Abstract
Introduction
There is significant unmet need for patient-centric remote monitoring of visual function for chronic retinal diseases, as demonstrated by the COVID-19 pandemic. The Macustat® central retinal function scan is a novel cloud-based digital health application for remote monitoring. The aim of this study is to assess the efficacy of the Macustat® compared to traditional in-office retinal evaluations.
Materials and methods
Patients with underlying macular pathology underwent office-based retinal and visual acuity examinations and OCT macula imaging followed by remote tele-monitoring assessment with the Macustat. Central visual function was assessed with the multi-modal Macustat test using dynamic virtual Amsler grid testing, hyperacuity perimetry and visual acuity testing. The results were compared to the findings of the in-office comprehensive retina exam and OCT evaluation.
Results
The foveal acuity potential registered with the Macustat test showed high correlation with the office Snellen acuity potential 96% of eyes registered Macustat acuity within 0.2 LogMAR of office acuity measurement. In Wet AMD eyes with CNV pathology documented on OCT, the Macustat foveal function scan showed a corresponding abnormality in 89% of any CNV eyes and 100% of all visually significant CNV. In normal eyes without any visually significant edema or CNV, more than 92% showed corresponding normal retinal function scan.
Conclusion
The Macustat demonstrates high concordance with clinical findings using traditional diagnostic devices. Home monitoring with the Macustat® may offer complementary clinical utility as a telehealth tool for the assessment of visual acuity and macular function in patients at high risk for macular disease.
Keywords: telemedicine, remote monitoring, ophthalmology, retina, Macustat
Background
There is a significant unmet need for remote monitoring of visual function with home-based, patient-centric technologies for chronic retinal diseases. There are more than 11 million patients with AMD in the US alone and 170 million worldwide.1 Diabetes has become an epidemic with 28.5% of diabetic patients having some degree of diabetic retinopathy (DR) and 4.4% having vision-threatening diabetic retinopathy (VTDR). Globally, the number of people with DR is expected to affect up to 191.0 million diabetics by 2030, with about 30% (56.3 million) having VTDR.2,3 From a population health perspective, these chronic retinal diseases can benefit from remote home monitoring during the interval between office visits to improve early detection and timely treatment. This is especially true during times of crisis, as was seen during the height of the COVID-19 pandemic.
The macula measures 5–6 mm in diameter of the posterior retina and centrally contains the eye's highest density of cone photoreceptors, accounting for the eye’s greatest visual acuity and resolution.4 Macular function is impacted in up to 10% of diabetic patients where leakage from microaneurysms causes retinal edema.5 In addition, the macula is the anatomic location of choroidal neovascularization and retinal atrophy associated with exudative and non-exudative AMD, respectively. Changes in the normal retinal architecture caused by macular diseases can result in profound loss of visual function and are associated with functional defects such as metamorphopsia and scotomas.6 Macular disorders such as diabetic macular edema, age-related macular degeneration (AMD), macular holes and central serous retinopathy (CSR) cause visual distortion in their early stage.7,8 Metamorphopsia and scotomas significantly correlate with a patient's vision-related quality of life, as demonstrated by the 25-item National Eye Institute Visual Function Questionnaire and a simple 10-question metamorphopsia questionnaire.9,10
Studies have previously demonstrated the clinical utility of macular function tests such as the Amsler grid and M-chart, whose efficacy can reach close to 90% in the detection of metamorphopsia.10–15 In fact, daily macular function monitoring with conventional Amsler testing is recommended by the American Academy of Ophthalmology and the American Optometric Association. Even though no significant relationship has been found between the degree of metamorphopsia and visual acuity and they are independent of one another, the combined monitoring of both should provide additive and potentially synergistic efficacy for central retinal monitoring.16
