Abstract
Accumulating evidence indicates a central role for epigenetic modifications in the progression of stroke pathology. These epigenetic mechanisms are involved in complex and dynamic processes that modulate post-stroke gene expression, cellular injury response, motor function, and cognitive ability. Despite decades of research, stroke continues to be classified as a leading cause of death and disability worldwide with limited clinical interventions. Thus, technological advances in the field of epigenetics may provide innovative targets to develop new stroke therapies. This review presents the evidence on the impact of epigenomic readers, writers, and erasers in both ischemic and hemorrhagic stroke pathophysiology. We specifically explore the role of DNA methylation, DNA hydroxymethylation, histone modifications, and epigenomic regulation by long non-coding RNAs in modulating gene expression and functional outcome after stroke. Furthermore, we highlight promising pharmacological approaches and biomarkers in relation to epigenetics for translational therapeutic applications.
Keywords: Biomarkers, cerebral ischemia, epigenomics, hemorrhagic stroke, neuroprotection
Introduction
Epigenetics refers to heritable and modifiable processes that regulate gene expression without altering the DNA sequence. 1 Epigenetic regulation is highly complex and can be influenced by environmental factors such as stress, diet, and aging that modulate physiological conditions. 2 Several epigenetic modifications have been shown to play critical roles in the developing brain and are important for the maintenance of brain function throughout the lifespan. 1 In addition, epigenetics are involved in various aspects of CNS pathologies and have been associated with the severity and progression of many neurological disorders. 2 Mounting evidence has indicated that epigenetic mechanisms regulate cerebrovascular pathology in both human and experimental stroke paradigms. Consequently, understanding the role of epigenetic alterations in stroke may improve our understanding of brain damage following stroke.
The majority of strokes are ischemic (>85%) resulting from arterial blockage that reduces or interrupts blood flow to the brain. Cerebral ischemia initiates a pathophysiological cascade that includes energy failure, excitotoxicity, mitochondrial dysfunction, apoptosis and blood-brain barrier disruption. 3 Reperfusion also leads to significant post-ischemic damage by promoting edema as well as inflammation, oxidative stress and endoplasmic reticulum stress. 3 The ischemic core (infarct) consisting of the dead or dying neurons is surrounded by the penumbra, hypoperfused tissue containing neurons destined to die, which may be salvaged with therapeutic intervention.
In hemorrhagic stroke, weakened blood vessels rupture causing bleeding in the brain. Hemorrhagic strokes can be classified as an intracerebral hemorrhage (ICH), when the bleeding is within the brain tissue, or a subarachnoid hemorrhage (SAH) when bleeding is within the subarachnoid space and ventricular cisterns.4,5 ICH comprise the majority of hemorrhagic strokes and also have the highest mortality of all strokes. 6 Both ICH and SAH lead to increased intracranial pressure and edema, excitotoxicity, reactive oxygen species (ROS) production, inflammation, apoptosis and necrosis. 5 Hemorrhagic strokes can cause further injury by promoting other cerebrovascular injuries such as vasospasms and hydrocephalus, as well as physiological distress in other organs such as the heart, lungs, liver and kidneys. 7
This review discusses epigenetic regulators that modulate cellular and molecular mechanisms involved in both ischemic and hemorrhagic stroke. We will focus on three major mechanisms including 1) DNA methylation and hydroxymethylation 2) histone modifications, 3) and regulation of the epigenome by long non-coding RNAs (lncRNAs). Furthermore, we will discuss the putative applications of epigenetic factors as therapeutic targets and biomarkers for stroke.
Epigenetic mechanisms
DNA methylation
DNA methylation occurs when DNA methyltransferases (DNMTs) transfer a methyl group from the metabolite S-adenosyl methionine (SAM) to the cytosine of DNA to produce 5-methylcytosine (5mC) (Figure 1). 8 The DNMT1 isoform is involved in the maintenance of 5mC during DNA replication, while the DNMT3 family (including DNMT3A and DNMT3B) are responsible for de novo methylation induction. 8 DNA methylation usually occurs on CpG islands (dense regions of CG dinucleotide repeats) located near the promoter region of genes and is involved in the stable silencing of gene expression. In recent years, the discovery that 5mC could undergo further oxidation by the ten-eleven translocation (TET1-3) family of dioxygenases led to the detection of oxidized methylcytosine epigenetic modifications. TETs add a hydroxyl group to 5mC to produce 5-hydroxymethylcytosine (5hmC) by using α-ketoglutarate and molecular oxygen as coenzymes. 9 The 5hmC modification is found in gene regulatory regions and is associated with gene activation (Figure 1). While 5hmC is considered a stable epigenetic modification, TETs can also perform further oxidization of 5hmC to form 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC), which are involved in DNA demethylation via thymine DNA glycosylase base pair removal. 9 Both DNA methylation and DNA hydroxymethylation are highly expressed in the mammalian brain and have been shown to be involved in brain development, neuronal maintenance, cognition, and aging. 10 Furthermore, 5mC and 5hmC levels are known to be altered in both neurodegenerative diseases and acute brain injury, including stroke. 2
Figure 1.
Epigenetic control of gene expression. Chemical modifications on the DNA and histones along with noncoding RNAs epigenetically regulate gene expression. (a) DNA methylation is mediated by DNA methyltransferases (DNMTs) that add the methyl group to the cytosine to generate 5-methylcytosine (5mC), which is subsequently converted to 5-hydroxymethylcytosine (5hmC) by ten-eleven translocation (TETs) dioxygenases. 5mC is a suppressive mark, whereas 5hmC is an activation mark for gene expression. (b) Histone acetyltransferases (HATs) mediate the transfer of the acetyl group to the lysine residues, whereas histone deacetylases reverse this mark. Histone acetylation loosens the chromatin and activates gene expression. (c) Histone methyltransferases (HMTs) mediate the transfer of the methyl groups to the lysine or arginine residues, whereas histone demethylases (KDMs) reverse this mark. The location and degree of methylation dictate its role in gene regulation. For example, trimethylation of histone 3, lysine residue 4 (H3K4me3) activates transcription, whereas H3K27me3 suppresses transcription. (d) Noncoding RNAs bind to the regulatory proteins in the nucleus, such as chromatin modifying proteins and transcription factors, and can act as scaffolds, decoys, or guides to control gene expression.
Histone modifications
Histones are the proteins that wrap and organize DNA into chromatin. Post-translational modifications to histones can alter the accessibility of chromatin to transcriptional regulatory elements. 11 Several types of histone modifications have been identified, and among them acetylation and methylation are well studied in neurological disorders. 12 Histone acetylation is regulated by histone acetyltransferases (HATs), which transfer an acetyl group at the lysine residue of histone proteins. Histone acetylation weakens the electrical bond between the histone and DNA to facilitate the binding of proteins such as transcription factors, thereby promoting gene expression. Histone deacetylases (HDACs) reverse this process by making the chromatin structure relatively tight, which promotes transcriptional repression (Figure 1). 11
In histone methylation, lysine and arginine residues can be methylated by two classes of histone methyltransferases (HMTs) known as lysine methyltransferases (KMTs) and protein arginine methyltransferases (PRMTs). 13 While the existence of a specific histone arginine demethylase has not yet been identified, histone demethylation for both KMT- and PRMT-mediated methylation can occur by histone lysine demethylases (KDMs). 13 Histone methylation has diverse effects on gene expression depending on the type of histone protein in which methylation occurs as well as the position of the amino acid residue used as a substrate. 12 For example, methylation occurring at lysine 4 or lysine 48 of histone H3 (H3K4me or H3K48me) is correlated with increased gene expression, whereas methylation of H3 lysine 27 (H3K27me) has been shown to mediate repression of gene expression (Figure 1).
Interestingly, both DNA methylation and histone modifications are part of a dynamic process to regulate gene expression. For example, methyl-CpG binding domain proteins (MBDs) such as MBD2 and methyl CpG-binding protein 2 (MeCP2) bind to 5mC and can recruit chromatin remodelers such as HDACs and HMTs to the methylated DNA to further promote gene silencing. 14 Moreover, crosstalk between chromatin-modifying proteins (CMPs) adds further complexity in the modulation of gene expression. For instance, lysine-specific demethylase 1 (LSD1) interacts with transcriptional co-repressor complexes such as HDAC1/2 or the RE1-silencing transcription factor (REST)/corepressors of REST (coREST) complex. 15 This further demonstrates the intricate nature of epigenetic regulation by chromatin remodeling which has been shown to play important roles in both neurodevelopmental processes and neurological diseases.1,2
Long noncoding RNAs
LncRNAs are noncoding RNA transcripts that are >200 nucleotides in length. In humans, the number of lncRNAs exceeds the number of protein-coding RNAs. 16 The biological functions of many lncRNAs are still evolving, but accumulating evidence reveals that lncRNAs play major roles in modulating the epigenome at nearly all stages of transcriptional regulation including imprinting genomic loci, transcriptional regulation, and gene masking. 17 LncRNAs can serve as a link between DNA and epigenetic factors by acting as guides and structural scaffolds for CMPs (Figure 1). For example, lncRNAs have been shown to recruit HMTs to promoter regions to repress transcription, mediate the assembly of lysine-specific histone demethylase complexes on chromatin, and act as molecular scaffolds for lysine acetyltransferases. 17 LncRNAs have also been shown to promote locus specific and genome wide DNA methylation by recruiting DNMTs and inhibiting DNMT inhibitors. 18 Thus, lncRNAs act as important mediators to target epigenetic machinery across the genome.
