Abstract
Indoor air pollution (IAP) is a recognized risk factor for various diseases. This paper examines the role of indoor solid fuel exposure in the risk of mycobacterium tuberculosis (TB) in Delhi Metropolitan, India. Using a cross-sectional design, subjects were screened for a history of active TB and lifelong exposure to IAP sources, such as solid fuel burning and kerosene. The TB prevalence rate in the study area was 1117 per 100 000 population. Every year, increase in solid fuel exposure was associated with a three percent higher likelihood of a history of active TB. Subjects exposed to solid fuel and kerosene use for both heating home and cooking showed significant associations with TB. Age, household expenditure (a proxy of income), lung function, and smoking also showed significant associations with TB. Smokers and solid fuel–exposed subjects were four times more likely to have a history of active TB than non-smoker and unexposed subjects. These finding calls strategies to mitigate solid fuel exposure, such as use of clean cookstove and ventilation, to mitigate the risk of TB which aligns with the United Nations’ goal of “End TB by 2030.”
Keywords: air pollution, Delhi, indoor air pollution, lifelong exposure, solid fuel, tuberculosis
1 |. INTRODUCTION
Tuberculosis (TB), caused by mycobacterium tuberculosis (M tb), is the second most prevalent infectious disease1,2 and accounts for about 1.5 million deaths annually.3 Low- and middle-income countries (LMIC) bear disproportionately higher burden of TB mortality and morbidity.4 Among LMIC, India has the highest incidence and prevalence rates of TB in the world.5 Many factors are shown to increase the risk of activation from latent TB infection to clinically manifested active TB and its severity, including host immunity, exposure to smear-positive pulmonary TB patients, malnutrition, and socioeconomic and environmental exposure and risk behavior (eg, smoking, alcohol consumption, sexual behavior).6–8
Among environmental risk factors, air pollution is of particular interest not only because air pollutants, such as particulate matter (PM), can serve as carriers of airborne M.tb but also because it affects lung immunity by inducing oxidative stress and inflammation, and impairs the host’s immunity.9,10 Literature shows epidemiological associations between air pollution exposure and tuberculosis, including in India.8,11–13 Although the mechanism of TB activation from air pollution is not fully understood,14 it has been proposed that air pollution affects TB activation through altering lung immunity of the host due to chronic oxidative stress followed by inflammation.15 Recent literature also shows that air pollution can cause accumulation of carbon in the bronchial tree, which increases the risk of TB by inactivating pulmonary macrophages.16,17 Although air pollution has long been recognized as a health hazard since the 1952 London Smog,18,19 LMIC countries account for more than 90% of the air pollution mortality burden, which does not include mortality due to TB.20
Given people spend most of their time indoors and a large number of households in LMIC continue to use solid fuel (including, coal, coke or wood, crop residue, and dung cake) for heating and cooking, indoor air pollution (IAP) from these sources is the main contributor of the total air pollution exposure. IAP is implicated in several diseases including cardiovascular disease, respiratory infections, chronic lung diseases, and even death.21–30 However, the role of IAP in TB disease is inconsistent. For example, Slama et al reviewed 994 articles and only found 6 studies on association between IAP and TB. Only 3 of these 6 showed a positive association between IAP and TB.31 Likewise, another study found a higher risk of liquid petroleum gas (LPG) exposure in Nepal rather than solid fuel. However, two other studies in Pune (India) and South Africa showed a higher prevalence rate of TB in homes with solid fuel exposure.32,33 This inconsistency in the epidemiological association between TB risk and IAP is the likely reason that IAP is still not recognized as one of the risk factors for TB by the World Health Organization (WHO).34 Using a large sample (n = 15 573), this research addresses this gap by quantifying the risk of TB with respect to short and lifelong IAP exposure in a megacity with hazardous levels of air pollution. Our central hypothesis is that current and long-term IAP exposure is associated with a history of active TB.
2 |. MATERIALS AND METHODS
2.1 |. Study setting
Using a cross-sectional design, data for this study were collected from Delhi Metropolitan, India. The city is home to 16.75 million people35 and identified one of the most polluted cities in the world. The annual PM10 level in the city is as high as 268.6 μg/m,36 five times higher than the WHO standards.37 A significant number of homes in the city continue to use solid fuel (wood, crops, dung cake, and charcoal) for heating and cooking, which results in the elevated concentration of fine particulate matter.38–41
2.2 |. Study definitions
History of TB disease was self-reported, which was cross-validated by the responses to the questions on the clinical assessment of the sputum culture, and other TB-related symptoms, including a history of hemoptysis, prolong cough. Data on latent TB were not collected for this study.
2.2.1 |. Obstructive lung disease
Obstructive lung disease (OLD) was defined based on spirometry. A ratio of forced expiratory volume after one second (FEV1) to forced vital capacity (FVC) < 0.7 was considered as OLD.42
2.2.2 |. Indoor air pollution
Indoor air pollution (IAP) was defined if households primarily used solid fuel (that included wood, coke, coal, crop residue, and dung cake) or kerosene for cooking and heating purposes. Indoor air pollutants were not monitored in this study, rather pollution sources were used as proxy of exposure. Since the emission types from solid fuel are different from kerosene, both were also examined separately.
2.2.3 |. Lifelong exposure
Individual survey module included questions on the history of cooking fuel use (see survey instrument in SOM, question I105 to I110). Using these data, the number of years of exposure to solid fuel and kerosene was computed, separately. Data on other covariates were also collected, such as separate kitchen in the house, exhaust fan in kitchen and its use while cooking, age, smoking, and gender.
2.3 |. Data collection
Graduate students from Jawaharlal Nehru University (JNU), Delhi, and physicians with MBBS (Bachelor of Medicine, Bachelor of Surgery) degree were recruited to administer the surveys. Four teams were recruited, and each team consisted of a male and female interviewer, and one physician. All four teams worked simultaneously. All teams were trained to administer the survey, and physicians were trained to perform anthropometric measurement and pulmonary function tests using a handheld portable spirometer (MicroDL, Vyaire, USA). A standard protocol and coding system (SPCS) was developed and enforced. All teams participated in a pilot survey to learn and practice how to enforce the SPCS.
Two rounds of surveys were conducted in the Delhi metropolitan in 2004 and 2009. Households were drawn using a probabilistic sampling design. Details on sampling methodology and sample selection are provided elsewhere.43 In the first round, 2000 random points were simulated in the residential areas. The locations were navigated with the aid of a global position system (GPS). If a point was in a multiple housing units building (such as an apartment complex), a list of all households was created and one household was randomly drawn without replacement. Of the 2000 households around the simulated locations, 1576 households consented to participate in the study (a response rate of 78.8%). Location coordinates of the households were recorded. In 2009, 1496 households within 50-m distance of the homes, which participated in 2004, were recruited in order to capture subjects from the same areas.
