Abstract
PURPOSE:
To examine associations between indoor airborne microbial concentration and dry eye (DE) measures.
DESIGN:
Prospective, observational, cross-sectional study.
METHODS:
A total of 157 individuals with normal external ocular anatomy were recruited from the Miami Veterans Affairs eye clinic. Subjects underwent a clinical evaluation that included assessment of DE symptoms and signs. Indoor air was sampled using bioaerosol impactors with nutrient and soy media, and samples were incubated for 48 hours at 37 C with 5% CO2. Number of microbial colonies (CFU) was recorded. Outcome measures were DE symptoms and signs.
RESULTS:
A total of 157 unique subjects participated in home and clinical visits and of these, 93 completed a 6-month follow-up of home and clinical visits. Older homes were found to have higher CFU compared to newer homes. A 1% increase in humidity was associated with a 3% increase in nutrient CFU (95% confidence interval [CI] = 0.01 to 0.04; P < .001). Instrumented CFU significantly associated with 2 DE measures: corneal epithelial disruption and lower eyelid meibomian gland (MG) dropout, adjusted for age and sex (odds ratio [OR] = 28.07, 95% CI = 1.8, 443.8, P < .05; OR = 39.6, CI = 1.8, 875.2, P < .05 for soy, respectively). After adjusting for other confounders, CFU and age remained significantly associated with MG dropout. Other DE measures did not significantly associate with CFU.
CONCLUSIONS:
Individuals with higher CFU counts in the home had more severe MG dropout, after adjusting for age and other confounders. This finding suggests that home CFU exposure may impact MG dropout, one of the DE measures, and may be a target for intervention.
Dry eye (DE) is one of the most frequently encountered ocular disorders in the United States, with a 6.8% prevalence of diagnosed DE among adults and a 25% frequency of symptom report by patients visiting an eye clinic.1 The complexity of the disorder is highlighted by the most updated definition: “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”2 DE can be initiated by intrinsic and/or extrinsic factors including older age, immunologic disorders, and environmental exposures. With respect to the latter, research suggests that exposure to particulate matter,3–5 ozone (O3),6 and humidity5,6 plays a role in the development and persistence of DE. While exposure to some bioaerosols, such as outdoor pollens,7 has been associated with related disorders like allergic conjunctivitis, exposure to indoor bioaerosols has not been fully evaluated as a risk factor in DE symptoms and signs.8
Bioaerosols are ubiquitous, airborne particles (0.001–100 μm in diameter) of biological origin such as endotoxins, glucans, mycotoxins, allergens, bacteria, and fungal and mold spores.9 Indoor and outdoor concentrations of bioaerosols vary by environmental conditions, seasonality, and human and animal activity. Generally, bioaerosol concentrations in indoor environments are lower than in outdoor environments, although the two are interrelated.10 There are currently no indoor, outdoor, or occupational bioaerosol concentration standards in the United States, likely owing to the lack of methodological and quantification standards. While it is known that some exposure to bioaerosols can be beneficial,11 their associations with the adverse health effects have been widely reported, including allergies, infections, and cancer. Of these, bioaerosol-related respiratory symptoms and lung function impairment are the most widely studied. For example, exposure to endotoxins has been linked to airway inflammation and decrease in lung function.12 Moreover, a dose-response relationship has been reported with exposure to beta-glucan and the development and persistence of asthma in children.13
Many factors can affect the composition and counts of bioaerosols, including environmental conditions, proximity to machinery, living in dense urban areas, and household characteristics. Major household sources of bioaerosols include domestic animals, plants, bedding or soft furniture, and poorly maintained air-conditioning systems.14 Outdoor sources of bioaerosols include soil, plants, and animals and, therefore, proximity to agricultural sites.15 Industrial activities such as waste sorting,16 agriculture,17 and composting18 are known occupational sources of bacteria and have been negatively linked with respiratory and ophthalmic health. For example, in a 5-year longitudinal study, 218 compost workers from 41 facilities were compared to 66 office workers in Germany. Compost workers experienced a higher risk of chronic bronchitis (relative risk 1.41; 95% confidence interval [95% CI] 1.28–1.55) compared to office workers. Over the 5-year observation period, compost workers also showed a decrease in forced vital capacity (−5.6%, P < .05), while the control group demonstrated a slight, yet insignificant decrease in forced vital capacity.19 Occupational exposure to cultivable microorganisms was reported for 6 of the industrial sites. However, because exposure was not reported at the control site, nor do industry standards exist, these data lack comparison.
While research linking bioaerosol exposure to DE is limited, indirect evidence suggests an association. Studies demonstrate that living in or commuting through metropolitan areas, which generally have higher concentrations of bioaerosols owing to human traffic and animal density,20,21 negatively impacts symptoms and signs of DE.22 In a case-control study in Delhi, India, DE measures were compared across 2 groups: healthy volunteers who commuted through the heavily polluted city (n = 250) and healthy volunteers who did not commute (n = 250).22 Commuters were found to have higher levels of eye symptoms compared to noncommuters, including redness (42% vs 20%) and irritation (50% vs 26%) (P < .05 for both comparisons). DE signs were also different, with lower Schirmer values (13 ± 7 mm vs 16 ± 6 mm, P < .001) and faster tear break-up time (TBUT) (13 ± 6 seconds vs 19 ± 6 seconds, P < .001) in commuters vs noncommuters. However, the clinical significance of the latter differences is unclear, as the mean values are within the normal range.22 One limitation of the study is that bioaerosols were not directly measured. Furthermore, other factors, such as inorganic air particulates, are also found in high concentrations in polluted cities and thus associations to any one exposure is can confound the effects of bioaerosols. In fact, exposure to ozone (O3) and particulate matter less than 2.5 μm in diameter (PM2.5) has been found to correlate both with bioaerosol concentration23,24 and DE.25 A study of 23,922 outpatients seen in ophthalmic clinics across 32 cities in China reported higher frequency of DE (self-reported) in cities with “extreme” levels (>75th quantile) of O3 (odds ratio [OR] = 3.97; 95% CI 3.67, 4.29) and PM2.5 (OR 2.01; 95% CI 1.79, 2.26), controlling for relative humidity, air pressure, and air temperature.25 However, outcome data were limited by a self-diagnosis of DE and outdoor environmental exposures were collected by extraction from 1 monitoring station in each city, which did not capture individual-level indoor exposure. None of the prior studies examined individual level bioaerosol exposure indoors, where the average person spends 90% of time.26 This paper examines associations between household indoor airborne microbial concentration and DE measures, considering other potential patient-related and environmental confounders.
METHODS
STUDY POPULATION:
Patients were recruited from the Miami Veterans Affairs (VA) eye clinic between October 2017 and October 2019. Upon enrollment, written informed consent was obtained. Subjects who completed participation in the study received $100 compensation for time and travel. The study was approved by the Miami VA Institutional Review Board and the University of Miami (IRB approval #3011.05, and CR00012905, respectively). The study was conducted in accordance to the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act.
