Abstract
Background:
Epidemiological studies have identified common risk factors for cerebral stroke worldwide. Some of these factors include hypertension, diabetes, smoking, excessive drinking, and dyslipidemia. It is important to note, however, that genetic factors can also contribute to the occurrence of stroke. Here, we evaluated the association of ischemic stroke with rs12514417 polymorphism of the alcohol metabolizing gene, aldehyde dehydrogenase 7A1 (ALDH7A1) and alcohol consumption.
Methods:
Taiwan Biobank (TWB) data collected between 2008 and 2015 were available for 17,985 subjects. The odd ratios for stroke were obtained using logistic regression models.
Results:
Among eligible subjects (n = 17,829), 897 had ischemic stroke and 70 had hemorrhagic stroke. Subjects with ischemic stroke were older (mean ± SE, 58.45 ± 8.19 years vs. 48.33 ± 10.89 years, p < 0.0001) and had a higher body mass index (BMI) than the stroke-free individuals. The risk of ischemic stroke was significantly higher among subjects with the ALDH7A1 rs12514417 TG + GG genotype who also consumed alcohol at least 150 ml/week (odds ratio (OR), 1.79; 95% confidence interval (CI), 1.18–2.72). We found that rs12514417 genotype and alcohol consumption (at least 150 ml/week) showed a significant interaction (p for interaction = 0.0266). Stratification based on alcohol exposure and ALDH7A1 rs12514417 genotypes indicated that ischemic stroke risk was significantly higher among alcohol drinkers with the TG + GG genotype than in those with the TT genotype (OR, 1.64, 95% CI: 1.15–2.33).
Conclusion:
Our study suggests that the combination of ALDH7A1 rs12514417 TG + GG genotype and alcohol exposure of at least 150 ml/week may increase the risk of ischemic stroke in Taiwanese adults.
Keywords: Cardiovascular disease, Stroke, Epidemiology, ALDH7A1, Polymorphism
Introduction
About 12 million people are affected by stroke worldwide[1]. In spite of being the second leading cause of death after coronary artery disease (CAD), it is associated with more disabilities than CAD [2]. Over 87% of all strokes are ischemic, with the remaining strokes being hemorrhagic, including cerebral and subarachnoid hemorrhages. There are a number of modifiable risk factors for stroke, including hypertension, smoking, diabetes, hyperlipidemia, high salt intake, binge alcohol drinking, high-fat diets, and inactivity [3]. However, the relationships between alcohol consumption and stroke vary between epidemiological studies. According to a meta-analysis on alcohol drinking and stroke types by Larsson et al., [4] light (less than 1 drink/day) and moderate (1–2 drinks/day) alcohol consumption were inversely associated with ischemic stroke, whereas heavy drinking (> 4 drinks/day) was associated with increased risk of all stroke types, with hemorrhagic stroke showing the strongest association.
Alcohol metabolism is genetically controlled and can significantly affect drinking behavior and alcohol-related organ damage [5]. Alcohol is first converted to acetaldehyde by alcohol dehydrogenase 1B (ADH1B). Acetaldehyde is further oxidized by aldehyde dehydrogenase (ALDH) isoenzymes to nontoxic acetate, using NAD + as a cofactor[6, 7]. ALDH2 is considered one of the most important enzymes for ethanol metabolism in vivo, and an inactivating mutation in ALDH2, the common East Asian ALDH2*2 (the rs671 E504K missense variant), is associated with a wide range of health complications, such as osteoporosis, cancer (about 20% increased cancer risk in inactive, ALDH2*2, homozygous individuals) and Alzheimer’s disease [8–10]. In addition, several studies suggest that ALDH2 protects against oxidative stress and could influence the onset of hypertension[11, 12]. ALDH2*2 has a lower NAD + coenzyme binding affinity, which reduces the clearance capacity of acetaldehyde, thereby increasing the risk of ischemic stroke[6].
We have previously reported that the ADH1B rs1229984 variant is associated with hemorrhagic stroke among alcohol drinkers, while the ALDH2*2 variant showed no association with either ischemic or hemorrhagic stroke among alcohol drinkers[13].
Chronic alcoholism frequently results in specific micronutrient deficiencies and can interfere with one-carbon metabolism, for which vitamin B12, B6, and folate all act as coenzymes[14]. In chronic alcoholics, serum pyridoxal 5’-phosphate (an active form of vitamin B6) and red blood cell folate concentrations were found to be significantly lower than in control subjects[14]. Following a 2-week intervention study with vodka or red wine, serum vitamin B12 and folate levels significantly decreased [15]. Folate, vitamin B6, and vitamin B12 deficiency are known to elevate homocysteine levels in blood [14, 16], which, in turn, may increase the risk of ischemic stroke.[17]. In addition, elevated levels of homocysteine in acute stroke were related to higher mortality.[18].
As a member of the ALDH gene family, Antiquitin (ALDH7) is not only involved in ethanol metabolism but also has α-aminoadipic semialdehyde (α-AASA) dehydrogenase activity [19]. Mutations of the antiquitin gene result in reduced pyridoxal-5-phosphate (PLP) activity and cause pyridoxine-dependent seizure, a recessive disorder that responds dramatically to the intravenous injection of pyridoxine (vitamin B6). Considering the importance of ALDH7A1 in both vitamin B6 and ethanol metabolism, we examined the potential correlations between the most common polymorphic locus in ALDH7A1 among East Asians (rs12514417; Lys439Gln missense variant allele frequency 12.9% in Asian [20]), stroke, and alcohol consumption.