A total 15 573 subjects participated in the study, and spirometry test and exposure history were restricted to subjects who were 15 years or older. Of the total 11 775 eligible subjects, 5257 (44.6%) consented to participate in the spirometry. The difference in the values of EFV1 and FVC values (of the healthy subjects) of the handheld spirometer MicroDL and those from the office spirometer (Model #Mir, Spirolab 3) was less than 5%. Participants were paid Rupees 100 (US $1.55) each for their cooperation. The household locations in both rounds were recorded using GPS.
The survey included questions on TB, home environment, types of fuel used for cooking and heating, and life course history of exposure to those fuels. For TB, participants were asked “Has anyone in the household been diagnosed with active Tuberculosis?” If response was “Yes,” subject was defined as a case, control otherwise. Follow-up questions concerning the clinical assessment of TB by sputum culture and associated symptoms, such as “coughing blood,” were asked for the cross-validation of cases.
Questions concerning other risk factors, such as tobacco smoking and income, were also extracted. The data related to tobacco smoking were self-reported. In case a subject answered “Yes” to a smoking question, follow-up questions about the types and frequency of smoking were asked (see the survey instrument in the supplement online data [SOM]). The lifetime exposure to various types of cooking fuel including types of fuel used at different ages was recorded (see questions I105 through I110). Type of fuel was categorized into (1) solid fuel (coal, coke, dung cake, crop residue, and wood), (2) gas (or liquefied petroleum gas (LPG), (3) electric heater, (4) kerosene, and (5) others. Indoor and outdoor air pollutants were not directly measured. However, outdoor particulate matter 2.5 ≤ μm in aerodynamic diameter (PM2.5) was estimated using a hybrid approach, which has been used for deriving PM2.5 estimates in different parts of the world including Delhi Metropolitan.44–47 Satellite-based aerosol optical depth (AOD) from MODerate resolution Imaging Spectroradiometer (MODIS) onboard Terra and Aqua satellite at 3-km spatial resolution were acquired from NASA from 2002 to 2009 for the study area.48 These data were collocated with the meteorological data. An empirical model was developed between in situ monitored PM2.5 at the American Embassy in New Delhi and collocated AOD, meteorological conditions, and seasonality. Using the parameter estimated from the empirical model, daily PM2.5 was predicted from 2002 to 2009 and aggregated at 1-km spatial resolution (Figure 1). Using the local time-space Kriging, PM2.5 was estimated at the location of each household and used in the final analyses.49
FIGURE 1.
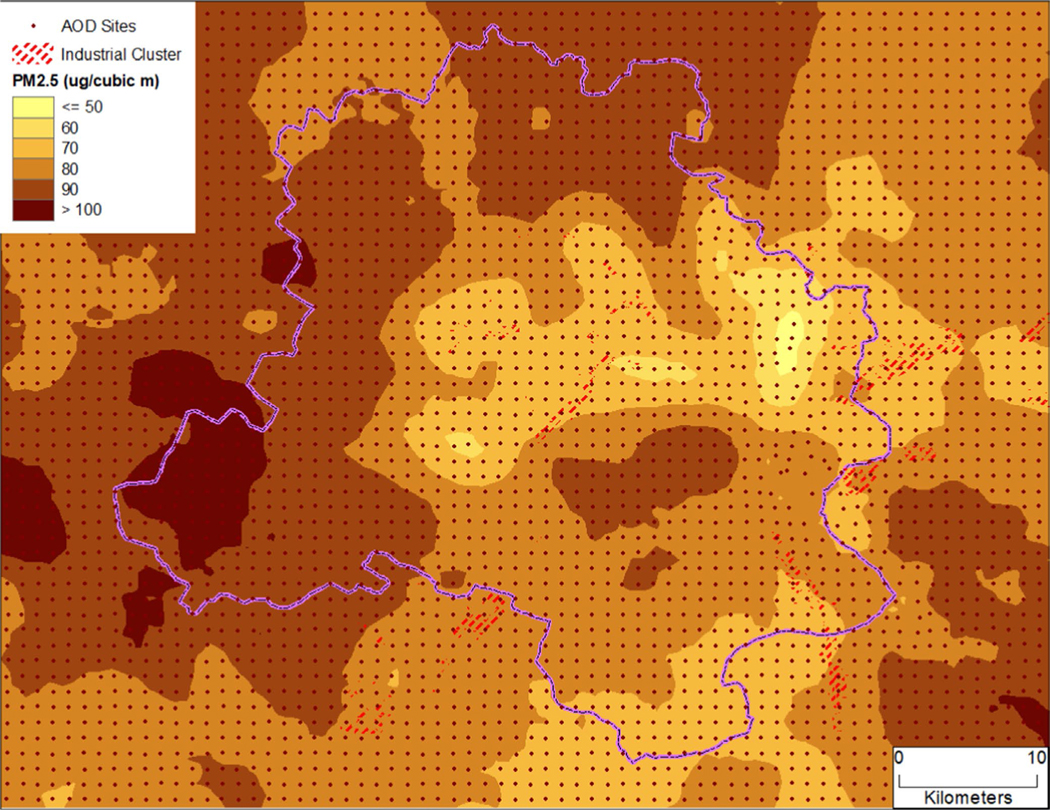
Ambient PM2.5 (μg/m3) concentration in and around Delhi Metropolitan from 2002 to 2009 estimated using a hybrid approach (see Sinha and Kumar44 for details)
This study was approved by the Institutional Review Boards of Brown University and the University of Iowa (IRB approval #0810992578 and #IRB00000100, respectively). All methods were performed in accordance with the relevant guidelines and regulations. Informed verbal consent was obtained from each subject before enrollment in study. However, reasons for not participating in the study were not noted.
2.4 |. Statistical analysis
Descriptive analyses were conducted to summarize outcome variable and associated risk factors. Univariate analysis was used to compare differences in demographic and clinical variables between subjects with and without a history of active TB. Comparisons were unpaired, and all tests of significance were two-tailed. Continuous variables were compared using Student’s t test for normally distributed variables and Wilcoxon’s rank-sum test for non-normally distributed variables. Since LPG is considered as a clean fuel, it was used as a reference compared to solid fuel and kerosene exposure. We used a multivariate logistic regression to examine risk of TB with respect to IAP adjusting for individual and household level confounders (logit function in STATA Ver 14.150 with cluster option, household being defined as the cluster). Interaction of exposure with smoking was used to assess their synergistic effect. Three models were run separately with all confounding variables. In Model 1, smoking and solid fuel exposure were included independently. In Model 2, smoking, solid fuel exposure, and their interaction were included. In Model 3, only the interaction of smoking and solid fuel exposure was included, as well as long-term outdoor exposure to PM2.5. Potential confounders included in all three models were age, gender, lung function, and per capita household expenditure, which served as proxy of household income (see SOM for details and its justification).
