Abstract
Transcranial magnetic stimulation (TMS) is a noninvasive neuromodulation tool currently used as a treatment in multiple psychiatric and neurologic disorders. Despite its widespread use, we have an incomplete understanding of the way in which acute and chronic sessions of TMS affect various neural and vascular systems. This systematic review summarizes the state of our knowledge regarding the effects TMS may be having on cerebral blood flow, glucose metabolism, and neurotransmitter release. Forty-five studies were identified. Several key themes emerged: 1) TMS transiently increases cerebral blood flow in the area under the coil; 2) TMS to the prefrontal cortex increases glucose metabolism in the anterior cingulate cortex of patients with depression; and 3) TMS to the motor cortex and prefrontal cortex decreases dopamine receptor availability in the ipsilateral putamen and caudate respectively. There is, however, a paucity of literature regarding the effects TMS may have on other neurotransmitter and neuropeptide systems of interest, all of which may shed vital light on existing biologic mechanisms and future therapeutic development.
Significance Statement
Transcranial magnetic stimulation (TMS) is a noninvasive neuromodulation tool currently used as a treatment in multiple psychiatric and neurologic disorders. This systematic review summarizes the state of our knowledge regarding the effects TMS on cerebral blood flow, glucose metabolism, and neurotransmitter release.
I. Introduction
Through technical and experimental advances in brain imaging research over the last 20 years, we have an increasingly sophisticated understanding of the neural circuit disruptions that are present in patients with various neurologic and psychiatric diseases. These circuit disruptions represent biologic targets that can be identified and harnessed for therapeutic use. There is correspondingly a strong momentum toward developing noninvasive neural circuit–based therapeutics for these patient populations. One of these promising approaches is transcranial magnetic stimulation (TMS).
TMS is a noninvasive neuromodulatory therapy that uses electromagnetic induction to depolarize cells in cortical areas directly affected by the magnetic field, as well as in monosynaptic afferent targets. Single-pulse TMS was first introduced in 1985 by Dr. Anthony Barker. He demonstrated that single pulses of TMS delivered to the left motor cortex generated motor-evoked potentials in the right hand with amplitudes proportional to the strength of the magnetic field. Single-pulse TMS (and the closely related paired-pulse TMS) is now routinely used as a probe of cortical excitability. Single-pulse TMS can be used to evaluate cortical remapping following a stroke and as a biomarker to determine the effects of various pharmacotherapies on the cortical inhibitory/excitatory balance (a surrogate of GABA/glutamate balance) (Ziemann et al., 2015). As a therapeutic tool, most TMS is delivered in a repetitive manner, known as repetitive TMS (rTMS), wherein a series of single pulses of TMS are delivered to a prespecified brain region over a period of seconds to minutes. There are three key parameters to understand when evaluating the effects of rTMS on the brain:
Frequency. This can be a fixed frequency, wherein the interpulse interval is consistent (e.g., 10 Hz, 5 seconds on, 10 seconds off, for a total of 3000 pulses) or a bursting frequency, wherein the interpulse interval varies [e.g., θ burst stimulation (TBS) consisting of three pulse bursts delivered five times/s, 2 seconds on and 8 seconds off for a total of 600 pulses]. The first rTMS studies demonstrated that 10 Hz rTMS over the motor cortex increased cortical excitability (Pascual-Leone et al., 1994), whereas 1 Hz rTMS decreased cortical excitability (Chen et al., 1997). More recent studies using bursting frequencies like TBS, however, they demonstrated that there is a lot more complexity to the effects of frequency and pulse number on cortical excitability than initially understood (McCalley et al., 2021; Hanlon et al., 2022). An influential clinical trial demonstrated that 10 Hz rTMS and intermittent TBS (using the protocols described above) have similar efficacy for treating major depressive disorder (Blumberger et al., 2018).
Intensity. This is typically measured as a percentage of the capacitor output. There is a dose-dependent relationship between the amplitude of the induced magnetic field and the amplitude of effects on the brain. This is most elegantly demonstrated in the motor cortex using motor-evoked potentials.
Location of stimulation. The brain regions modulated by TMS are directly related to the geometry of the TMS coil used and the location of the coil on the head. There are dozens of TMS coil designs currently available, but the two most common classes are figure-eight coils and Hesed coils (H-coils). Figure-eight coils have a relatively compact magnetic field distribution and are typically used in applications wherein the researcher is focusing on a specific cortical target. H-coils have larger magnetic field distributions, which enables modulation of many brain regions—a feature that is often desired when trying to modulate a neural network or the left and right sides of the brain synchronously.
The majority of TMS–positron emission tomography (PET) studies have used figure-eight coils, which can only stimulate one hemisphere at a time. The left hemisphere has been the primary area studied. Within the motor cortex, the choice of the left side for experimental design is merely because most people are right-handed. Within the prefrontal cortex (PFC), there are established functional asymmetries wherein greater activity in the left dorsolateral prefrontal cortex (DLPFC) relative to the right DLPFC is associated with positive effect (Harmon-Jones et al., 2010). Consistent with this, TMS studies in patients with depression have found that 10 Hz TMS to the left DLPFC (which should increase excitability) improves depressive symptoms, whereas 10 Hz TMS to the right DLPFC worsens depressive symptoms and induces anxiety. The influence of TMS on functional laterality is still underdeveloped, however, and represents a scientific gap that should be pursued.
A variety of frequencies, intensities, and coil geometries have been cleared by the US Food and Drug Administration as therapeutic tools for major depressive disorder (O'Reardon et al., 2007), obsessive-compulsive disorder (OCD) (Carmi et al., 2018), anxious depression (Pell et al., 2022), and smoking cessation (Zangen et al., 2021). A recent European consensus paper stated that there was also Level A evidence (definite efficacy) to support its use for neuropathic pain and motor recovery after stroke (Lefaucheur et al., 2020). Combined publication rates of peer-reviewed articles examining TMS as a therapeutic tool for depression, OCD, and substance use disorder have nearly doubled from 2016 to 2021. All of the currently approved clinical indications for repetitive TMS in the United States use high frequency stimulation with coils that reach superficial and/or deeper structures in the PFC.
However, despite increasingly widespread clinical indications for TMS, there are still large gaps in our knowledge regarding its mechanisms of action. In particular, the field has very little understanding of the effects of TMS on neurochemistry (the typical therapeutic target for most pharmacotherapeutics) or regional blood flow (which may be particularly important for stroke recovery). The purpose of this literature review is to summarize our existing knowledge regarding the effects of TMS on the brain and to identify gaps for future research to fill.
A. PET: Evaluating Neurometabolic and Neurochemical Changes
PET is a functional imaging approach in which a radioactive tracer is injected into a peripheral vein. These tracers are labeled with radioisotopes that decay over time and emit a positron. This positron collides with an electron in the body’s tissue and emits two high-energy photons traveling in opposite directions (Bailey et al., 2005). Both of these photons are detected by the PET scanner and are reconstructed into a three-dimensional image indicating the location of accumulated tracer in the body. Through this technique, it is possible to quantify changes in blood flow and metabolic processes and to indirectly measure the release of neurotransmitters in the brain. This review will first describe studies that have used PET methodologies to evaluate the effects of TMS on cerebral blood flow (CBF) and glucose metabolism, which make up most of the published literature on PET and TMS.
Next, we will highlight a particularly relevant gap in the field’s knowledge of TMS’s effects on the release of neurotransmitters, such as L-3,4-dihydroxyphenylalanine (L-DOPA), as well as various pre- and postsynaptic mechanisms. Perhaps the greatest promise of combined TMS-PET research, from a therapeutic development perspective, is that PET can be used to measure regional releases of specific neurotransmitters that may be induced by TMS. While neurologic and psychiatric research have revealed the influence of a variety of neurotransmitter systems on symptom severity and disease development, most of the published work focuses exclusively on dopamine. Additionally, most of these investigations have been carried out in healthy individuals, while clinical populations have been largely unstudied.
Following a description of the known literature and themes that emerge, the review will conclude with a discussion of several critical gaps in our existing knowledge and a forward-focusing viewpoint on the paradigm-shifting gains that we may have as a field if we can fill these gaps to design therapeutic strategies for patients that unite our understanding of neurochemical disruptions with neural circuit modulation approaches.
B. Standard Experimental Designs Using PET and TMS
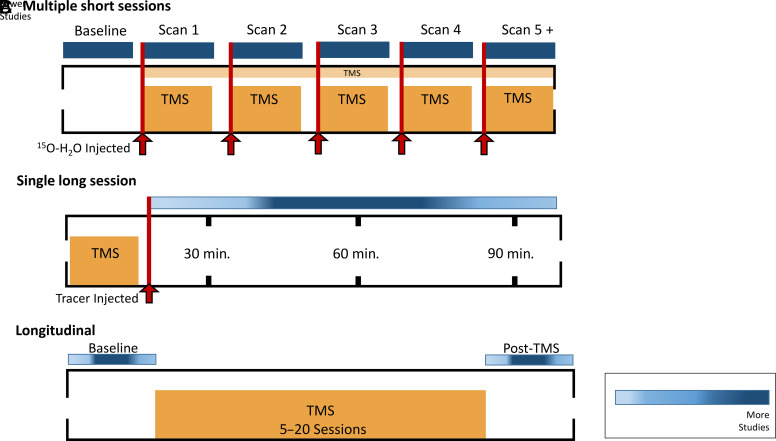
Before diving into the literature, it is important to highlight the three most common experimental designs that have been used in these studies to date (Fig. 1).
Fig. 1.
