Abstract
Accumulating evidence indicates that the glymphatic system has a critical role in maintaining brain homeostasis. However, the detailed anatomy of the glymphatic pathway is not well understood, mostly due to a lack of high spatial resolution 3D visualization. In this study, a fluorescence micro-optical sectioning tomography (fMOST) was used to characterize the glymphatic architecture in the mouse brain. At 30 and 120 min after intracisternal infusion with fluorescent dextran (Dex-3), lectin was injected to stain the cerebral vasculature. Using fMOST, a high-resolution 3D dataset of the brain-wide distribution of Dex-3 was acquired. Combined with fluorescence microscopy and microplate array, the heterogeneous glymphatic flow and the preferential irrigated regions were identified. These cerebral regions containing large-caliber penetrating arteries and/or adjacent to the subarachnoid space had more robust CSF flow compared to other regions. Moreover, the major glymphatic vessels for CSF influx and fluid efflux in the entire brain were shown in 3D. This study demonstrates the regional heterogeneity in the glymphatic system and provides an anatomical resource for further investigation of the glymphatic function.
Keywords: Glymphatic system, cerebrospinal fluid, paravascular spaces, vasculature, fluorescence micro-optical sectioning tomography
Introduction
Abundant evidence suggests that the cerebrospinal fluid (CSF) in the subarachnoid space (SAS) can move into the brain through the paravascular space (PVS) and then mix with interstitial fluid (ISF). The extensive CSF-ISF exchange is also known as the glymphatic system/pathway.1–4 According to the initial concept, the glymphatic pathway is composed of a para-arterial CSF inflow routes (glymphatic influx vessels) and para-venous fluid outflow routes (glymphatic efflux vessels), which are coupled by directional ISF flow from the para-arterial spaces to the para-venous spaces facilitated by the astrocytic aquaporin 4. 2 The presence of the glymphatic system in rodents,2,5–8 pigs 9 and the human brain,10–13 and its role in the removal of toxic molecules from the brain has been recently identified;2,14–17 yet, this concept has also been questioned by other researchers.4,18–20 Additionally, the detailed anatomical structure of the glymphatic system has not been well identified.
Following intrathecal injection of fluorescent tracer, the glymphatic system has been successfully visualized in vivo and ex vivo. Two-photon laser scanning microscopy has been initially employed to characterize the paravascular CSF–ISF exchange in rodent brain, providing high spatial resolution on local PVSs.2,7,21 Moreover, the visualization of CSF tracer distribution within thin brain sections provides detailed information on the glymphatic system in both the whole brain and specific regions.2,8,14,22–26 Observation of the local regions suggested that the glymphatic pathway represents a brain-wide anatomical structure. However, the above fluorescence-based imaging approaches fail to directly assess the brain-wide CSF flow in a three-dimensional (3D) manner. Since two-dimensional (2D) imaging has obvious limitations, it is imperative to conduct 3D visualization of the glymphatic pathway in the whole brain.
As an innovative technique, fluorescence micro-optical sectioning tomography (fMOST) has been developed to construct whole-brain imaging at the mesoscopic level. 27 By combining automated sequential sectioning and high-resolution optical imaging, this technique can map the whole mouse brain vasculature and neuronal projection.28–30 However, whether fMOST is suitable to depict CSF circulation has not yet been reported. In this study, we used the dual-color fMOST to demonstrate the glymphatic pathway in the mouse brain in 3D. These data provide an anatomical resource for further investigation of the glymphatic function.
Materials and methods
Animals
Adult male C57BL/6 mice (2-3 months old, obtained from Shanghai Laboratory Animal Center) were used in this study. All the animals were housed in an environment with a temperature of 22 ± 1°C, relative humidity of 50 ± 1%, and a light/dark cycle of 12/12 h and were given water and food ad libitum. All the experimental procedures conformed to the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Council of Europe No 123, Strasbourg 1985), and all experiments were approved by the Committee on Animal Resources of Soochow University. This study was performed in accordance with the ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines for reporting of animal experiments.
Anaesthesia
According to previous studies, some anesthetics can enhance the glymphatic activity by increasing cortical delta power in electroencephalogram (EEG) recordings.31,32 In this study, mice were anesthetized with a mixture of chloral hydrate (400 mg/kg) and xylazine (10 mg/kg) by a single intraperitoneal injection, for the reason that these two anesthetics can effectively induce or increase cortical delta power.33,34
CSF tracer infusion
Fluorescein isothiocyanate–conjugated dextran (D3306, Thermos, USA, lysine-fixable, molecular weight: 3 kDa, Dex-3) was mixed with phosphate-buffered saline (PBS) at a concentration of 0.5%. After anaesthesia induction, mice were fixed in a stereotaxic apparatus in a prone position, and the posterior atlanto-occipital membrane overlying the cisterna magna (CM) was surgically exposed, as previously described. 35 Then, a 30-gauge needle connected to a polyethylene tube was inserted into the CM. Using a syringe pump (LSP01-1A, Longer Precision Pump, China), Dex-3 was infused into the SAS at a controlled flow rate of 1 μl/min for 10 min (total amount of 10 μl). After infusion, Dex-3 was allowed to circulate for 20 or 110 min when mice were in a lateral position. Throughout tracer infusion, mice were placed on a regulated heating pad to maintain the core temperature at 37 ± 0.5°C.
