Abstract
Background and Objects: Bruton's tyrosine kinase inhibitors are commonly used and effective for lymphoma and chronic lymphocytic leukemia (CLL). Ibrutinib might improve the effect of anti-cluster of differentiation 19 (CD19) chimeric antigen receptor (CD19 CAR) T-cell therapy in lymphoma, but the effects of zanubrutinib combined with CAR-T cells is unclear. Methods: We selected a low effect-target ratio (E:T = 1:3) to study this synergistic effect in vitro. The programed cell death protein 1 (PD-1) expression in CD19 CAR-T cells and immune phenotype of T lymphocytes were analyzed by flow cytometry (FCM). We selected CD19 CAR-T cells of a patient with diffuse large B cell lymphoma (DLBCL) to study the synergistic effect of zanubrutinib with CAR-T cells by bioluminescence imaging monitoring. The CD19 CAR-T cells expansion in mice was compared by FCM. Results: Zanubrutinib and ibrutinib had dose-dependent toxicity on both CAR-T cells and lymphoma cells. But there was no significant synergistic effect of the CD19 CAR-T cells combined with zanubrutinib/ibrutinib in vitro. The PD-1 expression in CD19 CAR-T cells increased when the CD19 CAR-T cells were co-cultured with Raji cells and decreased when ibrutinib was added in culture, but zanubrutinib had no such effect. The extinction of luciferase expression was more obvious in the polytherapy group of ibrutinib and CD19 CAR-T cell than that in the other groups. Moreover, the proportion of CAR-T cells in the combination therapy group of CD19 CAR-T cells and ibrutinib was higher than that of the polytherapy group of CD19 CAR-T cells with zanubrutinib group. The synergistic effect could be observed obviously in mice receiving ibrutinib combined with CD19 CAR-T cells. But zanubrutinib cannot perform joint therapy effect either in vitro or in mice. Conclusion: Zanubrutinib might have no joint therapy effect with CD19 CAR-T cells neither in vitro nor in mice, but the mechanism of different curative effects requires our further research and exploration.
Keywords: Bruton tyrosine kinase, zanubrutinib, chimeric antigen receptor T-cell, lymphoma
Introduction
As a key mediator of B-cell receptor (BCR)-dependent cell growth, Bruton's tyrosine kinase (BTK) is involved in all aspects of B-cell development, such as proliferation, apoptosis, and cell migration.1 Therefore, BTK inhibitor have been enrolled in the treatment of various B-cell malignancies. Ibrutinib as a BTK inhibitor is currently approved for the treatment of chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL).2,3 Compared with ibrutinib, zanubrutinib was designed to be a pure BTK inhibitor for B-cell malignancies which has more target occupancy and less off-target binding.4 In vitro, zanubrutinib has similar potency to ibrutinib at inhibiting BTK but approximate 20-times less potent than ibrutinib at inhibiting interleukin-2-inducible tyrosine kinase (ITK).5 Although the efficacy of BTK inhibition as a single agent therapy is remarkable, its resistance might develop and could not be ignored.6
Chimeric antigen receptor (CAR) T-cell therapy is a highly effective salvage treatment in various of B-cell malignancies, especially for relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL). However, complete response (CR) rates of cluster of differentiation 19 (CD19) CAR-T cell therapy in R/R B-cell non-Hodgkin lymphoma (B-NHL) patients were lower than that of in R/R B-ALL patient.7,8 The presence of bulky masses might hinder the infiltration of T lymphocyte and impair the antitumor activity of T lymphocyte.9 Additionally, an abnormal tumor microenvironment (TME) which is characterized by dysfunctional blood vessels that hinder the delivery of immunotherapeutic agents and one that causes immunosuppression might also reduce the effectiveness of CD19 CAR-T cell therapy.10 Moreover, the efficacy of CD19 CAR-T cell therapy for CLL was not satisfactory owing to the dysfunction of T-cells.11 In a study before, long-term ibrutinib treatment might limit or reverse the dysfunction of T cells and CD19 CAR-T cells in CLL. A similar synergistic effect of ibrutinib and CD19 CAR-T cells was reported in MCL models.12
Our previous findings suggest that ibrutinib might improve the efficacy of CD19 CAR-T on B-NHL.13,14 Whether zanubrutinib combined with CD19 CAR-T cells has the same synergistic effect is not clear. In our study, we selected the same CD19 CAR-T cells of R/R B-NHL patients who failed in his CD19 CAR-T-cell therapy as our previous study.13 We did not observe the significant synergistic effect of CD19 CAR-T cells combined with zanubrutinib in vivo and in vitro.
Materials and Methods
Pharmacologic Agents and Cell Lines
Ibrutinib was provided by Xian Janssen pharmaceutical co. Ltd Zanubrutinib was provided by Bei Gene (Beijing, PR China). Reagents were dissolved in dimethyl sulfoxide (DMSO) and stored at − 20 °C. Raji cell line, EHEB cell line and JEKO-1 cell line (American Type Culture Collection, ATCC, Manassas, VA, USA) were cultured in RPMI-1640 medium (Gibco, Thermo Fisher Scientific, Inc. Waltham, MA, USA) containing 10% fetal bovine serum (FBS) (Gibco) and 50 UI/mL penicillin/streptomycin (Gibco, Life Technologies). The human embryonic kidney 293 (Lenti-X-293T) cells (ATCC) were maintained in Dulbecco's Modified Eagle Medium (Sigma-Aldrich, USA) and supplemented with 10% FBS and 50 UI/mL penicillin/streptomycin.
