Introduction
Non-Hispanic Black older adults are at elevated risk of Alzheimer’s disease and related dementias (ADRD) compared with non-Hispanic Whites (Mayeda et al., 2016; Tang et al., 2001). This inequality reflects racial differences in both brain and cognitive aging. For example, non-Hispanic Black older adults in the Washington Heights-Inwood Columbia Aging Project (WHICAP) exhibit less cortical thickness in brain regions affected most by early Alzheimer’s disease (L. Zahodne et al., 2015), more white matter hyperintensities (WMH; Zahodne et al., 2015), and lower cognitive scores across domains (L. B. Zahodne et al., 2016) compared with non-Hispanic Whites. Racial disparities in ADRD are not fully explained by socioeconomic status or physical health (Tang et al., 2001), and an emerging literature points to racially-patterned social stress as an independent contributor to Black-White disparities in cognitive aging (L. B. Zahodne, Sol, & Kraal, 2019). This literature reinforces the American Medical Association’s recent acknowledgment that racially-patterned social stress (e.g., discrimination) represents an important cause of health disparities (American Medical Association, 2020; O’Reilly, 2020).
Discrimination represents a particularly potent form of social stress with documented implications for cognitive aging. Greater discrimination has been linked to lower cross-sectional (L.L. Barnes et al., 2012) and more decline in longitudinal (L. B. Zahodne et al., 2016, 2020) cognitive performance in racially/ethnically diverse samples of older adults. While comparative (i.e., between-group) studies clearly show that non-Hispanic Blacks face a disproportionate level of discrimination than non-Hispanic Whites (Lisa L. Barnes et al., 2004), there is a relative lack of studies focused exclusively on non-Hispanic Black older adults that can examine within-group heterogeneity and clarify how the unique experiences of discrimination among Black older adults link to their ADRD risk. Further, much of the research regarding discrimination and ADRD-relevant health outcomes does not disaggregate experiences of discrimination by attribution (Beatty Moody et al., 2014; Beatty Moody, Chang, et al., 2019; L. B. Zahodne et al., 2020). Focusing specifically on experiences of discrimination due to race may help to advance our understanding of the role that this key form of discrimination plays in explaining disproportionate rates of ADRD among non-Hispanic Black older adults.
While links between discrimination and structural magnetic resonance imaging (MRI) markers of brain aging are not well-established, one cross-sectional study found that greater racial discrimination was associated with greater white matter lesion burden among African Americans aged 55–70 (Beatty Moody, Taylor, et al., 2019). This finding is in line with a growing body of research linking greater discrimination to higher cardiovascular (e.g., hypertension) and inflammatory (e.g., C-reactive protein) markers (Beatty Moody et al., 2014; Beatty Moody, Chang, et al., 2019; Forde et al., 2020; L. B. Zahodne, Kraal, Sharifian, et al., 2019; L. B. Zahodne, Kraal, Zaheed, et al., 2019). Cerebrovascular health in general, and white matter disease in particular, are important predictors of future ADRD risk (Brickman et al., 2009; Song et al., 2020). For example, greater initial WMH burden, as well as the progression of WMH over time, predict incident ADRD above and beyond hippocampal atrophy (Brickman et al., 2015). Despite preliminary cross-sectional evidence for an association between racial discrimination and white matter lesions (Beatty Moody, Taylor, et al., 2019), the extent to which discrimination predicts other aspects of brain health or longitudinal processes of brain aging is unknown.
Hippocampal volume can reflect a variety of developmental, experiential, and neurodegenerative processes and is a key marker of ADRD risk and progression (Jack et al., 1999). Hippocampal volume measured in late life is sensitive to neurodegenerative changes that disproportionately affect the medial temporal lobe early in the disease (Braak & Braak, 1995). While the potential influence of discrimination on hippocampal integrity is unknown, animal and human models demonstrate that both acute (Serra et al., 2018) and chronic (Lupien et al., 2018) stress is toxic to the hippocampus. Therefore, discrimination may have negative consequences not only for cerebrovascular health (Beatty Moody, Taylor, et al., 2019), but also the structural integrity of the hippocampus, both of which are important predictors of ADRD (Brickman et al., 2015; Jack et al., 1999).
The current study sought to advance the literature on psychosocial contributors to ADRD risk among non-Hispanic Black older adults by examining associations between discrimination and longitudinal changes in ADRD-relevant MRI outcomes in a sample of 221 non-Hispanic Black older adults living in northern Manhattan. Specifically, we tested whether aggregate discrimination (regardless of attribution), as well as discrimination attributed to race (racial discrimination), predicted hippocampal volume and/or WMH measured at two time points over an average of four years. We predicted that greater discrimination would be associated with lower hippocampal volume and greater WMH burden over time.
Methods
Participants and Procedures
The 221 individuals in this study were participants in the Washington Heights-Inwood Columbia Aging Project (WHICAP; Manly et al., 2005; Tang et al., 2001) a longitudinal, community-based study of aging and dementia in northern Manhattan. In brief, neighborhood-representative adults aged 65 and older living in northern Manhattan were identified from Medicare records or a commercial marketing company starting in 1992. Study visits occur approximately every 18–24 months in participants’ homes or at the Columbia University Medical Center and include a battery of cognitive, functional, and health measures.
