Abstract
Background
The world symposium on pulmonary hypertension (PH) has proposed that PH be defined as a mean pulmonary artery pressure (mPAP) > 20 mmHg as assessed by right heart catheterisation (RHC). Transthoracic echocardiography (TTE) is an established screening tool used for suspected PH. International guidelines recommend a multi-parameter assessment of the TTE PH probability although effectiveness has not been established using real world data.
Study aims
To determine accuracy of the European Society of Cardiology (ESC) and British Society of Echocardiography (BSE) TTE probability algorithm in detecting PH in patients attending a UK PH centre. To identify echocardiographic markers and revised algorithms to improve the detection of PH in those with low/intermediate BSE/ESC TTE PH probability.
Methods
TTE followed by RHC (within 4 months after) was undertaken in patients for suspected but previously unconfirmed PH. BSE/ESC PH TTE probabilities were calculated alongside additional markers of right ventricular (RV) longitudinal and radial function, and RV diastolic function. A refined IMPULSE algorithm was devised and evaluated in patients with low and/or intermediate ESC/BSE TTE PH probability.
Results
Of 310 patients assessed, 236 (76%) had RHC-confirmed PH (average mPAP 42.8 ± 11.7). Sensitivity and specificity for detecting PH using the BSE/ESC recommendations was 89% and 68%, respectively. 36% of those with low BSE/ESC TTE probability had RHC-confirmed PH and BSE/ESC PH probability parameters did not differ amongst those with and without PH in the low probability group. Conversely, RV free wall longitudinal strain (RVFWLS) was lower in patients with vs. without PH in low BSE/ESC probability group (− 20.6 ± 4.1% vs − 23.8 ± 3.9%) (P < 0.02). Incorporating RVFWLS and TTE features of RV radial and diastolic function (RVFAC and IVRT) within the IMPULSE algorithm reduced false negatives in patients with low BSE/ESC PH probability by 29%. The IMPULSE algorithm had excellent specificity and positive predictive value in those with low (93%/80%, respectively) or intermediate (82%/86%, respectively) PH probability.
Conclusion
Existing TTE PH probability guidelines lack sensitivity to detect patients with milder haemodynamic forms of PH. Combining additional TTE makers assessing RV radial, longitudinal and diastolic function enhance identification of milder forms of PH, particularly in those who have a low BSE/ESC TTE PH probability.
Supplementary Information
The online version contains supplementary material available at 10.1186/s44156-022-00010-9.
Keywords: Echocardiography, Pulmonary hypertension, RV free wall longitudinal strain
Introduction
Pulmonary hypertension (PH) is a relatively common complication of lung or heart disease and more rarely from pulmonary vascular abnormalities for example proliferative vasculopathy or thromboembolic obstruction. Irrespective of cause, PH is associated with increased mortality and morbidity [1]. Historically, PH has been defined as a mean pulmonary artery pressure (mPAP) of ≥ 25 mmHg at rest as assessed by right heart catheterization (RHC) [2–4]. At the World symposium on Pulmonary Hypertension in 2018 a proposal was made to lower the diagnostic threshold to a mPAP > 20 mmHg as this represents 2SD above normal [3].
Untreated PH leads to right ventricular (RV) dysfunction and ultimately failure resulting in exertional dyspnoea, presyncope, chest pain and peripheral oedema. Early identification of PH allows prompt intervention with increasingly effective therapeutic interventions. However, many years may pass between the onset of symptoms and correct diagnosis, delaying potential treatment [5, 6].
Transthoracic echocardiography (TTE) is an established screening tool to asses non-invasively for markers of PH. TTE is a key screening tool due to its wide availability, portability, and cost effectiveness [4, 7, 8].
Doppler TTE estimates of PASP are frequently discordant with RHC evaluation [9–11]. Doppler TTE PASP estimates show good correlations across patient populations but with only moderate precision on an individual basis. Such imprecision can lead to both under and overestimation of true PASP resulting in either a failure to identify PH or lead to unnecessary invasive diagnostic tests [4, 12].
For this reason, both European Society of Cardiology (ESC) [2] and British Society of Echocardiography (BSE) [4] recommend a multi parameter assessment of the TTE probability of PH that incorporates assessment of the peak tricuspid regurgitation velocity (TRV) together with three main categories (A: The ventricles B: Pulmonary artery C: Inferior vena cava (IVC) and right atrium). These measures help to evaluate RV size and pressure overload, the profile of blood flow velocity at the pulmonary valve, the diameter of the pulmonary artery (PA) and an estimate of right atrial pressure [2]. When determining the BSE/ESC TTE probability of PH, the TRV is used in conjunction with the TTE markers described in Table 1 to assign the probability of PH being present (see Fig. 1) [4]. Following ESC and BSE guideline implementation [2, 4] there has been limited research in real world populations to assess the effectiveness of these recommendations in detecting PH.
Table 1.
Echocardiographic parameters used to assess the echocardiographic probability of pulmonary hypertension
| A: The ventricles* | B: Pulmonary artery* | C: Inferior vena cava and right atrium* |
|---|---|---|
| Right ventricle/left ventricle basal diameter ratio > 1.0 | Right ventricular outflow Doppler acceleration time < 105 ms and/or mid systolic notching | Inferior vena cava diameter > 21 mm with decreased inspiratory collapse (< 50% with a sniff or < 20% with quiet respiration) |
| Flattening of the interventricular septum (left ventricular eccentricity index > 1.1 in systole or both systole and diastole) | Early diastolic pulmonary regurgitation velocity > 2.2 m/s | Right atrial area (end systole) > 18 cm2 |
| Pulmonary artery diameter > 25 mm |
Adapted from British Society of Echocardiography guidelines on the assessment of suspected pulmonary hypertension [4]. *Echocardiographic parameters from at least two different categories (A/B/C) from the list should be present to alter the level of echocardiographic probability of pulmonary hypertension
Fig. 1.
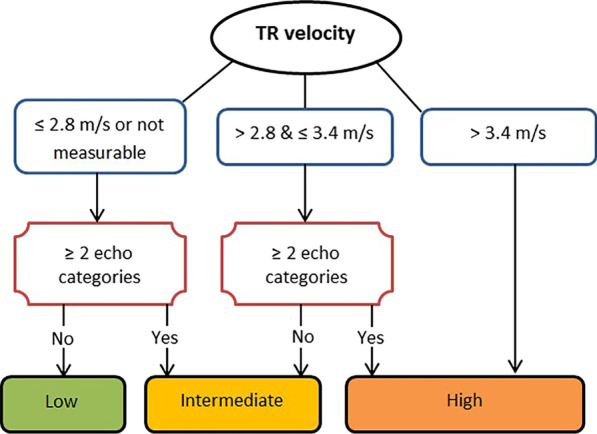
Flow chart to assess the probability of pulmonary hypertension using parameters identified from within ≥ 2 categories (the ventricles, pulmonary artery or the inferior vena cava and right atrium) in conjunction with tricuspid regurgitation velocity. Adapted from [3]
Research has demonstrated that right ventricular free wall longitudinal strain (RVFWLS) is reduced in those with PH compared to controls [13, 14]. Evidence also suggests that RVFWLS may provide important prognostic information in those with PH [13, 14]. It has also been hypothesised that in those with PH a reduction in radial RV function is one of the first parameters to deteriorate, therefore assessment by right ventricular fractional area of change (RVFAC) may be a particularly sensitive indicator of elevated pressures [15]. Furthermore, markers of RV diastolic dysfunction are abnormal in those with PH compared to controls [16]. However, to our knowledge no previous studies have looked at the added predictive value of markers including RVFWLS, RVFAC and RV diastolic function indices in the TTE screening of those with suspected PH.
The aims of this study were to determine the accuracy of the BSE/ESC TTE probability algorithm in detecting PH in a real-world population being assessed in a shared care UK PH centre. The study population was used as a derivation cohort to identify other echocardiographic markers which may help to improve identification of those with PH and either low or intermediate BSE/ESC TTE PH probability.
