Abstract
Nirmatrelvir-ritonavir (NMVr) is used to treat symptomatic, nonhospitalized patients with coronavirus disease-2019 (COVID-19) who are at high risk of progression to severe disease. Patients with cardiovascular risk factors and cardiovascular disease are at a high risk of developing adverse events from COVID-19 and as a result have a higher likelihood of receiving NMVr. Ritonavir, the pharmaceutical enhancer used in NMVr, is an inhibitor of the enzymes of CYP450 pathway, particularly CYP3A4 and to a lesser degree CYP2D6, and affects the P-glycoprotein pump. Co-administration of NMVr with medications commonly used to manage cardiovascular conditions can potentially cause significant drug-drug interactions and may lead to severe adverse effects. It is crucial to be aware of such interactions and take appropriate measures to avoid them. In this review, we discuss potential drug-drug interactions between NMVr and commonly used cardiovascular medications based on their pharmacokinetics and pharmacodynamic properties.
Key Words: cardiovascular medications, COVID-19, drug-drug interactions, nirmatrelvir-ritonavir, SARS-CoV-2
Abbreviations and Acronyms: AF, atrial fibrillation; AUC, area under the concentration-time curve; Cmax, maximum concentration; CVD, cardiovascular disease; CYP, cytochrome P; DDI, drug-drug interaction; NMVr, nirmatrelvir/ritonavir; OATP, organic anion transporter polypeptide; PCI, percutaneous coronary intervention; P-gp, P-glycoprotein; UGT, uridine diphosphate–glucuronosyltransferase; VTE, venous thromboembolism
Central Illustration
A 57-year-old man with ischemic cardiomyopathy and a history of percutaneous coronary intervention (PCI) a month before, presents with symptomatic coronavirus disease-2019 (COVID-19) despite being vaccinated with 3 doses of an mRNA COVID-19 vaccine. His home medications include aspirin, clopidogrel, high-dose atorvastatin, metoprolol succinate, sacubitril/valsartan, spironolactone, and dapagliflozin. He is prescribed nirmatrelvir-ritonavir (Paxlovid [Pfizer]). At the recommendation of his primary care physician, he calls his cardiologist to inquire about potential interactions with his medications. He is advised to continue aspirin, spironolactone, dapagliflozin, and metoprolol succinate. Clopidogrel is switched to prasugrel. Atorvastatin and sacubitril/valsartan are temporarily withheld. All cardiovascular medications are resumed after completion of a 5-day course of NMVr except atorvastatin, which is restarted 3 days after completion of treatment with NMVr.
Nirmatrelvir-ritonavir (NMVr) received emergency use authorization from the U.S. Food and Drug Administration in December 2021 as one of the first oral antiviral agents for the treatment of symptomatic nonhospitalized adults with mild to moderate SARS-CoV-2 infection who are at high risk for progression to severe disease.1 Compared with placebo, NMVr reduces progression to severe COVID-19 by 89% in nonvaccinated high-risk symptomatic patients.2 Patients with cardiovascular disease (CVD) and risk factors such as diabetes, hypertension, chronic kidney disease, and smoking constitute a large proportion of the high-risk population among whom NMVr is beneficial.2 In the EPIC-HR trial, patients with CVD benefitted the most from NMVr therapy (a difference of 24% in COVID-19–related hospitalizations or death from any cause when compared to placebo).2 In the trial, NMVr was tested exclusively in nonvaccinated patients; but real-world data suggest that it may be equally effective in those who have been vaccinated against COVID-19.3, 4, 5 Najjar-Debbiny et al3 retrospectively analyzed outcomes in unvaccinated and vaccinated patients who were prescribed NMVr, 32% of whom had CVD, and demonstrated a combined risk reduction of 46%, with subgroup analysis showing a similar magnitude of effectiveness in both populations. The use of NMVr reduces disease progression, time to achieve a lower viral load, emergency room visits, hospitalizations, and all-cause mortality.4 , 5 More than a million courses of NMVr have been prescribed thus far, and its use is predicted to increase with emerging variants of SARS-CoV-2 resistant to monoclonal antibody therapies. While NMVr has been shown to be very effective in patients with pre-existing CVD, it has significant drug-drug interactions (DDIs) with commonly used cardiovascular medications and therefore it is important that all clinicians are familiar with those DDIs.
Mechanism of Action and Pharmacokinetics of NMVr
Nirmatrelvir inhibits a protease enzyme produced by SARS-CoV-2 that is necessary for viral replication. Low-dose ritonavir is combined with nirmatrelvir as a pharmaceutical enhancer to delay its hepatic metabolism and prolong its duration of action, a concept well established in antiretroviral therapy for human immunodeficiency virus. Ritonavir is an inhibitor of cytochrome P (CYP) 450 enzymes, particularly CYP3A4 and to a lesser degree CYP2D6.6 These enzymes, primarily present in hepatocytes, are responsible for the oxidative metabolism of many medications. Ritonavir also induces other CYP450 enzymes to a weaker degree, leading to decreased levels of various medications when co-administered.6 P-glycoprotein (P-gp) is an efflux pump in the intestinal, hepatic, and renal epithelia that facilitates transport of drugs. The effect of ritonavir on P-gp by primary inhibition and subsequent induction with time can result in unpredictable DDIs. Nirmatrelvir does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2D6 in vitro at clinically relevant concentrations, but it does inhibit CYP3A4 and P-gp.7 With NMVr itself being a substrate of CYP450 enzymes, other potent enzyme inducers can render it ineffective. Organic anion transporter polypeptide (OATP) 1B1 is an uptake transporter expressed on the sinusoidal surface of hepatocytes, responsible for hepatic uptake of certain drugs. NMVr has a weak inhibitory effect on OATP1B1, and that can result in relevant DDIs.7
NMVr is renally cleared and is therefore not recommended for those with an estimated glomerular filtration rate <30 mL/min. Because no data are available in patients with severe hepatic impairment (Child-Pugh class C), it is contraindicated in patients with severe liver disease.7 No dose adjustment is required in mild (Child-Pugh class A) or moderate (Child-Pugh class B) liver dysfunction.7
Cardiovascular Drug Interactions With NMVr
We here review the potential interactions between commonly prescribed cardiovascular medications and NMVr (Figures 1 and 2 , Table 1 ). While there is limited clinical information regarding interaction-related adverse events, we use pharmacokinetics and pharmacodynamics data to provide guidance regarding potential interactions and the associated likely consequences based on the degree of interaction.
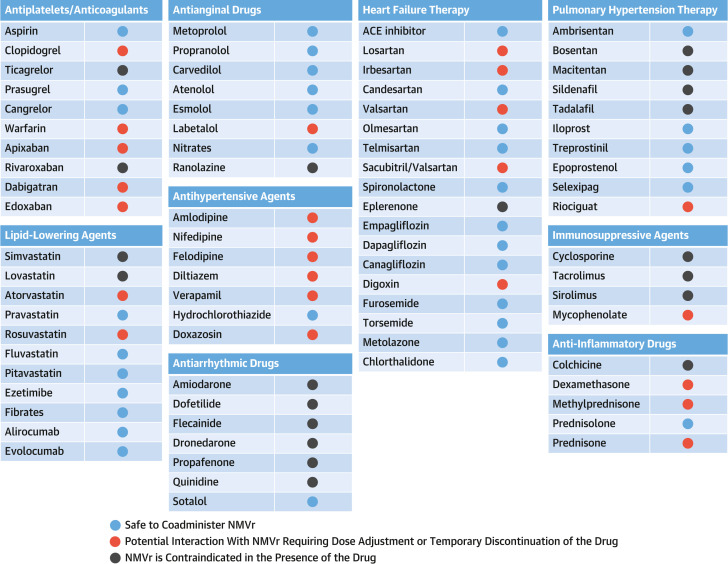
Figure 1.