Data on self-reported racial/ethnic identity in WHICAP is collected using the format of the 2000 U.S. Census. The three major racial and ethnic groups represented in the WHICAP sample are Hispanics, non-Hispanic Blacks, and non-Hispanic Whites. These identity categories correspond to social constructs. As such, they capture variation in life course experiences, opportunities, and environments, but there is also substantial heterogeneity within each group. The current study was restricted to non-Hispanic Black individuals due to its primary interest in racial discrimination as a mechanism of disproportionate ADRD incidence within this group (Mayeda et al., 2016; Tang et al., 2001), and due to preliminary evidence that links between discrimination and brain outcomes differ for Black versus White adults (Meyer et al., 2019). Of the 221 individuals included in the current study, 204 (92%) reported being born in the U.S., and 17 (8%) were born outside the U.S. (e.g., Jamaica, Virgin Islands). This study complied with the ethical rules for human experimentation stated in the Declaration of Helsinki and was approved by the local institutional review board. Informed consent was obtained from all participants.
Starting in 2011, a random subsample of WHICAP participants without dementia were invited to undergo 3T MRI. Follow-up MRIs were initiated in 2016. Starting in 2017, a series of psychosocial measures that included the discrimination scales described below was added to the WHICAP core battery. The current study was restricted to participants who (1) self-reported being Black/African American and non-Hispanic; (2) had at least one time point of usable MRI data; (3) completed the battery of psychosocial measures that included the discrimination questionnaires; and (4) did not have a consensus diagnosis of dementia at the time they completed psychosocial measures. In WHICAP, dementia diagnoses are made by a consensus group of neurologists and neuropsychologists based on Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition criteria (American Psychiatric Association, 1987) using all available neuropsychological, functional and medical data, but not neuroimaging data.
Two hundred and sixty-one non-Hispanic Black participants had available psychosocial data and at least one structural MRI scan. Of those, 16 were excluded who had a consensus diagnosis of dementia (n = 14) or lack of diagnosis information (n = 2) at the visit at which psychosocial measures were collected. An additional 24 participants were excluded for missing covariates. Therefore, our final analytic sample included 221 non-Hispanic Black older adults. Compared to the larger WHICAP sample of non-Hispanic Black older adults, individuals in the current analytic sample were younger (Cohen’s d = .66), had more education (Cohen’s d = .68) and income (0.58), had lower measured systolic blood pressure (Cohen’s d = .22) and reported more depressive symptoms (Cohen’s d = .23), but they did not differ in terms of sex/gender (phi = .03) or the number of cardiometabolic/vascular health conditions (Cohen’s d = .03).
Approximately 46% (n = 102) had a follow-up MRI scan. Compared to individuals with a follow-up scan, individuals without a follow-up scan did not differ in age, sex/gender, education, income, systolic blood pressure, cardiometabolic/vascular health, depressive symptoms, discrimination, or initial WMH volume. Small differences in age and initial hippocampal volume were found such that individuals with a follow-up scan were younger (Cohen’s d = .30) and had larger hippocampi than individuals without a follow-up scan (Cohen’s d = .35). However, these differences were no longer significant after correcting for Type 1 error using a Bonferroni correction. On average, follow-up MRIs occurred 3.59 years later (SD = 1.62 years, range = 1.58 – 8.33 years, median = 3.75 years, inter-quartile range = 3 years). Sample characteristics are shown in Table 1.
Table 1.
Sample characteristics at baseline
| M or % | SD | |
|---|---|---|
|
| ||
| Age | 73.46 | 5.99 |
| % Female | 66.10 | - |
| Years of Education (0–20) | 13.79 | 2.84 |
| Income (1–12) | 8.50 | 2.57 |
| Cardiometabolic Diseasesa (0–3) | 1.17 | 0.83 |
| Systolic Blood Pressure (44–191) | 136.14 | 20.43 |
| Depressive Symptoms (0–10) | 1.17 | 1.77 |
| Everyday Discrimination (10–60) | 14.48 | 5.54 |
| Everyday Racial Discrimination (10–60) | 12.88 | 5.80 |
| Lifetime Discrimination (0–9) | 1.33 | 1.72 |
| Lifetime Racial Discrimination (0–9) | 0.66 | 1.19 |
| Hippocampal Volume (3522–11484) | 7072.60 | 874.87 |
| White Matter Hyperintensities (0.1–30.7) | 5.13 | 5.44 |
Cardiometabolic diseases included hypertension, heart disease, and diabetes
Exposures
Experiences of discrimination were operationalized with the Everyday Discrimination (Williams et al., 1997) and Major Experiences of Lifetime Discrimination (Williams et al., 2008) scales, modified to be administered orally due to the wide range of literacy levels in WHICAP. Everyday discrimination included 10 items assessing how often participants experience unfair treatment in their day-to-day lives without reference to a specific timeframe (e.g., “You are treated with less respect than other people”). Responses are on a 6-point Likert-type scale ranging from Never (6) to Almost Every Day (1). Items were reverse-scored and summed so that higher scores correspond to greater everyday discrimination. Major Experiences of Lifetime Discrimination included 9 dichotomous items assessing the occurrence of unfair treatment in relation to a variety of major life events (e.g., “At any time in your life, have you ever been unfairly fired from a job?”). Items were summed so that higher scores correspond to greater lifetime discrimination. Initial models included both everyday and lifetime discrimination scores, regardless of attributions (i.e., “aggregate discrimination”).