Common experimental designs used in TMS-PET literature. Above is the representation of several different experimental designs used throughout the combined TMS and PET literature included in this review. These designs depict (A) studies examining CBF changes (measured with 15O-H2O) induced by TMS using multiple short TMS-PET sessions, (B) single long session studies mainly used to investigate changes in dopamine receptor availability following TMS protocols, and (C) a standard longitudinal design that is primarily used to measure changes in glucose metabolism using [18F]Fluoro-2-deoxy-D-glucose PET.
1. Design A: Multiple Short Sessions (Fig. 1A)
Most of the studies examining cerebral blood flow changes induced by TMS use this design. These studies use 15O-H2O, a radiotracer that has a half-life of approximately 2 minutes. This rapid decay rate enables investigators to evaluate the acute effects of TMS on CBF. In this design, participants lay supine in the PET scanner as each bolus of 15O-H2O is injected. Immediately following the injection, various TMS pulse sequences are delivered while the participant is in the scanner. This process can then be repeated (e.g., subsequent injections and TMS sessions). As many as nine short PET scans have been acquired using this design (Takano et al., 2004), with most studies acquiring at least five PET scans. In this design, each PET scan typically lasts 50–90 seconds.
2. Design B: Single Long Session (Fig. 1B)
The majority of TMS studies using PET have used Design B, wherein a radioligand of interest is injected immediately before or after a single TMS session. This allows investigators to evaluate the acute effects of TMS on an array of radioligands with varying decay rates. TMS can be given in the bore of the PET scanner (similar to Design A), but it is often given outside of the PET scanner—especially when using radioligands with a longer half-life, such as [11C]raclopride (half-life of approximately 20 minutes). The PET scanning procedures last between 30 and 90 minutes, depending on the study, with most studies using a 60-minute scan time.
3. Design C: Longitudinal (Fig. 1C)
While the effects of a single session of TMS are of interest from a mechanistic perspective, many research teams are interested in evaluating the effects of a therapeutic course of TMS (e.g., multiple daily sessions of TMS in patients with depression) on basal brain activity (as measured via various PET radioligands). Most of these studies are done in patient populations. For example, these studies have measured the effects of multiple TMS sessions on glucose metabolism via [18F]Fluoro-2-deoxy-D-glucose (FDG), CBF (Speer et al., 2000), dopamine receptor availability (Kuroda et al., 2006), and dopamine synthesis rate (Kuroda et al., 2010) in individuals with depression. Typically, there is a 30–90-minute PET scan at baseline. Following the initial scan, participants undergo anywhere from 5 to 20 TMS sessions over the course of several weeks. Following the TMS treatment sessions, another 30–90-minute PET scan is acquired. Most studies measure their post-TMS PET scan within one week following treatment.
C. Procedures for Article Selection
A comprehensive literature search was conducted on PubMed. A combination of several key terms was used to explore relevant TMS and PET articles. The exact combination used is shown in Supplemental Fig. 1. To assess the risk of bias, an additional search was also conducted on Google Scholar. The initial searches resulted in 753 records. During the early stage of screening, two independent reviewers (K.K. and C.H.) excluded 300 duplicate records, 242 records based on their titles and abstracts, and 81 review articles. After an in-depth full-text review, 130 articles were included in the final evaluation to identify eligible articles. The exclusion criteria at this stage included: 1) articles that were not full-text; 2) studies evaluating neurochemical changes in rodents using microdialysis; 3) studies evaluating neurochemical changes using single-photon emission computed tomography; 4) studies not in English; and 5) studies where “TMS” was an abbreviation for “trunk muscle strength.” The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) chart for the inclusion/exclusion procedures for TMS-PET studies are shown in Supplemental Fig. 1.
II. The Effects of TMS on CBF
Some of the earliest papers examining the effects of TMS using PET imaging techniques measured changes in regional cerebral blood flow (rCBF) in healthy control populations with the [15O]H2O radioligand (Table 1). [15O]H2O is water labeled with a radioactive [15O] molecule that is able to penetrate the blood-brain barrier (Raichle et al., 1983). Increases in [15O]H2O indicate elevations in oxygen consumption associated with increased blood supply to that cerebral region. It is similar to the blood oxygen level–dependent signal that is commonly investigated with functional magnetic resonance imaging, but [15O]H2O PET has several advantages, including a more quantifiable dependent measure, less sensitivity to movement, and less variability in longitudinal studies (Kameyama et al., 2016). Nearly all of these studies follow Design A (see Fig. 1).
TABLE 1.
The effect of TMS on CBF
These data highlight a common theme that low-frequency TMS to the motor cortex and high frequency TMS to the DLPFC tends to increase blood flow in the area underneath the coil.
| Author | Year | Subject Population | Site of Stimulation | Stimulation Frequency | PET Radiotracer | Effect on CBF | Regions Affected |
|---|---|---|---|---|---|---|---|
| Motor Cortex | |||||||
| Fox et al. | 1997 | HC | M1 - Left | 1 Hz | [15O]H2O | ⇧ | Directly under stimulated site |
| Paus et al. | 1998 | HC | M1 - Left | 10 Hz | [15O]H2O | ⇩ | Site of stimulation, R M1, premotor, SMA, medial parietal |
| Siebner et al. | 2001 | HC | M1 - Left | 1–5 Hz | [15O]H2O | ⇧ | Directly under stimulation site |
| Strafella and Paus | 2001 | HC | M1 - Left | 10 Hz | [15O]H2O | ⇧ | Site of stimulation, R M1, L lateral premotor cortex |
| Lee et al. | 2003 | HC | M1 - Left | 1 Hz | [15O]H2O | ⇧ and ⇩ |
Site of stimulation, bilateral dorsal premotor cortex, cerebellum R cingulate motor cortex, L ventral premotor area |
| Speer et al. | 2003 | HC | M1 - Left | 1 Hz | [15O]H2O | ⇧ and ⇩ |
Under stimulation site, L primary auditory cortex, R cerebellum, putamen, insula and red nucleus Contralateral frontal, parietal cortex, ACC, occipital cortex |
| Takano et al. | 2004 | HC | M1 - Left | 5 Hz | [15O]H2O | ⇧ and ⇩ |
Under stimulation site, temporal cortex and insula Medial frontal, occipital and cuneus |
| Rounis et al. | 2005 | HC | M1 - Left | 1 Hz and 5 Hz | [15O]H2O | 1 Hz ⇧ and ⇩ 5 Hz ⇧ and ⇩ |
Under the stimulated area, R M1, L SMA, R dorsal and ventral premotor areas, L ventromedial prefrontal cortex, caudal and rostral cingulate motor areas. R putamen, L head of caudate, R cerebellar vermis. Under the stimulation site, L ventral premotor area, prefrontal and somatosensory cortices, anterior cingulate, parietal areas. Superior cerebellum |
| Obeso et al. | 2013 | HC | Pre-SMA - Right | 50 Hz (cTBS) | [15O]H2O | ⇧ | L pre-SMA, L inferior frontal gyrus, R premotor and R inferior parietal cortex. |
| Prefrontal Cortex | |||||||
| Paus et al. | 1997 | HC | FEF- Left | 10 Hz | [15O]H2O | ⇧ | Directly under the coil, superior parietal cortex, L cuneus, R supplementary eye field |
| Speer et al. | 2000 | Depression | DLPFC- Left | 1 Hz and 20 Hz | [15O]H2O | 1 Hz ⇩ 20 Hz ⇧ |
R prefrontal cortex, L medial temporal cortex, L basal ganglia, L amygdala Directly under the stimulation site, R prefrontal cortex, L and R cingulate gyrus, L amygdala, bilateral insula, basal ganglia, uncus, hippocampus, thalamus, cerebellum |
| Paus et al. | 2001 | HC | DLPFC- Left | 10 Hz | [15O]H2O | ⇧ | Directly under the stimulation site, ACC, posterior cingulate |
| Speer et al. | 2003 | HC | PFC- Left | 1 Hz | [15O]H2O | ⇧ and ⇩ |
L ACC, cerebellum, R insula, primary auditory cortex, and somatosensory face area Stimulated region and in the R PFC, L medial temporal lobe, parahippocampus, and posterior middle temporal gyri |
| Ohnishi, Matsuda et al. |
2004 | HC | DLPFC- Left | 1 Hz | [15O]H2O | ⇧ | R ACC, R medial prefrontal cortex, L ventromedial PFC, and L ventral striatum |
| Barrett et al. | 2004 | HC | DLPFC- Left | 1 Hz and 10 Hz | [15O]H2O | 1 Hz – No effect 10 Hz ⇧ and ⇩ |
- Under stimulation site, perigenual ACC, insula, thalamus, parahippocampal gyrus, caudate nucleus Amygdala, middle frontal gyrus, gyrus rectus, inferior frontal gyrus, inferior and superior parietal lobes |
| Knoch et al. | 2005 | HC | DLPFC- Left and Right | 1 Hz and 10 Hz | [15O]H2O | 1 Hz R ⇧ and ⇩ 1 Hz L ⇧ and ⇩ 10 Hz R ⇧ and ⇩ 10 Hz L ⇧ and ⇩ |
L postcentral gyrus, R precentral gyrus, cingulate gyrus L posterior cingulate, L parahippocampal gyrus Directly under stimulation site, R middle frontal gyrus, anterior cingulate, R caudate nucleus L inferior frontal gyrus, L OFC Directly under stimulation site, L uncus, R caudate R middle and superior frontal gyrus regions, L middle frontal gyrus Directly under stimulation site, R inferior frontal gyrus, cingulate gyrus R middle frontal gyrus |
| Eisenegger et al. | 2008 | HC | DLPFC- Right | 1 Hz | [15O]H2O | ⇧ | L DLPFC, right ventrolateral PFC, inferior frontal cortex |
FEF, frontal eye field; HC, healthy control; L, left; MDL-FC, mid-dorsolateral frontal cortex; R, right; SM1 HAND, sensorimotor hand area.