Drug-Drug Interactions of Cardiovascular Medications With Nirmatrelvir-Ritonavir
This figure describes potential drug-drug interactions of nirmatrelvir-ritonavir (NMVr) with commonly used cardiovascular (CV) medications along with grade of interactions and recommendations regarding any necessary adjustment in the regimen of CV medications and suitability of NMVr. ACE = angiotensin-converting enzyme.
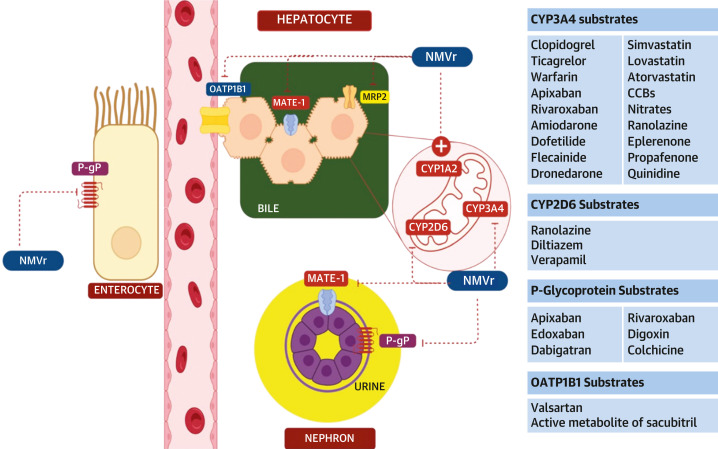
Figure 2.
Nirmatrelvir-Ritonavir Interaction With Enzymes Involved in Drug Metabolism
Ritonavir, the pharmaceutical enhancer in nirmatrelvir-ritonavir (NMVr), has significant interactions with other medications. NMVr inhibits CYP3A4 and to a lesser extent CYP2D6. It also inhibits the transmembrane efflux pump P-glycoprotein (P-gp) and the multidrug and toxin extrusion (MATE-1) transporter in the intestinal and renal cells. It inhibits the organic anion transporter polypeptide 1B1 (OATP1B1), which is a transporter on the sinusoidal membrane of the hepatocytes and MRP2 and an efflux transporter on the apical side of the bile canaliculus in the hepatocytes. It has weak inducing effects on CYP1A2, CYP2B6, CYP2C9, and CYP2C19. Some significant DDIs with the substrates of these clinically relevant enzymes are noted. An exhaustive list of all CV medications is not represented. The figure was prepared with the use of Biorender.
Table 1.
Drug-Drug Interactions With Nirmatrelvir-Ritonavir and Commonly Used Cardiovascular Medications
| Druga | NMVr Effect on Drug Level | Effect of Interaction | Recommendation |
|---|---|---|---|
| Antiplatelet agents | |||
| Aspirin | None | None | Safe to co-administer with NMVr. |
| Clopidogrel | Decrease | Increased risk of thrombosis | Recent PCI or history of high-risk PCI: Avoid NMVr or switch to prasugrel with a loading dose while on NMVr. Other patients: Safe to co-administer with NMVr. |
| Prasugrel | Decrease but with no clinical effect | None | Safe to co-administer with NMVr. |
| Ticagrelor | Increase | Increased risk of bleeding | Avoid NMVr or switch to prasugrel with a loading dose while on NMVr. |
| Anticoagulation (AC) | |||
| Apixabanc | Increase | Increased risk of bleeding | Cannot be safely adjusted/interrupted if patient is on dialysis: Avoid NMVr. Consider alternative therapies.b For AF: If on 5 mg apixaban twice daily: Start NMVr and decrease dose to 2.5 mg twice daily for a total of 8 days and then resume apixaban at the previous dose. If on 2.5 mg apixaban twice daily: Avoid NMVr or withhold apixaban for 12-24 hours before starting NMVr along with an alternative AC (eg, enoxaparin) for a total of 8 days and then resume apixaban at the previous dose. For VTE: If on 10 mg apixaban twice daily: Start NMVr and decrease apixaban to 5 mg twice daily for a total of 8 days and then resume apixaban at the previous dose. If on 5 mg apixaban twice daily: Start NMVr and decrease apixaban to 2.5 mg twice daily for a total of 8 days and then resume apixaban at the previous dose. If on 2.5 mg apixaban twice daily for VTE prophylaxis: Can continue the same dose while on NMVr (will likely achieve therapeutic levels). |
| Rivaroxabanc | Increase | Increased risk of bleeding | Cannot be safely adjusted/interrupted: Avoid NMVr. Consider alternative therapies.b Other patients: Withhold rivaroxaban for 24-36 hours before starting NMVr. Use an alternative AC (eg, enoxaparin) for a total of 8 days and then resume rivaroxaban. |
| Edoxabanc | Increase | Increased risk of bleeding | Cannot be safely adjusted or interrupted: Avoid NMVr. Consider alternative therapies.b If on 60 mg edoxaban daily: Decrease dose by 50% for a total of 8 days from the start of NMVr and then resume edoxaban at the previous dose. If on 30 mg edoxaban daily: Withhold edoxaban when starting NMVr. Use an alternative AC (eg, enoxaparin) for 8 days and then resume edoxaban. |
| Dabigatranc | Increase | Increased risk of bleeding | Cannot be safely adjusted/interrupted: Avoid NMVr. Consider alternative therapies.b For AF: CrCl >50 mL/min: Decrease dose to 110 mg twice daily for 8 days from the start of NMVr and then resume dabigatran at the previous dose (this dosage is not available in the United States). CrCl 30-50 mL/min: Decrease dose to 75 mg twice daily for 8 days from the start of NMVr and then resume dabigatran at the previous dose. CrCl <30 mL/min: Avoid co-administration of NMVr with dabigatran; consider switching to an alternative AC. For VTE: If on 150 mg dabigatran twice daily: Co-administration with NMVr is not recommended. Withhold dabigatran for 12-24 hours and then start NMVr with an alternative AC (eg, enoxaparin) for a total of 8 days, and then resume dabigatran at the previous dose. |
| Warfarin | Increase or decrease (most likely decrease) | Variable effect on thrombotic and bleeding risk | May co-administer NMVr with close INR monitoring. |
| Enoxaparin | None | None | Safe to co-administer with NMVr. |
| Lipid management | |||
| Atorvastatin | Increase | Increased myopathy, liver toxicity | Withhold atorvastatin while on NMVr. Can be resumed 3 days after last dose of NMVr. A lower dose of 10 mg daily is acceptable to continue with NMVr. |
| Rosuvastatin | Increase | Increased myopathy, liver toxicity | Withhold rosuvastatin while on NMVr. Can be resumed the day after last dose of NMVr. If co-administered with NMVr, a maximum daily dose of 10 mg rosuvastatin is acceptable. |
| Simvastatin | Increase | Increased myopathy, liver toxicity | Withhold simvastatin 12 hours before the first dose of NMVr. Can resume 5 days after the last dose of NMVr. |
| Lovastatin | Increase | Increased myopathy, liver toxicity | Withhold lovastatin 12 hours before the first dose of NMVr. Can resume 5 days after the last dose of NMVr. |
| Pravastatin | None | None | Safe to co-administer with NMVr. |
| Fluvastatin | None | None | Safe to co-administer with NMVr. |
| Pitavastatin | None | None | Safe to co-administer with NMVr. |
| Ezetimibe | Decrease | Decreased efficacy | Safe to co-administer with NMVr. |
| Fibrates | None | None | Safe to co-administer with NMVr. |
| Evolocumab | None | None | Safe to co-administer with NMVr. |