Lectin administration
According to previous studies,36,37 anesthetized mice were injected with DyLight® 594 labeled tomato lectin (DL-1177-1, Vector Laboratories, USA, Lectin) by tail vein in order to delineate the cerebral vasculature. The lectin was mixed with PBS at a concentration of 1 mg/ml, and injection was conducted before perfusion-fixation, at 0.4 ml/min for 5 min (total amount of 2 ml).
Tissue processing and fMOST imaging
Anesthetized mice were transcardially perfused with ice-cold PBS, followed by 4% paraformaldehyde (PFA), after which their brains were removed from the skull and post-fixed in 4% PFA at 4°C for 48 h. After fixation, brains were rinsed with PBS overnight and subsequently dehydrated in a graded ethanol solution of 50%, 75%, 95% and 100% (each gradient for 2 h), and then soaked in xylene overnight. Next, samples were immersed in an LR White resin solution with a solubility gradient, soaked for 32 h, and finally immersed in 100% LR White resin solution, and cured for 36 h in an oven at 60°C.
The resin-embedded brain specimens were imaged with the fMOST system (BioMapping5000, Wuhan OE-Bio Co., Ltd, Wuhan, China) to achieve the raw dataset, as previously described. 28 While working, the mouse brain was installed in the MOST data acquisition automatic system, using lasers (Cobolt, 473 nm and 561 nm) as excitation light source, adopting 40× (0.8 NA, LUMPLFLN, Olympus) water immersion eyepiece and TDI-CCD for signal detection. The brain was sequentially cut into coronal ultra-thin slices (2 μm) and simultaneously imaged on the slices from front to back. Data acquisition was continuous and lasted for more than 4-5 d for one sample. A total of about 6300 coronal sections constituted the whole brain data set.
The original fragment images (resolution: 0.35 μm ×0.35 μm × 2 μm) were collected by fMOST, based on the special preprocessing software of the equipment. After strip splicing and brightness homogenization, the dual-channel coronal images were processed for 2D and then 3D reconstruction using Amira (2020.1) and imaris (9.8) software. The images taken on consecutive 50 slices were reconstructed into one image showing a virtual thick slice (total thickness of 100 μm). A total of 125 reconstructed 2D whole-slice images were obtained from the olfactory bulb to the cerebellum. The virtual 3D representation of the glymphatic system on the brain surface, in the whole mouse brain, and along the branches of the middle cerebral artery, were reconstructed.
Ex vivo fluorescence imaging
After tracer infusion, mice were transcardially perfused with PBS and 4% PFA. After post-fixation in 4% PFA overnight at 4°C, brains were harvested and cut into 100 μm coronal sections on a vibratome. Dex-3 distribution within the slices was imaged under a fluorescence microscope (Eclipse TE 2000-U, Nikon, Japan). The exposure level was determined and maintained constant throughout the study, and whole-slice montages were integrated using the Virtual Slice module of Kolor Autopano Giga (V4.4). Dex-3 distribution was quantified as previously described. 26 Under a × 5 objective (0.12NA, ZEISS), the region of interest (ROI) was chosen and the mean fluorescence intensity was quantified using ImageJ software (NIH).
Immunohistochemistry
After fixation, brains were cut into 100 μm slices using a vibrating microtome. Free-floating slices were permeabilized and blocked in PBS consisting of 0.5% Triton X-100 and 5% normal donkey serum for 2 h. Slices were then incubated with rabbit anti-α-smooth muscle actin (19245, Cell Signaling Technology, USA, 1:500, α-SMA) overnight at 4°C. After 3 × 10 min washes in PBS, slices were incubated with Alexa Fluor 647-conjugated Affinipure Donkey Anti-Rabbit lgG (H + L) (711-605-152, Jackson Immunoresearch, USA, 1:500) for 2 h at room temperature. Slices were then washed in PBS with DNaseI, and 4′, 6-diamidine-2′-phenylindole dihydrochloride (Sigma-Aldrich, USA, 1:2000, DAPI) for 20 min, washed again and mounted. Immunofluorescence was imaged using a confocal laser scanning microscopy (FV1200, Olympus, Japan).
Tracer assays
Anesthetized mice were decapitated at 30 and 120 min after CM infusion. As previously described, 26 the olfactory bulb, dorsal cortex, lateral cortex, ventral cortex, hippocampus, striatum, subcortical region, substantia nigra, cerebellum and brainstem were carefully dissected. Brain tissues were immersed in the RIPA lysis (P0013C, Beyotime, China, 100 mg/mL), homogenized on ice, and then centrifuged at 12,000 rpm at 4°C for 20 min. The supernatants were shifted in the enzyme label plate, and the intensity of Dex-3 was spectrophotofluorometrically assessed using the microplate reader (Synergy NEO, BioTek, USA). The content of fluorescent tracer in the defined brain region was quantified from the standard curves and was expressed as ng/mg tissue.
