Abstract
Aim:
Initiating continuous glucose monitoring (CGM) shortly after Type 1 diabetes diagnosis has glycaemic and quality of life benefits for youth with Type 1 diabetes and their families. The SARS-CoV-2 pandemic led to a rapid shift to virtual delivery of CGM initiation visits. We aimed to understand parents' experiences receiving virtual care to initiate CGM within 30 days of diagnosis.
Methods:
We held focus groups and interviews using a semi-structured interview guide with parents of youth who initiated CGM over telehealth within 30 days of diagnosis during the SARS-CoV-2 pandemic. Questions aimed to explore experiences of starting CGM virtually. Groups and interviews were audio-recorded, transcribed and analysed using thematic analysis.
Results:
Participants were 16 English-speaking parents (age 43 ± 6 years; 63% female) of 15 youth (age 9 ± 4 years; 47% female; 47% non-Hispanic White, 20% Hispanic, 13% Asian, 7% Black, 13% other). They described multiple benefits of the virtual visit including convenient access to high-quality care; integrating Type 1 diabetes care into daily life; and being in the comfort of home. A minority experienced challenges with virtual care delivery; most preferred the virtual format. Participants expressed that clinics should offer a choice of virtual or in-person to families initiating CGM in the future.
Conclusion:
Most parents appreciated receiving CGM initiation education via telehealth and felt it should be an option offered to all families. Further efforts can continue to enhance CGM initiation teaching virtually to address identified barriers.
Keywords: children and adolescents, continuous glucose monitoring, healthcare delivery, psychological aspects, telehealth, type 1 diabetes
1 ∣. INTRODUCTION
The use of continuous glucose monitoring (CGM) in youth with Type 1 diabetes has been shown to have benefits for glycaemic outcomes (e.g., reduced HbA1c and/or reduced hypoglycaemia) and quality of life.1-3 The 2022 American Diabetes Association's Standards of Care recommend that CGM ‘should be offered for diabetes management in youth’ with Type 1 diabetes, regardless of the method of insulin administration.4
Initiating CGM shortly following Type 1 diagnosis is a relatively new clinical practice that has been shown to promote improved glucose management, particularly when combined with comprehensive care, education and insulin dosing adjustments.5,6 When introduced during the new onset period, CGM has been accepted by most families.7 Rather than feeling additionally overwhelmed by having to adopt a new device with its own learning curve during the new onset period, qualitative data showed that parents viewed early CGM initiation as essential for learning to manage their child's diabetes.8 In addition, CGM enables remote monitoring of glucose values, which provides parents with greater peace of mind, improved sleep and other psychosocial benefits.9
The SARS-CoV-2 pandemic prompted an accelerated shift to increased telehealth-based diabetes care delivery.10 As a result, CGM initiation education, previously carried out in-person, also shifted to telehealth-based delivery. However, little is known about how parents and their children experience CGM initiation over telehealth shortly following Type 1 diabetes diagnosis, amidst a period of significant adjustment with a large volume of education delivery.11-13 Thus, this follow-up qualitative study aimed to explore the experience of telehealth-based CGM initiation during the Type 1 diabetes new-onset period to guide clinical practice.
2 ∣. RESEARCH DESIGN AND METHODS
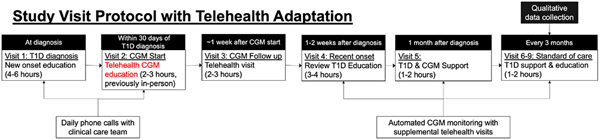
This qualitative study is part of a previously reported pragmatic research study5,14 and is a follow-up to previously reported qualitative results from in-person delivery of CGM initiation during the Type 1 diabetes new-onset period for families of youth with Type 1 diabetes.8 In brief, the main study delivers a clinical protocol to initiate CGM within 30 days of Type 1 diabetes diagnosis (mean time to CGM initiation = 7 days14) and provides 30 days of CGM supplies while insurance approval is sought. Families receive Dexcom G6 CGM (Dexcom Inc.); the rationale for which has been previously described.8 The SARS-CoV-2 pandemic necessitated a shift to delivering CGM initiation care over telehealth as a part of the clinical protocol; Figure 1 presents an overview of the flow of care delivery following diagnosis with pandemic-prompted changes highlighted in red.