3 |. RESULTS
3.1 |. Participants
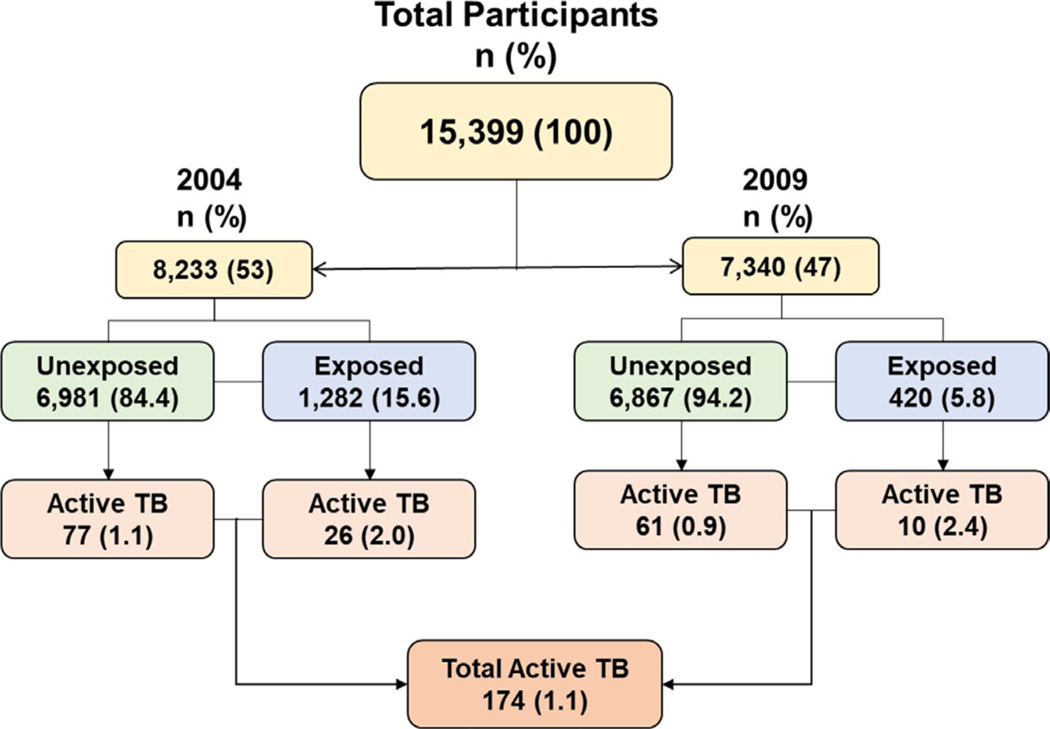
Of the 15 573 subjects, who participated in the study, 174 (1.12%) had a history of active TB, a prevalence rate of 1117 per 100 000 population). Of these 174 cases, 138 (79.31%) also confirmed clinical assessment of their sputum culture. TB prevalence decreased significantly from 1.135% in 2004 to 0.97% in 2009 (P < .05). Figure 2 shows the flowchart of the study.
FIGURE 2.
The flowchart of participants by TB and solid fuel exposure status
3.2 |. Demographics
Subjects with a history of active TB were significantly older than those without TB (mean = 43.8 years ± 18.7 years [95% confidence interval (CI)] and 30.5 years ± 19.3 years; P value < .0001). Although more than half (8126; 52%) of subjects were females, the history of TB prevalence did not vary significantly by gender (P = .760).
3.3 |. Univariate analysis of association between IAP and TB
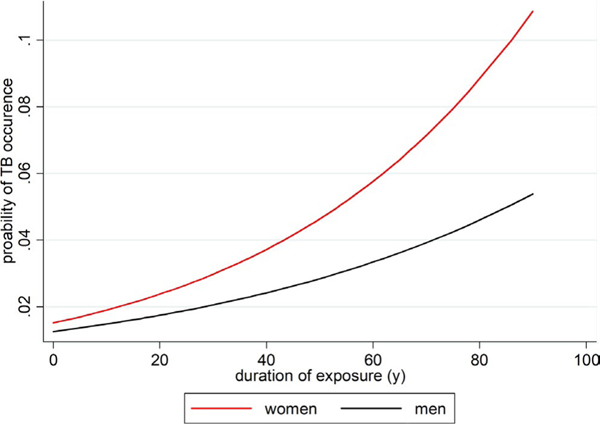
The TB prevalence rate was 2.67% among subjects 60 years or older, who were 3.61 times more likely to have a history of active TB than those in the age-group of 15 to 30 years (95% CI: 2.25–5.81; P < .01) (Table 1). The probability of the TB risk with respect to duration of exposure to solid fuel among men and women in Delhi is shown in Figure 3. TB prevalence was higher (n = 94,1.4%) among subjects who lived in homes without an exhaust fan as compared to those in homes with an exhaust fan (n = 79, 0.9%) (OR = 1.5, 95% CI: 1.6–3.48; P < .001). The frequency of TB among those exposed to solid fuel from cooking and heating in their home was 2.1% (n = 36) as compared to 1% among unexposed (n = 137; OR = 2.36; 95% CI 1.6–3.48; P < .0001). TB frequency also varied significantly with respect to household income, measured by household expenditure per capita. For example, frequency of TB was significantly higher among subjects in homes with <2000 Rupees (Rs.) (ie, ~US $30.00)/month per capita expenditure (n = 79, 1.6%) as compared to those with Rs. 2000–5000/month per capita household expenditure (n = 23, 0.56%) (OR = 0.3, 95% CI: 0.18–0.48; P < .0001). The frequency of TB was almost two times higher among subjects exposed to kerosene during lifetime as compared to those unexposed (OR = 1.88, 95% CI 1,10–3.268, P = .026) (Table 1). TB risk among past and current tobacco smokers was 3.27 and 2.88 times higher than among non-smokers, respectively (Table 1). TB was found to be higher in subjects with OLD (n = 11, 3.7%) than those without OLD (n = 91, 1.5%) (Table 1).
TABLE 1.