A. Motor Cortex
Nearly all of the studies that have evaluated the effect of motor cortex TMS on CBF have used low frequency (1 Hz) stimulation. Seven of these papers showed TMS-related increases in motor cortex CBF, while only one study demonstrated a decrease (Paus et al., 1998). This may seem counterintuitive given that 1 Hz TMS causes a decrease in cortical excitability (Chen et al., 1997). These findings, however, emphasize that CBF imaging is measuring something distinct from cortical excitability.
The first published TMS-PET study was done by Fox and colleagues in 1997. They applied 1 Hz rTMS to the left primary motor cortex concurrently with PET in three healthy controls. They found that there was an increase in CBF directly under the stimulated site (Fox et al., 1997). All additional research stimulating the left M1 of healthy controls with 1Hz rTMS replicated these 1997 results (Siebner, Takano, et al., 2001; Lee et al., 2003; Speer et al., 2003a; Rounis et al., 2005). These results were consistent despite differing experimental designs. PET scans acquired within the first hour after 1 Hz TMS was administered showed stable CBF increases in the motor cortex for up to an hour poststimulation (Lee et al., 2003; Rounis et al., 2005). These results suggest that a single session of 1 Hz TMS to the left M1 produced both acute and sustained increases (1 hour) in CBF under the stimulated site. This has also been demonstrated in several studies following 5 Hz TMS (Takano et al., 2004; Rounis et al., 2005). Siebner et al. (2001) demonstrated that left supplementary motor cortex stimulation (nine frequencies between 1 and 5 Hz) increased rCBF under the stimulation site for all frequencies.
There have been two studies investigating the effects of 10 Hz TMS to the motor cortex on CBF. During 10 Hz paired-pulse TMS to the left motor cortex, Strafella and Paus (2001) reported increases in CBF under the stimulation site. Conversely, Paus et al. (1998) demonstrated that 10 Hz TMS decreased CBF in the area under the coil (motor cortex). These differences could be attributed to differing TMS protocols and experimental designs. As 10 Hz TMS is frequently associated with an increase in motor cortex excitability, the 1998 findings seem counterintuitive. That said, the inverse relationship between 1 Hz and 10 Hz TMS on CBF mirrors the inverse relationship these frequencies have on cortical excitability (as measured with electromyography). It is interesting that the effects of 1 Hz versus 10 Hz TMS paradoxically go in the unexpected direction (increase for 1 Hz and a decrease for 10 Hz).
Overall, there has not been enough work done with high-frequency stimulation to draw a definitive conclusion. Additionally, while 1 Hz TMS to the motor cortex consistently increases CBF under the coil, the effects of TMS on blood flow in regions not directly affected by the magnetic field is more varied (Paus et al., 1998; Lee et al., 2003; Speer et al., 2003a; Takano et al., 2004; Rounis et al., 2005; Obeso et al., 2013) (Table 1).
Collectively, studies examining CBF changes with stimulation to the motor cortex are quite consistent. Most of the literature has used a stimulation frequency of 1 Hz and reported an increase in CBF under the site of stimulation (Fig. 2). Two studies have examined the effects of 10 Hz TMS to the motor cortex with opposing results at the stimulation site. The effect of TMS to the motor cortex on CBF in brain regions distal from the site of stimulation remains unclear.
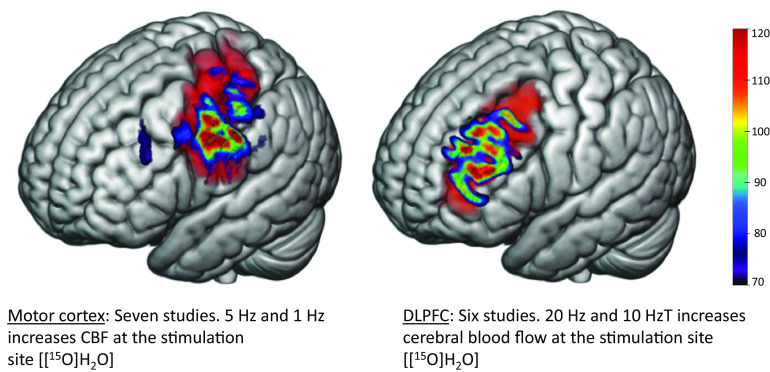
Fig. 2.
TMS transiently increases CBF at the site of stimulation. Above are representative models of electric fields following TMS to the motor (left) and dorsolateral prefrontal (right) cortices in standard space. Changes in rCBF were measured in vivo using PET scanning procedures via a [15O]H2O radioligand. In seven published studies, 5 Hz and 1 Hz (low-frequency) TMS to the motor cortex transiently increased CBF at the site of stimulation. Six published studies suggested a transient increase in CBF at the site of stimulation using 20 Hz and 10 Hz (high-frequency) TMS to stimulate the DLPFC. Modeling parameters include a Magstim B70 coil at 60% machine output and standard tissue conductivity values. The electric fields depicted range from 70 to 120 millivolts.
B. PFC
Eight studies have evaluated the effects of PFC TMS on CBF. 10 Hz rTMS increased CBF under the stimulation site in healthy controls (Paus et al., 1997; Paus et al., 2001; Barrett, Della-Maggiore et al., 2004; Knoch et al., 2006), while 1Hz TMS does not appear to change CBF in the area under the coil (Barrett, Della-Maggiore et al., 2004; Ohnishi, Matsuda, et al., 2004; Knoch et al., 2006; Eisenegger et al., 2008). These results were the opposite of what was observed in the motor cortex. Although it is not completely understood why these differences emerge, there are cytoarchitectural differences between the prefrontal and motor cortices. Additionally, there are also likely neurovascular differences contributing to these incongruent findings. These results confirm the importance of considering these regions independently and not generalizing TMS findings across cortical target sites.
As with motor cortex stimulation, the effects of prefrontal TMS on CBF in distal areas are more variable (Paus et al., 2001; Barrett, Della-Maggiore et al., 2004; Knoch et al., 2006). That said, several studies have shown that 10 Hz TMS to the left DLPFC decreased CBF in the medial PFC (Barrett, Della-Maggiore et al., 2004; Knoch et al., 2006) and increased CBF in the cingulate gyrus (Paus et al., 2001; Barrett, Della-Maggiore et al., 2004). This reciprocal change is important for the treatment of diseases and disorders wherein modulation of the medial prefrontal cortex (MPFC) and cingulate via DLPFC stimulation are part of the conceptual design for treatment development. This increased CBF in the cingulate gyrus following 10 Hz TMS has also been shown following 1 Hz TMS (Speer et al., 2003b; Ohnishi, Matsuda, et al., 2004; Knoch et al., 2006) (Table 1).
C. Insight from a Study in Patients with Depression
All of the studies presented to this point have been performed in healthy control populations. There has only been one study investigating the effects of TMS on CBF in patients. Speer et al. (2000) evaluated the effect of a single session of 20 Hz or 1 Hz TMS to the DLPFC in individuals with major depression. High-frequency rTMS increased CBF under the stimulation site (consistent with studies in healthy controls) and in the right PFC, bilateral cingulate, bilateral insula, amygdala, basal ganglia, hippocampus, thalamus, and cerebellum. In contrast, low-frequency rTMS did not change CBF under the stimulation site (also consistent with healthy controls), but decreased CBF in the right PFC, left medial temporal cortex, left basal ganglia and the left amygdala. The frequency-dependent effects on CBF in the distal areas may have therapeutic value given that 10 Hz rTMS to the left PFC is widely used to treat major depressive disorder.
D. Take-Home Points
Seventeen studies have evaluated the effects of TMS on regional CBF. Nearly all of these studies have been performed on healthy controls, with about half of them evaluating the effects of motor cortex stimulation and the other half evaluating the effect of DLPFC stimulation. The results of motor cortex stimulation are different than DLPFC stimulation (see Fig. 2). Consistent themes are that 1) 1 Hz rTMS to the motor cortex increases rCBF in the area under the coil and 10 Hz may decrease rCBF (though it hasn’t been widely studied); 2) 1 Hz rTMS to the DLPFC does not change rCBF, but 10 Hz rTMS to the DLPFC stimulation reliably increases rCBF; and 3) 10 Hz rTMS to the DLPFC simultaneously decreases rCBF in the MPFC while increasing rCBF at the DLPFC. This last point is particularly valuable, as it points to a biologic foundation that buttresses functional connectivity literature demonstrating a reciprocal relationship between engagement of the executive control network (which contains the DLPFC) and the default mode network (which contains the MPFC).
III. The Effects of TMS on Glucose Metabolism
Another area of interest has been the effect of TMS on glucose metabolism. [18F]FDG-PET scans use the radiotracer [18F]FDG, a glucose analog, to measure the rate of consumption of glucose in the brain (Phelps et al., 1979; Berger, 2003). Greater concentrations of [18F]FDG are indicative of greater glucose metabolic rates in that brain region. In the following studies, [18F]FDG-PET was used to measure changes in regional cerebral metabolic rate of glucose (rCMRglc) after TMS (Table 2). The majority of [18F]FDG studies presented follow a longitudinal design (Design C) outlined in Fig. 1.
TABLE 2.