FIGURE 1.
Flow of care delivery for early CGM initiation.
2.1 ∣. Sample and recruitment
Eligible participants were a convenience sample of English-speaking parents of youth who had started CGM within 1 month of their Type 1 diabetes diagnosis as part of the larger study and who received a telehealth-based CGM initiation visit following the start of the SARS-CoV-2 pandemic. Parents provided informed consent to participate. This study was approved by the Stanford University Institutional Review Board. Participants were compensated with a $50 gift card.
2.2 ∣. Data collection
Focus groups and interviews were held between July and October 2021 over a HIPAA-compliant videoconference platform (Zoom Video Communications Inc.). Data collection and analyses followed the methods described in the first qualitative study.8 To offer greater flexibility in scheduling, we offered both focus groups and interviews to parents. The purpose of the focus groups and interviews was to explore parents' experiences with initiating CGM through virtual care within 30 days of their child's Type 1 diabetes diagnosis. Groups and interviews were conducted by two researchers (M.L.T. and J.H.) using a semi-structured interview guide to ensure consistency. Groups and interviews were recorded and transcribed; transcripts were checked for quality and de-identified prior to analysis. Five groups (with between 2 and 5 participants) and three individual interviews were conducted. Interviews and groups lasted between 30 and 62 minutes.
2.3 ∣. Data analysis
Data from transcripts were analysed using thematic analysis.15 Coders began with open-coding, independently reviewing one transcript to develop initial codes. Given the narrow scope of this follow-up study, coders (A.A., M.L.T, D.P.Z.), who were the same multidisciplinary coding group (endocrinologist, clinical psychologist, exercise physiologist, all with qualitative research experience and Type 1 diabetes expertise) from the initial qualitative study, and drew upon the prior framework developed for the initial study.8 The coding team applied this framework to the new transcripts with a focus on content related directly to the experience of virtual CGM initiation. Two coders reviewed and assigned codes to each transcript; and a third coder reviewed codes and resolved discrepancies. Through regular meetings, group discussion and a third coder reviewing and resolving discrepancies, agreement was reached on all codes. The coding team maintained an audit trail throughout coding to track decision making, and to support dependability and confirmability.16 The Standards for Reporting Qualitative Research (SRQR) was used to support the rigour of qualitative methods.17
3 ∣. RESULTS
3.1 ∣. Participants
Participants were 16 parents (age 43 ± 6 years; 63% female) of 15 youth (age 9 ± 4 years; 47% female; 47% non-Hispanic White, 20% Hispanic, 13% Asian, 7% Black, 13% other; diabetes duration 9 ± 3 months). For one participating family, both parents took part in the focus group. Families initiated CGM at a mean of 3 days after diagnosis (range: 2–24 days). Table 1 presents participant characteristics.
TABLE 1.
Participant characteristics (parent N = 16; youth N = 15).
| N (%) | M ± SD | Range | |
|---|---|---|---|
| Parent age (years) | 43 ± 6 | 34–56 | |
| Parent gender | |||
| Female | 10 (62.5) | ||
| Male | 6 (37.5) | ||
| Parent race/ethnicity | |||
| Non-Hispanic White | 6 (37.5) | ||
| Asian/Pacific Islander | 2 (12.5) | ||
| Hispanic | 2 (12.5) | ||
| Non-Hispanic Black | 1 (6.25) | ||
| Other | 1 (6.25) | ||
| Not available | 4 (25) | ||
| Youth age (years) | 9 ± 4 | 2–17 | |
| Child gender | |||
| Female | 7 (47) | ||
| Male | 8 (53) | ||
| Child race/ethnicity | |||
| Non-Hispanic White | 7 (47) | ||
| Asian/Pacific Islander | 2 (13) | ||
| Hispanic | 3 (20) | ||
| Non-Hispanic Black | 1 (0.07 | ||
| Other | 2 (13) | ||
| Months since diagnosis | 7 ± 3 | 3–12 | |
| CGM start (days after diagnosis) | 3 (6.8) | 2–24 |
3.2 ∣. Themes
Table 2 presents example quotes for each of the following three themes: (1) advantages to starting CGM through telehealth-based care; (2) challenges to starting CGM through telehealth-based care and (3) preferences for CGM initiation.