Although there are few available technologies for home vision monitoring, recent prospective population health studies have demonstrated the high clinical utility of home monitoring of central retinal function for chronic conditions such as AMD.17 Home monitoring with preferential hyperacuity perimetry (PHP) testing of central retinal function showed that home monitoring plus standard care is capable of detecting the conversion of dry to wet AMD earlier than standard care alone in more than 1500 patients. Patients with home monitoring had a smaller average lesion size of CNV (0.23 DA vs. 0.7 DA) and a significantly higher percentage of these patients maintained 20/40 or better vision at the time of CNV detection (91–94% vs. 62%).18 Another study has also demonstrated the utility of macular function testing, using simple M-charts or perimetry to detect 50% of the patients with significant macular edema.19
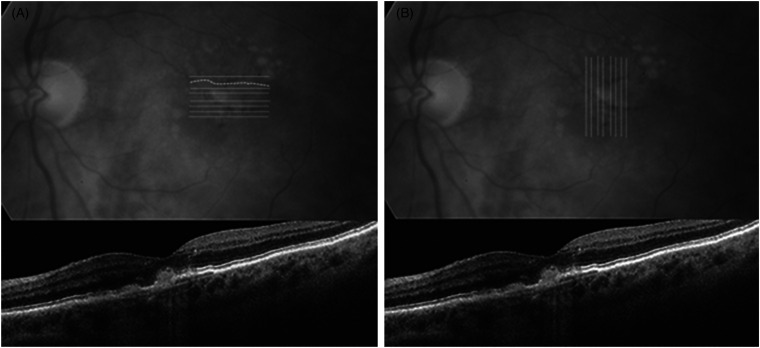
The Macustat® central retinal function scan is designed as an on-demand cloud-based digital health application for home monitoring that can be delivered to any web-connected device such as smartphone, tablet, laptop or desktop. The Macustat® hyperacuity testing uses both horizontal and vertical bi-linear scans of the central 10° (Figure 1) It is a 3-min multi-modal test with a foveal acuity test, dynamic photo-stress Amsler grid test and hyperacuity vernier central function scan to detect defects such as scotomas and metamorphopsia. Like Snellen visual acuity, hyperacuity reflects the eye's ability to see fine detail, but with much higher sensitivity. Hyperacuity testing is approximately 10 times more sensitive than Snellen visual acuity, with a range of 3–6 s of arc versus Snellen 30–60 s of arc. It is also less sensitive to age and blur which makes it particularly useful for macular function assessment.
Figure 1.
Macustat hyperacuity horizontal scan (A) and vertical scan (B) with corresponding OCT below the central 10° to detect subtle defects including metamorphopsia and scotomas.
This consecutive case-series study was designed to determine whether the multi-modal Macustat function retinal scan was able to detect clinically significant macular pathologies in patients at high risk for disease progression and to assess its feasibility for home monitoring and remote vision care for patients with chronic retina diseases.
Methods
A consecutive case series review of telehealth patient charts from two retina practices was performed. Patients had undergone central retinal function testing using the Macustat remote retina function scan test, which was self-administered and delivered on demand to patients’ smartphone, tablet and laptop (Figure 2) or desktop using a cloud-based interface. All subjects were all over 18 years of age, had visual acuity of 20/200 or better in at least one eye, were literate and had an updated distance and near eyeglasses prescription within the past 12 months to help ensure maximum best-corrected distance and near visual acuity potential.
Figure 2.
Macustat remote digital test interface on generic consumer devices (e.g. MacBook Air).
In the office, patients had undergone standard visual acuity testing using the Snellen test, as well as a full ophthalmologic retina eye examination with a slit lamp and fundoscopic assessment. In addition, all subjects had OCT imaging of the macula. The Macustat central retina function scan is a multi-modal test with four sequential steps:
Physiometric test of the central visual acuity using the Acustat®, a modified Rosenbaum digital acuity test of best-corrected near vision.
Dynamic virtual Amsler grid test from 0° to 5° of the central field with a photoreceptor super-saturation stress test.
Similar to Step 2 but extends the testing from 5° to 10° of the central field.