LncRNAs are highly expressed in the brain and have been studied extensively for their roles in neurodevelopment and neurological disorders. 19 As differential expression of several lncRNAs is linked to the pathophysiology of stroke, 20 we will discuss the evidence relating to lncRNAs and their interactions with epigenetic regulators of DNA and histone modifications, in both ischemic and hemorrhagic stroke.
Epigenetic mechanisms in stroke
Stroke and DNA methylation
Early epigenetic studies revealed that DNA methylation may play an important role in post-stroke secondary brain damage. Rodent studies showed robust induction of global hypermethylation after cerebral ischemia which was associated with exacerbated brain damage.21–23 For example, levels of 5mC were elevated in the mouse cortex and striatum following transient middle cerebral artery occlusion (MCAO). 21 Heterozygous knockout of DNMT1, or pharmacological inhibition of DNMT (with 5-aza-2′-deoxycitide (5-aza-dc)), was shown to reduce infarct volume following both mild or moderate transient MCAO.21,22 Alternatively, genetic deletion of DNMT did not prevent secondary brain damage following ischemia/reperfusion after severe transient MCAO, 21 which indicated a differential impact of 5mC on pathophysiological processes based on stroke severity. The expression of MBDs, such as MeCP2 and MBD1 were also increased in the rat hippocampus following global ischemia which further suggests that 5mC may also modulate chromatin modifications in the post-stroke brain. 23
Recent studies showed increased DNMT1 and 5mC levels in the peri-infarct region at both acute and chronic timepoints following transient MCAO or photothrombotic ischemic stroke in rodents.24–29 Furthermore, DNMT inhibition with 5-aza-dc treatment was shown to enhance axonal plasticity and improve motor function recovery up to 4 weeks following photothrombotic stroke. 25 Recent studies also showed increased DNMT1 expression in aged male mice and no difference in DNMT1 expression between adult and middle-aged female rats in the ischemic cortex following transient MCAO.30,31 This indicates that DNMT1 may be a stroke-responsive epigenetic regulator irrespective of age, but may also have sexual dimorphism in stroke. Interestingly, DNMT1 levels were increased in wild type mice, but not diabetic mice following transient MCAO. 32 Moreover, diabetic mice showed reduced levels of DNMT3A and 5mC compared to wild type mice following focal ischemia, 32 suggesting potential differences in post-stroke 5mC regulation in stroke comorbid disease conditions.
Although global methylation changes following cerebral ischemia have been shown to significantly impact outcome after stroke, genome-wide sequencing studies identifying specific 5mC-modulated genes are lacking. However, a few studies have shown the association of DNA methylation changes at specific loci with stroke injury. For example, transient MCAO in adult mice led to hypermethylation of the tissue inhibitor of metalloproteinases 2 (TIMP2) promoter and a reduction in mitochondrial TIMP2 levels in mice at 3 days of reperfusion. 33 Reduction of TIMP2 promoter hypermethylation with 5-aza-dc was associated with increased TIMP2 levels leading to superior extracellular matrix integrity and reduced vascular impairment after focal ischemia. 33 In rats, transient MCAO led to increased methylation of genes associated with axonal repair and neurogenesis including neurite outgrowth inhibitor protein-A (Nogo-A), Nogo receptor, ras homolog gene family member A, and rho-associated coiled-coil protein kinase 2 in the ipsilateral cortex between 1 to 7 days of reperfusion. 34 Mouse primary neuronal cultures treated with amorfrutin B led to hypermethylation of the peroxisome proliferator-activated receptor gamma (PPARγ) gene, reduced PPARγ expression, and prevented neuronal death following oxygen-glucose deprivation (OGD). 35 These examples indicate that DNA methylation is involved in regulating genes involved in stroke pathophysiology (Figure 2). However, more studies are needed to understand the extent to which global DNA methylation changes impact gene expression after cerebral ischemia.
Figure 2.
Epigenetic signatures controlling key post-stroke pathological processes. Multiple pathological processes synergistically potentiate the post-stroke outcome. Epigenetic modifications including 5mC, 5hmC, H3ac, H4ac, H3K9me2, H3K9me3, H3K27me3 and H3K4me3 are implicated in the cell damaging processes such as apoptosis, inflammation, autophagy and mitochondrial dysfunction as well as in recovery related processes such as neuroplasticity, angiogenesis and neurogenesis after stroke.
Recent studies also investigated the role of 5hmC in the post-ischemic brain damage. In mouse models of focal ischemia, global levels of 5hmC were rapidly induced and robustly sustained up to 3 days of reperfusion.24,28,29,36 The TET2 isoform was increased in the ischemic cerebral hemisphere where it was associated with increased BDNF promoter 5hmC and enhanced BDNF expression. 36 Within the peri-infarct region, the TET3 isoform was observed to be responsible for increased 5hmC and modulated neuroprotective genes associated with metabolism, DNA repair, oxidative stress, angiogenesis and cell survival.24,28 TET3 knockdown in both male and female mice was shown to increase edema, cerebral infarction, and motor function impairment, indicating TET3 and 5hmC may provide endogenous protection following ischemic stroke.24,28 The 5fC and 5caC modifications were also shown to increase in the peri-infarct region in male and female mice following focal ischemia, 29 but their roles in the post-ischemic brain are not clear at this time.
Decreased 5hmC after ischemic stroke has been associated with the promotion of pathology in several experimental stroke paradigms (Figure 2). For instance, in a rat model of perinatal hypoxia-ischemia, expression of Tet1 and Tet2 was decreased and 5hmC levels were reduced in genes related to neuronal development in the temporal cortex. 37 Post-stroke depression (PSD), modeled by transient MCAO followed by spatial restraint stress, decreased the expression of TET2 and 5hmC in the mouse brain. 38 Genome-wide 5hmC analysis indicated that the PSD-induced decrease in TET2 was associated with decreased 5hmC in genes involved in the Wnt/β-catenin/lymphoid enhancer factor 1 pathway, which promoted expression of the proinflammatory IL-18. 38 In cultured astrocytes, TET1 levels were reduced, while TET1 overexpression abrogated the induction of autophagy and apoptosis following OGD. 39 Thus, enhancing TETs and 5hmC may be associated with cell survival after ischemic stroke.
Post-stroke administration of high dose ascorbate led to robust induction of TET3 activity and 5hmC levels in the cortical peri-infarct region following mouse transient MCAO.28,29 Genome-wide sequencing analysis revealed that ascorbate-induced 5hmC was increased in genes related to oxidative stress, angiogenesis, apoptosis, mitochondrial function, and inflammation. 29 Furthermore, ascorbate decreased cerebral infarction in male and female aged, diabetic, and hypertensive mice, and ameliorated motor and cognitive deficits in a TET3-dependent manner.28,29 As ascorbate has several molecular targets, additional studies on TET3-specific targeting could further delineate its role in the post-stroke brain. Nevertheless, these studies revealed the therapeutic potential of pharmacologic activation of TETs and 5hmC after stroke.
Changes in 5mC and 5hmC on mitochondrial DNA (mtDNA) have also been observed in experimental models of focal ischemia. Within isolated mouse brain mitochondria, transient MCAO increased 5mC levels and DNMT1 and DNMT3A expression at 72 hours of reperfusion, which were inhibited with 5-aza-dc. 33 Alternatively, another study showed that 5mC levels were unchanged while 5hmC increased in mtDNA 24–48 h after mouse transient MCAO. 40 Furthermore, the increased mtDNA 5hmC was associated with increased mitochondrial gene expression. 40 The reason for the discrepancy of mtDNA 5mC changes between the two studies is not clear, but may be due to the timing of the post-stroke assessments or the region of ischemic brain tissue harvested (i.e. only cerebral cortex or whole cerebral hemisphere).
The role of DNA methylation in hemorrhagic stroke is just beginning to emerge. At this time, only one study has investigated the role of 5mC and 5hmC following ICH in mice. Global levels of 5hmC and the expression of TET1, TET2, and TET3 were reported to be dramatically decreased from 24 h to 72 h following ICH. 41 Genomic profiling revealed that 5hmC levels decreased, while 5mC levels increased at the promoters of Akt serine/threonine kinase 2 (AKT2), 3-phosphoinositide dependent protein kinase 1 (PDPK1), and vascular endothelial growth factor (VEGF), which are the genes known to play a key role in cell survival after ICH. 41 Expression of AKT2, PDPK1, and VEGF was also downregulated after ICH, suggesting that 5mC and 5hmC influence the mechanisms underlying ICH pathophysiology. 41 Future studies assessing genome-wide DNA methylation changes may reveal additional roles of 5mC and 5hmC in the ICH injury response.
Although mechanistic studies on the role of 5mC and 5hmC in hemorrhagic stroke are lacking, differential regulation of DNA methylation markers was reported in the peripheral blood from patients with SAH and ICH. For example, ICH patients displayed a higher ratio of hypermethylated genes compared to hypomethylated genes, many of which were associated with inflammatory pathways. 42 Similarly higher levels of DNA methylation and DNMT1, and lower levels of TET1, were observed in SAH patients with delayed cerebral ischemia (DCI).43,44 Furthermore, DCI patients showed hypermethylation in the inositol 1-,4-,5-trisphosphate receptor 3 (ITPR3) gene, a major mediator of cerebral vasospasm following SAH. 44 In patients with spontaneous ICH, hypomethylation of the dedicator of cytokinesis 1 (DOCK1) promoter was associated with increased DOCK1 expression, hematoma volume, and worsened rehabilitative outcomes. 45
In ischemic stroke patients, several studies showed significant correlation between blood DNA methylation and post-stroke outcome. In particular, blood methylation patterns in solute carrier family 6 member 4 (SLC6A4) have been associated with the risk of stroke recurrence, post-stroke depression, and neurologic deterioration.46–48 Additionally, blood methylation in dozens of genes have been associated with stroke risk and functional outcomes in stroke patients with comorbid diseases (hypertensive and obese).49–51 Furthermore, additional profiling studies showed that biological age (DNAm age), which is measured by circulating levels 5mC, can predict stroke risk, outcome, and recurrence more accurately than chronological age.52–56 Thus, these studies collectively indicate that blood levels of methylated DNA may serve as a biomarker for the diagnosis, prognosis and treatment of stroke.