TB status with respect to the selected demographic and household variables, Delhi 2004–2009
| Variables | % TB+ (n) | Odds Ratio | 95% CI |
|---|---|---|---|
| Year | |||
| 2004 (base) | 1.25 (8233) | 1 | |
| 2009 | 0.97 (7340) | 0.73 | 0.53–0.99 |
| Gender | |||
| Female (base) | 1.14 (8126) | 1 | |
| Male | 1.09 (7447) | 0.90 | 0.66–1.22 |
| Age (y) | |||
| 15–30 (base) | 0.74 (5106) | 1 | |
| 31–45 | 1.59 (3325) | 2.14*** | 1.39–3.27 |
| 46–60 | 1.88 (2236) | 2.52*** | 1.61–3.95 |
| >60 | 2.67 (1275) | 3.61*** | 2.25–5.81 |
| Exhaust fan in house | |||
| Yes(base) | 0.89 (8571) | 1 | |
| No | 1.40 (6729) | 1.50*** | 1.11–2.03 |
| Solid fuel use for heating house | |||
| No (base) | 0.99 (13 818) | 1 | |
| Yes | 2.12 (1702) | 2.36*** | 1.60–3.48 |
| Smoking status | |||
| No (base) | 1.30 (5916) | 1 | |
| Past smoker | 4.10 (242) | 3.27*** | 1.67–6.40 |
| Current smoker | 3.66 (465) | 2.88*** | 1.68–4.91 |
| Cooking fuel | |||
| LPG (base) | 1.02 (14 254) | 1 | |
| Solid fuel | 2.06 (1018) | 2.32*** | 1.44–3.74 |
| Kerosene | 2.71 (221) | 2.83*** | 1.14–7.02 |
| Household expenditure per capita | |||
| <1000 (base) | 1.60 (4945) | 1 | |
| 1000–2000 | 1.09 (6025) | 0.61*** | 0.44–0.86 |
| 2000–5000 | 0.56 (4074) | 0.30*** | 0.18–0.48 |
| ≥5000 | 0.85 (472) | 0.43*** | 0.16–1.19 |
| Lung function (100 x (FEV1/FVC)) | |||
| 90 to 100 | 1.28 (4522) | 1 | |
| 80 to 90 | 1.90 (1313) | 1.40 | 0.86–2.27 |
| 70 to 80 | 1.91 (419) | 1.49 | 0.71–3.16 |
| <70 (or OLD) | 3.67 (300) | 2.86*** | 1.49–5.57 |
| Solid fuel exposure duration during life course (year) | |||
| No exposure (base) | 1.00 (3664) | 1 | |
| <20 | 2.00 (1506) | 2.05*** | 1.26–3.33 |
| 20–40 | 2.50 (1284) | 2.57*** | 1.59–4.17 |
| 40+ | 3.55 (169) | 3.71*** | 1.53–8.94 |
| Kerosene exposure during life course | |||
| No exposure (base) | 1.47 (6071) | 1 | |
| <20 | 2.76 (326) | 1.91* | 0.95–3.82 |
| 20–40 | 2.61 (115) | 1.80 | 0.56–5.78 |
| 40+ | 2.70 (111) | 1.86 | 0.58–5.99 |
Note: No of of years of exposure was computed based on data from lifetime cooking matrix of the individual module of the survey instrument (question i105 to i110).
P < .1.
P < .05.
P < .1.
FIGURE 3.
Probability of the history of active TB with respect to duration of exposure to solid fuel among men and women in Delhi, 2004–2009
3.4 |. Univariate analysis for association between IAP and OLD
OLD was found in 297 (4.5%) out of 6554 subjects who were tested with spirometer. There was a significant difference in the age of subjects with OLD in this cohort. 108 (8.9%) were 50 years or older (OR = 2.68, 95% CI: 2.09–3.42, P < .0001). OLD did not differ significantly by gender. 135 (4.7%) and 164 (4.4%) subjects were female in OLD and non-OLD groups, respectively (P = .49). There was no significant difference in the history of tobacco smoking between subjects with and without OLD (75, 4.9% vs. 222, 4.4%, P = .42). However, there was a significant association between IAP exposure and diagnosis of OLD. 191 (5.5%) of subjects with OLD were exposed to IAP as compared to 103 (3.5%) unexposed subjects (OR = 1.61, 95% CI: 1.26–2.05, P < .0001). Duration of solid exposure also showed a significant association active risk of TB. With every year, increase in solid fuel exposure was associated with 3% higher likelihood of a history of active TB (odds ratio: ~1.03, 95% CI: 1.02–1.04; P < .01).
3.5 |. Smoking and exposure interaction
Smoking and solid fuel exposure interaction was examined (see Table S2 and Figure S2 in SOM). Interaction of cooking fuel and smoking was insignificant, and interaction of solid fuel exposure and smoking was marginally significant (Figure S1B).
3.6 |. Multivariate analysis
Three models were run separately for each cooking and heating exposure (Table 2).
TABLE 2.
Odds ratio of TB with respect to the use of solid fuel in comparison with clean fuel (ie, liquid petroleum gas) for heating home and cooking in Delhi, 2004–2009
| Covariate | Solid fuel use for cooking |
Solid fuel use for heating |
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| ln (household expenditure per capita (Rs.)) | 0.73** (0.55–0.96) |
0.73** (0.56–0.97) |
0.65*** (0.49–0.85) |
0.72** (0.54–0.95) |
0.72** (0.54–0.95) |
0.68*** (0.51–0.91) |
| Age (y) | 1.02*** (1.00–1.03) |
1.02*** (1.00–1.03) |
1.02*** (1.01–1.03) |
1.01** (1.00–1.03) |
1.01** (1.00–1.03) |
1.01** (1.00–1.03) |
| Gender (0 = female, 1 = male) | 0.87 (0.56–1.36) |
0.88 (0.56–1.37) |
0.71* (0.48–1.06) |
0.9 (0.58–1.42) |
0.91 (0.58–1.43) |
0.82 (0.54–1.23) |
| Lung function (%) | 0.98** (0.97–1.00) |
0.98** (0.97–1.00) |
0.98** (0.97–1.00) |
0.98** (0.97–1.00) |
0.98** (0.97–1.00) |
0.98** (0.97–1.00) |
| Smoking (1 = Yes, 0 = No) | 1.80** (1.07–3.02) |
2.04** (1.18–3.52) |
1.90** (1.14–3.18) |
1.54 (0.85–2.79) |
||
| Exposure (1 = Yes, 0 = No) | 1.95** (1.06–3.61) |
2.46*** (1.26–4.80) |
1.88** (1.13–3.11) |
1.39 (0.71–2.72) |
||
| Smoking × Exposure | 0.47 (0.14–1.59) |
1.59 (0.55–4.60) |
2.36 (0.81–6.90) |
4.15*** (1.95–8.79) |
||
| Ambient PM2.5 exposure (μg/m3) | 0.99 (0.96–1.03) |
1 (0.96–1.03) |
0.99 (0.96–1.03) |
1 (0.96–1.03) |
||
| Constant | 0.4 (0.04–3.71) |
0.53 (0.02–12.04) |
1.49 (0.07–31.58) |
0.41 (0.04–4.39) |
0.61 (0.02–14.98) |
0.96 (0.04–21.68) |
| Observations | 5187 | 5187 | 5187 | 5166 | 5166 | 5166 |
P < .1 (95% confidence interval in parenthesis).