Hyperacuity central perimetry defect registration scans with horizontal and vertical components of the central 10°.
Data collection and analysis
Data for patients in both eyes were collected and are available in both Snellen and logMAR scales. For data analysis, the median, mean and standard deviation (SD) for visual acuity between Macustat and office Snellen were calculated in R using the median, mean and SD functions, respectively. All data analyses were performed in the software package GraphPad Prism 8.4.3 (GraphPad Software, La Jolla, CA). Figures were also created in GraphPad Prism.
Paired Student's t tests and correlations between Macustat visual acuity evaluations and office Snellen evaluations were assessed. Bland–Altman plots were used to compare the agreement between Macustat and in-office VA.
Two-way contingency tables presenting Macustat diagnostic accuracy of CNV compared to in-office physician diagnoses were constructed for overall Macustat, Macustat AmslerHD and Macustat Hyperperimetry. Two-sided Fisher's exact tests were performed to assess Macustat diagnostic accuracy and the Wilson/Brown method to calculate 95% confidence intervals was used to assess Macustat sensitivity, specificity and predictive values.
Results
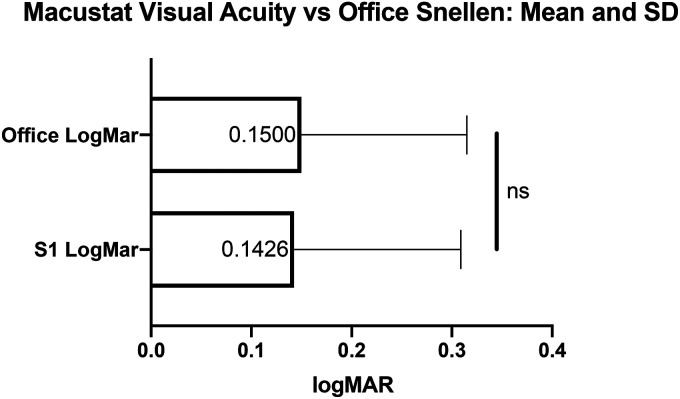
A total of 25 patients with 50 eyes were enrolled, 64% of subjects were female, and the mean age was 69.2 years. The top three diagnoses included AMD (72%), Myopic Degeneration (16%) and other (12%). All participants were literate with an educational level of high school or above. The foveal acuity potential registered with the Macustat test showed high agreement with the office Snellen acuity potential (logMAR Macustat 0.14 ± 0.17 vs. 0.15 ± 0.17 Snellen Office; Figure 3). A two-tailed paired Student's t test shows no statistical significance between the two valuation methods (p = 0.8997). In a scatterplot of Macustat versus office Snellen, a linear regression model with 95% confidence intervals revealed a strong correlation between Macustat logMAR and office Snellen logMAR (regression: Y = 0.7378X + 0.3950; R2 = 0.5707; Figure 4). The Bland–Altman plot of study eyes below showed that 95.7% of eyes had clinically significant agreement defined as ≤0.2 logMAR of difference from the mean difference. About 96% of eyes also demonstrated agreement within the 95% limits of agreement (−0.2278 to 0.2235 logMAR; Figure 5).
Figure 3.
Mean and SD of Macustat VA measurements (S1 logMAR) versus office Snellen (Office logMAR). Paired Student's t test reveals no significant difference between the means (p = 0.8997).
Figure 4.
Scatter plot of Macustat VA measurements (S1 logMAR) versus office Snellen (office logMAR) with simple linear regression model trendline and 95% confidence intervals. The darker data points represent greater overlapping data at a location.
Figure 5.
Bland–Altman plot of VA differences between Macustat versus office Snellen. Dotted lines represent 95% limits of agreement. The darker data points represent greater overlapping data at a location.