The effect of TMS on glucose metabolism
| Author | Year | Subject Population | Site of Stimulation | Stimulation Frequency | PET Radiotracer | Effect on Glutamate Metabolism | Regions Effected |
|---|---|---|---|---|---|---|---|
| Motor Cortex | |||||||
| Siebner, et al. | 1998 | HC | M1 - Left | 2 Hz | [18F]FDG | ⇧ | Directly under the coil and SMA |
| Siebner, et al. | 1999 | HC | M1 - Left | 2 Hz | [18F]FDG | ⇧ | Directly under the coil and primary auditory cortex |
| Siebner, et al. | 2000 | HC | M1 - Left | 5 Hz | [18F]FDG | ⇧ | Directly under the coil, R M1, caudal SMA |
| Siebner, et al. | 2001 | HC | M1 - Left | 2 Hz | [18F]FDG | ⇧ | Directly under the coil, caudal SMA, ACC |
| Hayashi, et al. | 2004 | NHP | M1 - Right | 5 Hz | [18F]FDG | ⇧ and ⇩ |
Anterior cingulate, posterior cingulate gyrus, L OFC Directly under the coil, L M1 |
| Prefrontal Cortex | |||||||
| Kimbrell, et al. | 2002 | HC | DLPFC - Left | 1 Hz | [18F]FDG | ⇧ and ⇩ |
Cuneus L Superior frontal gyrus |
| Baeken, et al. | 2009 | Depression | DLPFC - Left | 10 Hz | [18F]FDG | ⇧ | Anterior cingulate |
| Li, et al. | 2010 | Depression | DLPFC - Left | 10 Hz | [18F]FDG | ⇧ and ⇩ |
Anterior cingulate, somatosensory and precuneus L fusiform gyrus and middle temporal lobe |
| Baeken, et al. | 2015 | Depression | DLPFC - Left | 10 Hz | [18F]FDG | ⇧ and ⇩ |
Right prefrontal cortex Subgenual cingulate |
| Tastevin, et al. | 2020 | Depression | DLPFC - Left | 10 Hz | [18F]FDG | ⇧ | Bilateral precuneus, R temporal lobe, fusiform gyrus, hippocampus and amygdala |
| Li, et al. | 2018 | Depression | DLPFC - Left and Right | TBS | [18F]FDG | R cTBS⇧ L iTBS⇧ |
Anterior cingulate, medial PFC Anterior Cingulate, medial PFC |
| Other Regions | |||||||
| Cho, et al. | 2011 | HC | Cerebellum - Left | 1 Hz | [18F]FDG | ⇧ and ⇩ |
R SMA, R posterior parietal cortex, L frontal lobe and ACC L middle and inferior frontal lobes, temporal cortex, L pons, and L dentate nucleus |
| Nauczyciel, et al. | 2014 | OCD | OFC - Right | 1 Hz | [18F]FDG | ⇩ | Directly under stimulation area, R frontal lobe, L putamen and L caudate |
| Lee, et al. | 2013 | HC | Temporal - Right | 1 Hz | [18F]FDG | ⇧ | Directly under the coil, R frontal cortex, ACC and various motor cortical regions |
| Horacek, et al. | 2007 | Schizophrenia | Temporoparietal- Left | 0.9 Hz | [18F]FDG | ⇧ and ⇩ |
Middle frontal lobe, superior temporal lobe, and the supramarginal gyrus Directly under the coil, temporal cortex, cerebellum, insula, cuneus, uncus and the hippocampus |
HC, healthy control; iTBS, intermittent θ burst stimulation; L, left; NHP, nonhuman primate; R, right.
A. Motor Cortex
The first group to evaluate the effects of TMS on glucose metabolism, Siebner et al. (1998, 1999, 2000; Siebner, Takano et al., 2001), completed a series of studies examining the effects of 2–5 Hz rTMS in healthy controls. All four studies targeted the left primary sensorimotor cortex and reported an increase in rCMRglc in the area under the coil with this low frequency stimulation. Additionally, there were TMS-induced increases in glucose metabolism reported within the supplementary motor area (SMA) (Siebner, Peller, et al., 2001; Siebner et al., 2000; Siebner et al., 1998), the bilateral primary auditory cortex (Siebner et al., 1999), the right M1 (Siebner et al., 2000), and the anterior cingulate cortex (ACC) (Siebner, Peller, et al., 2001). The stimulated cortical area (left sensorimotor cortex), right M1 and caudal SMA were elevated acutely (Siebner et al., 1998, 1999, 2000) and lasted for at least 1 hour (Siebner et al., 2000). The elevated bilateral primary auditory cortex glucose metabolism was thought to be related to the sound of the TMS rather than the magnetic field, leading Siebner et al. (1999) to note the importance of taking rTMS-related acoustic input into consideration—a factor that is now widely considered in all TMS studies when developing high fidelity sham techniques.
In addition to these human studies, Hayashi et al. (2004) performed an innovative investigation of the effects of TMS on glucose metabolism in nonhuman primates. In these anesthetized animals, they demonstrated that 5 Hz TMS increased rCMRglc in the ACC and posterior cingulate gyrus. Interestingly, increased rCMRglc was found in the left orbitofrontal cortex (OFC) on post-TMS day 1 and day 8. This lasting increase in rCMRglc was no longer present at day 16.
To summarize, low-frequency TMS to the motor cortex consistently increased rCMRglc under the coil and in remote brain regions in healthy controls. Data suggests that these rCMRglc changes are both acute and can be sustained for at least an hour post-stimulation. Changes in rCMRglc following high-frequency TMS to the motor cortex remains largely unstudied in healthy populations.
B. PFC
While the majority of motor cortex rCMRglc research has been carried out in healthy controls, most PFC research has been done on individuals with major depressive disorder (see C. Insight from Clinical Populations).
C. Insight from Clinical Populations
The first study done in patients with major depressive disorder demonstrated that 10 daily sessions of 10 Hz TMS to the left DLPFC increased rCMRglc in the ACC (Baeken et al., 2009). Other studies demonstrate this may last for 1 week (Tastevin et al., 2020) to 3 months (Li et al., 2010). TBS to the left DLPFC (4 sessions per day for 5 days) decreased rCMRglc of the subgenual ACC, while TBS to the right DLPFC increased rCMRglc in the ACC (Baeken et al., 2015). While the neurobiologic rationale for the laterality differences is unclear, there are established functional asymmetries in the PFC, wherein the greater activity in the left DLPFC relative to the right DLPFC is associated with positive effect (Harmon-Jones et al., 2010).
While TMS strategies for depression typically aim to amplify activity in the executive control system (e.g., through 10 Hz TBS to the DLPFC), many clinical trials of TMS for OCD and schizophrenia have focused on dampening activity in areas that are typically overactive. Among individuals with schizophrenia, low-frequency rTMS (0.9 Hz) to the temporoparietal cortex decreased brain glucose metabolism in the area under the coil (Horacek et al., 2007), as well as in the temporal cortex, cerebellum, insula, cuneus, uncus, and the hippocampus (Horacek et al., 2007). Increased rCMRglc was found in the middle frontal lobe, superior temporal lobe, and the supramarginal gyrus.
In OCD, low-frequency rTMS (1 Hz) to the right OFC decreased rCMRglc in the area under the coil as well as the caudate and putamen (Nauczyciel et al., 2014) (Table 2).
D. Cerebellum and Temporal Cortex
While the bulk of the studies have focused on the motor cortex or PFC, four studies have examined unique brain targets—two in healthy controls and two in patient populations. Results of the two studies involving patient populations are outlined above in C. Insight from Clinical Populations. In healthy controls, when stimulating the left lateral cerebellum (Cho et al., 2012), 1 Hz rTMS [18F]FDG-PET scans revealed decreased glucose metabolism in the stimulated area and a number of distal targets (Cho et al., 2012). This is in contrast with the increased glucose metabolism observed following 1 Hz TMS to the motor cortex. Following 1 Hz rTMS to the right temporal cortex, healthy controls had no change in the area under the coil, but experienced increased glucose metabolism in remote regions such as the right frontal cortex, ACC, and various motor cortical regions (Lee et al., 2013).
E. Take-Home Points
In healthy controls, low-frequency TMS to the motor cortex increased glucose metabolism under the coil, but high-frequency TMS remains largely unstudied. In patient populations high-frequency stimulation has been evaluated however. Ten to 20 sessions of 10 Hz TMS appears to increase glucose metabolism in a network of distributed regions and may last for several weeks. Additionally, there appears to be a reciprocal relationship between DLPFC stimulation and glucose metabolism in the MPFC. This is consistent with the theme that was observed in studies looking at rCBF, further strengthening the biologic basis for previously observed patterns of reciprocal connectivity between the executive control network (of which the DLPFC is a node) and the default mode network (of which the MPFC is a node).
4. The Effects of TMS on Dopamine Receptor Availability
Studies of changes in specific neurotransmitters after TMS make up the smallest portion of combined TMS-PET literature, yet an online literature search of all papers included in this review suggests that this category is the most cited in the literature. In this section, we will examine studies that use various radiotracers to examine the effects that TMS can have on dopamine receptor availability in the brain (Table 3). The most commonly used radiotracer to measure dopamine receptor availability is [11C]raclopride, a dopamine type 2/3 (D2/D3) receptor antagonist. This has low affinity for dopamine type 1 receptors and high affinity for D2 receptors (Laruelle, 2000). When [11C]raclopride is injected into the body, it competes for binding sites with synaptic dopamine in the striatum (Laruelle, 2000). With this, one can indirectly measure dopamine release in the brain as lower [11C]raclopride binding is associated with an increase in synaptic dopamine release. Other radiotracers, such as [11C]FLB 457 and [11C]-(+)-PHNO, are also used to examine dopamine receptor availability in vivo. While [11C]FLB 457 has a high affinity for D2-type dopamine receptors (Farde et al., 1997), [11C]-(+)-PHNO is a D3-type dopamine receptor–preferring agonist (Narendran et al., 2006). A reduction in radiotracer binding potential is indicative of an increase in extracellular dopamine concentration (Laruelle et al., 1997; Endres et al., 2003). The dopamine receptor studies presented primarily follow Design B, outlined in Fig. 1.