Statistical analysis
All statistical analyses were performed with GraphPad Prism (La Jolla, CA, USA). Data were tested for normality using Shapiro-Wilk test. Analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for multiple comparisons. All statistical data were presented as the mean ± SD. P < 0.05 was considered to be statistically significant.
Results
Identification of the preferential brain regions for the glymphatic flow
At 30 and 120 min after CM infusion with Dex-3, mice were transcardially perfused with 4% PFA following the tail vein injection with lectin. After tissue fixing, processing and embedding, the brain specimens were imaged with the fMOST system. Through the automated sectioning and imaging, a mesoscopic 2D and then 3D imaging on Dex-3 distribution was constructed at the submicron level (experimental procedures are shown in Supplementary Figure 1).
The 3D visualizations of Dex-3 distribution on the entire brain surface 30 and 120 min after CM infusion are provided in Supplementary video 1. The glymphatic flow considerably differed among cerebral regions, and these regions containing large-caliber penetrating arteries exhibited strong Dex-3 staining (green). Screenshots displayed that at 30 min, the tracer signal was extremely strong at the base of the brain including the medulla, pons, hypothalamus, medial olfactory bulb, and ventral cortex. These regions included a basilar artery (Bas), olfactory artery (Olfa), anterior cerebral artery (Acer), middle cerebral artery (Mcer) and posterior cerebral artery (Pcer), most of them connecting the Circle of Willis (CW) (Figure 1(a)). The tracer signal was also strong in the lateral cortex surrounding the Mcer, superior colliculus, and cerebellum besides cerebellar fissures. In contrast, the tracer signal was minimal in the dorsal brain (Figure 1(b) and (c)).
Figure 1.
A 3D visualization of CSF tracer distribution on the whole-brain surface. Representative images of whole-brain ventral view (a and d), dorsal view (b and e), and lateral view (c and f) 3D reconstruction of CSF tracer (Dex-3, green) in mice brain. Dex-3 distribution 30 min (a–c) and 120 min (d–f) after CM infusion. The brain regions and blood vessels with strong tracer labeling are marked in each panel.
Olfa: olfactory artery; Acer: anterior cerebral artery; Mcer: middle cerebral artery; Ictd: internal carotid artery; Pcer: posterior cerebral artery; Scba: superior cerebellar artery; Bas: basilar artery; Aica: anterior inferior cerebellar artery; Sos: superior olfactory sinus; Rcs: rostral confluence of sinuses; Sss: Superior sagittal sinus; Ts: transverse sinus; Ss: sigmoid sinus; Rrhv: rostral rhinal vein; Crhv: caudal rhinal vein; Mcerv: middle cerebral vein; HYP: hypothalamus; PGN: pontine gray nuclei; MED: medulla; SC: superior colliculus.
At 120 min, the tracer signal in the whole brain strikingly declined from values at 30 min, considering that a large amount of tracer was removed from the brain (Figure 1(d) to (f)). Notably, Dex-3 was not totally cleared but still persisted at the base of the brain, medial olfactory bulb, superior colliculus and cerebellum (Figure 1(d) to (f)). We also found that the cerebral regions with strong Dex-3 staining at 120 min were the same regions with robust tracer penetration at 30 min, indicating that the glymphatic flow is brain-region dependent instead of homogenous throughout the brain. These cerebral regions with great CSF tracer dispersion represent the preferential areas for the glymphatic flow.
In addition to arteries, Dex-3 spreading was also evident along large-caliber veins located on the brain surface. This included the rostral rhinal vein (Rrhv), middle cerebral vein (Mcerv), a rostral confluence of sinuses (Rcs), superior olfactory sinus (Sos), superior sagittal sinus (Sss), caudal rhinal vein (Crhv), transverse sinus (Ts) and sigmoid sinus (Ss) at both time-points (Figure 1). These venous PVSs are believed to play an important role in the fluid efflux from the brain parenchyma.