P < .05.
P < .01.
3.6.1 |. Cooking related solid fuel exposure
Results of Model 1 suggest that cooking solid fuel–exposed subjects and smokers were 95% and 80% more likely to have a history of active TB than unexposed subjects and non-smokers, respectively. When the interaction term (smoking × exposure) was introduced in Model 2, the odds values further increased for both smokers (odds ratio: 2.03; 95% CI: 1.05 to 3.07; P < .001) and solid fuel exposure (odds ratio: 2.46; 95% CI: 1.24 to 4.79; P < .001). However, their synergistic (or interactive) effect was insignificant as in Model 2. Household expenditure, age, and lung function showed significant associations with a history of active TB, but it did not vary significantly by gender. Household income is a protective factor, for example, a unit increase in the log (per capita household expenditure (Rs.)) was associated with 30% less likelihood of a history of active TB (odds ratio: 0.73; 95% CI: 0.55–0.96; P < .05). In Model 3, the long-term ambient PM2.5 exposure did not show a significant association with the history of active TB.
Solid fuel use for heating home was significantly associated with TB risk as well, for example, exposed subjects were 88% more likely to have a history of active TB than unexposed subjects (odds ratio: 1.88; 95% CI: 1.13 to 3.11; P < .05). Smokers had a 90% greater likelihood to have a history of active TB than non-smokers (odds ratio: 1.90; 95% CI: 1.1 to 3.2; P < .05). Introducing the interaction term in Model 2, all three terms (smoking, exposure, and smoking × exposure) were insignificant, suggesting autocollinearity among them canceling out each other’s influence (ρ ≥ 0.1; P ≤ .05). However, when “smoking × exposure” is introduced independently without their main effects, smokers and exposed subjects were 4.1 times more likely to have a history of active TB than non-smokers and unexposed subjects (odd ratio: 4.14; 95% CI: 1.96 to 8.71; P < .01). In Model 3, lung function, age, and household expenditure also showed a significant association with the history of active TB. But ambient PM2.5 and gender did not show associations with the history of active TB (Table 2).
3.7 |. Uncertainty analysis
While 79% of the TB cases were identified based on the culture of their sputum, 21% (36 cases) were not, which can be susceptible to case misclassification. We conducted 100 simulations in which 50% of these non-sputum culture verified cases were randomly misclassified, and analyses were conducted on these misclassified cases and compared to the observed TB cases (Table 3). Although the TB risk of solid fuel exposure was slightly lower in the misclassified data, solid fuel exposure through cooking, heating, and heating and cooking still had a significant association with the history of active TB. For example, in the analysis of solid fuel exposure through cooking, the risk of TB was 66% higher among exposed subjects in the simulated data as compare to 93% in the observed data set. A similar trend was observed for solid fuel exposure through heating (Table 3).
TABLE 3.
Odds ratio of observed and 100 simulations of randomly misclassified 50% (ie, 18 cases) of the non-sputum culture verified TB cases with respect to different solid fuel exposures and confounders
| Variables | Observed |
With 50% randomly cases misclassified |
||||
|---|---|---|---|---|---|---|
| Cooking | Heating | Cooking and heating | Cooking | Heating | Cooking and heating | |
| ln (household expenditure per capita (Rs.)) | 0.70 (0.516–0.957) | 0.68 (0.500–0.933 | 0.75 (0.544–1.021 | 0.65 (0.64–0.66) | 0.64 (0.63–0.64) | 0.69 (0.68–0.70) |
| Age (y) | 1.03 (1.016–1.038) | 1.03 (1.015–1.037 | 1.03 (1.016–1.038 | 1.03 (1.03–1.03) | 1.03 (1.03–1.03) | 1.03 (1.03–1.03) |
| Gender (0 = female, 1 = male) | 0.87 (0.572–1.328) | 0.90 (0.586–1.371 | 0.87 (0.571–1.327 | 0.90 (0.89–0.92) | 0.93 (0.92–0.94) | 0.90 (0.89–0.91) |
| Lung function (%) | 0.99 (0.970–1.003) | 0.99 (0.971–1.004 | 0.99 (0.970–1.003 | 0.99 (0.99–0.99) | 0.99 (0.99–0.99) | 0.99 (0.99–0.99) |
| Smoking (1 = Yes, 0 = No) | 1.70 (0.952–3.053) | 1.77 (0.986–3.182 | 1.66 (0.924–2.977 | 1.54 (1.50–1.59) | 1.60 (1.55–1.64) | 1.50 (1.46–1.54) |
| Exposure (1 = Yes, 0 = No) | 1.93 (1.065–3.480) | 1.75 (1.038–2.948 | 1.99 (1.229–3.219 | 1.66 (1.62–1.71) | 1.57 (1.54–1.61) | 1.78 (1.75–1.82) |
| Observations | 6544 | 6521 | 6544 | 6544 | 6521 | 6544 |
Note: Odds ratios and 95% confidence interval in parenthesis.
4 |. DISCUSSION
Our study shows that 1.12% of participants had a history of TB disease and a majority of them were sputum positive, and IAP exposure is an independent risk factor for TB. The World Health Organization (WHO) reports that almost half of the world’s population cook and heat their home with open fire and unsafe stoves using different types of solid fuel, including animal dung, crop residue, wood, and coal.51 According to WHO, IAP is associated with the risk of pneumonia, stroke, ischemic heart disease, chronic obstructive lung diseases, and lung cancer. However, IAP is not recognized as one of the risk factors of TB.51
A few studies evaluated the association between IAP and TB previously. Pokhrel et al conducted a hospital-based case-control study on 125 TB subjects compared with 250 matched controls in Nepal. They found that exposure to IAP was 3.4 times more common in TB cases than controls.52 Slama et al reviewed 994 articles on the topics of IAP and TB and conducted a meta-analysis of 6 papers in 2010. Of these six studies, one included only urban area and the rest included both urban and rural areas. Three out of 6 papers showed a significant association between IAP and TB, and the rest did not, concluding insufficient evidence of the association between IAP and TB, and recommended for studies with larger sample size.31 Of these six studies, one study included national data set on women from the demographic and health survey.13 Slama’s team conclusion was in the line with Hsien et al study that conducted another meta-analysis in 2007.41 Since 2010, a meta-analysis showed a positive association between IAP and TB, which presented a systematic review of 13 papers.40 In the pooled analysis of the selected 13 studies, this meta-analysis found 30% higher risk of TB for IAP-exposed subjects (OR 1.30 with CI, 1.04–1.62 and P value = .02). A recent case-control study from South Africa found that TB was more common in 30% of the homes which used solid fuel.32 However, a recent study, conducted in Nepal, shows a lower risk of active TB among households using solid fuel as compared to those using LPG.53 Given this study was conducted based on self-reported data, it is important to use direct measures of different air pollutants to conduct epidemiological studies of the health effects of IAP.33
Likewise, a review of literature suggests that there is inconsistency in the association between outdoor air pollution and TB. A literature search by key terms “tuberculosis” and “ambient air pollution” in PubMed yielded 41 articles. Of these 42, five article presented empirical data on the association between tuberculosis and outdoor air pollution (or proximity sources). Two of these five were conducted in the United States (North Carolina and Los Angeles)8,54 and the remaining three in Asia.11,55,56 A study in Seoul Metropolitan areas suggested that citywide PM10, O3, CO, and NO2 did not show any association with smear-positive TB cases, but inter-quartile range of SO2 showed a significant association with TB in only males11 but not in women. However, the study conducted in LA showed no association between proximity to pollution sources (freeways and major arterial roads), but outdoor residential PM2.5 exposure showed a significant association with smear-positive TB cases.54 Study in Beijing and Hong Kong showed associations between seasonal changes in PM2.5 smear-positive TB cases.56 The sample size of most of these studies was small, and most of these studies lacked direct measurements of air pollution. Moreover, outdoor air pollution represented a small fraction of the total air pollution exposure, given people spend 90% of their time indoors. Thus, the possibility of outdoor air pollution exposure misclassification cannot be discounted.
