Abstract
The borreliacidal-antibody test has been used for the serological detection and confirmation of Lyme borreliosis. However, the presence of antimicrobial agents in serum can confound the accurate detection of borreliacidal antibodies. In this study, we developed a Bacillus subtilis agar diffusion bioassay to detect small concentrations of antimicrobial agents in serum. We also used XAD-16, a nonionic polymeric resin, to adsorb and remove high concentrations of amoxicillin, cefotaxime, ceftriaxone, cefuroxime, doxycycline, and erythromycin without significantly affecting even small concentrations of immunoglobulin M (IgM) or IgG borreliacidal antibodies. High concentrations of penicillin could also be removed by adding 1 U of penicillinase without significantly influencing the levels of borreliacidal antibodies. These simple procedures greatly enhance the clinical utility of the borreliacidal-antibody test.
Lyme borreliosis, caused by infection with Borrelia burgdorferi sensu lato, is the most prevalent tick-borne illness in the United States (12). The widespread availability of an accurate serodiagnostic test remains desirable because of shortcomings in the sensitivity and/or specificity of most diagnostic methods used today (4). Highly specific borreliacidal antibodies, especially against B. burgdorferi outer surface protein A (OspA) (9, 14, 23, 24), OspB (24), and OspC (22), are often present in Lyme borreliosis patient sera. Borreliacidal antibodies against OspA and/or OspB can be detected during early-localized infection (2, 7). However, high titers of borreliacidal antibodies against these Osps are detected primarily in sera from patients with Lyme arthritis (7, 9, 14, 23, 24). Similarly, borreliacidal antibodies against other B. burgdorferi proteins, especially OspC (22), have been demonstrated to be present in sera from patients recently infected with B. burgdorferi (7). High titers of anti-OspC borreliacidal antibodies are most often present during early-localized infection (7, 22). Thus, a standardized borreliacidal-antibody test should be useful for accurately detecting early and late infection with B. burgdorferi. Determining the protein specificity and titer of the borreliacidal-antibody response can also provide insight into the duration of illness, which can be important when selecting appropriate therapy. More detailed reviews of the clinical utility of the borreliacidal-antibody test have been published previously (6, 8).
Borreliacidal antibodies are detected by incubating viable B. burgdorferi organisms with serum and complement for 16 to 24 h. If serum contains borreliacidal antibodies, spirochetes will be readily killed. The use of live spirochetes increases the specificity by eliminating the detection of cross-reactive antibodies that bind to the organism but are incapable of killing the spirochetes. A limitation of the borreliacidal-antibody test has been the necessity of ensuring that antimicrobial agents are not present in the serum. Antimicrobial agents may kill B. burgdorferi and cause a false-positive test. In this study, we developed simple procedures for detection and elimination of antimicrobial agents before testing for borreliacidal antibodies. High concentrations of antimicrobial agents commonly used to treat Lyme borreliosis were removed from serum without affecting even small concentrations of immunoglobulin M (IgM) or IgG borreliacidal antibodies. These findings greatly enhance the clinical utility of the borreliacidal-antibody test.
MATERIALS AND METHODS
Organisms.
Low passage (<10 times) B. burgdorferi sensu stricto isolates 297 (human spinal fluid) and 50772 (Ixodes scapularis) were grown at 35°C (22) in Barbour-Stoenner-Kelly (BSK) medium until reaching a concentration of approximately 108 organisms per ml. Following incubation, 500-μl aliquots of each isolate were dispensed into sterile 1.5-ml screw-cap tubes (Sarstedt, Newton, N.C.) and stored at −70°C until used.
Lyme sera.
Human serum was obtained from separate patients with clinically documented early-localized (erythema migrans) or late-disseminated (arthritis) Lyme borreliosis. Serum was also obtained from a healthy individual with no previous history of Lyme borreliosis or serologic evidence of past exposure. Patients donating serum had not been treated with antimicrobial agents during the previous 60 days.
Antimicrobial agents.