Subsequent models focused on racial discrimination, defined as discrimination attributed to race or skin color. After participants reported the frequency or occurrence of discrimination, they were then asked to identify the reason for these experiences out of the following categories: Ancestry/National Origin, Gender, Race, Age, Height/Weight, Skin Color, Sexual Orientation, Religion, Financial Status, Physical Disability, or Other. For everyday discrimination, only those participants who reported that at least one of the 10 items occurred more than once a year were asked to specify the reason(s) for these experiences. For participants who attributed these experiences to race and/or skin color, everyday racial discrimination was quantified as the sum of all 10 items. Participants who reported no everyday discrimination or did not attribute their experiences to race or skin color were given the lowest possible score for everyday racial discrimination. For lifetime discrimination, participants were asked to specify the main reasons for each of the 9 items. For all participants, lifetime racial discrimination was quantified as the number of life events for which discrimination was attributed to race and/or skin color. Note that the Everyday Discrimination Scale queries attributions once for all 10 items, while the Major Experiences of Lifetime Discrimination Scale queries attributions separately for each of the 9 items. This may be because the individual items of the Everyday Discrimination Scale can be conceptualized as separate indicators of the same underlying construct (e.g., interpersonal discrimination or microaggressions), whereas the individual items of the Major Experiences of Lifetime Discrimination Scale can be conceptualized as indicators of separate underlying constructs relevant to structural racism. For example, discrimination in lending may reflect the historical legacy of redlining, whereas police harassment may reflect racist criminal justice practices (e.g., stop and frisk).
Outcomes
Outcomes were measured using structural MRI obtained on a 3.0T Philips Achieva scanner at Columbia University Medical Center. T1-weighted (repetition time = 6.6 ms, echo time = 3.0 ms, field of view 256 cm, 256 × 256 matrix, 1.0 mm slice thickness) images were acquired in the axial orientation. Left and right hippocampal volumes were quantified with FreeSurfer (version 6.0; http://surfer.nmr.mgh.harvard.edu/) using T1-weighted images, and total hippocampal volume was summed across left and right hemispheres. Whole-brain WMH volumes were derived from T2-weighted FLAIR images using previously described procedures (Brickman et al., 2009, 2011, 2012). In brief, images were skull stripped, and a Gaussian curve was fit to map voxel intensity values. Voxels above a standardized study-specific threshold of the image mean were labeled as WMH. Labeled images were also visually inspected and corrected if errors were detected. Total WMH volume was log-transformed before analysis.
Covariates
Models controlled for age, sex/gender, education, income, total intracranial volume (ICV) at the initial scan, and time between scans. Age was a continuous variable reflecting age in years at the time of initial MRI scan. Sex/gender was self-reported and was represented by a binary variable (men as the reference group). Education (0–20 years) was measured via self-report. Self-reported monthly household income corresponding to the initial scan was operationalized as a 12-category variable ranging from $450 or less to more than $4,000, which was treated as a continuous variable. Total intracranial volume (ICV) was derived from T1-weighted images obtained during the initial scan using FreeSurfer version 6.0 (http://surfer.nmr.mgh.harvard.edu/). Time between scans was calculated as the number of years between the initial and follow-up MRI scans. All covariates were included as controls on both the exposure (i.e., discrimination) and outcome (i.e., hippocampal and WMH volumes) variables except for total intracranial volume, which was only included as a control on the outcome variables, and time between scans, which was only included as a control on latent change in the outcome variables.
Sensitivity analyses additionally controlled for vascular/cardiometabolic health and depressive symptoms, which are known ADRD risk factors that represent potential mediators of associations between discrimination and the MRI outcomes. Vascular/cardiometabolic health corresponding to the initial scan was indexed by two variables: (1) the sum of self-reported presence of hypertension, diabetes, and heart disease; and (2) systolic blood pressure, which was the average of three measurements. Depressive symptoms corresponding to the initial scan were quantified with a 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D; Irwin et al., 1999) Both of these additional covariates were included as controls on both exposure and outcome variables.
Analytic Strategy
Analyses were conducted in Mplus version 8. Two minimally adjusted latent difference score (LDS) models controlling only for age and sex/gender were conducted to describe changes in (1) hippocampal volume and (2) WMH over the study period (McArdle & Nesselroade, 2019). Rather than calculating raw difference scores, the LDS model defined a latent variable corresponding to the residual of the follow-up value above and beyond what is predicted by the baseline value. In the LDS model, features of change that are of interest (e.g., mean change, inter-individual variability in change, relationship between the baseline value and change) are modeled as explicit parameters (McArdle, 2009). The primary outcomes of interest in each model was the baseline values of the MRI variable (i.e., hippocampal volume or WMH), as well as latent change in the MRI variable (controlling for the baseline value).
Next, both aggregate measures of discrimination (i.e., everyday and lifetime discrimination) and primary covariates were added to the models to quantify associations (i.e., model coefficients) between aggregate discrimination and the MRI outcomes above and beyond the covariates. In subsequent models, the everyday and lifetime aggregate discrimination variables were replaced with everyday and lifetime racial discrimination variables. Finally, sensitivity analyses added the vascular/cardiometabolic health and depressive symptom covariates. In all models, missing data were managed using full information maximum likelihood. Adequate model fit was evaluated with the comparative fit index (CFI > 0.95), the root-mean-square error of approximation (RMSEA < 0.08), and the standardized root-mean square residual (SRMR < 0.08; Hu & Bentler, 1999).
Results
Descriptive statistics are listed in Table 1. As expected, aggregate discrimination scores were higher than racial discrimination scores, which were computed as a subset of aggregate scores, for both everyday (t(211)=−9.22; p<.001) and lifetime (t(217)=−10.29; p<.001) discrimination. With regard to everyday discrimination, 69.4% of participants reported experiencing any discrimination (regardless of attribution), and 50.3% of those had follow-up MRI, while 23.8% reported racial discrimination per se, and 45.3% of those had follow-up MRI.
With regard to lifetime discrimination, 53.1% reported experiencing discrimination in at least one major life event (regardless of attribution), and 52.1% of those had follow-up MRI, while 34.6% reported racial discrimination in at least one life event, and 47.4% of those had follow-up MRI. Specifically, 42 individuals reported racial discrimination in one type of life event, 17 individuals reported racial discrimination in two types of life events, seven individuals reported racial discrimination in three types of life events, six individuals reported racial discrimination in four types of life events, two individuals reported racial discrimination in five types of life events, one individual reported racial discrimination in six types of life events, and one individual reported racial discrimination in 7 types of life events.