Table 1 presents two-way contingency tables of overall Macustat, Macustat Amsler HD (steps 2 and 3) and Macustat Hyperperimetry (step 4) diagnostic accuracies compared to physician diagnosis in the office. Fisher exact tests on each contingency table (Figure 6) reveal that overall Macustat and Macustat AmslerHD have a sensitivity of 1 while Macustat Hyperperimetry also has a high sensitivity of 0.8333. All three contingency tables have a high calculated specificity of 0.9231. The Macustat has a high positive predictive value of 0.75 (positive predictive values of individual components of the Macustat range between 0.7143 and 0.75) and an even higher negative predictive value of 1 (negative predictive values of individual components of the Macustat range between 0.96 and 1).
Table 1.
2 × 2 contingency tables of overall Macustat, Macustat Amsler HD and Macustat hyperperimetry versus actual physician diagnoses of CNV at the office (Total n = 32).
| MACUSTAT Results (n) | Visually Significant CNVa | Normal |
|---|---|---|
| MACUSTAT Abnormal (8) | 6 | 2 |
| MACUSTAT Normal (24) | 0 | 24 |
| AMSLER HD Abnormal (8) | 6 | 2 |
| AMSLER HD Normal (24) | 0 | 24 |
| MACUSTAT HYPERPERIM Abnormal (7) | 5 | 2 |
| MACUSTAT HYPERPERIM Normal (25) | 1 | 24 |
Visually significant CNV is any symptomatic CNV impacting central vision with BCVA worse than 20/25.
Figure 6.
The sensitivity, specificity and predictive values of overall Macustat, Macustat AmslerHD and Macustat hyperperimetry calculated using Fisher's exact test error bars show 95% confidence intervals calculated using the Wilson/Brown method.
Discussion
This is the first study to report on the clinical performance and feasibility of the FDA-registered Macustat multi-modal macular function scan as a home monitoring test of central retina function in a real-world retina practice setting. Our results demonstrate a high correlation between two measures of central retinal function and acuity potential. We found 96% of eyes registering a Best-Corrected Near Visual Acuity with the digitally modified Rosenbaum self-test within 0.2 logMAR of office Snellen acuity measurement.
These results demonstrate the potential feasibility of using digital near visual acuity testing as a surrogate and correlate of office BCVA. This correlation is important because remote home monitoring of visual function is difficult to perform at a distance of 20 feet as most consumer electronic devices in the home setting do not provide such a controlled environment to evaluate distance acuity. Near acuity on the other hand can be easily accessed on smartphones and tablets during daily activities such as reading and writing. The ability to measure visual potential equivalent to Snellen BCVA in the office through near digital acuity can enable not only remote diagnostics and monitoring but population digital health applications and e-screening.
Previous efforts to develop a near acuity digital home test as a surrogate of the office Snellen BCVA have been met with varying success. The Sightbook near acuity test showed a 7.7 mean letter difference from the office Snellen, but the variability and standard deviation were relatively large to provide useful clinical utility.20 The Bland–Altman plots of the difference between the SightBook and Snellen acuities showed that the disparity between SightBook and Snellen acuities was consistent across the range of acuities measured. Another study looked at an alternative near visual acuity tool, the Runge Card. The Runge card has been designed for administration in primary and urgent care settings. The Runge card uses Sloan letters of similar legibility like those utilized by ETDRS charts, a known standard for clinical research. The study evaluated the differences in visual acuity between the Runge and ETDRS, between Runge and Snellen and between ETDRS and Snellen testing in patients with various retinal pathologies, including AMD, DR, DME and others. The mean logMAR visual acuities were similar across the different measurements with Runge, ETDRS and Snellen, and were 0.66 ± 0.50, 0.59 ± 0.51 and 0.67 ± 0.62, respectively. The mean difference was 0.07 ± 0.20 logMAR for the Runge–ETDRS comparison, −0.01 ± 0.29 logMAR for Runge–Snellen and −0.07 ± 0.23 logMAR for ETDRS–Snellen. For the group overall, the Runge card appeared to correlate well with the ETDRS and similarly with Snellen.