| Alirocumab | None | None | Safe to co-administer with NMVr. |
| Antianginal drugs | |||
| Metoprolol, propranolol, carvedilol, atenolol, esmolol | None | None | Safe to co-administer with NMVr. |
| Labetolol | Decrease | Reduce antihypertensive effect | Can co-administer NMVr with home blood pressure monitoring. |
| Nitrates | Decrease | Decreased effect | Safe to co-administer with NMVr. |
| Ranolazine | Increase | QT prolongation, torsades de pointes | Avoid co-administration of NMVr or temporarily withhold ranolazine while on NMVr. |
| Heart failure therapy | |||
| Lisinopril, enalapril, quinapril, captopril | None | None | Safe to co-administer with NMVr. |
| Irbesartan | Decrease | Reduced antihypertensive effect | Safe to co-administer with NMVr; interaction is mild. Blood pressure monitoring may be helpful. |
| Losartan | Increase | Hypotension | Safe to co-administer with NMVr; interaction is mild. Blood pressure monitoring may be helpful. |
| Valsartan | Increase | Hypotension | Can co-administer with NMVr with blood pressure monitoring. Stop valsartan if hypotension ensues. |
| Sacubitril/valsartan | Increase | Hypotension | Can co-administer with NMVr with blood pressure monitoring. Stop sacubitril/valsartan if hypotension ensues. |
| Candesartan | None | None | Safe to co-administer with NMVr. |
| Telmisartan | None | None | Safe to co-administer with NMVr. |
| Olmesartan | None | None | Safe to co-administer with NMVr. |
| Spironolactone | None | None | Safe to co-administer with NMVr. |
| Eplerenone | Increase | Hyperkalemia | Avoid co-administration and temporarily withhold eplerenone while on NMVr. |
| Canagliflozin | Decrease | Decreased glycemic control | Safe to co-administer with NMVr, because NMVr is prescribed for a short duration. |
| Empagliflozin | None | None | Safe to co-administer with NMVr. |
| Dapagliflozin | None | None | Safe to co-administer with NMVr. |
| Ivabradine | Increase | Bradycardia | Avoid co-administration or temporarily withhold ivabradine while on NMVr. |
| Digoxin | Increase | Toxic levels | Based on the patient’s renal function, dose reduction can be advised; however, extreme caution should be exercised with appropriate monitoring of digoxin levels. Alternatively, digoxin can be temporarily withheld while on NMVr. |
| Furosemide | None | None | Safe to co-administer with NMVr. |
| Torsemide | Mildly decrease | None | Weak interaction, so safe to co-administer with NMVr. |
| Hydrochlorothiazide, metolazone, chlorthalidone | None | None | Safe to co-administer with NMVr. |
| Other antihypertensive agents | |||
| Amlodipine | Increase | Hypotension, flushing, and edema | A 50% reduction in the dose of amlodipine is recommended for 8 days when starting NMVr. |
| Nifedipine, felodipine | Increase | Hypotension, flushing, and edema | Close blood pressure monitoring and/or dose reduction if co-administered with NMVr. Temporarily discontinue if needed. Can be restarted 3 days after the last dose of NMVr. |
| Diltiazem, verapamil | Increase | Bradycardia, dizziness, hypotension, edema | Close blood pressure monitoring and/or dose reduction if co-administered with NMVr. Temporarily discontinue if needed. Can be restarted 3 days after the last dose of NMVr. |
| Doxazosin/Terazosin | Increase | Hypotension | Close blood pressure monitoring or temporary discontinuation while on NMVr. |
| Antiarrhythmic drugs | |||
| Amiodarone, dofetilide, flecainide, dronedarone, propafenone, quinidine | Increase | Life-threatening arrhythmias | Co-administration of NMVr is contraindicated. Consider alternative COVID-19 therapiesb or consider withholding antiarrhythmic therapy temporarily if feasible. Amiodarone has prolonged half-life of ∼100 days which precludes the option of temporarily stopping the drug and initiating NMVr. |
| Sotalol | None | None | Safe to co-administer with NMVr. |
| Pulmonary hypertension therapy | |||
| Ambrisentan | None | None | Safe to co-administer with NMVr. |
| Bosentan | Increase | Headaches, nausea, and vomiting, liver toxicity | Co-administration with NMVr is contraindicated. Discontinue for at least 36 hours before initiation of NMVr. |
| Macitentan | Increase | Headaches, nausea, and vomiting, liver toxicity | Co-administration with NMVr is not advised. Discontinue for at least 36 hours before initiation of NMVr. |
| Sildenafil | Increase | Hypotension, persistent erection, visual disturbances | Do not co-administer NMVr. |
| Tadalafil | Increase | Hypotension, persistent erection, visual disturbances | Do not co-administer NMVr. |
| Prostacyclin analogs | |||
| Selexipag | None | None | Safe to co-administer with NMVr. |
| Riociguat | Increase | None | Reduce dose of riociguat to 0.5 mg 3 times a day while co-administering NMVr along with close monitoring for side-effects. |
| Antiinflammatory drugs | |||
| Colchicine | Increase | Toxic levels | Avoid co-administration of NMVr or temporarily withhold colchicine while on NMVr. |
| Dexamethasone | Increase | Cushing’s syndrome, adrenal suppression | Reduce dose to 50% while taking NMVr and resume full dose 3 days after completion of NMVr. Consider switching to prednisolone or beclomethasone. |
| Methylprednisone | Increase | Cushing’s syndrome, adrenal suppression | Continue with caution and monitor for side-effects. Consider switching to prednisolone or beclomethasone. |
| Prednisone | Increase | Cushing’s syndrome, adrenal suppression | Continue with caution and monitor for side-effects. Consider switching to prednisolone or beclomethasone. |
| Prednisolone | None | No clinically significant effects with short duration of NMVr | Safe to co-administer with NMVr. |
| Immunosuppressive therapy in heart transplant patients | |||
| Cyclosporine | Increase | Toxic levels | NMVr is not recommended in these patients.d |
| Tacrolimus | Increase | Toxic levels | NMVr is not recommended in these patients.d |
| Sirolimus | Increase | Toxic levels | NMVr is not recommended in these patients.d |
AF = atrial fibrillation; CrCl = creatinine clearance; INR = international normalized ratio; NMVr = nirmatrelvir-ritonavir; PCI = percutaneous coronary intervention; VTE = venous thromboembolism.