TABLE 2.
Feedback on experience of CGM virtual start—advantages, challenges and preferences.
| Sub-theme | Example quote |
|---|---|
| Theme 1: Advantages to starting CGM through telehealth-based care | |
| Access to high-quality care |
It was the same as if I were in the room. (FG5, father, daughter aged 5)
I think it went as well as it would have if we'd been there in person, you know? I do not think anything would have been different, really. (Int1, mother, son aged 17) They sent us the equipment and it was fine. I wasn't sure how it was gonna go…but it was relatively easy…we learned what we needed to. (FG3, mother, daughter aged 10) |
| Convenience: lack of travel & ease of scheduling |
For us, we would have had to drive three hours…being at home was much more convenient, and I think it's just as effective. (FG2, mother, son aged 9)
[My son] was having finals week…he had some stuff we could not pull him away from. We were able to do it over a lunch hour from school. If we had to drive [to clinic] we could not have gone up and made it back in time for his school stuff. [Telehealth] made it possible to squeeze it in instead of delaying it for weeks until [my son] was gonna be free (Int1, mother, son aged 17) |
| Being in the comfort of home |
It was very comforting for me to be able to just sit and do it in our house where [my child is] comfortable. (FG1, mother, daughter aged 3)
Having a comfort of home helps, especially given that this procedure will have to continue and happen on a day-to-day basis (FG1, son aged 2) |
| Type 1 diabetes care as part of life at home |
It was great that we were able to be at home…we were forced to do it where, maybe had we been in the office I cannot help but wonder if we would've relied on the nurse and then probably would've had to do a follow-up call going, ‘How do you do this again?’ (FG1, mother, son aged 16)
This [telehealth] is the way the world is now. We were just used to it. [My daughter] just put [the CGM] on and got a lot of esteem out of it. She feels very accomplished when she's able to do things. (FG4, father, daughter aged 10) |
| Easy to do |
It's pretty user friendly for the most part (FG4, mother, daughter aged 10)
There was good peace of mind and plenty of information provided, that it was easy. (FG2, mother, son aged 9) |
| Provider also used CGM |
It's a little more helpful, having that personal interaction where somebody who is actually dealing with it can tell you ‘This is what works for me.’ (FG2, father, son aged 9)
Just the fact that she went through it with him step by step, and she did it too, I think that was important to both of us. You're not a stranger in a strange world. (Int2, mother, son aged 12) |
| Theme 2. Challenges of starting CGM through telehealth-based care | |
| Intimidating & challenging, felt less supported |
I was really scared. I did not think I would be able to do it. (FG1, mother, daughter aged 5)
It's a little intimidating at first, thinking we are gonna insert something on our kid's body. (FG2, mother, son aged 9) The box [of supplies] showed up in front of my house, and I did not know anything about what was in it. (Int2, mother, son aged 12) |
| It was long, overwhelming amount of information |
I feel like they are all long when you are learning new technology…but I do not think there's a way to shorten it. (FG3, mother, daughter aged 11)
It was information overload. At the time I do not think I could have processed anything else. (FG1, mother, daughter aged 3) |
| Theme 3. Preferences for care delivery and suggestions for the future | |
| Prefer telehealth | At the time, I probably would have chosen in-person, but I'm appreciative it was at home because it forced me to learn on the spot. I did not have anyone else to rely on. (FG1, mother, daughter aged 3) |
| Prefer in person | I love in-person visits. I'm a face-to-face person. (Int3, father, son aged 9) |
| Clinic should offer choice | I think everybody's different. The parents may not be comfortable. The child may not be comfortable. I think it's just based on that statement, would you rather have hands-on assistance or would you rather us guide you through it online and if something goes sideways, you could come in. I think that's just good stewardship. (FG4, father, son aged 10) |
Note: FG, focus group; Int, Interview.
3.3 ∣. Theme 1. ‘Same as if I were in the room’: Advantages to starting CGM through telehealth-based care
The majority of parents described a range of positive aspects to receiving CGM initiation via telehealth. Within this overarching theme, we identified several subthemes on the advantages of telehealth to start CGM including: (a) access to high-quality care; (b) convenience of not having to travel and ease of scheduling; (c) being in the comfort of home; (d) Type 1 diabetes care as part of daily life at home; (e) it was easy to do and (f) some families appreciated having a provider who also used CGM in the visit.