Methods
Data source
We included data from two data resources: (1) The National Health Insurance Research Database (NHIRD), with medical data available from 1998 to 2015 and (2) Taiwan Biobank, a national health resource that is open to researchers and contains basic demographic and genotype data on ethnic Taiwanese residents (aged 30 to 70 years) from 2008 to 2015. The biobank aims to facilitate the development of better prevention and treatment strategies for chronic diseases such as cerebrovascular disease, cancer, liver cirrhosis, or other conditions listed among the ten leading causes of death in Taiwan. We linked the two databases at the Health and Welfare Data Science Center (HWDC) and obtained information about stroke occurrences through personal identification numbers. The Institutional Review Board of Chung Shan Medical University Hospital (CS1-20009) approved this study.
Patient identification
The current analyses included 17,985 TWB subjects. The information included sex, age, body mass index (BMI), lifestyle exposures (regular exercise, smoking, and alcohol consumption), and the rs12514417 polymorphism. The outcome variable was an ischemic stroke. We excluded subjects with incomplete questionnaires and genotyping data (n = 103), those with both ischemic and hemorrhagic stroke (n = 41) as well as those who started to drink after developing stroke (n = 12). After the exclusions, the final enrollment included 897 patients with ischemic stroke, 70 hemorrhagic stroke patients, and 16,862 stroke-free controls.
We defined alcohol drinkers as persons who reported drinking more than 150 ml of alcohol per week during the past six months and were still drinking during assessment visits. Exercise included any amount of physical activity at least 3 times a week and lasting for at least 30 min each time. Other lifestyle exposures have already been discussed in our previous publication [21].
Genetic variant selection
A literature search using Pub Med, ScienceDirect, Google Scholar, SNPedia, and the GWAS Catalog was carried out to identify common ALDH7A1 gene variants. The most common polymorphism, rs12514417, was chosen after searching these databases [20]. SNP genotyping was carried out using the custom TWB chips and run on the Axiom™ Genome-Wide Array Plate System (Affymetrix, Santa Clara, CA, USA). The rs12514417 polymorphism passed the quality control test: the call rate was > 95%, the p-value for the Hardy-Weinberg equilibrium test was > 1.0 × 10− 3, and the minor allele frequency was > 0.05.
Definition of outcomes
Patients were defined as having an ischemic or hemorrhagic stroke if they had either two outpatient visits or one-time hospitalization from 1998 to 2015 with reported International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) 433–437 and 430–432, respectively. Potential covariates included hypertension (ICD-9-CM: 401–405), diabetes mellitus (ICD-9-CM: 250), and hyperlipidemia (ICD-9-CM: 272). Since metabolic syndrome is more prevalent among those with stroke, we added the metabolic syndrome in the model to analyze. Participants with any three of the following risk factors were considered to have metabolic syndrome: (1) central obesity (waist circumference (WC) 90 cm for men, ≥80 cm for women); (2) high blood pressure (SBP≥ 130 mmHg and/or DBP ≥85mmHg); (3) low HDL-C (HDL-C < 40 mg/dL for men, < 50 mg/dL for women); (4) increased fasting plasma glucose ( ≥ 100 mg/dL); and (5) elevated TG level ( ≥ 150 mg/dL).
Statistical analysis
We used the PLINK 1.09 beta and SAS 9.4 software (SAS Institute, Cary, NC) for data management and statistical analyses. We performed descriptive statistics using chi-square and t-tests. We ran logistic regression models to determine the ORs and 95% CI for developing ischemic stroke/hemorrhagic stroke among the rs12514417 individuals based on alcohol exposure. We also used logistic regression analysis to determine the interaction between alcohol exposure and ALDH7A1 rs12514417 on ischemic stroke/hemorrhagic, followed by stratified analyses based on these variables. The covariates included the sex, age, education, smoking, physical activity, BMI, diabetes, hypertension and hyperlipidemia. A two-sided P value less than 0.05 was considered statistically significant.
Results
The basic characteristics of the study subjects are shown in Table 1. The study comprised 897 individuals with ischemic stroke, 70 with hemorrhagic stroke, and 16,862 with neither, whom we refer to as control subjects. Subjects with ischemic stroke were older (mean ± SE, 58.45 ± 8.19 years vs. 48.33 ± 10.89 years, p < 0.0001) and had a higher body mass index (BMI) than control individuals. The distributions of risk factors for cerebrovascular disease such as hypertension, hyperlipidemia, and diabetes were significantly different between cases and controls (p < 0.0001, Table 1). We found that ALDH7A1 rs12514417 was not associated with hemorrhagic stroke based on alcohol intake (Table 2). However, importantly, the risk for ischemic stroke was higher among carriers of the ALDH7A1, rs12514417 TG + GG who drank alcohol (OR, 1.70; 95% CI, 1.16–2.50). In addition, significant associations with ischemic stroke were found for hypertension and hyperlipidemia among patients with both rs12514417 TT and TG + GG genotypes. Among subjects with the TT genotype, the OR (95% CI) was 1.54 (1.27–1.87), 2.53 (2.07–3.09), and 1.62 (1.32–1.98) for those with diabetes, hypertension, and hyperlipidemia, respectively. There was an interaction between alcohol consumption and rs12514417 (p = 0.0266).
Table 1.