Only commonly used drugs are listed here, and it is by no means an exhaustive list of all CV drugs.
Monoclonal antibodies against SARS-CoV-2, molnupiravir, remdesivir can be considered based on the clinical scenario after screening for DDIs.
When considering interrupting oral anticoagulation without substitution, it should be kept in mind that this should be reserved only for those at very low risk for thromboembolism. Low thromboembolic risk: CHA2DS2-VASc <3 and/or no history of stroke, VTE more than 1 year earlier, no history of recurrent clots, and no history of severe thrombophilia. Caution must be exercised because active COVID-19 infection, may in itself increase the risk of thromboembolism.
Significant dose reduction is required if co-administered with NMVr. Repeated checks of drug levels of the immunosuppressive agents are required during the 5-day course of NMVr. This is logistically difficult in the setting of active COVID-19 infection and therefore alternative therapies for COVID-19 should be considered.
Antiplatelet agents
Aspirin is metabolized by hepatic conjugation with glycin or glucuronic acid. Ritonavir weakly induces glucuronidation and can theoretically increase aspirin metabolism. However, there are no reported clinical adverse events, and it is safe to take with NMVr.8
CYP3A4 and CYP2B6 are responsible for the bioactivation of prasugrel, a prodrug. Although there is a 2-fold decrease in the maximum concentration (Cmax) of prasugrel in patients on ritonavir, this does not affect its antiplatelet activity.9 The preserved potency of platelet inhibition with prasugrel, when administered with ritonavir, is poorly understood, but it is considered safe to continue prasugrel in patients on NMVr.
Ritonavir decreases the production of the active metabolite of clopidogrel, reducing the area under the concentration-time curve (AUC) by 51% and platelet inhibition by 20%.9 Ticagrelor is a direct P2Y12 antagonist that does not require activation for its antiplatelet effect. Ticagrelor is a CYP3A4 substrate, and co-administration of NMVr therefore poses an increased risk of bleeding.10 Clinicians should avoid prescribing NMVr to patients on ticagrelor or clopidogrel unless these agents can be replaced by prasugrel. Alternatively, other COVID-19 therapies can be considered (Central Illustration ).
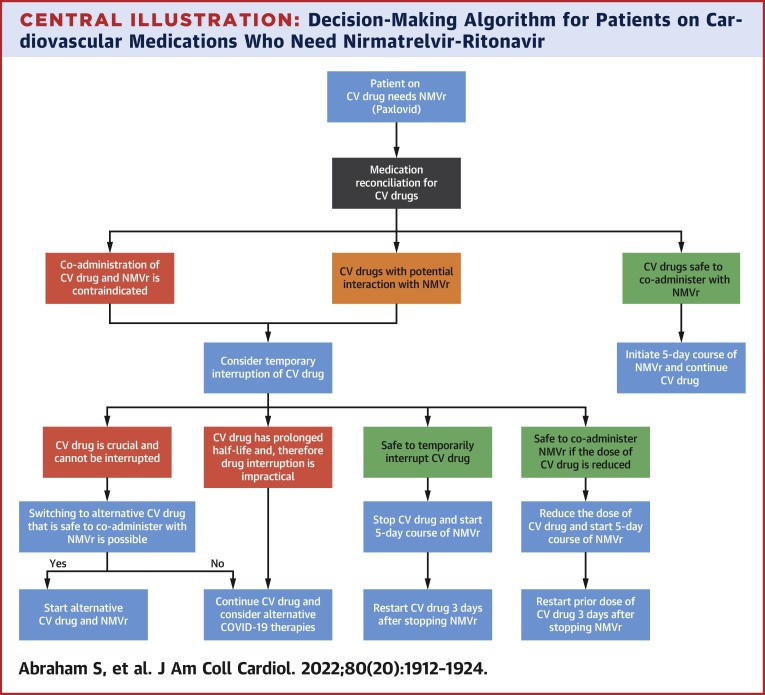
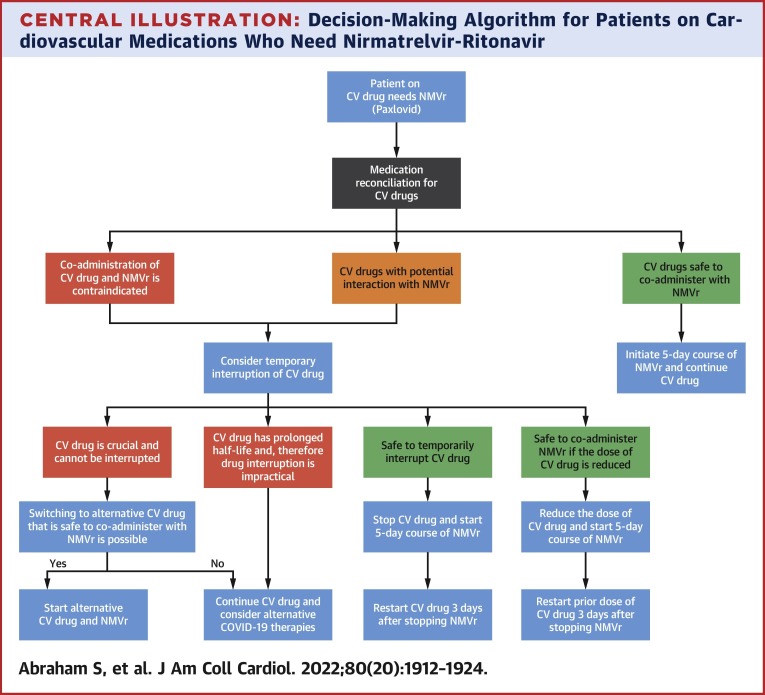
Central Illustration.
Decision-Making Algorithm for Patients on Cardiovascular Medications Who Need Nirmatrelvir-Ritonavir
This figure illustrates the proposed algorithm to guide the management of cardiovascular (CV) medications for those who require nirmatrelvir-ritonavir (NMVr) for the management of COVID-19.
Given this higher risk, we suggest that NMVr not be prescribed in patients on clopidogrel or ticagrelor with a recent PCI (within 6 months) or to those who have had a high-risk PCI (unprotected left main or proximal left anterior descending artery stent, history of in-stent thrombosis, intervention of the last patent vessel, left ventricular ejection fraction <35%, or complex 3-vessel disease) unless they can be switched to prasugrel, starting with a loading dose. Contraindications to prasugrel use include previous transient ischemic attack/stroke, age ≥75 years, and weight <60 kg.
Cangrelor is an intravenous direct-acting P2Y12 inhibitor that is rapidly inactivated by ADPase on the surface of vascular endothelial cells.11 Its metabolism does not involve CYP450 enzymes, and it is therefore safe to administer with NMVr.
Anticoagulants
Unfractionated and low-molecular-weight heparin are not metabolized by CYP450 enzymes and can be co-administered with NMVr. In contrast, warfarin is differently metabolized by various CYP450 enzymes. Its S-enantiomer is metabolized by CYP2C9, and its R-enantiomer is metabolized by CYP3A4 and CYP1A2.12 Ritonavir inhibits CYP3A4 but induces the other 2 enzymes. Because the initial predominant effect of ritonavir is CYP inhibition followed by CYP induction about a week later, it is more likely to result in a reduction in international normalized ratio (INR), owing to the short course of NMVr, warranting frequent INR monitoring.
