Abstract
A complex cellular cascade characterizes the pathophysiological response following spinal cord injury (SCI) limiting regeneration. Biomaterial and stem cell combination therapies together have shown synergistic effects, compared to the independent benefits of each intervention, and represent a promising approach towards regaining function after injury. In this study, we combine our polyethylene glycol (PEG) cell delivery platform with lentiviral-mediated overexpression of the anti-inflammatory cytokine interleukin (IL)-10 to improve mouse embryonic day 14 (E14) spinal progenitor transplant survival. Immediately following injury in a mouse SCI hemisection model, five PEG tubes were implanted followed by direct injection into the tubes of lentivirus encoding for IL-10. Two weeks after tube implantation, mouse E14 spinal progenitors were injected directly into the integrated tubes, which served as a soft substrate for cell transplantation. Together, the tubes with the IL-10 encoding lentivirus improved E14 spinal progenitor survival, assessed at two weeks post-transplantation (four weeks post-injury). On average 8.1% of E14 spinal progenitors survived in mice receiving IL-10 lentivirus-laden tubes compared to 0.7% in mice receiving transplants without tubes, an 11.5-fold difference. Surviving E14 spinal progenitors gave rise to neurons when injected into tubes. Axon elongation and remyelination were observed, in addition to a significant increase in functional recovery in mice receiving IL-10 lentivirus-laden tubes with E14 spinal progenitor delivery compared to the injury only control by 4 weeks post-injury. All other conditions did not exhibit increased stepping until 8 or 12 weeks post-injury. This system affords increased control over the transplantation microenvironment, offering the potential to improve stem cell-mediated tissue regeneration.
Keywords: spinal cord injury, neural stem cells, biomaterials, gene delivery, tissue engineering
1. Introduction
Spinal cord injury (SCI) is an incredibly devastating condition that can leave patients both physically and financially burdened for the remainder of their lives. North America alone reports approximately 17,000 new cases of traumatic SCI annually, with lifetime healthcare costs averaging about $2 million per patient (“National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance,” 2020). Difficulties in treating SCI arise as both the innate and adaptive immune responses attempt to protect the spinal cord from further damage. The immune response, while necessary for removing dead cells and debris, creates an inhospitable milieu characterized by a cascade of cellular and biochemical signals, highlighting a delicate balance between neuroplasticity and further damage. Known as the secondary injury, this induced damage persists through acute, sub-acute, and chronic stages of injury, spanning hours to months post-SCI (Dalamagkas, Tsintou, Seifalian, & Seifalian, 2018; Donnelly & Popovich, 2008; Dumont, Margul, & Shea, 2016) resulting in glial and fibrotic scarring (Bradbury & Burnside, 2019; Hackett & Lee, 2016; Soderblom et al., 2013; Zhu, Soderblom, Trojanowsky, Lee, & Lee, 2014), axonal dieback and demyelination (Busch, Horn, Silver, & Silver, 2009; Hill, 2017), apoptosis (X. Z. Liu et al., 1997), and oxidative stress (Hall, 2011) severely limiting regenerative potential.
Exogenous stem cell transplantation is a promising technique that is highly dependent on the transplantation microenvironment (Tejeda, Ciciriello, & Dumont, 2021). Cell transplantations have shown initial promise both broadly in tissue engineering (Kwon, Kwon, Lee, Park, & Kim, 2018) and specifically in SCI repair models (Dulin & Lu, 2014). On a cellular level, neural stem cell (NSC) transplants repopulate lost neural and glial cells in damaged tissue (Dulin & Lu, 2014; Mortazavi et al., 2015) in addition to working on a molecular level through cytokine and chemokine release post-SCI (P. Lu, Jones, Snyder, & Tuszynski, 2003). Furthermore, transplanted NSCs can integrate with endogenous neural circuitry (Ceto, Sekiguchi, Takashima, Nimmerjahn, & Tuszynski, 2020; Kumamaru et al., 2018) and can significantly decrease local expression of pro-inflammatory cytokines (Cheng et al., 2016). Altogether, NSCs exhibit numerous pro-regenerative functions that are highly contingent on cell survival post-transplantation. Transplantation method, medium, location, and cell age all play pivotal roles in influencing survival (Chen, Bernreuther, Dihné, & Schachner, 2005; Karimi-Abdolrezaee, Eftekharpour, Wang, Morshead, & Fehlings, 2006; Nagoshi et al., 2018), but perhaps the most important factor to consider with cell therapies is their transplantation microenvironment. In SCI, the persisting secondary injury complicates NSC delivery. As the immune response clears damaged cells and cell debris, inhospitable conditions for transplanted cells lead to poor survival and limited regenerative effect. SCI cell transplantation strategies commonly delay delivery to occur after an initial wave of post-injury inflammation (Tetzlaff et al., 2011). Starting hours after injury, inflammatory cytokines including interleukin (IL)-1β, tumor necrosis factor α (TNFα), IL-6, and leukemia inhibitory factor (LIF) are strongly activated in the wound area, but their concentrations decrease by 1 week post-injury. Similarly, reactive oxygen species peak in concentration approximately 12 hours after injury, but by 1 week post-injury, their concentration drops precipitously (Donnelly & Popovich, 2008; Dumont et al., 2016; Lacroix, Chang, Rose-John, & Tuszynski, 2002; Shamash, Reichert, & Rotshenker, 2002). Avoiding these inflammatory mediators by delaying transplantation has the potential to improve survival, but cell transplants require further intervention to have their intended regenerative effects.
Following SCI, biomaterial interventions have proven to be effective in improving regeneration by creating a more permissive environment for tissue growth (Shuo Liu, Xie, & Wang, 2019; Straley, Foo, & Heilshorn, 2010) making them promising candidates for combination therapies with stem cell transplants (Shengwen Liu, Schackel, Weidner, & Puttagunta, 2018; Shrestha et al., 2014) with the goal of creating a more hospitable transplantation environment to improve transplant survival. Highly porous, polymeric scaffold implants can facilitate regeneration in the spinal cord, where the porosity allows for endogenous progenitor infiltration, aiding in axonal regrowth and myelination (Pawar et al., 2015; Shahriari, Koffler, Tuszynski, Campana, & Sakamoto, 2017; Straley et al., 2010; Tuinstra et al., 2012; Tuinstra et al., 2013; Y. Yang et al., 2009). Furthermore, implantable scaffolds provide a defined architecture that can direct axonal elongation. Softer, hydrogel based implants fabricated from polyethylene glycol (PEG) microspheres also support regeneration (Dumont et al., 2019). Macroporous tubes, molded from PEG microspheres, function similar to rigid, polymeric scaffolds, but better match native tissue mechanical properties with the potential to conform to individual injury anatomy, making them advantageous. Biomaterial strategies for SCI can additionally support stem cell transplantation through ex vivo culture on a rigid scaffold (Dumont et al., 2018; Li et al., 2016; Mothe, Tam, Zahir, Tator, & Shoichet, 2013), suspension in hydrogel injections (Assunção-Silva, Gomes, Sousa, Silva, & Salgado, 2015; Cai, Dewi, & Heilshorn, 2015; Marquardt & Heilshorn, 2016), or direct injection into an integrated implant (Ciciriello et al., 2020). Alone, biomaterials passively modify immune cell infiltration and inflammation, resulting in improved stem cell survival post-transplantation, but anti-inflammatory factors can take a more active role in immune modulation.
Lentiviral vectors are a highly effective method for active therapeutic treatment with presently ongoing clinical trials (Milone & O’Doherty, 2018). Lentivirus-mediated therapies integrate within a host genome, resulting in long-term overexpression of the selected gene of interest. Implantable biomaterials can serve as a medium for loading and release of lentivirus. Both SCI models (Abdellatif et al., 2006; Boehler et al., 2014; Margul et al., 2016; Park, Decker, Margul, et al., 2018; Park, Decker, Smith, et al., 2018; Dominique R. Smith et al., 2020; D. R.Smith et al., 2019) and other tissue engineering applications like islet transplantation (Chou & Sytwu, 2009; Jimenez-Moreno et al., 2015; J. M. H. Liu, Zhang, Joe, Luo, & Shea, 2018), cancer immunotherapies (Arce, Breckpot, Collins, & Escors, 2011; Liechtenstein, Perez-Janices, & Escors, 2013; Milone & O’Doherty, 2018), and cardiac tissue repair (Di Pasquale, Latronico, Jotti, & Condorelli, 2012; Niwano et al., 2008; Zhao et al., 2002) have investigated this heavily. Lentiviral-mediated over-expression of cytokines from the IL family, specifically those targeting macrophages, has shown promise in altering immune response post-injury. Macrophages are a well-studied cell population, often viewed as an attractive therapeutic target. Infiltrating macrophages are traditionally grouped into two general phenotype classes, pro-inflammatory M1 and pro-regenerative M2, each with important functions post-injury. In reality, the behavior of these phenotypes is much more nuanced than the binary options presented here (Mosser & Edwards, 2008), but this provides a frame of reference for distinguishing between pro-inflammatory and pro-regenerative roles. Furthermore, this described phenotypic difference opens the opportunity for lentiviral-mediated overexpression of an anti-inflammatory cytokine, like IL-10, to facilitate the polarization of M1 to M2 macrophages to create a more pro-regenerative microenvironment.
In this work, we expand the passive microenvironment modulation of our macroporous PEG tubes to actively modify the transplantation microenvironment through lentiviral-mediated delivery of IL-10. Previously, we demonstrated the short-term feasibility of the temporally flexible transplantation of enhanced green fluorescent protein (EGFP+) embryonic day 14 (E14) spinal progenitors into integrated PEG tubes (Ciciriello et al., 2020). Expanding on this prior work, in our current study we will implant IL-10 encoding lentivirus-laden tubes into a cervical (C5) lateral hemisection, and two weeks later inject EGFP+ E14 spinal progenitors directly into the tubes at the lesion epicenter. This two week period between tube implantation and E14 spinal progenitor transplantation affords the tubes time to integrate and the IL-10 encoding lentivirus to modulate the immune response, ultimately improving transplantation. Alone, the tubes served as a privileged cell transplantation site, so we anticipate that adding lentivirus-mediated IL-10 delivery will further improve transplantation survival, resulting in long term benefits in regeneration and functional restoration through both endogenous and exogenous repair mechanisms, marking an important step forward in the development of a comprehensive cell delivery platform for SCI repair.
2. Methods
2.1. Fabrication of hydrogel tubes
Hydrogel tubes were generated as previously described (Dumont et al., 2019). Briefly, 20% w/v 8-arm polyethylene glycol maleimide (PEG-MAL, 20 kDa; JenKem, Plano, TX) was crosslinked with 5 mM slow-degrading-plasmin-sensitive YKND cross-linking peptide (Ac-GCYKNDGCYKNDCG; Genscript, Piscataway, NJ) (Shikanov, Smith, Xu, Woodruff, & Shea, 2011) to form microspheres through water-oil emulsion with diameter ranging between 15 and 150 μm and an average of 45 μm. The PEG-YKND solution was homogenized in silicone oil (Fisher, Hampton, NH) with 2% Tween-20 (Sigma, St. Louis, MO) at a speed of 4000 rpm for 1 minute. Microspheres were rinsed by centrifugation three times. Irgacure 2959 photoinitiator (Sigma) dissolved in N-vinylpyrrolidinone (660 mg/mL; Sigma) was added to the microspheres at a final concentration of 1% w/v. The resulting microspheres were then packed into polydimethylsiloxane (PDMS, Dow Corning, Midland, MI) molds to generate PEG tubes (approximate OD: 600 μm, ID: 250 μm, porosity 66%) and exposed to an ultraviolet lamp for 3 minutes to initiate free radical polymerization. Tubes were rinsed three times, dehydrated, and stored at −80 until use. Tubes were cut to length during surgery to ensure fit within the defect.
2.2. Spinal progenitor isolation
All animal work pertaining to progenitor cell isolation was performed with prior approval and in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines at the University of Michigan and University of Miami. Embryonic spinal progenitors were isolated from the spinal cords of embryonic day 14 (E14) C57BL/6-Tg(CAG-EGFP)10sb/J mice (Jackson Laboratory, Bar Harbor, ME), enzymatically dissociated with 10 U/mL papain (Worthington, Lakewood, NY) and 37 μg/mL DNase (Sigma) into single cells, and expanded as neurospheres in ultralow attachment flasks (Corning, Corning, NY), as described previously (Dumont et al., 2018). Embryonic spinal progenitors were expanded in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, NY) supplemented with 1X B27 (Gibco), 1X N2 (Gibco), N-acetyl cysteine (NAC; Sigma), and 20 ng/mL basic fibroblast growth factor (FGF2; Peprotech, Rocky Hill, NJ) and leukemia inhibitory factor (LIF; Peprotech). E14 cell colonies were passaged with papain as needed and not used beyond the second passage for transplantation studies.