The current study reports a significant association between IAP and TB with some advantages as compared to previous studies. First, a large sample size with a face-to-face interview. Second, adjustment for a few confounding factors, including smoking tobacco, installation of an exhaust fan, and OLD data, that was directly collected during the interviews. Third, to define accurate history of TB and reduce the risk of recall bias, 79% of the TB cases were cross-validated using their response to questions on their sputum exam and history of hemoptysis.
Findings of this research have important policy implications. First, given that the frequency of TB in our study was 1.12% and considering the population of Delhi as 16.4 million, 183 188 people are likely to have/had active TB in the city, extrapolated an incidence rate to 1117 in 100 000 population. However, if the risk is adjusted to population in age-group 15 years or older, the prevalence of the history of active TB will be 1.48, which will translate to about 242 228 cases of history of active TB. This rate is even higher than the estimated TB cases in India in 1997. Dye and colleagues reported incidence and prevalence rates in India in 1997 as 187 and 505 in 100 000 population, respectively.57 This is higher than the TB rates of Delhi in 2016 (62 706 cases with an incidence rate of 348 in 100 000 population).58
Second, Delhi is the capital of India and many households continue to rely on solid fuel and kerosene for both cooking and heating.59 We observed a significant decrease in solid fuel use from 2004 to 2009 (34% exposed to IAP in 2004% vs. 23% in 2009). Given that IAP increases the risk for other diseases including cardiovascular diseases, the government should promote the use of cleaner fuels, which would help in reducing IAP-related diseases.60 Considering that many households are still below the poverty line61 and cannot afford cleaner fuel for cooking and heating an international collaboration is needed to provide subsidized or low-interest loans for purchasing and installing clean fuel technology. Initiation of an awareness campaign would be an important way to provide information on the risk of solid fuel exposure for different pulmonary and cardiac diseases, including TB. Improving harm reduction measurements and incorporating shear opening windows and doors, or a portable fan facing a window may decrease IAP. In new home designs, the provision of an appropriate exhaust fan in the kitchen and air circulation must be mandatory. Based on the current study findings, we suggest that healthcare professionals in settings similar to India should interrogate cooking- and ventilation-related history for all TB cases, and engage their patients in mitigating their exposure to emission from solid fuel burning. We also suggest that any patient with respiratory symptoms for more than 2–3 weeks and a history of IAP exposure must be screened for TB.
Finally, given the association between solid fuel exposure and TB, mitigation of solid fuel exposure should be considered in strategies aimed at eliminating TB by 2030, as articulated in the UN sustainable development goal.62
This study documents the epidemiological association of the risk of TB with long-term solid fuel exposure. However, the findings of the study should be interpreted in light of its limitations. First, due to lack of data availability, results were not adjusted for all comorbidities, including immunological disorders and diabetes, and other risk behaviors, such as alcohol consumption, which are shown to elevate the risk of TB.63–66 Second, given the nature of the survey data we relied on self-reported measures of TB. The accuracy of self-reported TB was studied before by Hessol and her colleagues.67 They found that self-reporting of TB disease had 100% accuracy among women with acquired immunodeficiency syndrome (AIDS). The future research is warranted to cross-validate survey-based assessments with the clinical data on TB to determine bias in the self-reported TB in the non-AIDS population. Third, we did not collect data on contact of our subjects with a known TB case and/or history of TB infection among other family members, which can increase the risk of contracting TB. Thus, the results were not adjusted for this risk factor. Fourth, we did not record reasons for not participating in the study. However, the response rate was 78%, which diminished the probability of non-response bias. In our simulation analysis, we show that even if 50% of the unverified cases are misclassified, solid fuel exposure still showed significant association with the history of active TB. Fifth, we did not measure post-bronchodilator (BD) FEV1. Therefore, we could not determine the proportion of chronic obstructive lung disease with fixed post-BD obstructions in our study population with OLD. Finally, this research measured exposure to IAP, not the levels of IAP exposure. We did not measure particulate matter size, their type, and concentrations that might be diverse among participants’ homes. Therefore, future research should be geared toward (short- and long-term) direct measurements of exposure with respect to different fuel usages, household characteristics, duration, and frequency of household activities that affect IAP. This can help develop precise TB burden associated with indoor exposure, and their associated sources, which can support evidence-based strategies to target the main sources of IAP. The same strategies should be incorporated in the United Nation (UN) and WHO to the reach goal of “End TB by 2030.”68
In this study, we concluded that IAP was associated with the risk of OLB and TB. Exposure to IAP should be routinely examined in patients with TB in LMIC countries. Clean stove projects69 that substitute clean with the unsafe fuels should be prompted in LMIC to mitigate the risk of TB.
Supplementary Material
Practical implications.
Indoor solid fuel exposure is a risk factor of TB.
Efforts must be made to mitigate solid fuel exposure by installing clean cook stove and exhaust fan.
Clinicians need to screen their patients for the history of solid fuel exposure.
Indoor air pollution must be incorporated in the strategies to end TB, such as the UN goal to End TB.
ACKNOWLEDGMENT
The authors thank Dr Golnaz Ebrahimi (University of Miami) for her technical assistance. This work in part was supported by the National Institute of Environmental Health Sciences (NIEHS: ES014004-01A2) and Population Studies and Training Center (seed grant).