Apixaban is a substrate of both CYP3A4 and P-gp, and the risk of bleeding increases when administered with NMVr. For patients on 10 mg or 5 mg twice daily dosing, the dose should be reduced by 50% when co-administered with ritonavir.8 , 10 For patients on 2.5 mg twice daily for extended venous thromboembolism (VTE) prophylaxis, it is reasonable to continue apixaban at the same dose with the expectation that this will likely achieve therapeutic levels of anticoagulation for 8 days after initiating NMVr owing to the interaction with ritonavir. For patients on 2.5 mg twice daily for atrial fibrillation (AF), case-by-case management is recommended. In those with a high thrombotic risk, apixaban should be withheld for 12 to 24 hours, followed by initiation of both NMVr and an alternative anticoagulant, such as enoxaparin, which should be continued for 3 additional days after NMVr treatment completion (total of 8 days) before switching back to apixaban.8 , 10
Metabolism and excretion of rivaroxaban involves CYP3A4, CYP2J2, and P-gp. The AUC of rivaroxaban over time increases to 153% while on ritonavir.13 Therefore, concomitant administration of rivaroxaban with NMVr should be avoided. If NMVr treatment is needed, rivaroxaban should be withheld for 24 to 36 hours based on its elimination half-life, after which both NMVr and an alternative anticoagulant (eg, enoxaparin) can be initiated and which should be continued for 3 additional days (total of 8 days) after completion of NMVr, before switching back to rivaroxaban.8 A physiologically based pharmacokinetics modeling study suggested that a decreased dose of 10 mg daily of rivaroxaban during NMVr treatment and 3 days after completion of treatment could ensure safe systemic levels of rivaroxaban. However, the risk of bleeding was noted to be higher in elderly patients and those with at least moderate renal impairment.14
Edoxaban is a substrate of P-gp. Ritonavir inhibits P-gp and can potentially increase the concentration of edoxaban.15 In the ENGAGE AF-TIMI 48 (Global Study to Assess the Safety and Effectiveness of Edoxaban [DU-176b] vs Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation-Thrombolysis In Myocardial Infarction 48; NCT00781391) study, edoxaban dose was reduced by 50% in patients on other P-gp inhibitors.15 However, ritonavir was not studied. For patients on 60 mg edoxaban daily for AF, the dose can be reduced by 50% for a total of 8 days from the start of NMVr. For those on 30 mg daily, switching to an alternative anticoagulant, such as enoxaparin, while taking NMVr is a viable option.8 When prescribed for acute or recent VTE, edoxaban should be stopped and enoxaparin started while on NMVr for a total of 8 days. Edoxaban can be restarted 3 days after completing NMVr.
Dabigatran is a P-gp substrate, and its active metabolite is a substrate of the multidrug and toxin extrusion protein 1 transporter,8 , 10 which mediates elimination of certain drugs through the apical membrane of hepatic and renal cells. Both of these transporters are inhibited by ritonavir. Dabigatran Cmax and AUC are increased by 133% and 95%, respectively, when co-administered with ritonavir.8 However, in the absence of renal dysfunction, no dose adjustment is required when dabigatran is co-administered with ritonavir.8 If the creatinine clearance (CrCl) is <30 mL/min, NMVr should not be given with dabigatran. For patients with AF and CrCl 30-50 mL/min, the dose can be decreased to 75 mg twice daily and continued for a total of 8 days.8 In those with CrCl >50 mL/min, the dose can be reduced to 110 mg twice daily when co-administered with NMVr; however, this dosage is not approved in the United States.
When direct oral anticoagulants cannot be interrupted or dose adjusted, NMVr should not be administered. Complete withdrawal of anticoagulation while on NMVr should be reserved for extremely low-risk patients (AF with CHA2DS2-VASc <3 and/or no history of stroke, VTE more than a year earlier, or no history of recurrent clots or severe thrombophilia [antiphospholipid antibody syndrome, homozygous factor V Leiden, etc]), knowing that COVID-19 infection can itself increase the risk of a thromboembolic event.
Lipid management
The interaction of statins with ritonavir has been extensively described in the context of antiretroviral therapy.16 In general, lipophilic statins (eg, simvastatin, atorvastatin, lovastatin) are metabolized by CYP3A4 and hydrophilic statins (eg, pravastatin, rosuvastatin) are not affected by CYP3A4.17 Exceptions are elaborated below.
Co-administration of ritonavir/saquinavir and simvastatin increases its 24-hour AUC by 3,059%.18 Therefore, NMVr should not be administered with simvastatin or lovastatin owing to risk of myopathy and rhabdomyolysis.18 They should be discontinued 12 hours before the initiation of NMVr and restarted 3 to 5 days after completion of the antiviral course.
Ritonavir/saquinavir increases atorvastatin exposure by 343% but atorvastatin activity increases by only 79%.18 Atorvastatin can be either discontinued or reduced to a maximum daily dose of 10 mg while on NMVr.7 , 8
Pitavastatin is metabolized primarily by glucuronidation. Although ritonavir induces glucuronidation and theoretically reduces pitavastatin levels, there is no significant interaction between pitavastatin and darunavir/ritonavir.19 Fluvastatin is metabolized by CYP2C9 and CYP2C8. While ritonavir induces CYP2C9, neither nirmatrelvir nor ritonavir affects CYP2C8.7 Because the metabolism of fluvastatin, pitavastatin, and pravastatin does not involve CYP3A4, they can be considered if a statin must be continued while on NMVr. Rosuvastatin is metabolized primarily by CYP2C9. Ritonavir induces CYP2C9 and therefore one would expect decreased plasma levels of rosuvastatin on co-administration of NMVr, but on the contrary, although the mechanism remains unclear, increased levels of rosuvastatin (up to 3-fold) have been reported with co-administration of ritonavir.18 Rosuvastatin can either be discontinued or if co-administered with NMVr, a dose higher than 10 mg daily should not be administered.18
Ezetimibe and fibrates are thought to be safe while on ritonavir.20 Monoclonal antibodies such as evolocumab and alirocumab and small interfering RNA–based therapeutics, such as inclisiran, are metabolized primarily by proteolysis in the liver and reticuloendothelial system and generally have no significant DDIs,21 making them safe to co-administer with NMVr.
Antianginal agents
Beta-blockers that are metabolized by the liver (metoprolol, propranolol, carvedilol) are CYP2D6 substrates,22 with metoprolol being highly dependent on the enzyme. However, this does not result in clinical DDIs when administered with ritonavir.22 Atenolol is excreted unchanged by the kidneys. Esmolol is metabolized in red blood cells. Therefore, concomitant use of CYP450 or P-gp inhibitors such as NMVr should not affect the efficacy of either. Glucuronidation is the major metabolic pathway for labetalol. By the induction of uridine diphosphate–glucuronosyltransferase (UGT), ritonavir could potentially decrease the concentration of labetalol.23 Because the effect of induction is delayed, coadministration with NMVr is considered to be safe with close monitoring of blood pressure.