Antimicrobial agents commonly used to treat Lyme borreliosis (20) were obtained from the manufacturer or distributor (ceftriaxone [Roche Laboratories, Belvidere, N.J.]; cefotaxime [Hoechst-Roussel Pharmaceuticals, Sommerville, N.J.]; doxycycline [Pfizer, Inc., Groton, Conn.]; cefuroxime [Glaxo Pharmaceuticals, Research Triangle Park, N.C.]; amoxicillin, erythromycin, and penicillin G [Sigma Chemical, St. Louis, Mo.]). Stock solutions of ceftriaxone, cefotaxime, doxycycline, cefuroxime, amoxicillin, and erythromycin were prepared by dissolving each antimicrobial agent in distilled water such that the final concentration was 3,200 μg/ml (3). Penicillin G was prepared at 200,000 U/ml with distilled water. Stock solutions were then filter-sterilized by using a 0.20-μm syringe-tip filter, and 500-μl aliquots were dispensed into 1.5-ml microcentrifuge tubes (Sarstedt) and stored at −70°C until used.
Detection of borreliacidal antibodies.
Borreliacidal antibodies were detected with a flow cytometer as previously described (7, 10, 22). Briefly, a frozen aliquot of B. burgdorferi sensu stricto isolate 297 or 50772 was thawed, 200 μl was inoculated into 6 ml of fresh BSK medium, and the cultures were incubated at 35°C for 72 h. Following incubation, the concentration of B. burgdorferi organisms was adjusted to 106 spirochetes per ml with fresh BSK medium. A 100-μl amount of serum diluted 1:5 with BSK medium and sterilized by passage through a 0.2-μm centrifuge filter tube (Corning Costar, Cambridge, Mass.) was transferred to a sterile 1.5-ml microcentrifuge tube (Sarstedt) and heat inactivated at 56°C for 10 min. Subsequently, 100 μl of the B. burgdorferi suspension (105 organisms) and 10 μl of sterile guinea pig complement (≥210 50% hemolytic complement units; Gibco Laboratories, Grand Island, N.Y.) were added to the diluted serum. Assays were gently mixed and incubated at 35°C for 16 to 24 h.
Following incubation, 100 μl of each assay suspension was diluted 1:5 in phosphate-buffered saline (PBS) containing acridine orange (5 × 10−9 M) and analyzed by using a FACScan flow cytometer (Becton-Dickinson Immunocytometry Systems, San Jose, Calif.). For each sample, events were acquired in the list mode for 60 s. The sample flow rate was set to 12 μl/min to reduce signal variability. Analysis of data was accomplished by using LYSYS II research software (Becton-Dickinson Immunocytometry Systems). Live gating with side-scatter and FL1 fluorescence dot plots was used to identify spirochetes for analysis. All signals were logarithmically amplified and converted to a linear scale for comparison after analysis. A sample was considered to be positive for borreliacidal antibody if the increase in fluorescence intensity was ≥13% (7). A detailed technical description of the flow cytometric borreliacidal-antibody test with examples of histograms and dot plots has recently been published (5).
Removal of borreliacidal antibodies.
A 125-μl volume of goat anti-human IgM or IgG (heavy and light chain; Kallestad, Chaska, Minn.) was added to 25 μl of control or Lyme borreliosis patient serum sample and the mixture was incubated for 2 h at 37°C. After incubation, samples were centrifuged at 5,000 × g for 10 min (Surespin; Helena Laboratories, Beaumont, Tex.). Supernatants (100 μl) were then removed, diluted threefold with fresh BSK medium, and sterilized by passage through a 0.2-μm centrifuge filter tube (Corning Costar). After sterilization, samples were tested for borreliacidal activity as described. The borreliacidal activity of control and Lyme borreliosis patient sera before removal of IgM or IgG antibodies was determined after the addition of 125 μl of PBS.
Western blotting.