Stroke and histone acetylation
Experimental animal models indicate that stroke increases the cerebral expression of HDACs in a spatial and temporal manner.57–59 Hypoacetylation of histone proteins, from early to late timepoints following stroke was observed to be associated with exacerbated injury and poor outcomes.57–59 Therefore, the majority of studies on the role of histone deacetylation in stroke investigated the effect of post-stroke pharmacological inhibition of HDACs in various models of experimental stroke.
For instance, the pan-HDAC inhibitor scriptaid reduced hematoma volume, edema and white matter injury following ICH in mice. 60 In rodent photothrombotic stroke, treatment with the pan-HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) increased peri-infarct transcription of neuroplasticity genes, enhanced spine density, and dendritic complexity, 61 and the HDAC2/3 inhibitor MI192 prevented peri-infarct apoptosis. 62 Moreover, both SAHA and MI192 reduced infarct volume and promoted motor function recovery after photothrombotic stroke.61,62 Similarly in rodent transient MCAO, administration of SAHA abrogated H3 deacetylation, increased expression of anti-apoptotic genes Hsp70 and Bcl-2, improved functional recovery, and decreased secondary brain damage in rodents following transient MCAO.58,63 Moreover, treatment with pan-HDAC inhibitor sodium butyrate reduced the expression of inflammatory molecules, infarct volume and motor function impairment following transient MCAO in rodents.64,65 Rodent hypoxia-ischemia models showed that the pan-HDAC inhibitor 4-sodium phenylbutyrate (PBA) reduced inflammation, edema, apoptosis, and infarct volume, 66 while treatment with the pan-HDAC inhibitor Trichostatin A (TSA) stimulated BDNF expression and prevented neuroblast and oligodendrocyte progenitor cell loss. 67 TSA treatment following rat permanent MCAO reduced histone H3 acetylation, prevented neurological deficits, and decreased brain damage, 68 while treatment with sodium butyrate reduced microglial activation and stimulated neurogenesis in the subventricular zone, dentate gyrus, striatum, and frontal cortex after permanent MCAO.68,69 Thus, post-stroke HDAC inhibition has shown potential as a therapeutic agent in several models and types of experimental stroke.
Exposure to the class I HDAC (HDACs 1,2,3 and 8) inhibitor valproic acid (VPA) has been associated with decreased risk of stroke in clinical settings. 70 Experimental animal studies have further shown neuroprotection when VPA was administered after stroke. Following global cerebral ischemia in rodents, VPA decreased microglial activation and increased the density of surviving neurons in the hippocampal CA1 region, stimulated neurogenesis in the subventricular zone, and ameliorated cognitive deficits.71,72 Following permanent MCAO in rats, VPA treatment stimulated white matter repair and neurogenesis in the peri-ischemic region, reduced microglial activation, enhanced Hsp70 levels, and improved neurological outcomes up to 28 days following stroke induction.68,73 Post-ischemic treatment of VPA in rodent transient MCAO models increased Hsp70, decreased caspase-3 activation, attenuated BBB disruption and tight junction degradation, mitigated inflammatory markers and activated microglia in the peri-infarct cortex, and reduced infarct volume.74–76 In a rat model of ICH, post-stroke VPA treatment reduced genes associated with apoptosis and inflammation, and reduced hematoma expansion and perihematomal cell death. 77 Post-insult VPA treatment also upregulated H3 acetylation, decreased the expression of apoptosis proteins, and lowered the incidence of vasospasm after SAH in rats, 78 further indicating that VPA protection may extend to hemorrhagic stroke as well.
The specific role of pharmacological HDAC inhibition is unclear since some HDAC inhibitors can modulate neuroprotection independent of HDAC inhibition, such as through their ability to bind metals or function as chemical chaperones.66,79,80 However, several recent studies investigated the role of HDACs after stroke via genetic manipulation. Conditional microglial HDAC2 knockout modulated microglia/macrophage polarization and reduced the secretion of proinflammatory cytokines after ICH in mice. 81 Similarly, shRNA-mediated HDAC2 knockdown or HDAC2 conditional knockout reduced inflammation and improved motor function recovery after photothrombotic stroke in mice.61,82 After transient MCAO in rats, overexpression or knockdown of HDAC4 and HDAC5, was shown to decrease or increase the expression of the neuroprotective Na+/Ca2+ exchanger 3 (NCX3), respectively. 83 RNA silencing of HDAC9 reduced endothelial injury and improved BBB integrity, thereby reduced inflammation, edema, and infarct following transient MCAO in rats. 84 HDAC9 expression was increased in the carotid plaques of stroke patients 85 and a single nucleotide polymorphism (SNP) in the HDAC9 gene has been associated with large artery atherosclerotic stroke in multiple stroke patient studies.85–87 This indicates that some individuals carry a genetic susceptibility to stroke via HDAC9-mediated histone acetylation. Furthermore, this suggests that in addition to the critical role of histone acetylation in modulating cerebral ischemic injury (Figure 2), polymorphisms in histone acetylation markers might mediate genetic risk to stroke.
Stroke and histone methylation
As histone methylation marks are diverse and abundant, the role of histone methylation in stroke is still emerging. Nevertheless, ischemic stroke has been shown to modulate the activity and expression of several HMTs and KDMs. For example, mild ischemia using an internal carotid artery occlusion model (ICAO) in mice, showed dysregulation of several HMTs (including G9a, G9a-like protein, SUV39H1, SUV39H2, enhancer of zeste homolog 2 (EZH2), and SUV420H2) along with a significant decrease in the transcriptionally repressive mark H3K9me2 in the striatum from 3 h to 15 days of reperfusion. 88 Knockdown or pharmacological inhibition of SUV39H1 or G9a, involved in establishing repressive H3K9me3, enhanced BDNF expression and improved the survival of rat cortical neurons following OGD. 89 Levels of SUV39H1 and G9a were increased in the penumbra following photothrombotic stroke in rats, and pharmacological inhibition of G9a reduced apoptosis in the penumbra and decreased infarct volume in the cortex. 27
The Jumonji C (JmjC) domain (JMJD)-containing histone demethylases (JHDMs) are active under hypoxic conditions and can be induced via hypoxia inducible factor 1-alpha (HIF1α)-dependent or -independent mechanisms. 90 In a range of cultured human cell lines, hypoxia was shown to rapidly modulate various H3 histone methylation marks via JmjC-containing KDMs. 90 KDM4A (aka JHDM3A) expression was increased in cultured neurons after OGD or in the brain following transient MCAO in mice. 91 Lentiviral-mediated KDM4A overexpression was shown to exacerbate neuroinflammation, while KDM4A knockdown abrogated the activation of nuclear factor kappa B (NFκB) signaling in microglia and improved cognitive and motor function recovery after mouse transient MCAO. 91 Expression of JMJD3, which demethylates H3K27me3, was increased following OGD, and knockout of JMJD3 was shown to inhibit OGD-induced apoptosis by altering transcriptional activation of p53 and its downstream genes in cultured mouse cortical neurons. 92 Furthermore, JMJD3 knockout mice exhibited reduced neurological deficits and cerebral infarction after transient MCAO. 92 Pharmacological inhibition of KDM4 or JMJD2, using dimethyloxalyglycine (DMOG) restored striatal levels of H3K9me2 and reduced neurological deficits following ICAO, 88 providing another example that inhibition of JHDMs mediate protection against focal ischemia.
H3K4 modulation is the most well-studied histone methylation process in ischemic stroke. LSD1, which induces transcriptional repression via removal of H3K4 methyl groups, was increased in the dentate gyrus, amygdala and cortex from 1 h to 3 days of reperfusion in a rat model of transient global cerebral ischemia. 93 The protein methyltransferase SET and MYND domain-containing protein 2 (SMYD2) histone methyltransferase that methylates H3K4 or H3K36 was shown to increase in the cortical peri-infarct region and SMYD2 knockout reduced BBB breakdown, edema, neurological deficits, and secondary brain damage following transient MCAO in mice. 94 In vitro analysis of primary rat brain microvascular endothelial cells showed that SMYD2 regulates the sphingosine kinases involved in endothelial cell barrier integrity via methylation-mediated ubiquitin-dependent degradation after OGD, indicating that SMYD2 potentiates post-ischemic BBB disruption. 94 In the astrocytes of middle-aged female mice, H3K4 histone methyltransferase activity was decreased compared to adult female mice following transient MCAO. 30 Concomitantly, adult female mice displayed higher enrichment of H3K4me3 (transcriptional enhancer) versus H3K9me3 (transcriptional repressor) compared to middle-aged mice, indicating less active chromatin in the astrocytes of aged females after stroke. 30 Interestingly, H3K4me3 was enriched at the VEGF gene following ischemia and increased VEGF expression was observed in the astrocytes of adult female mice. 30 As VEGF has been shown to be important for the induction of neurogenesis, angiogenesis and post-stroke recovery, decreased H3K4me3 may promote age-related vulnerability to ischemic injury in female mice. 30 In middle-aged male mice H3K4me3 was increased, while the H3K27me3 (transcriptional repressor) was decreased and associated with astrogliosis in cerebral cortex following transient MCAO, 31 however a comparison to adult mice was not performed in this study. Collectively, these studies indicate sex- and age-specific differential regulation of histone modifications after focal ischemia.