The results of the Macustat modified e-Rosenbaum near vision assessment versus office Snellen are similar to the performance of a modified home distance ETDRS print chart performed at 5 feet.21 The mean office-based VA was 0.15 logMAR (SD 0.16), and the mean home VA was 0.17 logMAR (SD 0.15). The office-based VA was slightly better than the home vision though this was not statistically significant. Of the eyes tested, 91% had clinically significant agreement defined as ≤0.2 logMAR of difference from the mean difference and 60% of eyes had ≤0.1 logMAR of difference between the two methods.
The second and the third components of the Macustat are designed to detect, measure and quantify parafoveal and sub-foveal scotomas and metamorphopsia. The self-assessment algorithm of the digital Amsler grid scan was successful in detecting all patients with visually significant CNV (N6) and had a false-positive rate of 7.7%. In the two subjects with false-positive results, one had an epiretinal membrane and the other had moderate dry AMD, both confounding macular pathologies. These results are consistent with the experience from prior studies of Amsler gird and M-charts whose sensitivity for the detection of metamorphopsia can reach 90%.10–13 A unique aspect of the Macustat test is the hyperacuity vernier perimetry scan which is designed not only to detect scotomas and metamorphopsias but also to quantify and localize their extent.
Remote monitoring of central retinal function with the Macustat scan demonstrates a high degree of clinical and technical feasibility on ubiquitous consumer electronic peripherals without the need for specialized hardware and correlates well with office-based retinal exams and testing. Limitations of this study are its small sample size, its non-randomized design and lack of longitudinal performance assessment over time. It was also limited by the scope to patients with relatively mild disease and high-to-mid level visual acuity that will need further validation in a larger cohort with a greater range of baseline acuity and central retinal function. In addition, this study compared the Macustat visual acuity self-assessment to Snellen Visual Acuity in the office, rather than ETDRS which could be a point for further investigation. Even though ETDRS visual acuity is preferred over Snellen visual acuity in clinical studies, Snellen remains the primary method for VA testing in clinical practice. In addition, at visual acuity levels of 20/200 or better, Snellen and ETDRS correlate well.22 Despite this, a parallel comparison to ETDRS would be informative in the future. Further investigations will need to validate the digital macular function scan for large-scale population health applications in order to determine its clinical efficacy for disease monitoring, screening and early detection.
Remote self-monitoring of retinal function using on-demand ubiquitous digital health applications offers significant advantages to extend the clinical practice outreach beyond the medical office into the patients home. This could impact the disease progression and detection curve of some of the leading causes of blindness, especially age-related macular degeneration and DR. This study demonstrated the feasibility of a remote retina function scan using a multi-modal retinal function scan and further studies are needed to validate its clinical and public health utility.
Conclusion
This is the first study on the Macustat retina function scan and one of the first remote monitoring studies in ophthalmology using digital health devices. It can have a significant impact on population health and chronic disease management, particularly during the COVID-19 pandemic whereby many patients with AMD, diabetic retinopathy and other retina diseases cannot get access to care and need home monitoring.
Footnotes
Author's note: Earnest Chen, Gentile Retina Practice, New York City, NY, USA and Ophthalmology, New York Eye and Ear of Mount Sinai, New York, NY, USA.
Contributorship: All authors contributed substantially to the design of the study and the review of the data and the writing of the manuscript. Dr. RCG contributed clinical data from his practice.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Sean Ianchulev and Dr. Peter Pham are inventors and patent application holders. Dr. Jasper is a consultant for KYS Vision.
Ethical approval: No ethical approval – this is an anonymized chart review study.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Supported by the clinic and staff of Dr. Gentile.
Guarantor: EC.