Results from LDS models adjusted only for age and sex/gender are provided in Table 2. On average, the sample exhibited declines in hippocampal volume and increases in WMH burden over the study period. In addition, random effects revealed sufficient interindividual variability to examine predictors of both initial levels and subsequent change in both MRI outcomes.
Table 2.
Unstandardized results from LDS models adjusted only for age and sex/gender
| Initial | Latent change | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Estimate | SE | p | Estimate | SE | p | |
|
| ||||||
| Model 1: Hippocampal volume | ||||||
| Fixed effects | 7.28 | .10 | < .001 | −0.24 | .04 | < .001 |
| Random effects | 0.71 | .07 | < .001 | 0.05 | .01 | < .001 |
| Model 2: WMH volume | ||||||
| Fixed effects | 0.50 | .06 | < .001 | 0.07 | .04 | .050 |
| Random effects | 0.24 | .02 | < .001 | 0.04 | .01 | < .001 |
Note. LDS = Latent difference score; SE = Standard error; WMH = White matter hyperintensities (log-transformed)
Hippocampal Volume
Models examining aggregate discrimination fit the data well (χ2 (9) = 9.78, CFI = .99, RMSEA = .02 [.00, .08]). As shown in Table 3, neither aggregate everyday nor aggregate lifetime discrimination predicted initial hippocampal volume or change in hippocampal volume.
Table 3.
Standardized results for aggregate discrimination (regardless of attributions)
| Initial | Latent change | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Estimate | SE | p | Estimate | SE | p | |
|
| ||||||
| Model 1: Hippocampal volume | ||||||
| Everyday aggregate discrimination | .02 | .07 | .731 | .13 | .11 | .248 |
| Lifetime aggregate discrimination | −.12 | .07 | .078 | −.13 | .10 | .185 |
| Initial hippocampal volume | - | - | - | .16 | .10 | .122 |
| Total intracranial volume | .32 | .08 | < .001 | −.07 | .12 | .528 |
| Time between scans | - | - | - | −.30 | .09 | .001 |
| Age | −.27 | .07 | < .001 | −.10 | .11 | .381 |
| Sex/Gender | .00 | .08 | .995 | −.04 | .12 | .716 |
| Education | .08 | .07 | .269 | .24 | .10 | .014 |
| Income | −.09 | .07 | .166 | −.18 | .10 | .063 |
| Model 2: WMH volume | ||||||
| Everyday aggregate discrimination | .00 | .08 | .960 | .06 | .13 | .674 |
| Lifetime aggregate discrimination | .06 | .08 | .417 | −.01 | .11 | .931 |
| Initial WMH | - | - | - | −.14 | .10 | .148 |
| Total intracranial volume | .21 | .08 | .009 | −.06 | .14 | .669 |
| Time between scans | - | - | - | .10 | .10 | .318 |
| Age | .17 | .07 | .014 | .22 | .12 | .053 |
| Sex/Gender | .09 | .08 | .253 | −.09 | .13 | .490 |
| Education | −.02 | .07 | .771 | .10 | .11 | .391 |
| Income | −.05 | .07 | .527 | −.20 | .11 | .070 |
Note. WMH = White matter hyperintensities (log-transformed)
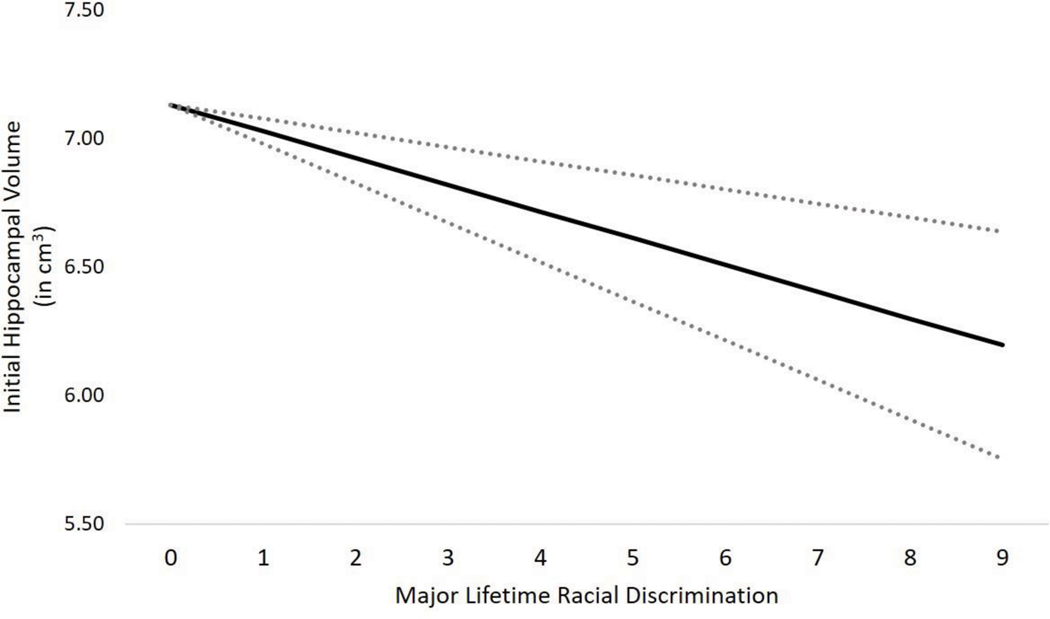
Models that replaced the aggregate discrimination variables with the specific racial discrimination variables also fit well (χ2(9) = 6.47, CFI = 1.00, RMSEA = .00 [.00, .06]). As shown in Table 4 and Figure 1, greater lifetime racial discrimination was associated with lower initial hippocampal volume. A comparison of raw (unstandardized) estimates revealed that the effect of racial discrimination in a single life event was equivalent to 2.6 years of hippocampal aging. Everyday racial discrimination was not associated with initial hippocampal volume. Neither everyday nor lifetime racial discrimination was associated with subsequent changes in hippocampal volume.