2.3. Production of lentiviral vectors
Lentivirus was produced as described previously (D. R. Smith et al., 2019). HEK-293FT cells (80–90% confluent, American Type Culture Collection, Manassas, VA) were transfected with third generation lentiviral packaging vectors and pLenti-CMV-Luciferase or pLenti-CMV-hIL10. Correct insertion was validated via DNA sequencing. Plasmids were incubated in OptiMEM (Life Technologies, Carlsbad, CA) with Lipofectamine 2000 (Life Technologies) for 20 minutes prior to being added to cells. After 48 hours of incubation, supernatant was collected, centrifuged to remove cellular debris, and then incubated with PEG-It (System Biosciences, Palo Alto, CA) for 16–24 hours at 4°C. Virus was centrifuged at 1500g at 4°C for 30 min, supernatant was removed, and the pellet was re-suspended in sterile phosphate buffered saline (PBS; Life Technologies). Viral solution was aliquoted and frozen at −80°C until use. Viral titers used throughout the study were 4E9 IU/mL as determined by the Lentivirus qPCR Titer Kit (Applied Biological Materials, Richmond, BC, Canada). Viral vectors used in this study were from the same batch as described by Smith et. al. (Dominique R. Smith et al., 2020), thus all in vivo protein expression data would be comparable between these studies. However, it should be noted that additional vector expression analysis was performed using the In Vivo Imaging System (IVIS; Perkin Elmer, Waltham, MA) with the pLenti-CMV-Luciferase vector as described below.
2.4. SCI surgeries
All animal work was performed with prior approval and in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines at the University of Michigan. A C5 lateral hemisection SCI was created in adult C57BL/6J female mice aged 6–8 weeks, as previously described (Anzalone et al., 2018; Pawar et al., 2015; Dominique R. Smith et al., 2020). Briefly, mice were anesthetized with 2% isoflurane and provided preemptive local pain management (1 mg/kg bupivacaine). After confirmation of anesthesia via toe pinch, a 2 cm incision was made in the skin to facilitate the laminectomy performed at C5. A 1.15 mm lateral hemisection was excised in the left side of the spinal cord. This ensures any functional deficits are confined to the left forepaw, providing an internal control with the right paw. PEG tubes were cut to size, allowed to dehydrate for 30 s, and implanted individually into the injury site, which accommodated 5 tubes in total. A subset of these mice received 2 μL of 4E9 IU/mL pLenti-CMV-hIL10 viral vector injected directly into the tubes. Gelfoam was used to secure the injury site in all conditions, after which the muscles were sutured and skin stapled. A subset of mice (no-treatment control group) did not receive an implant but did receive gelfoam over the injured spinal cord. Mice were immediately provided post-operative antibiotics (enrofloxacin 2.5 mg/kg once a day for 2 weeks), analgesics (0.1 mg/kg buprenorphine twice a day for 3 days), and supportive hydration (1 mL/20 g lactated ringer solution once a day for 5 days). Bladders were expressed twice daily until function recovered and staples were removed after 10 days. Surgical controls were put in place to limit lesion size variance, including the order of the incisions made to limit the effects of swelling to cut lines, measuring the distance between the rostral and caudal cuts, and verifying the absence of bruising to the contralateral tissue. Exclusion criteria include any deviations to the surgical controls, as well as any variance to the recovery timeline, including an inability to ambulate by post-operative day 3. No mice met these exclusion criteria for this study.
All mice received a second surgery 14 days after the primary injury. Protocols for anesthesia and post-operative care were the same as described with the initial surgery. After anesthesia induction, an incision in the skin was made, sutures in the muscle were removed, and any remaining gelfoam was carefully removed from atop the spinal cord. Two injections of E14 EGFP spinal progenitors (2 μL of 150,000 cells in PBS – 300,000 cells/mouse) or PBS were injected in the rostral-medial and caudal-lateral regions of the hydrogel implant using a 33G 10μL Hamilton syringe at a rate of 1 μL/minute. Multiple injections allows for greater spread throughout the injury and is commonly performed both pre-clinically and clinically (Platt, David, Fessler, & Richard, 2020; Tetzlaff et al., 2011). A dose of 300,000 total cells was chosen based on work presented by Piltti et al. to balance proliferation with sufficient cell densities to quantify via immunohistochemistry (Piltti, Salazar, Uchida, Cummings, & Anderson, 2013). Gelfoam was placed back over the injury, muscles were sutured, and wound clips applied. Mice were euthanized and spinal cord segments (C4–6) were collected after 4 or 12 weeks (2 or 10 weeks after cell transplantation). For each condition, n = 4–12 mice at each time point: n = 4 for histology at week 4 (N=24), n = 6 for bioluminescent imaging (N=12), n = 6 for histology at week 12 (N=36), and n = 12 for ladder beam analysis (N=72).
2.5. Bioluminescent imaging
Bioluminescent signal was evaluated with IVIS. Mice used to assess bioluminescent signal received a single surgery, in which the injury was made, tubes were implanted, and 2 μL of 4E9 IU/mL lentivirus with a luciferase reporter was injected directly into the tubes. A second set of mice did not receive the viral injection and served as controls. Gelfoam was placed over the injury, muscles were sutured, and wound clips applied. After 7 days the wound clips were removed. Mice were injected with 150 mg/kg D-Luciferin (Promega, Madison, WI) intraperitoneally 10 minutes prior to imaging. Mice were sedated 5 minutes prior to imaging and transferred to the IVIS chamber under continuous isoflurane sedation. D-Luciferin injections and IVIS imaging were repeated at weeks 4, 6, 8, and 12. Living Image (Perkin Elmer) software was used to evaluate total flux based on bioluminescent signal within the injury site.
2.6. Endogenous progenitor identification
Bromodeoxyuridine (BrdU) was used to identify proliferating cells in the first 7 days post-SCI, as described previously (Ciciriello et al., 2020). Intraperitoneal injections of 50 mg/kg BrdU (Roche, Basel, Switzerland) were pulsed every day for 7 days, allowing 7 additional days for unbound BrdU to wash out before exogenous spinal progenitors were implanted, as done by others (Ciciriello et al., 2020; Z. Yang et al., 2015). Using this protocol, overlap of BrdU and exogenous EGFP cell transplants was not observed. Any cells that co-localized with BrdU and neuronal lineage markers described below were considered to arise from endogenous spinal progenitors giving rise to new cells along the neuronal lineage. Tissue sections requiring BrdU identification were first denatured in 2N hydrochloric acid for 1 hour at 37 °C followed by neutralization in 2 five minute rinses of 0.1M borate buffer to facilitate antigen retrieval. Samples were incubated for 1 hour at room temperature in rat anti-BrdU (1:200, Abcam, Cambridge, UK) antibody followed by appropriate fluorophore-conjugated goat anti-rat secondary antibody before proceeding with additional immunohistochemistry of specific cell phenotypes described below.
2.7. Immunohistochemistry
Isolated spinal cords were flash frozen, and then cryosectioned transversely (4 or 12 week tissue) in 12 μm sections. Immunohistochemistry analysis was performed using nine transverse sections evenly spaced across the rostral, middle, and caudal regions of the tubes and average across each animal allowing for comparisons between animals while considering regional differences (Ciciriello et al., 2020; Dumont et al., 2019). Samples were fixed, permeabilized (0.5% triton-X for 10 minutes) and/or prepared with BrdU staining (described above) as necessary, and incubated overnight at 4°C with primary antibodies. The following antibodies were used for primary detection: rat anti-F4/80 (1:200, Abcam, Cambridge, United Kingdom), goat anti-arginase (1:100, Santa Cruz, Dallas, TX, USA), rabbit anti-neurofilament-200 (NF-200; 1:200, Sigma), goat anti-myelin basic protein (MBP; 1:500, Santa Cruz), chicken anti-myelin protein zero (P0; 1:250, Aves Labs, Tigard, OR), chicken anti-GFP (1:200, Aves Labs), rabbit anti-beta-III tubulin (Tuj1; 1:500, Sigma), mouse anti-neuronal nuclear protein (NeuN; 1:500, Millipore, Burlington, MA), mouse anti-Nestin (1:200, Millipore), rabbit anti-glial fibrillary acidic protein (GFAP 1:500, Thermo Scientific, Waltham, MA), and rat anti-IL-10 (1:100, Thermo Scientific). Species-specific fluorescent secondary antibodies were used for detection at 1:1000 (Life Technologies, Carlsbad, CA, USA). Hoechst 33342 (Life Technologies) was used as a counterstain in all tissue sections. Immunostained tissue sections were imaged using an AxioObserver inverted fluorescent microscope (Zeiss, Oberkochen, Germany) using a 10X dry objective.
Progenitor survival, proliferation, and fate:
Immunohistochemistry was used to verify no overlap between BrdU+ and EGFP+ cell types. Subpopulations of exogenous EGFP+ cells and endogenous BrdU+ cells were counted manually by two blinded researchers to evaluate progenitor-driven neurogenesis. The following cell populations were quantified: exogenous progenitors (EGFP+BrdU−Nestin+), endogenous progenitors (BrdU+EGFP−Nestin+), exogenous neuroblasts (EGFP+BrdU−Nestin+Tuj1+), endogenous neuroblasts (BrdU+EGFP−Nestin+Tuj1+), exogenous early neurons (EGFP+BrdU−Tuj1+Nestin−), endogenous early neurons (BrdU+EGFP−Tuj1+Nestin−), exogenous neurons (EGFP+BrdU−NeuN+), and endogenous neurons (BrdU+EGFP−NeuN+). Cells were quantified within the injury and normalized to the implant or injury (empty control) area. Nine tissues evenly distributed throughout the rostrocaudal axis of the injury were averaged for each animal.
Axon Density and Myelination:
Semi-automated counting software, previously described by McCreedy et al.(McCreedy et al., 2016), was used to quantify axons and the co-localization of myelin with axons in transverse sections Briefly, the software was calibrated using manual NF-200+ (axons), NF-200+MBP+ (myelinated axons), and NF-200+MBP+P0+ (Schwann cell myelinated axons) counts from a subset of transverse 10X images taken from different animals and regions of the implant. The software then used a series of Hessian filters and threshold functions within the bridge region to reduce noise for selected NF-200, MBP, and P0 images (McCreedy et al., 2016). The software then output total axon counts, as well as the myelinated axon counts based on the curvilinear MBP co-localizing with axons with or without P0 co-localization; image acquisition and analysis was performed by investigators blinded to treatment condition. ImageJ (NIH, Bethesda, MD, USA) was used to analyze all other fluorescent images and define the bridge area. Cells were quantified within the injury and normalized to the implant or injury (empty control) area. Nine tissues evenly distributed throughout the rostrocaudal axis of the injury were averaged for each animal.
Immune Cell Density and Glial Scar Thickness:
Cells positive for F4/80+ (macrophages) and F4/80+arginase+ (M2 macrophages) containing Hoechst+ nuclei were counted manually by two blinded researchers to quantify macrophage infiltration. Additionally, researchers were blinded to condition during image acquisition and quantification to eliminate bias for all histological analyses. Cells were quantified within the injury and normalized to the implant or injury (empty control) area. Nine tissues evenly distributed throughout the rostrocaudal axis of the injury were averaged for each animal. Glial scar thickness was quantified via GFAP+ staining at the injury as previously reported (Dumont et al., 2019). Reactive astrocytes strongly express GFAP when forming the glial scar post-injury. Two manual thickness measurements were averaged within each tissue, while nine tissues evenly distributed throughout the rostrocaudal axis of the injury were averaged for each animal.
2.8. Locomotor assessment
Ladder beam was used to evaluate mouse locomotor and coordination over a 12 week period post-SCI, as previously described for all conditions (N=72, 12 per condition) (B. J. Cummings, Engesser-Cesar, Cadena, & Anderson, 2007). Each of the 50 rungs were numbered and equally spaced along the length of the beam with a dark enclosure containing bedding at the far end of the apparatus. An HD Handycam camcorder (Sony, Tokyo, Japan) was used to record mouse ambulation across ladderbeam. Mice were acclimated to the ladder beam over three sessions in the two weeks preceding the initial surgery. Baseline scores were determined to separate animals into equal groups (tubes or gelfoam) prior to the initial surgery. Mice were evaluated on the ladder beam every 2 weeks over the course of the experiment. The 2 week evaluation occurred prior to the second surgery in which the mice received spinal progenitors or vehicle (PBS) injections. This was to allow researchers to separate animals in equal groups prior to the addition of cell transplants. Observation and ladder beam scoring were performed by two blinded observers for 3 trials per animal. Animals were scored by average forepaw full placement on the ladder beam during the task.
2.9. Statistics
Data normality was assessed using a Shapiro-Wilk normality test with an α value of 0.05, which determined parametric statistical tests were appropriate for our analyses. Multiple comparison pairs were analyzed using a one-way or two-way ANOVA with Tukey post-hoc test. All statistics test significance using an α value of 0.05. For all graphs, * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.005, and **** denotes p < 0.001, unless otherwise specified in the figure caption. All values are reported as mean +/− standard error of the mean (SEM). Prism 7 (GraphPad Software, La Jolla, CA) software was used for all data analysis.