Methods
Subjects and study procedures
Patients attending for initial evaluation of PH between August 2010 and March 2020 were assessed. Exclusion criteria included those with known PH; RHC not performed within 4-months of TTE or RHC performed prior to TTE. A clinical diagnosis of pre-capillary PH was defined as a RHC mPAP > 20 mmHg and PVR ≥ 3 woods units (WU). Post-capillary PH was defined as a mPAP > 20 mmHg and a PCWP > 15 mmHg, as per ESC and WSPH guidelines [3]. PH aetiologies were categorised according to the WHO classifications of PH [3].
RHC was only performed in those with a strong clinical suspicion of PH. RHC in those with low TTE PH probability was only performed in those with symptoms consistent with suspected PH, strong risk factors or suspicion of PH from other imaging modalities.
Relevant demographic and clinical characteristics were recorded. Any echocardiographic parameters required to calculate the BSE/ESC TTE PH probability that had not been reported at the time of the initial test were retrospectively re-measured by two experienced echocardiographers blinded to PH status. PH TTE probabilities were calculated following BSE/ESC [2, 4] guidance.
Echocardiographic measures of right heart size and function were performed in accordance with BSE guidelines [17]. LV dedicated AFI speckle-tracking software was used to measure RVFWLS. An average of the basal, mid and apical RV free wall segments was used to quantify RVFWLS. For the purposes of this study more positive RVFWLS values are referred to as ‘lower RVFWLS’ to emphasise abnormality.
Ethics approval was obtained from both the RUH Bath NHS Foundation Trust R&D department and Manchester Metropolitan University Ethos ethics committee.
Development of IMPULSE algorithm
The IMPULSE algorithm (Fig. 2) was developed to see whether including additional markers of RV longitudinal, radial and diastolic function could help identify PH in those with a low or intermediate TTE probability. Markers including RVFWLS, RVFAC and RV TDI IVRT were chosen as they could be measured retrospectively and had acceptable diagnostic accuracy when evaluated by ROC analysis.
Fig. 2.
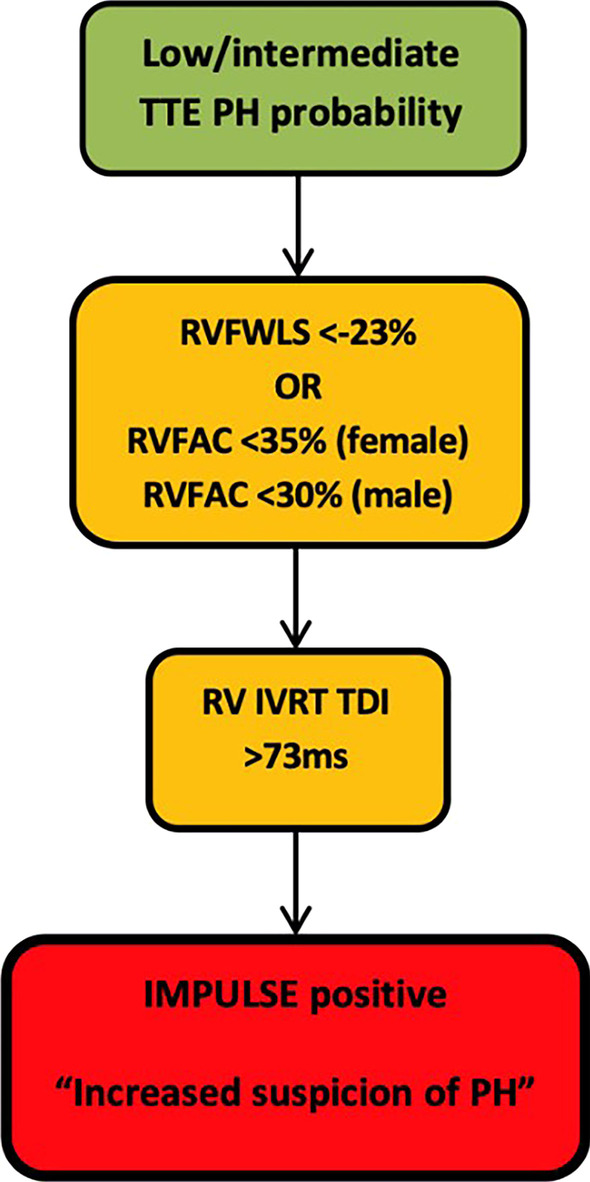
Proposed additional algorithm for those with low or intermediate TTE probability to help identify those with PH and low TTE probability. RVFAC normal cut-off values taken from (Harkness et al., 2020). RVFWLS (right ventricular free wall longitudinal strain); RVFAC (right ventricular fractional area change); IVRT TDI (isovolumetric relaxation time by tissue Doppler imaging); IMPULSE (improving pulmonary hypertension screening by echocardiography)
Statistical analysis
All statistical analysis was performed using SPSS version-26. Categorical variables are summarised as percentage frequencies and continuous variables as mean ± SD. The dataset was tested for normality using the Shapiro–Wilk test. Proportions were compared using the Chi-squared test and Fisher’s exact test. Comparisons of means were analysed using the Mann–Whitney U tests and Kruskal–Wallis tests. Receiver operating characteristic (ROC) curve analysis was used to establish optimal sensitivities and specificities for the individual PH TTE parameters.
When calculating sensitivities and specificities of the ESC/BSE TTE PH probability algorithm those with either high or intermediate TTE probability were deemed as positives for PH whilst those with low TTE PH probability were deemed as negative for PH.
Univariate Cox proportional hazard ratios were determined to assess the predictive value of RVFWLS for all-cause mortality in PH. Kaplan–Meier survival curve analysis was used to estimate the distribution of time from PH diagnosis to all-cause mortality. ROC analysis was used to determine the optimal cut-off RVFWLS value for predicting all-cause mortality.
Results
Clinical and functional characteristics
Of 498 patients undergoing TTE assessment, 310 eligible patients were identified for this analysis. Their clinical characteristics are described in Additional file 1: Table S1. There was a female predominance (62%) with a mean age of 67 ± 14 years. The median time from TTE to RHC was 24 days (IQR, 8–46 days).
A summary of clinical and haemodynamic parameters in those with PH according to BSE/ESC TTE PH probability is shown in Table 2. Seventy-six percent of the cohort (N = 236) had RHC PH (average mPAP of 42.8 ± 11.7 mmHg) with the most frequent aetiology being chronic thromboembolic PH (CTEPH). Average NT-proBNP levels were higher in those with PH (1817.6 ± 2123 ng/L) when compared to those without (286 ± 459.8 ng/L) (P < 0.001). Those with PH covered significantly lower six-minute walk test (6MWT) distance (299.9 ± 153.2 m) compared to those without PH (372.9 ± 127.2 m, P < 0.001).
Table 2.
Clinical and haemodynamic parameters in those with either low, intermediate or high BSE/ESC TTE PH probability (N = 310)
| TTE parameters | Low (N = 78) | Intermediate (N = 68) | High (N = 164) |
|---|---|---|---|
| PAH | 4 | 17 | 42 |
| LHD | 19 | 14 | 28 |
| CTEPH | 5 | 11 | 64 |
| Lung disease | 2 | 6 | |
| Multifactorial/unclear | 6 | 18 | |
| No PH | 50 | 18 | 6 |
| Haemodynamic data | (N = 78) | (N = 68) | (N = 164) |
| mPAP (mmHg) | 31.3 ± 6.9 | 37.4 ± 9.7 | 46.3 ± 11.2 |
| PVR (mmHg) | 3.0 ± 1.6 | 5.4 ± 3.2 | 7.7 ± 3.8 |
| PCWP (mmHg) | 15.1 ± 4.8 | 14.1 ± 5.4 | 16.9 ± 6.1 |
| PA sats (%) | 71.6 ± 4.0 | 66.1 ± 8.2 | 63.0 ± 8.7 |
| CO (L/min) | 5.1 ± 1.4 | 4.7 ± 1.3 | 4.3 ± 1.2 |
| CI ((L/min/m2) | 2.8 ± 0.7 | 2.5 ± 0.7 | 2.4 ± 0.6 |
| NT Pro BNP (ng/L) | 321.1 ± 341.6 | 1039.7 ± 1393.9 | 2282.5 ± 2995.5 |
| 6MWT (m) | 318.9 ± 178.7 | 298.6 ± 164.4 | 262.7 ± 144.7 |
PAH pulmonary arterial hypertension, LHD left heart disease, CTEPH chronic thromboembolic pulmonary hypertension, mPAP mean pulmonary artery pressure, PVR pulmonary vascular resistance, PCWP pulmonary capillary wedge pressure, PA sats pulmonary artery saturations, CO cardiac output, CI cardiac index, NT pro BNP N-terminal pro hormone of brain natriuretic peptide, 6MWT six-min walk test
ESC/BSE TTE probability of PH
TTE PH probabilities (high N = 164; 53%, intermediate N = 68; 22% and low 78; 25%) and aetiologies of PH are further detailed in Table 2. Ninety-six percent and 75%, of those with high or intermediate TTE probability had RHC PH, respectively. Thirty-six percent (N = 28) of those with low TTE probability had PH definitively diagnosed at RHC. Their PH aetiologies are depicted in Fig. 3. The four patients with PAH all had systemic scleroderma which accounted for 9% of all those in the cohort with systemic scleroderma associated PH. The calculated sensitivity and specificity of the ESC/BSE TTE algorithm for detecting PH was 88% and 68%, respectively.