Source of T Cells for CD19 CAR-T Cell Therapy
Seven patients (Male: female = 4:3, Age:25-68 years) were enrolled in a clinical trial at the Department of Hematology in Tianjin First Center Hospital (Tianjin, China) and received CD19 CAR-T cell expressing anti-CD19 scFv and 4-1BB-CD3ζ costimulatory-activation domains therapy (ChiCTR1800018059). The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Medical Ethics Committee of the Department of Hematology, Tianjin First Center Hospital (Tianjin, China) (Approval number: 2018N105KY). All the patients or their representatives provided informed consent before enrollment. They agreed with the use of their specimens and data for our study. Generation and detection of CD19 CAR-T cells has been described in detail in our previous study.13
The Proliferation of CD19 CAR-T Cells and the Cell Lines
CD19 CAR-T cell (2 × 104) or cell lines (1 × 104) were treated with various doses (0 µM, 1 µM, 15 µM) of ibrutinib or zanubrutinib for 0, 24, and 48 h in the absence of supplemented cytokines in 96-well plates. The proliferations of different cells were determined by Cell Counting Kit-8 (Dojindo Molecular Technologies Inc., Kumamoto, Japan). The absorbances were measured at 450 nm with an enzyme standard instrument at 0, 24, and 48 h. Each triplicate experiment was performed no less than 3 times to construct the cell survival curve.
Annexin V/propidium iodide Apoptosis Assay
Raji cells and CD19 CAR-T cells were co-cultured with zanubrutinib/ibrutinib at 5 µM for 24 h, 48 h. The dosage of zanubrutinib was based on one of our previous studies.13 Apoptosis was analyzed by flow cytometry (FCM). BGB-treated cells were fixed and stained with Annexin V and propidium iodide (Abcam, Cambridge, UK).
The Expression of Programed Cell Death Protein 1 on CD19 CAR-T Cells and Programed Cell Death 1 Ligand 1 on Raji Cells
In the preparation of CD19 CAR-T cells, the expression of programed cell death protein 1 (PD-1) in CD19 CAR-T cells was analyzed by FCM. When the CD19 CAR-T cells were harvested, the expression of PD-1 in CD19 CAR-T cells was analyzed in the co-culture system of CD19 CAR-T cells, Raji cells, and with/without zanubrutinib/ibrutinib (5 µM) at 24 h and 48 h.
The Cytotoxicity of CD19 CAR-T Cells to Cell Lines
The cytotoxicity was carried out with the effective target ratio of 1:3 for 24 and 48 h in the absence of supplemented cytokines in 96-well plates. The concentration of zanubrutinib at 5 µM was combined with CD19 CAR-T cells respectively. As controls, targets (1.5 × 104 cells per well) and effectors (0.5 × 104 cells per well) alone were simultaneously incubated to determine spontaneous cell death. Cytotoxicity of CD19 CAR-T cells combined with zanubrutinib was detected using an LDH cytotoxicity test kit (Dojindo Molecular Technologies, Inc.) at 490 nm at 0, 24, and 48 h.
Immune Phenotype of T Lymphocyte Assay
After cultured with/without zanubrutinib/ibrutinib at 5 µM for 72 h, the PBMCs were isolated by Ficoll density gradient centrifugation and analyzed by FCM. Cells were stained for surface markers for 15 min at room temperature. The dilution of these antibodies was performed according to the instructions. The T lymphocyte functions were analyzed using anti-CD4-FITC, CD3-Percp, CD8-APCCY7, CXCR3-PE, CCR4-BV421, CD62L-APC, CCR7-PE, CCR6-PC7, CD45RO-PC7, and CD45RA-V500. We evaluated the percentage and absolute numbers of T cells of various subsets. The T cell subsets we examined include naive T cells (45RA + 62L + CCR7 + ), Central Memory T Cell (T-CM, 45RA-62L + CCR7 + ), Effector Memory T Cell (T-EM, 45RA-62L-CCR7-), T-helper 1 (Th1) (CD3 + CXCR3+CCR4-), Th2 (CCR4 + CXCR3-CCR6-), T-helper 17 (Th17) (CCR4 + CXCR3-CCR6 + ).