Subjects were excluded from the study if they presented with conditions or factors that could confound DE, including contact lens wear, cataract surgery (within last 6 months), use of ocular medications (except for artificial tears), an active external ocular process, or a history of refractive, glaucoma, or retinal surgery. Exclusion criteria also include prior diagnosis of human immunodeficiency virus, sarcoidosis, Sjögren syndrome, and graft-vs-host disease or a collagen vascular disease. Eligibility criteria based on home characteristics were living on or below the third floor of a residential building (including single-story and multi-story homes), having a central air conditioning system in home, and having a permanent residence with no plans to move in the next 6 months. All eligible patients were offered participation regardless of DE symptoms, signs, or past diagnosis.
QUESTIONNAIRES:
Patients were administered questionnaires to collect demographic and health data such as age, sex, race, ethnicity, medication information, nutritional supplements, medical history, and pain. Patients also completed a series of validated questionnaires characterizing DE symptoms, including the Dry Eye Questionnaire 5 (DEQ5)27 and Ocular Surface Disease Index.28
OCULAR SURFACE EVALUATION:
Each patient underwent a clinical assessment at the Miami VA Eye Center to examine overall ocular surface health. A series of examinations were administered by a designated staff member in the same room for each subject. The average temperature and humidity in the room was 20.3 C to 21.1 C and 50%, respectively. All tests were performed in the following order: (1) tear osmolarity (TearLAB Osmolarity System; TearLAB, San Diego, California, USA) (once in each eye); (2) inflammation via InflammaDry (measure of matrix metallopeptidase 9 [MMP-9] (Quidel, San Diego, California, USA) graded as absent or present (as an added [unvalidated] level of interpretation, we qualitatively graded the intensity of the pink stripe on a scale of 0–3 [none, faint, pink, fuchsia]); (3) tear evaporation via TBUT (5 μL fluorescein instilled in the superior conjunctivae, seconds measured by a stopwatch until the first black spot appeared in the tear film, 3 measurements taken with 5-second blink interval between measurements and averaged); (4) fluorescein corneal staining to assess corneal epithelial cell disruption (CED) (National Eye Institute scale, 5 areas of cornea assessed; score 0–3 in each, total 15); (5) Schirmer score with anesthesia measured as mm of wetting at 5 minutes; (6) eyelid parameters including eyelid vascularity (0 = none, 1 = mild, 2 = moderate, 3 = severe), lower eyelid meibomian gland (MG) dropout graded via the Meiboscale, 0–4, and meibum quality (0 = clear liquid, 1 = white liquid, 2 = granular, 3 = toothpaste, 4 = no visible meibum extracted) expressed from the lower meibomian glands.29 This protocol was developed to balance a comprehensive assessment of ocular surface status with patient comfort. Schirmer test was repeated a second time during the home visit following the same procedures. In the analysis, data from the eye with the more abnormal value (higher value for corneal staining, lower value for TBUT and Schirmer) were used. All procedures are detailed in the Supplemental Material (available at AJO.com), which includes the description of each procedure, the instrument used, and data/measurement scale used for each test.
ENVIRONMENTAL MEASURES COLLECTION AND PROCESSING:
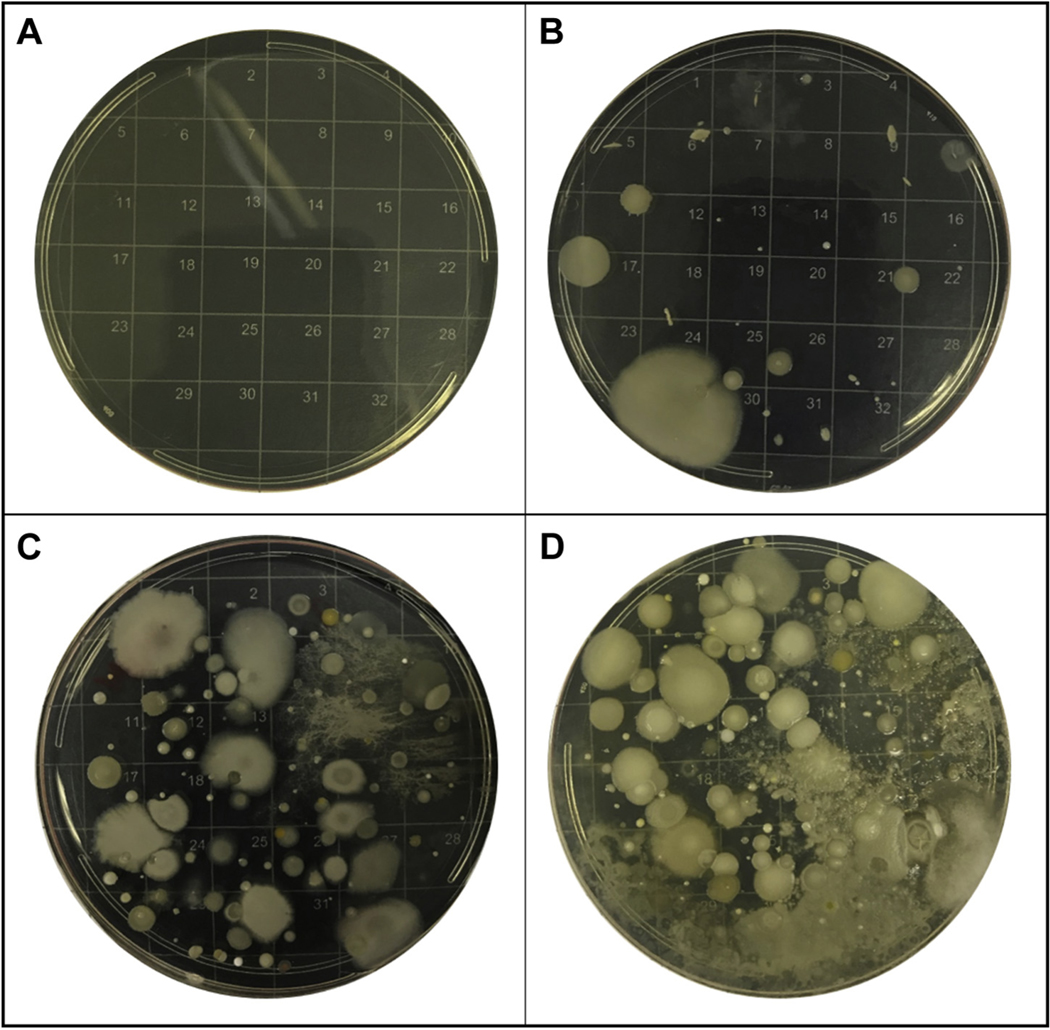
Within 7 days of the clinic visit, indoor environmental monitoring was conducted by the trained research personnel (see Supplemental Material for details). Air was sampled at a flow rate of 28.5 L/min, according to standard methods, using single-stage Biostage samplers with 400 0.25-mm holes (Standard Biostage single-stage cascade impactor #225–9611) and 90 mm agar plates. Two different samples were collected for 45 minutes each, using agar plates with (1) nutrient media (bovine serum used for bacteria) and (2) soy media (trypticase soy agar used for fungi and mold). Impactor was positioned in the living room of the home, within 4 feet of the home’s air conditioning (AC) closet or 8 feet of an AC vent while the AC or AC fan was running for the representative air inside the home. Following collection, samples were incubated for 48 hours at 37 C with 5% CO2. Petri dishes were removed from the incubator and microbial colonies were counted by a trained team member following a standard operating procedure our team has developed in hypertext preprocessor (PHP) programming langugage. Each dish was placed on a 32-box grid (Figure 1). Random numbers were generated by our online data capturing system using the rand function in PHP between 1 and 32 (Supplemental Figure S1, available at AJO.com). Numbers of colonies in all randomly selected boxes were manually counted. These values were summed and multiplied by 4 to calculate the total number of microbial communities, or colony-forming units (CFU). These data and images of each dish were uploaded online in our secure database. Images and their counts were then verified by another staff member. Results were recorded in CFU (nutrient CFU for CFU on nutrient media, and soy CFU for CFU on the soy media from here to after).