3. Results
3.1. Lentiviral expression is sustained in PEG hydrogel tubes over 12 weeks
A cohort of mice received a C5 lateral hemisection injury followed by immediate implantation of PEG hydrogel tubes with a subsequent injection of firefly luciferase (FLuc) encoding lentivirus directly into the tube. A subset of these mice did not receive lentiviral injections to serve as a background control. Bioluminescent signal from the FLuc reporter gene was measured over 12 weeks post-implantation to assess lentiviral expression. Lentiviral expression of FLuc was observed in the injured area (Fig. 1A). The average bioluminescent signal was assessed across 12 weeks for mice receiving lentivirus and compared to those that did not receive lentivirus (Fig. 1B). Increased FLuc expression was observed across the 12 weeks in lentivirus-laden PEG hydrogel tubes, with a significant increase observed at weeks 4, 6, and 8. These results matched lentiviral-mediated IL-10 presence at the injury demonstrated with immunohistochemistry (Fig. S1). IL-10 was strongly observed at 4 weeks post-injury in the tubes + IL-10 encoding lentivirus condition (Fig. S1A), but there was a noticeable drop by 12 weeks (Fig. S1B) that coincides with the decrease in signal measured via IVIS (Fig. 1B). Conversely, no IL-10 was observed at 4 (Fig. S1C) or 12 weeks (Fig. S1D) in the tubes alone condition.
Figure 1:
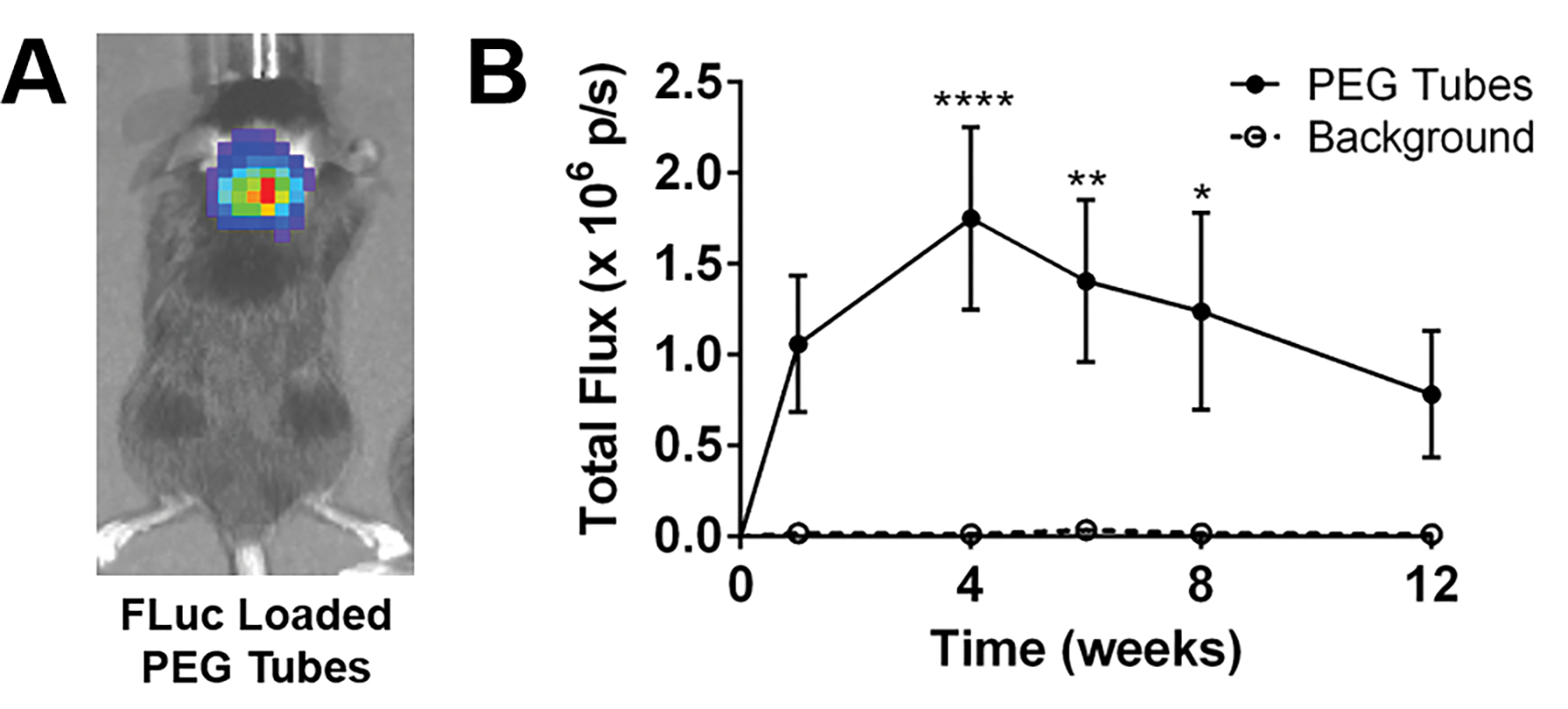
FLuc loaded PEG tubes sustained expression over a course of 12 weeks. Qualitatively, (A) bioluminescence was detected in highest concentrations at the injured spinal cord. (B) Bioluminescent signal of lentivirus-laden hydrogel tubes was assessed over a course of 12 weeks with higher expression compared to background across all times evaluated. n=6 per condition, * p<0.05, ** p<0.01, **** p<0.001.
3.2. Macrophage infiltration is not significantly changed among experimental groups
This study included six total conditions tested, 3 without E14 spinal progenitors (SCI, tubes, tubes + IL-10) and 3 with E14 spinal progenitors (E14 only, E14 + tubes, E14 + tubes + IL-10). Each individual tube has an approximate inner diameter of 250 μm and outer diameter of 600 μm (Fig. S2A). Upon implantation, the tubes integrate with surrounding tissue for two weeks prior to transplanting E14 spinal progenitors. At the two week point, microvasculature can be observed growing through the implant, indicating robust cell infiltration (Fig. S2B). Four weeks following implantation tube lumens are visible when evaluating tissue sections transversely (Fig. S2C), and immune cell infiltration was quantified through F4/80 staining for macrophages (Fig. 2A–F). Identified F4/80+ macrophages were further classified through arginase staining where F4/80+arginase+ macrophages were a pro-regenerative M2-like phenotype. No significant differences were observed in total (Fig. 2G) or M2-specific (Fig. 2H) macrophage density observed across all conditions at 4 weeks. M2 classified macrophages were then reported as a percent of total macrophages, and there were no significant differences observed in M2 fraction across all conditions (Fig. 2I).
Figure 2:
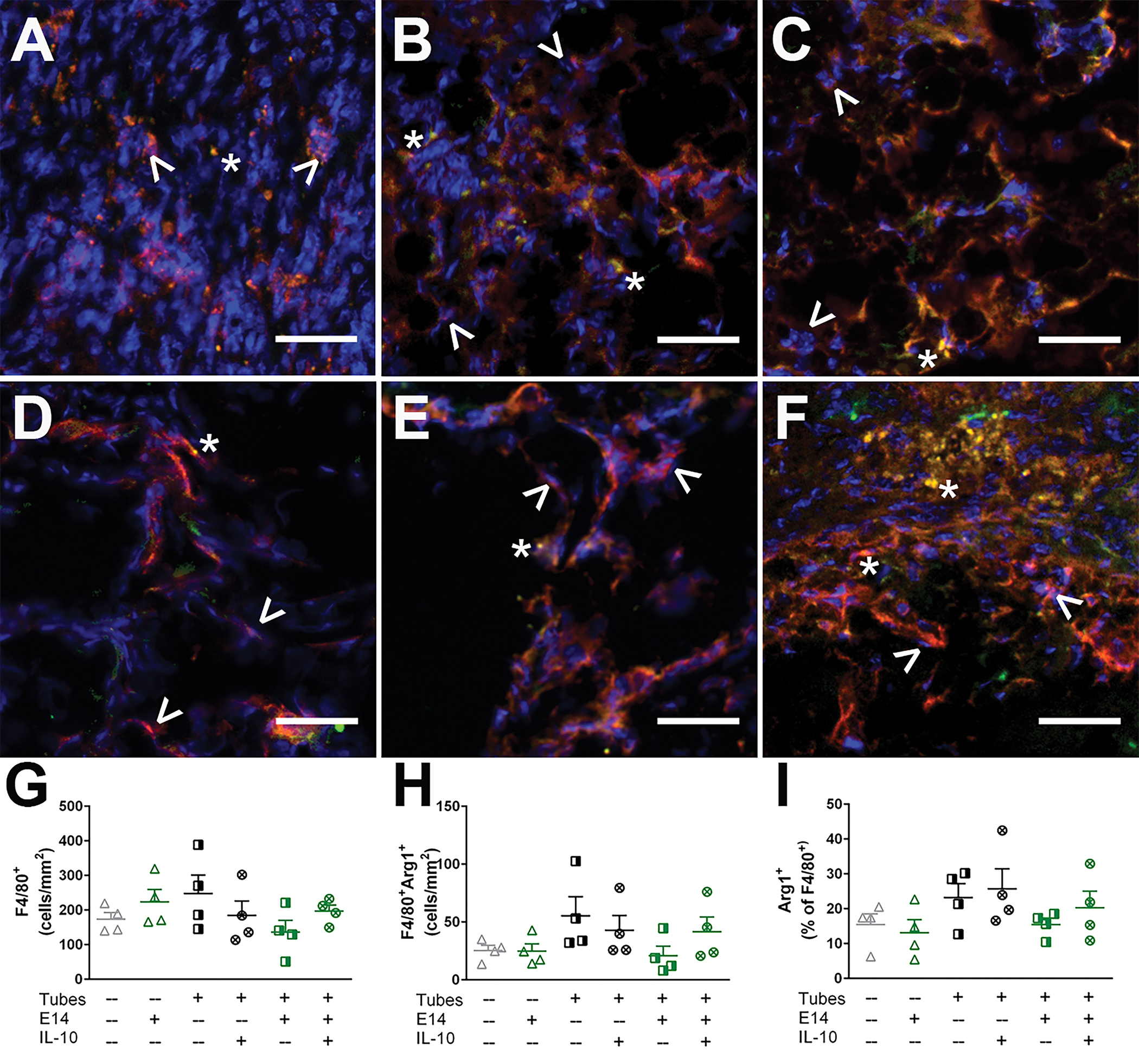
Macrophage infiltration is not exacerbated by tube implantation or E14 spinal progenitor transplantation. Macrophage densities in (A) SCI only, (B) Tubes only, (C)Tubes+IL-10, (D) E14 spinal progenitors only, (E) Tubes+E14, and (F) Tubes+E14+IL-10 in transverse tissue cross sections exhibit both M1 macrophages (F4/80+, red) denoted by ^ and M2 macrophages (F4/80+arginase+, red and green) denoted by *. At 4 weeks after implantation, there were no differences in (G) total macrophage density, (H) M2 macrophage density, and (I) M2 percent across all conditions. Data are presented as mean ± SEM. n=4 per condition, 50 μm scale bars.
3.3. Implanted hydrogel tubes attenuate glial scar thickness
Four weeks post-tube implantation, a glial scar was observed in all conditions. The scar was quantified by measuring the thickness of the GFAP+ astrocyte layer at the injury margin in transversely cross-section tissue (Fig. S3A). Formation of the scar is important for remediating the integrity of the blood-brain barrier, but it can also act as a physical barrier limiting axon elongation into the injury site (T. Yang, Dai, Chen, & Cui, 2020). Attenuation, rather than total ablation, of the glial scar can improve functional recovery (M. A. Anderson et al., 2016; Gu et al., 2019; Liddelow & Barres, 2016). In all mice that received hydrogel tube implants (tubes, tubes + IL-10, E14 + tubes, E14 + tubes + IL-10) a significant decrease in average scar thickness at the medial interface was observed compared to both SCI only and E14 spinal progenitors only (Fig. S3B).
3.4. E14 progenitor survival increases when combined with IL-10 encoding lentivirus loaded hydrogel tubes
Following hemisection injury, five tubes either loaded with IL-10 encoding lentivirus or empty, were implanted into the lesion. Additionally, an injury only condition with no tube implantation served as a control. These mice recovered for two weeks, at which point a second surgery was performed where mice were injected with two doses of 150,000 EGFP+ E14 spinal progenitors or PBS as a control, delivered into the integrated tubes (Fig. 3A). A subset of mice (n = 4) were sacrificed after another two weeks (4 weeks post-injury) to assess the short-term survival of transplanted E14 spinal progenitors. EGFP+ cells were observed in all conditions receiving transplants (Fig. 3B–D). Survival was assessed as both a density normalized to the injury area and as a percent of transplanted cells. E14 spinal progenitor transplantation alone resulted in the lowest density (20 ± 8 cells/mm2) compared to E14 spinal progenitor transplants with tubes (44 ± 4 cells/mm2) and tubes with IL-10 encoding lentivirus (67 ± 19 cells/mm2) (Fig. 3E). Similarly, mice receiving E14 spinal progenitor transplants with no additional treatments resulted in 0.7% of cells surviving, and those with transplants directly into tubes had 4.3% survival. When the E14 spinal progenitors were transplanted into IL-10 lentivirus loaded tubes, cell survival increased to 8.1%, an 11.5-fold increase over the E14 spinal progenitor alone condition (Fig. 3F).