Table 4.
Standardized results for racial discrimination
| Initial | Latent change | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Estimate | SE | p | Estimate | SE | p | |
|
| ||||||
| Model 1: Hippocampal volume | ||||||
| Everyday racial discrimination | −.01 | .07 | .874 | .11 | .11 | .316 |
| Lifetime racial discrimination | −.14 | .07 | .035 | −.07 | .09 | .445 |
| Initial hippocampal volume | - | - | - | .17 | .10 | .097 |
| Total intracranial volume | .32 | .07 | < .001 | −.06 | .12 | .613 |
| Time between scans | - | - | - | −.30 | .09 | .001 |
| Age | −.28 | .06 | <.001 | −.09 | .11 | .339 |
| Sex/Gender | .00 | .08 | .956 | −.01 | .11 | .901 |
| Education | .09 | .07 | .190 | .22 | .10 | .029 |
| Income | −.09 | .07 | .174 | −.16 | .10 | .097 |
| Model 2: WMH volume | ||||||
| Everyday racial discrimination | −.01 | .07 | .918 | .25 | .11 | .038 |
| Lifetime racial discrimination | .05 | .07 | .496 | .03 | .10 | .781 |
| Initial WMH | - | - | - | −.12 | .10 | .234 |
| Total intracranial volume | .22 | .08 | .008 | −.07 | .14 | .604 |
| Time between scans | - | - | - | .10 | .10 | .279 |
| Age | .17 | .07 | .018 | .26 | .11 | .018 |
| Sex/Gender | .09 | .08 | .259 | −.09 | .13 | .481 |
| Education | −.02 | .08 | .825 | .06 | .11 | .569 |
| Income | −.05 | .07 | .452 | −.17 | .10 | .102 |
Note. WMH = White matter hyperintensities (log-transformed)
Figure 1.
Association between major lifetime racial discrimination and initial hippocampal volume, adjusted for covariates. Note. Dotted lines correspond to standard error of the estimate.
A sensitivity analysis additionally controlling for vascular/cardiometabolic diseases, systolic blood pressure, and depressive symptoms revealed an identical pattern of results (Supplementary Table 1), with no evidence for attenuation of the association between lifetime racial discrimination and initial hippocampal volume (standardized effect of −.14 versus −.15). None of the three added covariates was associated with initial hippocampal volume or change in hippocampal volume.
White Matter Hyperintensities
Models examining the two aggregate discrimination variables fit well (χ2(9) = 9.89, CFI = 1.00, RMSEA = .02 [.00, .08]). As shown in Table 3, neither aggregate everyday nor aggregate lifetime discrimination predicted initial WMH volume or change in WMH volume.
Models that replaced the aggregate discrimination variables with the specific racial discrimination variables also fit well (χ2 (9)= 6.27, CFI = 1.00, RMSEA = .00, [.00, .06]). As shown in Table 4, neither everyday nor lifetime racial discrimination was associated with initial WMH volume. Greater everyday racial discrimination, but not lifetime racial discrimination, was associated with faster accumulation of WMH over time (Figure 2). A comparison of raw (unstandardized) estimates revealed that the effect of each additional point of everyday racial discrimination was equivalent to one year of WMH aging. In other words, experiencing everyday racial discrimination “at least once a week” versus “less than once a year” was equivalent to three years of aging.
Figure 2.
Changes in WMH as a function of everyday racial discrimination, adjusted for covariates. Note. WMH = White matter hyperintensities; SD = Standard deviation.
A sensitivity analysis additionally controlling for vascular/cardiometabolic diseases, systolic blood pressure, and depressive symptoms revealed an identical pattern of results (Supplementary Table 1), with no evidence for attenuation of the association between everyday racial discrimination and WMH change (standardized effect of .25 versus .25). None of the three added covariates was associated with initial WMH volume. Only systolic blood pressure was associated with change in WMH volume, with higher blood pressure predicting more rapid WMH accumulation.
Discussion
This study of non-Hispanic Black older adults living in northern Manhattan provides evidence that racial discrimination is associated with hippocampal and WMH volumes even after accounting for socioeconomic status, vascular/cardiometabolic health, and depressive symptoms. Specifically, reporting more instances of racial discrimination across major life domains (e.g., labor market, housing) was associated with lower initial hippocampal volume, while reporting more frequent everyday racial discrimination was associated with a faster accumulation of WMH over four years. These associations were specific to racial discrimination, as opposed to aggregate reports of discrimination regardless of attribution. This study adds to a growing body of evidence suggesting that racially-patterned social stress may contribute to the disproportionate rates of ADRD seen among non-Hispanic Black older adults over and above socioeconomic status.