FIGURE 1.
Example blank, nutrient, and soy agar petri dishes with low and high concentration of microbial communities. A. Field blank nutrient agar petri dish. B. Nutrient agar petri dish after 48 hours of incubation with moderate concentration. C. Soy agar petri dish with very high concentration. D. Nutrient agar petri dish with medium-high concentration.
Data on indoor temperature (degrees Celsius), relative humidity (%), total suspended particles count (TSP), and particulate matter <2.5 μm diameter (PM2.5) and <10 μm in diameter (PM10) were collected using MetOne Aerocet 531, a handheld particle counter (Met One Instruments Inc., Grants Pass, Oregon, USA). Aerocet was deployed for 1.5 hours total, capturing both the mass and count of particles.30 Temperature and relative humidity were also recorded by PRECISE, a portable hand-held instrument developed by our team, and a hand-held monitor (CVS Health Humidity Monitor, Item # 521064). Data on home characteristics, such as floor type, year built, number and type of indoor plants, and number and type of pets were also collected during this visit.
STATISTICAL ANALYSIS:
Descriptive analysis was first conducted to describe patient demographics, medication use, comorbidities, and DE and environmental measures. Certain environmental measures including number of microbial communities (soy and nutrient), PM2.5, and PM10 were log-transformed to adjust for left skew. Plates with CFU >350 were considered outliers and excluded from analysis (n = 2). Missing values for indoor temperature and indoor relative humidity (collected via Aerocet) were replaced with measures taken by other devices: PRECISE or hand-held monitor. We used standards from the US Centers of Disease Control31 as reference to categorize elevated relative humidity and temperature. We next examined which home factors impacted the number of microbial communities (eg, year built, humidity, temperature, pets, and floor type).
Our main analysis focused on associations between indoor microbial communities and DE symptoms and signs using multivariate linear and logistic regressions (depending on the data scale of each variable). Outcome variables found to be significant in correlation analyses were selected for the final analyses. Outcome variables were categorized as 0 or 1, based on clinical cutoffs, with 1 being present and 0 being absent. CED was categorized as present if score ≥ 2 and MG dropout was categorized as present if score ≥ 1. Covariates were selected a priori, based on previous research.32 Model 1 included covariates age and sex while model 2 additionally adjusted for TSP at loge scale, smoke exposure (0 = no, 1 = yes), allergy status (0 = none, 1 = yes), fish oil supplement, pets and plants in home, and visit number (visit 1 or visit 2). Next, we evaluated associations between CFU and measures of DE severity using ordinal logistic regression analyses (with outcome variables treated as ordinal scales). All analyses were conducted in STATA IC 16.1 (StataCorp, College Station, Texas, USA).
RESULTS
STUDY POPULATION AND ENVIRONMENTAL VARIABLES:
A total of 157 unique subjects participated in clinical and home monitoring visits and of these, 93 patients completed 6-month follow-up visits. Most subjects were male (86.6%) with a mean age of 59.9 years (standard deviation [SD] = 11.4 years) (Table 1). Approximately 53.5% were white and most reported being either current or past smokers (31.2% and 55.5%, respectively). Common comorbidities included history of depression (66%), osteoarthritis (55%), and hypertension (56%). DE symptoms in the population were in the moderate range27 with a mean DEQ5 score of 10.8 ± 5.3 (Table 1).
TABLE 1.
Demographic and Clinical Characteristics, Unique number of subjects = 157
| Variable | N | % |
|---|---|---|
|
| ||
| Unique subjects | 157 | 100 |
| Subjects with 2 clinic examinations and home visits | 93 | 59.2 |
| Demographics (unique subjects at the time of first visit) | ||
| Mean age (years) ± SD | 59.9 ± 11.4 | |
| Sex, male | 136 | 86.6 |
| Race, white | 84 | 53.5 |
| Ethnicity, Hispanic | 50 | 31.8 |
| Smoking | ||
| Past | 87 | 55.4 |
| Current | 49 | 31.2 |
| Comorbidities (at the time each clinical visit) | ||
| Depression | 175 | 66.0 |
| Osteoarthritis | 144 | 54.6 |
| Hypercholesteremia | 120 | 45.5 |
| Hypertension | 148 | 55.9 |
| Diabetes mellitus | 86 | 33.2 |
| Sleep apnea | 91 | 34.6 |
| Post-traumatic stress disorder | 50 | 21.9 |
| Hepatitis C | 39 | 14.7 |
| Traumatic brain injury | 4 | 1.5 |
| Medications (at the time each clinical visit) | ||
| Analgesics | 154 | 58.1 |
| Antianxiety | 131 | 49.4 |
| Antidepressant | 133 | 50.2 |
| Cholesterol-lowering agent | 122 | 46.0 |
| Nonsteroidal anti-inflammatory agent | 47 | 17.7 |
| Dry eye symptoms at the time of each clinical visit | ||
| DEQ5 (0–22, <6 normal) | 10.8 ± 5.3 | |
| OSDI (0–100, <12 normal) | 33.5 ± 24.6 | |
| Intensity of ocular pain, average over 1 week (0–10) | 3.0 ± 2.6 | |
| Total NSPI (0–100) | 26.0 ± 20.5 | |
| Dry eye signs (at the time each clinical visit) | ||
| Osmolarity (mOsm/L) | 313.1 ± 18.7 | |
| Inflammation+, n (%) | 66 (26.4) | |
| Tear break-up time (seconds) | 8.1 ± 5.0 | |
| Corneal epithelial disruption (0–15) | 1.8 ± 2.8 | |
| Schirmer score (mm wetting at 5 minutes) | 16.5 ± 9.3 | |
| Eyelid vascularity (0–3) | 0.7 ± 1.0 | |
| Meibomian gland dropout (0–4) | 1.7 ± 1.2 | |
| Meibum quality (0–4) | 1.5 ± 1.2 | |
DEQ5 = Dry Eye Questionnaire 5; NSPI = Neuropathic Pain Symptom Inventory; OSDI = Ocular Surface Disease Index.