Figure 3:
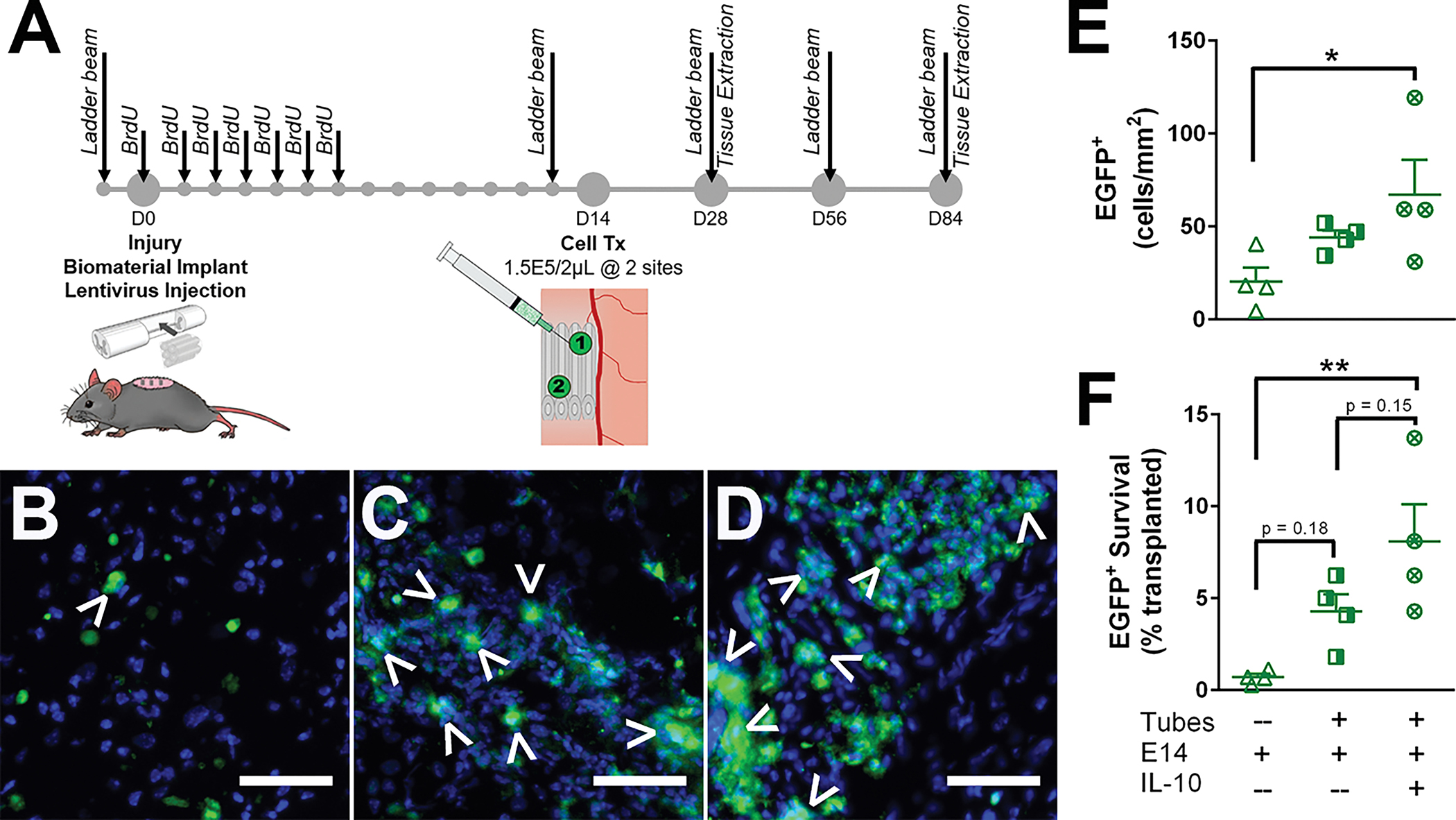
(A) Experimental timeline detailed with major interventions. Delayed EGFP+ spinal progenitor transplantation into IL-10 encoding lentivirus loaded tubes 2 weeks post-injury improves survival. Five hydrogel tubes, either blank or IL-10 lentivirus loaded, were implanted directly after lateral C5 hemisection injury, and EGFP+ spinal progenitors were transplanted 2 weeks post-injury into (B) SCI only, (C) blank tubes, or (D) IL-10 lentivirus loaded tubes. Quantitatively, survival was assessed 4 weeks post-injury (2 weeks post-transplantation), and IL-10 lentivirus loaded tubes exhibited an increase in (E) cell density and (F) percent survival over the other two conditions. Data are presented as mean ± SEM. n=4 per condition, * p<0.05, ** p<0.01, 50 μm scale bars.
3.5. Implanted tubes support exogenous-sourced mature neuron formation by 12 weeks
E14 spinal progenitor commitment to a neuronal lineage was assessed in surviving transplant populations at both 4 and 12 weeks post-injury. At 4 weeks post-injury (Fig. S4A–D), no significant differences were observed in exogenous progenitor (EGFP+Nestin+) or neuroblast (EGFP+Nestin+Tuj1+) densities (Fig. S4E–F), but there was a significant increase in exogenously sourced immature neurons (EGFP+Nestin−Tuj1+) in mice receiving E14 spinal progenitor transplants into tubes (Fig. S4G). No significant differences were observed in mature neuron formation (EGFP+NeuN+) at this early time point (Fig. S4H). Similarly, endogenous progenitors were quantified at 4 weeks post-injury. To label endogenous progenitors, BrdU was injected for 7 days post-injury, followed by 7 days with no injections to allow sufficient clearance of BrdU prior to exogenous E14 spinal progenitor transplantation. Endogenous progenitor (BrdU+Nestin+) (Fig. S5A), neuroblast (BrdU+Nestin+Tuj1+) (Fig. S5B), immature neuron (BrdU+Nestin−Tuj1+) (Fig. S5C), and mature neuron (BrdU+NeuN+) (Fig. S5D) densities had no significant differences across all conditions (Fig. S5E–H).
Neuron formation at 12 weeks post-injury was assessed from both exogenous and endogenous progenitor cells (Fig. 4A). For the conditions receiving transplanted EGFP+ E14 spinal progenitors, neurons were identified as EGFP+NeuN+ cells and quantified as a density normalized to the injury cross section and as a percent of total EGFP+ positive cells. No significant differences were observed in EGFP+NeuN+ densities between E14 spinal progenitors only (0.12 ± 0.1 cells/mm2), tubes with E14 spinal progenitors (1.7 ± 0.4 cells/mm2), and IL-10 lentivirus loaded tubes with E14 spinal progenitors (1.9 ± 0.8 cells/mm2) (Fig. 4B). When expressed as a percent of the total EGFP+ cells mice receiving transplanted E14 spinal progenitors only had on average 12.8 ± 10% of their transplants form neurons. This was significantly lower than the average percentages for mice receiving E14 spinal progenitors with blank tubes (38.9 ± 5.1%) and with IL-10 lentivirus loaded tubes (44.3 ± 1.7%) (Fig. 4C). Endogenous-derived neurons were assessed as a density, and there were no significant differences across any of the conditions tested (Fig. 4D).
Figure 4:
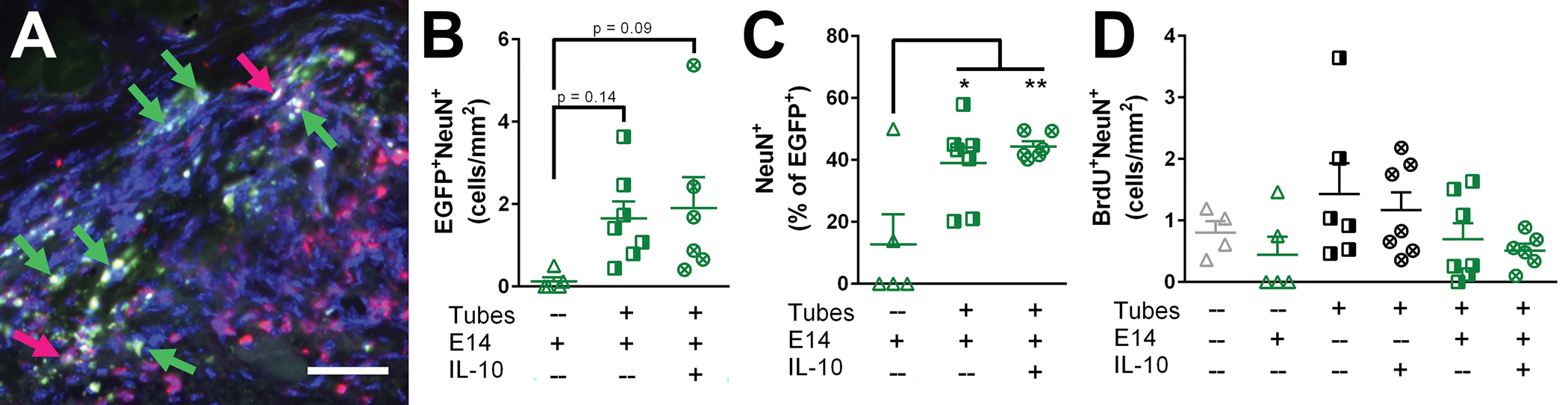
Neuron formation at 12 weeks post-injury is increased when hydrogel tubes are implanted. (A) Formed neurons were observed at 12 weeks post-injury arising from both exogenous (EGFP+NeuN+|green+gray+, green arrow) and endogenous (BrdU+NeuN+|red+gray+, pink arrow) sources. (B) No statistically significant differences was observed in exogenous-sourced neuron density. (C) A significant increase in NeuN+ cells as a percent of total EGFP+ cells was observed when E14 spinal progenitors were transplanted into either blank tubes or IL-10 lentivirus-loaded tubes. (D) There were no statistically significant differences observed in endogenous-sourced neuron formation. Data are represented as mean ± SEM, n = 4–7 animals per conditions, * p<0.05, ** p<0.01, 50 μm scale bars.
3.6. Axon elongation increases with IL-10 lentivirus-laden hydrogel tubes and E14 spinal progenitors
Transverse cross sections of isolated spinal cords were evaluated to assess axon elongation in the lesion space at 12 weeks post-injury. Facilitating and encouraging axon elongation through the injury is crucial for any SCI treatment. Axon counts quantified using NF-200 staining were normalized to the injury area (Fig. S6), shown as the solid white border (Fig. 5A–F) to give an axon density for each condition. Assessed samples were evenly spaced across rostral, middle, and caudal regions of the injuries and averaged as no significant differences in axon density were observed between individual regions within each condition. All conditions exhibited axon elongation into the injury (Fig. 5A–F) at 12 weeks. Infiltrating axons were typically observed growing in fascicle-like bundles (Fig. 5G) or as individual, elongated structures (Fig. 5H). Mice receiving IL-10 lentivirus loaded tubes with E14 spinal progenitors had significantly greater axon formation (490 ± 41 axons/mm2) compared to all other conditions. No other significant differences were observed between conditions at this time point (Fig. 5I).