Raji Xenograft Model
This study was carried out in parallel with the previous research.13 Six-week-old female CAnN.Cg-Foxn1nu/CrlVR (BALB/c) mice weighing 20.12 ± 1.45 g (n = 25, Beijing Viton Lihua Experimental Animal Technology Co, Ltd, Beijing, China) received 1 × 107 Raji cells transduced with luciferase (purchased from Shanghai Super Biotechnology Co.) by subcutaneous injection. Following bioluminescence imaging monitoring once a week, the mice were confirmed of tumor engraftment after 25 days. Then the mice were randomized into zanubrutinib (2.5 mg/kg, twice a day) monotherapy group, ibrutinib (8 mg/kg, once a day) monotherapy group, CD19 CAR-T cell (2 × 107/kg) monotherapy group, zanubrutinib combined with CD19 CAR-T cell group and ibrutinib combined with CD19 CAR-T cell group. The CD19 CAR-T cells generated from a germinal center B-cell (GCB) diffuse large B cell lymphoma (DLBCL) patient who suffered from CD19 CAR-T cell therapy failure. Zanubrutinib and ibrutinib were administered daily by oral gavage. On 0, 14 and 28 days, mice were monitored with bioluminescent imaging and tumor volume measuring for disease progression. Mice were anesthetized via a nose cone with 2% isoflurane (Zoetis, UK)/medical oxygen and maintained under inhalational anesthesia, following intraperitoneal injection with D-luciferin (Goldbio, 150 mg/kg) 10 min before scanning. ALL mice were cared for during the study in accordance with the Guide for the Care and Use of Laboratory Animals, eighth Edition.15 All mice were sacrificed through broken neck method when either experimental or humane endpoints were reached. The reporting of this study conforms to ARRIVE 2.0 guidelines.16 All mice were sacrificed through broken neck when either experimental or human endpoints were reached.
Change of the Proportion of CD19 CAR-T Cells in Mice
The proportions of CD19 CAR-T cells in mice were analyzed by FCM in inner canthus blood from the mice of CD19 CAR-T cell group and anubrutinib/ibrutinib combined with CD19 CAR-T cell group on 0, 7, 14 and 28 days.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as the mean ± Standard Deviation (SD) or standard errors of the mean (SEM). When appropriate, t test and 2-way ANOVA of variance were used to compare experimental groups. A difference was considered significant if P-value <.05.
Results
Transduction Efficiency of CD19 CAR-T Cells
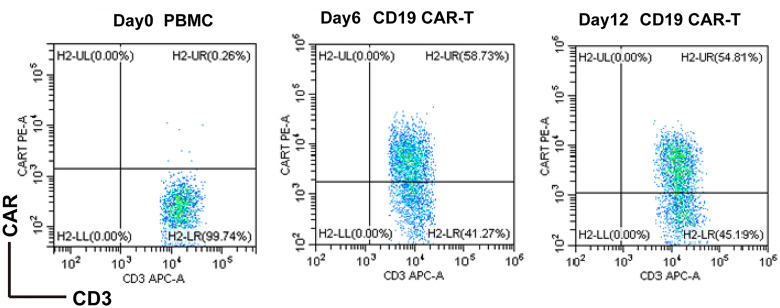
The titer of anti-CD19-CAR virus was 3 × 108 TU/ml. The mean anti-CD19-CAR transduction efficiency of the 7 relapse/refractory B-cell lymphoma patients was 58.62 ± 6.18%. While, the anti-CD19-CAR transduction efficiency of the CD19 CAR-T cell using in mouse model was 48.28%. We presented the FACS data of Patient #1 (Figure 1).
Figure 1.
FACS quantitative results of CD19 CAR-T cells of patient#1, the positive rate on the 12th day (d12) after transfection reached 54.81%. Abbreviations: CD19, cluster of differentiation 19; CAR-T, chimeric antigen receptor T cell.
The Effect of Zanubrutinib/Ibrutinib on the Proliferation of Lymphoma Cell Lines and CD19 CAR-T Cells
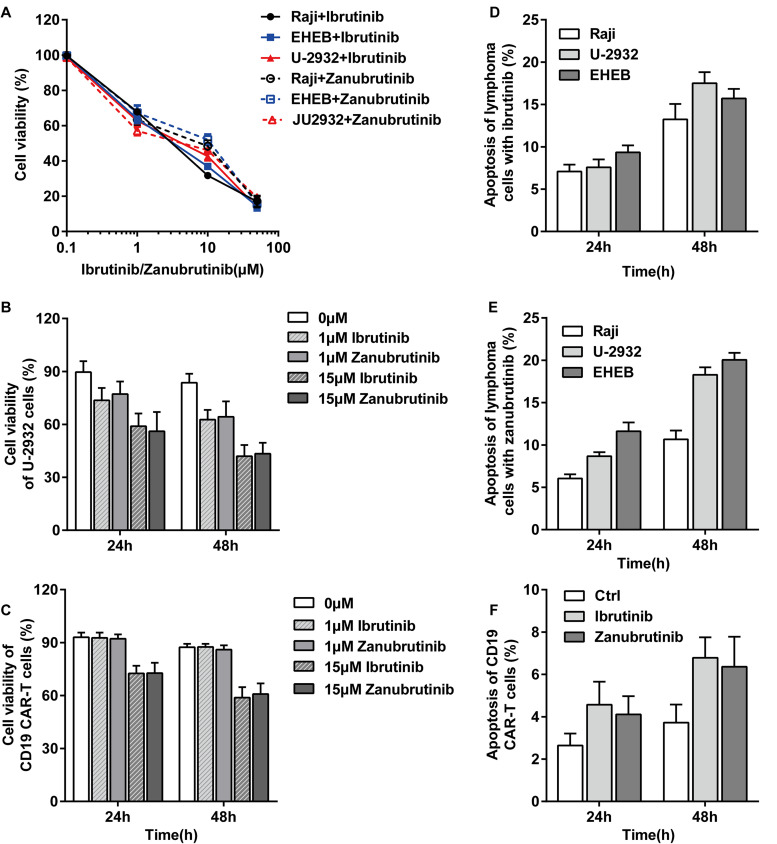
We selected 3 lymphoma cell lines to observe whether different lymphoma subsets perform the dependent effect of zanubrutinib or ibrutinib. Proliferations of the 3 lymphoma cells were inhibited by zanubrutinib/ ibrutinib for 48 h of co-culture. The inhibition of proliferations was in a dose-dependent manner of the 2 BTK inhibitors (Figure 2A). We compared the ability of the 2 agents to inhibit Raji cells and CD19 CAR-T cells proliferation. Inhibition to cell proliferation was readily detectable following 24 h, 48 h incubation at 0 µM, 1 µM, and 15 µM. The cytotoxicity to Raji cells at a different dose of zanubrutinib was similar to ibrutinib (Figure 2B). Furthermore, the 2 BTK inhibitors performed slight inhibition in CD19 CAR-T cells at low concentrations and obvious suppression at high concentrations (Figure 2C).