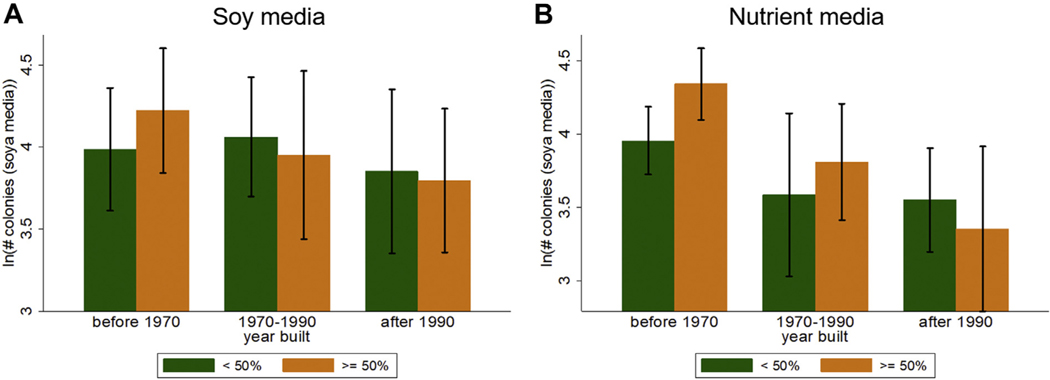
The average temperature inside the home was 23.9 C (SD = 2.2 C) and the average relative humidity was 51.3% (SD = 5.5%) (Table 2). We observed a significant difference in the mean number of microbial communities on plates with soy and nutrient media (85.9 on soy vs 67.3 on nutrient, difference ~16.3, P < .01) (Table 2). Home age and humidity were associated with the CFU, especially on the nutrient media (Figure 2). Homes built before 1970 also showed significantly elevated concentration of microbial communities (nutrient CFU: mean = 75.8, SD = 5.5; soy CFU: mean = 87.4, SD = 9.0) compared to newer homes (nutrient CFU: mean = 52.3, SD = 4.9; P < .01; soy CFU: mean = 64.7, SD = 5.8; P < .001). With a year increase in home/building construction year was associated with a 1% decrease in nutrient CFU (coefficient ~−0.01; 95 % CI = −0.01 to 0.00; P < .001), meaning newer homes have low concentrations of CFU. A 1% increase in relative humidity was associated with 3% increase in CFU (coefficient ~0.03; 95% CI = 0.01 to 0.04; P < .01) (model 3 in Table 3). Home construction year, humidity, and temperature explained 15% of the total variance in nutrient CFU.
TABLE 2.
Indoor Environment Conditions and Home Characteristics
| Variable | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|
|
| |||||
| CFU (soy) | 200 | 85.9 | 90.2 | 0 | 932 |
| CFU (nut) | 202 | 67.3 | 51.7 | 0 | 288 |
| Temperature (F) | 240 | 23.9 | 2.2 | 18.4 | 29.7 |
| Relative humidity (%) | 215 | 51.3 | 8.5 | 34.0 | 77.8 |
| PM2.5 | 239 | 4.0 | 9.2 | 0 | 63.7 |
| PM10 | 239 | 11.4 | 14.4 | 0 | 84.3 |
| Total suspended particles | 239 | 18.1 | 19.4 | 0 | 98.7 |
| Year built | 172 | 1975.8 | 22.9 | 1920 | 2018 |
| Square footage | 162 | 1660.6 | 1105.3 | 150 | 7500 |
CFU = colony-forming units, PM2.5 = particulate matter ≤2.5 μm, PM10 = particulate matter ≤10 μm.
FIGURE 2.
Number of colonies (log) by year built and % humidity: A. Concentration on soy media. B. Concentration on nutrient media.
TABLE 3.
Regression of Microbial Communities (Nutrient Media and Selected Home Environmental Conditions)
| Covariates | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
|
| ||||
| Year home was built (YYYY) | −0.01*** (−0.01 to −0.00) | −0.01*** (−0.02 to −0.00) | −0.01*** (−0.02 to −0.00) | −0.01*** (−0.02 to −0.01) |
| Indoor humidity (%) | 0.02*** (0.01 to 0.04) | 0.03*** (0.01 to 0.04) | 0.03*** (0.01 to 0.04) | |
| Indoor temperature (C) | 0.04 (−0.03 to 0.11) | 0.04 (−0.04 to 0.12) | ||
| Pet(s) in home (0 = no, 1 = yes) | 0.11 (−0.24 to 0.46) | |||
| Plant(s) in home (0 = no, 1 = yes) | −0.04 (−0.40 to 0.32) | |||
| Floor type (0 = tile/concrete, 1 = other) | 0.14 (−0.22 to 0.50) | |||
| Constant | 19.89*** (7.83–31.94) | 23.72*** (11.92–35.51) | 22.07*** (10.29–33.85) | 24.46*** (11.97 to 36.95) |
| Observations | 135 | 113 | 112 | 109 |
| R-squared | 0.05 | 0.15 | 0.15 | 0.17 |
Robust confidence interval in parentheses;
P < .01
P < .05
P < .1.
Numbers of microbial communities on soy and nutrient agar were significantly correlated (r = 0.48, P < .001) and thus analyzed independently in regression analyses. TSP was found to be highly correlated with PM2.5 (0.69, P < .001) and PM10 (0.83, P < .001). Therefore, TSP served as a proxy for PM. We found that indoor relative humidity was inversely correlated with DE symptoms (DEQ5) and signs (tear osmolarity, meibum quality) and positively correlated with ocular surface inflammation. Indoor temperature was positively correlated only with osmolarity. Since humidity and temperature were correlated with microbial concentration (although not significant), CFU was instrumented on the interaction of indoor humidity and temperature (for both soy and nutrient) in the final analyses.
REGRESSION ANALYSIS:
Multivariate regressions showed significant associations between indoor nutrient and soy CFU (instrumented on temperature and humidity) with 2 DE measures, namely CED and MG dropout. In model 1, nutrient CFU (log-transformed) was a significant predictor of CED (OR: 17.3, 95% CI = 1.63, 183.0) and MG dropout (OR = 23.1, 95% CI = 1.6, 327.0) adjusting for sex and age (Table 4). Age also showed a significant association with both CED and MG dropout.
TABLE 4.