Figure 5:
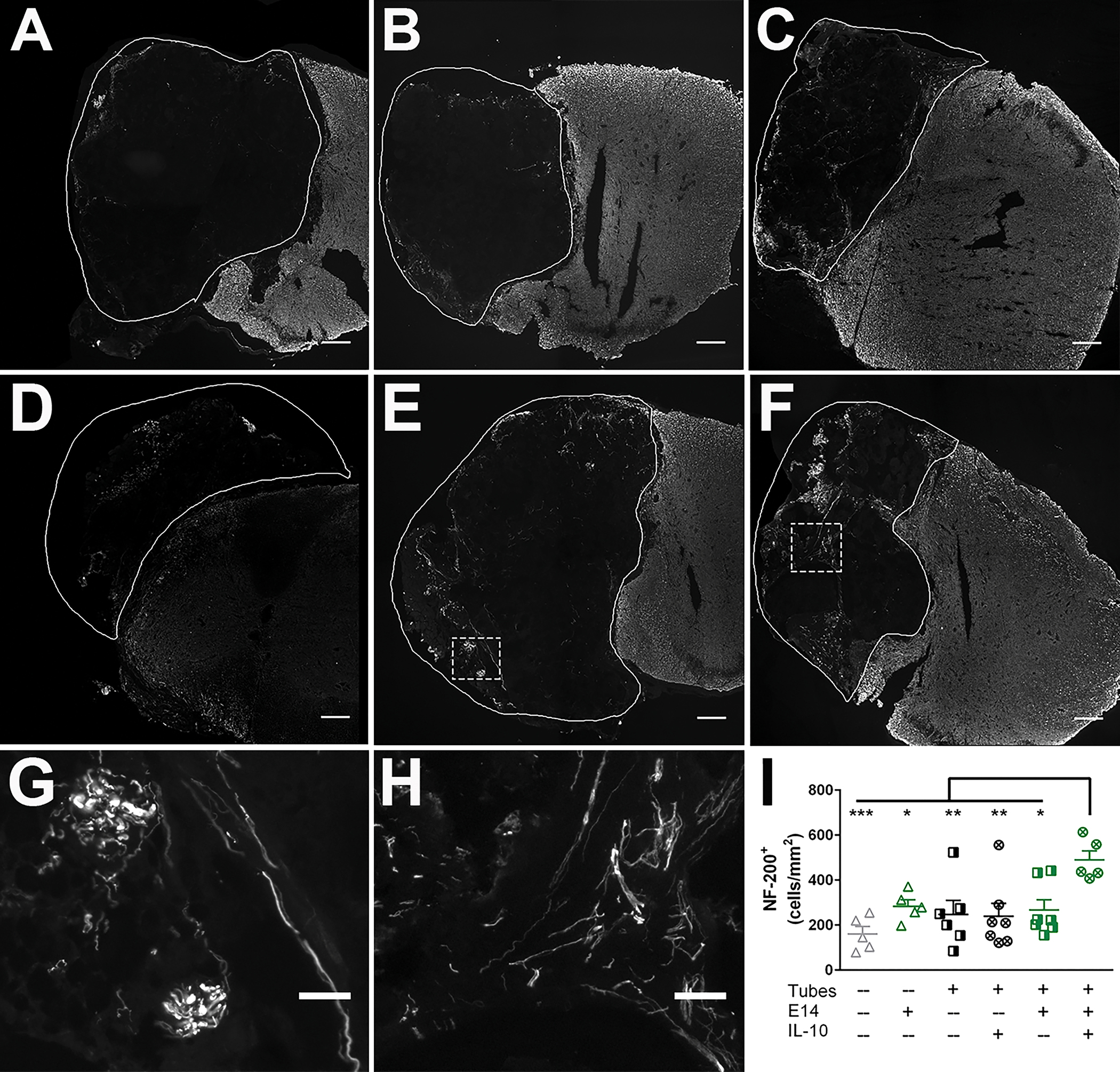
E14 spinal progenitor transplants into IL-10 lentivirus-laden tubes improve new axon formation at 12 weeks post-injury. Axon expression (NF-200+) in (A) SCI only, (B) Tubes only, (C) Tubes+IL-10, (D) E14 spinal progenitors only, (E) Tubes+E14, and (F) Tubes+E14+IL-10. Examples of (G) bundles and (H) elongated axons observed in the ipsilateral tissue. Quantitatively, animals receiving Tubes+E14+IL-10 exhibited a higher elongated axon density (I) in the ipsilateral tissue. Data are presented as mean ± SEM. n=6 animals per condition, * p<0.05, ** p<0.01, *** p<0.005, 200 μm scale bars (A-F), 50 μm scale bars (G-H).
3.7. Axon remyelination increases with E14 spinal progenitor delivery
Elongated axons were further characterized 12 weeks post-injury by the extent and source of their myelination to indicate functional axonal regrowth. Myelination was quantified as a density of NF-200+MBP+ axons normalized to the injury area of a transverse cross section (Fig. S6). Mice not receiving tubes (SCI only and E14 only) exhibited a decreased cross-sectional area compared to the other conditions, most likely due to tissue collapse with no support from the implanted biomaterial. All conditions had myelinated axons present in the injury (Fig. 6A–F). Mice receiving IL-10 lentivirus loaded tubes with E14 spinal progenitors had 83 ± 13 myelinated axons/mm2 which was a significantly greater density compared to the injury only control (24 ± 6 myelinated axons/mm2), but they were not different compared to any of the other experimental conditions. Similarly, the E14 spinal progenitor only condition had a significant increase in myelin density (105 ± 12 myelinated axons/mm2) compared to the injury only but not any of the other conditions (Fig. 6G). It is possible that the density calculations for the E14 spinal progenitor only condition are artificially inflated as a result of lower ipsilateral tissue area to normalize to, which might be attributable to tissue collapse with no biomaterial. Ipsilateral tissue in the E14 spinal progenitor only condition was approximately 67%, 70%, and 39% lower by comparison to tubes + IL-10, E14 + tubes, and E14 + tubes + IL-10, respectively (Fig. S6). The percent of regenerated axons was determined by normalizing the number of NF-200+MBP+ axons to the total number of NF-200+ axons and reported as a percent. Mice receiving E14 spinal progenitors had a significant increase in myelination percent compared to all other conditions tested, with 42 ± 3% of their axons having myelin. No other significant differences were observed for myelination percent (Fig. 6H).
Figure 6:
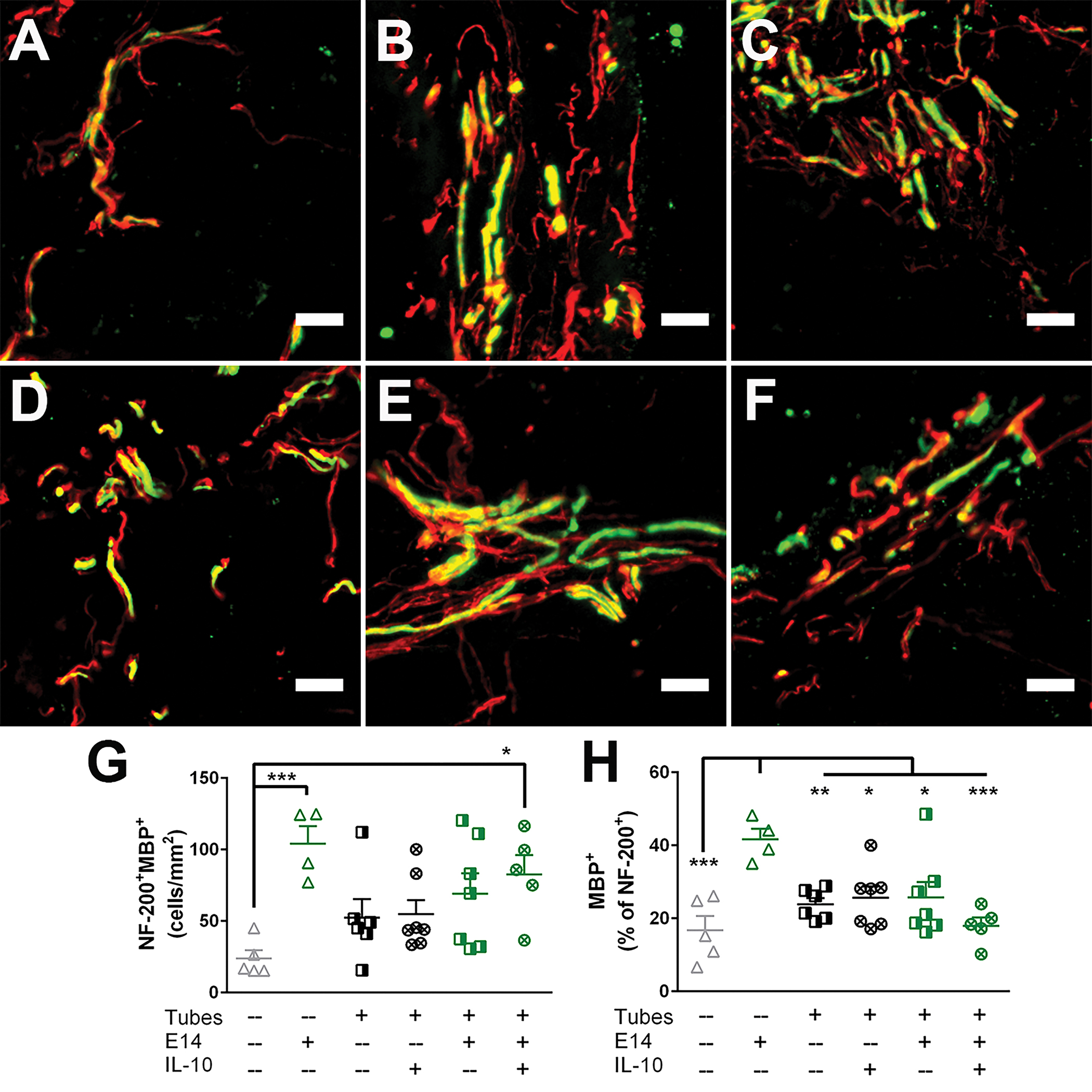
E14 spinal progenitor delivery increases axon remyelination 12 weeks post-injury. Axon (NF-200+, red) co-localization with myelin (MBP+, green) was assessed in (A) SCI only, (B) Tubes only, (C) Tubes+IL-10, (D) E14 spinal progenitors only, (E) Tubes+E14, and (F) Tubes+E14+IL-10. (G) E14 only transplants and Tubes+E14+IL-10 exhibited an increase in overall myelinated axon density compared to the SCI only condition. (H) As a percent, myelination was significantly increased in the E14 only condition compared to all other conditions. Data are represented as mean ± SEM, n = 4–7 animals per condition, * p<0.05, ** p<0.01, *** p<0.005, **** p<0.001, 20 μm scale bars.
Myelination source was determined where axons expressing MBP+P0+ were identified as Schwann cell-derived myelinated axons and MBP+P0− were assumed to be oligodendrocyte-derived. Both longitudinally and transversely myelinated axons were observed in the tissue (Fig. S7A). Mice receiving E14 spinal progenitors alone and delivered into tubes had significant increases in Schwann cell-derived myelinated axon densities compared to the injury only control (Fig. S7B). The percent of Schwann cell-derived myelinated axons was determined by normalizing the NF-200+MBP+P0+ axons to all NF-200+MBP+ axons and reported as a percent. The E14 spinal progenitor only condition had a significant increase in Schwann-cell derived myelin compared to all other conditions with 31 ± 4% of axons coming from Schwann cells (Fig. S7C). No other significant differences were observed.
3.8. IL-10 lentivirus loaded tubes with E14 spinal progenitors increase rate of functional recovery
The horizontal ladder beam test assessed the functional recovery of the left forelimb of mice at 2, 4, 8, and 12 weeks post-injury. An average left paw placement score out of 50 possible rungs was assessed at each time point for each condition (Fig. 7). At 4 weeks post-injury the mice receiving IL-10 lentivirus loaded tubes and E14 spinal progenitors exhibited increased function compared to the injury only control. By 8 weeks, any combination of condition where the mice had tubes implanted demonstrated increased function, and at 12 weeks, all conditions had significantly more successful placements compared to the injury only control.
Figure 7:
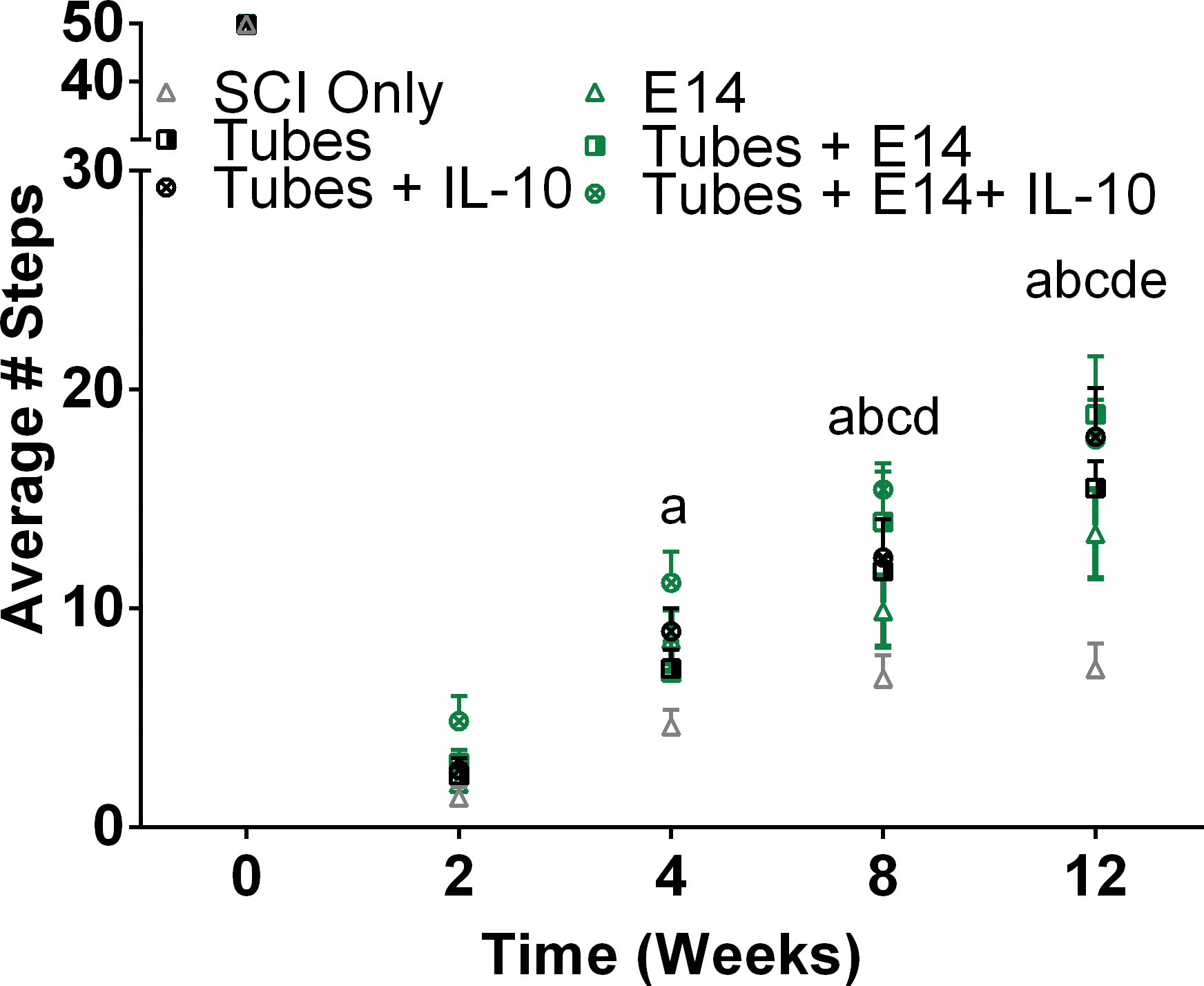
Animals receiving E14 spinal progenitors transplanted into IL-10 lentivirus-laden tubes exhibited a quicker return of forelimb motor function. To assess mobility, a horizontal ladder beam test was used. Mice were trained on walking across the ladder prior to injury. Mobility was assessed through successful placements as a score out of 50 possible rungs. By 4 weeks post-injury Tubes+E14+IL-10 (a, p<0.005) had improved stepping compared to SCI only. At 8 weeks all conditions with tubes had improved stepping compared to (Tubes+E14, b, p<0.005; Tubes+IL-10, c, p<0.05; Tubes, d, p<0.05). At 12 weeks all conditions exhibited improved stepping (E14 only, e, p<0.01).