Currently, studies on the role of histone methylation in hemorrhagic stroke are sparse. Using a SAH model in rats, EZH2 and H3K27me3 levels were shown to be increased in astrocytes, neurons and microglial cells from 3 h to 3 days following insult. 95 This study further showed that pharmacological inhibition of EZH2 with EPZ6438 decreased H3K27me3 and proinflammatory cytokines and increased the expression of suppressor of cytokine signaling 3, 95 suggesting an important role for H3K27me3 in modulating inflammation following hemorrhagic stroke. In addition, various histone methylation changes were implicated in vascular hyperplasia, aortic aneurysms, arterial hypertension, and endothelial dysfunction, 96 which further suggests a role for histone methylation in hemorrhagic stroke.
Long noncoding RNAs as epigenetic regulators in stroke
Aberrant expression of hundreds of lncRNAs have been reported following stroke in both humans and rodent models of experimental stroke. 20 Furthermore, lncRNAs have been evaluated as potential diagnostic and prognostic biomarkers in stroke patients 97 as well as mechanistic mediators of pathophysiology in rodent models of ischemic and hemorrhagic stroke.98,99 As lncRNAs can function as scaffolds for histone modification complexes, 17 we will discuss several studies that have investigated the role of lncRNAs as regulators of the epigenome in the post-stroke brain (Figure 3).
Figure 3.
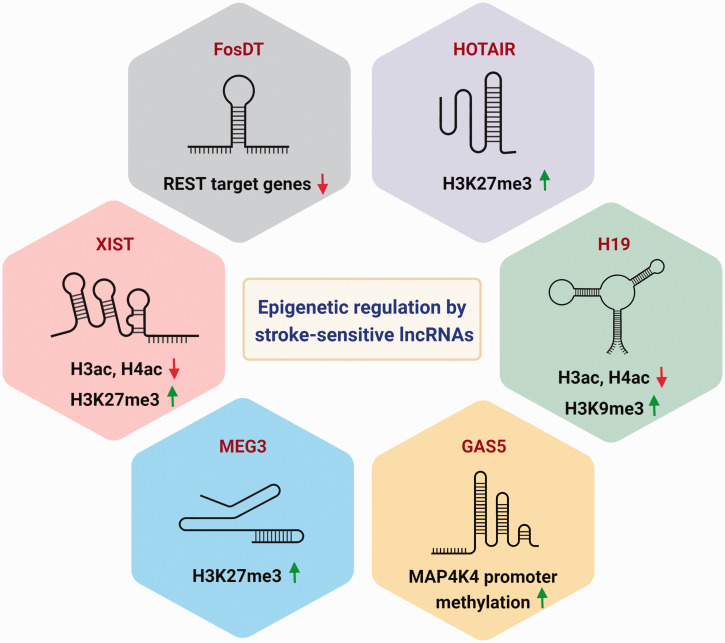
Epigenomic regulation by stroke-sensitive lncRNAs. LncRNAs interact with epigenetic regulators of DNA and histone modifications to fine tune the post-stroke transcriptome. Predominant stroke-sensitive lncRNAs that are involved in the epigenetic regulation include FosDT, HOTAIR, H19, GAS5, MEG3 and XIST.
LncRNAs are known to interact with CMPs to regulate gene expression which may significantly impact stroke outcome. For instance, REST binds to the RE1 element of target genes and recruits its corepressors Sin3A and coREST to mediate gene repression. 100 REST was shown to fuel epigenetic silencing of several genes in hippocampal CA1 neurons after transient global ischemia in rats. 101 Following transient MCAO, REST inhibition was shown to derepress synaptic plasticity-related genes, decrease infarct volume and improve functional outcome in rats. 102 Nearly 200 lncRNAs in the rat cerebral cortex were shown to bind to Sin3A or coREST, and many of them were shown to be upregulated following rat transient MCAO. 103 One of these lncRNAs, the Fos downstream transcript (FosDT), was shown to bind to both Sin3a and coREST and repress REST-target genes involved in synaptic plasticity. 104 Conversely, knockdown of FosDT resulted in derepression of the REST-downstream genes and attenuated motor deficits and infarct volume. 104 Similarly, FosDT knockout rats showed decreased sensorimotor deficits and brain damage after transient MCAO, suggesting that FosDT promotes post-stroke brain damage by epigenetic mechanisms. 105
Another lncRNA called Hox transcript antisense intergenic RNA (HOTAIR) acts as a scaffold to tether Polycomb Repressive Complex 2 (PRC2) comprised of H3K27 methylase EZH2 on its 5′ domain and LSD1/CoREST/REST protein complex on its 3′ domain. 106 The HOTAIR/PRC2/LSD1 complex promotes histone H3 lysine 27 methylation and lysine 4 demethylation to epigenetically repress homeobox D cluster (HOXD) genes. 106 Following permanent MCAO in mice, HOTAIR expression increased from 6 h to 24 h after ischemia induction.107,108 HOTAIR knockdown was further shown to decrease inflammation, apoptosis, and infarct volume and improve motor and cognitive function in mice after permanent MCAO. 108 HOTAIR was also upregulated in human brain microvascular endothelial cells (hBMVECs) following OGD and knockdown of HOTAIR reduced apoptosis and improved hBMVEC layer integrity by increasing the expression of tight junction proteins in a EZH2-dependent manner. 109 The HOTAIR/EZH2 axis was further shown to regulate cell migration and angiogenesis in hBMVECs after OGD. 109 As HOTAIR increases in the serum of neonatal hypoxic-ischemic encephalopathy (HIE) patients, 109 it may also serve as a potential biomarker for ischemic injury.
The lncRNA H19 has been shown to modulate several chromatin modifications and was increased in the rodent brain after focal ischemia.110–113 For example, H19 interacts with MBD1 that mediates gene repression by enhancing the repressive histone H3K9me3 mark. 114 In cultured mouse microglial cells subjected to OGD, H19 induced upregulation of HDAC1 with concomitant downregulation of acetyl-histone H3 and acetyl-histone H4. 115 In rat neural stem cells subjected to OGD, H19 was shown to interact with the EZH2 and SUZ12 chromatin remodelers to modulate the transcription of genes involved in hypoxia response, proliferation, and neurogenesis. 113 Following transient MCAO in rats, H19 promoted secondary brain damage by inducing C1q and tumor necrosis factor 6 expression, secretion of IL-1β and TNF-α from leukocytes, and BBB disruption. 116 H19 knockdown increased the expression of increased insulin like growth factor 1 receptor, neurogenesis-related proteins such as Notch1, and mTOR pathway genes which promoted axon sprouting after transient MCAO in mice.111,117 H19 knockdown further decreased cerebral infarction and neurological deficits, and improved cortical-dependent motor task performance after focal ischemia in several rodent studies.111–113,115,117 Similarly, H19 was shown to increase in the perivascular hematoma tissue and H19 knockdown decreased neurological impairment, levels of ROS, and inflammatory markers such as TNF-α, IL-6 and IL-1β following ICH in rats. 118 Interestingly, in stroke patients, H19 was increased in the leukocytes, blood, and serum and circulating H19 levels were associated with increased risk for ischemic stroke.110,112,116,119–122 This indicates the potential of H19 to serve as a biomarker for the prognosis for ischemic stroke.
The lncRNA growth-arrest-specific transcript 5 (GAS5) was shown to induce methylation of the mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4) promoter via recruitment of DNMT3B, which reduced MAP4K4 expression and apoptosis in primary cortical neurons subjected to OGD.123,124 GAS5 overexpression reduced infarct and improved cognitive functional recovery via inhibition of MAP4K4 following transient MCAO in mice. 123 Alternately, in a rat model of global ischemia, GAS5 knockdown reduced proapoptotic Bax, increased anti-apoptotic Bcl2 and reduced neuronal death in the hippocampus and prefrontal cortex. 125 Similarly, GAS5 knockdown suppressed apoptosis and decreased survival via inactivation of Notch 1 in primary neuronal cultures subjected to OGD. 126 These conflicting results on the role of GAS5 on ischemic brain damage indicates that additional factors such as stroke subtype should be considered when assessing the role of a lncRNA in pathological processes. GAS5 increased in the blood of stroke patients where it was associated with increased stroke risk and poor rehabilitative outcomes in hypertensive, diabetic, and aged stroke patients.127,128
The lncRNA maternally expressed gene 3 (MEG3) was upregulated in the core, but downregulated in the penumbra following transient MCAO in rodents.129,130 MEG3 has been shown to play a role in the recruitment of EZH2 and the PRC2 component jumonji and AT-rich interaction domain containing 2 (JARID2) and histone H3 methylation. 131 MEG3 also directly binds to the DNA binding domain of p53 leading to its activation and apoptosis following in vitro and in vivo models of ischemia.130,132,133 MEG3 knockdown reduced the expression of Bax and cleaved caspase-3 in hBMVECs and enhanced the expression of pro-angiogenic genes VEGF and HIF-1α in rat BMVECs following OGD. 132 Inhibition of MEG3 decreased apoptosis, promoted Notch1 signaling, angiogenesis, and neurogenesis, and decreased neurological deficits and infarct volume following transient MCAO in rodents.129,134,135 In an ICH rat model, MEG3 downregulation reduced caspase-3 activity and apoptosis, and ameliorated inflammation and oxidative stress. 136 Expression of MEG3 was increased in the cerebrospinal fluid of SAH patients, and in the blood of ischemic stroke patients, which was associated with the severity of post-stroke brain damage.137,138 These clinical studies further indicate that MEG3 may be involved in exacerbation of brain injury after stroke.
