, Zhang N, et al. The newly emerged SARS-like coronavirus HCoV-EMC also has an “Achilles' heel”: current effective inhibitor targeting a 3C-like protease. Protein & Cell. 2013;4:248–50.. 10.1007/s13238-013-2841-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang L, Lin D, Sun X, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–12.. 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mody V, Ho J, Wills S, et al. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Communications Biology. 2021;4:93. 10.1038/s42003-020-01577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cvetkovic RS, Goa KL.. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63:769–802.. 10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]
- 61. Chan JF-W, Yao Y, Yeung M-L, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–13.. 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399–406. [PubMed] [Google Scholar]
- 63. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–99.. 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet North Am Ed. 2020;396:1345–52.. 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reis G, Moreira Silva EADS, Medeiros Silva DC, et al. Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the together randomized clinical trial. JAMA Network Open. 2021;4:e216468. 10.1001/jamanetworkopen.2021.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Di Castelnuovo A, Costanzo S, Antinori A, et al. Lopinavir/ritonavir and darunavir/cobicistat in hospitalized COVID-19 patients: findings from the multicenter Italian CORIST study. Frontiers in Medicine2021;8:639970. 10.3389/fmed.2021.639970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen J, Xia L, Liu L, et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infectious Diseases. 2020;7:ofaa241. 10.1093/ofid/ofaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bahadur Gurung A, Ajmal Ali M, Lee J, et al. Structure-based virtual screening of phytochemicals and repurposing of FDA approved antiviral drugs unravels lead molecules as potential inhibitors of coronavirus 3C-like protease enzyme. Journal of King Saud University - Science. 2020;32:2845–53.. 10.1016/j.jksus.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beck BR, Shin B, Choi Y, et al. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Computational and Structural Biotechnology Journal. 2020;18:784–90.. 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fintelman-Rodrigues N, Sacramento CQ, Ribeiro Lima C, et al. Atazanavir, alone or in combination with ritonavir, inhibits SARS-CoV-2 replication and proinflammatory cytokine production. Antimicrob Agents Chemother. 2020;64. 10.1128/AAC.00825-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kalantari S, Fard SR, Maleki D, et al. Comparing the effectiveness of atazanavir/ritonavir/dolutegravir/hydroxychloroquine and lopinavir/ritonavir/hydroxychloroquine treatment regimens in COVID-19 patients. J Med Virol. 2021;93:6557–65.. 10.1002/jmv.27195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boras B, Jones RM, Anson BJ, et al. Discovery of a novel inhibitor of coronavirus 3CL protease for the potential treatment of COVID-19. bioRxiv. 2021. 10.1101/2020.09.12.293498. [DOI] [Google Scholar]
- 73. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3.. 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 M inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–93.. 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 75. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–408.. 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Niesor EJ, Boivin G, Rhéaume E, et al. Inhibition of the 3CL protease and SARS-CoV-2 replication by dalcetrapib. ACS Omega. 2021;6:16584–91.. 10.1021/acsomega.1c01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hamed MIA, Darwish KM, Soltane R, et al. β-Blockers bearing hydroxyethylamine and hydroxyethylene as potential SARS-CoV-2 Mpro inhibitors: rational based design, in silico, in vitro, and SAR studies for lead optimization. RSC Adv. 2021;11:35536–58.. 10.1039/d1ra04820a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. El-Masry RM, Al-Karmalawy AA, Alnajjar R, et al. Newly synthesized series of oxoindole-oxadiazole conjugates as potential anti-SARS-CoV-2 agents: in silico and in vitro studies. New J Chem. 2022;46:5078–90.. 10.1039/d1nj04816c. [DOI] [Google Scholar]
- 79. Shin D, Mukherjee R, Grewe D, et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–62.. 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Báez-Santos YM, Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38.. 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rut W, Lv Z, Zmudzinski M, et al. Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti-COVID-19 drug design. Sci Adv. 2020;6. 10.1126/sciadv.abd4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Báez-Santos YM, Barraza SJ, Wilson MW, et al. X-ray structural and biological evaluation of a series of potent and highly selective inhibitors of human coronavirus papain-like proteases. J Med Chem. 2014;57:2393–412.. 10.1021/jm401712t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Klemm T, Ebert G, Calleja DJ, et al. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020;39:e106275. 