Descriptive data of the subjects
| Control n = 16,862 | Ischemic n = 897 | Hemorrhagic n = 70 | p-valuea | p-valueb | ||||
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||||||
| ALDH7A1 rs12514417 | 0.316 | 0.212 | ||||||
| TT | 11,959 | (70.92) | 615 | (68.56) | 54 | (77.14) | ||
| TG | 4496 | (26.66) | 259 | (28.87) | 13 | (18.57) | ||
| GG | 407 | (2.41) | 23 | (2.56) | 3 | (4.29) | ||
| Sex | 0.099 | 0.211 | ||||||
| Female | 8729 | (51.77) | 439 | (48.94) | 31 | (44.29) | ||
| Male | 8133 | (48.23) | 458 | (51.06) | 39 | (55.71) | ||
| Smoking | 0.517 | 0.103 | ||||||
| No | 12,737 | (75.54) | 669 | (74.58) | 47 | (67.14) | ||
| Yes | 4125 | (24.46) | 228 | (25.42) | 23 | (32.86) | ||
| Alcohol intake | 0.085 | 0.022 | ||||||
| No | 15,135 | (89.76) | 789 | (87.96) | 57 | (81.43) | ||
| Yes | 1727 | (10.24) | 108 | (12.04) | 13 | (18.57) | ||
| Diabetes | < 0.001 | 0.008 | ||||||
| No | 14,783 | (87.67) | 573 | (63.88) | 54 | (77.14) | ||
| Yes | 2079 | (12.33) | 324 | (36.12) | 16 | (22.86) | ||
| Hypertension | < 0.001 | 0.008 | ||||||
| No | 13,269 | (78.69) | 353 | (39.35) | 46 | (65.71) | ||
| Yes | 3593 | (21.31) | 544 | (60.65) | 24 | (34.29) | ||
| Hyperlipidemia | < 0.001 | 0.007 | ||||||
| No | 12,087 | (71.68) | 326 | (36.34) | 40 | (57.14) | ||
| Yes | 4775 | (28.32) | 571 | (63.66) | 30 | (42.86) | ||
| Age (mean ± SD) | 48.32 | ± 10.89 | 58.45 | ± 8.19 | 50.66 | ± 10.64 | < 0.001 | 0.073 |
| BMI (mean ± SD) | 24.23 | ± 3.63 | 24.83 | ± 3.65 | 24.51 | ± 4.16 | < 0.001 | 0.531 |
Table 2.
Association of ischemic and hemorrhagic stroke with alcohol intake in rs12514417 individuals
| Ischemic stroke | Hemorrhagic stroke | |||||||
|---|---|---|---|---|---|---|---|---|
| rs12514417(TT) | rs12514417(TG + GG) | rs12514417(TT) | rs12514417(TG + GG) | |||||
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Alcohol intake (ref: No) | ||||||||
| Yes | 0.85 | (0.64–1.15) | 1.7 | (1.16–2.50) | 1.9 | (0.86–4.05) | 1.1 | (0.29–4.36) |
| Sex (ref: Female) | ||||||||
| Male | 0.99 | (0.81–1.22) | 1.1 | (0.81–1.50) | 1 | (0.53–1.98) | 2.2 | (0.61–7.80) |
| Age | 1.07 | (1.06–1.08) | 1.1 | (1.06–1.10) | 1 | (0.98–1.04) | 1 | (0.93–1.04) |
| Education (ref: Elementary School) | ||||||||
| Junior & Senior High School | 1.01 | (0.77–1.32) | 1.3 | (0.84–1.99) | 0.7 | (0.24–1.85) | 1.7 | (0.20-14.18) |
| University & above | 0.8 | (0.60–1.07) | 1.2 | (0.73–1.81) | 0.6 | (0.23–1.83) | 0.6 | (0.06–5.40) |
| Smoking (ref: No) | ||||||||
| Yes | 1.04 | (0.83–1.32) | 0.7 | (0.52–1.03) | 1.1 | (0.52–2.20) | 1.4 | (0.42–4.46) |
| Physical activity (ref: No) | ||||||||
| Yes | 1.08 | (0.90–1.29) | 1 | (0.77–1.30) | 0.7 | (0.39–1.26) | 1.5 | (0.51–4.19) |
| BMI | 1.01 | (0.98–1.04) | 1 | (0.94–1.01) | 1 | (0.88–1.04) | 1 | (0.90–1.18) |
| Diabetes (ref: No) | ||||||||
| Yes | 1.54 | (1.27–1.87) | 1.3 | (0.96–1.75) | 0.8 | (0.33–1.72) | 9 | (2.54–32.09) |
| Hypertension (ref: No) | ||||||||
| Yes | 2.53 | (2.07–3.09) | 1.9 | (1.45–2.59) | 1.5 | (0.76–2.98) | 1 | (0.27–3.46) |
| Hyperlipidemia (ref: No) | ||||||||
| Yes | 1.62 | (1.32–1.98) | 1.7 | (1.27–2.27) | 1.7 | (0.90–3.27) | 0.7 | (0.19–2.29) |
Alcohol intake*rs12514417 in Ischemic stroke, p = 0.0266.; Alcohol intake*rs12514417 in Hemorrhagic stroke, p = 0.9101
Furthermore, we wanted to see if genes would influence the stroke risk among drinkers. After stratification by alcohol consumption (Table 3), the odds of having ischemic stroke remained significantly higher among ALDH7A1 rs12514417 TG + GG subjects who consumed alcohol (OR = 1.80, 95% CI: 1.19–2.73). No significant association was found between the rs12514417 genotype and hemorrhagic stroke risk regardless of whether the subjects consumed alcohol or not. Using the TT genotype and no alcohol intake as a reference group (Table 4), the risk for ischemic stroke was significantly higher in people with TG + GG genotype who consumed alcohol (OR = 1.67; 95% CI: 1.18–2.38).