The lncRNA X-inactive specific transcript (XIST) recruits repressive histone modifications such as HDAC3 and subunits of PRC2 to regulate gene expression. 139 Following transient MCAO, XIST expression increased and XIST knockdown impaired angiogenesis and increased cerebral vascular injury in mice.140,141 In vitro, XIST knockdown abrogated caspase-3 activity and apoptosis in OGD-treated cultured neurons 140 and inhibited hypoxia-induced angiogenesis by suppressing VEGF in cultured brain hBMECs. 142 In ischemic stroke patients, XIST levels decreased in the early stage (48 h), but increased in the late stage (7d) after stroke, yet overall XIST levels were negatively correlated with neurological impairment severity. 141
The functional landscape of lncRNAs is still evolving. However, the evidence above indicates that exploring the epigenetic mechanisms mediated by lncRNAs that interact with CMPs may hold therapeutic value. Furthermore, investigating the circulating levels of these lncRNAs in stroke patients highlights the prognostic and diagnostic potential of lncRNAs involved in epigenomic regulation after stroke.
Epigenetic regulators as therapeutic targets in stroke
The overarching goal of stroke therapies is to salvage the hypo-perfused ischemic penumbra that has not yet evolved into infarcted tissue. 143 Currently, the standard of care for ischemic stroke patients involves early recanalization to restore blood flow by intravenous thrombolysis with tissue plasminogen activator and/or by direct clot removal with mechanical thrombectomy. 143 So far, over 1,000 neuroprotective agents have been tested at the preclinical phase and nearly 70 agents have undergone clinical trials. 144 However, none of the candidate neuroprotectants has entered the clinic. This translational failure is partially attributed to their nature of action directed against a single pathway. 144 Given the clinical heterogeneity and complex post-stroke sequelae, it is may be advantageous to identify and test pleiotropic agents that act against multiple pathways of the ischemic cascade. Epigenetic regulators qualify as a novel class of druggable pleiotropic targets, as they sharply fine-tune the expression of thousands of genes to remodel the injured brain. 2
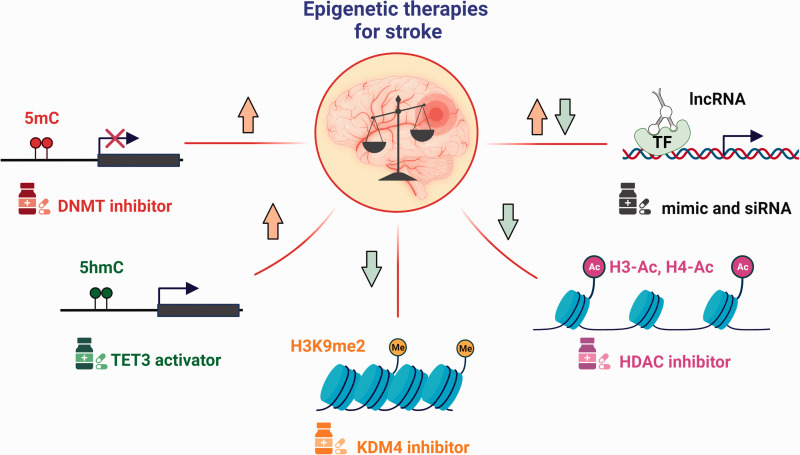
As reviewed here, pharmacological modulation of epigenetic enzymes involved in DNA methylation, DNA hydroxymethylation, and histone acetylation robustly improved functional outcome after experimental stroke. Notable examples of epigenetic drugs that produced beneficial effects in rodents include DNMT inhibitors, the TET activator ascorbate, and various HDAC inhibitors such as sodium butyrate, VPA, TSA, and SAHA (Figure 4). As per recent recommendations on clinical trial design, epigenetic therapies need to be tested as adjunctive treatments to thrombolysis and thrombectomy. 144 To prioritize the candidate epigenetic drugs for testing in stroke patients, a multi-center randomized preclinical assessment based on the guidelines set by the Stroke Preclinical Assessment Network (SPAN) is necessary. 145 The US FDA has approved 7 epigenetic drugs that are now commercially available for the treatment of various types of cancers, however none have entered the clinical phase testing for stroke.
Figure 4.
Epigenetic therapies for stroke. Epigenetic imbalance is a well-understood pathological hallmark of stroke. Specifically, DNA methylation (5mC) and hydroxymethylation (5hmC) are elevated, whereas histone methylation (H3K9me2) and acetylation (H3-Ac and H4-Ac) are suppressed in the ischemic brain. Drugs/Molecules targeting the epigenetic enzymes (DNMTs, TETs, KDMs and HDACs) and noncoding RNAs showed promising therapeutic benefits in experimental stroke models, underscoring the dire need for clinical trials.
Over a dozen clinical trials are currently testing the efficacy of the DNMT inhibitors hydralazine and procainamide for CVDs such as hypertension and heart failure, but not for stroke. Interestingly, post-stroke activation of the DNA hydroxymethylase TET3 by high-dose ascorbate was shown to ameliorate secondary brain damage and accelerated motor and cognitive function recovery in young, aged, hypertensive and diabetic mice of both sexes. 29 Currently, as many as 28 clinical trials are in place testing the efficacy of high dose ascorbate against various diseases, including sepsis, cancer, cardiovascular diseases (CVDs) and diabetes. Several phase 1 clinical trials have already validated the safety and pharmacokinetic profiles of ascorbic acid in both healthy and diseased subjects. Of note, a phase 1 clinical trial has launched recently to evaluate the efficacy of a tri combo therapy involving ascorbate (as well as deferoxamine and N-acetylecysteine) in patients with acute myocardial infarction (NCT05215743). Epigenetic drugs if used in high doses, modulate the expression of thousands of genes globally, which may produce off-target effects. Programming epigenetic drugs to direct them to specific genomic regions will also be critical for attaining specificity. One potential strategy is to conjugate these drugs with polyamides that can target specific motifs in the genome. 146 However, none of the designer molecules are yet tested in the preclinical stroke studies.
The use of pan-HDAC inhibitors provided robust protection in experimental stroke models. However, both spatial and temporal brain expression of several HDACs were shown to be differentially affected by cerebral ischemia in mice,57–59 indicating that therapeutic approaches to HDAC inhibition may benefit from considering timing of administration and HDAC subtype. The groundbreaking development of a PET tracer to evaluate class I HDAC expression in the living human brain 147 opened the possibility for future investigations into HDAC subtypes in the post-stroke brain, which may facilitate drug-target specificity. Furthermore, not all HDAC inhibitors may be effective against stroke as the pan-HDAC inhibitors panobinostat and MS-275 (aka entinostat) failed to enhance motor function recovery after photothrombotic stroke in mice. 148 Collectively, this suggests that additional studies are needed to confirm and delineate the epigenetic mechanism of HDAC inhibition in preclinical stroke models before clinical testing. Nevertheless, there are some HDAC inhibitors that already showed significant promise in clinical stroke settings. For example, the class I HDAC inhibitor VPA is currently utilized as a pharmacological treatment in a number of neurological disorders due to its ability to increase levels of the inhibitor neurotransmitter GABA in the brain. 149 In human clinical case studies, exposure to VPA was associated with decreased risk of stroke and with lower risk of recurrent stroke. 70 Moreover, a recently completed phase 3 clinical trial showed that apabetanol, an inhibitor of the acetylated lysine residue reader Bromodomain Containing 4 (BRD4), significantly reduced cardiovascular death in patients with diabetes and acute coronary syndrome (NCT02586155), which may suggest that BRD4 inhibition may also lower cerebrovascular risk in these susceptible populations with stroke comorbidity.
The measurement of blood markers has transformed the management of patients suffering from myocardial infarction, 150 but identification of a specific and reliable biomarker for stroke patients has not yet been determined. Epigenetic studies showed that a plethora of lncRNAs and methylated DNA, are differentially altered in the blood of acute and chronic stroke patients. In particular, both DNAm age and methylation of SLC6A4 hold promise as biomarkers for stroke risk, recurrence, and outcome.46–48,52–56 The current evidence indicates that epigenetic modulators have tremendous potential as biomarkers for both injury and response to therapy.