Fig. 3.
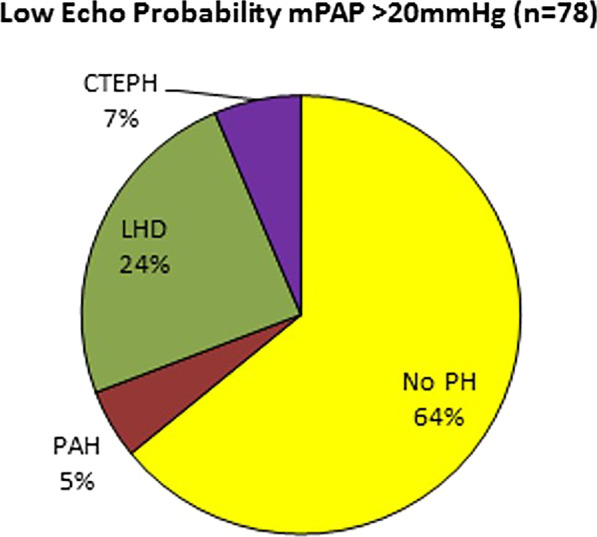
Comparison of the frequency of WHO Classification of PH in those with an low echocardiographic probability of PH (N = 78)
The peak TRV was measurable in 83% of the overall cohort and in 92% of those with RHC PH. However, the peak TRV was only measurable in 63% of those with a low TTE probability of PH. Most other conventional TTE parameters were frequently measurable (Table 3). However, the main pulmonary artery (MPA) diameter and pulmonary regurgitation velocity at beginning of diastole (PRVBD) were frequently unmeasurable (measurements able to be made in 40% and 30%, respectively).
Table 3.
Comparison of TTE PH parameters in those with and without PH
| TTE parameters | PH (N = 236) | No PH (N = 74) | Statistical significance |
|---|---|---|---|
| Peak TRV (m/s) (N = 257) | 3.6 ± 0.84 | 2.56 ± 0.4 | P < 0.001 |
| RV/LV BASAL diameter ratio (N = 249) | 1.01 ± 0.31 | 0.78 ± 0.21 | P < 0.001 |
| End systolic eccentricity index (N = 274) | 1.28 ± 0.36 | 0.99 ± 0.18 | P < 0.001 |
| End diastolic eccentricity index (N = 267) | 1.12 ± 0.28 | 0.90 ± 0.17 | P < 0.001 |
| RVOT acceleration time (ms) (N = 290) | 81 ± 25 | 109 ± 31 | P < 0.001 |
| MPA diameter (mm) (N = 125) | 24 ± 6 | 20 ± 5 | P < 0.01 |
| PRVBD (m/s) (N = 92) | 2.34 ± 0.41 | 1.5 ± 0.36 | P < 0.001 |
| Right atrial area (cm2) (N = 310) | 20.9 ± 8.4 | 15.4 ± 5.8 | P < 0.001 |
| Frequency with IVC diameter > 21 mm and reduced collapsibility (N = 273) | N = 41 (17%) | N = 2 (3%) | P < 0.001 |
| RVFWLS (-%) (N = 155) | − 17.4 ± 6.8 | − 24.1 ± 5.1 | P < 0.001 |
| RVFAC (%) (N = 81) | 27.2 ± 12 | 39.1 ± 9.7 | P < 0.001 |
| Indexed right atrial area (cm2/m2) (N = 268) | 11.16 ± 4.6 | 8 ± 3.4 | P < 0.001 |
| RV TDI E’ (N = 228) | 9.02 ± 3.45 | 10.63 ± 3.19 | P < 0.01 |
| RV TDI E’/A’ ratio (N = 219) | 0.71 ± 0.52 | 0.82 ± 0.36 | P < 0.01 |
| RV TDI IVRT (N = 164) | 98.1 ± 39.2 | 76.5 ± 26.2 | P < 0.001 |
TTE transthoracic echocardiography, TRV tricuspid regurgitant velocity, RV right ventricle, LV left ventricle, PRVBD pulmonary regurgitant velocity at begging of diastole, MPA main pulmonary artery, RVOT right ventricular outflow tract, MSN mid-systolic notching, IVC inferior vena cava, RVFWLS right ventricular free wall longitudinal strain, TDI tissue doppler imaging, IVRT isovolumetric relaxation time, RVFAC right ventricular fractional area of change
Accuracy of echocardiographic parameters in those with PH
A comparison of TTE markers in those with and without PH can be seen in Table 3. Table 4 demonstrates ROC analysis of the TTE PH parameters. The peak TRV displayed the greatest diagnostic accuracy. The PRVBD similarly demonstrated good diagnostic accuracy with very high specificity but low sensitivity.
Table 4.
Receiver operating characteristic analysis of the TTE PH parameters
| TTE parameter | BSE Cut-off value | AUC | 95% CI | P-value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Lower peak TRV (m/s) (N = 257) | > 2.8 | 0.87 | 0.83–0.91 | P < 0.001 | 83 | 73 |
| Upper peak TRV (m/s) (N = 257) | > 3.4 | 0.87 | 0.83–0.91 | P < 0.001 | 60 | 98 |
| End systolic eccentricity index (N = 274) | > 1.1 | 0.78 | 0.72–0.84 | P < 0.001 | 64 | 83 |
| End diastolic eccentricity index (N = 267) | > 1.1 | 0.75 | 0.69–0.81 | P < 0.001 | 46 | 93 |
| RV/LV basal diameter ratio (N = 249) | > 1.0 | 0.74 | 0.67–0.81 | P < 0.001 | 47 | 91 |
| RVOT acceleration time (ms) (N = 290) | < 105 | 0.78 | 0.71–0.84 | P < 0.001 | 84 | 54 |
| MPA diameter (mm) (N = 125) | > 25 | 0.71 | 0.60–0.81 | P < 0.01 | 40 | 91 |
| PRVBD (m/s) (N = 92) | > 2.2 | 0.82 | 0.73– 0.91 | P < 0.001 | 61 | 94 |
| Right atrial area (cm2) (N = 310) | > 18 | 0.73 | 0.66–0.79 | P < 0.001 | 57 | 81 |
| RVFWLS (-%) (N = 155) | < − 23 | 0.78 | 0.71–0.85 | P < 0.001 | 81 | 58 |
| RVFAC (%) (male) (N = 33) | < 30 | 0.72 | 0.61–0.83 | P < 0.001 | 65 | 69 |
| RVFAC (%) (female) (N = 48) | < 35 | 0.72 | 0.61–0.83 | P < 0.001 | 64 | 70 |
| Indexed right atrial area (cm2/m2) (N = 268) | > 11 | 0.74 | 0.67–0.81 | P < 0.001 | 42 | 89 |
| RV TDI E’ (N = 228) | < 8.5 | 0.67 | 0.60–0.74 | P < 0.001 | 55 | 74 |
| RV TDI E’/A’ ratio (N = 219) | < 0.65 | 0.66 | 0.58–0.73 | P < 0.001 | 86 | 28 |
| RV TDI IVRT (N = 164) | > 73 | 0.72 | 0.62–0.82 | P < 0.001 | 81 | 62 |
TTE Transthoracic echocardiography, AUC area under curve, CI confidence interval, BSE British Society of Echocardiography, TRV tricuspid regurgitant velocity, RV right ventricle, LV left ventricle, PRVBD pulmonary regurgitant velocity at begging of diastole, MPA main pulmonary artery, RVOT right ventricular outflow tract, MSN mid-systolic notching, IVC inferior vena cava, RVFWLS right ventricular free wall longitudinal strain, TDI tissue doppler imaging, IVRT isovolumetric relaxation time, RVFAC right ventricular fractional area of change, RVOT right ventricular outflow tract, PRVBD pulmonary regurgitation velocity beginning of diastole, RVFWLS right ventricular free wall longitudinal strain, RVFAC right ventricular fractional area of change
Other conventional TTE parameters used to assess BSE/ESC PH probability exhibited acceptable diagnostic accuracy typically demonstrating low sensitivity but high specificity for detecting PH (Table 4). A re-audit of the TTE PH probabilities using ROC defined cut-off values did not significantly increase sensitivity or specificity of the BSE/ESC TTE PH probability algorithm (Additional file 1: Tables S2, S3).