TABLE 3.
The effect of TMS on dopamine receptor availability
| Author | Year | Subject Population | Site of TMS | Stimulation Frequency | PET Radiotracer | Effect on Dopamine Receptor Binding | Regions Effected |
|---|---|---|---|---|---|---|---|
| Motor Cortex | |||||||
| Strafella et al. | 2003 | HC | M1 - Left | 10 Hz | [11C]raclopride | ⇩ | L putamen |
| Ohnishi, Hayashi et al. |
2004 | NHP | M1 - Right | 5 Hz | [11C]raclopride | ⇧ and |
R putamen Bilateral ventral striatum |
| Strafella et al. | 2005 | PD | M1 - Left and Right | 10 Hz | [11C]raclopride | ⇩ | Ipsilateral putamen from stimulated side |
| Kim et al. | 2008 | PD | M1 | 5 Hz | [11C]raclopride | ⇩ | Contralateral caudate from stimulated side |
| Lamusuo et al. | 2017 | HC | M1 - Right | 10 Hz | [11C]raclopride | No Effect | - |
| Prefrontal Cortex | |||||||
| Strafella et al. | 2001 | HC | DLPFC - Left | 10 Hz | [11C]raclopride | ⇩ | L dorsal caudate |
| Kuroda et al. | 2006 | Depression | DLPFC - Left | 10 Hz | [11C]raclopride | No Effect | - |
| Ko et al. | 2008 | HC | DLPFC - Left and Right | 10 Hz | [11C]raclopride | L ⇩ R – No Effect |
L caudate, putamen - |
| Cho and Strafella | 2009 | HC | DLPFC - Left and Right | 10 Hz | [11C]FLB 457 | L ⇩ R – No Effect |
L subgenual, pregenual cingulate, L medial OFC - |
| Cho et al. | 2015 | HC | MPFC (BA 10) - Double cone coil | 10 Hz | [11C] PHNO | ⇩ | Bilateral dorsal putamen, bilateral dorsal and ventral globus pallidus |
| Other Regions | |||||||
| Malik et al. | 2018 | HC | Insula | 1 Hz and 10 Hz | [11C] PHNO | 1 Hz ⇧ 10 Hz – No Effect |
Substantia nigra, sensorimotor striatum - |
DLPFC, dorsolateral prefrontal cortex; HC, healthy control; L, left; NHP, nonhuman primate; R, right.
A. Motor Cortex
There are five studies examining the effects of rTMS to the motor cortex on dopamine receptor availability. Two of these studies were done in healthy controls (Strafella et al., 2003; Lamusuo et al., 2017), one in healthy nonhuman primates (Ohnishi, Hayashi, et al., 2004), and two explored dopamine changes after TMS in individuals with Parkinson’s disease (PD) (Strafella et al., 2005; Kim et al., 2008). With one exception (Lamusuo et al., 2017), these studies all reported significant changes in dopamine receptor availability induced by TMS to the motor cortex. For example, Strafella et al. (2003) demonstrated that 10 Hz rTMS to the left M1 reduced [11C]raclopride binding in the left putamen in healthy controls—an indication that TMS is induced dopamine release. A study in patients with PD demonstrated that 10 Hz rTMS decreased [11C]raclopride binding in the ipsilateral putamen, again suggesting that TMS increased dopamine release in these patients (Strafella et al., 2005). In another study of PD, Kim et al. (2008) demonstrated that 5 Hz TMS to the motor cortex also reduced dopamine receptor availability in the contralateral caudate.
Similarly, Ohnishi, Hayashi et al. (2004) reported decreased dopamine receptor availability in the bilateral striatum in nonhuman primates. In contrast, this study was the only one to observe increases in dopamine receptor availability following stimulation to the motor cortex. As all human studies described above were done in awake humans, Ohnishi, Hayashi et al. (2004) measured changes in anesthetized nonhuman primates. Thus, it is possible that anesthesia could be responsible for slight differences in results. Pentobarbital, the anesthesia used in this study, has been found to inhibit both ketamine-induced dopamine release (Masuzawa et al., 2003) and L-DOPA–induced dopamine release (Adachi et al., 2006) in the rat striatum. Perhaps the anesthetic used suppressed TMS-induced dopamine release in the putamen—part of the dorsal striatum—leading to an increase in dopamine receptor availability in this region.
Overall, these studies in patients and in healthy controls suggest that high-frequency TMS to the motor cortex tends to reduce [11C]raclopride binding, suggesting a TMS-induced increase in dopamine release.
B. DLPFC
Four studies investigated the effects of DLPFC TMS on dopamine receptor availability. In healthy controls, 10 Hz rTMS to the left DLPFC decreased dopamine receptor availability, as measured by [11C]raclopride, in the left dorsal caudate (Strafella et al., 2001). Using [11C]FLB 457, decreased dopamine receptor availability was also reported in the left subgenual and pregenual ACC and the left medial OFC following 10 Hz rTMS to the left DLPFC in healthy controls (Cho and Strafella, 2009). There was no change in dopamine receptor availability after 10 Hz rTMS to the right DLPFC (Cho and Strafella, 2009). In individuals with major depressive disorder, 10 sessions of 10 Hz rTMS to the left DLPFC did not change [11C]raclopride binding (Kuroda et al., 2006). Further, in an attempt to measure changes in dopamine synthesis rates after TMS to the left DLPFC, the L-[β-11C]DOPA radiotracer was used in patients with depression. As endogenous dopamine is produced by enzymatic decarboxylation of L-DOPA, radioactively labeled L-DOPA can be used to assess the rate of endogenous dopamine synthesis (Ito et al., 2006). The rate of endogenous dopamine synthesis can be used to estimate presynaptic function of the dopaminergic system (Ito et al., 2006). Results suggested that 10 sessions of TMS treatment over a 2-week period does not change the dopamine synthesis rate in individuals with major depressive disorder (Kuroda et al., 2010). Despite the growing list of clinical indications for TMS beyond depression, there are no other published reports evaluating the effect of TMS on dopamine receptor availability in diseases with known dopamine disruptions, including drug and alcohol abuse and schizophrenia.
One study evaluated the effects of DLPFC TBS (a patterned form of TMS) on striatal dopamine release during a task that required planning and set-shifting (Ko et al., 2008). As this task involves executive processes, it likely engages the DLPFC while concurrently inducing dopamine release in the brain. Participants first received continuous TBS (cTBS; 600 pulses) to either the left or right DLPFC. Within 5 minutes of stimulation, participants performed the Montreal Card Sorting Task (Monchi et al., 2006) while in the PET scanner. TBS of the left DLPFC increased [11C]raclopride binding (ipsilateral caudate, anterior putamen, and contralateral caudate nucleus), indicating that cTBS decreased typical dopamine release that occurs during the set-shifting task (Ko et al., 2008). cTBS of the right DLPFC did not change dopamine receptor availability (Ko et al., 2008). This study has interesting implications for patients with schizophrenia, for example, who are known to have elevated PFC dopamine. There are also interesting implications for individuals with alcohol and substance use disorders, as elevated dopaminergic tone in the presence of drug cues can lead to relapse.
C. Insula and MPFC
After stimulating the MPFC at 10Hz, one study used an [11C]-(+)-PHNO radiotracer to examine changes in D3 receptor availability in healthy controls (Cho et al., 2015). In agreement with previous literature, Cho et al. (2015) reported a reduction in dopamine receptor binding potential in the bilateral dorsal putamen and the bilateral dorsal and ventral globus pallidus, indicating a TMS-induced increase in dopamine. Another study, measuring dopamine changes with [11C]-(+)-PHNO, used a specially designed coil, the H-coil, to bilaterally target the insula (Malik et al., 2018). No changes were observed after 10 Hz rTMS to the bilateral insula. Conversely, 1 Hz rTMS to the insula caused an increase in [11C]-(+)-PHNO binding, suggesting a decrease in dopamine levels in the substantia nigra and the sensorimotor striatum (Malik et al., 2018).
D. Take-Home Points
Eleven studies have evaluated the effects of potential TMS-induced changes in dopamine receptor availability. Most of these studies use higher frequency stimulation (5–10 Hz) in healthy participants. Following TMS to both the frontal and motor cortices, there are consistent reductions in dopamine receptor availability in the striatum in healthy controls. This reduction in dopamine receptor availability is thought to be indicative of endogenous dopamine release induced by TMS. Similar results have been shown in individuals with PD, as stimulation to the motor cortex was reportedly able to decrease dopamine receptor availability in two studies. Only one study examined a patient population other than individuals with PD. This study found that individuals with depression, who were stimulated at the left DLPFC, had no change in dopamine receptor availability following TMS.
5. Less Studied Areas: μ-Opioid and Serotonin Binding
Lamusuo et al. (2017) examined changes in μ-opioid receptor binding in healthy controls after rTMS to the right M1. Using [11C]carfentanil, a highly potent and selective μ-opioid receptor agonist (Endres et al., 2003), widespread significant decreases in [11C]carfentanil binding in the right ACC, MPFC, medial OFC cortex, ventral striatum, left insula, DLPFC, precentral gyrus, superior temporal gyrus, and precentral gyrus were indicated (Lamusuo et al., 2017). These results suggest that 10 Hz rTMS to the right M1 activated the endogenous opioid system.