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kuo C-J, Chao T-L, Kao H-C, et al. Kinetic characterization and inhibitor screening for the proteases leading to identification of drugs against SARS-CoV-2. Antimicrob Agents Chemother. 2021;65. 10.1128/AAC.02577-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Osipiuk J, Azizi S-A, Dvorkin S, et al. Structure of papain-like protease from SARS-CoV-2 and its complexes with non-covalent inhibitors. Nat Commun. 2021;12:743. 10.1038/s41467-021-21060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stasiulewicz A, Maksymiuk AW, Nguyen ML, et al. SARS-CoV-2 papain-like protease potential inhibitors-in silico quantitative assessment. Int J Mol Sci. 3957, 2021;22. 10.3390/ijms22083957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nogara PA, Omage FB, Bolzan GR, et al. In silico studies on the interaction between Mpro and PLpro from SARS-CoV-2 and ebselen, its metabolites and derivatives. Mol Inf. 2021;40:2100028. 10.1002/minf.202100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Weglarz-Tomczak E, Tomczak JM, Talma M, et al. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci Rep. 2021;11:3640. 10.1038/s41598-021-83229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shoemark DK, Colenso CK, Toelzer C, et al. Molecular simulations suggest vitamins, retinoids and steroids as ligands of the free fatty acid pocket of the SARS-CoV-2 spike protein*. Angew Chem Int Ed. 2021;60:7098–110.. 10.1002/anie.202015639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen J, Malone B, Llewellyn E, et al. Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell. 1560–1573.e13., 2020;182. 10.1016/j.cell.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xiang R, Yu Z, Wang Y, et al. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharmaceutica Sinica B. 2022;12:1591–623.. 10.1016/j.apsb.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yan L, Yang Y, Li M, et al. Coupling of N7-methyltransferase and 3'-5' exoribonuclease with SARS-CoV-2 polymerase reveals mechanisms for capping and proofreading. Cell. 3474–3485.e11., 2021;184. 10.1016/j.cell.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Basu S, Mak T, Ulferts R, et al. Identifying SARS-CoV-2 antiviral compounds by screening for small molecule inhibitors of Nsp14 RNA cap methyltransferase. Biochem J. 2021;478:2481–97.. 10.1042/BCJ20210219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bobiļeva O, Bobrovs R, Kaņepe I, et al. Potent SARS-CoV-2 mRNA cap methyltransferase inhibitors by bioisosteric replacement of methionine in SAM cosubstrate. ACS Med Chem Lett. 2021;12:1102–7.. 10.1021/acsmedchemlett.1c00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Otava T, Šála M, Li F, et al. The structure-based design of SARS-CoV-2 nsp14 methyltransferase ligands yields nanomolar inhibitors. ACS Infectious Diseases. 2021;7:2214–20.. 10.1021/acsinfecdis.1c00131. [DOI] [PubMed] [Google Scholar]
- 96. El Hassab MA, Ibrahim TM, Shoun AA, et al. In silico identification of potential SARS COV-2 2'-O-methyltransferase inhibitor: fragment-based screening approach and MM-PBSA calculations. RSC Adv. 2021;11:16026–33.. 10.1039/d1ra01809d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Krafcikova P, Silhan J, Nencka R, et al. Structural analysis of the SARS-CoV-2 methyltransferase complex involved in RNA cap creation bound to sinefungin. Nat Commun. 2020;11:3717. 10.1038/s41467-020-17495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shrimp JH, Kales SC, Sanderson PE, et al. An enzymatic TMPRSS2 assay for assessment of clinical candidates and discovery of inhibitors as potential treatment of COVID-19. ACS Pharmacology & Translational Science. 2020;3: 997–1007. 10.1021/acsptsci.0c00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gunst JD, Staerke NB, Pahus MH, et al. Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial. EClinicalMedicine. 2021;35:100849. 10.1016/j.eclinm.2021.100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71.. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yamamoto M, Kiso M, Sakai-Tagawa Y, et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12: 629. 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hoffmann M, Hofmann-Winkler H, Smith JC, et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine. 2021;65:103255. 10.1016/j.ebiom.2021.103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lucas JM, Heinlein C, Kim T, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–25.. 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Samuel RM, Majd H, Richter MN, et al. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27:876–89..e12 10.1016/j.stem.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Qiao Y, Wang X-M, Mannan R, et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci. 2021;118. 10.1073/pnas.2021450118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Trigunaite A, Dimo J, Jørgensen TN.. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294:87–94.. 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 107. Zhou T, Xu W, Zhang W, et al. Preclinical profile and phase I clinical trial of a novel androgen receptor antagonist GT0918 in castration-resistant prostate cancer. Eur J Cancer. 