Funding information
This study, in part, was supported by the Population Studies and Training Center, Brown University, and NIH (HD046571; ES014004).
Footnotes
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ina.12756.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTEREST
All authors declare no competing interests.
DATA AVAILABILITY STATEMENT
Due to IRB restriction, the original data cannot be shared with anyone. But the de-identified original electronic data may be made available to the editor of this journal upon a reasonable request.
REFERENCES
- 1.Sandhu GK. Tuberculosis: current situation, challenges and overview of its control programs in India. J Glob Infect Dis. 2011;3(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsh AE, Tsolaki AG, DeRiemer K, Feldman MW, Small PM. Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc Natl Acad Sci U S A. 2004;101(14):4871–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Global Tuberculosis Report 2019. WHO; 2019. https://www.who.int/tb/publications/factsheet_global.pdf?ua=1 [Google Scholar]
- 4.Pescarini JM, Rodrigues LC, Gomes MG, Waldman EA. Migration to middle-income countries and tuberculosis-global policies for global economies. Global Health. 2017;13(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhanaraj B, Papanna MK, Adinarayanan S, et al. Prevalence and risk factors for adult pulmonary tuberculosis in a metropolitan city of South India. PLoS One. 2015;10(4):e0124260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai JW, Ruan QL, Liu QH, Zhang WH. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect. 2016;5:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara G, Murray M, Winthrop K, et al. Risk factors associated with pulmonary tuberculosis: smoking, diabetes and anti-TNFalpha drugs. Curr Opin Pulm Med. 2012;18(3):233–240. [DOI] [PubMed] [Google Scholar]
- 8.Smith GS, Schoenbach VJ, Richardson DB, Gammon MD. Particulate air pollution and susceptibility to the development of pulmonary tuberculosis disease in North Carolina: an ecological study. Int J Environ Health Res. 2014;24(2):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snow SJ, De Vizcaya-Ruiz A, Osornio-Vargas A, et al. The effect of composition, size, and solubility on acute pulmonary injury in rats following exposure to Mexico city ambient particulate matter samples. J Toxicol Environ Health A. 2014;77(19):1164–1182. [DOI] [PubMed] [Google Scholar]
- 10.Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ Health Perspect. 2006;114(4):627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang SS, Kang S, Lee JY, et al. Impact of outdoor air pollution on the incidence of tuberculosis in the Seoul metropolitan area, South Korea. Korean J Intern Med. 2014;29(2):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J. Is ambient air pollution another risk factor of tuberculosis? Korean J Intern Med. 2014;29(2):170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra VK, Retherford RD, Smith KR. Cooking with biomass fuels increases the risk of tuberculosis. Natl Fam Health Surv Bull. 1999;13:1–4. [PubMed] [Google Scholar]
- 14.Simkovich SM, Goodman D, Roa C, et al. The health and social implications of household air pollution and respiratory diseases. NPJ Prim Care Respir Med. 2019;29(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghio AJ. Particle exposures and infections. Infection. 2014;42(3):459–467. [DOI] [PubMed] [Google Scholar]
- 16.Mirsadraee M, Saffari A, Sarafraz Yazdi M, Meshkat M. Frequency of tuberculosis in anthracosis of the lung: a systematic review. Arch Iran Med. 2013;16(11):661–664. [PubMed] [Google Scholar]
- 17.Ghanei M, Aslani J, Peyman M, Asl MA, Pirnazar O. Bronchial anthracosis: a potent clue for diagnosis of pulmonary tuberculosis. Oman Med J. 2011;26(1):19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samet JM, Marbury MC, Spengler JD. Health effects and sources of indoor air pollution. Part II. Am Rev Respir Dis. 1988;137(1):221–242. [DOI] [PubMed] [Google Scholar]
- 19.Bell ML, Davis DL, Fletcher T. A retrospective assessment of mortality from the London smog episode of 1952: the role of influenza and pollution. Environ Health Perspect. 2004;112(1):6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Ambient air pollution: A global assessment of exposure and burden of disease. World Health Organization; 2016. http://www.who.int/iris/bitstream/10665/250141/1/9789241511353-eng.pdf?ua=1 [Google Scholar]
- 21.Abtahi M, Koolivand A, Dobaradaran S, et al. National and sub-national age-sex specific and cause-specific mortality and disability-adjusted life years (DALYs) attributable to household air pollution from solid cookfuel use (HAP) in Iran, 1990–2013. Environ Res. 2017;156:87–96. [DOI] [PubMed] [Google Scholar]
- 22.Assad NA, Kapoor V, Sood A. Biomass smoke exposure and chronic lung disease. Curr Opin Pulm Med. 2016;22(2):150–157. [DOI] [PubMed] [Google Scholar]
- 23.Fatmi Z, Coggon D. Coronary heart disease and household air pollution from use of solid fuel: a systematic review. Br Med Bull. 2016;118(1):91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naz S, Page A, Agho KE. Household Air Pollution and Under-Five Mortality in Bangladesh (2004–2011). Int J Environ Res Public Health. 2015;12(10):12847–12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naz S, Page A, Agho KE. Household air pollution and under-five mortality in India (1992–2006). Environ Health. 2016;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naz S, Page A, Agho KE. Household air pollution from use of cooking fuel and under-five mortality: The role of breastfeeding status and kitchen location in Pakistan. PLoS One. 2017;12(3):e0173256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naz S, Page A, Agho KE. Attributable risk and potential impact of interventions to reduce household air pollution associated with under-five mortality in South Asia. Glob Health Res Policy. 2018;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neogi SB, Pandey S, Sharma J, et al. Association between household air pollution sand neonatal mortality: an analysis of Annual Health Survey results, India. WHO South East Asia J Public Health. 2015;4(1):30–37. [DOI] [PubMed] [Google Scholar]
- 29.Rylance J, Fullerton DG, Semple S, Ayres JG. The global burden of air pollution on mortality: the need to include exposure to household biomass fuel-derived particulates. Environ Health Perspect. 2010;118(10):424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55(6):518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slama K, Chiang CY, Hinderaker SG, Bruce N, Vedal S, Enarson DA. Indoor solid fuel combustion and tuberculosis: is there an association? Int J Tuberc Lung Dis. 2010;14(1):6–14. [PubMed] [Google Scholar]
- 32.Elf JL, Eke O, Rakgokong M, et al. Indoor air pollution from secondhand tobacco smoke, solid fuels, and kerosene in homes with active tuberculosis disease in South Africa. BMC Res Notes. 2017;10(1):591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elf JL, Kinikar A, Khadse S, et al. The association of household fine particulate matter and kerosene with tuberculosis in women and children in Pune, India. Occup Environ Med. 2019;76(1):40–47. [DOI] [PubMed] [Google Scholar]