ORCID iD: Sean Ianchulev https://orcid.org/0000-0002-9893-5909
References
- 1.Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vision (London, England) 2016; 3: 34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempen JH, O’Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004; 122: 552–563. [DOI] [PubMed] [Google Scholar]
- 4.Provis JM, Penfold PL, Cornish EE, et al. Anatomy and development of the macula: specialisation and the vulnerability to macular degeneration. Clin Exp Optom 2005; 88: 269–281. [DOI] [PubMed] [Google Scholar]
- 5.Rahman R, Stephenson J. Early surgery for epiretinal membrane preserves more vision for patients. Eye (London, England) 2014; 28: 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MM, Brown GC, Sharma S, et al. The burden of age-related macular degeneration: a value-based analysis. Curr Opin Ophthalmol 2006; 17: 257–266. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004; 122: 564–572. 10.1001/archopht.122.4.564 [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol 2008; 86: 126–145. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda S, Okamoto F, Yuasa M, et al. Vision-related quality of life and visual function in patients undergoing vitrectomy, gas tamponade and cataract surgery for macular hole. Br J Ophthalmol 2009; 93: 1595–1599. [DOI] [PubMed] [Google Scholar]
- 10.Arimura E, Matsumoto C, Nomoto H, et al. Correlations between M-CHARTS and PHP findings and subjective perception of metamorphopsia in patients with macular diseases. Invest Ophthalmol Visual Sci 2011; 52: 128–135. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto F, Sugiura Y, Okamoto Y, et al. Associations between metamorphopsia and foveal microstructure in patients with epiretinal membrane. Invest Ophthalmol Visual Sci 2012; 53: 6770–6775. [DOI] [PubMed] [Google Scholar]
- 12.Nowomiejska K, Oleszczuk A, Brzozowska A, et al. M-charts as a tool for quantifying metamorphopsia in age-related macular degeneration treated with the bevacizumab injections. BMC Ophthalmol 2013; 13: 13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achiron A, Lagstein O, Glick M, et al. Quantifying metamorphopsia in patients with diabetic macular oedema and other macular abnormalities. Acta Ophthalmol 2015; 93: e649–e653. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto C, Arimura E, Okuyama S, et al. Quantification of metamorphopsia in patients with epiretinal membranes. Invest Ophthalmol Visual Sci 2003; 44: 4012–4016. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa T, Harino S, Iwahashi Y. [Quantification of metamorphopsia in the course of branch retinal vein occlusion with M-CHARTS] Nippon Ganka Gakkai Zasshi 2007; 111: 331–335. [PubMed] [Google Scholar]
- 16.Matsumoto C, Tsuboi S, Okuyama S, et al. Otori T: quantification of metamorphopsia. Method of evaluation. Rinsho Ganka 1990; 44: 271–274. [Google Scholar]
- 17.Faes L, Bachmann LM, Sim DA. Home monitoring as a useful extension of modern tele-ophthalmology. Eye (London, England) 2020; 34: 1950–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AREDS2-HOME Study Research Group, Chew EY, Clemons TE, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the eye (HOME) study. Ophthalmology 2014; 121: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinowska A, Nowomiejska K, Brzozowska A, et al. Metamorphopsia score and central visual field outcomes in diabetic cystoid macular edema. BioMed Res Int, 2018; 2018: 4954532. 10.1155/2018/4954532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phung L, Gregori NZ, Ortiz A, et al. Reproducibility and comparison of visual acuity obtained with sightbook mobile application to near card and Snellen chart. Retina (Philadelphia, PA) 2016; 36: 1009–1020. [DOI] [PubMed] [Google Scholar]
- 21.Chen TA, Li J, Schallhorn JM, et al. Comparing a home vision self-assessment test to office-based Snellen visual acuity. Clin Ophthalm (Auckland, N.Z.) 2021; 15: 3205–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser PK. Prospective evaluation of visual acuity assessment: a comparison of Snellen versus ETDRS charts in clinical practice (an AOS thesis). Trans Am Ophthalmol Soc 2009; 107: 311–324. [PMC free article] [PubMed] [Google Scholar]