RVFWLS analysis
RVFWLS was measurable in 50% (n = 155) and was significantly lower in those with PH (− 17.4 ± 6.8%) compared to those without (− 24.1 ± 5.1%), (P < 0.001), (Additional file 1: Fig. S1a). RVFWLS was also lower in those with a high TTE PH probability compared to both intermediate and low probability groups (P < 0.001), (Additional file 1: Fig. S1b). RVFWLS demonstrated acceptable diagnostic accuracy with sensitivity and specificity of 81% and 58%, respectively, using a cut-off value of < − 23%. Subgroup analysis in those with pre-capillary PH exhibited sensitivity and specificity of 84% and 60%, respectively.
Interestingly, RVFWLS was significantly lower in those with low TTE probability and PH (− 20.6 ± 4.1%) compared to those without PH (− 23.8 ± 3.9%), (P < 0.02), (Additional file 1: Fig. S2a, b). RVFWLS was also lower in those with intermediate TTE probability and PH (− 20.14 ± 7.48%) compared to those without (− 25 ± 7.34%) (p = 0.09). RVFWLS was only measurable in 1 patient with high TTE probability and no PH with an RVFWLS value of − 23.7%.
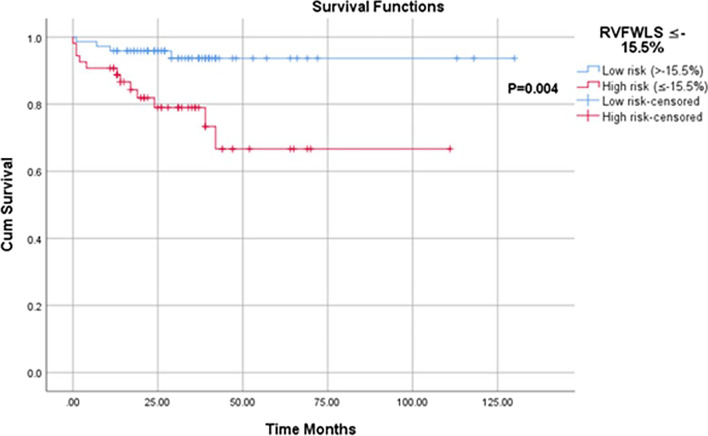
There were 38 documented deaths (all-causes) during a median follow-up of 2 years (IQR 1–3 years). Of these deaths, 37 occurred in those with PH and of these 84% (N = 31) had a high TTE PH probability. RVFWLS was significantly lower in those with PH that died (− 14 ± 8% vs − 17.8 ± 6.6%, P < 0.05). Univariate hazard ratios demonstrated an increased risk of all-cause mortality in those with an RVWLS ≤ − 15.5% [4.517, 95% CI 1.454–14.038]. Kaplan–Meier survival analysis demonstrated those with PH and an RVFWLS ≤ − 15.5% had an increased risk of all-cause mortality (p < 0.01) (Fig. 4).
Fig. 4.
Kaplan–Meier survival curve analysis in 127 PH patients for RVFWLS over a follow-up period of 29 [19–40] months. RVFWLS was categorised into low risk (blue line, n = 73) and high risk (red line, n = 54). ROC derived cut-off RVFWLS value was used to categorise those at low (> − 15%) or high (≤ − 15.5%) risk of all-cause mortality. Kaplan–Meier survival curve analysis demonstrated a significant difference in survival between those with low risk RVFWLS scores and high risk RVFWLS scores (P < 0.01)
RV diastolic parameters
Markers of RV diastolic function including indexed RAA, TDI E’, TDI E’:A’ and IVRT by TDI were significantly worse in those with PH compared to those without (all, P < 0.05) (Table 3; Additional file 1: Table S4). A meaningful assessment of RV E:A ratios, deceleration times and E/e’ was not possible as trans-tricuspid PW Doppler was only used in 8% (N = 25).
Of the RV diastolic parameters studied, the TDI IVRT demonstrated the highest diagnostic accuracy when using a cut-off value of > 73 ms (Table 4; Additional file 1: Fig. S3). RV diastolic parameters are further described in Additional file 1: Fig. S4a–c. There was a trend for higher average IVRT values in those with intermediate and low TTE probability and PH compared to those without PH (Additional file 1: Fig. S5a, b).
RVFAC analysis
RVFAC was significantly lower in those with PH compared to those without (P < 0.001). RVFAC also demonstrated acceptable diagnostic accuracy using a cut-off RVFAC of < 30% in males and < 35% in females. RVFAC was retrospectively measurable in 45% of those with low and intermediate TTE PH probability. In subgroup analysis of those with low TTE probability, RVFAC trended to be lower in those with PH compared to those without (32.7 ± 11.1% vs 38.6 ± 9.7%, P = 0.05) and was significantly lower in those with pre-capillary PH (25.9 ± 8.7% vs 38.6 ± 9.7%, P < 0.01). In those with intermediate TTE probability RVFAC was significantly lower in those with PH compared to those without (27.46 ± 13.1% vs 42.33 ± 10%, P < 0.02).
Low TTE probability subgroup analysis
Of those with low TTE probability and PH, mPAP and PVR were significantly lower compared to those with intermediate or high TTE probability (Fig. 5a, b). In those with low TTE probability, there was no significant difference in 6MWT distances, BORG scores or NYHA classifications between those with PH and those without. NT-proBNP values were significantly higher in those with LHD PH compared to those without (462.6 ± 428.1 ng/L vs 148.4 ± 107.7 ng/L, P < 0.01) but not significantly different between those with pre-capillary PH and no PH (152.8 ± 113.2 vs 169.1 ± 298.1, P = 0.78).
Fig. 5.