The longitudinal finding that greater everyday racial discrimination was associated with faster accumulation of WMH over four years extends a recent cross-sectional study in which greater lifetime racial discrimination was associated with greater white matter lesion burden among African Americans aged 55–70 in the Healthy Aging in Neighborhoods of Diversity study (Beatty Moody, Taylor, et al., 2019). The link between racial discrimination and WMH may reflect known associations between discrimination and worse cardiovascular health(Beatty Moody, Chang, et al., 2019; Forde et al., 2020), as well as greater systemic inflammation (Beatty Moody et al., 2014; L. B. Zahodne, Kraal, Sharifian, et al., 2019; L. B. Zahodne, Kraal, Zaheed, et al., 2019). Indeed, both cardiovascular health (Moroni et al., 2018) and inflammation (Low et al., 2019) have been linked to WMH accumulation. However, the fact that racial discrimination predicted WMH changes above and beyond the number of vascular/cardiometabolic diseases and systolic blood pressure may suggest that discrimination has direct neurotoxic effects on white matter integrity regardless of systemic vascular health. Future research incorporating more specific indicators of vascular health that better capture extent, severity, and duration of conditions, as well as data on inflammation, is needed to clarify physiological mechanisms linking racial discrimination to the accumulation of WMH.
While the current study found a longitudinal association between everyday racial discrimination and WMH accumulation, there were no associations between lifetime racial discrimination and WMH. The lack of association between lifetime racial discrimination and WMH contrasts with the study by Beatty Moody and Taylor et al. (2019), which did not include measures of everyday discrimination. Thus, the lack of association between lifetime racial discrimination and WMH in the current study may relate to shared variance between everyday and lifetime racial discrimination, which were moderately correlated in the current study. Another potential explanation for the discrepant results regarding lifetime racial discrimination relates to the ages of the samples. Specifically, participants in the current study (mean age of 73) were older than those in the study by Beatty Moody and Taylor et al. (2019) (mean age of 51). It may be that the consequences of discrimination in major life domains (e.g., labor market, housing) are more obvious for the initial appearance of WMH earlier in life, whereas the accumulation of other sources of individual differences across older adulthood could overshadow these initial effects. Of note, the included measure of lifetime racial discrimination may be more likely to reflect cumulative experiences of systemic racism than day-to-day interpersonal racism, and many of the queried life events are likely to have been encountered prior to older adulthood. In contrast, the continued experience of everyday discrimination throughout late life may be more consequential for the rate of ongoing accumulation of WMH. Another difference between the current study and that of Beatty Moody and Taylor et al. (2019) is that the current study included both everyday discrimination and lifetime experiences of discrimination in major life events, while that previous study only examined the latter.
The finding that greater lifetime racial discrimination was associated with lower initial hippocampal volume is in line with a substantial body of research demonstrating the specific neurotoxic effects of stress on hippocampal integrity (Lupien et al., 2018). The current study extends this work by highlighting racial discrimination as a unique and potent social stressor that may have similar negative effects on the hippocampus among non-Hispanic Black older adults. However, physiological stress pathways represent only one potential set of mechanisms by which lifetime racial discrimination could affect hippocampal integrity. Racial discrimination in domains such as the labor market, housing, and the criminal justice system could all have long-lasting effects on an individual’s life course opportunities and environments, which could ultimately affect brain health independent of perceived stress. As just one example, racial discrimination in housing could result in greater lifetime exposure to air pollution or other environmental toxicants that are particularly deleterious to hippocampal development (Costa et al., 2017). More research is needed to understand all of the potential mechanisms linking systemic racism to brain health.
Interestingly, the association between racial discrimination and initial hippocampal volume did not extend to subsequent changes in hippocampal volume. This pattern of results may suggest that racial discrimination in major life events has a stronger influence on hippocampal development and maintenance earlier in life than on rate of hippocampal atrophy during late life. However, because of the limited follow-up in the current study (i.e., four years, on average), additional follow-up is needed to determine whether the effects of discrimination on hippocampal atrophy may be measurable over a longer time period.
In the current study, measures of discrimination were only associated with the MRI outcomes once the attributions of discrimination were disaggregated. That is, discrimination attributed to race and/or skin color was linked to brain measures, while aggregate discrimination regardless of attribution was not. This pattern of results suggests that discrimination based on race and/or skin color may be particularly detrimental to brain health among non-Hispanic Black older adults, which is consistent with research on discrimination and other health outcomes.38 Race-based discrimination may be particularly harmful for non-Hispanic Black older adults when race is a salient aspect of self-identity, as it has been theorized that stressors that threaten individuals’ most central self-conceptions are most damaging (Thoits, 2013). Future work on discrimination and ADRD inequalities should consider disaggregating reports of discrimination by attribution, as disregarding attributions could result in underestimations of the negative effects of racial discrimination per se.
Limitations of this study include the timing of the discrimination assessments, which typically occurred after the first MRI due to the timing of grant funding, making our results more vulnerable to reverse causation. However, it should be noted that the measure of lifetime discrimination is cumulative rather than time-locked. Further, extant longitudinal research (Paradies, 2006) suggests that everyday racial discrimination, which has been shown to be relatively stable over 5 years (Lewis et al., 2006), leads to WMH rather than vice versa. Another limitation relates to the relatively low proportion of participants who had follow-up MRIs, which reflects a disruption in neuroimaging data collection due to COVID-19. In the current study, we chose to include all participants who had at least one MRI in order to maximize power and minimize potential attrition bias. Importantly, there were no reliable differences between individuals with and without a follow-up scan, which supports the adequacy of the missing data procedure (i.e., full information maximum likelihood). While we were able to detect longitudinal changes in both brain outcomes, as well as an association between everyday racial discrimination and change in WMH burden, future studies are needed to determine whether additional longitudinal associations would have been evident with more follow-up data. It should be noted that the current study included a regional sample of non-Hispanic Black older adults living in northern Manhattan. Therefore, results may not be generalizable to individuals in other geographic regions or individuals living in non-urban settings. Because the current study only included non-Hispanic Black individuals without dementia who were able to undergo MRI, it is possible that the current study underestimated the negative impacts of discrimination on brain health.