Western blotting was performed as previously described (22). Briefly, B. burgdorferi sensu stricto isolate 50772 or 297 spirochetes were boiled in sample buffer for 5 min and 150 μg of total protein was loaded onto a 0.1% sodium dodecyl sulfate–12% polyacrylamide gel (4% polyacrylamide stacking gel without comb). Protein concentrations were determined with a protein determination kit (Bio-Rad Inc., Richmond, Calif.). Two gels were run simultaneously in an electrophoresis unit (SE600; Hoefer Scientific Instruments, San Francisco, Calif.) at 55 mA for 3 h with the buffer system of Laemmli (16). After electrophoresis, proteins were transferred to nitrocellulose for 3 h at 300 mA under conditions described by Towbin et al. (30). The nitrocellulose was cut into strips and blocked with PBS–0.3% Tween 20 for 30 min at 22°C. Strips were incubated for 1 h at 22°C with human serum diluted 1:100 and washed three times with PBS–0.05% Tween 20. Horseradish peroxidase-labeled anti-human IgM or IgG (heavy and light chains; Organon Teknika Cappel, Malvern, Pa.) was added, and the strips were incubated for 30 min at 22°C. After incubation, strips were washed and developed (TMB membrane peroxidase substrate system; Kirkegaard & Perry Laboratories, Gaithersburg, Md.).
Detection of antimicrobial agents.
We used an agar diffusion bioassay (13) to detect antimicrobial agents. Mueller-Hinton agar plates containing 106 Bacillus subtilis spores per ml were prepared. Wells (diameter, 5 mm) were cut into the agar by using the nondispensing end of a sterile 2-ml serological pipette. Agar plugs were removed and discarded prior to loading serum samples. Fifty-microliter amounts of sera containing serial dilutions of each antimicrobial agent were loaded into individual wells, and the plates were incubated for 4 to 6 h at 37°C. After incubation, clear zones of inhibition (no B. subtilis growth) were measured to determine the minimum detectable concentration. The minimum detectable concentration was considered the smallest concentration (in micrograms per milliliter) of antimicrobial agent yielding a zone of inhibition that was >8 mm in diameter.
Removal of antimicrobial agents from sera.
Amberlite XAD-16 nonionic polymeric adsorbent beads (Aldrich Chemical Co., Milwaukee, Wis.) were washed three times with 25-ml volumes of sterile PBS (0.01 M, pH 7.2). Doxycycline, cefotaxime, ceftriaxone, cefuroxime, amoxicillin, and erythromycin were removed from serum by combining 1 g of washed XAD-16 and 500 μl of serum diluted fivefold in PBS and incubating the combination at room temperature for 20 min with occasional mixing. Following adsorption, the diluted serum was removed from the beads and tested for borreliacidal activity. Penicillin G was removed by adding 1 U of penicillinase to the serum and incubating the mixture at ambient temperature for 10 min prior to analysis.
RESULTS
Detection of antimicrobial agents.
Antimicrobial agents were added to normal serum samples to determine the minimum detectable concentrations. Distinct zones of inhibition were observed at concentrations of 0.5 μg/ml or less for all antimicrobial agents except ceftriaxone (Table 1). B. subtilis organisms were killed only when the ceftriaxone concentration was >2 μg/ml. Zones of inhibition were also not detectable at lower concentrations of ceftriaxone when several other organisms, such as Escherichia coli, were used.
TABLE 1.
Minimum concentrations of various antimicrobial agents detectable by a B. subtilis agar diffusion bioassay
| Antimicrobial agent | Minimum detectable concentration (μg/ml)a |
|---|---|
| Amoxicillin | 0.125 |
| Cefotaxime | 0.5 |
| Ceftriaxone | 2.0 |
| Cefuroxime | 0.5 |
| Doxycycline | ≤0.03 |
| Erythromycin | 0.25 |
| Penicillin | 0.125 |
Zone of clearing, ≥8 mm.
Effect of removal of IgM or IgG borreliacidal antibodies on borreliacidal activity.