2020;134:29–40.. 10.1016/j.ejca.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 108. McCoy J, Goren A, Cadegiani FA, et al. Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial. Frontiers in Medicine2021;8:668698. 10.3389/fmed.2021.668698. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109. Cadegiani FA, Zimerman RA, Fonseca DN, et al. Final results of a randomized, placebo-controlled, two-arm, parallel clinical trial of proxalutamide for hospitalized COVID-19 patients: a multiregional, joint analysis of the proxa-rescue AndroCoV trial. Cureus. 2021;13:e20691. 10.7759/cureus.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rodon J, Muñoz-Basagoiti J, Perez-Zsolt D, et al. Identification of plitidepsin as potent inhibitor of SARS-CoV-2-Induced cytopathic effect after a drug repurposing screen. Frontiers in Pharmacology. 2021;12:646676. 10.3389/fphar.2021.646676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sacco MD, Ma C, Lagarias P, et al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M and cathepsin L. Sci Adv. 2020;6. 10.1126/sciadv.abe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Steuten K, Kim H, Widen JC, et al. Challenges for targeting SARS-CoV-2 proteases as a therapeutic strategy for COVID-19. ACS Infectious Diseases. 2021;7:1457–68.. 10.1021/acsinfecdis.0c00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Maisonnasse P, Guedj J, Contreras V, et al. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–7.. 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 114. Cheng Y-W, Chao T-L, Li C-L, et al. Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Cell Rep. 2020;33:108254. 10.1016/j.celrep.2020.108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hoffmann M, Mösbauer K, Hofmann-Winkler H, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585:588–90.. 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 116. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–8.. 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chorin E, Dai M, Shulman E, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26:808–9.. 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 118. Gorshkov K, Chen CZ, Bostwick R, et al. The SARS-CoV-2 cytopathic effect is blocked by lysosome alkalizing small molecules. ACS Infectious Diseases. 2021;7:1389–408.. 10.1021/acsinfecdis.0c00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kang Y-L, Chou Y-Y, Rothlauf PW, et al. Inhibition of PIKfyve kinase prevents infection by Zaire ebolavirus and SARS-CoV-2. Proc Natl Acad Sci. 2020;117:20803–13.. 10.1073/pnas.2007837117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bojkova D, Klann K, Koch B, et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–72.. 10.1038/s41586-020-2332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. White KM, Rosales R, Yildiz S, et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science. 2021;371:926–31.. 10.1126/science.abf4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Braga L, Ali H, Secco I, et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature. 2021;594:88–93.. 10.1038/s41586-021-03491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Backer V, Sjöbring U, Sonne J, et al. A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19. The Lancet Regional Health - Europe. 2021;4:100084. 10.1016/j.lanepe.2021.100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang SNY, Atkinson SC, Wang C, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- 127. Rajter JC, Sherman MS, Fatteh N, et al. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in covid nineteen study. Chest. 2021;159:85–92.. 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325:1426–35.. 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Han Y, Duan X, Yang L, et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 2021;589:270–5.. 10.1038/s41586-020-2901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet North Am Ed. 2020;395:497–506.. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–60.. 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–502.. 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet North Am Ed. 2021;397:1637–45.. 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117:10970–5.. 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–36.. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 136. Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–93.. 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 137. Spinelli FR, Conti F, Gadina M.. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Science Immunology. 2020;5. 10.1126/sciimmunol.abc5367. [DOI] [PubMed] [Google Scholar]