Multivariate Linear Regression of Indoor Microbial Communities (Nutrient Media) and Selected Dry Eye Signs, Odds Ratio (95% Confidence Interval)
| Corneal Epithelial Disruption | Meibomian Gland Dropout | |||
|---|---|---|---|---|
|
|
|
|||
| Variables | Model 1 | Model 2 | Model 1 | Model 2 |
|
| ||||
| loge(# colonies) | 17.29** (1.63–183.02) | 9.61 (0.49–189.88) | 23.18** (1.64–327.01) | 36.56** (1.15–1,163.72) |
| Age (y) | 1.04*** (1.01–1.07) | 1.03 (0.99–1.06) | 1.05*** (1.02–1.08) | 1.05** (1.01–1.08) |
| Sex (1 = male; 2 = female) | 1.18 (0.54–2.55) | 1.17 (0.44–3.10) | 0.47* (0.20–1.09) | 0.39* (0.15–1.05) |
| loge(TSP) | 1.05 (0.73–1.51) | 1.22 (0.81–1.85) | ||
| Smoke exposure (0 = no, 1 = yes) | 0.87 (0.43–1.76) | 1.03 (0.45–2.36) | ||
| Allergy status (0 = no, 1 = yes) | 0.62 (0.30–1.30) | 1.23 (0.50–3.06) | ||
| Fish oil supplement (0 = no, 1 = yes) | 2.89 (0.66–12.75) | 0.83 (0.19–3.71) | ||
| Race (1 = white, 0 = otherwise) | 0.98 (0.38–2.48) | 1.24 (0.46–3.33) | ||
| Ethnicity (1 = Hispanic, 0 = otherwise) | 1.16 (0.39–3.48) | 0.83 (0.28–2.49) | ||
| Pet(s) in home (0 = no, 1 = yes) | 2.50** (1.14–5.48) | 1.47 (0.67–3.22) | ||
| Observations | 214 | 162 | 212 | 161 |
TSP = total suspended particles.
P < .01
P < .05
P < .1.
Model 1 adjusts for age and sex; model 2 adjusts for age, sex, log(TSP), smoke exposure, allergy status, and number of observations.
However, CED did not show a significant association with nutrient CFU when adjusted for additional confounders, including age and pet(s) in home (Table 4). Among all covariates included in the analysis (model 2, Table 4), only pet(s) in home showed a significant association with CED. Subjects without a pet(s) in their home were 2.5 times more likely to have positive detection of CED as compared to those with a pet.
However, the odds of MG dropout increased to 36.6 with a unit increase in nutrient CFU when adjusted for all confounders (OR = 36.6, 95% CI = 1.15 to 1,163.7; P < .05), including age, fish supplement, and pets. Age showed a significant association with MG dropout, suggesting every year increase in age was associated with a 5% increase in the risk of MG dropout. Sex also showed a marginal association with MG dropout, suggesting female subjects were less likely to have MG dropout as compared to male. Soy CFU was similarly associated with both CED and MG dropout (model 1: OR = 28.7, 95% CI = 1.8, 443.8 and OR = 39.6, 95% CI = 1.8, 875.2, respectively) (Table 5). Like for nutrient CFU, it did not show a significant association with CED and its association with MG dropout increased significantly when adjusted for all confounders (model 2, Table 5). Besides CED and MG dropout, we did not find other DE signs or symptoms to be significantly associated with nutrient or soy CFUs.
TABLE 5.
Multivariate Linear Regression of Indoor Microbial Communities (Soy Media) and Selected Dry Eye Signs, Odds Ratio (95% Confidence Interval)
| Corneal Epithelial Disruption | Meibomian Gland Dropout | |||
|---|---|---|---|---|
|
|
|
|||
| Variables | Model 1 | Model 2 | Model 1 | Model 2 |
|
| ||||
| loge(# colonies) | 28.07** (1.78–443.79) | 14.12 (0.43–463.29) | 39.55** (1.79–875.15) | 67.42** (1.18–3,864.59) |
| Age (y) | 1.04*** (1.01–1.07) | 1.03 (0.99–1.06) | 1.05*** (1.02–1.08) | 1.05** (1.01–1.08) |
| Sex (1 = male; 2 = female) | 1.18 (0.54–2.55) | 1.17 (0.44–3.10) | 0.47* (0.20–1.09) | 0.39* (0.15–1.05) |
| loge(TSP) | 1.05 (0.73–1.51) | 1.22 (0.81–1.85) | ||
| Smoke exposure (0 = no, 1 = yes) | 0.87 (0.43–1.76) | 1.03 (0.45–2.36) | ||
| Allergy status (0 = no, 1 = yes) | 0.62 (0.30–1.30) | 1.23 (0.50–3.06) | ||
| Fish oil supplement (0 = no, 1 = yes) | 2.89 (0.66–12.75) | 0.83 (0.19–3.71) | ||
| Race (1 = white, 0 = otherwise) | 0.98 (0.38–2.48) | 1.24 (0.46–3.33) | ||
| Ethnicity (1 = Hispanic, 0 = otherwise) | 1.16 (0.39–3.48) | 0.83 (0.28–2.49) | ||
| Pet(s) in home (0 = no, 1 = yes) | 2.50** (1.14–5.48) | 1.47 (0.67–3.22) | ||
| Observations | 214 | 162 | 212 | 161 |
TSP = total suspended particles.
P < .01
P < .05
P < .1.
Model 1 adjusts for age and sex; model 2 adjusts for age, sex, log(total suspended particles), smoke exposure, allergy status, and number of observations.
In the ordered logistic regression, outcome variables were on an ordinal scale. The maximum score for corneal staining in this population was 11. All values ≥7 were combined as category 7 (n = 13). MG dropout was scored 0–4 in clinic, with a score of 4 indicating highest level of dropout. These categories were maintained in this analysis. Our results show that number of CFU showed significant associations with both CED and MG dropout in ordered logistic regression (Table 6). A 1-unit increase in log(# colonies) soy and nutrient CFU was associated with increase in odds of higher MG dropout (nutrient CFU: OR = 85.0, 95% CI = 4.0 to 1821.0, P < .01; soy CFU: OR = 44.6, 95% CI = 3.3, 611.0). However, CFU did not show a significant association with CED. The severity (measured by their respective scales) of both CED and MG dropout were associated with age. A year increase in age was associated with 3% and 6% higher likelihood of CED and MG dropout severity, respectively (for CED: OR = 1.03, 95% CI = 1.00–1.05, P < .05; for MG dropout: OR = 1.06; 95% CI = 1.03–1.09; P < .01; Table 6).
TABLE 6.