Strengths of this study include the large, regionally representative sample of non-Hispanic Black older adults with neuroimaging data. Other strengths are the inclusion of two measures of discrimination (lifetime and everyday) and the consideration of discrimination attributions, which allowed for preliminary conclusions about the relative effects of different types of discrimination and the generation of specific recommendations for future research on discrimination and ADRD.
In conclusion, this study found that racial discrimination was associated with hippocampal and WMH volumes in non-Hispanic Black older adults. Future research should clarify specific mechanisms underlying these associations, such as physiological stress pathways, environmental exposures, and life course opportunities. Mechanisms may differ for different types of institutional versus interpersonal racism, as well as for neurodegenerative versus cerebrovascular markers of brain health. Given that both hippocampal volume (Jack et al., 1999) and WMH (Brickman et al., 2015) predict incident ADRD, future research should incorporate measures of racial discrimination to understand how racial disparities in brain and cognitive aging contribute to ADRD inequalities.
Supplementary Material
References
- American Medical Association. (2020). AMA Board of Trustees pledges action against racism, police brutality | American Medical Association. https://www.ama-assn.org/press-center/ama-statements/ama-board-trustees-pledges-action-against-racism-police-brutality
- American Psychiatric Association. (1987). Diagnostic and Statistical Manual of Mental Disorders - Third Revised Version (DSM III-R). [Google Scholar]
- Barnes LL, Lewis TT, Begeny CT, Yu L, Bennett DA, & Wilson RS (2012). Perceived Discrimination and Cognition in Older African Americans. Journal of the International Neuropsychological Society, 18(5), 856–865. 10.1017/S1355617712000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes Lisa L., Leon De, M CF, Wilson RS, Bienias JL, Bennett DA, & Evans DA (2004). Racial Differences in Perceived Discrimination in a Community Population of Older Blacks and Whites. Journal of Aging and Health, 16(3), 315–337. 10.1177/0898264304264202 [DOI] [PubMed] [Google Scholar]
- Beatty Moody DL, Brown C, Matthews KA, & Bromberger JT (2014). Everyday Discrimination Prospectively Predicts Inflammation across 7-Years in Racially Diverse Midlife Women: Study of Women’s Health across the Nation. Journal of Social Issues, 70(2), 298–314. 10.1111/josi.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Chang YF, Pantesco EJ, Darden TM, Lewis TT, Brown C, Bromberger JT, & Matthews KA (2019). Everyday discrimination prospectively predicts blood pressure across 10 years in racially/ethnically diverse midlife women: Study of women’s health across the nation. Annals of Behavioral Medicine, 53(7), 608–620. 10.1093/abm/kay069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Taylor AD, Leibel DK, Al-Najjar E, Katzel LI, Davatzikos C, Gullapalli RP, Seliger SL, Kouo T, Erus G, Rosenberger WF, Evans MK, Zonderman AB, & Waldstein SR (2019). Lifetime discrimination burden, racial discrimination, and subclinical cerebrovascular disease among African Americans. Health Psychology, 38(1), 63–74. 10.1037/hea0000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, & Braak E (1995). Staging of alzheimer’s disease-related neurofibrillary changes. Neurobiology of Aging, 16(3), 271–278. 10.1016/0197-4580(95)00021-6 [DOI] [PubMed] [Google Scholar]
- Brickman AM, Muraskin J, & Zimmerman ME (2009). Structural neuroimaging in Altheimer’s disease: do white matter hyperintensities matter? Dialogues in Clinical Neuroscience, 11(2), 181–190. 10.31887/DCNS.2009.11.2/ambrickman [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, Stern Y, Brown TR, Luchsinger JA, & Mayeux R (2012). Regional White Matter Hyperintensity Volume, Not Hippocampal Atrophy, Predicts Incident Alzheimer Disease in the Community. Archives of Neurology, 69(12), 1621. 10.1001/archneurol.2012.1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Sneed JR, Provenzano FA, Garcon E, Johnert L, Muraskin J, Yeung L-K, Zimmerman ME, & Roose SP (2011). Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Research: Neuroimaging, 193(2), 101–106. 10.1016/j.pscychresns.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, Provenzano FA, Schupf N, Manly JJ, Stern Y, Luchsinger JA, & Mayeux R (2015). Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiology of Aging, 36(1), 27–32. 10.1016/j.neurobiolaging.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang Y-C, Dao K, & Roqué PJ (2017). Neurotoxicity of traffic-related air pollution. NeuroToxicology, 59, 133–139. 10.1016/j.neuro.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde AT, Sims M, Muntner P, Lewis T, Onwuka A, Moore K, & Diez Roux AV (2020). Discrimination and Hypertension Risk Among African Americans in the Jackson Heart Study. Hypertension, 76(3), 715–723. 10.1161/HYPERTENSIONAHA.119.14492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Irwin M, Artin KH, & Oxman MN (1999). Screening for Depression in the Older Adult. Archives of Internal Medicine, 159(15), 1701. 10.1001/archinte.159.15.1701 [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, & Kokmen E (1999). Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology, 52(7), 1397–1397. 10.1212/WNL.52.7.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, Jacobs E, & Wesley D (2006). Chronic Exposure to Everyday Discrimination and Coronary Artery Calcification in African-American Women: The SWAN Heart Study. Psychosomatic Medicine, 68(3), 362–368. 10.1097/01.psy.0000221360.94700.16 [DOI] [PubMed] [Google Scholar]