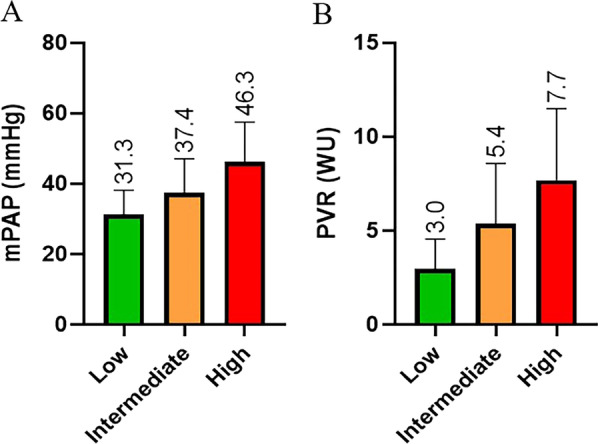
a Comparison of mPAP (mmHg) relative to TTE probability of PH. Kruskal–Wallis analysis demonstrated mPAP values were significantly different across TTE PH probability groups; Kruskal–Wallis H = 138.2 (2) (P < 0.0001). Pairwise analysis with adjusted P-values demonstrated mPAP was significantly higher in those with either high or intermediate TTE probability compared to low (both P < 0.0001). mPAP was also significantly higher in those with intermediate compared to low TTE probability (P < 0.0001). mPAP (mean pulmonary artery pressure). b Comparison of PVR (woods units) relative to TTE probability of PH. Kruskal–Wallis analysis demonstrated PVR was significantly different across TTE PH probability groups; H = 138.3(2) (P < 0.0001). Pairwise analysis with adjusted P-values demonstrated PVR was significantly higher in those with high or intermediate TTE probability compared to low probability (both P < 0.0001). PVR was also significantly elevated in those with intermediate compared to low TTE probability (P < 0.0001). PVR (pulmonary vascular resistance)
Table 5 compares TTE PH parameters in those with a low TTE probability and RHC PH to those with no RHC PH. None of the BSE/ESC TTE PH probability parameters were significantly different between those with PH and without. RVFWLS however was significantly lower in those with PH (P < 0.02). In addition to RVFWLS, RVFAC was lower in those with PH (p = 0.05) and significantly lower when excluding those with LHD (P < 0.01). Three with PH had an RV wall thickness > 5 mm compared to none without PH (P < 0.03). Those with PH and low TTE PH probability had significantly higher biplane LA volumes (67.9 ± 39.4 mL vs 49.4 ± 18.6 mL) (P < 0.05) and E/e′ (9.3 ± 3.5 vs 6.5 ± 2.3) (P < 0.05) reflecting the higher proportion of PH due to LHD. There was also a trend towards higher IVRT values in those with PH (NS).
Table 5.
Summary of PH Echocardiographic parameters in those with low TTE probability and with PH or no PH (N = 78)
| Echocardiographic parameter | No Pulmonary Hypertension (N = 51) | Pulmonary hypertension (N = 28) | Statistical significance |
|---|---|---|---|
| Average Peak TRV (m/s) | 2.34 ± 0.31 | 2.36 ± 0.43 | NS |
| Average RV/LV basal diameter ratio | 0.72 ± 0.12 | 0.77 ± 0.14 | NS |
| End-Diastolic Eccentricity index | 0.91 ± 0.12 | 0.91 ± 0.13 | NS |
| End-Systolic Eccentricity index | 0.97 ± 0.12 | 1.0 ± 0.17 | NS |
| Early Peak Diastolic PRV (m/s) | 1.58 ± 0.31 | 1.7 ± 0.44 | NS |
| Average MPA diameter (mm) | 19.4 ± 4 | 20.6 ± 7.2 | NS |
| RVOT acceleration time (ms) | 114.6 ± 31.6 | 102.6 ± 29.8 | NS |
| Frequency of MSN notching (%) | 6 | 7 | NS |
| Frequency of IVC diameter > 21 mm with < 50% Respiratory collapse | 0 | 4 | NS |
| RAA (cm2) | 14 ± 4 | 14.6 ± 6.5 | NS |
| RVFWLS (–%) | − 23.8 ± 3.9 | − 20.6 ± 4.1 | P < 0.02 |
| RV TDI IVRT (ms) | 71.5 ± 23.9 | 80.3 ± 22 | NS |
| RV TDI E’ | 10.3 ± 3.1 | 10.1 ± 3.7 | NS |
| RV TDI E’:A’ ratio | 0.83 ± 0.38 | 0.8 ± 0.39 | NS |
| RV wall thickness > 5 mm (n = 14) | 0/9 | 3/5 (60) | P < 0.03 |
| RAAI (cm2/m2) | 7.1 ± 2.1 | 7.65 ± 3 | NS |
| RVD-1 | 3.38 ± 0.64 | 3.59 ± 0.63 | NS |
| RVEDAI (cm2/m2) | 9.34 ± 2.1 | 8.41 ± 3.45 | NS |
| RVFAC (%) | 38.6 ± 9.7 | 32.7 ± 11.1 | P = 0.05 |
| TAPSE (cm) | 2.17 ± 0.45 | 2.08 ± 0.4 | NS |
| RV S’ (cm/s) | 14.04 ± 3.28 | 13.08 ± 3.25 | NS |
| Pericardial effusion present (%) | 4 | 8 | NS |
TTE transthoracic echocardiography, PH pulmonary hypertension, TRV tricuspid regurgitant velocity, RV right ventricle, LV left ventricle, PRV pulmonary regurgitant velocity, MPA main pulmonary artery, RVOT right ventricular outflow tract, MSN mid-systolic notching, IVC inferior vena cava, RAA right atrial area, RVFWLS right ventricular free wall longitudinal strain, TDI tissue doppler imaging, IVRT isovolumetric relaxation time, RAAI right atrial area indexed, RVFAC right ventricular fractional area of change, RVEDAi right ventricular end-diastolic area indexed, TAPSE tricuspid annular plane excursion
IMPULSE algorithm
The three markers in the IMPULSE algorithm (Fig. 2) were developed because they either demonstrated a significant difference between those with/without PH or suggested a trend towards abnormality in those with PH. ROC curve analysis of markers including RVFWLS, TDI IVRT and RVFAC also demonstrated comparable diagnostic accuracy when compared to conventional PH TTE markers.
TTE PH probabilities in those with low or intermediate probability were re-evaluated using the IMPULSE algorithm which included RVFWLS, RVFAC and IVRT as additional markers of PH probability. Those who had either an RVFWLS < − 23% or reduced RVFAC (< 35% female or < 30% male) in combination with an IVRT TDI > 73 ms were deemed IMPULSE positive and therefore hypothesised to be more likely to have PH than those IMPULSE negative. Cut-off values are reflective of current BSE guidance [18].
The IMPULSE algorithm however could only be retrospectively used in 52% of those with low or intermediate TTE probability. The number of false negatives in those who had low BSE/ESC TTE PH probability (N = 28) was reduced by 29% (N = 8). Of these, 4 had pre-capillary PH (N = 2 WHO group 4, N = 2 WHO group 1). The remaining 4 had PH secondary to LHD.
In those with intermediate TTE probability and PH, IMPULSE correctly identified 85% (N = 11) of those with pre-capillary PH. IMPULSE was achievable in N = 13 (43%) of those with intermediate TTE probability and pre-capillary PH (N = 30). IMPULSE was negative in all of the 5 patients with LHD (N = 5) where IMPULSE was measurable.
The IMPULSE algorithm had excellent specificity and positive predictive value in those with a low (93% / 80%, respectively) or intermediate (82% / 86%, respectively) BSE/ESC TTE PH probability (Table 6). This compares to a positive predictive value of 74% with a BSE/ESC intermediate TTE probability only.
Table 6.
Calculated sensitivity, specificity, negative predictive value and positive predictive value of using the IMPULSE algorithm in those with low or intermediate TTE probability
| Low TTE probability (%) | Intermediate TTE probability (5) | |
|---|---|---|
| Sensitivity | 44 | 46 |
| Specificity | 93 | 82 |
| NPV | 74 | 39 |
| PPV | 80 | 86 |
Discussion
BSE/ESC TTE PH probability
PH is a progressive and life-limiting disease. Early recognition and treatment are key in determining long-term prognosis [1]. Optimal screening methods for PH involve early identification of risk factors as well as a multi-modality approach with TTE often being the first line investigation [2]. Our findings support the use of a multi-parameter TTE approach to the screening of suspected PH. In our population of patients referred to a PH centre ESC/BSE TTE probability algorithm [2, 4] provides high sensitivity but moderate specificity for identifying individuals at risk of PH. We have shown that whilst 96% of those with a high BSE/ESC TTE PH probability had RHC PH, 22% of those with an intermediate BSE/ESC TTE PH probability did not have RHC PH. Strikingly, 36% of patients with a low BSE/ESC TTE PH probability had PH. These patients typically displayed milder forms of PH haemodynamics at RHC and therefore are less likely to have positive BSE/ESC TTE PH echocardiographic markers. No clinical markers were able to distinguish those who had pre-capillary PH in this group. Only those with LHD associated PH and low TTE probability had significantly higher NT-proBNP compared to those without PH. In addition, none of the TTE parameters used in the BSE/ESC TTE PH probability algorithm were significantly different between those with or without PH. Therefore, additional echocardiographic techniques are needed to identify patients earlier in the ‘PH cascade’.