Conclusion
The advances in computational biology tools have increased the ability to study the role of epigenetic regulators and their involvement in stroke. The impact of epigenetic mechanisms in stroke is still emerging, however the studies described indicate that epigenetic imbalance of DNA and histone modifications plays a clear role in the pathoetiologic phenomenon after stroke. Furthermore, modulation of several epigenetic processes from molecular readers, writers, and erasers to lncRNA-mediated targeting of epigenetic machinery are associated with significant changes in brain damage and functional recovery after stroke. Collectively, this evidence highlights both the translational potential of epigenetic enzymes as therapeutic targets for stroke, as well the challenge in defining the intricate and dynamic processes involved in post-stroke epigenetic regulation. Overall, epigenetic therapies are a rapidly evolving niche area with promising outcomes for clinical stroke research.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in part by National Institutes of Health grants R01NS099531, R01NS101960, R01NS109459 and Department of Neurological Surgery, University of Wisconsin. Dr. Vemuganti is the recipient of a Research Career Scientist award (# IK6BX005690) from the US Department of Veterans Affairs.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs
Kahlilia C Morris-Blanco https://orcid.org/0000-0003-1459-2871
Anil K Chokkalla https://orcid.org/0000-0002-0101-0417
Samantha M Probelsky https://orcid.org/0000-0002-8316-6203
References
- 1.Isles AR. Epigenetics, chromatin and brain development and function. Brain Neurosci Adv 2018; 2: 2398212818812011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertogliat MJ, Morris-Blanco KC, Vemuganti R. Epigenetic mechanisms of neurodegenerative diseases and acute brain injury. Neurochem Int 2020; 133: 104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith WS. Pathophysiology of focal cerebral ischemia: a therapeutic perspective. J Vasc Interv Radiol 2004; 15: S3–12. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014; 10: 44–58. [DOI] [PubMed] [Google Scholar]
- 5.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 2011; 42: 1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 167–176. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Li Q, Wu H, et al. The harmful effects of subarachnoid hemorrhage on extracerebral organs. Biomed Res Int 2014; 2014: 858496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology 2013; 38: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu H, Zhang Y. Mechanisms and functions of tet protein-mediated 5-methylcytosine oxidation. Genes Dev 2011; 25: 2436–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres RF, Kouro R, Kerr B. Writers and readers of DNA methylation/hydroxymethylation in physiological aging and its impact on cognitive function. Neural Plast 2019; 2019: 5982625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev 1998; 12: 599–606. [DOI] [PubMed] [Google Scholar]
- 12.Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol 2007; 14: 1008–1016. [DOI] [PubMed] [Google Scholar]
- 13.Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 2012; 13: 343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Q, Luu PL, Stirzaker C, et al. Methyl-CpG-binding domain proteins: readers of the epigenome. Epigenomics 2015; 7: 1051–1073. [DOI] [PubMed] [Google Scholar]
- 15.Lan F, Nottke AC, Shi Y. Mechanisms involved in the regulation of histone lysine demethylases. Curr Opin Cell Biol 2008; 20: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao J. The functional role of long non-coding RNAs and epigenetics. Biological Procedures Online 2014; 16: 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol 2013; 20: 300–307. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Sun H, Wang H. Long noncoding RNAs in DNA methylation: new players stepping into the old game. Cell Biosci 2016; 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Policarpo R, Sierksma A, De Strooper B, et al. From junk to function: LncRNAs in CNS health and disease. Front Mol Neurosci 2021; 14: 714768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan J, Saft M, Sadanandan N, et al. LncRNAs stand as potent biomarkers and therapeutic targets for stroke. Front Aging Neurosci 2020; 12: 594571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endres M, Meisel A, Biniszkiewicz D, et al. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci 2000; 20: 3175–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endres M, Fan G, Meisel A, et al. Effects of cerebral ischemia in mice lacking DNA methyltransferase 1 in post-mitotic neurons. Neuroreport 2001; 12: 3763–3766. [DOI] [PubMed] [Google Scholar]
- 23.Jung BP, Zhang G, Ho W, et al. Transient forebrain ischemia alters the mRNA expression of methyl DNA-binding factors in the adult rat hippocampus. Neuroscience 2002; 115: 515–524. [DOI] [PubMed] [Google Scholar]
- 24.Morris-Blanco KC, Chokkalla AK, Bertogliat MJ, et al. TET3 regulates DNA hydroxymethylation of neuroprotective genes following focal ischemia. J Cereb Blood Flow Metab 2021; 41: 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi IA, Lee CS, Kim HY, et al. Effect of inhibition of DNA methylation combined with task-specific training on chronic stroke recovery. IJMS 2018; 19: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asada M, Hayashi H, Murakami K, et al. Investigating the relationship between neuronal cell death and early DNA methylation after ischemic injury. Front Neurosci 2020; 14: 581915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharifulina S, Dzreyan V, Guzenko V, et al. Histone methyltransferases SUV39H1 and G9a and DNA methyltransferase DNMT1 in penumbra neurons and astrocytes after photothrombotic stroke. IJMS 2021; 22: 12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris-Blanco KC, Kim THee, Lopez MS, et al. Induction of DNA hydroxymethylation protects the brain after stroke. Stroke 2019; 50: 2513–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris-Blanco KC, et al. High-dose vitamin C prevents secondary brain damage after stroke via epigenetic reprogramming of neuroprotective genes. Transl Stroke Res 2022. doi: 10.1007/s12975-022-01007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chisholm NC, Henderson ML, Selvamani A, et al. Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics 2015; 10: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H, Li G, Wang R, et al. MiR-424 prevents astrogliosis after cerebral ischemia/reperfusion in elderly mice by enhancing repressive H3K27me3 via NFIA/DNMT1 signaling. FEBS J 2019; 286: 4926–4936. [DOI] [PubMed] [Google Scholar]
- 32.Kalani A, Kamat PK, Tyagi N. Diabetic stroke severity: epigenetic remodeling and neuronal, glial, and vascular dysfunction. Diabetes 2015; 64: 4260–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondal NK, Behera J, Kelly KE, et al. Tetrahydrocurcumin epigenetically mitigates mitochondrial dysfunction in brain vasculature during ischemic stroke. Neurochem Int 2019; 122: 120–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong L, Chen W, He L, et al. Effect of naoluoxintong on the NogoA/RhoA/ROCK pathway by down-regulating DNA methylation in MCAO rats. J Ethnopharmacol 2021; 281: 114559. [DOI] [PubMed] [Google Scholar]
- 35.Wnuk A, Przepiórska K, Pietrzak BA, et al. Post-treatment with amorfrutin B evokes PPARγ-mediated neuroprotection against hypoxia and ischemia. Biomedicines 2021; 9: 854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao Z, He Y, Xin N, et al. Altering 5-hydroxymethylcytosine modification impacts ischemic brain injury. Hum Mol Genet 2015; 24: 5855–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhang Y, Chen D, et al. Genome-wide alteration of 5-hydroxymethylcytosine in hypoxic-ischemic neonatal rat model of cerebral palsy. Front Mol Neurosci 2019; 12: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei X, Yu L, Zhang Y, et al. The role of Tet2-mediated hydroxymethylation in poststroke depression. Neuroscience 2021; 461: 118–129. [DOI] [PubMed] [Google Scholar]
- 39.Zhou D, Huang Z, Zhu X, et al. Circular RNA 0025984 ameliorates ischemic stroke injury and protects astrocytes through miR-143-3p/TET1/ORP150 pathway. Mol Neurobiol 2021; 58: 5937–5953. [DOI] [PubMed] [Google Scholar]
- 40.Ji F, Zhao C, Wang B, et al. The role of 5-hydroxymethylcytosine in mitochondria after ischemic stroke. J Neurosci Res 2018; 96: 1717–1726. [DOI] [PubMed] [Google Scholar]
- 41.Tang Y, Han S, Asakawa T, et al. Effects of intracerebral hemorrhage on 5-hydroxymethylcytosine modification in mouse brains. Neuropsychiatr Dis Treat 2016; 12: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Genome-wide DNA methylation pattern in whole blood associated with primary intracerebral hemorrhage. Front Immunol 2021; 12: 702244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim BJ, Kim Y, Youn DH, et al. Genome-wide blood DNA methylation analysis in patients with delayed cerebral ischemia after subarachnoid hemorrhage. Sci Rep 2020; 10: 11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim BJ, Kim Y, Hong EP, et al. Correlation between altered DNA methylation of intergenic regions of ITPR3 and development of delayed cerebral ischemia in patients with subarachnoid hemorrhage. World Neurosurg 2019; 130: e449–e456. [DOI] [PubMed] [Google Scholar]
- 45.Gao Y, Fu X, Yu L, et al. DNA hypomethylation of DOCK1 leading to high expression correlates with neurologic deterioration and poor function outcomes after spontaneous intracerebral hemorrhage. Evid Based Complement Alternat Med 2021; 2021: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Kim J-M, Stewart R, Kang H-J, et al. A longitudinal study of SLC6A4 DNA promoter methylation and poststroke depression. J Psychiatr Res 2013; 47: 1222–1227. [DOI] [PubMed] [Google Scholar]
- 47.Santoro M, Siotto M, Germanotta M, et al. Association study of SLC6A4 (5-HTTLPR) polymorphism and its promoter methylation with rehabilitation outcome in patients with subacute stroke. Genes (Basel) 2021; 12: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang H-J, Lee E-H, Kim J-W, et al. Association of SLC6A4 methylation with long-term outcomes after stroke: focus on the interaction with suicidal ideation. Sci Rep 2021; 11: 2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu S, et al. High MTHFR promoter methylation levels in men confer protection against ischemic stroke. Bosn J Basic Med Sci 2020; 20: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang A, Zhang M, Ding Y, et al. Associations of B-type natriuretic peptide and its coding gene promoter methylation with functional outcome of acute ischemic stroke: a mediation analysis. J Am Heart Assoc 2020; 9: e017499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gómez-Úriz AM, Milagro FI, Mansego ML, et al. Obesity and ischemic stroke modulate the methylation levels of KCNQ1 in white blood cells. Hum Mol Genet 2015; 24: 1432–1440. [DOI] [PubMed] [Google Scholar]