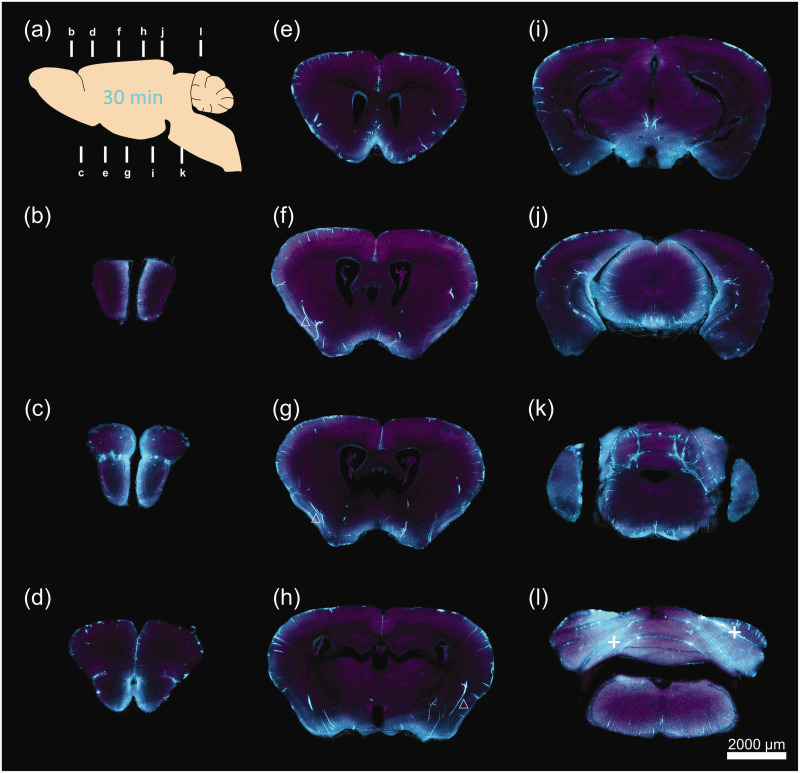
Next, we further determined the regional heterogeneity of the glymphatic flow on the 100 μm sections obtained from fMOST and vibratome slicing. Representative images of coronal sections were obtained from fMOST (Figures 2 and 3), and the mean fluorescence intensity of Dex-3 in the defined regions was quantified on the vibratome sections (Figure 3(m) and (n); Representative images are shown in Supplementary Figure 2). Representative images of the coronal sections showed that at 30 min post-infusion, Dex-3 (blue) was mainly located in the superficial tissues such as the pial surface (71.50 ± 5.131), cerebral fissures (43.74 ± 11.37), and ventral cortex (17.21 ± 1.411), which were connected with or close to the SAS. The strong fluorescence of the tracer was also observed along the penetrating blood vessels (30.64 ± 3.040) throughout the brain. In contrast, tracer signal was minimal in the deep tissues such as cortical white matter (5.863 ± 0.6070) and striatum (6.974 ± 0.8292) (Figure 2). Tracer signal was also present in the choroid plexus surrounding the ventricles, mostly due to a little reflux of tracers from the CM (Figure 2(e) to (g)). Moreover, the dispersion of tracer in the ventral brain, such as the hypothalamus (25.14 ± 3.580), substantia nigra (16.61 ± 2.195), and medial olfactory bulb (21.52 ± 4.316) was significantly greater than that in the dorsal cortex (7.388 ± 0.6724), suggesting that CSF influx primarily occurs in the ventral brain (Figure 2(d) to (i)). Additionally, the tracer signal in the cerebellum (19.25 ± 4.689) and brainstem (19.39 ± 2.593) was stronger than in some other regions, considering that these regions were close to the injection site (Figure 2(l)). At 120 min post-infusion, Dex-3 (blue) signal in the whole brain was considerably reduced compared to 30 min values, even though residual tracers persisted in the ventral brain like the hypothalamus (15.60 ± 4.133) and medial olfactory bulb (16.26 ± 3.714), and cerebral regions close to the injection site such as the cerebellum (13.79 ± 4.752) and brainstem (16.87 ± 4.402) (Figure 3).
Figure 2.
A 2D demonstration of CSF tracer penetration into the mouse brain 30 min after CM infusion. (a) Schematic diagram of sagittal plane showing the locations of slicing for coronal sections. (b-l) Representative images of reconstructed coronal brain slices demonstrating the penetration of Dex-3 (blue) into the brain at 11 different locations. The inner wall of blood vessels was stained by lectin (purple). (b) +4.28, (c) +3.08, (d) +2.68, (e) +1.10, (f) −0.10, (g) −0.22, (h) −1.58, (i) −2.80, (j) −4.04, (k) −4.72 and (l) −5.68 mm from bregma. △ tracers along arteries. + tracers along cerebellar fissures.
Figure 3.
A 2 D demonstration of tracer distribution in the mouse brain 120 min and quantification analysis of tracer intensity 30 and 120 min after CM infusion. (a) Schematic diagram of sagittal plane exhibiting the locations of slicing for coronal sections. (b-l) Representative images of reconstructed coronal brain slices demonstrating the residual Dex-3 (blue) in the brain at 11 different locations. (b) +4.28, (c) +3.08, (d) +2.68, (e) +1.10, (f) −0.10, (g) −0.22, (h) −1.58, (i) −2.30, (j) −3.28, (k) −4.72, (l) −5.68 mm from bregma. (m–n) Quantification of tracer fluorescence within defined brain regions on the slices obtained from conventional vibratome slicing 30 min (m) and 120 min (n) after CM infusion (representative images and ROI see Supplementary Figure 2). N = 5 for all groups, *P < 0.05, **P < 0.01, ***P < 0.001 vs DC. ▲ tracers along veins, + tracers along cerebellar fissures.