Figure 2.
(A) Ibrutinib and zanubrutinib inhibited cell proliferation in a dose-dependent manner. (B-C) Ibrutinib and zanubrutinib were strongly cytostatic toward Raji cell, and the 2 BTK inhibitors performed slight inhibition in CD19 CAR-T cells at low concentration but obvious suppression at high concentration. (D-E) Lymphoma cell lines exhibited dose-dependent increases in apoptosis by ibrutinib and zanubrutinib. (F) The 2 BTK inhibitors induced slight apoptosis in CD19 CAR-T cell. Abbreviations: CD19, cluster of differentiation 19; CAR-T, chimeric antigen receptor T cell; BTK, Bruton's tyrosine kinase
Apoptosis of Raji Cells Induced by Zanubrutinib and Ibrutinib
Lymphoma cells exhibited time-dependent increases in apoptosis with a certain dose ibrutinib, suggesting that apoptosis might partially underlie the observed decrease in cell proliferation (Figure 2D). Similarly, zanubrutinib-induced apoptosis in the 3 lymphoma cell lines (Figure 2E). Although ibrutinib and zanubrutinib-inhibited proliferation of CD19 CAR-T cell, the 2 BTK inhibitors could not induce apoptosis in CD19 CAR-T cells obviously (Figure 2F). We observed that the 3 lymphoma cells are similarly sensitive to the 2 BTK inhibitors, and we selected Raji cell for the subsequent experiments in vivo and in vitro.
The PD-1 Expression in CD19 CAR-T Cells Effected by the 2 BTK Inhibitors
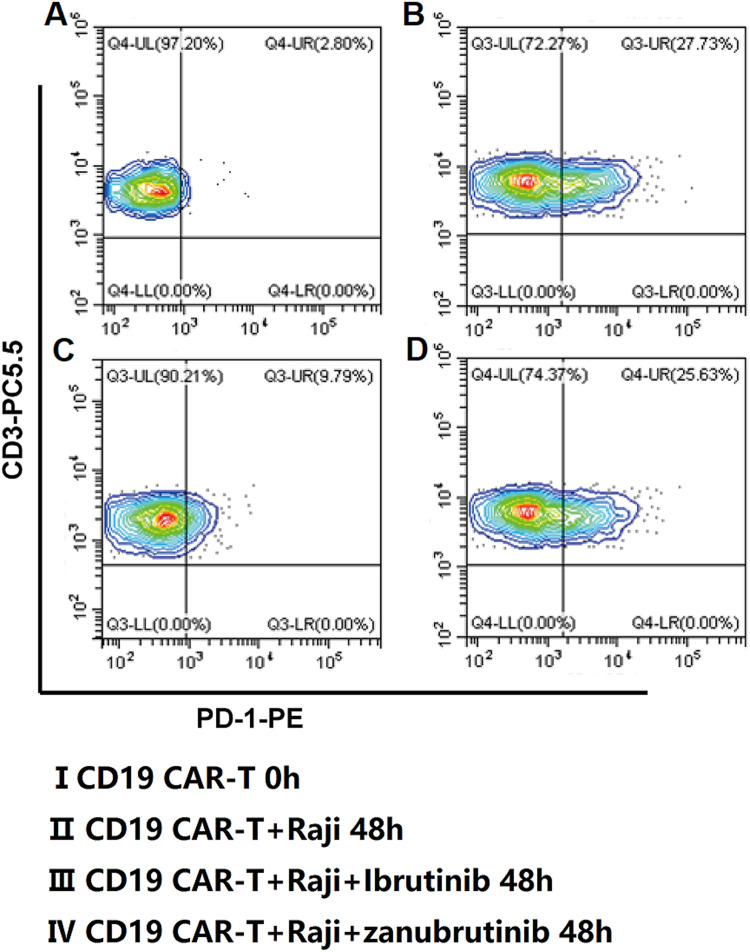
The mean expression of PD-1 in CD19 CAR-T cells of the patients was 1.53 ± 0.69% on the harvest day. The PD-1 expression in CD19 CAR-T cells increased when the CD19 CAR-T cells were co-cultured with Raji cells. While the PD-1 expression in CD19 CAR-T cells decreased when ibrutinib was added in the culture system. With the prolongation of co-culture time, this effect was weakened gradually. But this phenomenon could not be observed in the zanubrutinib groups (Figures 3 and 4A).