Obtaining an accurate peak TRV measurement is crucial to assessing the TTE PH probability. Peak TRV’s were unmeasurable in 8% of PH patients; 53% of whom had low TTE probability. However, even in those with a measurable TRV just under half of those with a peak TRV ≤ 2.8 m/s had PH. The most likely explanation is measurement of suboptimal Doppler signals [18], adding further evidence to suggest a cut-off TRV alone would be unreliable in detecting PH [2, 4]. Accurate measurement of the peak TRV can however be improved with the use of agitated saline [4].
The MPA diameter was frequently unmeasurable due to suboptimal imaging. There is a lack of evidence demonstrating that MPA dilatation is a sensitive marker at detecting PH [18]. Our data suggests MPA dilatation shows poor sensitivity but high specificity in detecting PH. It could be concluded that more sensitive markers of PH could replace MPA dilation in the TTE algorithm. Retrospective measurement of the PRVBD was frequently hampered by poor PR Doppler optimisation.
The remaining TTE parameters were frequently measurable but were often only abnormal in those with higher mPAP at RHC. These markers often provided good specificity but low sensitivity for detecting milder forms of PH. This is perhaps explained by the fact that RV hypertrophy (RVH) precedes dilatation during progressive RV pressure overload in PH [18, 19]. Right atrial area indexed was significantly higher in those with PH further increasing specificity however at the expense of sensitivity. These markers were highly specific for PH but were unable to identify those earlier in the disease process.
We have shown that the existing cut-offs for the currently used BSE/ESC echocardiographic parameters to assess the probability of PH are similar to the optimal values in our real-world cohort. This reinforces our findings that additional echocardiographic markers to assess the probability of PH are needed in those with milder forms of PH which current echocardiographic measures may not be sufficient to detect. We have found that using a combination of RVFWLS, RVFAC and RV IVRT may help to identify those with milder forms of PH. Theses markers represent sensitive indices of RV longitudinal, radial and diastolic function parameters.
RVFAC
Measuring RVFAC is recommended when evaluating RV function in PH [18]. Gender specific cut-off values of normality are recommended [20]. We found RVFAC to be significantly lower in those with low BSE/ESC TTE PH probability and pre-capillary PH. It was also significantly lower in those with intermediate BSE/ESC TTE PH probability and PH compared to those without PH. It is thought that radial function is more predictive of global RV function in states of increased afterload such as PH [21]. In addition, there is an increased contribution of circumferential and radial shortening to RV function in PH patients due to RVH [19]. Conventional measures of RV longitudinal function including TAPSE and RV S’ therefore may not reflect global RV dysfunction in PH, especially in milder forms of PH. RVFAC is a simple method of assessing RV systolic function that has been shown to correlate with prognosis and response to treatment in PH patients [22, 23]. However, accurate measurements can be limited by poor endocardial definition and off-axis imaging [24]. We found that RVFAC was retrospectively measurable in 65% of those with low TTE probability. However, with an increased focus on obtaining suitable imaging to accurately measure RVFAC the proportion in whom this measurement is available in would likely increase.
RVFWLS
Whilst RVFAC incorporates RV radial assessment, RVFWLS is a sensitive measure of longitudinal function. LV GLS has been routinely utilised to identify early markers of LV dysfunction [25, 26]. Previous studies have shown RVFWLS is reduced in those with PH compared to controls and also adds prognostic value [13, 14]. RVFWLS is also closely correlated to RV ejection fraction by CMR [27]. However, to our knowledge no studies have evaluated RVFWLS in screening for PH. We have demonstrated that RVFWLS is able to help distinguish those with PH from those without in a cohort of low BSE/ESC TTE PH probability patients. RVFWLS was also lower in those with PH and an intermediate TTE probability although did not reach statistical significance. This may have been due to low number of patients with no PH and measurable RVFWLS in this group (N = 12). It is important to note however that no one TTE marker in isolation can identify PH [18]. In addition, baseline RVFWLS can provide prognostic information. RVFWLS is believed to be less susceptible to the assumptions and limitations of conventional parameters of RV function including angle dependency [14, 28]. Including RVFWLS in those with a low or intermediate TTE probability may help identify those with PH.
There are limitations associated with RVFWLS. It relies on good endocardial definition and therefore isn’t achievable in every patient. Retrospective measurements were made in just over half of those with low or intermediate TTE probability. However, prospective measurements are likely to be more feasible [29, 30]. Previous literature has suggested good reproducibility in RVFWLS measurements [14, 30]. In addition, there is no definitive cut-off value of normality across vendor platforms for RVFWLS. A provisional cut-off of -23% has been suggested which was the level that provided optimal sensitivity and specificity for detecting PH in our cohort [17, 31]. The routine adoption of RVFWLS has been previously limited by inter-vendor variability [17]. Guidance on the standardisation of RVFWLS measurement has been published including the use of the RV focused A4C view and dedicated RVFWLS analysis software [17]. [31].
RV diastolic dysfunction
We hypothesised that markers of RV diastolic dysfunction may help identify those with PH and low or intermediate TTE PH probability. To our knowledge no previous studies have assessed the use of RV diastolic markers in screening for PH. RV E′ and RV TDI E′/A′ ratios were significantly lower in those with PH. TDI IVRT was significantly prolonged in those with PH, displaying good sensitivity but moderate specificity in detecting PH. There was a trend for higher average IVRT values in those with intermediate and low BSE/ESC TTE PH probability and PH compared to those without PH. An increased IVRT reflects poor myocardial relaxation [18]. Studies have demonstrated a reduction in IVRT durations in PH patients using Sildenafil therapy [17]. A comprehensive assessment of RV diastolic dysfunction was limited given PW Doppler interrogation of tricuspid inflow was used infrequently as it is not part of the standard minimum dataset [32].
IMPULSE algorithm
Our findings suggest a combination of additional TTE makers such as RVFWLS, RVFAC and IVRT in those with low or intermediate BSE/ESC determined probability may increase the detection of PH in these groups. Using the IMPULSE algorithm reduced the number of false negatives with pre-capillary PH and low TTE probability by 44%. It also had a superior PPV compared to intermediate BSE/ESC TTE PH probability alone. The IMPULSE algorithm however could only be retrospectively used in 52% of those with low or intermediate TTE probability. IMPULSE was often unmeasurable due to no RV TDI imaging being stored or endocardial definition being suboptimal for RVWLS or RVFAC measurements. Prospective measurements however are likely more feasible. Further prospective research is needed to confirm the improved sensitivity of the IMPUSLE algorithm amongst patients with low/intermediate probability PH using existing tools.
Limitations
Our findings represent those of a single centred retrospective study in a cohort of patients referred to a shared care national PH service, thus the number of patients without PH is low compared to screening populations. Therefore, the clinical suspicion of PH is increased. RVFAC and RVFWLS were not measurable in all those with a low or intermediate TTE probability and PH. Retrospective RVFWLS measurements were not performed using dedicated RVFWLS software which may have influenced the accuracy of some measurements [18]. Differences in RVFWLS values may exist between vendors however all measurements in this study used the same vendor software. 2D RVFWLS measurements in our study did not consider the complex 3D geometry of the RV with future studies including 3D RVFWLS needed. Typically, retrospective measurements of PRVBD could not be performed due to poor Doppler optimisation of the PR jet. The TTE parameters assessed were limited by those that could be retrospectively measured from the stored data and therefore did not evaluate all possible right heart indices such as hepatic venous flow or trans-tricuspid Doppler for example.
TTE was not performed simultaneously with RHC. The median interval from TTE to RHC was 24 days and therefore comparisons between echocardiographic and invasive measures may have been influenced by varying haemodynamic loading conditions.
Conclusion
Current international guidelines for the echocardiographic probability of PH may not be sensitive enough to detect patients with milder haemodynamic forms of PH. Our findings suggest a combination of additional TTE makers assessing RV radial, longitudinal and diastolic function are helpful in identifying those with milder forms of PH, particularly in those who have a low BSE/ ESC TTE PH probability. Incorporating RVFWLS, RVFAC and IVRT in those with low or intermediate BSE/ESC determined probability may reduce the number of false negatives, improve specificity and positive predictive value when compared to current guidelines.