Ordered Logistic Regression of Indoor Microbial Communities on Soy and Nutrient Media and Selected Dry Eye Signs, Odds Ratio (95% Confidence Interval)
| Nutrient Media | Soy Media | |||
|---|---|---|---|---|
|
|
|
|||
| Variables | Corneal Staining | Meibomian Gland Dropout | Corneal Staining | Meibomian Gland Dropout |
|
| ||||
| loge(# colonies) | 5.56 (0.33–92.65) | 85.03*** (3.97–1,821.96) | 4.34 (0.39–47.98) | 44.59*** (3.25–611.98) |
| Age (y) | 1.03** (1.00–1.05) | 1.06*** (1.03–1.09) | 1.03** (1.00–1.05) | 1.06*** (1.03–1.09) |
| Sex (1 = male; 2 = female) | 0.88 (0.39–2.00) | 0.39***(0.19–0.77) | 0.88 (0.39–2.00) | 0.39*** (0.19–0.77) |
| loge(TSP) | 1.1 (0.80–1.49) | 1.18 (0.88–1.59) | 1.1 (0.80–1.49) | 1.18 (0.88–1.59) |
| Smoke exposure (0 = no, 1 = yes) | 0.88 (0.48–1.62) | 1.31 (0.71–2.41) | 0.88 (0.48–1.62) | 1.31 (0.71–2.41) |
| Allergy status (0 = no, 1 = yes) | 0.79 (0.40–1.55) | 0.72 (0.39–1.35) | 0.79 (0.40–1.55) | 0.72 (0.39–1.35) |
| Fish oil supplement (0 = no, 1 = yes) | 1.9 (0.81–4.46) | 0.96 (0.30–3.03) | 1.9 (0.81–4.46) | 0.96 (0.30–3.03) |
| Race (1 = white, 0 = otherwise) | 1.13 (0.50–2.56) | 1.74 (0.85–3.54) | 1.13 (0.50–2.56) | 1.74 (0.85–3.54) |
| Ethnicity (1 = Hispanic, 0 = otherwise) | 0.87 (0.37–2.06) | 1.08 (0.55–2.14) | 0.87 (0.37–2.06) | 1.08 (0.55–2.14) |
| Pet(s) in home (0 = no, 1 = yes) | 2.46*** (1.26–4.81) | 1.36 (0.73–2.54) | 2.46*** (1.26–4.81) | 1.36 (0.73–2.54) |
| Observations | 162 | 161 | 162 | 161 |
TSP = total suspended particles.
P < .01
P < .05
P < .1.
DISCUSSION
IN THIS STUDY, WE FOUND NOVEL RELATIONSHIPS BETWEEN microbial counts in the home (quantified via soy and nutrient CFU instrumented on humidity and temperature) and 2 signs of DE, namely CED and MG dropout. The association between microbial counts and MG dropout was particularly robust, and remained significant with both soy and nutrient CFU when examined at binary or ordinal scales in models that adjusted for demographics, particulate matter, smoke exposure, allergy status, fish oil supplement, pets in home, and visit number. Further information gleaned from our study is that there is a wide range of microbial indoor environments in Miami homes, and that several factors including home age and humidity impact the indoor microbial concentration. Furthermore, we replicated the observations of a number of studies by demonstrating that DE measures (both CED and MG dropout) increased in severity with increasing age.8,33 Being that the average age of the study population is 59 years, this is an important consideration when interpreting results of this study. However, even when adjusting for age, both nutrient and soy CFU showed significant association with MG dropout, suggesting elevated concentration of CFU can exacerbate MG dysfunction even in an older population.
The direct comparison of our findings with the literature was not possible because indoor microbial exposure and DE measures are largely unexplored owing to lack of indoor monitoring. Much of the prior work has focused on outdoor6 or occupational19 bioaerosol exposures, while indoor studies focused on eye symptoms in office or hospital settings, but did not directly measure bioaerosol concentration in the residential setting.34,35 For example, in a study of 7,441 office workers from 167 buildings across 8 European countries, 91.2% of the 2,530 reporting eye dryness while working at the office reported improvements in symptoms on days away from the office.35 This study also examined relationships between office exposures and DE symptoms reporting an increased risk for proximity (<100 m) to potential sources of outdoor air pollution (OR = 1.41, 95% CI = 1.06, 1.88), absence of operable windows (OR = 1.70, 95% CI = 1.34, 2.16), and exposed concrete and/or plaster (OR: 1.29, 95% CI = 1.02, 1.62), and a negative association for cleaning (once per week) (OR: 0.75, 95% CI: 0.61, 0.91); however, indoor bioaerosol concentration was not specifically tested.35
Other studies have evaluated associations between conjunctival microbial load and DE symptoms and signs.34,36 In 1 study of 66 patients with DE (1 DE symptom experienced “often” or “constantly” from the McMonnies Survey, TBUT <10 seconds, staining >3 using the Oxford scale) and 18 controls, swabs were taken from the inferior conjunctival fornix and the lid margin. This study found higher total CFU (sum CFU on horse blood agar, chocolate agar, MacConkey, and anaerobic agar) in individuals with DE compared to controls (mean ± SD = 106 ± 82 vs 12 ± 18, P < .0001).36 This study further sub-grouped DE into individuals with and without MG dysfunction (eyelid thickening, irregularity, telangiectasia, MG loss, capping, or abnormal meibum quality) (n = 15 and n = 51, respectively) and interestingly, also found higher CFU in the DE group with MG dysfunction compared to non-DE controls (mean ± SD = 95 ± 66, P = .0002 vs 12 ± 18, P < .0001).36
While there is a paucity of research on the association between airborne exposure to microorganisms and DE, studies of related outcomes such as respiratory illness and “sick building syndrome” (SBS) may provide evidence to support our findings. SBS can be described as “a group of symptoms of unclear etiology which commonly include the itchy or watery eyes, blocked or stuffed nose, dry throat, dry skin, and general symptoms of headache and lethargy.”37 SBS is largely observed in the office environment, where people report improvement of symptoms within hours of leaving the building.38 However, similar observations have also been reported in schools,39,40 hospitals,41,42 and home environments.40,43 In indoor settings, SBS has been associated with the indoor environment conditions, such as high humidity and water damage,44 which are optimal growth conditions for microorganisms.45 Likewise, elevated levels of microorganisms have also been correlated with symptoms of SBS.46,47 In 1 study of 48 schools in the United States, building indoor air, outdoor air, and indoor surface samples were analyzed for fungal concentration and species. Staff at each school self-reported symptoms of SBS via questionnaire. Based on responses from the questionnaire, certain rooms were categorized as complaint areas (if there was a complaint from a staff member primarily working at this location) and noncomplaint areas (if no complaints from a staff member at this location). From the 622 staff member responses, complaints of nasal congestion (incidence per 100 employees, 95% CI: 19.8 ± 1.3) and itchy or watery eyes (incidence per 100 employees, 95% CI: 14.3 ± 1.1) were the most frequently reported. At 20 schools, there were significantly higher CFU of propagules of Penicillium species in the air samples from complaint areas compared with noncomplaint areas (P < .0001).47 Similar results have been found in studies examining symptoms of SBS and indoor bacteria46 and mold.48 Thus, it is hypothesized that increased airborne microbial concentrations, including bacteria and fungi, as well as highly allergenic endotoxins and mold, are a contributing factor in SBS. The many parallels between symptoms of DE and SBS, including irritation, dryness, or wateriness of the eyes,37 may substantiate our results.