Figure 3.
Representative FACS plots of PD-1 expression changes in CD19 CAR-T cells. Abbreviations: CD19, cluster of differentiation 19; CAR-T, chimeric antigen receptor T cell; PD-1, programed cell death protein 1
Figure 4.
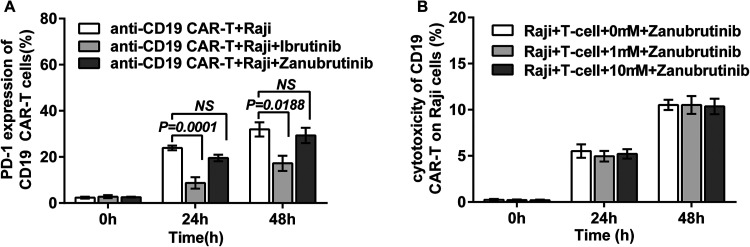
(A) The PD-1 expression on CD19 CAR-T cells obviously increased when co-cultured with U-2932 cells, the PD-1 expression can’t be decreased when ibrutinib added in the culture system. But zanubrutinib can’t reduce the PD-1 expression increase. (B) There was no combined effect of zanubrutinib with CD19 CAR-T cell on cytotoxicity in Raji cell in vitro. Abbreviations: CD19, cluster of differentiation 19; CAR-T, chimeric antigen receptor T cell; PD-1, programed cell death protein 1
The Synergistic Effect of the CD19 CAR-T Cells Combined With Zanubrutinib on Raji Cells in Vitro
The cytotoxicity of CD19 CAR-T cells combined with zanubrutinib at 24 h and 48 h after co-culture with Raji cells was analyzed. We selected a relatively low effect-target ratio (E:T = 1:3) in our study in vitro. There was no synergistic effect of the CD19 CAR-T cells combined with zanubrutinib in vitro (Figure 4B).
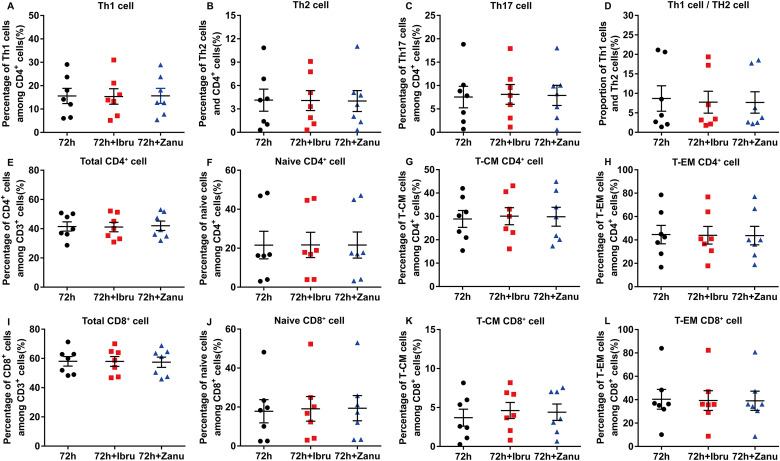
Changes of T Lymphocyte Subsets Affected by Ibrutinib/Zanubrutinib in Vitro
We evaluated the percentage of the various subsets in T cells cultured with/without ibrutinib or zanubrutinib for 72 h among the 7 patients. The CD3+CD8+CD4– and CD3+CD4+CD8– T cell expression in peripheral blood was detected using FCM methods. There was no difference between the percentage of the Th1 cell, Th2 cell, naive T cells, effector T cells, central memory T cells and effector memory T cells under the influence of ibrutinib or zanubrutinib in vitro (Figure 5).
Figure 5.