3.3.1 ∣. Access to high-quality care
For the most part, parents did not feel that care had been diminished by being over telehealth instead of in the clinic. Some expressed that they had initial reservations about having this visit via telehealth instead of in-person, but most shared that they were pleasantly surprised and glad they were able to receive care over telehealth. Parents made statements that normalized the practice of telehealth-based care given that so much of healthcare delivery, work and schooling at the time had shifted to a virtual format during the pandemic. One parent pointed out that a particular advantage of the telehealth format was it increased ease of note-taking during the visit to be able to retain information better.
3.3.2 ∣. Convenience due to lack of travel and ease of scheduling
Parents expressed feeling glad that they were spared additional driving to be able to attend this appointment. Virtual care was of benefit to families, as participants in the current study lived up to 3 h away from clinic, and also helped busy families squeeze in the CGM initiation visit between work and school commitments. In addition, some parents pointed out that they had been attending other Type 1 diabetes new onset visits in-person, so having the CGM initiation visit via telehealth complemented the in-person care and offered a break from time spent in clinic.
3.3.3 ∣. Being in the comfort of home
Parents of young children shared that one benefit of having a telehealth visit was being able to initiate CGM in the comfort of home rather than in a potentially anxiety-producing clinic setting: At home, children had easy access to their own room, toys and comforting objects.
3.3.4 ∣. Type 1 diabetes care as part of life at home
Parents of older children and adolescents noted that being able to start CGM at home served to boost the confidence of their teen. Participants shared that they might have been more dependent on clinic staff if they started CGM in the clinic. However, being ‘forced’ to start on their own at home increased self-efficacy for continuing the practice at home which is more in line with the majority of Type 1 diabetes management that occurs outside of the clinic office and in day-to-day life.
3.3.5 ∣. Easy to do
Overall, parents felt the process was relatively easy to do and felt they were supported by clinic staff who walked them through setting up their CGM step-by-step.
3.3.6 ∣. Provider also used CGM
Finally, two families pointed out that they appreciated that the Certified Diabetes Care and Education Specialist (CDCES) who trained them to use CGM also lives with Type 1 diabetes and uses CGM. Being able to show how to do it and provide personal experiences with CGM increased families' sense of being supported.
3.4 ∣. Theme 2. Challenges of starting CGM through telehealth-based care
While most families found the telehealth CGM visit to be the same as in-person care and to provide additional advantages, three families shared challenges they experienced with the telehealth visit. Within this overarching theme, we identified two subthemes on the disadvantages of telehealth to start CGM including the visit being: (a) intimidating and challenging, with less support; and (b) long with an overwhelming amount of information.
3.4.1 ∣. Intimidating, challenging visit with less support
Some families described feeling more intimidated and scared through the process and expressed worries about getting it wrong and felt that these feelings were intensified by having to be at home without the support of clinic staff in the room. A family of a toddler described the visit as a negative experience with having to hold their child down for the initial CGM placement and expressed that they would have wanted more in-person clinical support for starting CGM. Another family found it ‘awkward’ to figure out the step-by-step placement of CGM over telehealth with trying to stay in the computer screen while inserting a sensor for the first time.
3.4.2 ∣. Long visit with an overwhelming amount of information
Five families shared that the visit was long and contained an overwhelming amount of information. However, most families did not think less information could be presented or that there would be a way to cut down the visit.
3.5 ∣. Theme 3. Preferences for care delivery and suggestions for the future
3.5.1 ∣. Preference for virtual or in-person CGM initiation
During the interview or focus group, parents were asked what option they would pick if they had the choice: virtual or in-person. Of participants in the focus groups who expressed a preference, eight shared that they would want a telehealth-based CGM visit. Several parents who ultimately said they preferred a telehealth visit stated that going into the experience they would have expected to prefer it to be in-person. Three parents shared that they would choose in-person if they could do it again based on their personal preference for in-person care.
3.5.2 ∣. Clinics should offer a choice in the future
Regardless of their own preference, many parents expressed that they thought the choice of telehealth or in-person should be offered to families in the future, recognizing that each family may have different preferences, priorities and comfort with initiating a new diabetes device at home.