Supplementary Information
Additional file 1: Figure S1a: Comparison of average RV free wall longitudinal strain (RVFWLS) in those with a high, intermediate or low TTE probability as well as those without PH. Kruskal–Wallis analysis demonstrated RVFWLS was significantly different relative to TTE PH probability group; H = 51.73 (3) (P < 0.001). Pairwise analysis with adjusted P-values demonstrated that RVFWLS values were significantly lower in those with high TTE probability compared to those with low intermediate TTE probability and those without PH (all P < 0.001). There was no significant difference in strain values between low and intermediate probabilities (p > 0.05). Figure S1b: Comparison of RVFWLS values relative to WHO classification of PH. Kruskal–Wallis analysis showed no statistically significant difference in RVFWLS values relative to WHO classification in either group (p > 0.05). RVFWLS values however were significantly higher in those without PH compared to all WHO classifications of PH, H = 31.12 (5) (all P < 0.05). Figure S2a: Comparison of frequencies of PH in those with a low TTE probability (n = 78) (left) and average RVFWLS in those Low TTE probability with PH (= 23) and without (n = 27) (right). RVFWLS was significantly lower in those with PH and low TTE probability (P < 0.02). Figure S2b: Comparison of frequencies of PH in those with an intermediate (n = 36) echocardiographic probability (left) and average RVFWLS in those with intermediate TTE probability in those with PH (n = 24) and without (n = 12) (right). Figure S3: ROC curve analysis of RV TDI IVRT (ms) N = 164. AUC 0.72 (95% CI 0.62–0.82). Using an IVRT cut-off value of > 73 ms demonstrated sensitivity and specificity of 81% and 62%, respectively for detecting PH. Figure S4a: Comparison of RV TDI e’ (cm/s) relative to WHO classifications of PH. Kruskal–Wallis analysis demonstrated RV TDI e’ values were different across WHO classifications of PH; H = 31.7(5) P < 0.001. Pairwise analysis with adjusted p-values showed that RV e’ values were significantly lower in those with CTEPH (P < 0.01) and PAH (P < 0.02) compared to those with PH due to LHD. RV e’ values were not significantly different between those with no PH and LHD or multifactorial PH (p > 0.05). Figure S4b: Comparison of RV TDI E’:A’ ratios relative to WHO classifications of PH. Kruskal–Wallis analysis demonstrated E/A ratios were significantly different across WHO classifications of PH H = 33.1(5), P < 0.001). RV TDI E’:A’ ratios were significantly lower in those with PAH (P < 0.02), CTEPH (P < 0.01) and lung disease (P < 0.02) compared to those with LHD. E/A ratios were not significantly different between those without PH and those with LHD or multifactorial PH (p > 0.05). Figure S4c: Comparison of RV TDI IVRT (ms) relative to WHO classifications of PH. Kruskal–Wallis analysis demonstrated IVRT values were not statistically significantly different across WHO classifications of PH. However, IVRT values were significantly higher in those with PAH (P < 0.001) and CTEPH (P < 0.01) compared to those without PH (H = 24.3(5) P < 0.001). Figure S5a: Comparison of average RV TDI IVRT values (ms) in those with low TTE probability with PH (N = 17) and without (N = 28). RV TDI IVRT (right ventricular tissue Doppler imaging isovolumetric relaxation times). Figure S5b: Comparison of average RV TDI IVRT values (ms) in those with intermediate TTE probability in those with PH (N = 27) and without N = 13). RV TDI IVRT (right ventricular tissue Doppler imaging isovolumetric relaxation times). Table S1: Population characteristics (N = 310). Table S2: Comparison of echocardiographic markers of RV diastolic dysfunction in those with and without PH. Table S3: Receiver operating characteristic derived cut-off values for the TTE PH parameters. Table S4: Re-audit of the TTE PH probabilities using ROC curved defined cut-off values compared to BSE/ESC algorithm.
Abbreviations
- A4C
Apical four chamber
- AFI
Automated function imaging
- BSA
Body surface area
- BSE
British Society of Echocardiography
- CAD
Coronary artery disease
- CI
Confidence interval
- CMR
Cardiac magnetic resonance imaging
- COPD
Chronic obstructive pulmonary disease
- CTD
Connective tissue disease
- CTEPH
Chronic thromboembolic pulmonary hypertension
- CW
Continuous wave
- ESC
European Society of Cardiology
- GE
General electric
- GLS
Global longitudinal strain
- IMPULSE
Improving pulmonary hypertension screening by echocardiography
- IVC
Inferior vena cava
- IVRT
Isovolumetric relaxation time
- IQR
Inter quartile range
- LA
Left atrium
- LHD
Left heart disease
- LV
Left ventricular
- MPA
Main pulmonary artery
- mPAP
Mean pulmonary artery pressure
- MSN
Mid systolic notching
- NYHA
New York Heart Association
- NT pro BNP
N-terminal pro hormone of brain natriuretic peptide
- PAH
Pulmonary arterial hypertension
- PASP
Pulmonary artery systolic pressure
- PCWP
Pulmonary capillary wedge pressure
- PH
Pulmonary hypertension
- PR
Pulmonary regurgitation
- PRVBD
Pulmonary regurgitation velocity at beginning of diastole
- PVR
Pulmonary vascular resistance
- PW
Pulsed wave
- RAA
Right atrial area
- RAAI
Right atrial area indexed
- R&D
Research and development
- RAP
Right atrial pressure
- RHC
Right heart catheterisation
- ROC
Receiver operating characteristic
- RV
Right ventricle
- RVFAC
Right ventricular fractional area of change
- RVFWLS
Right ventricular free wall longitudinal strain
- RVH
Right ventricular hypertrophy
- RVOT
Right ventricular outflow tract
- RVSP
Right ventricular systolic pressure
- SPSS
Statistical package for social sciences
- TAPSE
Tricuspid annular peak systolic excursion
- TDI
Tissue Doppler imaging
- TRV
Tricuspid regurgitant velocity
- TTE
Transthoracic echocardiography
- UK
United Kingdom
- WHO
World Health Organisation
- WU
Woods units
- 6MWT
Six-minute walk test
Author contributions
Authors OGS and DXA wrote the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The data that support the findings of this study are available on reasonable request from the corresponding author DXA.