There was no difference between the percentage of the Th1 cell, Th2 cell, Th17 cell and the proportion of Th1 cell and Th2 cell after incubation with ibrutinib or zanubrutinb for 72 h. Similarly, the 2 BTK inhibitors played no any positive or negative effect on naïve cell, central memory T cells, effector memory T cells and other T cell subsets. Th1, T-helper 1; Th2, T-helper 2 ;Th17, T-helper 17; BTK, Bruton's tyrosine kinase
Synergistic Effect of the CD19 CAR-T Cells Combined With the Zanubrutinib on Raji Cells in Vivo
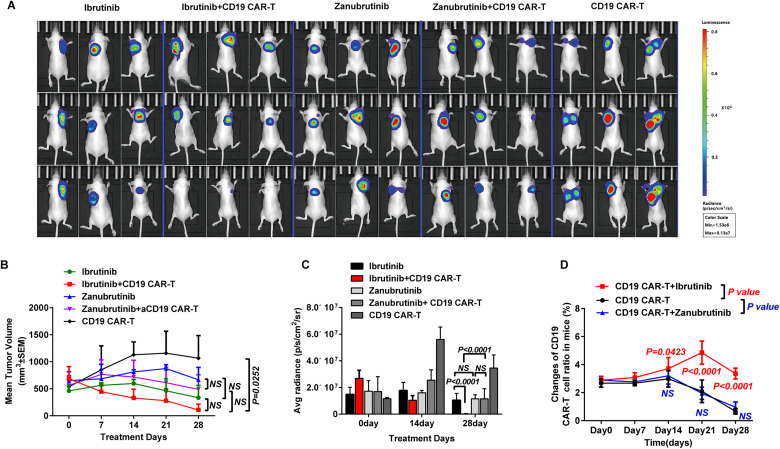
The effects of zanubrutinib combined with CD19 CAR-T cells in vivo were evaluated using the subcutaneous Raji cell xenogeneic tumor model. In this study, the single agent zanubrutinib/ibrutinib, CD19 CAR-T cell, and CD19 CAR-T cell combined with zanubrutinib group mice presented no detectable adverse effects, only the CD19 CAR-T cell combined with ibrutinib group mice presented satisfactory treatment effect (Figure 6A). The BTKi or CD19 CAR-T cell alone could not inhibit tumor development, but the reduction of tumor size was greater in the polytherapy group of ibrutinib with CD19 CAR-T cell than that of the other 4 groups (Figure 6B). There was no difference of the extinction in tumor volume in the CD19 CAR-T cell alone group, or in the zanubrutinib group and the polytherapy group of zanubrutinib and CD19 CAR-T cell (Figure 6B). All 5 group mice maintained overall weight. The luciferase expression extinction was the more obvious in the combined group of ibrutinib and CD19 CAR-T cell than that of the ibrutinib group (P < .0001) and the polytherapy group of zanubrutinib and CD19 CAR-T cell (P < .0001) on day28. And there was no difference of the extinction in luciferase expression in the ibrutinib or zanubrutinib alone group (Figure 6A and C). The extinction of luciferase expression in the zanubrutinib combined with CD19 CAR-T-cell group was greater than that in the CD19 CAR-T cell group, but there was no statistical difference (Pday28>0.05). In other words, CD19 CAR-T cells could not inhibit tumor development, and zanubrutinib could not significantly improve the effect of CAR-T cell on Raji cell in vivo.
Figure 6.
(A-B) Ibrutinib improved the effect of CD19 CAR-T cells in inhibiting tumor growth obviously. The tumor size reduction of the ibrutinib combined with CD19 CAR-T cell polytherapy group was greatest, and the reduction in tumor size of the zanubrutinib combined with CD19 CAR-T cell polytherapy group was slighter than the CD19 CAR-T cell monotherapy group. (C) The extinction of luciferase expression was greatest in the polytherapy group of ibrutinib with CAR-T cells than other 4 groups. (D) Ibrutinib improved CD19 CAR-T cell expansion while zanubrutinib had a slight impact on CAR-T cell expansion. Abbreviations: CD19, cluster of differentiation 19; CAR-T, chimeric antigen receptor T cell.
Expansion of CD19 CAR-T Cells in Vivo
The proportions of CAR-T cells in the CD19 CAR-T cell monotherapy group and the CD19 CAR-T cell combined with zanubrutinib/ibrutinib polytherapy group were analyzed. There was no difference of the CD19 CAR-T cell proportions in these 3 groups within the first 7 days of treatment. The proportion of CD19 CAR-T cells in the polytherapy group of CD19 CAR-T cells and ibrutinib was higher than that of the CD19 CAR-T cell group on 14, 21 and 28 days of the treatment ((Pday14 = 0.0423, Pday21<0.0001, Pday28<0.0001). However, there were no difference of the CD19 CAR-T cell proportions between CD19 CAR-T cell group and the polytherapy group of CD19 CAR-T cells and zanubrutinib (Figure 6D).
Discussion
CD19 CAR-T cell therapy is a promising treatment for patients with R/R CD19+ B-cell malignancies.17 CD19 CAR-T cell therapy achieved high response rates in parts of B-NHL.18,19 However, opportunities exist to potentiate the promising clinical responses of CAR-T therapy in R/R B-NHL by improving the durable response rates of CD19 CAR-T cell therapy and improving dysfunction of TME.20 Ibrutinib has been approved for the treatment of several B-cell malignancies and shown best responses evidenced in patients with chronic active BCR signaling.21 Zanubrutinib is a next-generation, highly potent, selective, irreversible BTK inhibitor and shows excellent efficacy in MCL22 and CLL/SLL.23 We selected 2 BTK inhibitors to study their synergistic effects with CD19 CAR-T cell therapy in vitro and in vivo in order to find a better combination therapy method in R/R B-NHL therapy.
In addition to the inhibition of oncogenic BCR signaling, ibrutinib could also modulate the dysfunctional microenvironment and improve T-cell function.24 Programed cell death 1 ligand 1 (PD-L1) expression in CLL cells might deliver an inhibitory signal to T cells that could suppresses T-cell function.25 Ibrutinib has been found to enhance the antitumor immune response in CLL by down-regulating the expression of PD-1/PD-L1 pathway induced by signal transducers and activators of transcription-3.26 We observed that ibrutinib had an effect on decreased PD-1 expression in CD19 CAR-T cells which was increased by co-cultured with lymphoma cell line in vitro. In a previous study, the ibrutinib treatment to CLL patients for more than 5 months could improve the proliferation and cytotoxicity of their T cells and CD19 CAR-T cells.27 This is consistent with results in our study in vitro.