- 52.Soriano-Tárraga C, Lazcano U, Jiménez-Conde J, et al. Biological age is a novel biomarker to predict stroke recurrence. J Neurol 2021; 268: 285–292. [DOI] [PubMed] [Google Scholar]
- 53.Soriano-Tárraga C, Giralt-Steinhauer E, Mola-Caminal M, et al. Biological age is a predictor of mortality in ischemic stroke. Sci Rep 2018; 8: 4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallego-Fabrega C, Muiño E, Cullell N, et al. Biological age acceleration is lower in women with ischemic stroke compared to men. Stroke 2022; 53: 2320–2330. [DOI] [PubMed] [Google Scholar]
- 55.Soriano-Tárraga C, Mola-Caminal M, Giralt-Steinhauer E, et al. Biological age is better than chronological as predictor of 3-month outcome in ischemic stroke. Neurology 2017; 89: 830–836. [DOI] [PubMed] [Google Scholar]
- 56.Soriano-Tárraga C, Giralt-Steinhauer E, Mola-Caminal M, et al. Ischemic stroke patients are biologically older than their chronological age. Aging (Albany NY) 2016; 8: 2655–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demyanenko S, Berezhnaya E, Neginskaya M, et al. Сlass II histone deacetylases in the post-stroke recovery period-expression, cellular, and subcellular localization-promising targets for neuroprotection. J Cell Biochem 2019; 120: 19590–19609. [DOI] [PubMed] [Google Scholar]
- 58.Kassis H, Shehadah A, Li C, et al. Class IIa histone deacetylases affect neuronal remodeling and functional outcome after stroke. Neurochem Int 2016; 96: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baltan S, Bachleda A, Morrison RS, et al. Expression of histone deacetylases in cellular compartments of the mouse brain and the effects of ischemia. Transl Stroke Res 2011; 2: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang H, Ni W, Jiang H, et al. Histone deacetylase inhibitor scriptaid alleviated neurological dysfunction after experimental intracerebral hemorrhage in mice. Behav Neurol 2018; 2018: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang Y, Lin Y-H, Ni H-Y, et al. Inhibiting histone deacetylase 2 (HDAC2) promotes functional recovery from stroke. Jaha 2017; 6: e007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demyanenko SV, Nikul VV, Uzdensky AB. The neuroprotective effect of the HDAC2/3 inhibitor MI192 on the penumbra after photothrombotic stroke in the mouse brain. Mol Neurobiol 2020; 57: 239–248. [DOI] [PubMed] [Google Scholar]
- 63.Faraco G, Pancani T, Formentini L, et al. Pharmacological inhibition of histone deacetylases by suberoylanilide hydroxamic acid specifically alters gene expression and reduces ischemic injury in the mouse brain. Mol Pharmacol 2006; 70: 1876–1884. [DOI] [PubMed] [Google Scholar]
- 64.Park MJ, Sohrabji F. The histone deacetylase inhibitor, sodium butyrate, exhibits neuroprotective effects for ischemic stroke in middle-aged female rats. J Neuroinflammation 2016; 13: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patnala R, Arumugam TV, Gupta N, et al. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol Neurobiol 2017; 54: 6391–6411. [DOI] [PubMed] [Google Scholar]
- 66.Qi X, Hosoi T, Okuma Y, et al. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol 2004; 66: 899–908. [DOI] [PubMed] [Google Scholar]
- 67.Zalewska T, Jaworska J, Sypecka J, et al. Impact of a histone deacetylase inhibitor-trichostatin A on neurogenesis after hypoxia-ischemia in immature rats. IJMS 2020; 21: 3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HJ, Rowe M, Ren M, et al. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 2007; 321: 892–901. [DOI] [PubMed] [Google Scholar]
- 69.Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem 2009; 110: 1226–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brookes RL, Crichton S, Wolfe CDA, et al. Sodium valproate, a histone deacetylase inhibitor, is associated with reduced stroke risk after previous ischemic stroke or transient ischemic attack. Stroke 2018; 49: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xuan A, Long D, Li J, et al. Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sci 2012; 90: 463–468. [DOI] [PubMed] [Google Scholar]
- 72.George S, Kadam SD, Irving ND, et al. Impact of trichostatin A and sodium valproate treatment on post-stroke neurogenesis and behavioral outcomes in immature mice. Front Cell Neurosci 2013; 7: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu XS, Chopp M, Kassis H, et al. Valproic acid increases white matter repair and neurogenesis after stroke. Neuroscience 2012; 220: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ren M, Leng Y, Jeong M, et al. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem 2004; 89: 1358–1367. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z, Leng Y, Tsai LK, et al. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 2011; 31: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuo TT, Wang V, Wu JS, et al. Post-stroke delivery of valproic acid promotes functional recovery and differentially modifies responses of peri-infarct microglia. Front Mol Neurosci 2021; 14: 639145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sinn D-I, Kim S-J, Chu K, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis 2007; 26: 464–472. [DOI] [PubMed] [Google Scholar]
- 78.Wu CH, et al. Valproic acid reduces vasospasm through modulation of akt phosphorylation and attenuates neuronal apoptosis in subarachnoid hemorrhage rats. Int J Mol Sci 2021; 22: 5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sleiman SF, Olson DE, Bourassa MW, et al. Hydroxamic acid-based histone deacetylase (HDAC) inhibitors can mediate neuroprotection independent of HDAC inhibition. J Neurosci 2014; 34: 14328–14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iannitti T, Palmieri B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D 2011; 11: 227–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang H, Ni W, Wei P, et al. HDAC inhibition reduces white matter injury after intracerebral hemorrhage. J Cereb Blood Flow Metab 2021; 41: 958–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin Y-H, Dong J, Tang Y, et al. Opening a new time window for treatment of stroke by targeting HDAC2. J Neurosci 2017; 37: 6712–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Formisano L, Laudati G, Guida N, et al. HDAC4 and HDAC5 form a complex with DREAM that epigenetically down-regulates NCX3 gene and its pharmacological inhibition reduces neuronal stroke damage. J Cereb Blood Flow Metab 2020; 40: 2081–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi W, Wei X, Wang Z, et al. HDAC9 exacerbates endothelial injury in cerebral ischaemia/reperfusion injury. J Cell Mol Med 2016; 20: 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Markus HS, Mäkelä K-M, Bevan S, et al. Evidence HDAC9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke 2013; 44: 1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu Y, Haessler JW, Manansala R. Whole-genome sequencing association analyses of stroke and its subtypes in ancestrally diverse populations from trans-omics for precision medicine project. Stroke 2022; 53: 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang M, Gu M, Li Z, et al. HDAC9 polymorphisms predict susceptibility, severity, and short-term outcome of large artery atherosclerotic stroke in Chinese population. J Mol Neurosci 2019; 67: 165–171. [DOI] [PubMed] [Google Scholar]
- 88.Chakravarty S, Jhelum P, Bhat UA, et al. Insights into the epigenetic mechanisms involving histone lysine methylation and demethylation in ischemia induced damage and repair has therapeutic implication. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 152–164. [DOI] [PubMed] [Google Scholar]
- 89.Schweizer S, Harms C, Lerch H, et al. Inhibition of histone methyltransferases SUV39H1 and G9a leads to neuroprotection in an in vitro model of cerebral ischemia. J Cereb Blood Flow Metab 2015; 35: 1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Batie M, Frost J, Frost M, et al. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science 2019; 363: 1222–1226. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, Zhao L, Zhang J, et al. Histone demethylase KDM4A inhibition represses neuroinflammation and improves functional recovery in ischemic stroke. Curr Pharm Des 2021; 27: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 92.Zhang H, Wang J, Huang J, et al. Inhibiting Jumoji domain containing protein 3 (JMJD3) prevent neuronal apoptosis from stroke. Exp Neurol 2018; 308: 132–142. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y-Z, Zhang Q-H, Ye H, et al. Distribution of lysine-specific demethylase 1 in the brain of rat and its response in transient global cerebral ischemia. Neurosci Res 2010; 68: 66–72. [DOI] [PubMed] [Google Scholar]
- 94.Wang J, Zhong W, Cheng Q, et al. Histone methyltransferase Smyd2 contributes to blood-brain barrier breakdown in stroke. Clin Transl Med 2022; 12: e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo Y, Fang Y, Kang R, et al. Inhibition of EZH2 (enhancer of zeste homolog 2) attenuates neuroinflammation via H3k27me3/SOCS3/TRAF6/NF-κB (trimethylation of histone 3 lysine 27/suppressor of cytokine signaling 3/tumor necrosis factor receptor family 6/nuclear factor-κB) in a rat model of subarachnoid hemorrhage. Stroke 2020; 51: 3320–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei X, Yi X, Zhu XH, et al. Histone methylation and vascular biology. Clin Epigenetics 2020; 12: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao J, Wang Q, Zhu R, et al. Circulating non-coding RNAs as potential biomarkers for ischemic stroke: a systematic review. J Mol Neurosci 2022. doi: 10.1007/s12031-022-01991-2. [DOI] [PubMed] [Google Scholar]
- 98.Akella A, Bhattarai S, Dharap A. Long noncoding RNAs in the pathophysiology of ischemic stroke. Neuromolecular Med 2019; 21: 474–483. [DOI] [PubMed] [Google Scholar]
- 99.Gareev I, Beylerli O, Aliev G, et al. The role of long non-coding RNAs in intracranial aneurysms and subarachnoid hemorrhage. Life (Basel) 2020; 10: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grimes JA, Nielsen SJ, Battaglioli E, et al. The co-repressor mSin3A is a functional component of the REST-CoREST repressor complex. J Biol Chem 2000; 275: 9461–9467. [DOI] [PubMed] [Google Scholar]