Isosorbide dinitrate and isosorbide mononitrate are substrates of CYP3A4 leading to the formation of their active metabolite, nitric oxide.24 Their clinical effect may be diminished by the concomitant use of NMVr, but this has not been studied.24 No dose adjustment is required.
Ranolazine is a CYP3A4 and P-gp substrate.25 Its plasma concentration is exponentially increased in the presence of CYP450 inhibitors, thereby increasing the risk of QT prolongation and torsades de pointe. Coadministration of NMVr is, therefore, contraindicated.7 , 25
Heart failure therapy
Angiotensin-converting enzyme inhibitors, such as lisinopril, enalapril, quinapril, and captopril, are excreted unchanged in the urine, translating into no significant interactions with NMVr, making them safe to continue.23
As a weak inducer of CYP2C9, ritonavir could reduce the antihypertensive effects of irbesartan, which is oxidized by CYP2C9. In contrast, losartan is converted to its active metabolite via the CYP2C9 pathway,26 and coadministration with NMVr can lead to hypotension. However, because the inducing properties of ritonavir are delayed, no dose adjustment is advised.26 Weak inhibition of the hepatic uptake transporter OATP1B1 by NMVr may increase the concentration of both valsartan and the active metabolite of sacubitril, warranting close blood pressure monitoring.27 , 28 For this reason, one may consider stopping sacubitril/valsartan temporarily while on NMVr. Candesartan29 and olmesartan30 are excreted unaltered in the urine and do not interact with NMVr. Telmisartan is not metabolized by the CYP450 enzymes and therefore, has no interaction with NMVr.31 Azilsartan is primarily metabolized by CYP2C9. Ritonavir, as a weak inducer of CYP2C9, could theoretically reduce azilsartan plasma levels, but the clinical relevance is unknown.
Beta-blockers are covered under the antianginal section.
Spironolactone is metabolized by monooxygenases to sulfur-containing active metabolites.23 The lack of overlapping metabolic pathways makes interaction with NMVr unlikely. Conversely, eplerenone is primarily eliminated by CYP3A4,32 and concurrent NMVr administration can significantly increase the risk of hyperkalemia and therefore, is contraindicated (Central Illustration).
The metabolism of sodium-glucose cotransporter-2 inhibitors (SGLT2is) is dependent on UGT in the liver.33 Coadministration with UGT inducers such as ritonavir can decrease the plasma levels of all SGLT2is; however, this effect is clinically appreciated only with canagliflozin. Theoretically, the dose of canagliflozin should be increased from 100 to 300 mg for glycemic control when administered with ritonavir.33 However, given the short duration of NMVr, no dose adjustment is required. Empagliflozin is also a substrate of P-gp and OATP1B1, but clinically pertinent effects are not seen.34 Therefore, no dose adjustments are required for empagliflozin and, similarly, dapagliflozin.
Ivabradine is a CYP3A4 substrate. When given with NMVr, significant bradycardia is expected and therefore, ivabradine should be stopped before NMVr initiation; NMVr should be avoided if temporary discontinuation of ivabradine is deemed to be unsafe.35 Digoxin is a substrate of P-gp. Ritonavir decreases its renal and nonrenal clearance by 35% and 48%, respectively.36 Dose reduction by 30% to 50% or interruption of therapy, based on renal function along with close monitoring is recommended when co-administered with NMVr.7
Furosemide undergoes glucuronidation in the kidneys and liver, but is mostly excreted unchanged in urine. Hydrochlorothiazide, metolazone, and chlorthalidone also are excreted largely unchanged. They are considered safe to take with NMVr. Torsemide is metabolized mainly by CYP2C9. Although ritonavir is a weak inducer of CYP2C9, its delayed onset of induction warrants no dose adjustments for torsemide.23
Other antihypertensive agents
Amlodipine, nifedipine, and felodipine are metabolized by CYP3A4.37 Studies on DDIs between amlodipine and indinavir/ritonavir have shown an approximately 2-fold increase in amlodipine levels.37 A 50% reduction in the dose of amlodipine is recommended for 8 days when starting NMVr ,with close monitoring for side-effects such as hypotension, flushing, and edema, which may warrant temporary discontinuation until the inhibitory effect of ritonavir is fully attenuated (about 3 days after the last dose).7
Diltiazem and verapamil are metabolized by CYP3A4 and CYP2D6. Concomitant administration with indinavir/ritonavir increases the AUC of diltiazem by 26.5%.37 Careful monitoring for adverse effects such as bradycardia, dizziness, and hypotension that may warrant temporary discontinuation is recommended when given with NMVr.7
Alpha-blockers such as doxazosin and terazosin are metabolized by CYP3A4, and coadministration with NMVr can increase plasma concentration of the agents causing hypotension.38 Therefore, close monitoring or temporary discontinuation is recommended. Alfuzosin is contraindicated with NMVr owing to a risk of profound hypotension.7
Antiarrhythmic agents
Antiarrhythmic agents including amiodarone, dofetilide, dronedarone and quinidine are metabolized by CYP3A4. Flecainide is metabolized by CYP2D6. Propafenone is metabolized by CYP2D6 and, to a lesser extent, CYP3A4. Coadministration of ritonavir with these agents is contraindicated owing to an increased risk of life-threatening arrhythmias (Central Illustration).39 Sotalol is renally cleared and is safe to co-administer with NMVr.40 If NMVr therapy is deemed to be essential, agents such as dofetilide, propafenone, and quinidine, that have a relatively short half-life, can be withheld for 2.0 to 2.5 days (4 to 5 half-lives) before NMVr is initiated. However, this approach may be impractical owing to the risk of recurrent arrhythmias while therapy is interrupted, the need for hospitalization to restart certain antiarrhythmics, and the required time delay in initiating NMVr, which lowers its effectiveness. Drug interruption is not feasible with flecainide, dronedarone, or amiodarone, because the time to elimination of these agents is more than 5 days, beyond which NMVr may not be effective; therefore, NMVr should be avoided (Central Illustration).7
Pulmonary hypertension drugs
Among the endothelin receptor antagonists (ERAs), ambrisentan is safe to co-administer with NMVr. It is primarily metabolized by glucuronidation, and although it is also a substrate of OATP1B1, P-gp, and CYP3A4 to a lesser extent,41 no clinically relevant interactions are noted with ritonavir.41 The active metabolites of bosentan are formed via CYP2C9 and CYP3A4 and excreted in bile via OATP. Its concentration increases up to 48-fold when administrated with lopinavir/ritonavir.41 Therefore, concomitant use of bosentan and NMVr is contraindicated. Bosentan should be discontinued for at least 36 hours before initiating NMVr.7 Unlike other ERAs, hepatic uptake of macitentan is not dependent on OATP,42 but macitentan does inhibit CYP3A4, so, although not studied, the manufacturers recommend against co-administration of macitentan with strong CYP inhibitors such as ritonavir.41
Concomitant administration of phosphodiesterase-5 inhibitors such as sildenafil and tadalafil is contraindicated with NMVr. Ritonavir increases the AUC and Cmax of sildenafil by 11-fold and 3.9-fold, respectively, leading to prolonged erection, hypotension, blurred vision, and syncope.43 The AUC of tadalafil also is increased by 124% when administered with ritonavir.44
Prostacyclin analogs such as iloprost, treprostinil, and epoprostenol are considered safe to co-administer with NMVr. Iloprost is metabolized primarily by β-oxidation of the carboxyl side chain, and epoprostenol is rapidly hydrolyzed in blood and undergoes enzymatic degradation.41 Neither of them are CYP450 substrates. Treprostinil is metabolized by CYP2C8 and to a lesser degree CYP2C9.41 Because ritonavir inhibits CYP2C8 and induces CYP2C9, its overall effect on treprostinil is minimal.