4. Discussion
Biomaterial-based strategies are increasingly relevant in SCI tissue engineering with numerous clinical trials investigating scaffold-based therapies (Tsintou, Dalamagkas, & Seifalian, 2015). As a singular therapy, biomaterial implants can promote endogenous repair mechanisms and modulate immune responses, but combination therapies involving biomaterials, stem cells, and therapeutic factors are more effective as they can have therapeutic synergism, improving the potential to facilitate repair (Ashammakhi et al., 2019). In this work, we modified our PEG hydrogel tube platform to incorporate lentiviral-mediated anti-inflammatory overexpression while also serving as a soft substrate for delayed E14 spinal progenitor transplantation. Alone, the PEG tubes are soft enough to conform to injury anatomy while maintaining an aligned structure for axonal elongation guidance in an acute SCI (Dumont et al., 2019). Once implanted, the tubes create a more pro-regenerative environment, serving as a privileged injection site for E14 spinal progenitors 2 weeks after injury. Our previous work showed that transplanted cell survival is enhanced when injected into the tubes, compared to direct transplantation into the injury, demonstrating the feasibility of using this as a cell-biomaterial system for SCI (Ciciriello et al., 2020). While survival did improve with the integrated tubes, the stem cell-influenced results of that study were minimized as functional and histological assessments occurred only 2 weeks after cell transplantation (4 weeks after injury). Axon elongation and myelination were comparable between the tubes only and tubes with E14 spinal progenitor conditions, indicating the tubes played a bigger role at this early time point. For the work presented here, we hypothesized that adding lentivirus encoding for the anti-inflammatory cytokine IL-10 to our implanted tubes would allow for active immune modulation to complement the immunomodulatory properties of the integrated tubes, further remediating the cell transplantation site. Herein, we developed a combinatorial therapy that both improved cell transplantation survival while also exhibiting synergistic regenerative gains at 12 weeks post-injury in an acute cervical SCI.
Lentiviral-mediated therapies present an attractive option for local therapeutic delivery as the virus genome integrates into the host genome and gene-of-interest expression is sustained up to 12 weeks (Sakuma, Barry, & Ikeda, 2012; Thomas, Gomez, Palma, Yap, & Shea, 2014; Thomas, Palma, & Shea, 2015; Thomas & Shea, 2013). Delivery via lentiviral-mediated overexpression also provides a clinically-relevant, robust method to rapidly test host response to different therapeutics of interest with extended expression and without needing to redesign the platform for each therapeutic of interest. Moreover, lentivirus can easily be loaded onto a biomaterial scaffold and implanted directly at an injury site (Avilés & Shea, 2011; Shin, Salvay, & Shea, 2010). For this work, we added lentivirus encoding the gene for the anti-inflammatory cytokine IL-10 to our hydrogel PEG tubes once implanted in an acute cervical SCI model. Previous reports have similarly used lentiviral delivery of anti-inflammatory cytokines from multi-channel poly(lactide-co-glycolide) (PLG) bridges in SCI models (Park, Decker, Margul, et al., 2018; Park, Decker, Smith, et al., 2018; Dominique R. Smith et al., 2020; D. R. Smith et al., 2019). In these studies, rigid PLG bridges were loaded with multiple rounds of virus ex vivo prior to implantation. Bridges loaded with IL-10 lentivirus significantly increased polarization to M2 macrophage phenotype density in the injured region, compared to control conditions, demonstrating the efficacy of lentiviral-mediated IL-10 overexpression in facilitating a shift towards a more pro-regenerative microenvironment. For our study, we injected IL-10 encoding lentivirus directly into our tubes immediately after implantation to modulate the immune response after injury and create a more hospitable microenvironment for transplanting E14 spinal progenitors two weeks later. Macrophage phenotype was assessed four weeks post-injury, and we observed no significant differences across all conditions or total macrophage density or M2 macrophage density and fraction. It should be noted, however, that mice receiving IL-10 lentivirus injections did have a trending increase in M2 density and fraction. Other work with IL-10 lentivirus biomaterials has shown that the elevated M2 concentrations begin to resolve by 4 weeks post-injury, indicating an earlier assessment point for our work would likely show a significant increase in M2 density (Park, Decker, Margul, et al., 2018). Additionally, our viral load was lower by comparison to the dose used with rigid PLG bridges. Park et al. loaded their bridges with 2 μL of 2E9 IU/mL of virus and incubated for 2 minutes to allowing for absorption. This process was repeated three additional times for four total doses (Park, Decker, Margul, et al., 2018; Park, Decker, Smith, et al., 2018). By comparison, our viral loading conditions were somewhat restricted, as the tubes are dried prior to implantation for handling ease, and upon implantation, they swell to fit an injury. As a result, we could not load the tubes with virus ex vivo prior to implanting, potentially limiting the total viral load absorbed by the bridge. Loading concentrations were restricted to a single 2 μL dose of 4E9 IU/mL, half of the total dose used by Park et al. Moving forward, we will need to optimize viral loading concentrations and conditions to potentially observe a greater anti-inflammatory effect when used in conjunction with stem cell transplants. Additionally, incorporating multiple factors based on the host response post-injury could further improve the system. A system that has additional factors beyond IL-10 alone could create a more robust therapy to first target early inflammation with an anti-inflammatory and later stimulate regeneration with a neurotrophic factor (Dominique R. Smith et al., 2020). Furthermore, affinity peptides for lentivirus incorporated into hydrogel tubes could improve its retention and localization at the injury (Skoumal, Seidlits, Shin, & Shea, 2016).
Stem cell transplantation into the injured spinal cord is a treatment option with high potential, but cell sourcing and scale-up have met difficulty (A. J. Anderson, Piltti, Hooshmand, Nishi, & Cummings, 2017; Levi et al., 2018; Temple & Studer, 2017). In research, a cytotoxic transplantation site at the injury is a notable barrier contributing to low survival post-transplantation. As the post-injury immune response clears out dead cells and debris, it also targets transplanted cells, limiting their durability and potential to promote repair (Pereira, Marote, Salgado, & Silva, 2019). Improving exogenous cell transplant survival would allow spinal progenitors to integrate with endogenous circuitry (Lien, Tuszynski, & Lu, 2019; Paul Lu et al., 2017; Paul Lu et al., 2019) and remediate extracellular space (Hawryluk et al., 2012; P. Lu et al., 2003). Biomaterial strategies have long proven to be effective vehicles for transplanting stem cells to central nervous system (CNS) injuries as they can be functionalized for cell adhesion and offer a protective role against transplantation conditions (Higuchi et al., 2019; Katoh, Yokota, & Fehlings, 2019). Bulk biomaterial implants, like porous PLG bridges (Dumont et al., 2018) or 3D printed scaffolds (Koffler et al., 2019), can be cultured ex vivo with stem cells and implanted into the injury. Rigid implants with a defined architecture also provide directional cues for axon elongation once in the injury. Alternatively, injections of cells suspended in synthetic (Marquardt et al., 2020) or natural (Cerqueira et al., 2018; Mothe et al., 2013) hydrogels can form a biomimetic extracellular matrix once in the injury. Soft hydrogel implants are advantageous as they better mimic the mechanical properties of the surrounding native tissue and can swell to fit unique injury shapes, advancing their translatability.
Unfortunately, both ex vivo culturing and hydrogel suspension techniques for cell transplantation offer little control over delivery time relative to biomaterial implantation as cell transplants are dependent on the biomaterial as a carrier. The temporal dependence on the biomaterial limits the opportunity for addressing regenerative barriers, such as inflammation, prior to cell transplantation. Alternatively, a previously implanted biomaterial has time to integrate and modulate the immune response and can additionally serve as a platform for directly injecting stem cells. In our previous report, we saw approximately 4.3% of transplanted E14 spinal progenitors survive two weeks after transplantation when injected into integrated PEG tubes (Ciciriello et al., 2020). For the present study, we similarly delayed E14 spinal progenitor transplantation for 2 weeks, but we additionally incorporated IL-10 encoding lentivirus to our PEG tubes at implantation. A buffer period between 1 and 2 weeks is common in SCI-cell transplant strategies (Brian J. Cummings et al., 2005; Führmann et al., 2016; Mothe et al., 2013), and we chose 2 weeks for delaying E14 spinal progenitor delivery to coincide with IL-10 lentivirus-attributable M2 polarization shown by others (Park, Decker, Margul, et al., 2018). Additionally, we chose to inject our E14 spinal progenitors in two locations along the length of the implanted biomaterial. The rationale here was to improve their spread through the injury, and multiple injection locations is a strategy commonly used both pre-clinically and clinically (Platt et al., 2020; Tetzlaff et al., 2011). We assessed transplant survival after another 2 weeks, 4 weeks after tube implantation, and 8.1% of E14 spinal progenitors transplanted into IL-10 lentivirus loaded tubes survived, an 11.5-fold difference compared to the transplantation only control in this study. The 8.1% survival for the E14 spinal progenitors presented here is a significant increase in transplant engraftment compared to survival previously demonstrated in other immunocompetent models (Tejeda et al., 2021). Combining the independent immunomodulatory properties of the tubes with lentiviral-mediated overexpression of IL-10 resulted in synergistic gains for the transplants, significantly improving their survival.
Exogenous NSC transplants can facilitate de novo neurogenesis, improving regenerative potential post-SCI (Ogawa et al., 2002). We assessed neurogenesis from surviving exogenous E14 spinal progenitors at both 4 and 12 weeks post-injury (2 and 10 weeks post-transplantation, respectively) using NeuN staining, a marker for mature neuron formation. At 4 weeks post-injury, we observed very little staining for EGFP+NeuN+ cells, indicating low levels of neurogenesis from exogenous cells, however we observed increase in neuronal lineage commitment with Tuj staining in EGFP+ cells transplanted with tubes or IL-10 lentivirus-laden tubes. Ogawa et al., transplanted E14.5 spinal progenitors 9 days after a contusive injury, and they observed neurogenesis beginning at 5 weeks post-transplantation, albeit in a rat model (Ogawa et al., 2002), so it is unsurprising that we saw few mature neurons at our earlier time point. By 12 weeks post-injury, we did observe an increase in NeuN staining densities across conditions with a significant increase in the percentage of EGFP+ cells co-staining for NeuN+ cells in conditions that received tube implants.
In a hemisection injury model, as used in the present study, all tissue on the ipsilateral side of the injury is completely removed, thus any axons identified histologically are attributable to post-injury regrowth. Increasing axon density and elongation is an important factor when designing a treatment for SCI, as this will bridge the healthy tissue rostral and caudal to the injury. Additionally, remyelination of these new axons is important for signal propagation along axons in the CNS, a process largely attributed to the myelin sheath. We observed a significantly higher axon density at the injury in mice receiving IL-10 lentivirus loaded tubes with E14 spinal progenitors compared to all other experimental conditions. With that in mind, we would like to note that sparing, plasticity, axonal growth, and remyelination likely occurred early after implantation and transplantation. We suspect that by 12 weeks post-injury, the anti-inflammatory effects of the tubes and E14 spinal progenitors have resolved. Benefits from the tubes and E14 spinal progenitors have an early impact as we previously saw in mice with E14 spinal progenitors alone, tubes alone, and tubes with E14 spinal progenitors had increased axonal densities and myelinated axon densities at 4 weeks post-injury (Ciciriello et al., 2020). In this study, mice receiving E14 spinal progenitors with IL-10 lentivirus-laden tubes possibly had a longer therapeutic regime compared to all other conditions, resulting in the significant increase in axon density. Directly comparing densities between conditions can be misleading if the overall context is not considered, and this is more apparent with the myelin data. The mice that received E14 spinal progenitors only had an average injury area of 1.10 ± 0.5 mm2 compared to the expected area of 1.75 mm2 based on the cross-sectional area of resected tissue. These mice may have experienced some tissue collapse as there was no biomaterial to fill the lesion, effectively lowering the area to which cell counts are normalized and artificially inflating densities. Axonal remyelination results in the E14 spinal progenitor only condition indicate significantly improved remyelination compared to other conditions, but this might be attributable to a lower cross-sectional area resulting in a higher apparent density. This discrepancy is also highlighted when comparing axon myelination to functional data. The E14 only condition had the most myelin of all the conditions, but it was the last treatment group to reach significantly more successful placements compared to the SCI only group. This disparity between histological and functional data raises a point of disconnect that could possibly be attributable to improper synapse formation or poor myelin quality, which could be investigated further to better understand this relationship.