Zanubrutinib has complete and durable BTK inhibition comparing with off-target kinase inhibition and toxicities by them of ibrutinib.28 Ibrutinib treatment was associated with increased effector-memory CD4+ and CD8+ T cells in some patients.29 Treatment with ibrutinib was reported to show the promotion of T cells toward type 1 Th1 immune phenotype through ITK inhibition pathways, while the inhibition of Th2 cell differentiation at the same time.30,31 The down-regulated expression of ITK activity by ibrutinib maybe a possible mechanism in the enhancement of the efficacy of immunotherapy.31,32 In another report recently33 suggested that the presence of ibrutinib or acalabrutinib improved the CD19 CAR-T cell effector function after long-term agonistic stimulation by CD19+ tumor cells. In this study, we selected to investigate effects of ibrutinib and zanubrutinib on T cells in vitro. Neither ibrutinib nor zanubrutinib had any influence on the percentage and absolute numbers of effector memory T cells in CD3+CD4−CD8+ T cells and CD3+CD4+CD8− T cells or Th1, Th2 cell in vitro. And another previous study also did not prove the changes in T-cell polarization or memory subsets in the combination therapy in the MCL mice model.12 Additionally, the significant suppression to natural killer cell toxicity of ibrutinib but not zanubrutinib in patients with MCL, could be the effect through ITK inhibition pathways.5 In our study, zanubrutinib only sligntly weakened the tumor size after 21 days combination therapy, and significantly promote the amplification of CD19 CAR-T cell.
The potential reasons for the lack of synergy between zanubrutinib and CD19 CAR-T cells were initially investigated and explored in our previous study. Firstly, ibrutinib can significantly reduce the high expression of PD-1 triggered by tumor cells in vitro, while zanubrutinib had no such effect (Figures 3 and 4A). Ibrutinib obviously promoted the proliferation of dysfunctional CAR-T from lymphoma patients in mice, while zanubrutinib does not promote the expansion of CAR-T cells in mice, we believe that this maybe significantly related to ibrutinib reduced the PD-1 expression of CAR-T cells. Secondly, ibrutinib did not improve the function of CAR-T in vitro, but significantly improved the efficacy of CAR-T in lymphoma mice (Figure 6). We speculate that this is probably because ibrutinib improved the TME of lymphoma. In recent years, several studies have shown that ibrutinib could improve the TME of solid tumors, possible mechanism includes regulating the polarization of tumor-associated macrophages, which is an important direction for our future research.18,34,35
Conclusion
In this study, zanubrutinib showed no significant association with CD19 CAR-T in lymphoma cells neither in vitro or in vivo. Given the limitations of the data, the synergistic effects of Zanubrutinib combined with CD19 CAR-T cells in lymphoma need our further in vivo and clinical studies.
Acknowledgments
We thank the patients for their participation in our experimental studies and clinical trials.
Footnotes
Authors’ Notes: Concept and design were handled by QD; the manuscript was drafted or revised by XPY and MJL; acquisition of data was done by MJL, CCL; analysis and interpretation of data were done by MJL, CCL, JM; writing, review and/or revision of manuscript was handled by all authors; study supervision was taken by QD.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Medical Ethics Committee of the Department of Hematology, Tianjin First Center Hospital of Nankai University (Tianjin, China) (Approval No. of ethics committee: 2018N105KY). Patients agreed to the use of specimens and data for our study. All animal procedures were approved by the institutional animal and care use committee of Nankai University (Approval number: 13612055872).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Tianjin Municipal Science and Technology Commission Grant, Key R & D projects of Ningxia Hui Autonomous Region (grant numbers 21JCQNJC00070, 2021BEG03036).
Informed Consent: All subjects gave their informed consent for inclusion before they participated in the study.
Trial Registration: The Clinical trial is registered at http://www.chictr.org.cn/index.aspx as ChiCTR1800018059.