Considering associations between microbial counts and MG dropout, lipolytic enzymes and polar lipids secreted by bacteria are among the factors that influence meibum composition.49,50 In turn, altered meibum with decreased fluidity and increased viscosity can enhance microbial growth,51,52 perhaps setting up a vicious cycle that underlies our noted observations. In addition, the introduction of bacterial enzymes can generate free fatty acids that can lead to inflammation.53 However, our cross-sectional design cannot substantiate directionality, nor can we identify species of microorganisms, as our study did not sequence the culture. Thus, it is not clear if exposure to microorganisms precludes ocular surface abnormalities or if microorganisms exacerbate an already compromised surface. It is interesting that despite our hypothesis, we did not observe associations between ocular surface inflammation (via InflammaDry) and microbial counts. However, we did not measure tear cytokines and thus cannot discount the possibility of the relationships between other inflammatory mediators and microbial counts.
As with all studies, our results must be considered in light of their limitations. While our environmental monitoring captured data on number of microbial communities, particulate matter, temperature, and relative humidity, we do not have data on other exposures such as ozone or nitrogen dioxide, which have been previously associated with DE.23 Furthermore, our analysis of bioaerosols is limited to quantification of CFU, and we do not have data on species of microorganisms, which may unveil the importance of commensal and pathogenic bacteria. However, it is important to note that cultures that preferentially grow bacteria (nutrient) and fungus (soy)54 both demonstrated associations with MG dropout when adjusted for potential confounders. Another limitation is the inclusion of patients who used artificial tears (Natural Balance Tears [dextran 70/hypromellose] or preservative-free Refresh [carboxy-methylcellulose 0.5%]) prescribed by our clinic. Thus, this factor may present a possible bias in our findings. Furthermore, we assessed MG dropout in the lower eyelid. However, the literature suggests that there may be differences between lower and upper MG status.55 Nonetheless, others document lower lid MG drop with different conditions, including severity of keratitis sicca and chronic blepharitis.56,57 In addition, we excluded individuals with a known diagnosis of autoimmune disease but did not conduct laboratory screening for this potential confounder in the present study. Finally, the study is limited by specificity of the study population and geographic location. Miami is a subtropical environment with relatively higher-than-average temperature and humidity compared to the rest of the country. The focus of our study on US veterans, who have higher-than-normal comorbidities as well as distinct home characteristics, constrains the scope of generalizability of our results. Future studies will need to address these gaps and focus on whether reducing indoor microbial concentration will have a beneficial effect on MG dysfunction.
Despite these limitations, this study yields a novel relationship between indoor microbial concentration and MG dropout of the lower eyelid. The breadth of previous research including occupational exposure, indoor sources, association to human diseases, and notable parallels between SBS and DE warrants the need to further explore bioaerosol exposure indoors, where the average American spends 90% of time.26 This study provides a key indication of how the indoor environment conditions, namely building age and humidity, affect indoor microbial concentrations. Thus, improving such conditions may mitigate microbial concentration and hence its associated MG disorders.
Supplementary Material
FUNDING/SUPPORT:
SUPPORTED BY THE DEPARTMENT OF VETERANS AFFAIRS, VETERANS HEALTH ADMINISTRATION, OFFICE of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr Galor) and Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (Dr Galor) and Vision Research Program (VRP) W81XWH2010820 (Dr Galor), National Eye Institute R01EY026174 (Drs Kumar and Galor) and R61EY032468 (Dr Galor), NIH Center Core Grant P30EY014801 (institutional), and Research to Prevent Blindness Unrestricted Grant (institutional).
Footnotes
Financial Disclosures: The authors have no financial relationships to disclose. The authors have no proprietary or commercial interest in any materials discussed in this article. All authors attest that they meet the current ICMJE criteria for authorship.
REFERENCES
- 1.Farrand KF, Fridman M, Stillman IO¨ , Schaumberg DA. prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol 2017;182:90–98. [DOI] [PubMed] [Google Scholar]
- 2.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017;15(3): 276–283. [DOI] [PubMed] [Google Scholar]
- 3.Huang A, Janecki J, Galor A, et al. Association of the indoor environment with dry eye metrics. JAMA Ophthalmol 2020; 138(8):867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan G, Li J, Yang Q, et al. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci Rep 2018;8(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galor A, Kumar N, Feuer W, Lee DJ. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology 2014;121(4):972–973.e971. [DOI] [PubMed] [Google Scholar]
- 6.Hwang SH, Choi Y-H, Paik HJ, Wee WR, Kim MK, Kim DH. Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmol 2016;134(5):503–510. [DOI] [PubMed] [Google Scholar]
- 7.Bacsi A, Dharajiya N, Choudhury BK, Sur S, Boldogh I. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J Allergy Clin Immunol 2005; 116(4):836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf 2017;15(3):334–365. [DOI] [PubMed] [Google Scholar]
- 9.Georgakopoulos DG, Després V, Fröhlich-Nowoisky J, et al. Microbiology and atmospheric processes: biological, physical and chemical characterization of aerosol particles. Biogeosciences 2009;6(4):721–737. [Google Scholar]
- 10.Flannigan BE, Samson R, Miller J, eds. Microorganisms in Home and Indoor Work Environments. London: CRC Press; 2001. [Google Scholar]
- 11.Severson KM, Mallozzi M, Driks A, Knight KL. B cell development in GALT: role of bacterial superantigen-like molecules. J Immunol 2010;184(12):6782–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoppin JA, Umbach DM, Long S, et al. Respiratory disease in United States farmers. Occup Environ Med 2014;71(7): 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maheswaran D, Zeng Y, Chan-Yeung M, et al. Exposure to beta-(1,3)-D-glucan in house dust at age 7–10 is associated with airway hyperresponsiveness and atopic asthma by age 11–14. PLoS One 2014;9(6):e98878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prussin AJ 2nd, Marr LC. Sources of airborne microorganisms in the built environment. Microbiome 2015;3:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas P, Robertson S, Gay R, Hansell AL, Gant TW. A systematic review of the public health risks of bioaerosols from intensive farming. Int J Hyg Environ Health 2018;221(2): 134–173. [DOI] [PubMed] [Google Scholar]
- 16.Bowers RM, Sullivan AP, Costello EK, Collett JL, Knight R, Fierer N. Sources of bacteria in outdoor air across cities in the midwestern United States. Appl Environ Microbiol 2011; 77(18):6350–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson C, Littlewood E, Douglas P, Robertson S, Gant TW, Hansell AL. Exposures and health outcomes in relation to bioaerosol emissions from composting facilities: a systematic review of occupational and community studies. J Toxicol Environ Health B Crit Rev 2015;18(1):43–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albrecht A, Witzenberger R, Bernzen U, Jäckel U. Detection of airborne microbes in a composting facility by cultivation based and cultivation-independent methods. Ann Agric Environ Med 2007;14(1):81–85. [PubMed] [Google Scholar]