We determined whether IgM and/or IgG borreliacidal antibodies were present in sera from patients with early-localized or late-disseminated disease. Anti-B. burgdorferi sensu stricto isolate 50772 borreliacidal antibodies were detectable with a dilution of up to 1:5,120 in serum from a patient with erythema migrans lesions. The borreliacidal-antibody titer remained at 1:5,120 after removal of IgG antibodies. When IgM was removed without adsorbing IgG antibodies, the anti-B. burgdorferi sensu stricto isolate 50772 borreliacidal-antibody titer was reduced to 1:320. In an additional study, IgG antibodies were removed first followed by removal of IgM antibodies. After removing IgG, the borreliacidal-antibody titer was 1:5,120. However, borreliacidal antibodies were no longer detectable (titer, <1:40) when IgM antibodies were removed. In subsequent studies, IgG antibodies were removed from early-stage Lyme borreliosis patient serum so that the effects on IgM borreliacidal antibodies could be more accurately determined. Anti-B. burgdorferi sensu stricto isolate 297 borreliacidal antibodies were detectable at a dilution of 1:2,560 in serum from the patient with Lyme arthritis. After removing the IgM antibodies, the borreliacidal activity remained detectable with a dilution of up to 1:2,560. However, the borreliacidal activity was completely abrogated (titer, <1:40) after removing IgG antibodies.
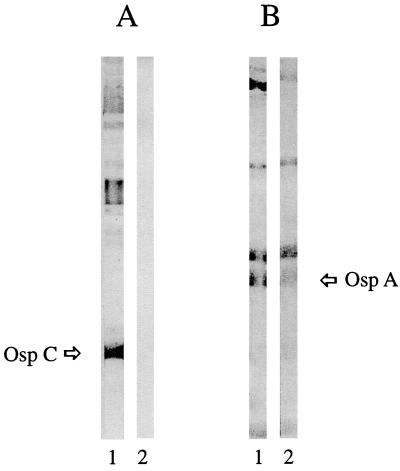
We next determined the protein specificities of the IgM and IgG borreliacidal antibodies. Anti-OspC and anti-OspA antibodies were readily detectable by Western blotting in the sera from the patients with early-localized and late-disseminated Lyme borreliosis, respectively (Fig. 1). After removing IgM antibodies from the early-stage Lyme disease patient serum and IgG antibodies from the late-stage patient serum, anti-OspC and anti-OspA antibodies were no longer detectable. These results confirmed our previous observations (9, 22) that OspC and OspA induce borreliacidal antibodies.
FIG. 1.
Western blots of Lyme borreliosis patient sera. (A) Early-stage serum before (lane 1) and after (lane 2) removal of IgM antibodies. (B) Late-stage serum before (lane 1) and after (lane 2) removal of IgG antibodies.
Removal of antimicrobial agents from serum.
We added increasing amounts of each of the six antimicrobial agents to the control serum to determine the maximum concentrations which could be removed by treatment with XAD-16. To confirm complete removal of the antimicrobial agents, sera were tested before and after adsorption. Concentrations of 80, 640, 80, 1,280, 40, and 40 μg/ml of amoxicillin, cefotaxime, ceftriaxone, cefuroxime, doxycycline, and erythromycin were removed from serum after adsorption with XAD-16 (Table 2). In contrast, penicillin G was not removed by treatment with XAD-16. However, 20,480 U of penicillin G were inactivated by adding 1 U of penicillinase to serum. Subsequently, all serum samples tested for borreliacidal activity were treated with XAD-16.
TABLE 2.
Maximum concentrations of various antimicrobial agents which could be removed by adsorption with XAD-16
| Antimicrobial agent | Maximum removable concentration (μg/ml) |
|---|---|
| Amoxicillin | 80 |
| Cefotaxime | 640 |
| Ceftriaxone | 80 |
| Cefuroxime | 1,280 |
| Doxycycline | 40 |
| Erythromycin | 40 |
| Penicillina | 0 |
Addition of 1 U of penicillinase inactivated ≥20,480 U of penicillin.
Effect of removal of antimicrobial agents on borreliacidal activity.
To determine whether removing the antimicrobial agents also removed borreliacidal antibodies, we diluted the Lyme borreliosis patient sera with control serum so that IgM or IgG borreliacidal antibodies were only detectable with a serum dilution of up to 1:40. We then added antimicrobial agents to the peak concentrations obtainable in vivo (19), removed them with XAD-16 or penicillinase, and determined the effect on borreliacidal-antibody detection. In all instances, borreliacidal antibodies were still detectable, although the borreliacidal-antibody titer had decreased by a dilution (1:20) in some samples (Table 3). Similar results were obtained when these experiments were repeated. Thus, the removal of antibacterial agents by using XAD-16 or penicillinase did not significantly decrease the ability to detect borreliacidal antibodies.