3.5.3 ∣. Suggestions for improvement
Some families shared suggestions for how virtual care delivery of CGM initiation could be improved in the future. One parent suggested that the visit could start with an orientation to all of the components of the CGM before jumping into starting on the system. Families also requested that additional tips and tricks be shared early on specifically with strategies for adhesives and adhesive removal to be able to decrease the potential for pain; and education on what kinds of things can impact the accuracy of CGM (e.g., compression lows, rapid changes in glucose, etc.). One parent requested wanting to record the telehealth visit to be able to review later.
4 ∣. DISCUSSION
This qualitative study reports on families' experiences of initiating CGM remotely via telehealth following their child's Type 1 diabetes diagnosis. This study is a follow-up to the initial qualitative study that reported on CGM initiation experiences within 30 days of Type 1 diabetes diagnosis. The initial study found that early CGM initiation—delivered with in-person care—was overwhelmingly accepted and viewed as essential, providing a range of benefits to parents and youth such as improved sleep and peace of mind, and facilitating adjustment to living with Type 1 diabetes.8 The aims of the current study focused on the experience of starting CGM virtually following the rapid shift to telehealth-based care during the SARS-CoV-2 pandemic. Most parents in the current study appreciated receiving virtual care to start their child on CGM. Many felt that despite being a virtual visit, they still had access to high-quality care and received guidance that was easy to follow to initiate CGM. Parents appreciated the convenience of scheduling and lack of travel, particularly during the new-onset period during which they received other Type 1 diabetes education and care in-person. In addition, some parents of young children noted a particular advantage of the virtual visit that their child could be in the comfort of home rather than in a doctor's office which may provoke more anxiety or stress. Finally, some parents noted that starting CGM through a remote visit boosted a sense of self-efficacy and confidence that they could continue to do it on their own at home without the in-person support from a clinical provider. This modality, some felt, was more in line with what daily life with Type 1 diabetes would be and increased their sense of preparedness for the first sensor change. These observations are in line with past literature that has noted the increased ‘ecological validity’ of providing care to youth in their own homes and life contexts.18
A smaller number of participants in the current study experienced barriers and challenges to receiving virtual care to start CGM, which are important to note. Some felt the visit was long and created a sense of information overload, though this observation is not unique to virtual delivery.8 Specific to the virtual nature of the visit, one family found it challenging to learn how to apply the first sensor while following instructions from a provider on a computer screen. A small number of parents felt that the virtual visit led to more anxiety and fear that they would do something wrong in initiating the first sensor. In the end, most families stated that they would prefer a virtual CGM initiation visit while some said they would have preferred in-person to get started.
Findings from this study are in line with recent literature demonstrating the acceptability and benefits of telehealth-based delivery of diabetes care, which is increasingly becoming a recommended avenue to increase access to care, increase frequency of visits while minimizing travel and time burden, and to monitor patients remotely between visits.19-22 The SARS-CoV-2 pandemic led to a rapid acceleration of expanding the use of telehealth-based care23,24 and highlighted its potential as major paradigm shift in diabetes care25,26 as well as some challenges (e.g., risk of widening disparities and gaps in access particularly with rural populations; concern about less personalized care over time; lower rates of anthropometric and lab measurements).27-29 Recent research has demonstrated that most patients and providers have been satisfied with telehealth-based diabetes care delivery.23,30 Telehealth-based diabetes care has shown the ability to increase self-efficacy and engagement in care in adolescents and young adults with Type 1 diabetes and other health conditions.31,32
Participants in the current study agreed that regardless of their personal preference, clinics should offer a choice of virtual or in-person to families moving forward so that those who want a provider present can opt for that in the future. More research may be needed to better understand what factors drive the preference for in-person or telehealth-based care as well as to understand who may benefit from one modality over the other and for what type of visit (e.g., device initiation versus routine check-ups). Recent work in other healthcare contexts (e.g., primary care, mental healthcare) suggests that several different priorities may inform patient preference for telehealth or in-person including time until the appointment; severity of the problem; relationship with their provider; and lack of time for an in-person visit, among others.33,34 Specific to diabetes device initiation, comfort with technology may play an important role in determining a family's preference for visit type. A better understanding of these factors will be important to ensure that proliferation of telehealth-based care can help to expand access to timely, high-quality care rather than widen existing health disparities.18,35,36
The current study has limitations that are important to note. These include that all participants received CGM start remotely during the SARS-CoV-2 pandemic, and the study did not actively seek to interview families who may have declined virtual delivery or had barriers to opting for this method of receiving care (e.g., lack of reliable Internet connection). In addition, families were asked to share their experiences retrospectively and therefore may not be able to recall all study details and feedback as clearly. The small sample size may also limit generalizability to all families initiating CGM virtually following Type 1 diabetes diagnosis. Further, as these interviews and focus groups were held with parents, more research is needed to fully capture the experience of initiating CGM virtually for newly diagnosed youth with Type 1 diabetes from their own perspective. Participants in the current study were English-speaking, which may limit generalizability to non-English-speaking participants. Finally, the study did not explicitly assess parents' pre-diagnosis comfort and fluency with technology and/or past experiences with receiving healthcare through telehealth. When determining who may benefit from in-person versus virtual visits in the future, it may be important to explore whether overall comfort with technology is a useful indicator of preference for mode of care delivery.