- Low A, Mak E, Rowe JB, Markus HS, & O’Brien JT (2019). Inflammation and cerebral small vessel disease: A systematic review. Ageing Research Reviews, 53, 100916. 10.1016/j.arr.2019.100916 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Juster R-P, Raymond C, & Marin M-F (2018). The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Frontiers in Neuroendocrinology, 49, 91–105. 10.1016/j.yfrne.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Manly JJ, Bell-McGinty S, Tang M-X, Schupf N, Stern Y, & Mayeux R (2005). Implementing Diagnostic Criteria and Estimating Frequency of Mild Cognitive Impairment in an Urban Community. Archives of Neurology, 62(11), 1739. 10.1001/archneur.62.11.1739 [DOI] [PubMed] [Google Scholar]
- Mayeda ER, Glymour MM, Quesenberry CP, & Whitmer RA (2016). Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s & Dementia, 12(3), 216–224. 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ (2009). Latent Variable Modeling of Differences and Changes with Longitudinal Data. Annual Review of Psychology, 60(1), 577–605. 10.1146/annurev.psych.60.110707.163612 [DOI] [PubMed] [Google Scholar]
- McArdle JJ, & Nesselroade JR (2019). Using Multivariate Data to Structure Developmental Change. In Life-Span Developmental Psychology (pp. 223–267). Psychology Press. 10.4324/9781315792712-10 [DOI] [Google Scholar]
- Meyer CS, Schreiner PJ, Lim K, Battapady H, & Launer LJ (2019). Depressive Symptomatology, Racial Discrimination Experience, and Brain Tissue Volumes Observed on Magnetic Resonance Imaging. American Journal of Epidemiology, 188(4), 656–663. 10.1093/aje/kwy282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Ammirati E, Rocca MA, Filippi M, Magnoni M, & Camici PG (2018). Cardiovascular disease and brain health: Focus on white matter hyperintensities. IJC Heart & Vasculature, 19, 63–69. 10.1016/j.ijcha.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzon DM, Taylor RJ, Woodward AT, & Chatters LM (2017). Everyday Racial Discrimination, Everyday Non-Racial Discrimination, and Physical Health Among African-Americans. Journal of Ethnic & Cultural Diversity in Social Work, 26(1–2), 68–80. 10.1080/15313204.2016.1187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KB (2020). American Medical Association: Racism is a threat to public health | American Medical Association. https://www.ama-assn.org/delivering-care/health-equity/ama-racism-threat-public-health
- Paradies Y (2006). A systematic review of empirical research on self-reported racism and health. International Journal of Epidemiology, 35(4), 888–901. 10.1093/ije/dyl056 [DOI] [PubMed] [Google Scholar]
- Serra M, Poddighe L, Boi M, Sanna F, Piludu M, Sanna F, Corda M, Giorgi O, & Quartu M (2018). Effect of Acute Stress on the Expression of BDNF, trkB, and PSA-NCAM in the Hippocampus of the Roman Rats: A Genetic Model of Vulnerability/Resistance to Stress-Induced Depression. International Journal of Molecular Sciences, 19(12), 3745. 10.3390/ijms19123745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Xu H, Pan K, Qi X, Buchman AS, Bennett DA, & Xu W (2020). Association of cerebrovascular risk burden with mild cognitive impairment, dementia and brain vascular pathologies. Alzheimer’s & Dementia, 16(S10). 10.1002/alz.040528 [DOI] [Google Scholar]
- Tang M-X, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, & Mayeux R (2001). Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology, 56(1), 49–56. 10.1212/WNL.56.1.49 [DOI] [PubMed] [Google Scholar]
- Thoits PA (2013). Self, Identity, Stress, and Mental Health. In Aneshensel CS, Phelan JC, & Bierman A (Eds.), Handbook of the Sociology of Mental Health (pp. 357–377). Springer; Netherlands. 10.1007/978-94-007-4276-5_18 [DOI] [Google Scholar]
- Williams DR, Gonzalez HM, Williams S, Mohammed SA, Moomal H, & Stein DJ (2008). Perceived discrimination, race and health in South Africa. Social Science and Medicine, 67(3), 441–452. 10.1016/j.socscimed.2008.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Yan Yu, Jackson JS, & Anderson NB (1997). Racial Differences in Physical and Mental Health. Journal of Health Psychology, 2(3), 335–351. 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Kraal AZ, Sharifian N, Zaheed AB, & Sol K (2019). Inflammatory mechanisms underlying the effects of everyday discrimination on age-related memory decline. Brain, Behavior, and Immunity, 75, 149–154. 10.1016/j.bbi.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Kraal AZ, Zaheed A, Farris P, & Sol K (2019). Longitudinal effects of race, ethnicity, and psychosocial disadvantage on systemic inflammation. SSM - Population Health, 7, 100391. 10.1016/j.ssmph.2019.100391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Azar M, Brickman AM, & Glymour MM (2016). Racial Disparities in Cognitive Performance in Mid- and Late Adulthood: Analyses of Two Cohort Studies. Journal of the American Geriatrics Society, 64(5), 959–964. 10.1111/jgs.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Morris EP, Sharifian N, Zaheed AB, Kraal AZ, & Sol K (2020). Everyday discrimination and subsequent cognitive abilities across five domains. Neuropsychology, 34(7), 783–790. 10.1037/neu0000693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Sol K, & Kraal Z (2019). Psychosocial Pathways to Racial/Ethnic Inequalities in Late-Life Memory Trajectories. The Journals of Gerontology: Series B, 74(3), 409–418. 10.1093/geronb/gbx113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne L, Manly J, Narkhede A, Griffith E, DeCarli C, Schupf N, Mayeux R, & Brickman A (2015). Structural MRI Predictors of Late-Life Cognition Differ Across African Americans, Hispanics, and Whites. Current Alzheimer Research, 12(7), 632–639. 10.2174/1567205012666150530203214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.