Sibon et al. (2007) worked to start forming an answer regarding potential changes in serotonin synthesis in healthy controls after TMS by targeting the left DLPFC. Using [11C]-α-methyl-tryptophan, a radioactively labeled precursor to serotonin (Diksic and Young, 2001), they were able to measure the rate of serotonin synthesis in vivo (Sibon et al., 2007). After 10 Hz rTMS to the left DLPFC, there were lower [11C]-α-methyl-tryptophan levels in the left parahippocampal gyrus and the right insula, while increased values were noted in the right cingulate gyrus and the cuneus (Sibon et al., 2007). Given these early results, it is possible that rTMS to the left DLPFC modulates aspects of 5-hydroxytryptamine (5-HT) metabolism in limbic areas.
6. Themes and Future Directions
TMS has gained momentum as a therapeutic tool in clinical research as well as clinical practice. Various TMS protocols (e.g., different frequencies, cortical targets) are currently approved for therapeutic use in major depressive disorder, OCD, smoking cessation, pain, and headaches. That said, we still have an incomplete understanding of how TMS is affecting rCBF, glucose metabolism, and dopamine binding. This review highlights several prominent themes from the literature: 1) 5–20 Hz TMS transiently increases CBF in the area under the coil; 2) multiple sessions of 10 Hz TMS to the PFC in depression patients increases glucose metabolism in the anterior cingulate; and 3) 10 Hz TMS to the motor and prefrontal cortices decreased dopamine receptor availability in the ipsilateral putamen and caudate, respectively. The potential therapeutic implications of these findings are discussed below.
A. Selective Amplification of Blood Flow to a Cortical Target: Implications for Multimodal Therapy
Among the 17 studies reviewed here, the majority demonstrated that high-frequency stimulation increased CBF to the area directly affected by the electrical field. All of these studies used 15O-H2O, a radioligand that has a very short half-life (approximately 120 seconds). The increase in CBF could be detected within a few seconds, and may accumulate. Fox and colleagues (1997), for example, demonstrated that acute TMS resulted in a 12% increase in local blood flow (approximately 400 mm3), which was maintained for 1 minute and had decayed to 6% about 10 minutes later. A subsequent bolus of TMS at 10 minutes further increased CBF to 14%, while a third TMS bolus at 20 minutes increase blood flow by 20% compared with baseline.
Although the biologic mechanism for this blood flow change is not clear, there are intriguing therapeutic implications. These include the potential to direct pharmacotherapeutics to a brain target of interest (e.g., TMS-optimized drug infusions for patients with psychiatric/neurologic/oncologic disease). Additionally, while the bulk of the literature to date regarding the biologic mechanism of TMS has focused on changes in the blood oxygen level–dependent signal and connectivity, it is possible that regional blood flow changes may be even more critical. This could be particularly true in headaches, for example, wherein many of the successful migraine treatments act on the vasculature and induction of migraine can be accomplished with vasoactive agent (Mason and Russo, 2018).
B. Modulating Dopamine Binding through TMS
Although there are now many radioligands that could easily be paired with TMS, most studies that have focused on neurotransmitter changes with TMS have focused on dopamine. They almost all use higher (5–10 Hz) stimulation frequencies and report reductions in dopamine receptor availability, particularly in striatal regions and related circuitry, after TMS to the frontal and motor cortices (Fig. 3). This reduction likely reflects an increase in endogenous dopamine release induced by TMS and is consistent across both healthy control populations and individuals with PD. These reductions in binding were not replicated in individuals with depression, however, suggesting that underlying differences in dopamine availability/function in these patients may alter TMS effects. For individuals with PD, administering TMS to the motor cortex to induce endogenous dopamine release could be a promising therapeutic as a lack of dopamine is a hallmark of the disease.
Fig. 3.
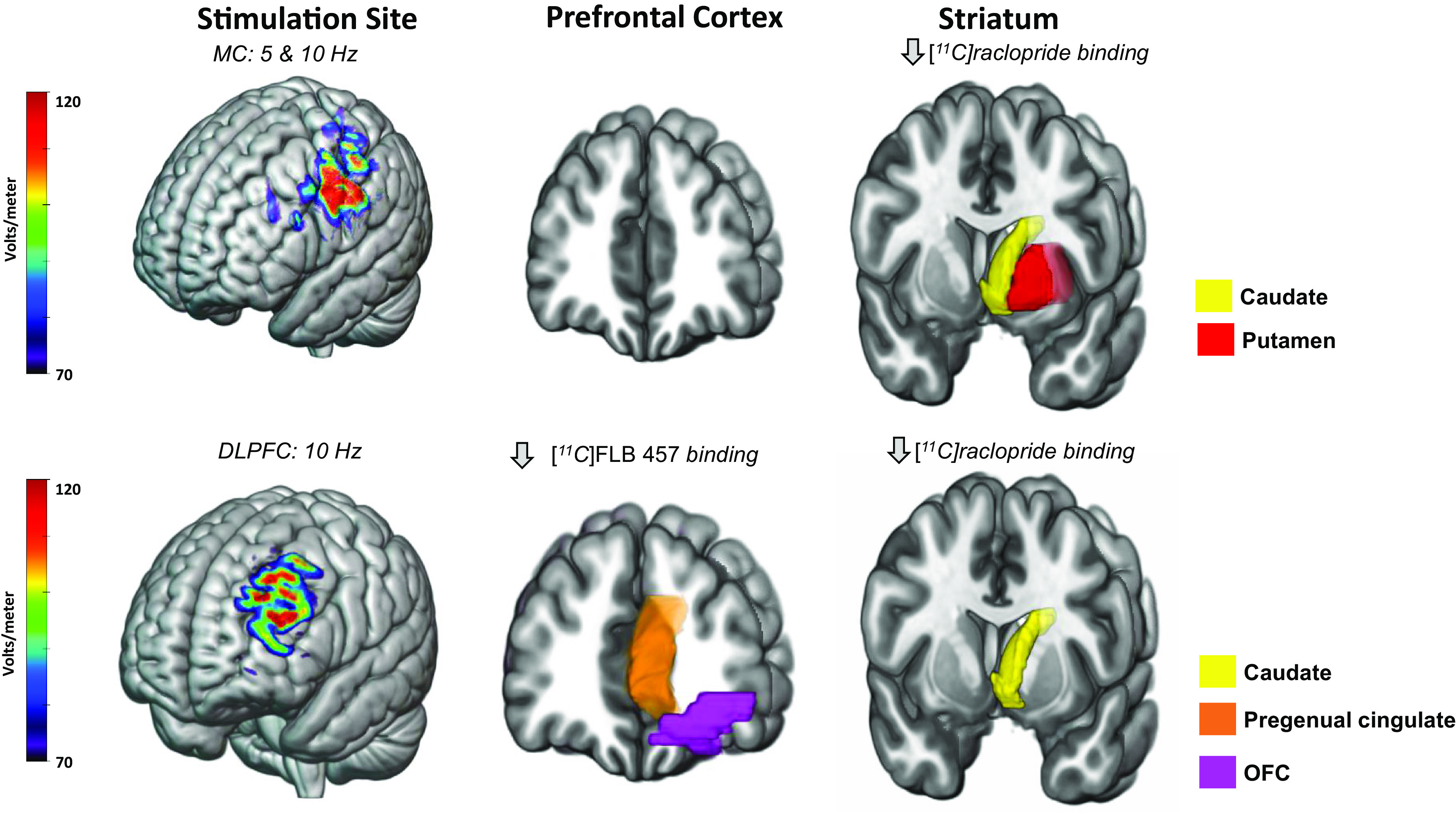
TMS to the motor cortex and the DLPFC influences dopamine receptor availability in a region-specific manner. Above are representative models of electric fields following TMS to the motor (top) and dorsolateral prefrontal (bottom) cortices in standard space. D2 dopamine receptor availability was measured in vivo using [11C]raclopride, a radiotracer detectible through PET scanning procedures. Reported above are regions exhibiting a decrease in [11C]raclopride binding in at least two publications. One study used [11C]FLB 457. Overall, 5 Hz and 10 Hz TMS to the left motor cortex decreases [11C]raclopride binding in the caudate (yellow) and putamen (red). 10 Hz TMS to the left dorsolateral prefrontal cortex (DLPFC) decreases [11C]FLB 457 binding in the pregenual cingulate (orange) and orbitofrontal cortex (OFC) (purple). Additionally, 10 Hz TMS to the left DLPFC decreases [11C]raclopride binding in the caudate (yellow) and putamen (red). Decreases in dopamine receptor availability suggest a TMS-induced dopamine release. Modeling parameters include a Magstim B70 coil at 60% machine output and standard tissue conductivity values. The electric fields depicted range from 70 to 120 millivolts.
The uniformity of results across dopamine receptor work is encouraging, but there is an obvious gap in the literature: there is only one study examining low-frequency (1 Hz) TMS on dopamine receptor availability. This study used an H-coil, which is different from a regular TMS coil as it has a deeper magnetic field and can reach beyond superficial cortical areas and target deeper regions, like the insula (Malik et al., 2018). In healthy controls, an increase in dopamine receptor availability was observed in the striatum after 1 Hz rTMS to the insula, suggesting a decrease in striatal dopamine levels following low-frequency rTMS. These results have promising therapeutic implications, especially in clinical areas in which there is significant dysregulation of dopamine. For example, in the addiction field, previous studies have shown that various drugs of abuse, including tobacco, amphetamine, cannabis, ketamine, and alcohol (Vollenweider et al., 2000; Boileau et al., 2003; Barrett, Boileau et al., 2004; Bossong et al., 2009) result in increased striatal dopamine release in humans, but many of these individuals have low tonic dopamine levels in the striatum (Volkow et al., 2004). If 10 Hz rTMS can increase dopamine receptor availability in substance-using populations, it is possible that it could be used as an agonist therapy or as a tool to reset the homeostatic balance of dopamine in the striatum.