Declarations
Ethics approval and consent to participate
Ethics approval was obtained from both the RUH Bath NHS Foundation Trust R&D department and Manchester Metropolitan University Ethos ethics committee.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery J, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension’. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustine DX, Coates-Bradshaw LD, Willis J, Harkness A, Ring L, Grapsa J, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2018;5(3):G11–G24. doi: 10.1530/ERP-17-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suntharalingam J, Ross RM, Easaw J, Robinson G, Coghlan G. Who should be referred to a specialist pulmonary-hypertension centre—a referrer's guide. Clin Med. 2016;16(2):135–141. doi: 10.7861/clinmedicine.16-2-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 7.Kiely DG, Lawrie A, Humbert M. Screening strategies for pulmonary arterial hypertension. Eur Heart J Suppl J Eur Soc Cardiol. 2019;21(Suppl K):K9–K20. doi: 10.1093/eurheartj/suz204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janda S, Shahidi N, Gin K, Swiston J. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart (Br Cardiac Soc) 2011;97(8):612–622. doi: 10.1136/hrt.2010.212084. [DOI] [PubMed] [Google Scholar]
- 9.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest. 2011;139(5):988. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 11.D'Alto M, Romeo E, Argiento P, D'Andrea A, Vanderpool R, Correra A, et al. Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int J Cardiol. 2013;168(4):4058–4062. doi: 10.1016/j.ijcard.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JD, Forfia PR. Diagnosis and assessment of pulmonary vascular disease by Doppler echocardiography. Pulmon Circul. 2011;1(2):160–181. doi: 10.4103/2045-8932.83446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasselberg NE, Kagiyama N, Soyama Y, Sugahara M, Goda A, Ryo-Koriyama K, et al. The prognostic value of right atrial strain imaging in patients with pre-capillary pulmonary hypertension. J Am Soc Echocardiogr. 2021 doi: 10.1016/j.echo.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circul Cardiovasc Imag. 2013;6(5):711–721. doi: 10.1161/CIRCIMAGING.113.000640. [DOI] [PubMed] [Google Scholar]
- 15.Pica S, Ghio S, Tonti G, Visconti LO. Right ventricular radial function in patients with and without pulmonary hypertension. Ultrasound Med Biol. 2014 doi: 10.1016/j.ultrasmedbio.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Gan CT-J, Holverda S, Marcus JT, Paulus WJ, Marques KM, Bronzwaer JGF, et al. Right ventricular diastolic dysfunction and the acute effects of sildenafil in pulmonary hypertension patients. Chest. 2007;132(1):11–17. doi: 10.1378/chest.06-1263. [DOI] [PubMed] [Google Scholar]
- 17.Zaidi A, Knight DS, Augustine DX, Harkness A, Oxborough D, Pearce, , et al. Echocardiographic assessment of the right heart in adults: a practical guideline from the British Society of Echocardiography. Echo Res Pract. 2020;7(1):G19–G41. doi: 10.1530/ERP-19-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard LS, Grapsa J, Dawson D, Bellamy M, Chambers JB, Masani ND, et al. Echocardiographic assessment of pulmonary hypertension: standard operating procedure. Eur Respir Rev. 2012;21(125):239–248. doi: 10.1183/09059180.00003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peluso D, Tona F, Muraru D, Romeo G, Cucchini U, Marra M, et al. Right ventricular geometry and function in pulmonary hypertension: non-invasive evaluation. Diseases (Basel, Switzerland) 2014;2(3):274–295. doi: 10.3390/diseases2030274. [DOI] [Google Scholar]
- 20.Harkness A, Ring L, Augustine DX, Oxborough D, Robinson S, Sharma V. Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: a guideline from the British Society of Echocardiography. Echo Res Pract. 2020;7(1):G1–G18. doi: 10.1530/ERP-19-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kind T, Mauritz G-J, Marcus JT, van de Veerdonk M, Westerhof N, Vonk-Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Resonan. 2010;12(1):35. doi: 10.1186/1532-429X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowe T, Jayasekera G, Peacock AJ. Non-invasive imaging of global and regional cardiac function in pulmonary hypertension. Pulmon Circul. 2018;8(1):2045893217742000. doi: 10.1177/2045893217742000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghio S, Klersy C, Magrini G, D’Armini AM, Scelsi L, Raineri C, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140(3):272–278. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 24.Maeder MT, Ehl NF. When the right is not doing right: The role of strain imaging in pulmonary arterial hypertension. Can J Cardiol. 2018;34(8):962–964. doi: 10.1016/j.cjca.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Zito C, Longobardo L, Citro R, Galderisi M, Oreto L, Carerj S, et al. Ten years of 2D longitudinal strain for early myocardial dysfunction detection: a clinical overview. BioMed Res Int. 2018;2018(2018):8979407. doi: 10.1155/2018/8979407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy treated patients. Am J Cardiol. 2011;107(9):1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leong DP, Grover S, Molaee P, Chakrabarty A, Shirazi M, Cheng YH, et al. Nonvolumetric echocardiographic indices of right ventricular systolic function: validation with cardiovascular magnetic resonance and relationship with functional capacity: Echocardiographic assessment of RV function. Echocardiography (Mount Kisco N.Y.) 2012;29(4):455–463. doi: 10.1111/j.1540-8175.2011.01594. [DOI] [PubMed] [Google Scholar]
- 28.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imag. 2017;18(12):1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 29.Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta P, et al. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circul Cardiovasc Imag. 2016;9(2):e003866. doi: 10.1161/CIRCIMAGING.115.003866. [DOI] [PubMed] [Google Scholar]
- 30.Chow P-C, Liang X-C, Cheung EWY, Lam WWM, Cheung Y-F. New two-dimensional global longitudinal strain and strain rate imaging for assessment of systemic right ventricular function. Heart (British Cardiac Society) 2008;94(7):855–859. doi: 10.1136/hrt.2007.131862. [DOI] [PubMed] [Google Scholar]
- 31.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imag. 2018;19(6):591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 32.Robinson S, Rana B, Oxborough D, Steeds R, Monaghan M, Stout M, Pearce K, Harkness A, Ring L, Paton M, Akhtar W, Bedair R, Bhattacharyya S, Collins K, Oxley C, Sandoval J, Schofield MBB, Siva A, Parker K, Willis J, Augustine DX. A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: the British Society of Echocardiography minimum dataset. Echo Res Pract. 2020;7(4):G59–G93. doi: 10.1530/ERP-20-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1a: Comparison of average RV free wall longitudinal strain (RVFWLS) in those with a high, intermediate or low TTE probability as well as those without PH. Kruskal–Wallis analysis demonstrated RVFWLS was significantly different relative to TTE PH probability group; H = 51.73 (3) (P < 0.001). Pairwise analysis with adjusted P-values demonstrated that RVFWLS values were significantly lower in those with high TTE probability compared to those with low intermediate TTE probability and those without PH (all P < 0.001). There was no significant difference in strain values between low and intermediate probabilities (p > 0.05). Figure S1b: Comparison of RVFWLS values relative to WHO classification of PH. Kruskal–Wallis analysis showed no statistically significant difference in RVFWLS values relative to WHO classification in either group (p > 0.05). RVFWLS values however were significantly higher in those without PH compared to all WHO classifications of PH, H = 31.12 (5) (all P < 0.05). Figure S2a: Comparison of frequencies of PH in those with a low TTE probability (n = 78) (left) and average RVFWLS in those Low TTE probability with PH (= 23) and without (n = 27) (right). RVFWLS was significantly lower in those with PH and low TTE probability (P < 0.02). Figure S2b: Comparison of frequencies of PH in those with an intermediate (n = 36) echocardiographic probability (left) and average RVFWLS in those with intermediate TTE probability in those with PH (n = 24) and without (n = 12) (right). Figure S3: ROC curve analysis of RV TDI IVRT (ms) N = 164. AUC 0.72 (95% CI 0.62–0.82). Using an IVRT cut-off value of > 73 ms demonstrated sensitivity and specificity of 81% and 62%, respectively for detecting PH. Figure S4a: Comparison of RV TDI e’ (cm/s) relative to WHO classifications of PH. Kruskal–Wallis analysis demonstrated RV TDI e’ values were different across WHO classifications of PH; H = 31.7(5) P < 0.001. Pairwise analysis with adjusted p-values showed that RV e’ values were significantly lower in those with CTEPH (P < 0.01) and PAH (P < 0.02) compared to those with PH due to LHD. RV e’ values were not significantly different between those with no PH and LHD or multifactorial PH (p > 0.05). Figure S4b: Comparison of RV TDI E’:A’ ratios relative to WHO classifications of PH. Kruskal–Wallis analysis demonstrated E/A ratios were significantly different across WHO classifications of PH H = 33.1(5), P < 0.001). RV TDI E’:A’ ratios were significantly lower in those with PAH (P < 0.02), CTEPH (P < 0.01) and lung disease (P < 0.02) compared to those with LHD. E/A ratios were not significantly different between those without PH and those with LHD or multifactorial PH (p > 0.05). Figure S4c: Comparison of RV TDI IVRT (ms) relative to WHO classifications of PH. Kruskal–Wallis analysis demonstrated IVRT values were not statistically significantly different across WHO classifications of PH. However, IVRT values were significantly higher in those with PAH (P < 0.001) and CTEPH (P < 0.01) compared to those without PH (H = 24.3(5) P < 0.001). Figure S5a: Comparison of average RV TDI IVRT values (ms) in those with low TTE probability with PH (N = 17) and without (N = 28). RV TDI IVRT (right ventricular tissue Doppler imaging isovolumetric relaxation times). Figure S5b: Comparison of average RV TDI IVRT values (ms) in those with intermediate TTE probability in those with PH (N = 27) and without N = 13). RV TDI IVRT (right ventricular tissue Doppler imaging isovolumetric relaxation times). Table S1: Population characteristics (N = 310). Table S2: Comparison of echocardiographic markers of RV diastolic dysfunction in those with and without PH. Table S3: Receiver operating characteristic derived cut-off values for the TTE PH parameters. Table S4: Re-audit of the TTE PH probabilities using ROC curved defined cut-off values compared to BSE/ESC algorithm.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author DXA.