DC: dorsal cortex; LC: lateral cortex; VC: ventral cortex; WM: white matter; STR: striatum; HIP: hippocampus; TH: thalamus; HTH: hypothalamus; MID: midbrain; SN: substantia nigra; OB(m): olfactory bulb (medial); OB(l): olfactory bulb (lateral); CERE: cerebellum; BS: brainstem.
Slices appeared in a sequential way and showed 100 μm reconstructions on coronal, sagittal and horizontal plane in turn for Dex-3 (blue) distribution in the whole brain (Supplementary video 2). Representative images of the sagittal and horizontal plane showed the same pattern of Dex-3 (blue) distribution as the coronal plane images (Figure 4(a) to (d)). Furthermore, the distribution of Dex-3 in the defined brain regions was quantitatively determined via the microplate assay. Data demonstrated that the tracer content in the whole brain at 120 min considerably decreased compared to 30 min values, and tracer distribution in these cerebral regions (ng/mg tissue) exhibited the same pattern at the two time-points. These regions close to the injection site such as the brainstem (30 min, 21.51 ± 1.579; 120 min, 11.83 ± 1.299) and cerebellum (30 min,15.17 ± 1.207; 120 min, 11.00 ± 0.6512) showed the great tracer uptake, and regions located in the ventral brain also showed great tracer uptake such as ventral cortex (30 min, 12.24 ± 1.143; 120 min, 9.343 ± 1.007), substantia nigra (30 min, 11.65 ± 2.553; 120 min, 6.149 ± 1.178) and olfactory bulb (30 min, 14.47 ± 1.298; 120 min, 11.99 ± 0.7108). In contrast, the regions distant from the base of the brain had minimal uptake of tracer like the dorsal cortex (30 min, 1.239 ± 0.4675; 120 min, 1.351 ± 0.2952), and the deep brain regions also had a low content of tracer such as the striatum (30 min, 2.315 ± 0.4400; 120 min, 2.338 ± 0.8345) (Figure 4(e) and (f)). Quantification data were consistent with the spatial profile obtained on 2D and 3D visualization, confirming that the glymphatic flow was brain-region dependent.
Figure 4.
Regional preference of the glymphatic flow. (a-b) Representative images of reconstructed sagittal brain slices (lateral 0.60 mm) showing the distribution of Dex-3 (blue) in the brain 30 min (a) and 120 min (b) after CM infusion. (c–d) Representative images of reconstructed horizontal brain slices exhibiting the distribution of Dex-3 (blue) in the brain 30 min (interaural 4.56, 3.44, 0.44 mm from left to right) (c) and 120 min (interaural 4.40, 3.44, 0.44 mm from left to right) (d) after CM infusion. (e–f) The content of Dex-3 in 10 defined brain regions assessed using the microplate assay 30 min (e) and 120 min (f) after CM infusion. N = 5 for all regions, **P < 0.01, ***P < 0.001 vs DC. DC, dorsal cortex.
LC: lateral cortex; VC: ventral cortex; OB: olfactory bulb; STR: striatum; SN: substantia nigra; SCR: subcortical region; HIP: hippocampus; CERE: cerebellum; BS: brainstem.
Visualization of the major glymphatic vessels throughout the brain
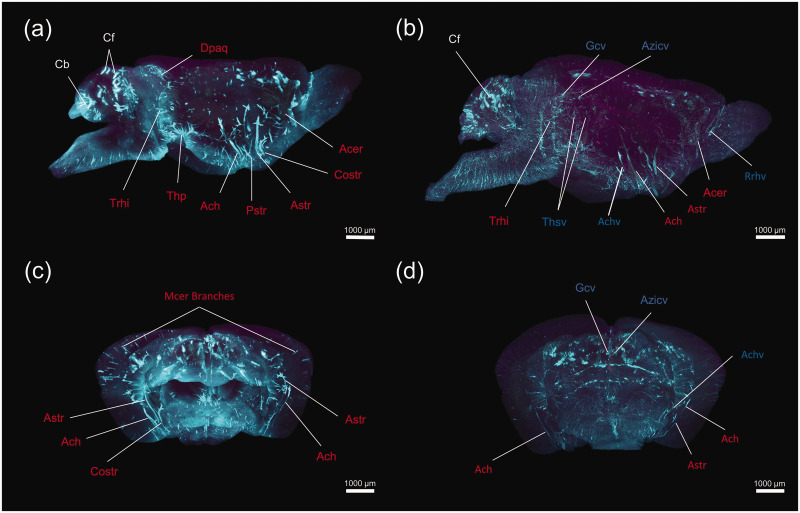
The PVS (or glymphatic vessel) functions as a conduit allowing the glymphatic transport. In order to observe the glymphatic vessels more clearly, we performed a 3D reconstruction of PVSs in the entire brain (Supplementary videos 3 and 4). Screenshots taken from Supplementary video 3 displayed the distribution of Dex-3 (blue) in the PVSs throughout the brain 30 min after CM infusion. In the ventral brain, intense Dex-3 labeling was observed along large-caliber arteries originating from the CW, such as Acer, corticostriate artery (Costr), anterior striate artery (Astr), posterior striate artery (Pstr), anterior choroidal artery (Ach), and thalamo-perforating arteries (Thp). Along these large arteries and their branches, Dex-3 extended into deep brain tissue like the striatum, hippocampus, and ventricles. In the dorsal cortex, the penetrating arteries originating from Mcer were smaller and shorter than those originating from the ventral brain surface. Along these small cortical arterioles, the tracer only reached the superficial cortex (Figure 5(a) and (c)). Our findings indicate that PVSs surrounding these large-caliber arteries constitute the major glymphatic influx vessels for CSF circulation.