- 34.Desai MA, Mehta S, Smith KR. Indoor smoke from solid fuels: Assessing the environmental burden of disease at national and local levels. Geneva: World Health Organization; 2004. [Google Scholar]
- 35.Census of India. Provisional Population Totals Paper 1 : 2011. Office of the Registrar General and Census Commissioner, Government of India; 2011. https://censusindia.gov.in/ [Google Scholar]
- 36.Shandilya KK, Khare M, Gupta AB. Suspended particulate matter distribution in rural-industrial Satna and in urban-industrial South Delhi. Environ Monit Assess. 2007;128(1–3):431–445. [DOI] [PubMed] [Google Scholar]
- 37.WHO. Air quality guidelines. World Health Organization; 2005. [Google Scholar]
- 38.Jafta N, Jeena PM, Barregard L, Naidoo RN. Childhood tuberculosis and exposure to indoor air pollution: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19(5):596–602. [DOI] [PubMed] [Google Scholar]
- 39.Lin HH, Suk CW, Lo HL, Huang RY, Enarson DA, Chiang CY. Indoor air pollution from solid fuel and tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2014;18(5):613–621. [DOI] [PubMed] [Google Scholar]
- 40.Sumpter C, Chandramohan D. Systematic review and meta-analysis of the associations between indoor air pollution and tuberculosis. Trop Med Int Health. 2013;18(1):101–108. [DOI] [PubMed] [Google Scholar]
- 41.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Medicine. 2007;4(1):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Culver BH, Graham BL, Coates AL, et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med. 2017;196(11):1463–1472. [DOI] [PubMed] [Google Scholar]
- 43.Kumar N. Spatial sampling design for a demographic and health survey. Popul Res Policy Rev. 2007;26(5–6):581–599. [Google Scholar]
- 44.Sinha J, Kumar N. Mortality and Air Pollution Effects of Air Quality Interventions in Delhi and Beijing. Front Environ Sci. 2019;7:1–10. [Google Scholar]
- 45.Kumar N, Chu A, Foster A. An empirical relationship between PM2.5 and aerosol optical depth in Delhi Metropolitan. Atmos Environ. 2007;41(21):4492–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar N, Chu AD, Foster AD, Peters T, Willis R. Satellite remote sensing for developing time and space resolved estimates of ambient particulate in Cleveland, OH. Aerosol Sci Technol. 2011;45(9):1090–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutierrez MP, Zuidema P, Mirsaeidi M, Campos M, Kumar N. Association between African dust transport and acute exacerbations of COPD in Miami. J Clin Med. 2020;9(8):2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.NASA. MODIS Atmosphere. NASA; 2020. https://atmosphere-imager.gsfc.nasa.gov/. Accessed April 24, 2013. [Google Scholar]
- 49.Kumar N, Liang D, Comellas A, Chu AD, Abrams T. Satellite-based PM concentrations and their application to COPD in Cleveland, OH. J Expo Sci Environ Epidemiol. 2013;23(6):637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.STATA/MP 14.2. Data Analysis and Statistical Software [computer program]. College Station, TX: Stata Corp LP; 2017. [Google Scholar]
- 51.WHO. Indoor Air Pollution: National Burden of Disease Estimates. World Health Organization; 2014. https://www.who.int/airpollution/publications/indoor_air_national_burden_estimate_revised.pdf?ua=1. Accessed October 10, 2014. [Google Scholar]
- 52.Pokhrel AK, Bates MN, Verma SC, Joshi HS, Sreeramareddy CT, Smith KR. Tuberculosis and indoor biomass and kerosene use in Nepal: a case-control study. Environ Health Perspect. 2010;118(4):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bates MN, Pope K, Sijali TR, et al. Household fuel use and pulmonary tuberculosis in western Nepal: A case-control study. Environ Res. 2019;168:193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn M, Kim H, Sung H, Lee Y, Choi H, Chung H. Association of social deprivation and outdoor air pollution with pulmonary tuberculosis in spatiotemporal analysis. Int J Environ Health Res. 2019;29(6):657–667. [DOI] [PubMed] [Google Scholar]
- 55.Chen KY, Chuang KJ, Liu HC, et al. Particulate matter is associated with sputum culture conversion in patients with culture-positive tuberculosis. Ther Clin Risk Manag. 2016;12:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You S, Tong YW, Neoh KG, Dai Y, Wang CH. On the association between outdoor PM2.5 concentration and the seasonality of tuberculosis for Beijing and Hong Kong. Environ Pollut. 2016;218:1170–1179. [DOI] [PubMed] [Google Scholar]
- 57.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 1999;282(7):677–686. [DOI] [PubMed] [Google Scholar]
- 58.Government of India. TB India 2017 - Revised National Tuberculosis Control Programme: Annual Report. Directorate General of Health Services, Ministry of Health and Family Welfare; 2017. https://www.tbfacts.org/wp-content/uploads/2017/12/TB-India-2017.pdf [Google Scholar]
- 59.Rizwan S, Nongkynrih B, Gupta SK. Air pollution in Delhi: its magnitude and effects on health. Indian J Community Med. 2013;38(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sood A. Indoor fuel exposure and the lung in both developing and developed countries: an update. Clin Chest Med. 2012;33(4):649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sood N, Bendavid E, Mukherji A, Wagner Z, Nagpal S, Mullen P. Government health insurance for people below poverty line in India: quasi-experimental evaluation of insurance and health outcomes. BMJ. 2014;349:g5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.UNESC. Report of the Inter-Agency and Expert Group on Sustainable Development Goal Indicators. United Nations Economic and Social Council; 2016. http://undocs.org/E/CN.3/2016/2/Rev.1. Accessed 2017. [Google Scholar]
- 63.Steinbrook R. Tuberculosis and HIV in India. N Engl J Med. 2007;356(12):1198–1199. [DOI] [PubMed] [Google Scholar]
- 64.Sester M, van Leth F, Bruchfeld J, et al. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med. 2014;190(10):1168–1176. [DOI] [PubMed] [Google Scholar]
- 65.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9(12):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hessol NA, Schwarcz S, Ameli N, Oliver G, Greenblatt RM. Accuracy of self-reports of acquired immunodeficiency syndrome and acquired immunodeficiency syndrome-related conditions in women. Am J Epidemiol. 2001;153(11):1128–1133. [DOI] [PubMed] [Google Scholar]
- 68.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6(4):299–314. [DOI] [PubMed] [Google Scholar]
- 69.Simkovich SM, Williams KN, Pollard S, et al. A systematic review to evaluate the association between clean cooking technologies and time use in low- and middle-income countries. Int J Environ Res Public Health. 2019;16(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to IRB restriction, the original data cannot be shared with anyone. But the de-identified original electronic data may be made available to the editor of this journal upon a reasonable request.