TABLE 3.
Effect of removal of various antimicrobial agentsc on small concentrationsa of IgM or IgG borreliacidal antibodies
| Antimicrobial agent removed | Borreliacidal antibody
titer (reciprocal dilution)
|
|
|---|---|---|
| Anti-OspC IgM | Anti-OspA IgG | |
| Amoxicillin | 40 | 20 |
| Cefotaxime | 20 | 20 |
| Ceftriaxone | 40 | 20 |
| Cefuroxime | 20 | 20 |
| Doxycycline | 20 | 20 |
| Erythromycin | 40 | 20 |
| Penicillinb | 40 | 40 |
Borreliacidal-antibody titers in Lyme borreliosis serum samples were adjusted to 1:40 with the addition of control serum.
Penicillin was removed by the addition of 1 U of penicillinase.
Amoxicillin, 4 μg/ml; cefotaxime, 21 μg/ml; ceftriaxone, 62 μg/ml; cefuroxime, 27 μg/ml; doxycycline, 3 μg/ml; erythromycin, 0.5 μg/ml; and penicillin, 2 μg/ml.
DISCUSSION
To improve the specificity of the serodiagnosis of Lyme borreliosis, the Centers for Disease Control and Prevention recommends that sera be tested by an enzyme-linked immunosorbent assay or indirect fluorescent-antibody (IFA) test followed by confirmation of equivocal and positive results with Western blotting (15). This approach is time-consuming and may yield inaccurate results (4). In addition, the recent approval and anticipated widespread use of commercial OspA Lyme vaccines (27, 28) will further confound diagnosis by this two-tiered testing procedure. A single laboratory test with high sensitivity, specificity, and the ability to discriminate between vaccination and Lyme borreliosis cases is still needed.
Detection of borreliacidal antibodies may be more accurate. Previously, we showed that the borreliacidal-antibody test can be sensitive and highly specific for serodiagnosis of Lyme borreliosis, especially when performed with a flow cytometer (7, 9–11, 22). In this investigation, IgM and IgG borreliacidal antibodies were present at high concentrations in sera from two patients with early-localized and late-disseminated Lyme borreliosis, respectively. The IgM antibodies primarily recognized OspC, while the IgG antibodies were specific for OspA. These results affirm our previous observations (7, 22) that borreliacidal antibodies against OspA are primarily associated with Lyme arthritis patients, while anti-OspC borreliacidal antibodies are common in sera from patients with erythema migrans lesions. These results are not surprising, especially since B. burgdorferi organisms up-regulate OspC and concomitantly down-regulate OspA shortly before tick inoculation of the host with spirochete (26, 29). Collectively, these results provide additional compelling evidence that detection of highly specific borreliacidal antibodies against OspC and other proteins besides OspA can be an accurate indicator of B. burgdorferi infection regardless of the Lyme borreliosis vaccination history.
Although the borreliacidal-antibody test is sensitive and highly specific for detection of Lyme borreliosis, its inability to discriminate borreliacidal antibodies from killing of B. burgdorferi by antimicrobial agents has been a concern. Members of our group (18) previously showed that a flow cytometric IFA test could be used for differentiation. However, this procedure was labor-intensive and could be inaccurate, like the enzyme-linked immunosorbent assay or IFA procedure, if serum contained cross-reactive antibodies. Thus, borreliacidal antibodies cannot always be accurately detected when serum contains antimicrobial agents.
In the current study, we developed a simple procedure for detecting small serum concentrations of antimicrobial agents commonly used to treat Lyme borreliosis. Distinct zones of inhibition were detectable at concentrations significantly below previously reported (1, 17, 21) MICs of all of the antimicrobial agents except ceftriaxone for B. burgdorferi. Ceftriaxone was detectable at concentrations of ≥2.0 μg/ml. In addition, we developed a simple procedure for adsorbing and removing ceftriaxone, cefotaxime, cefuroxime, doxycycline, erythromycin, and amoxicillin from serum prior to testing for the presence of borreliacidal antibodies by using XAD-16, a nonionic polymeric resin. The concentrations of antimicrobial agents removed greatly exceeded the maximum amounts of these agents attainable in serum after treatment (19, 25). Penicillin could not be removed by using XAD-16. Thus, it will be important to know when patients are taking penicillin. However, high concentrations of penicillin were readily removed by adding 1 U of penicillinase.