Table 3.
Association of ischemic and hemorrhagic stroke with rs12514417 based on alcohol intake
| Ischemic stroke | Hemorrhagic stroke | |||||||
|---|---|---|---|---|---|---|---|---|
| No alcohol intake | Alcohol intake | No alcohol intake | Alcohol intake | |||||
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Rs12514417(ref: TT) | ||||||||
| TG + GG | 1.08 | (0.92–1.27) | 1.79 | (1.18–2.72) | 0.72 | (0.39–1.33) | 0.83 | (0.23–3.08) |
| Sex (ref: Female) | ||||||||
| Male | 1 | (0.84–1.19) | 1.33 | (0.62–2.85) | 1.35 | (0.73–2.48) | 0.43 | (0.11–1.69) |
| Age | 1.07 | (1.06–1.08) | 1.04 | (1.02–1.07) | 1.01 | (0.98–1.05) | 0.96 | (0.90–1.02) |
| Education (ref: Elementary School) | ||||||||
| Junior & Senior High School | 1.11 | (0.87–1.41) | 0.84 | (0.40–1.76) | 1.12 | (0.38–3.33) | 0.25 | (0.04–1.41) |
| University & above | 0.93 | (0.72–1.20) | 0.67 | (0.31–1.45) | 0.9 | (0.29–2.77) | 0.13 | (0.02–0.96) |
| Smoking (ref: No) | ||||||||
| Yes | 0.97 | (0.79–1.21) | 0.71 | (0.46–1.11) | 1.2 | (0.61–2.34) | 0.94 | (0.27–3.27) |
| Physical activity (ref: No) | ||||||||
| Yes | 1.08 | (0.92–1.27) | 0.83 | (0.54–1.29) | 0.69 | (0.38–1.22) | 1.9 | (0.59–6.17) |
| BMI | 1 | (0.98–1.03) | 0.97 | (0.91–1.03) | 0.99 | (0.92–1.07) | 0.93 | (0.78–1.10) |
| Diabetes (ref: No) | ||||||||
| Yes | 1.48 | (1.25–1.76) | 1.48 | (0.93–2.35) | 1.28 | (0.62–2.64) | 2.88 | (0.69–12.10) |
| Hypertension (ref: No) | ||||||||
| Yes | 2.25 | (1.89–2.68) | 3.03 | (1.85–4.96) | 1.32 | (0.68–2.56) | 1.61 | (0.39–6.66) |
| Hyperlipidemia (ref: No) | ||||||||
| Yes | 1.63 | (1.37–1.94) | 1.63 | (1.02–2.61) | 1.55 | (0.82–2.92) | 0.87 | (0.21–3.68) |
Abbreviations: OR = odds ratio, CI = 95% confidence interval, BMI = body mass index, TT, TG, and GG = rs12514417 genotypes
Table 4.
Association of ischemic and hemorrhagic stroke with alcohol intake and rs12514417 genotypes
| Ischemic stroke | Hemorrhagic stroke | |||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| TT, alcohol intake | 0.91 | (0.68–1.22) | 1.59 | (0.75–3.38) |
| TG + GG, no alcohol intake | 1.08 | (0.92–1.27) | 0.72 | (0.39–1.34) |
| TG + GG, alcohol intake | 1.64 | (1.15–2.33) | 1.24 | (0.37–4.17) |
| Sex (ref: Female) | ||||
| Male | 1.03 | (0.87–1.22) | 1.19 | (0.67–2.11) |
| Age | 1.07 | (1.06–1.08) | 1 | (0.98–1.03) |
| Education (ref: Elementary School) | ||||
| Junior & Senior High School | 1.08 | (0.86–1.36) | 0.85 | (0.34–2.10) |
| University & above | 0.89 | (0.70–1.14) | 0.64 | (0.25–1.66) |
| Smoking (ref: N0) | ||||
| Yes | 0.93 | (0.77–1.13) | 1.14 | (0.62–2.11) |
| Physical activity (ref: No) | ||||
| Yes | 1.05 | (0.91–1.22) | 0.83 | (0.50–1.38) |
| BMI | 1 | (0.98–1.02) | 0.98 | (0.91–1.05) |
| Diabetes (ref: No) | ||||
| Yes | 1.47 | (1.25–1.74) | 1.47 | (0.77–2.79) |
| Hypertension (ref: No) | ||||
| Yes | 2.33 | (1.98–2.74) | 1.37 | (0.75–2.49) |
| Hyperlipidemia (ref: No) | ||||
| Yes | 1.64 | (1.39–1.93) | 1.42 | (0.80–2.54) |
Abbreviations: OR = odds ratio, CI = 95% confidence interval, BMI = body mass index, TT, TG, and GG = rs12514417 genotypes
Discussion
The pathogenesis of cerebral stroke is complex; various risk factors of for cerebral stroke identified by epidemiological studies mainly included aging, hypertension, hyperlipidemia, diabetes, smoking, excessive drinking, among others[1].
Stroke is also likely to be caused by genetic factors; a synergistic effect of genetic and environmental factors can lead to stroke in some individuals. Combining two data sources, we demonstrated the relationship between stroke and the rs12514417 polymorphism and its impact on alcohol consumption. Our main finding suggests that the ALDH7A1 rs12514417 TG + GG genotype may be associated with a 1.6-fold increased risk of ischemic stroke in subjects who drink more than 150 ml of alcohol per week[6, 7, 22–24]. This is the first study to identify the relationship between ALDH7A1 mutation and ischemic stroke, suggesting a change in lifestyle, specifically a reduction in alcohol consumption could reduce disease risk in carriers of this variant.