This qualitative study has strengths including valuable patient-centred feedback on the current 4T Study design. For example parents recommended more structured tips and tricks for CGM adhesion/removal, and education around factors that may impact the accuracy of CGM devices. Future iterations of the 4T Study will also incorporate the feedback from the present interviews and focus groups and we intend to offer the choice between in-person and virtual telehealth appointments to all families. In sum, most parents in the current study appreciated virtual delivery of CGM initiation, despite initial reservations and expressed a preference for the virtual modality. Parents also advocated for offering a choice of in-person or virtual, which may be effective to optimize care delivery and tailor new onset education based on families' particular comfort level with technology and preferred learning method. Our findings may encourage other clinical practices to consider virtual delivery of CGM initiation education as a feasible option which can also serve to increase flexibility within diabetes care teams.37
What's new?
Continuous glucose monitoring (CGM) can be initiated through virtual care in a paediatric Type 1 diabetes population shortly after Type 1 diabetes diagnosis and was acceptable for most families.
Most parents of newly diagnosed youth with Type 1 diabetes in this study felt that starting CGM through virtual care provided convenient access to high-quality care and offered unique benefits such as reducing anxiety for young children due to being in the comfort of their home.
A small number of parents would have preferred in-person delivery to increase their comfort with the new technology; study participants advocated for clinics offering a choice of virtual or in-person care to families in the future.
ACKNOWLEDGMENTS
The authors acknowledge the parents who provided their valuable insights, experiences and feedback regarding initiating CGM remotely during the SARS-CoV-2 pandemic. The authors also thank the other members of the 4T Study Group for their help with this project. Study team members include: David Scheinker, PhD, Manisha Desai, PhD, Korey Hood, PhD, Ramesh Johari, PhD, Brianna Leverenz, BS, Julie Hooper MPH, RD, Ana Cortes, BS, Nora Arrizon-Ruiz, BS, Erica Pang, BS, Carolyn Herrera, BS, Rachel Tam, BA, Dom Mitchell, BS, Liz Heckard, BS, Andrea Bonilla Ospina, BS, Franziska Bishop, MS, CDCES, Natalie Pageler, MD, Jeannine Leverenz, RN, CDCES, Piper Sagan, RN, CDCES, Anjoli Martinez-Singh, RD, CDCES, Barry Conrad RD, CDCES, Annette Chmielewski, RD, CDCES, Julie Senaldi RN, CDCES, Victoria Ding, MS, Rebecca Gardner, MS, Victor Ritter, PhD, Kim Clash, NP, Erin Hodgson, RD, CDCES, Diana Naranjo, PhD, Johannes Ferstad, BS, Ryan Pei, MS, Michael Gao, BS, Annie Chang, BS, Ransalu Senanayake, PhD, Anastasiya Vitko, MS, Simrat Ghuman, PhD, Esli Osmanlliu, MD, Emily Fox, PhD, Carlos Guestrin, PhD, Alex Wang, MS, Paul Dupenloup, MS, Arpita Singhal, BS and Juan Langlois, MS. The authors also thank our collaborators Dr. Mark Clements, MD, PhD and Dr. Michael Riddell, PhD.