- 19.Bünger J, Schappler-Scheele B, Hilgers R, Hallier E. A 5-year follow-up study on respiratory disorders and lung function in workers exposed to organic dust from composting plants. Int Arch Occup Environ Health 2007; 80(4):306–312. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer BT, Lighthart B. Survey of culturable airborne bacteria at four diverse locations in Oregon: urban, rural, forest, and coastal. Microb Ecol 1997;34(3):167–177. [DOI] [PubMed] [Google Scholar]
- 21.Cho EM, Hong HJ, Park SH, Yoon DK, Nam Goung SJ, Lee CM. Distribution and influencing factors of airborne bacteria in public facilities used by pollution-sensitive population: a meta-analysis. Int J Environ Res Public Health 2019; 16:1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta SK, Gupta SC, Agarwal R, Sushma S, Agrawal SS, Saxena R. A multicentric case-control study on the impact of air pollution on eyes in a metropolitan city of India. Indian J Occup Environ Med 2007;11(1):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adhikari A, Reponen T, Grinshpun SA, Martuzevicius D, LeMasters G. Correlation of ambient inhalable bioaerosols with particulate matter and ozone: a two-year study. Environ Pollut 2006;140(1):16–28. [DOI] [PubMed] [Google Scholar]
- 24.Rahman A, Luo C, Khan MHR, Ke J, Thilakanayaka V, Kumar S. Influence of atmospheric PM2.5, PM10, O3, CO, NO2, SO2, and meteorological factors on the concentration of airborne pollen in Guangzhou, China. Atmos Environ 2019; 212:290–304. [Google Scholar]
- 25.Yu D, Deng Q, Wang J, et al. Air pollutants are associated with dry eye disease in urban ophthalmic outpatients: a prevalence study in China. J Transl Med 2019;17:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adgate JL, Church TR, Ryan AD, et al. Outdoor, indoor, and personal exposure to VOCs in children. Environ Health Perspect 2004;112(14):1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalmers RL, Begley CG, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye 2010;33(2):55–60. [DOI] [PubMed] [Google Scholar]
- 28.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118(5):615–621. [DOI] [PubMed] [Google Scholar]
- 29.Heiko P. Dr Heiko Pult - Optometry & Vision Research. Available at 2019. https://www.heiko-pult.de/downloads-links.html.
- 30.Met One Instruments. AEROCET 531S Manual [computer program] Grants Pass, OR: Met One Instrument Inc; 2014. [Google Scholar]
- 31.NIOSH, Jacklitsch B, Williams WJ, Musolin K, Coca A, Kim J-H, Turner N. NIOSH criteria for a recommended standard: occupational exposure to heat and hot environments106. Cincinnati, OH: U.S. Department of Health and Human Services; 2016. [Google Scholar]
- 32.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 2000;118(9): 1264–1268. [DOI] [PubMed] [Google Scholar]
- 33.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008; 115(5):911–915. [DOI] [PubMed] [Google Scholar]
- 34.Smedbold HT, Ahlen C, Norback D, Hilt B. Sign of eye irritation in female hospital workers and the indoor environment. Indoor Air 2001;11(4):223–231. [DOI] [PubMed] [Google Scholar]
- 35.de Kluizenaar Y, Roda C, Dijkstra NE, et al. Office characteristics and dry eye complaints in European workers–The OFFICAIR study. Build Environ 2016;102:54–63. [Google Scholar]
- 36.Albietz JM, Lenton LM. Effect of antibacterial honey on the ocular flora in tear deficiency and meibomian gland disease. Cornea 2006;25(9):1012–1019. [DOI] [PubMed] [Google Scholar]
- 37.Burge PS. Sick building syndrome. Occup Environ Med 2004; 61(2):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. Indoor air quality: biological contaminants: report on a WHO meeting, Rautavaara, 29 August −2 September 1988 Copenhagen: WHO, Regional Office for Europe; 1987. [Google Scholar]
- 39.Saijo Y, Nakagi Y, Ito T, Sugioka Y, Endo H, Yoshida T. Dampness, food habits, and sick building syndrome symptoms in elementary school pupils. Environ Health Prev Med 2010; 15(5):276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takaoka M, Suzuki K, Norback D. Sick building syndrome among junior high school students in Japan in relation to the home and school environment. Glob J Health Sci 2015;8(2):165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalender Smajlovic S, Kukec A, Dovjak M. Association between sick building syndrome and indoor environmental quality in Slovenian hospitals: a cross-sectional study. Int J Environ Res Public Health 2019;16(17):3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Acebo I, Llorca J, Ortiz-Revuelta C, Angulo B, Gomez-Alvarez S, Dierssen-Sotos T. Sick building syndrome in a general hospital and the risks for pregnant workers. Int J Gynaecol Obstet 2011;113(3):241–242. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y. Indoor air quality, ventilation and their associations with sick building syndrome in Chinese homes. Energ Build 2019;197:112–119. [Google Scholar]
- 44.Engvall K, Norrby C, Norback D. Sick building syndrome in relation to building dampness in multi-family residential buildings in Stockholm. Int Arch Occup Environ Health 2001;74(4):270–278. [DOI] [PubMed] [Google Scholar]
- 45.WHO. WHO Guidelines for Indoor Air Quality: Dampness and Mould Copenhagen: World Helath Organization; 2009. [PubMed] [Google Scholar]
- 46.Teeuw KB, Vandenbroucke-Grauls CM, Verhoef J. Airborne gram-negative bacteria and endotoxin in sick building syndrome. A study in Dutch governmental office buildings. Arch Intern Med 1994;154(20):2339–2345. [PubMed] [Google Scholar]
- 47.Cooley JD, Wong WC, Jumper CA, Straus DC. Correlation between the prevalence of certain fungi and sick building syndrome. Occup Environ Med 1998;55(9):579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakayama K, Morimoto K. Relationship between, lifestyle, mold and sick building syndromes in newly built dwellings in Japan. Int J Immunopathol Pharmacol 2007;20(2 Suppl 2):35–43. [DOI] [PubMed] [Google Scholar]
- 49.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci 2011;52(4):1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci 1986;27(4):486–491. [PubMed] [Google Scholar]
- 51.Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol 2016;100(3):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang X, Deng A, Yang J, et al. Pathogens in the Meibomian gland and conjunctival sac: microbiome of normal subjects and patients with Meibomian gland dysfunction. Infect Drug Resist 2018;11:1729–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci 2011; 52(4):1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basu S, Bose C, Ojha N, et al. Evolution of bacterial and fungal growth media. Bioinformation 2015;11(4): 182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arita R, Suehiro J, Haraguchi T, Shirakawa R, Tokoro H, Amano S. Objective image analysis of the meibomian gland area. Br J Ophthalmol 2014;98(6):746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Paiva CS, Lindsey JL, Pflugfelder SC. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology 2003;110(6):1102–1109. [DOI] [PubMed] [Google Scholar]
- 57.Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV. Meibomian gland dysfunction in chronic blepharitis. Cornea 1991;10(4):277–285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.