5. Conclusion
Stem cell transplantations are a promising therapy across tissue engineering, especially in CNS damage and disease. Unfortunately, poor survival minimizes their therapeutic effect as a result from poor flexibility in transplantation strategies and subjecting the cells to inhospitable conditions upon injection. Biomaterials and therapeutic factors can facilitate improved conditions, but they are often delivered simultaneously with cell transplants, limiting the perceived immunomodulatory effect on the cells. In this study, our hydrogel tube system affords temporal independence between cell transplantation and biomaterial implantation, an advantage absent in many other cell-biomaterial strategies. In addition to temporal independence, we enhanced the passive immunomodulatory properties of our hydrogel tube system with active immunomodulation via IL-10 encoding lentivirus release from the tubes. In a 2 week course of combined passive and active immunomodulation prior to transplantation, the IL-10 lentivirus loaded tubes effectively created a privileged cell transplantation site for E14 spinal progenitors. To that end, we observed a significant increase in transplant survival and axon elongation in addition to a more rapid functional recovery. Altogether, this therapy synergizes the therapeutic benefits of biomaterials, stem cells, and therapeutic factor delivery, representing a combinatorial approach capable of addressing several of the challenges that limit recovery post-SCI.
Supplementary Material
Figure S1: IL-10 expression at 4 and 12 weeks
Figure S2: PEG implants facilitate tissue ingrowth
Figure S3: Glial scarring is decreased in mice receiving tubes
Figure S4: Exogenous progenitor characterization at 4 weeks
Figure S5: Endogenous progenitor characterization at 12 weeks
Figure S6: Ipsilateral tissue are is lower in conditions not receiving PEG implants
Figure S7: Remyelinated axons have Schwann cell derived myelin
Table S1: Primary Antibodies Used for Immunohistochemistry
Funding Information:
This work was supported by the NIH (R01EB005678).
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Supporting Information: Additional supporting information may be found in the online version of this article.
Data Availability:
The datasets generated during and/or analyzed during the present study are available from the corresponding author upon reasonable request.
References
- Abdellatif AA, Pelt JL, Benton RL, Howard RM, Tsoulfas P, Ping P, . . . Whittemore SR (2006). Gene delivery to the spinal cord: comparison between lentiviral, adenoviral, and retroviral vector delivery systems. Journal of Neuroscience Research, 84(3), 553–567. doi: 10.1002/jnr.20968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AJ, Piltti KM, Hooshmand MJ, Nishi RA, & Cummings BJ (2017). Preclinical Efficacy Failure of Human CNS-Derived Stem Cells for Use in the Pathway Study of Cervical Spinal Cord Injury. Stem Cell Reports, 8(2), 249–263. doi: 10.1016/j.stemcr.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, . . . Sofroniew MV (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature, 532(7598), 195–200. doi: 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A, Chacko J, Nishi R, Dumont C, Smith D, Shea L, . . . Anderson A (2018). Feasibility study on mouse live imaging after spinal cord injury and poly(lactide-co-glycolide) bridge implantation. Journal of Biomedical Optics, 23(6), 065007. Retrieved from 10.1117/1.JBO.23.6.065007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce F, Breckpot K, Collins M, & Escors D (2011). Targeting lentiviral vectors for cancer immunotherapy. Current cancer therapy reviews, 7(4), 248–260. doi: 10.2174/157339411797642605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashammakhi N, Kim H-J, Ehsanipour A, Bierman RD, Kaarela O, Xue C, . . . Seidlits SK (2019). Regenerative Therapies for Spinal Cord Injury. Tissue Engineering Part B: Reviews, 25(6), 471–491. doi: 10.1089/ten.teb.2019.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assunção-Silva RC, Gomes ED, Sousa N, Silva NA, & Salgado AJ (2015). Hydrogels and Cell Based Therapies in Spinal Cord Injury Regeneration. Stem cells international, 2015, 948040–948040. doi: 10.1155/2015/948040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilés MO, & Shea LD (2011). Hydrogels to modulate lentivirus delivery in vivo from microporous tissue engineering scaffolds. Drug delivery and translational research, 1(1), 91–101. doi: 10.1007/s13346-010-0011-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler RM, Kuo R, Shin S, Goodman AG, Pilecki MA, Leonard JN, & Shea LD (2014). Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnology and Bioengineering, 111(6), 1210–1221. doi: 10.1002/bit.25175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, & Burnside ER (2019). Moving beyond the glial scar for spinal cord repair. Nature Communications, 10(1), 3879. doi: 10.1038/s41467-019-11707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Horn KP, Silver DJ, & Silver J (2009). Overcoming macrophage-mediated axonal dieback following CNS injury. Journal of Neuroscience, 29(32), 9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Dewi RE, & Heilshorn SC (2015). Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Advanced functional materials, 25(9), 1344–1351. doi: 10.1002/adfm.201403631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira SR, Lee Y-S, Cornelison RC, Mertz MW, Wachs RA, Schmidt CE, & Bunge MB (2018). Decellularized peripheral nerve supports Schwann cell transplants and axon growth following spinal cord injury. Biomaterials, 177, 176–185. doi: 10.1016/j.biomaterials.2018.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceto S, Sekiguchi KJ, Takashima Y, Nimmerjahn A, & Tuszynski MH (2020). Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury. Cell stem cell. doi: 10.1016/j.stem.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Bernreuther C, Dihné M, & Schachner M (2005). Cell Adhesion Molecule L1–Transfected Embryonic Stem Cells with Enhanced Survival Support Regrowth of Corticospinal Tract Axons in Mice after Spinal Cord Injury. Journal of neurotrauma, 22(8), 896–906. doi: 10.1089/neu.2005.22.896 [DOI] [PubMed] [Google Scholar]
- Cheng Z, Zhu W, Cao K, Wu F, Li J, Wang G, . . . He X, (2016). Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury. International journal of molecular sciences, 17(9), 1380. doi: 10.3390/ijms17091380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou F-C, & Sytwu H-K (2009). Overexpression of thioredoxin in islets transduced by a lentiviral vector prolongs graft survival in autoimmune diabetic NOD mice. Journal of Biomedical Science, 16(1), 71–71. doi: 10.1186/1423-0127-16-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciciriello AJ, Smith DR, Munsell MK, Boyd SJ, Shea LD, & Dumont CM (2020). Acute Implantation of Aligned Hydrogel Tubes Supports Delayed Spinal Progenitor Implantation. ACS Biomaterials Science & Engineering, 6(10), 5771–5784. doi: 10.1021/acsbiomaterials.0c00844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Engesser-Cesar C, Cadena G, & Anderson AJ (2007). Adaptation of a ladder beam walking task to assess locomotor recovery in mice following spinal cord injury. Behav Brain Res, 177(2), 232–241. doi: 10.1016/j.bbr.2006.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, . . . Anderson AJ (2005). Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proceedings of the National Academy of Sciences of the United States of America, 102(39), 14069. doi: 10.1073/pnas.0507063102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalamagkas K, Tsintou M, Seifalian A, & Seifalian AM (2018). Translational Regenerative Therapies for Chronic Spinal Cord Injury. International journal of molecular sciences, 19(6), 1776. doi: 10.3390/ijms19061776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Latronico MVG, Jotti GS, & Condorelli G (2012). Lentiviral vectors and cardiovascular diseases: a genetic tool for manipulating cardiomyocyte differentiation and function. Gene Therapy, 19(6), 642–648. doi: 10.1038/gt.2012.19 [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, & Popovich PG (2008). Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Experimental Neurology, 209(2), 378–388. doi: 10.1016/j.expneurol.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin JN, & Lu P (2014). Bridging the injured spinal cord with neural stem cells. Neural regeneration research, 9(3), 229–231. doi: 10.4103/1673-5374.128212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont CM, Carlson MA, Munsell MK, Ciciriello AJ, Strnadova K, Park J, . . . Shea LD (2019). Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater, 86, 312–322. doi: 10.1016/j.actbio.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont CM, Margul DJ, & Shea LD (2016). Tissue Engineering Approaches to Modulate the Inflammatory Milieu following Spinal Cord Injury. Cells Tissues Organs, 202(1–2), 52–66. doi: 10.1159/000446646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont CM, Munsell MK, Carlson MA, Cummings BJ, Anderson AJ, & Shea LD (2018). Spinal Progenitor-Laden Bridges Support Earlier Axon Regeneration Following Spinal Cord Injury. Tissue Eng Part A, 24(21–22), 1588–1602. doi: 10.1089/ten.TEA.2018.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Führmann T, Tam RY, Ballarin B, Coles B, Elliott Donaghue I, van der Kooy D, . . . Shoichet MS (2016). Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials, 83, 23–36. doi: 10.1016/j.biomaterials.2015.12.032 [DOI] [PubMed] [Google Scholar]
- Gu Y, Cheng X, Huang X, Yuan Y, Qin S, Tan Z, . . . Su Z (2019). Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain, Behavior, and Immunity, 80, 394–405. doi: 10.1016/j.bbi.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Hackett AR, & Lee JK (2016). Understanding the NG2 Glial Scar after Spinal Cord Injury. Frontiers in Neurology, 7(199). doi: 10.3389/fneur.2016.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED (2011). Antioxidant therapies for acute spinal cord injury. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics, 8(2), 152–167. doi: 10.1007/s13311-011-0026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk GWJ, Mothe A, Wang J, Wang S, Tator C, & Fehlings MG (2012). An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells and Development, 21(12), 2222–2238. doi: 10.1089/scd.2011.0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi A, Suresh Kumar S, Benelli G, Ling Q-D, Li H-F, Alarfaj AA, . . . Murugan K (2019). Biomaterials used in stem cell therapy for spinal cord injury. Progress in Materials Science, 103, 374–424. doi: 10.1016/j.pmatsci.2019.02.002 [DOI] [Google Scholar]
- Hill CE (2017). A view from the ending: Axonal dieback and regeneration following SCI. Neuroscience Letters, 652, 11–24. doi: 10.1016/j.neulet.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Jimenez-Moreno CM, Herrera-Gomez I. d. G., Lopez-Noriega L, Lorenzo PI, Cobo-Vuilleumier N, Fuente-Martin E, . . . Martin-Montalvo A (2015). A Simple High Efficiency Intra-Islet Transduction Protocol Using Lentiviral Vectors. Current gene therapy, 15(4), 436–446. doi: 10.2174/1566523215666150630121557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, & Fehlings MG (2006). Delayed Transplantation of Adult Neural Precursor Cells Promotes Remyelination and Functional Neurological Recovery after Spinal Cord Injury. The Journal of Neuroscience, 26(13), 3377. doi: 10.1523/JNEUROSCI.4184-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Yokota K, & Fehlings MG (2019). Regeneration of Spinal Cord Connectivity Through Stem Cell Transplantation and Biomaterial Scaffolds. Frontiers in cellular neuroscience, 13, 248–248. doi: 10.3389/fncel.2019.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, . . . Tuszynski MH (2019). Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nature medicine, 25(2), 263–269. doi: 10.1038/s41591-018-0296-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamaru H, Kadoya K, Adler AF, Takashima Y, Graham L, Coppola G, & Tuszynski MH (2018). Generation and post-injury integration of human spinal cord neural stem cells. Nature Methods, 15(9), 723–731. doi: 10.1038/s41592-018-0074-3 [DOI] [PubMed] [Google Scholar]
- Kwon SG, Kwon YW, Lee TW, Park GT, & Kim JH (2018). Recent advances in stem cell therapeutics and tissue engineering strategies. Biomaterials research, 22, 36–36. doi: 10.1186/s40824-018-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Chang L, Rose-John S, & Tuszynski MH (2002). Delivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growth. J Comp Neurol, 454(3), 213–228. doi: 10.1002/cne.10407 [DOI] [PubMed] [Google Scholar]
- Levi AD, Anderson KD, Okonkwo DO, Park P, Bryce TN, Kurpad SN, . . . Gant K (2018). Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. Journal of neurotrauma, 36(6), 891–902. doi: 10.1089/neu.2018.5843 [DOI] [PubMed] [Google Scholar]
- Li X, Liu S, Zhao Y, Li J, Ding W, Han S, . . . Dai J, (2016). Training Neural Stem Cells on Functional Collagen Scaffolds for Severe Spinal Cord Injury Repair. Advanced functional materials, 26(32), 5835–5847. doi: 10.1002/adfm.201601521 [DOI] [Google Scholar]
- Liddelow SA, & Barres BA (2016). Not everything is scary about a glial scar. Nature, 532(7598), 182–183. doi: 10.1038/nature17318 [DOI] [PubMed] [Google Scholar]