FUNDING INFORMATION
Funding was provided in part by the Stanford Diabetes Research Center (P30DK116074) and R18DK122422. CGM supplies for the first month (transmitter, three sensors and receiver per patient) were donated by Dexcom. Funding for iOS devices was provided by a grant through the Lucile Packard Children's Hospital Auxiliaries Endowment. MLT is supported by K23DK119470. AA is supported by the Maternal Child Health Research Institute and by Stanford University's K12 (K12DK122550). DPZ is supported by Leona M. and Harry B. Helmsley Charitable Trust and ISPAD-JDRD Research Fellowship.
National Institutes of Health; ISPAD-JDRD Research Fellowship; Helmsley Charitable Trust; Stanford University's, Grant/Award Number: K12 (K12DK122550); Maternal Child Health Research Institute; Lucile Packard Children's Hospital Auxiliaries Endowment; Stanford Diabetes Research Center, Grant/Award Number: R18DK122422 and P30DK116074
Footnotes
CONFLICT OF INTEREST
MLT, AA, PP, JH and BL have no conflicts to disclose. DPZ has received speaking honoraria from Medtronic Diabetes, Ascensia Diabetes, Insulet and American Diabetes Association. DMM has had research support from the NIH, JDRF, NSF and the Helmsley Charitable Trust and his institution has had research support from Medtronic Diabetes, Dexcom, Insulet, Bigfoot Biomedical, Tandem and Roche. DMM has also consulted for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, Medtronic and Insulet.
REFERENCES
- 1.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group. A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care. 2021;44(2):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association Professional Practice Committee. 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Supplement_1):S97–S112. [DOI] [PubMed] [Google Scholar]
- 5.Prahalad P, Zaharieva DP, Addala A, et al. Improving clinical outcomes in newly diagnosed pediatric type 1 diabetes: teamwork, targets, technology, and tight control-the 4T study. Front Endocrinol. 2020;11:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prahalad P, Ding VY, Zaharieva DP, et al. Teamwork, targets, technology, and tight control in newly diagnosed type 1 diabetes: pilot 4T study. J Clin Endocrinol Metabol. 2022;107(4):998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prahalad P, Addala A, Scheinker D, Hood KK, Maahs DM. CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM use and wear time. Diabetes Care. 2020;43(1):e3–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanenbaum ML, Zaharieva DP, Addala A, et al. ‘I was ready for it at the beginning’: parent experiences with early introduction of continuous glucose monitoring following their child's Type 1 diabetes diagnosis. Diabet Med. 2021;38(8):e14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burckhardt MA, Abraham MB, Mountain J, et al. Improvement in psychosocial outcomes in children with type 1 diabetes and their parents following subsidy for continuous glucose monitoring. Diabetes Technol Ther. 2019;21(10):575–580. [DOI] [PubMed] [Google Scholar]
- 10.Prahalad P, Leverenz B, Freeman A, et al. Closing disparities in pediatric diabetes telehealth care: lessons from telehealth necessity during the COVID-19 pandemic. Clin Diabetes. 2022;40(2):153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rankin D, Harden J, Waugh N, Noyes K, Barnard KD, Lawton J. Parents' information and support needs when their child is diagnosed with type 1 diabetes: a qualitative study. Health Expect. 2016;19(3):580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whittemore R, Jaser S, Chao A, Jang M, Grey M. Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educ. 2012;38(4):562–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rankin D, Harden J, Waugh N, et al. Pathways to diagnosis: a qualitative study of the experiences and emotional reactions of parents of children diagnosed with type 1 diabetes. Pediatr Diabetes. 2014;15(8):591–598. [DOI] [PubMed] [Google Scholar]
- 14.Prahalad P, Ding VY, Zaharieva DP, et al. Teamwork, targets, technology, and tight control in newly diagnosed type 1 diabetes: the Pilot 4T study. J Clin Endocrinol Metabol. 2022;107(4):998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. [Google Scholar]
- 16.Russell CK, Gregory DM. Evaluation of qualitative research studies. Evid Based Nurs. 2003;6(2):36–40. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–1251. [DOI] [PubMed] [Google Scholar]