C. Moving Beyond Dopamine
In the last 20 years there has been a prominent expansion in the radionuclides available for study with PET imaging. While most clinical PET usage (and research) is in the field of oncology (e.g., detecting glucose metabolism in a primary tumor and metastases), there are now many widely available radioligands with relevance to neurologic and psychiatric disease (e.g., amyloid-β and -τ, serotonin, nicotine, endogenous opioids, inflammatory markers, synaptic density). To date however, there are very few studies that have gone beyond basic assessment of TMS effects on dopamine, glucose metabolism, and CBF. More research in this area may not only help us understand the biologic basis through which TMS is acting as a therapeutic agent in clinical populations (e.g., effect of TMS on serotonin), but it will also help the field develop more innovative therapeutic strategies that integrate pharmacotherapeutic approaches with brain stimulation interventions. Filling in the gaps between neural circuit–based therapeutics and neurochemistry-based therapeutics will produce a more complete therapeutic picture for the future.
D. Limitations and Future Directions
Unfortunately, there are still prominent gaps in our knowledge that cannot be filled by the existing literature. For example, most studies involve healthy control participants and use only a single frequency of stimulation. It is not clear that the patterns observed in healthy individuals will generalize to those with psychiatric diseases. This may be particularly true of disorders that are known to effect endogenous dopamine (e.g., substance use disorders, psychosis, Huntington’s chorea, PD), or diseases that are chronically treated with dopamine modulators (e.g., agonists, antagonists, prodrugs). More studies in these populations would be fruitful additions to the therapeutic development pipeline. Likewise, there are very few within-subjects designs in the TMS-PET field. This is due in part to safety concerns associated with repeated doses of radiation. This may be an excellent place for animal research, particularly large animals like nonhuman primates, to fill our knowledge gap.
There is also relatively little information regarding the effect of TBS on the brain using PET imaging. This is unfortunate given the growing usage of TBS as a brain stimulation intervention. Future research should develop combined TBS-PET protocols. Moreover, as most combined TMS-PET imaging studies have been conducted in control subjects and not in a patient population, exploring the effects of TMS on neurochemistry in various psychiatric populations is an essential future direction. As discussed above, moving forward, it will be important to evaluate how TMS may be changing other neural systems including serotonin receptors, opioid receptors, and inflammatory markers. Future studies should work to incorporate these and other neural systems into the TMS-PET body of literature.
Acknowledgments
The authors thank Daniel McCalley for the electric field maps on Figs. 2 and 3 and Hilary Smith and Daniel McCalley for editorial feedback.
Abbreviations
- 5-HT
5-hydroxytryptamine
- ACC
anterior cingulate cortex
- CBF
cerebral blood flow
- cTBS
continuous θ burst stimulation
- D2/3
dopamine type 2/3 receptor
- DLPFC
dorsolateral prefrontal cortex
- H-coil
Hesed coil
- L-DOPA
L-3, 4-dihydroxyphenylalanine
- M1
primary motor cortex
- MPFC
medial prefrontal cortex
- OCD
obsessive-compulsive disorder
- OFC
orbitofrontal cortex
- PD
Parkinson’s disease
- PET
positron emission tomography
- PFC
prefrontal cortex
- rCBF
regional cerebral blood flow
- rCMRglc
regional cerebral metabolic rate of glucose
- rTMS
repetitive transcranial magnetic stimulation
- SMA
supplementary motor area
- TBS
θ burst stimulation
- TMS
transcranial magnetic stimulation.
Authorship Contributions
Participated in research design: Kinney, Hanlon.
Performed data analysis: Kinney, Hanlon.
Wrote or contributed to the writing of the manuscript: Kinney, Hanlon.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant T32-AA007565], [Grant R01-AA027705], [Grant P50-AA026117]; and the National Institute on Drug Abuse [Grant R01-DA044471] (to C.A.H.).
C.A.H. serves on a scientific advisory board for Welcony-Magstim and as a consultant to the Roswell Park Cancer Center. K.R.K. has no conflict of interest to declare.
 This article has supplemental material available at pharmrev.aspetjournals.org.
This article has supplemental material available at pharmrev.aspetjournals.org.
References
- Adachi YU, Yamada S, Satomoto M, Watanabe K, Higuchi H, Kazama T, Doi M, Sato S (2006) Pentobarbital inhibits L-DOPA-induced dopamine increases in the rat striatum: An in vivo microdialysis study. Brain Res Bull 69:593–596. [DOI] [PubMed] [Google Scholar]
- Baeken C, De Raedt R, Van Hove C, Clerinx P, De Mey J, Bossuyt A (2009) HF-rTMS treatment in medication-resistant melancholic depression: results from 18FDG-PET brain imaging. CNS Spectr 14:439–448. [DOI] [PubMed] [Google Scholar]
- Baeken C, Marinazzo D, Everaert H, Wu GR, Van Hove C, Audenaert K, Goethals I, De Vos F, Peremans K, De Raedt R (2015) The Impact of Accelerated HF-rTMS on the Subgenual Anterior Cingulate Cortex in Refractory Unipolar Major Depression: Insights From 18FDG PET Brain Imaging. Brain Stimul 8:808–815. [DOI] [PubMed] [Google Scholar]
- Bailey DL, Townsend DW, Valk PE, Maisey MN (2005) Positron Emission Tomography, 1st ed, Springer, London. [Google Scholar]
- Barrett J, Della-Maggiore V, Chouinard PA, Paus T (2004) Mechanisms of action underlying the effect of repetitive transcranial magnetic stimulation on mood: behavioral and brain imaging studies. Neuropsychopharmacology 29:1172–1189. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A (2004) The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse 54:65–71. [DOI] [PubMed] [Google Scholar]
- Berger A (2003) How does it work? Positron emission tomography. BMJ 326:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, Knyahnytska Y, Kennedy SH, Lam RW, Daskalakis ZJ, et al. (2018) Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391:1683–1692. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS (2009) Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 34:759–766. [DOI] [PubMed] [Google Scholar]
- Carmi L, Alyagon U, Barnea-Ygael N, Zohar J, Dar R, Zangen A (2018) Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul 11:158–165. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG (1997) Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48:1398–1403. [DOI] [PubMed] [Google Scholar]
- Cho SS, Koshimori Y, Aminian K, Obeso I, Rusjan P, Lang AE, Daskalakis ZJ, Houle S, Strafella AP (2015) Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology 40:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Strafella AP (2009) rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One 4:e6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Yoon EJ, Bang SA, Park HS, Kim YK, Strafella AP, Kim SE (2012) Metabolic changes of cerebrum by repetitive transcranial magnetic stimulation over lateral cerebellum: a study with FDG PET. Cerebellum 11:739–748. [DOI] [PubMed] [Google Scholar]
- Diksic M, Young SN (2001) Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J Neurochem 78:1185–1200. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Treyer V, Fehr E, Knoch D (2008) Time-course of “off-line” prefrontal rTMS effects--a PET study. Neuroimage 42:379–384. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Bencherif B, Hilton J, Madar I, Frost JJ (2003) Quantification of brain mu-opioid receptors with [11C]carfentanil: reference-tissue methods. Nucl Med Biol 30:177–186. [DOI] [PubMed] [Google Scholar]
- Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J, Halldin C (1997) A PET-study of [11C]FLB 457 binding to extrastriatal D2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacology (Berl) 133:396–404. [DOI] [PubMed] [Google Scholar]
- Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, Martin C, Jerabek P (1997) Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport 8:2787–2791. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Smith HR, Epperly PM, Collier M, Galbo LK, Czoty PW (2022) Priming the pump? Evaluating the effect of multiple intermittent theta burst sessions on cortical excitability in a nonhuman primate model. Brain Stimul 15:676–677. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK (2010) The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol Psychol 84:451–462. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ohnishi T, Okabe S, Teramoto N, Nonaka Y, Watabe H, Imabayashi E, Ohta Y, Jino H, Ejima N, et al. (2004) Long-term effect of motor cortical repetitive transcranial magnetic stimulation [correction]. Correction Ann Neurol 56:77–85. [DOI] [PubMed] [Google Scholar]
- Horacek J, Brunovsky M, Novak T, Skrdlantova L, Klirova M, Bubenikova-Valesova V, Krajca V, Tislerova B, Kopecek M, Spaniel F, et al. (2007) Effect of low-frequency rTMS on electromagnetic tomography (LORETA) and regional brain metabolism (PET) in schizophrenia patients with auditory hallucinations. Neuropsychobiology 55:132–142. [DOI] [PubMed] [Google Scholar]
- Ito H, Ota M, Ikoma Y, Seki C, Yasuno F, Takano A, Maeda J, Nakao R, Suzuki K, Suhara T (2006) Quantitative analysis of dopamine synthesis in human brain using positron emission tomography with L-[beta-11C]DOPA. Nucl Med Commun 27:723–731. [DOI] [PubMed] [Google Scholar]
- Kameyama M, Murakami K, Jinzaki M (2016) Comparison of [(15)O] H2O Positron Emission Tomography and Functional Magnetic Resonance Imaging in Activation Studies. World J Nucl Med 15:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Chung EJ, Lee WY, Shin HY, Lee GH, Choe YS, Choi Y, Kim BJ (2008) Therapeutic effect of repetitive transcranial magnetic stimulation in Parkinson’s disease: analysis of [11C] raclopride PET study. Mov Disord 23:207–211. [DOI] [PubMed] [Google Scholar]