Resin beads have often been used to remove antimicrobial agents from blood cultures (31–33). Therefore, it is not surprising that this procedure is also effective at removing antimicrobial agents from serum. What is important, however, is that the removal of antimicrobial agents by XAD-16 or penicillinase did not also remove even small concentrations of IgM or IgG borreliacidal antibodies. The borreliacidal-antibody test may solve many of the current problems associated with serodiagnosis of Lyme borreliosis. A major obstacle has been overcome by eliminating false-positive tests due to the presence of antimicrobial agents. The ability to easily detect and remove antimicrobial agents without significantly affecting the borreliacidal-antibody concentration greatly improves the clinical utility of borreliacidal-antibody testing.
REFERENCES
- 1.Agger W A, Callister S M, Jobe D A. In vitro susceptibilities of Borrelia burgdorferi to five oral cephalosporins and ceftriaxone. Antimicrob Agents Chemother. 1992;36:1788–1790. doi: 10.1128/aac.36.8.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agger W A, Case K L. Clinical comparison of borreliacidal-antibody test with indirect immunofluorescence and enzyme-linked immunosorbent assays for diagnosis of Lyme disease. Mayo Clin Proc. 1997;72:510–514. doi: 10.4065/72.6.510. [DOI] [PubMed] [Google Scholar]
- 3.Anhalt J P, Washington J A., II . Preparation and storage of antimicrobial solutions. In: Balows A, Hausler W J Jr, Hermann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1991. pp. 1199–1200. [Google Scholar]
- 4.Bakken L L, Callister S M, Wand P J, Schell R F. Interlaboratory comparison of test results for the detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists proficiency testing program. J Clin Microbiol. 1997;35:537–543. doi: 10.1128/jcm.35.3.537-543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callister S M, Jobe D A, Schell R F. Detection of borreliacidal antibodies by flow cytometry. In: Robinson J P, Darzynkiewicz Z, Dean P N, Orfao A, Rabinovitch P S, Stewart C C, Tanke H S, Wheeless L L, editors. Current protocols in cytometry. New York, N.Y: John Wiley and Sons, Inc.; 1999. pp. 11.5.1–11.5.11. [DOI] [PubMed] [Google Scholar]
- 6.Callister S M, Jobe D A, Schell R F. The impact of vaccination against Lyme borreliosis on laboratory serodiagnosis. Clin Microbiol Newsl. 1999;21:129–134. [Google Scholar]
- 7.Callister S M, Jobe D A, Schell R F, Pavia C S, Lovrich S D. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a “gold standard”? Clin Diagn Lab Immunol. 1996;3:399–402. doi: 10.1128/cdli.3.4.399-402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callister S M, Schell R F. Laboratory serodiagnosis of Lyme borreliosis. J Spirochetal Tickborne Dis. 1998;5:4–10. [Google Scholar]
- 9.Callister S M, Schell R F, Case K L, Lovrich S D, Day S P. Characterization of the borreliacidal antibody response to Borrelia burgdorferi in humans: a serodiagnostic test. J Infect Dis. 1993;167:158–164. doi: 10.1093/infdis/167.1.158. [DOI] [PubMed] [Google Scholar]
- 10.Callister S M, Schell R F, Lim L C L, Jobe D A, Case K L, Bryant G L, Molling P E. Detection of borreliacidal antibodies by flow cytometry. Arch Intern Med. 1994;154:1625–1632. [PubMed] [Google Scholar]
- 11.Callister S M, Schell R F, Lovrich S D. Lyme disease assay which detects killed Borrelia burgdorferi. J Clin Microbiol. 1991;29:1773–1776. doi: 10.1128/jcm.29.9.1773-1776.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Lyme disease—United States, 1996. Morbid Mortal Weekly Rep. 1997;46:531–535. [PubMed] [Google Scholar]
- 13.Chapin-Robertson K, Edberg S C. Measurement of antibiotics in human body fluids: techniques and significance. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: Williams and Wilkins; 1991. pp. 295–366. [Google Scholar]