- 101.Noh K-M, Hwang J-Y, Follenzi A, et al. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci U S A 2012; 109: E962–E971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morris-Blanco KC, Kim TH, Bertogliat MJ, et al. Inhibition of the epigenetic regulator REST ameliorates ischemic brain injury. Mol Neurobiol 2019; 56: 2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dharap A, Pokrzywa C, Vemuganti R. Increased binding of stroke-induced long non-coding RNAs to the transcriptional corepressors Sin3A and coREST. ASN Neuro 2013; 5: AN20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mehta SL, Kim T, Vemuganti R. Long noncoding RNA FosDT promotes ischemic brain injury by interacting with REST-associated chromatin-modifying proteins. J Neurosci 2015; 35: 16443–16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mehta SL, Chokkalla AK, Kim THee, et al. Long noncoding RNA Fos downstream transcript is developmentally dispensable but vital for shaping the poststroke functional outcome. Stroke 2021; 52: 2381–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsai M-C, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010; 329: 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang L, Lu ZN. Long non-coding RNA HOTAIR promotes ischemic infarct induced by hypoxia through up-regulating the expression of NOX2. Biochem Biophys Res Commun 2016; 479: 186–191. [DOI] [PubMed] [Google Scholar]
- 108.Huang Y, Wang Y, Liu X, et al. Silencing lncRNA HOTAIR improves the recovery of neurological function in ischemic stroke via the miR-148a-3p/KLF6 axis. Brain Res Bull 2021; 176: 43–53. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y, Mao J, Li X, et al. lncRNA HOTAIR mediates OGD/R-induced cell injury and angiogenesis in a EZH2-dependent manner. Exp Ther Med 2022; 23: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang J, Cao B, Han D, et al. Long non-coding RNA H19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis 2017; 8: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu S, Zheng J, Du Z, et al. Knock down of lncRNA H19 promotes axon sprouting and functional recovery after cerebral ischemic stroke. Brain Res 2020; 1732: 146681. [DOI] [PubMed] [Google Scholar]
- 112.Xiao Z, Qiu Y, Lin Y, et al. Blocking lncRNA H19-miR-19a-Id2 axis attenuates hypoxia/ischemia induced neuronal injury. Aging (Albany NY) 2019; 11: 3585–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fan B, Pan W, Wang X, et al. Long noncoding RNA mediates stroke-induced neurogenesis. Stem Cells 2020; 38: 973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Monnier P, Martinet C, Pontis J, et al. H19 lncRNA controls gene expression of the imprinted gene network by recruiting MBD1. Proc Natl Acad Sci U S A 2013; 110: 20693–20698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Zhao H, Fan Z, et al. Long noncoding RNA H19 promotes neuroinflammation in ischemic stroke by driving histone deacetylase 1-dependent M1 microglial polarization. Stroke 2017; 48: 2211–2221. [DOI] [PubMed] [Google Scholar]
- 116.Li G, et al. Long non-coding RNA H19 promotes leukocyte inflammation in ischemic stroke by targeting the miR-29b/C1QTNF6 axis. CNS Neurosci Ther 2022; 28: 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J, Cao B, Zhao H, et al. Long noncoding RNA H19 prevents neurogenesis in ischemic stroke through p53/Notch1 pathway. Brain Res Bull 2019; 150: 111–117. [DOI] [PubMed] [Google Scholar]
- 118.Mao S, Huang H, Chen X. lncRNA H19 aggravates brain injury in rats following experimental intracerebral hemorrhage via NF-κB pathway. Comput Math Methods Med 2022; 2022: 3017312. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Rezaei M, Mokhtari MJ, Bayat M, et al. Long non-coding RNA H19 expression and functional polymorphism rs217727 are linked to increased ischemic stroke risk. BMC Neurol 2021; 21: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang J, Yang J, Li J, et al. Association of long noncoding RNA H19 polymorphisms with the susceptibility and clinical features of ischemic stroke in Southern Chinese Han population. Metab Brain Dis 2019; 34: 1011–1021. [DOI] [PubMed] [Google Scholar]
- 121.Huang Y, Wang L, Mao Y, et al. Long noncoding RNA-H19 contributes to atherosclerosis and induces ischemic stroke via the upregulation of acid phosphatase 5. Front Neurol 2019; 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu R, Liu X, He Z. Long non-coding RNA H19 and MALAT1 gene variants in patients with ischemic stroke in a Northern Chinese Han population. Mol Brain 2018; 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deng Y, Chen D, Gao F, et al. Silencing of long non-coding RNA GAS5 suppresses neuron cell apoptosis and nerve injury in ischemic stroke through inhibiting DNMT3B-dependent MAP4K4 methylation. Transl Stroke Res 2020; 11: 950–966. [DOI] [PubMed] [Google Scholar]
- 124.Liu YZ, Sun Y. High expression of GAS5 promotes neuronal death after cerebral infarction by regulating miR-365a-3p. Eur Rev Med Pharmacol Sci 2018; 22: 5270–5277. [DOI] [PubMed] [Google Scholar]
- 125.Zhao JH, Wang B, Wang XH, et al. Effect of lncRNA GAS5 on the apoptosis of neurons via the notch1 signaling pathway in rats with cerebral infarction. Eur Rev Med Pharmacol Sci 2019; 23: 10083–10091. [DOI] [PubMed] [Google Scholar]
- 126.Chen F, Zhang L, Wang E, et al. LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA for miR-137 to regulate the Notch1 signaling pathway. Biochem Biophys Res Commun 2018; 496: 184–190. [DOI] [PubMed] [Google Scholar]
- 127.Deng F, Zhu P, Liao C, et al. Genetic variants of lncRNA GAS5 contribute to susceptibility of ischemic stroke among Southern Chinese population. Biomed Res Int 2021; 2021: 6634253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fang P, et al. The clinical value of long noncoding RNA GAS5 in acute ischemic stroke: correlation with disease risk, inflammation, severity, and risk of recurrence. J Clin Lab Anal 2022; 36: e24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu J, Li Q, Zhang K-S, et al. Downregulation of the long non-coding RNA Meg3 promotes angiogenesis after ischemic brain injury by activating notch signaling. Mol Neurobiol 2017; 54: 8179–8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan H, Yuan J, Gao L, et al. Long noncoding RNA MEG3 activation of p53 mediates ischemic neuronal death in stroke. Neuroscience 2016; 337: 191–199. [DOI] [PubMed] [Google Scholar]
- 131.Terashima M, Tange S, Ishimura A, et al. MEG3 long noncoding RNA contributes to the epigenetic regulation of epithelial-mesenchymal transition in lung cancer cell lines. J Biol Chem 2017; 292: 82–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhan R, Xu K, Pan J, et al. Long noncoding RNA MEG3 mediated angiogenesis after cerebral infarction through regulating p53/NOX4 axis. Biochem Biophys Res Commun 2017; 490: 700–706. [DOI] [PubMed] [Google Scholar]
- 133.Chen C, Huang Y, Xia P, et al. Long noncoding RNA Meg3 mediates ferroptosis induced by oxygen and glucose deprivation combined with hyperglycemia in rat brain microvascular endothelial cells, through modulating the p53/GPX4 axis. Eur J Histochem 2021; 65: 3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.You D, You H. Repression of long non-coding RNA MEG3 restores nerve growth and alleviates neurological impairment after cerebral ischemia-reperfusion injury in a rat model. Biomed Pharmacother 2019; 111: 1447–1457. [DOI] [PubMed] [Google Scholar]
- 135.Yan H, Rao J, Yuan J, et al. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate ischemic neuronal death by targeting miR-21/PDCD4 signaling pathway. Cell Death Dis 2017; 8: 3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie B, Qiao M, Xuan J. lncRNA MEG3 downregulation relieves intracerebral hemorrhage by inhibiting oxidative stress and inflammation in an miR-181b-Dependent manner. Med Sci Monit 2021; 27: e929435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liang Z, Chi Y-J, Lin G-Q, et al. LncRNA MEG3 participates in neuronal cell injury induced by subarachnoid hemorrhage via inhibiting the Pi3k/akt pathway. Eur Rev Med Pharmacol Sci 2018; 22: 2824–2831. [DOI] [PubMed] [Google Scholar]
- 138.Wang M, Chen W, Geng Y, et al. Long non-coding RNA MEG3 promotes apoptosis of vascular cells and is associated with poor prognosis in ischemic stroke. J Atheroscler Thromb 2020; 27: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McHugh CA, Chen C-K, Chow A, et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 2015; 521: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weng S, Wang S, Jiang J. Long noncoding RNA X-inactive specific transcript regulates neuronal cell apoptosis in ischemic stroke through miR-98/BACH1 axis. DNA Cell Biol 2021; 40: 979–987. [DOI] [PubMed] [Google Scholar]
- 141.Wang C, Dong J, Sun J, et al. Silencing of lncRNA XIST impairs angiogenesis and exacerbates cerebral vascular injury after ischemic stroke. Mol Ther Nucleic Acids 2021; 26: 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hu C, Bai X, Liu C, et al. Long noncoding RNA XIST participates hypoxia-induced angiogenesis in human brain microvascular endothelial cells through regulating miR-485/SOX7 axis. Am J Transl Res 2019; 11: 6487–6497. [PMC free article] [PubMed] [Google Scholar]
- 143.Baron JC. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat Rev Neurol 2018; 14: 325–337. [DOI] [PubMed] [Google Scholar]
- 144.Lyden PD. Cerebroprotection for acute ischemic stroke: Looking ahead. Stroke 2021; 52: 3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol 2021; 335: 113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kawamoto Y, Bando T, Sugiyama H. Sequence-specific DNA binding pyrrole-imidazole polyamides and their applications. Bioorg Med Chem 2018; 26: 1393–1411. [DOI] [PubMed] [Google Scholar]
- 147.Wey H-Y, Gilbert TM, Zürcher NR, et al. Insights into neuroepigenetics through human histone deacetylase PET imaging. Sci Transl Med 2016; 8: 351ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Al Shoyaib A, Alamri FF, Syeara N, et al. The effect of histone deacetylase inhibitors panobinostat or entinostat on motor recovery in mice after ischemic stroke. Neuromolecular Med 2021; 23: 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Singh D, Gupta S, Verma I, et al. Hidden pharmacological activities of valproic acid: a new insight. Biomed Pharmacother 2021; 142: 112021. [DOI] [PubMed] [Google Scholar]
- 150.Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med 2010; 8: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]