Figure 5.
A 3D visualization of the glymphatic vessels in the whole mouse brain. Representative images of lateral view (a, b) and coronal view (c, d) demonstrating the brain-wide 3D reconstruction of Dex-3 (blue) distribution in the PVSs 30 min (a, c) and 120 min (b, d) after CM infusion. The major arteries for the glymphatic influx and veins for the glymphatic efflux are marked in each panel.
Dpaq: dorsal periaqueductal arteries; Trhi: transverse hippocampal arteries; Thp: thalamo-perforating arteries; Acer: anterior cerebral artery; Costr: corticostriate artery; Astr: anterior striate artery; Pstr: posterior striate artery; Ach: anterior choroidal artery; Gcv: great cerebral vein of Galen; Azicv: azygos internal cerebral vein; Achv: anterior choroidal vein; Thsv: thalamostriate veins; Cb: cerebellar lobule; Cf: cerebellar fissures.
Screenshots taken from Supplementary video 4 display- the distribution of Dex-3 (blue) along the blood vessels 120 min after CM infusion. Fluorescence labeling along large arteries like Astr and Ach markedly declined compared to 30 min values. In contrast, the tracer still persisted along large veins such as the great cerebral vein of Galen (Gcv), azygos internal cerebral vein (Azicv), thalamostriate vein (Thsv), anterior choroidal vein (Achv), and Rrhv (Figure 5(b) and (d)). The 3D analysis revealed that ISF could move out along these venous PVSs (namely glymphatic efflux vessels) from the brain parenchyma.
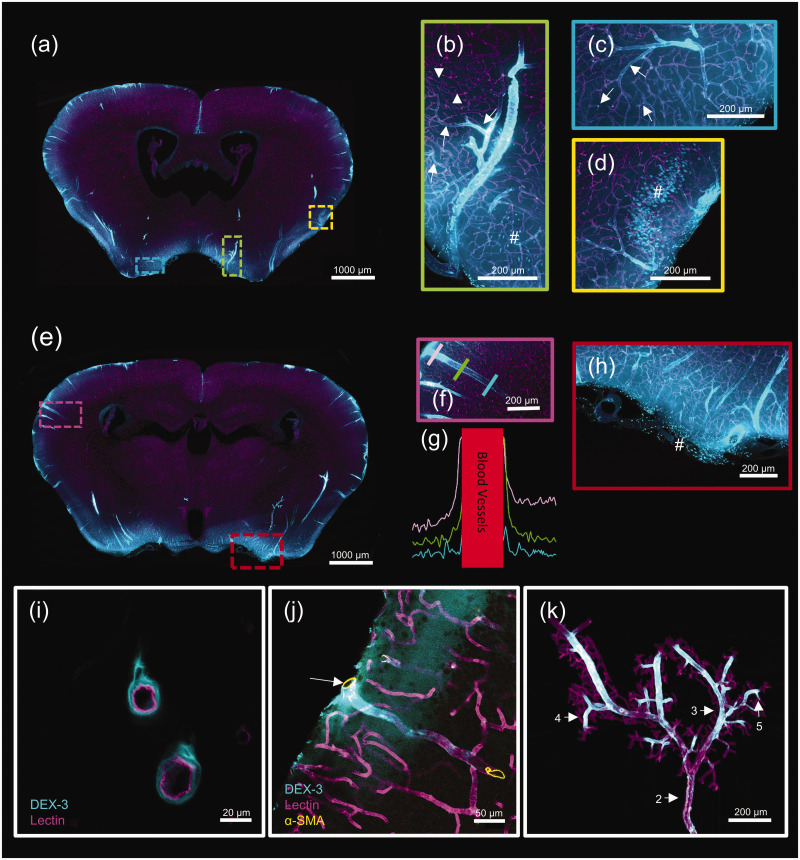
Next, we observed the detailed distribution pattern of CSF tracer along the blood vessels. Representative images of coronal sections showed that at 30 min post-infusion, Dex-3 (blue) was accumulated along the large-caliber penetrating arteries, their main branches, capillaries (the inner wall stained with lectin, purple) and cell bodies located in the superficial tissue (Figure 6(a) to (h)). On the cross-section of blood vessels, Dex-3 was accumulated in the PVSs outside the blood vessel (Figure 6(i)). Immunostaining results further showed that the penetrating blood vessel accumulated with Dex-3 was the artery, which was positive for SMA staining (brown) (Figure 6(j)). More interestingly, the tracer signal along the PVSs gradually reduced as blood vessel branched off, and no tracer was present around the arterioles and capillaries located in the deep tissue (Figure 6(b) to (d)). Meanwhile, tracer fluorescence was evaluated in linear ROS extending outward from the penetrating blood vessels. The curves showed that the tracer signal in the PVS gradually reduced along the penetrating artery from the pial surface to the deep tissue (Figure 6(f) and (g)). Furthermore, a 3D visualization of the tracer distribution along the Mcer branches was constructed (Supplementary video 5). Screenshots showed that Dex-3 (blue) was visible around the multi-level branches of Mcer (from level 2 to level 5). However, no tracer was present in tiny arterioles or capillaries (Figure 6(k)). It indicates that there is a gradient reduction of CSF flow along the arterial PVSs, mostly because CSF continually leaves PVS as it dives into the deep brain. The gradient reduction of CSF influx may account for the fact that the deep brain tissues receive less CSF than the superficial tissue.