- Liechtenstein T, Perez-Janices N, & Escors D (2013). Lentiviral vectors for cancer immunotherapy and clinical applications. Cancers, 5(3), 815–837. doi: 10.3390/cancers5030815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien BV, Tuszynski MH, & Lu P (2019). Astrocytes migrate from human neural stem cell grafts and functionally integrate into the injured rat spinal cord. Experimental Neurology, 314, 46–57. doi: 10.1016/j.expneurol.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Liu JMH, Zhang X, Joe S, Luo X, & Shea LD (2018). Evaluation of biomaterial scaffold delivery of IL-33 as a localized immunomodulatory agent to support cell transplantation in adipose tissue. Journal of Immunology and Regenerative Medicine, 1, 1–12. doi: 10.1016/j.regen.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Schackel T, Weidner N, & Puttagunta R (2018). Biomaterial-Supported Cell Transplantation Treatments for Spinal Cord Injury: Challenges and Perspectives. Frontiers in cellular neuroscience, 11(430). doi: 10.3389/fncel.2017.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Xie Y-Y, & Wang B (2019). Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural regeneration research, 14(8), 1352–1363. doi: 10.4103/1673-5374.253512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, . . . Choi DW (1997). Neuronal and glial apoptosis after traumatic spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience, 17(14), 5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Ceto S, Wang Y, Graham L, Wu D, Kumamaru H, . . . Tuszynski MH (2017). Prolonged human neural stem cell maturation supports recovery in injured rodent CNS. The Journal of clinical investigation, 127(9), 3287–3299. doi: 10.1172/JCI92955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Gomes-Leal W, Anil S, Dobkins G, Huie JR, Ferguson AR, . . . Tuszynski M (2019). Origins of Neural Progenitor Cell-Derived Axons Projecting Caudally after Spinal Cord Injury. Stem Cell Reports, 13(1), 105–114. doi: 10.1016/j.stemcr.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, & Tuszynski MH (2003). Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Experimental Neurology, 181(2), 115–129. doi: 10.1016/S0014-4886(03)00037-2 [DOI] [PubMed] [Google Scholar]
- Margul DJ, Park J, Boehler RM, Smith DR, Johnson MA, McCreedy DA, . . . Seidlits SK (2016). Reducing neuroinflammation by delivery of IL-10 encoding lentivirus from multiple-channel bridges. Bioengineering & Translational Medicine, 1(2), 136–148. doi: 10.1002/btm2.10018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt LM, Doulames VM, Wang AT, Dubbin K, Suhar RA, Kratochvil MJ, . . . Heilshorn SC (2020). Designer, injectable gels to prevent transplanted Schwann cell loss during spinal cord injury therapy. Science Advances, 6(14), eaaz1039. doi: 10.1126/sciadv.aaz1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt LM, & Heilshorn SC (2016). Design of Injectable Materials to Improve Stem Cell Transplantation. Current stem cell reports, 2(3), 207–220. doi: 10.1007/s40778-016-0058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreedy DA, Margul DJ, Seidlits SK, Antane JT, Thomas RJ, Sissman GM, . . . Shea LD (2016). Semi-automated counting of axon regeneration in poly(lactide co-glycolide) spinal cord bridges. J Neurosci Methods, 263, 15–22. doi: 10.1016/j.jneumeth.2016.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone MC, & O’Doherty U (2018). Clinical use of lentiviral vectors. Leukemia, 32(7), 1529–1541. doi: 10.1038/s41375-018-0106-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi MM, Jaber M, Adeeb N, Deep A, Hose N, Rezaei M, . . . Tubbs RS (2015). Engraftment of neural stem cells in the treatment of spinal cord injury. Translational Research in Anatomy, 1, 11–16. doi: 10.1016/j.tria.2015.10.002 [DOI] [Google Scholar]
- Mosser DM, & Edwards JP (2008). Exploring the full spectrum of macrophage activation. Nature reviews. Immunology, 8(12), 958–969. doi: 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe AJ, Tam RY, Zahir T, Tator CH, & Shoichet MS (2013). Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials, 34(15), 3775–3783. doi: 10.1016/j.biomaterials.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Nagoshi N, Khazaei M, Ahlfors J-E, Ahuja CS, Nori S, Wang J, . . . Fehlings MG (2018). Human Spinal Oligodendrogenic Neural Progenitor Cells Promote Functional Recovery After Spinal Cord Injury by Axonal Remyelination and Tissue Sparing. Stem cells translational medicine, 7(11), 806–818. doi: 10.1002/sctm.17-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. (2020). Retrieved from https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202020.pdf
- Niwano K, Arai M, Koitabashi N, Watanabe A, Ikeda Y, Miyoshi H, & Kurabayashi M (2008). Lentiviral Vector–mediated SERCA2 Gene Transfer Protects Against Heart Failure and Left Ventricular Remodeling After Myocardial Infarction in Rats. Molecular Therapy, 16(6), 1026–1032. doi: 10.1038/mt.2008.61 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Sawamoto K, Miyata T, Miyao S, Watanabe M, Nakamura M, . . . Okano H (2002). Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. Journal of Neuroscience Research, 69(6), 925–933. doi: 10.1002/jnr.10341 [DOI] [PubMed] [Google Scholar]
- Park J, Decker JT, Margul DJ, Smith DR, Cummings BJ, Anderson AJ, & Shea LD (2018). Local Immunomodulation with Anti-inflammatory Cytokine-Encoding Lentivirus Enhances Functional Recovery after Spinal Cord Injury. Molecular Therapy, 26(7), 1756–1770. doi: 10.1016/j.ymthe.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Decker JT, Smith DR, Cummings BJ, Anderson AJ, & Shea LD (2018). Reducing inflammation through delivery of lentivirus encoding for anti-inflammatory cytokines attenuates neuropathic pain after spinal cord injury. Journal of Controlled Release, 290, 88–101. doi: 10.1016/j.jconrel.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar K, Cummings BJ, Thomas A, Shea LD, Levine A, Pfaff S, & Anderson AJ (2015). Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: Association with recovery of forelimb function. Biomaterials, 65, 1–12. doi: 10.1016/j.biomaterials.2015.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira IM, Marote A, Salgado AJ, & Silva NA (2019). Filling the Gap: Neural Stem Cells as A Promising Therapy for Spinal Cord Injury. Pharmaceuticals (Basel, Switzerland), 12(2), 65. doi: 10.3390/ph12020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltti KM, Salazar DL, Uchida N, Cummings BJ, & Anderson AJ (2013). Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem cells translational medicine, 2(3), 204–216. doi: 10.5966/sctm.2012-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt A, David BT, Fessler, & Richard G (2020). Stem Cell Clinical Trials in Spinal Cord Injury: A Brief Review of Studies in the United States. Medicines (Basel, Switzerland), 7(5), 27. doi: 10.3390/medicines7050027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Barry MA, & Ikeda Y (2012). Lentiviral vectors: basic to translational. Biochem J, 443(3), 603–618. doi: 10.1042/bj20120146 [DOI] [PubMed] [Google Scholar]
- Shahriari D, Koffler JY, Tuszynski MH, Campana WM, & Sakamoto JS (2017). Hierarchically Ordered Porous and High-Volume Polycaprolactone Microchannel Scaffolds Enhanced Axon Growth in Transected Spinal Cords. Tissue Eng Part A, 23(9–10), 415–425. doi: 10.1089/ten.TEA.2016.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamash S, Reichert F, & Rotshenker S (2002). The cytokine network of Wallerian degeneration: tumor necrosis factor-alpha, interleukin-1alpha, and interleukin-1beta. The Journal of neuroscience : the official journal of the Society for Neuroscience, 22(8), 3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Smith RM, Xu M, Woodruff TK, & Shea LD (2011). Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials, 32(10), 2524–2531. doi: 10.1016/j.biomaterials.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Salvay DM, & Shea LD (2010). Lentivirus delivery by adsorption to tissue engineering scaffolds. Journal of biomedical materials research. Part A, 93(4), 1252–1259. doi: 10.1002/jbm.a.32619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Coykendall K, Li Y, Moon A, Priyadarshani P, & Yao L (2014). Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell Research & Therapy, 5(4), 91. doi: 10.1186/scrt480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoumal M, Seidlits S, Shin S, & Shea L (2016). Localized lentivirus delivery via peptide interactions. Biotechnology and Bioengineering, 113(9), 2033–2040. doi: 10.1002/bit.25961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Dumont CM, Park J, Ciciriello AJ, Guo A, Tatineni R, . . . Shea LD (2020). Polycistronic Delivery of IL-10 and NT-3 Promotes Oligodendrocyte Myelination and Functional Recovery in a Mouse Spinal Cord Injury Model. Tissue Engineering Part A. doi: 10.1089/ten.tea.2019.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Margul DJ, Dumont CM, Carlson MA, Munsell MK, Johnson M, . . . Shea LD (2019). Combinatorial lentiviral gene delivery of pro-oligodendrogenic factors for improving myelination of regenerating axons after spinal cord injury. Biotechnol Bioeng, 116(1), 155–167. doi: 10.1002/bit.26838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, . . . Lee JK (2013). Perivascular Fibroblasts Form the Fibrotic Scar after Contusive Spinal Cord Injury. The Journal of Neuroscience, 33(34), 13882. doi: 10.1523/JNEUROSCI.2524-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley KS, Foo CWP, & Heilshorn SC (2010). Biomaterial design strategies for the treatment of spinal cord injuries. Journal of neurotrauma, 27(1), 1–19. doi: 10.1089/neu.2009.0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda G, Ciciriello AJ, & Dumont CM (2021). Biomaterial Strategies to Bolster Neural Stem Cell-Mediated Repair of the Central Nervous System. Cells Tissues Organs(In press). [DOI] [PubMed] [Google Scholar]
- Temple S, & Studer L (2017). Lessons Learned from Pioneering Neural Stem Cell Studies. Stem Cell Reports, 8(2), 191–193. doi: 10.1016/j.stemcr.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, . . . Kwon BK (2011). A systematic review of cellular transplantation therapies for spinal cord injury. Journal of neurotrauma, 28(8), 1611–1682. doi: 10.1089/neu.2009.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Gomez AJ, Palma JL, Yap WT, & Shea LD (2014). Heparin–chitosan nanoparticle functionalization of porous poly(ethylene glycol) hydrogels for localized lentivirus delivery of angiogenic factors. Biomaterials, 35(30), 8687–8693. doi: 10.1016/j.biomaterials.2014.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Palma JL, & Shea LD (2015). Sponge-mediated lentivirus delivery to acute and chronic spinal cord injuries. Journal of Controlled Release, 204, 1–10. doi: 10.1016/j.jconrel.2015.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, & Shea LD (2013). Polysaccharide-modified scaffolds for controlled lentivirus delivery in vitro and after spinal cord injury. Journal of Controlled Release, 170(3), 421–429. doi: 10.1016/j.jconrel.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsintou M, Dalamagkas K, & Seifalian AM (2015). Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural regeneration research, 10(5), 726–742. doi: 10.4103/1673-5374.156966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, . . . Shea LD (2012). Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials, 33(5), 1618–1626. doi: 10.1016/j.biomaterials.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinstra HM, Margul DJ, Goodman AG, Boehler RM, Holland SJ, Zelivyanskaya ML, . . . Shea LD (2013). Long-Term Characterization of Axon Regeneration and Matrix Changes Using Multiple Channel Bridges for Spinal Cord Regeneration. Tissue Engineering Part A, 20(5–6), 127–1037. doi: 10.1089/ten.tea.2013.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Dai Y, Chen G, & Cui S (2020). Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Frontiers in cellular neuroscience, 14(78). doi: 10.3389/fncel.2020.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Laporte LD, Zelivyanskaya ML, Whittlesey KJ, Anderson AJ, Cummings BJ, & Shea LD (2009). Multiple Channel Bridges for Spinal Cord Injury: Cellular Characterization of Host Response. Tissue Engineering Part A, 15(11), 3283–3295. doi: 10.1089/ten.tea.2009.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Zhang A, Duan H, Zhang S, Hao P, Ye K, . . . Li X (2015). NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci U S A, 112(43), 13354–13359. doi: 10.1073/pnas.1510194112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pettigrew GJ, Thomas J, Vandenberg JI, Delriviere L, Bolton EM, . . . Lever AM (2002). Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res Cardiol, 97(5), 348–358. doi: 10.1007/s00395-002-0360-0 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Soderblom C, Trojanowsky M, Lee D-H, & Lee JK (2014). Fibronectin Matrix Assembly after Spinal Cord Injury. Journal of neurotrauma, 32(15), 1158–1167. doi: 10.1089/neu.2014.3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: IL-10 expression at 4 and 12 weeks
Figure S2: PEG implants facilitate tissue ingrowth
Figure S3: Glial scarring is decreased in mice receiving tubes
Figure S4: Exogenous progenitor characterization at 4 weeks
Figure S5: Endogenous progenitor characterization at 12 weeks
Figure S6: Ipsilateral tissue are is lower in conditions not receiving PEG implants
Figure S7: Remyelinated axons have Schwann cell derived myelin
Table S1: Primary Antibodies Used for Immunohistochemistry
Data Availability Statement
The datasets generated during and/or analyzed during the present study are available from the corresponding author upon reasonable request.