- 14.Fikrig E, Bockenstedt L K, Barthold S W, Chen M, Tao H, Ali-Salaam P, Telford S R, Flavell R A. Sera from patients with chronic Lyme disease protect mice from Lyme borreliosis. J Infect Dis. 1994;169:568–574. doi: 10.1093/infdis/169.3.568. [DOI] [PubMed] [Google Scholar]
- 15.Johnson B J B, Robbins K E, Bailey R E, Cao B L, Sviat S L, Craven R B, Mayer L W, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Levin J M, Nelson J A, Segreti J, Harrison B, Benson C A, Strle F. In vitro susceptibility of Borrelia burgdorferi to 11 antimicrobial agents. Antimicrob Agents Chemother. 1993;37:1444–1446. doi: 10.1128/aac.37.7.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y F, Lim L C L, Schell K, Lovrich S D, Callister S M, Schell R F. Differentiation of borreliacidal activity caused by immune serum or antimicrobial agents by flow cytometry. Clin Diagn Lab Immunol. 1994;1:145–149. doi: 10.1128/cdli.1.2.145-149.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandell G L, Sande M A. Antimicrobial agents: penicillins, cephalosporins, and other beta-lactam antibiotics. In: Gilman A G, Rale T W, Nies A S, Taylor P, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 8th ed. New York, N.Y: Pergamon Press; 1990. pp. 1065–1097. [Google Scholar]
- 20.Minnesota Department of Health. Lyme disease: guidelines for Minnesota clinicians: epidemiology, microbiology, treatment and prevention. Dis Control Newslett. 1995;23:56–67. [Google Scholar]
- 21.Mursic V P, Wilske B, Schierz G, Holmburger M, Suß E. In vitro and in vivo susceptibility of Borrelia burgdorferi. Eur J Clin Microbiol. 1987;6:424–426. doi: 10.1007/BF02013102. [DOI] [PubMed] [Google Scholar]
- 22.Rousselle J C, Callister S M, Schell R F, Lovrich S D, Jobe D A, Marks J A, Wieneke C A. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J Infect Dis. 1998;178:733–741. doi: 10.1086/515382. [DOI] [PubMed] [Google Scholar]
- 23.Sadziene A, Thompson P A, Barbour A G. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- 24.Sambri V, Armati S, Cevinini R. Animal and human antibodies reactive with the outer surface protein A and B of Borrelia burgdorferi are borreliacidal, in vitro, in the presence of complement. FEMS Immunol Med Microbiol. 1993;7:67–72. doi: 10.1111/j.1574-695X.1993.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 25.Sande M A, Mandell G L. Antimicrobial agents: tetracyclines, chloramphenicol, erythromycin, and miscellaneous antibacterial agents. In: Gilman A G, Rale T W, Nies A S, Taylor P, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. New York, N.Y: Pergamon Press; 1990. pp. 1117–1145. [Google Scholar]
- 26.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Malawista S E the Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 28.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buskarino C, Krause D S the Lyme Disease Study Group. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallis C, Melnick J L, Wende R D, Riely P E. Rapid isolation of bacteria from septicemic patients by use of an antimicrobial agent removal device. J Clin Microbiol. 1980;11:462–464. doi: 10.1128/jcm.11.5.462-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright A J, Thompson R L, McLimans C A, Wilson W R, Washington J A. The antimicrobial removal device: a microbiological and clinical evaluation. Am J Clin Pathol. 1982;78:173–177. doi: 10.1093/ajcp/78.2.173. [DOI] [PubMed] [Google Scholar]
- 33.Zabinski R A, Larsson A J, Walker K J, Gilliland S S, Rotschafer J C. Elimination of quinolone antibiotic carryover through use of antibiotic-removal beads. Antimicrob Agents Chemother. 1993;37:1377–1379. doi: 10.1128/aac.37.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]