Studies have found a link between stroke and ALDH2, another aldehyde dehydrogenase [6, 7, 22–24]. ALDH2 is the most studied aldehyde dehydrogenase[25], which are associated with decreased enzymatic activity, liver disease, cirrhosis, or pancreatitis in alcoholics[25]. Studies have suggested that the ALDH2*2 allele is an important risk factor for ischemic stroke in Taiwanese [23] and Korean [26] men, as well as in Chinese women (OR = 2.207, 95% CI 1.416–3.439)[22], since it increases dyslipidemia, hypertension, and diabetes, which may contribute to cerebral arteriosclerosis [27–29].
Besides the inflammatory mechanism, oxidative stress plays a critical role in the development of ischemic stroke. As oxidative stress develops, most aldehydes are produced by lipid peroxidation. Reactive aldehydes can further cause structural damage to biological macromolecules, such as DNA, lipids, and proteins caused by reactive oxygen species, including 4-Hydroxynonenal (4-HNE), malondialdehyde (MDA) and others [30]. The ALDH2 protein can detoxify these reactive aldehydes and is critical to the health of cells and therefore serves as an important shield against damage occurring under oxidative stress[30]. A previous studies have shown that expression of 4-HNE-protein was elevated in the ischemic cerebral cortex when measured within two hours of stroke induction in genetically stroke-prone mice [31]. Moreover, Lee et al. [32] demonstrated that plasma 4-HNE levels were elevated in patients with stroke and genetic stroke-prone mice (stroke-prone spontaneously hypertensive rats), as well as in experimental stroke rats with middle cerebral artery occlusion (MCAO). Intravenous administration of 4-HNE before stroke not only enlarged the cerebral ischemia-induced infarct area but also increased oxidative stress in mice. Transgenic mice overexpressing ALDH2 after 12 weeks of alcohol consumption displayed significantly reduced levels of brain damage and neuronal cell death [33]. To sum up the above, mutation of the ALDH2 gene (ALDH2*2), which results in a reduced enzyme activity, may increase oxidative stress and cause cerebral damage[33].
Unlike ALDH2, the function of ALDH7A1 is not well understood and no literature has described its association with stroke so far. ALDH7A1 is also considered to play a role in detoxifying aldehydes generated by alcohol metabolism and lipid peroxidation. Although most ALDH isozymes are expressed exclusively in the liver, extra-hepatic tissues may also express a relatively large amount of a specific ALDH isozyme, depending on their specific functions[34]. According to tissue distribution studies in mice, ALDH7A1 was most highly expressed in the brain, liver, and kidney [35]. The ALDH organ-specific distribution may indicate a greater likelihood of neurotoxicity caused by reduced activity of ALDH7A1 compared with ALDH2. Perhaps a more important function of ALDH7A1 in relation to stroke risk is its role in lysine catabolism through the oxidation of AASA. Lysine catabolism is essential for maintaining cellular nitrogen stores and for producing ketone bodies(KBs) [36]. KBs are an important source of energy for the brain in a nutrient deprivation state. Growing evidence indicates that ketone bodies have beneficial effects on stroke treatment, but the mechanisms are not clear[37]. After transient middle cerebral artery (MCA) occlusion in mice, ketones improved mitochondrial function and reduced oxidative stress by promoting NAD+-dependent Sirtuin 3 (SIRT3) and its downstream substrates in the penumbra, thereby reducing the infarct volume and improving neurological function after ischemic stroke [38].
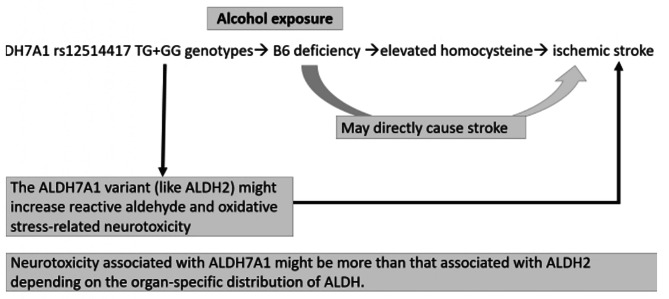
Homozygous mutations in ALDH7A1 decrease antiquitin acitivity, which can result in pyridoxine-dependent and folic acid-responsive seizure[39]. Deficiency of this activity leads to the accumulation of AASA and piperideine-6-carboxylate (P6C), the coenzyme form of vitamin B6 which activates PLP, leading to lifelong pyridoxine (Vit B6) demands[40]. Individuals with chronic alcoholism may further exhibit lower plasma levels of PLP, made even more severe by vitamin B6 deficiency[41]. Homocysteine metabolism-related vitamins (HMRV), including Vitamin B6, folic acid, and vitamin B12 are all cofactors in homocysteine metabolism. Vitamin B6 deficiency may elevate plasma homocysteine[42, 43]. There is growing evidence to suggest that hyperhomocysteinaemia is a risk factor for stroke, especially among hypertensive individuals[17]. Studies have suggested that hyperhomocysteinemia is closely correlated with ischemic stroke, as it induces atherosclerosis through the pathophysiologic mechanisms of intracranial small-vessel and extracranial large-artery diseases[44–46]. The severity of carotid atherosclerosis was inversely associated with plasma concentrations of folate and PLP after adjusting for age, sex, and other risk factors[44]. HMRV supplementation may lower the incidence of stroke-induced hyperhomocysteinemia resulting from HMRV deficiency. In a randomized controlled trial (HOPE-2 study)[47], HMRV supplementation combined with folic acid, vitamins B6 and B12 reduced the risk of stroke by 25%. Despite these, more studies are needed to ascertain whether homocysteine, folic acid, and vitamins B6 and B12 metabolism differ among subjects carrying the ALDH7A1 variant. A summary of possible mechanisms through which the rs12514417 TG + GG genotype and alcohol consumption may result in stroke is shown in Fig. 1.