Figure 6.
Detailed demonstration of the glymphatic influx vessels. Representative images of coronal sections demonstrating Dex-3 (blue) distribution on the PVSs (the inner wall of blood vessels stained by lectin, purple). (a) The coronal section at bregma −0.22 mm. The three regions inside the rectangles are the positions of (b), (c), and (d). (e) The coronal section at bregma −1.58 mm. The two regions inside the rectangles are the positions of (f) and (h). (g) Evaluation of the fluorescence intensity in linear ROS extending outward from penetrating blood vessels showed in panel (f). Lines of different colors representing different depths. (i) Representative images of a cross-section of blood vessels demonstrating Dex-3 surrounding the outside of blood vessels. (j) Immunostaining on vibratome sections confirming Dex-3 accumulating around the arteries (positive for α-SMA staining, brown) and (k) A 3 D reconstruction of Mcer branches, in which the branch levels are marked. ↑ Blood vessels surrounded by tracers, ▲ Blood vessels without tracer signal, # Cellular uptake of tracers.
According to the prior study, CSF tracers were not observed around parenchymal veins at early time-points, but visible at longer time-points (>1 h). 2 Thus, the time-point of 120 min following the CM infusion was chosen to observe the glymphatic efflux pathway in the current study. Representative images of coronal sections showed that at this time-point, Dex-3 (blue) was accumulated along some large veins including Rrhv located in the rostral forebrain, Achv located in the ventral brain and Crhv located in the caudal brain (Figure 7(a) to (f)). Furthermore, the immunostaining experiment confirmed that the blood vessels accumulated with Dex-3 (blue) were veins, which were negative for SMA staining (brown) (Figure 7(j)). Interestingly, along the blood vessels and pial surface, there were many cells containing Dex-3, which possibly were macrophages or microglia (Figure 7(c), (f) and (i)). Moreover, many cells contained Dex-3 in the brain parenchyma, which were possibly neurons (Figure 7(h)). Notably, cellular uptake of CSF tracers was observed both at 30 and 120 min after CM infusion, suggesting that CSF-derived solutes can also be degraded by cells besides elimination via CSF-ISF exchange.
Figure 7.
Detailed demonstration of the glymphatic efflux vessels. Representative images demonstrating Dex-3 (blue) distribution on the PVSs (the inner wall of blood vessels stained by lectin, purple). (a) The coronal section at bregma 2.68 mm. The two regions inside the rectangles are the positions of (b) and (c). (d) The coronal section at bregma −0.82 mm. The two regions inside the rectangles are the positions of (e) and (f). (g) The coronal section at bregma −4.47 mm. The two regions inside the rectangles are the positions of (h) and (i). (j) Immunostaining on vibratome sections exhibiting Dex-3 accumulation around veins (negative for α-SMA staining, brown).
Rrhv: rostral rhinal vein; Crhv: caudal rhinal vein; Achv: anterior choroidal vein. ↑ Blood vessels surrounded by tracers: # Cellular uptake of tracers.
Discussion
Studies on the glymphatic system are commonly based on in vivo two-photon and ex vivo fluorescence imaging on brain slices.2,14,22,38,39 Analysis of the local brain suggested that the glymphatic pathway represents a brain-wide anatomical system. However, traditional fluorescence-based imaging cannot map the brain-wide CSF flow in a 3D manner. For 3D visualization, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is an ideal method, which has been widely used in both basic and clinical studies of the glymphatic system.5,6,10,12,40,41 Being the least invasive approach, the MRI scan is suitable for 3D imaging of CSF flow in the entire brain. However, due to the low spatial resolution (cm level), MRI fails to acquire the 3D visualization of the glymphatic pathway at the microscopic level. Besides MRI, some ex vivo imaging techniques such as automated imaging cryomicrotome and optical clearing followed by light-sheet fluorescence microscopy have also been used to 3D demonstrate the glymphatic pathway in rodent brain.42,43 However, due to the severe invasiveness, these methods are not applicable in clinical work. Moreover, only one time-point (i.e. 30 min after CM infusion) is explored in these studies, which is mainly used to characterize the glymphatic influx pathway.