- 138. Cantini F, Niccoli L, Matarrese D, et al. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect. 2020;81:318–56.. 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Haagmans BL, Kuiken T, Martina BE, et al. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10:290–3.. 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Loutfy MR, Blatt LM, Siminovitch KA, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–8.. 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 141. Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b treatment for COVID-19. Front Immunol. 2020;11:1061. 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yuan J, Zou R, Zeng L, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020;69:599–606.. 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rossignol J-F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110: 94–103. 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Haffizulla J, Hartman A, Hoppers M, et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial.Lancet Infect Dis. 2014;14:609–18.. 10.1016/S1473-3099(14)70717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rocco PRM, Silva PL, Cruz FF, et al. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021;58: 2003725. 10.1183/13993003.03725-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369:712–7.. 10.1126/science.abc2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Telenti A, Arvin A, Corey L, et al. After the pandemic: perspectives on the future trajectory of COVID-19. Nature. 2021;596:495–504.. 10.1038/s41586-021-03792-w. [DOI] [PubMed] [Google Scholar]
- 148. Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–98.. 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Singh AK, Singh A, Singh R, et al. Molnupiravir in COVID-19: A systematic review of literature. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2021;15:102329. 10.1016/j.dsx.2021.102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tian L, Pang Z, Li M, et al. Molnupiravir and its antiviral activity against COVID-19. Front Immunol. 2022;13:855496. 10.3389/fimmu.2022.855496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Padhi AK, Shukla R, Saudagar P, et al. High-throughput rational design of the remdesivir binding site in the RdRp of SARS-CoV-2: implications for potential resistance. iScience. 2021;24:101992. 10.1016/j.isci.2020.101992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Mótyán JA, Mahdi M, Hoffka G, et al. Potential resistance of SARS-CoV-2 main protease (Mpro) against protease inhibitors: lessons learned from HIV-1 protease. Int J Mol Sci. 2022;23:3507. 10.3390/ijms23073507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Szemiel AM, Merits A, Orton RJ, et al. In vitro selection of remdesivir resistance suggests evolutionary predictability of SARS-CoV-2. PLoS Pathog. 2021;17:e1009929. 10.1371/journal.ppat.1009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mari A, Roloff T, Stange M, et al. Global genomic analysis of SARS-CoV-2 RNA dependent RNA polymerase evolution and antiviral drug resistance. Microorganisms. 2021;9: 1094. 10.3390/microorganisms9051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Martin R, Li J, Parvangada A, et al. Genetic conservation of SARS-CoV-2 RNA replication complex in globally circulating isolates and recently emerged variants from humans and minks suggests minimal pre-existing resistance to remdesivir. Antiviral Res. 2021;188:105033. 10.1016/j.antiviral.2021.105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Focosi D, Maggi F, McConnell S, et al. Very low levels of remdesivir resistance in SARS-COV-2 genomes after 18 months of massive usage during the COVID19 pandemic: A GISAID exploratory analysis. Antiviral Res. 2022;198:105247. 10.1016/j.antiviral.2022.105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Agostini ML, Pruijssers AJ, Chappell JD, et al. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. 2019;93. 10.1128/JVI.01348-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Abdelnabi R, Foo CS, Kaptein SJF, et al. The combined treatment of molnupiravir and favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine. 2021;72:103595. 10.1016/j.ebiom.2021.103595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Terada J, Fujita R, Kawahara T, et al. Favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia with/without oxygen therapy: an open-label, single-center phase 3 randomized clinical trial. EClinicalMedicine. 2022;49:101484. 10.1016/j.eclinm.2022.101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tan YL, Tan KSW, Chu JJH, et al. Combination treatment with remdesivir and ivermectin exerts highly synergistic and potent antiviral activity against murine coronavirus infection. Frontiers in Cellular and Infection Microbiology. 2021;11:700502. 10.3389/fcimb.2021.700502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Singh AK, Singh A, Singh R, et al. An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2022;16:102396. 10.1016/j.dsx.2022.102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. National COVID-19 Clinical Evidence Taskforce. Australian guidelines for the clinical care of people with COVID-19. 2022 [version 57]. Available from: https://covid19evidence.net.au/.
- 163. Prikis M, Paxlovid Cameron A. (Nirmatelvir/Ritonavir) and tacrolimus drug-drug interaction in a kidney transplant patient with SARS-2-CoV infection: A Case Report. Transplant Proc. 2022. 10.1016/j.transproceed.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhou S, Hill CS, Sarkar S, et al. β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021;224:415–9.. 10.1093/infdis/jiab247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.