- 18.Comer JS, Myers K. Future directions in the use of telemental health to improve the accessibility and quality of children's mental health services. J Child Adolesc Psychopharmacol. 2016;26(3):296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crossen S, Raymond J, Neinstein A. Top 10 tips for successfully implementing a diabetes telehealth program. Diabetes Technol Ther. 2020;22(12):920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood CL, Clements SA, McFann K, Slover R, Thomas JF, Wadwa RP. Use of telemedicine to improve adherence to American Diabetes Association standards in pediatric type 1 diabetes. Diabetes Technol Ther. 2016;18(1):7–14. [DOI] [PubMed] [Google Scholar]
- 21.Giani E, Laffel L. Opportunities and challenges of telemedicine: observations from the wild west in pediatric type 1 diabetes. Diabetes Technol Ther. 2016;18(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kompala T, Neinstein AB. Telehealth in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2021;28(1):21–29. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Carlson E, Albanese-O'Neill A, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. 2021;23(9):642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giani E, Dovc K, Dos Santos TJ, et al. Telemedicine and COVID-19 pandemic: The perfect storm to mark a change in diabetes care. Results from a world-wide cross-sectional web-based survey. Pediatr Diabetes. 2021;22(8):1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klonoff AN, Lee W-A, Xu NY, Nguyen KT, DuBord A, Kerr D. Six digital health technologies that will transform diabetes. J Diabetes Sci Technol. 2021;19322968211043498. 10.1177/19322968211043498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crossen SS, Bruggeman BS, Haller MJ, Raymond JK. Challenges and opportunities in using telehealth for diabetes care. Diabetes Spectr. 2022;35(1):33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes S, Marcin J. Pediatric telemedicine: lessons learned during the COVID-19 pandemic and opportunities for growth. Adv Pediatr. 2022. Epub ahead of print. 10.1016/j.yapd.2022.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva C, Zhang Q, Bone JN, Amed S. Anthropometric measurements and laboratory investigations in children and youth with type 1 diabetes before and during the COVID-19 pandemic. Can J Diabetes. 2022. Epub ahead of print. 10.1016/j.jcjd.2022.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott SN, Fontana FY, Helleputte S, et al. Use and perception of telemedicine in people with type 1 diabetes during the COVID-19 pandemic: a 1-year follow-up. Diabetes Technol Ther. 2022;24(4):276–280. [DOI] [PubMed] [Google Scholar]
- 30.Crossen SS, Romero CC, Loomba LA, Glaser NS. Patient perspectives on use of video telemedicine for type 1 diabetes care in the United States during the COVID-19 pandemic. Endocrine. 2021;2(4):449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakhach M, Reid MW, Pyatak EA, et al. Home telemedicine (CoYoT1 Clinic): a novel approach to improve psychosocial outcomes in young adults with diabetes. Diabetes Educ. 2019;45(4):420–430. [DOI] [PubMed] [Google Scholar]
- 32.Wood SM, White K, Peebles R, et al. Outcomes of a rapid adolescent telehealth scale-up during the COVID-19 pandemic. J Adolesc Health. 2020;67(2):172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozes I, Mossinson D, Schilder H, Dvir D, Baron-Epel O, Heymann A. Patients' preferences for telemedicine versus inclinic consultation in primary care during the COVID-19 pandemic. BMC primary care. 2022;23(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amini B, Kalankesh LR, Ferdousi R, Farahbakhsh M, Fatehi F. Willingness and preference for using telehealth services among patients with depression. Med Sci. 2021;25:1133–1139. [Google Scholar]
- 35.Khairat S, Haithcoat T, Liu S, et al. Advancing health equity and access using telemedicine: a geospatial assessment. J Am Med Inform Assoc. 2019;26(8–9):796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairchild RM, Ferng-Kuo S-F, Rahmouni H, Hardesty D. Telehealth increases access to care for children dealing with suicidality, depression, and anxiety in rural emergency departments. Telemed J E Health. 2020;26(11):1353–1362. [DOI] [PubMed] [Google Scholar]
- 37.Scheinker D, Prahalad P, Johari R, Maahs DM, Majzun R. A new technology-enabled care model for pediatric type 1 diabetes. NEJM Catal Innov Care Deliv. 2022;3(5):CAT.21.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]