The prostacyclin receptor agonist selexipag is metabolized by CYP3A4. However, based on studies on co-administration with lopinavir/ritonavir, the pharmacokinetics of selexipag is likely not affected to a clinical degree41 and it is, therefore, considered safe to administer with NMVr.
The soluble guanylate cyclase stimulator riociguat is metabolized by CYP3A4 and P-gp. Because of the possible interaction with ritonavir, it is recommended to reduce the dose of riociguat to 0.5 mg 3 times a day while on NMVr,41 with close monitoring for hypotension.
Anti-inflammatory agents
Colchicine, prescribed for pericarditis, is metabolized by CYP3A4 and P-gp and has biliary and renal excretion.45 Ritonavir increases the Cmax and AUC of colchicine by 2.7-fold and 3.5-fold, respectively, and therefore these agents should not be co-administered.45
Corticosteroids are recommended in some hospitalized patients with COVID-19 infection and is used in myocarditis. Dexamethasone is a CYP3A4 substrate. A 2.6-fold increase in its AUC during coadministration of azoles could potentially lead to Cushing’s syndrome and adrenal insufficiency.46 Expecting similar effects with NMVr, it is suggested to reduce the dose to 50% while taking NMVr and to resume full dose of dexamethasone 3 days after completion of NMVr. Similar effects are seen with methylprednisone and prednisone. The NMVr label recommends switching to prednisolone or beclomethasone, which have less interactions with NMVr.7 Overall, the incidence of side-effects such as Cushing’s syndrome is relatively low with a short duration of NMVr.7
Immunosuppressive therapy in heart transplant recipients
Tacrolimus and cyclosporine are frequently prescribed in heart transplant recipients. They are metabolized by CYP3A4. Tacrolimus is also a substrate of P-gp. Co-administration with ritonavir can lead to dangerously increased plasma levels of tacrolimus/cyclosporine.47 Lange et al47 recommended discontinuation of tacrolimus with a drug level assessment on day 3 while on NMVr. Frequent drug level monitoring was recommended for up to 2 weeks after completion of NMVr. For cyclosporine, they recommended empiric dose reduction to 80% of total daily dose and consideration of a once daily dosing.47 However, a substantial reduction in dosing and frequent monitoring of drug levels make the use of NMVr logistically difficult, and NMVr is therefore not recommended in these patients; alternative COVID-19 therapies should be considered (Central Illustration). The mammalian target of rapamycin inhibitor sirolimus is also a CYP3A4 substrate leading to toxic levels when co-administered with NMVr.48
Conclusions
We have described the many known and potential DDIs between various cardiovascular medications and NMVr. The importance of medication reconciliation before initiation of NMVr cannot be overemphasized to avoid serious DDIs. Dose adjustment or discontinuation of cardiovascular medications may be required for the duration of NMVr treatment and 3 to 5 days after completion. If co-administration of NMVr with certain cardiovascular medications is not advisable or if their temporary discontinuation is impractical, NMVr should be avoided and other treatments used. Awareness and availability of other COVID-19 therapies such as remdesivir, molnupiravir, and anti–SARS-COV-2 monoclonal antibodies enables clinicians to offer alternative treatment options to patients who are unable to take NMVr due to DDIs.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Listen to this manuscript's audio summary by Editor-in-Chief Dr Valentin Fuster onwww.jacc.org/journal/jacc.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.U.S. Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19
- 2.Hammond J., Leister-Tebbe H., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Najjar-Debbiny R, Gronich N, et al. Effectiveness of paxlovid in reducing severe covid-19 and mortality in high risk patients. Clin Infect Dis. Published online June 2, 2022. https://doi.org/10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed]
- 4.Wong CKH, Au ICH, et al. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among covid-19 inpatients during Hong Kong’s omicron BA.2 wave: an observational study. medRxiv. Lancet Infect Dis. Published online August 24, 2022. https://www.sciencedirect.com/science/article/pii/S1473309922005072?via%3Dihub [DOI] [PMC free article] [PubMed]
- 5.Ganatra S, Dani S, Ahmad J, et al. Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with covid-19. Clin Infect Dis. Published online August 20, 2022. https://doi.org/10.1093/cid/ciac673. [DOI] [PMC free article] [PubMed]
- 6.Yeh R.F., Gaver V.E., et al. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42(1):52–60. doi: 10.1097/01.qai.0000219774.20174.64. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration Fact sheet for healthcare providers: emergency use authorization for Paxlovid. https://www.fda.gov/media/155050/download
- 8.Liverpool Drug Interactions Group Interactions with essential medicines and nirmatrelvir/ritonavir. https://www.covid19-druginteractions.org/
- 9.Marsousi N., Daali Y., et al. Impact of boosted antiretroviral therapy on the pharmacokinetics and efficacy of clopidogrel and prasugrel active metabolites. Clin Pharmacokinet. 2018;57(10):1347–1354. doi: 10.1007/s40262-018-0637-6. [DOI] [PubMed] [Google Scholar]
- 10.Egan G., Hughes C.A., Ackman M.L. Drug interactions between antiplatelet or novel oral anticoagulant medications and antiretroviral medications. Ann Pharmacother. 2014;48(6):734–740. doi: 10.1177/1060028014523115. [DOI] [PubMed] [Google Scholar]
- 11.Ferri N., Corsini A., Bellosta S. Pharmacology of new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties. Drugs. 2013;73(15):1681–1709. doi: 10.1007/s40265-013-0126-z. [DOI] [PubMed] [Google Scholar]
- 12.Kaminsky L.S., Zhang Z.Y. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73(1):67–7412. doi: 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 13.Mueck W., Kubitza D., Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76(3):455–466. doi: 10.1111/bcp.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z., Chan E.C.Y. Physiologically-based pharmacokinetic modeling-guided dose management of oral anticoagulants when initiating nirmatrelvir/ritonavir (Paxlovid) for covid-19 treatment. Clin Pharmacol Ther. 2022;112(4):803–807. doi: 10.1002/cpt.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giugliano R.P., Ruff C.T., et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 16.Périard D., Telenti A., et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. Circulation. 1999;100(7):700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 17.Chastain D.B., Stover K.R., Riche D.M. Evidence-based review of statin use in patients with HIV on antiretroviral therapy. J Clin Transl Endocrinol. 2017;8:6–14. doi: 10.1016/j.jcte.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiggins B.S., Lamprecht D.G., Page R.L., 2nd, Saseen J.J. Recommendations for managing drug–drug interactions with statins and HIV medications. Am J Cardiovasc Drugs. 2017;17(5):375–389. doi: 10.1007/s40256-017-0222-7. [DOI] [PubMed] [Google Scholar]