Fig. 1.
A possible mechanism through which the rs12514417 TG + GG genotype and alcohol consumption may lead to stroke
The main limitation of our study is the uncertainty about alcohol exposure. The amount of alcohol consumed was measured in millimeters (ml), rather than milligrams (mg). Data on alcohol consumption were self-reported and are subject to recall bias. The second limitation is the lack of homocysteine data for those subjects. However, vitamin B6 deficiency could be an independent risk factor for ischemic stroke in the presence or absence of hyperhomocysteinemia. According to Kelly et al., low PLP was associated with chronic inflammation and elevated C-reactive protein (CRP) in addition to the acute phase response. There was no association between CRP and homocysteine, despite a significant correlation between homocysteine and PLP[48]. Clinical evidence demonstrated that low levels of vitamin B6, but not homocysteine were strongly associated with ischemic stroke and transient ischemic attack[49, 50].
The main finding of our study suggests that the ALDH7A1 rs12514417 TG + GG genotype is associated with a 1.6-fold increased risk of ischemic stroke in subjects who drink more than 150 ml of alcohol per week. The underlying mechanism is that ALDH7A1 mutations can cause oxidative stress, inflammation, and vitamin B6 deficiency and therefore result in ischemic stroke. It remains to be determined if the common rs12514417; Lys439Gln missense mutation would cause any change in the enzymatic function of ALDH7A1 in vitro and in vivo. We suggest that a large study on gene-environment interactions in diverse populations be conducted and also functional assessment using animal models or human cell lines to examine the underlying mechanisms behind this potential association.
Acknowledgements
The authors would like to thank the Ministry of Science and Technology of Taiwan and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) for funding the research.
Abbreviations
- ALDH7A1
aldehyde dehydrogenase 7A1
- ALDH
Aldehyde dehydrogenase
- ADH1B
Alcohol dehydrogenase 1B
- TWB
Taiwan Biobank
- NHIRD
National Health Insurance Research Database
- MOST
Ministry of Science and Technology
- CAD
Coronary artery disease
- BMI
Body mass index
- HWDC
Health and Welfare Data Science Center
- SE
Standard error
- OR
Odds ratio
- CI
Confidence interval
Funding
This work was funded by the Ministry of Science and Technology (MOST 109-2121-M-040-002; 109-2811-M-040-500; 110-2811-M-040-001;110-2121-M-040-002; 111-2811-M-040-001; 111-2121-M-040-002; 111-2811-M-040-001). The authors acknowledge the support of NIAAA1147 to DM-R and C-H Chen. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Data availability
The data that support the findings of this study are available from the Taiwan Biobank data source but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Taiwan Biobank.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (CS1-20009). Taiwan Biobank participants had provided written informed consent during enrollment. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
C-HL, ONN, C-CH, S-YH, DMT, Y-CL, M-RD, C-HC, and Y-PL conceptualized and designed the study. S-YH, ONN, C-CH, DMT, Y-CL, and M-RD participated in the management, analysis, and interpretation of data. C-HL and ONN participated in drafting the manuscript. Y-PL and C-HC supervised the whole process. All authors reviewed and approved the final manuscript as submitted.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Che-Hong Chen, Email: chehong@stanford.edu.
Yung-Po Liaw, Email: Liawyp@csmu.edu.tw.
References
- 1.Avan A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G, Amiri A, Tabrizi R, Mokhber N, Spence JD, Azarpazhooh MR. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17:191. doi: 10.1186/s12916-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehme AK, Esenwa C, Elkind MSV: Stroke Risk Factors, Genetics, and Prevention. 2017, 120:472–495. [DOI] [PMC free article] [PubMed]
- 4.Larsson SC, Wallin A, Wolk A, Markus HS. Differing association of alcohol consumption with different stroke types: a systematic review and meta-analysis. BMC Med. 2016;14:178. doi: 10.1186/s12916-016-0721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin S-J. Functional Polymorphism of Alcohol and Aldehyde Dehydrogenases. In; 2001: 1–26.
- 6.Gueldner J, Sayes C. Emerging Associations of the ALDH2*2 Polymorphism with Disease Susceptibility. Journal of Drug Metabolism & Toxicology 2016, 7.