ORCID iD: Xiupeng Ye https://orcid.org/0000-0003-3087-3956
References
- 1.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2(12):945‐956. doi: 10.1038/nri955 [DOI] [PubMed] [Google Scholar]
- 2.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507‐516. doi: 10.1056/NEJMoa1306220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425‐2437. doi: 10.1056/NEJMoa1509388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimopoulos M, Opat S, Lee H-P, et al. Major responses in Myd88 wildtype (Myd88wt) Waldenstrom macroglobulinemia (WM) patients treated with Bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111). HemaSphere. 2019;3:196. [Google Scholar]
- 5.Flinsenberg TWH, Tromedjo CC, Hu N, et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK cell effector function in patients with mantle cell lymphoma. Haematologica. 2020;105(2):e76-ee79. doi: 10.3324/haematol.2019.220590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pula B, Golos A, Gorniak P, Jamroziak K. Overcoming ibrutinib resistance in chronic lymphocytic leukemia. Cancers (Basel). 2019;11(12):1834. doi: 10.3390/cancers11121834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385(9967):517‐528. doi: 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagley S, O'Rourke D. Clinical investigation of CAR T cells for solid tumors: Lessons learned and future directions. Pharmacol Ther. 2020;205:107419. doi: 10.1016/j.pharmthera.2019.107419 [DOI] [PubMed] [Google Scholar]
- 9.Fisher DT, Chen Q, Appenheimer MM, et al. Hurdles to lymphocyte trafficking in the tumor microenvironment: Implications for effective immunotherapy. Immunol Invest. 2006;35(3-4):251‐277. doi: 10.1080/08820130600745430 [DOI] [PubMed] [Google Scholar]
- 10.Mpekris F, Voutouri C, Baish JW, et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci U S A. 2020;117(7):3728‐3737. doi: 10.1073/pnas.1919764117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter DL, Hwang W-T, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruella M, Kenderian SS, Shestova O, et al. The addition of the BTK inhibitor ibrutinib to anti-CD19 chimeric antigen receptor T cells (CART19) improves responses against mantle cell lymphoma. Clin Cancer Res. 2016;22(11):2684‐2696. doi: 10.1158/1078-0432.CCR-15-1527 [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Wang X, Li Z, et al. Synergistic effect of ibrutinib and CD19 CAR-T cells on Raji cells in vivo and in vitro. Cancer Sci. 2020;111(11):4051‐4060. doi: 10.1111/cas.14638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu M, Deng H, Mu J, et al. Ibrutinib improves the efficacy of anti-CD19-CAR T-cell therapy in patients with refractory non-Hodgkin lymphoma. Cancer Sci. 2021;112(7):2642‐2651. doi: 10.1111/cas.14915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Animals NRCUCftUotGftCaUoL. Guide for the care and use of laboratory animals. National Academies Press (US); 2011. doi: 10.17226/12910. [DOI] [PubMed] [Google Scholar]
- 16.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. doi: 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45‐56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 18.Yan ZX, Li L, Wang W, et al. Clinical efficacy and tumor microenvironment influence in a dose-escalation study of anti-CD19 chimeric antigen receptor T cells in refractory B-cell non-hodgkin's lymphoma. Clin Cancer Res. 2019;25(23):6995‐7003. doi: 10.1158/1078-0432.CCR-19-0101 [DOI] [PubMed] [Google Scholar]
- 19.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531‐2544. doi: 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cianciaruso C, Beltraminelli T, Duval F, et al. Molecular profiling and functional analysis of macrophage-derived tumor extracellular vesicles. Cell Rep. 2019;27(10):3062‐3080.e11. doi: 10.1016/j.celrep.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922‐926. doi: 10.1038/nm.3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CJ, Jiang C, Liu Y, et al. Pleiotropic action of novel bruton's tyrosine kinase inhibitor BGB-3111 in mantle cell lymphoma. Mol Cancer Ther. 2019;18(2):267‐277. doi: 10.1158/1535-7163.MCT-18-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bond DA, Woyach JA. Targeting BTK in CLL: Beyond ibrutinib. Curr Hematol Malig Rep. 2019;14(3):197‐205. doi: 10.1007/s11899-019-00512-0 [DOI] [PubMed] [Google Scholar]
- 24.Stiff A, Trikha P, Wesolowski R, et al. Myeloid-Derived suppressor cells express bruton's tyrosine kinase and can be depleted in tumor-bearing hosts by ibrutinib treatment. Cancer Res. 2016;76(8):2125‐2136. doi: 10.1158/0008-5472.CAN-15-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207‐212. doi: 10.1016/j.coi.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo K, Shaim H, Thompson PA, et al. Ibrutinib modulates the immunosuppressive CLL microenvironment through STAT3-mediated suppression of regulatory B-cell function and inhibition of the PD-1/PD-L1 pathway. Leukemia. 2018;32(4):960‐970. doi: 10.1038/leu.2017.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117‐1127. doi: 10.1182/blood-2015-11-679134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam CSL, Trotman J, Opat S, et al. Phase 1 study of selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851‐859. doi: 10.1182/blood.2019001160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052‐3064. doi: 10.1172/JCI89756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539‐2549. doi: 10.1182/blood-2013-06-507947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagiv-Barfi I, Kohrt HEK, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci USA. 2015;112(9):966‐972. doi: 10.1073/pnas.1500712112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kokhaei P, Jadidi-Niaragh F, Sotoodeh Jahromi A, Osterborg A, Mellstedt H, Hojjat-Farsangi M. Ibrutinib-A double-edge sword in cancer and autoimmune disorders. J Drug Target. 2016;24(5):373‐385. doi: 10.3109/1061186X.2015.1086357 [DOI] [PubMed] [Google Scholar]
- 33.Qin JS, Johnstone TG, Baturevych A, et al. Antitumor potency of an anti-CD19 chimeric antigen receptor T-cell therapy, lisocabtagene maraleucel in combination with ibrutinib or acalabrutinib. J Immunother. 2020;43(4):107‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Garcia A, Lynn RC, Poussin M, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021;12(1):877. doi: 10.1038/s41467-021-20893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu Q, Li C, Song Y, et al. Targeted delivery of ibrutinib to tumor-associated macrophages by sialic acid-stearic acid conjugate modified nanocomplexes for cancer immunotherapy. Acta Biomater. 2019;92:184‐195. doi: 10.1016/j.actbio.2019.05.030 [DOI] [PubMed] [Google Scholar]