- 19.Malvestutto C.D., Ma Q., et al. Lack of pharmacokinetic interactions between pitavastatin and efavirenz or darunavir/ritonavir. J Acquir Immune Defic Syndr. 2014;67(4):390–396. doi: 10.1097/QAI.0000000000000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon L.A., Malati C.Y., et al. Lack of an effect of ritonavir alone and lopinavir-ritonavir on the pharmacokinetics of fenofibric acid in healthy volunteers. Pharmacotherapy. 2016;36(1):49–56. doi: 10.1002/phar.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keizer R.J., Huitema A.D., et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Shin J., Johnson J.A. Pharmacogenetics of beta-blockers. Pharmacotherapy. 2007;27(6):874–887. doi: 10.1592/phco.27.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal S., Agarwal S.K. Lopinavir-ritonavir in SARS-CoV-2 infection and drug-drug interactions with cardioactive medications. Cardiovascular Drugs and Therapy. 2021;35(3):427–440. doi: 10.1007/s10557-020-07070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minamiyama Y., Takemura S., et al. Isoforms of CYPP450 on organic nitrate–derived nitric oxide release in human heart vessels. FEBS Lett. 1999;452(3):165–169. doi: 10.1016/s0014-5793(99)00612-2. [DOI] [PubMed] [Google Scholar]
- 25.Jerling M. Clinical pharmacokinetics of ranolazine. Clin Pharmacokinet. 2006;45(5):469–491. doi: 10.2165/00003088-200645050-00003. [DOI] [PubMed] [Google Scholar]
- 26.Fichtenbaum C.J., Gerber J.G. Interactions between antiretroviral drugs and drugs used for the therapy of the metabolic complications encountered during HIV infection. Clin Pharmacokinet. 2002;41(14):1195–1211. doi: 10.2165/00003088-200241140-00004. [DOI] [PubMed] [Google Scholar]
- 27.Annaert P., Ye Z.W., et al. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica. 2010;40(3):163–176. doi: 10.3109/00498250903509375. [DOI] [PubMed] [Google Scholar]
- 28.Hanna I., Alexander N., et al. Transport properties of valsartan, sacubitril and its active metabolite (LBQ657) as determinants of disposition. Xenobiotica. 2018;48(3):300–313. doi: 10.1080/00498254.2017.1295171. [DOI] [PubMed] [Google Scholar]
- 29.Gleiter C.H., Mörike K.E. Clinical pharmacokinetics of candesartan. Clin Pharmacokinet. 2002;41(1):7–17. doi: 10.2165/00003088-200241010-00002. [DOI] [PubMed] [Google Scholar]
- 30.Yoshihara K., Gao Y., Shiga H., et al. Population pharmacokinetics of olmesartan following oral administration of its prodrug, olmesartan medoxomil. Clin Pharmacokinet. 2005;44:1329–1342. doi: 10.2165/00003088-200544120-00011. [DOI] [PubMed] [Google Scholar]
- 31.Stangier J., Schmid J., Türck D., et al. Absorption, metabolism, and excretion of intravenously and orally administered telmisartan in healthy volunteers. J Clin Pharmacol. 2000;40(12 Pt 1):1312–1322. [PubMed] [Google Scholar]
- 32.Cook C.S., Berry L.M., et al. Involvement of CYP3A in the metabolism of eplerenone in humans and dogs: differential metabolism by CYP3A4 and CYP3A5. Drug Metab Dispos. 2002;30(12):1344–1351. doi: 10.1124/dmd.30.12.1344. [DOI] [PubMed] [Google Scholar]
- 33.Sarnoski-Brocavich S., Hilas O. Canagliflozin (invokana), a novel oral agent for type-2 diabetes. P T. 2013;38(11):656–666. [PMC free article] [PubMed] [Google Scholar]
- 34.Boehringer Ingelheim Pharmaceuticals Prescribing information: Jardiance (empagliflozin tablets), for oral use. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf
- 35.Tse S., Mazzola N. Ivabradine (corlanor) for heart failure: the first selective and specific If inhibitor. P T. 2015;40(12):810–814. [PMC free article] [PubMed] [Google Scholar]
- 36.Ding R., Tayrouz Y., et al. Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther. 2004;76(1):73–84. doi: 10.1016/j.clpt.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Hughes C.A., Tseng A., Cooper R. Managing drug interactions in HIV-infected adults with comorbid illness. CMAJ. 2015;187(1):36–43. doi: 10.1503/cmaj.131626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gervasoni C., Resnati C., et al. The relevance of drug–drug interactions in clinical practice: the case of concomitant boosted protease inhibitors plus alpha-1 blocker administration. Antivir Ther. 2018;23(5):467–469. doi: 10.3851/IMP3214. [DOI] [PubMed] [Google Scholar]
- 39.Pastori D., Mezzaroma I., et al. Atrial fibrillation and human immunodeficiency virus type-1 infection: a systematic review. Implications for anticoagulant and antiarrhythmic therapy. Br J Clin Pharmacol. 2019;85(3):508–515. doi: 10.1111/bcp.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Food and Drug Administration Betapace AF (sotalol HCl). NDA 09865. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021151s010lbl.pdf
- 41.Narechania S, Malesker MA. Drug interactions associated with therapies for pulmonary arterial hypertension. J Pharm Technol. Published online August 8, 2022. https://doi.org/10.1177/87551225221114001. [DOI] [PMC free article] [PubMed]
- 42.Treiber A., Aanismaa P., et al. Macitentan does not interfere with hepatic bile salt transport. J Pharmacol Exp Ther. 2014;350:130–143. doi: 10.1124/jpet.114.214106. [DOI] [PubMed] [Google Scholar]
- 43.Muirhead G.J., Wulff M.B., et al. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000;50(2):99–107. doi: 10.1046/j.1365-2125.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loulergue P., Gaillard R., Mir O. Interaction involving tadalafil and CYP3A4 inhibition by ritonavir. Scand J Infect Dis. 2011;43(3):239–240. doi: 10.3109/00365548.2010.526139. [DOI] [PubMed] [Google Scholar]
- 45.Terkeltaub R.A., Furst D.E., et al. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthrit Rheumat. 2011;63(8):2226–2237. doi: 10.1002/art.30389. [DOI] [PubMed] [Google Scholar]
- 46.Li M., Zhu L., et al. Assessment of drug-drug interactions between voriconazole and glucocorticoids. J Chemother. 2018;30(5):296–303. doi: 10.1080/1120009X.2018.1506693. [DOI] [PubMed] [Google Scholar]
- 47.Lange N.W., Salerno D.M., et al. Nirmatrelvir/ritonavir use: managing clinically significant drug-drug interactions with transplant immunosuppressants. Am J Transplant. 2022;22(7):1925–1926. doi: 10.1111/ajt.16955. [DOI] [PubMed] [Google Scholar]
- 48.Zijp T.R., Toren-Wielema M.L., et al. Important interactions of immunosuppressants with experimental therapies for novel coronavirus disease (covid-19) Ther Drug Monit. 2020;42(4):652–653. doi: 10.1097/FTD.0000000000000766. [DOI] [PubMed] [Google Scholar]