- 7.Sun S, He J, Zhang Y, Xiao R, Yan M, Ren Y, Zhu Y, Jin T, Xia Y. Genetic polymorphisms in the ALDH2 gene and the risk of ischemic stroke in a Chinese han population. Oncotarget. 2017;8:101936–43. doi: 10.18632/oncotarget.21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao PP, Chen YG, Wang JL, Wang XL, Zhang Y. Meta-analysis of aldehyde dehydrogenase 2 gene polymorphism and Alzheimer’s disease in East Asians. Can J Neurol Sci. 2011;38:500–6. doi: 10.1017/S0317167100011938. [DOI] [PubMed] [Google Scholar]
- 9.Cai Q, Wu J, Cai Q, Chen EZ, Jiang ZY. Association between Glu504Lys polymorphism of ALDH2 gene and cancer risk: a meta-analysis. PLoS ONE. 2015;10:e0117173. doi: 10.1371/journal.pone.0117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuchiya T, Sakai A, Menuki K, Mori T, Takeuchi Y, Kanoh S, Utsunomiya H, Murai T, Isse T, Kawamoto T, Nakamura T. Disruption of aldehyde dehydrogenase 2 gene results in altered cortical bone structure and increased cortical bone mineral density in the femoral diaphysis of mice. Bone. 2013;53:358–68. doi: 10.1016/j.bone.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen CH, Zhang Y, Yamamoto K, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43:531–8. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto C, Miedema MD, Ofman P, Gaziano JM, Sesso HD. An expanding knowledge of the mechanisms and effects of alcohol consumption on cardiovascular disease. J Cardiopulm Rehabil Prev. 2014;34:159–71. doi: 10.1097/HCR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 13.Lin CH, Nfor ON, Ho CC, Hsu SY, Tantoh DM, Liaw YC, Daria MR, Chen CH, Liaw YP. Association of ADH1B polymorphism and alcohol consumption with increased risk of intracerebral hemorrhagic stroke. J Transl Med. 2021;19:227. doi: 10.1186/s12967-021-02904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cravo ML, Glória LM, Selhub J, Nadeau MR, Camilo ME, Resende MP, Cardoso JN, Leitão CN, Mira FC. Hyperhomocysteinemia in chronic alcoholism: correlation with folate, vitamin B(-1)2, and vitamin B-6 status. Am J Clin Nutr. 1996;63:220–4. doi: 10.1093/ajcn/63.2.220. [DOI] [PubMed] [Google Scholar]
- 15.Gibson A, Woodside JV, Young IS, Sharpe PC, Mercer C, Patterson CC, McKinley MC, Kluijtmans LA, Whitehead AS, Evans A. Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers–a randomized, crossover intervention study. QJM. 2008;101:881–7. doi: 10.1093/qjmed/hcn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cravo ML, Camilo ME. Hyperhomocysteinemia in chronic alcoholism: relations to folic acid and vitamins B(6) and B(12) status. Nutrition. 2000;16:296–302. doi: 10.1016/S0899-9007(99)00297-X. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Wang X, He M, Qin X, Tang G, Huo Y, Li J, Fu J, Huang X, Cheng X, et al. Homocysteine and Stroke Risk. 2017;48:1183–90. doi: 10.1161/STROKEAHA.116.015324. [DOI] [PubMed] [Google Scholar]
- 18.Shi Z, Liu S, Guan Y, Zhang M, Lu H, Yue W, Zhang B, Li M, Xue J, Ji Y. Changes in total homocysteine levels after acute stroke and recurrence of stroke. Sci Rep. 2018;8:6993. doi: 10.1038/s41598-018-25398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 20.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–11. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin C-H, Nfor ON, Ho C-C, Hsu S-Y, Tantoh DM, Liaw Y-C, Daria M-R, Chen C-H, Liaw Y-P. Association of ADH1B polymorphism and alcohol consumption with increased risk of intracerebral hemorrhagic stroke. J Translational Med. 2021;19:227. doi: 10.1186/s12967-021-02904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li QY, Zhao NM, Ma JJ, Duan HF, Ma YC, Zhang W, Zhao HW, Qin YH. ALDH2*2 Allele is a Negative Risk Factor for Cerebral Infarction in Chinese Women. Biochem Genet. 2015;53:260–7. doi: 10.1007/s10528-015-9686-9. [DOI] [PubMed] [Google Scholar]
- 23.Sung YF, Lu CC, Lee JT, Hung YJ, Hu CJ, Jeng JS, Chiou HY, Peng GS. Homozygous ALDH2*2 Is an Independent Risk Factor for Ischemic Stroke in Taiwanese Men. Stroke. 2016;47:2174–9. doi: 10.1161/STROKEAHA.116.013204. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Zhang Y, Ren J. ALDH2 and Stroke: A Systematic Review of the Evidence. Adv Exp Med Biol. 2019;1193:195–210. doi: 10.1007/978-981-13-6260-6_11. [DOI] [PubMed] [Google Scholar]
- 25.Danquah KO, Gyamfi D: Chap. 3 - Alcohol and Aldehyde Dehydrogenases: Molecular Aspects. In Molecular Aspects of Alcohol and Nutrition. Edited by Patel VB. San Diego: Academic Press; 2016: 25–43.