- Knoch D, Treyer V, Regard M, Müri RM, Buck A, Weber B (2006) Lateralized and frequency-dependent effects of prefrontal rTMS on regional cerebral blood flow. Neuroimage 31:641–648. [DOI] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP (2008) Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task: a TMS-[(11)C]raclopride PET study. Eur J Neurosci 28:2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Motohashi N, Ito H, Ito S, Takano A, Nishikawa T, Suhara T (2006) Effects of repetitive transcranial magnetic stimulation on [11C]raclopride binding and cognitive function in patients with depression. J Affect Disord 95:35–42. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Motohashi N, Ito H, Ito S, Takano A, Takahashi H, Nishikawa T, Suhara T (2010) Chronic repetitive transcranial magnetic stimulation failed to change dopamine synthesis rate: preliminary L-[β-11C]DOPA positron emission tomography study in patients with depression. Psychiatry Clin Neurosci 64:659–662. [DOI] [PubMed] [Google Scholar]
- Lamusuo S, Hirvonen J, Lindholm P, Martikainen IK, Hagelberg N, Parkkola R, Taiminen T, Hietala J, Helin S, Virtanen A, et al. (2017) Neurotransmitters behind pain relief with transcranial magnetic stimulation - positron emission tomography evidence for release of endogenous opioids. Eur J Pain 21:1505–1515. [DOI] [PubMed] [Google Scholar]
- Laruelle M (2000) Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 20:423–451. [DOI] [PubMed] [Google Scholar]
- Laruelle M, D’Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, et al. (1997) Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology 17:162–174. [DOI] [PubMed] [Google Scholar]
- Lee L, Siebner HR, Rowe JB, Rizzo V, Rothwell JC, Frackowiak RS, Friston KJ (2003) Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J Neurosci 23:5308–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Kim SE, Kim WS, Han J, Kim HJ, Kim BS, Kim JY, Hong SB, Kim BG, Lee HW (2013) Cortico-cortical modulation induced by 1-Hz repetitive transcranial magnetic stimulation of the temporal cortex. J Clin Neurol 9:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, et al. (2020) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin Neurophysiol 131:474–528. [DOI] [PubMed] [Google Scholar]
- Li CT, Wang SJ, Hirvonen J, Hsieh JC, Bai YM, Hong CJ, Liou YJ, Su TP (2010) Antidepressant mechanism of add-on repetitive transcranial magnetic stimulation in medication-resistant depression using cerebral glucose metabolism. J Affect Disord 127:219–229. [DOI] [PubMed] [Google Scholar]
- Malik S, Jacobs M, Cho SS, Boileau I, Blumberger D, Heilig M, Wilson A, Daskalakis ZJ, Strafella AP, Zangen A, et al. (2018) Deep TMS of the insula using the H-coil modulates dopamine release: a crossover [11C] PHNO-PET pilot trial in healthy humans. Brain Imaging Behav 12:1306–1317. [DOI] [PubMed] [Google Scholar]
- Mason BN, Russo AF (2018) Vascular Contributions to Migraine: Time to Revisit? Front Cell Neurosci 12:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzawa M, Nakao S, Miyamoto E, Yamada M, Murao K, Nishi K, Shingu K (2003) Pentobarbital inhibits ketamine-induced dopamine release in the rat nucleus accumbens: a microdialysis study. Anesth Analg 96:148–152. [DOI] [PubMed] [Google Scholar]
- McCalley DM, Lench DH, Doolittle JD, Imperatore JP, Hoffman M, Hanlon CA (2021) Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci Rep 11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J (2006) Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 59:257–264. [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M (2006) Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse 60:485–495. [DOI] [PubMed] [Google Scholar]
- Nauczyciel C, Le Jeune F, Naudet F, Douabin S, Esquevin A, Vérin M, Dondaine T, Robert G, Drapier D, Millet B (2014) Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: a double-blind, crossover study. Transl Psychiatry 4:e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, et al. (2007) Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 62:1208–1216. [DOI] [PubMed] [Google Scholar]
- Obeso I, Cho SS, Antonelli F, Houle S, Jahanshahi M, Ko JH, Strafella AP (2013) Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul 6:769–776. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Hayashi T, Okabe S, Nonaka I, Matsuda H, Iida H, Imabayashi E, Watabe H, Miyake Y, Ogawa M, et al. (2004) Endogenous dopamine release induced by repetitive transcranial magnetic stimulation over the primary motor cortex: an [11C]raclopride positron emission tomography study in anesthetized macaque monkeys. Biol Psychiatry 55:484–489. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Imabayashi E, Okabe S, Takano H, Arai N, Ugawa Y (2004) rCBF changes elicited by rTMS over DLPFC in humans. Suppl Clin Neurophysiol 57:715–720. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117:847–858. [DOI] [PubMed] [Google Scholar]
- Paus T, Castro-Alamancos MA, Petrides M (2001) Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14:1405–1411. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC (1997) Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 17:3178–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC (1998) Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol 79:1102–1107. [DOI] [PubMed] [Google Scholar]
- Pell GS, Harmelech T, Zibman S, Roth Y, Tendler A, Zangen A (2022) Efficacy of Deep TMS with the H1 Coil for Anxious Depression. J Clin Med 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE (1979) Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 6:371–388. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J (1983) Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med 24:790–798. [PubMed] [Google Scholar]
- Rounis E, Lee L, Siebner HR, Rowe JB, Friston KJ, Rothwell JC, Frackowiak RS (2005) Frequency specific changes in regional cerebral blood flow and motor system connectivity following rTMS to the primary motor cortex. Neuroimage 26:164–176. [DOI] [PubMed] [Google Scholar]
- Sibon I, Strafella AP, Gravel P, Ko JH, Booij L, Soucy JP, Leyton M, Diksic M, Benkelfat C (2007) Acute prefrontal cortex TMS in healthy volunteers: effects on brain 11C-alphaMtrp trapping. Neuroimage 34:1658–1664. [DOI] [PubMed] [Google Scholar]
- Siebner H, Peller M, Bartenstein P, Willoch F, Rossmeier C, Schwaiger M, Conrad B (2001) Activation of frontal premotor areas during suprathreshold transcranial magnetic stimulation of the left primary sensorimotor cortex: a glucose metabolic PET study. Hum Brain Mapp 12:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Auer C, Bartenstein P, Drzezga A, Schwaiger M, Conrad B (1999) Imaging functional activation of the auditory cortex during focal repetitive transcranial magnetic stimulation of the primary motor cortex in normal subjects. Neurosci Lett 270:37–40. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P (2000) Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology 54:956–963. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Takano B, Peinemann A, Schwaiger M, Conrad B, Drzezga A (2001) Continuous transcranial magnetic stimulation during positron emission tomography: a suitable tool for imaging regional excitability of the human cortex. Neuroimage 14:883–890. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Willoch F, Peller M, Auer C, Boecker H, Conrad B, Bartenstein P (1998) Imaging brain activation induced by long trains of repetitive transcranial magnetic stimulation. Neuroreport 9:943–948. [DOI] [PubMed] [Google Scholar]
- Speer AM, Kimbrell TA, Wassermann EM, Repella JD, Willis MW, Herscovitch P, Post RM (2000) Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 48:1133–1141. [DOI] [PubMed] [Google Scholar]
- Speer AM, Willis MW, Herscovitch P, Daube-Witherspoon M, Shelton JR, Benson BE, Post RM, Wassermann EM (2003a) Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: I. Effects of primary motor cortex rTMS. Biol Psychiatry 54:818–825. [DOI] [PubMed] [Google Scholar]
- Speer AM, Willis MW, Herscovitch P, Daube-Witherspoon M, Shelton JR, Benson BE, Post RM, Wassermann EM (2003b) Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: II. Effects of prefrontal cortex rTMS. Biol Psychiatry 54:826–832. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O (2005) Corticostriatal functional interactions in Parkinson’s disease: a rTMS/[11C]raclopride PET study. Eur J Neurosci 22:2946–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T (2001) Cerebral blood-flow changes induced by paired-pulse transcranial magnetic stimulation of the primary motor cortex. J Neurophysiol 85:2624–2629. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A (2001) Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21:RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A (2003) Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126:2609–2615. [DOI] [PubMed] [Google Scholar]
- Takano B, Drzezga A, Peller M, Sax I, Schwaiger M, Lee L, Siebner HR (2004) Short-term modulation of regional excitability and blood flow in human motor cortex following rapid-rate transcranial magnetic stimulation. Neuroimage 23:849–859. [DOI] [PubMed] [Google Scholar]
- Tastevin M, Richieri R, Boyer L, Fond G, Lançon C, Guedj E(2020) Brain PET metabolic substrate of TMS response in pharmaco-resistant depression. Brain Stimul 13:683–685. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9:557–569. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL (2000) Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res 34:35–43. [DOI] [PubMed] [Google Scholar]
- Zangen A, Moshe H, Martinez D, Barnea-Ygael N, Vapnik T, Bystritsky A, Duffy W, Toder D, Casuto L, Grosz ML, et al. (2021) Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 20:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, Müller-Dahlhaus F (2015) TMS and drugs revisited 2014. Clin Neurophysiol 126:1847–1868. [DOI] [PubMed] [Google Scholar]