- 26.Shin C, Kwack K, Cho NH, Kim SH, Baik I. Sex-specific differences in the association of a common aldehyde dehydrogenase 2 gene polymorphism and alcohol consumption with stroke risk in a Korean population: a prospective cohort study. Nutr Res Pract. 2015;9:79–86. doi: 10.4162/nrp.2015.9.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong D, Zhang H, Hu S. Mitochondrial aldehyde dehydrogenase 2 activation and cardioprotection. J Mol Cell Cardiol. 2013;55:58–63. doi: 10.1016/j.yjmcc.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 28.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–38. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pizzimenti S, Ciamporcero E, Daga M, Pettazzoni P, Arcaro A, Cetrangolo G, Minelli R, Dianzani C, Lepore A, Gentile F, Barrera G. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okun E, Arumugam TV, Tang SC, Gleichmann M, Albeck M, Sredni B, Mattson MP. The organotellurium compound ammonium trichloro(dioxoethylene-0,0’) tellurate enhances neuronal survival and improves functional outcome in an ischemic stroke model in mice. J Neurochem. 2007;102:1232–41. doi: 10.1111/j.1471-4159.2007.04615.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee WC, Wong HY, Chai YY, Shi CW, Amino N, Kikuchi S, Huang SH. Lipid peroxidation dysregulation in ischemic stroke: plasma 4-HNE as a potential biomarker? Biochem Biophys Res Commun. 2012;425:842–7. doi: 10.1016/j.bbrc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Ren J, Babcock SA, Li Q, Huff AF, Li SY, Doser TA. Aldehyde dehydrogenase-2 transgene ameliorates chronic alcohol ingestion-induced apoptosis in cerebral cortex. Toxicol Lett. 2009;187:149–56. doi: 10.1016/j.toxlet.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song B-J, Abdelmegeed MA, Yoo S-H, Kim B-J, Jo SA, Jo I, Moon K-H. Post-translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J Proteom. 2011;74:2691–702. doi: 10.1016/j.jprot.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brocker C, Lassen N, Estey T, Pappa A, Cantore M, Orlova VV, Chavakis T, Kavanagh KL, Oppermann U, Vasiliou V. Aldehyde dehydrogenase 7A1 (ALDH7A1) is a novel enzyme involved in cellular defense against hyperosmotic stress. J Biol Chem. 2010;285:18452–63. doi: 10.1074/jbc.M109.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alnouti Y, Klaassen CD. Tissue Distribution, Ontogeny, and Regulation of Aldehyde Dehydrogenase (Aldh) Enzymes mRNA by Prototypical Microsomal Enzyme Inducers in Mice. Toxicol Sci. 2007;101:51–64. doi: 10.1093/toxsci/kfm280. [DOI] [PubMed] [Google Scholar]
- 37.Yang H, Shan W, Zhu F, Wu J, Wang Q: Ketone Bodies in Neurological Diseases: Focus on Neuroprotection and Underlying Mechanisms. 2019, 10. [DOI] [PMC free article] [PubMed]
- 38.Yin J, Han P, Tang Z, Liu Q, Shi J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab. 2015;35:1783–9. doi: 10.1038/jcbfm.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mills PB, Struys E, Jakobs C, Plecko B, Baxter P, Baumgartner M, Willemsen MA, Omran H, Tacke U, Uhlenberg B, et al. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat Med. 2006;12:307–9. doi: 10.1038/nm1366. [DOI] [PubMed] [Google Scholar]
- 40.Stockler S, Plecko B, Gospe SM, Jr, Coulter-Mackie M, Connolly M, van Karnebeek C, Mercimek-Mahmutoglu S, Hartmann H, Scharer G, Struijs E, et al. Pyridoxine dependent epilepsy and antiquitin deficiency: clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol Genet Metab. 2011;104:48–60. doi: 10.1016/j.ymgme.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Vech RL, Lumeng L, Li TK. Vitamin B6 metabolism in chronic alcohol abuse The effect of ethanol oxidation on hepatic pyridoxal 5’-phosphate metabolism. J Clin Investig. 1975;55:1026–32. doi: 10.1172/JCI108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller JW, Ribaya-Mercado JD, Russell RM, Shepard DC, Morrow FD, Cochary EF, Sadowski JA, Gershoff SN, Selhub J. Effect of vitamin B-6 deficiency on fasting plasma homocysteine concentrations. Am J Clin Nutr. 1992;55:1154–60. doi: 10.1093/ajcn/55.6.1154. [DOI] [PubMed] [Google Scholar]
- 43.Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic Acid in stroke prevention: a meta-analysis. Stroke. 2010;41:1205–12. doi: 10.1161/STROKEAHA.109.573410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selhub J, Jacques PF, Bostom AG, D’Agostino RB, Wilson PW, Belanger AJ, O’Leary DH, Wolf PA, Schaefer EJ, Rosenberg IH. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–91. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 45.Jeon SB, Kang DW, Kim JS, Kwon SU. Homocysteine, small-vessel disease, and atherosclerosis: an MRI study of 825 stroke patients. Neurology. 2014;83:695–701. doi: 10.1212/WNL.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 46.Wu GH, Kong FZ, Dong XF, Wu DF, Guo QZ, Shen AR, Cheng QZ, Luo WF. Association between hyperhomocysteinemia and stroke with atherosclerosis and small artery occlusion depends on homocysteine metabolism-related vitamin levels in Chinese patients with normal renal function. Metab Brain Dis. 2017;32:859–65. doi: 10.1007/s11011-017-9978-3. [DOI] [PubMed] [Google Scholar]
- 47.Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E. Homocysteine-Lowering Therapy and Stroke Risk, Severity, and Disability. Stroke. 2009;40:1365–72. doi: 10.1161/STROKEAHA.108.529503. [DOI] [PubMed] [Google Scholar]
- 48.Kelly PJ, Kistler JP, Shih VE, Mandell R, Atassi N, Barron M, Lee H, Silveira S, Furie KL. Inflammation, Homocysteine, and Vitamin B6 Status After Ischemic Stroke. Stroke. 2004;35:12–5. doi: 10.1161/01.STR.0000106481.59944.2F. [DOI] [PubMed] [Google Scholar]
- 49.Robinson K, Arheart K, Refsum H, Brattström L, Boers G, Ueland P, Rubba P, Palma-Reis R, Meleady R, Daly L, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation. 1998;97:437–43. doi: 10.1161/01.CIR.97.5.437. [DOI] [PubMed] [Google Scholar]
- 50.Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, Furie KL. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke. 2003;34:e51–4. doi: 10.1161/01.STR.0000071109.23410.AB. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Taiwan Biobank data source but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Taiwan Biobank.