The primary goal of this study was to obtain 3D atlases of the glymphatic pathway for the whole mouse brain at high spatial resolution. High resolution and large size of sample are mutually exclusive in conventional imaging approaches, given that the detailed imaging restricts the detection range. Fortunately, the innovative technique fMOST enables whole-brain imaging at the submicron level, and has been successfully used to map the entire vasculature and neuronal projection in rodent’s brain.27–30 Yet, whether fMOST is suitable to depict the CSF circulation pathway has not been reported. In this study, we used fMOST to perform visual reconstruction of the whole-mouse-brain glymphatic pathway at a high resolution. Two-time-points, including 30 and 120 min following intracisternal infusion, were used to characterize the dynamic CSF flow. According to the previous studies, the distribution pattern of Dex-3 at 30 min majorly represents the stage of robust CSF influx into the brain, while the distribution at 120 min represents the elimination of ISF tracer from the brain.2,26 Using fMOST, we obtained a 3D whole-brain dataset of the glymphatic flow and achieved full morphological reconstructions of the brain-wide glymphatic vessels. Compared with DCE-MRI, the obvious advantage of fMOST is the high spatial resolution owing to the whole imaging on thin slices (2 μm) under high objective power (40×). Thus, the fine structures such as small blood vessels and even cell bodies were evident in the reconstructed slices using fMOST.
Using an automated imaging cryomicrotome after CM infusion with Dex-500, Beatrice et al. obtained a combined 3D representation of the vasculature and CSF compartments of the whole rat’s brain. They found a strong co-localization of tracer with large arteries such as the CW, dorsocerebellar arteries, and Ach, as well as the large veins anatomically embedded in the SAS. They also found that the tracer signal is strong at the base of the brain around the CW and in several large cisterns. 42 Using optical clearing followed by light-sheet fluorescence microscopy, Bechet et al. provided 3D images of the glymphatic system in the whole mouse brain. They found that CSF tracer influx primarily occurs in the ventral brain, cerebellum and hindbrain. 43 These findings are consistent with our observations. However, Beatrice et al. suggested that tracers are only located around the arteries but not the veins in the brain parenchyma, 42 which is inconsistent with our data. In our experiments, we observed that the tracer signal was apparent around the parenchymal veins at both 30 and 120 min after CM infusion. The discrepancy was probably caused by the different tracer used. The tracer used by Beatrice et al. was Dex-500 (500 kDa), which was much larger than Dex-3 (3 kDa) used in our study. According to previous studies, large tracers like Dex-500 remain confined to the arterial PVSs and fail to expand into the ISF and then into the venous PVS, while small tracers like Dex-3 exchange broadly with the brain ISF and accumulate in the PVSs around both arteries and veins in the brain parenchyma.2,44
According to the glymphatic concept, the PVSs constitute a network equivalent to the lymphatics in the brain. 2 The PVSs are fluid-filled tunnels, restricted by a vessel wall at the inner boundary and by astrocytic endfeet on the exterior.45,46 The structural disorder of PVS is related to some neurological diseases. For instance, PVSs enlargement occurring in cerebral amyloid angiopathy and leukoencephalopathy could influence PVS bulk flow.47,48 Interestingly, PVSs closure caused by cortical spreading depression also reduces the glymphatic flow. 21 The present study yielded the high-resolution 3D atlas of the PVSs (glymphatic vessels) in the whole mouse brain. In the ventral brain, tracers extended into deep brain tissue along the large-caliber arteries, while in the dorsal cortex, tracers only reached superficial tissue along the small penetrating arteries. Mounting evidence supports that the paravascular pumping is a major driver of paravascular CSF influx and subsequent CSF–ISF exchange.38,39 Furthermore, large-caliber arteries may offer greater driving force than small penetrating arteries due to more vascular smooth muscles inside the wall. Consequently, the PVSs along the ventral large-caliber arteries constituted the main glymphatic influx vessels, and correspondingly the ventral brain was the preferential irrigated region for the glymphatic flow. Moreover, we observed a gradient reduction of CSF influx along the arterial PVSs, which could explain why the deep tissues obtain less CSF irrigation than the superficial tissues. It remains unclear about the role of the heterogeneous glymphatic flow in normal brain function and neurological diseases. Theoretically, a low level of the regional glymphatic flow may lead to inefficient clearance for toxic proteins such as Aβ and tau, and predispose some brain regions to proteins accumulation and neuronal vulnerability. For instance, Harrison et al. found that the greater tau pathology occurs in rostral compared to caudal cortex during early tau deposition in rTg4510 mice. Consistently, they found that the glymphatic activity is lower in the rostral compared to the caudal cortex of wide-type mice. It suggests that the heterogeneous nature of glymphatic flow may play a critical role in regional difference of tau deposition. 49 However, the implication of the regional heterogeneity of glymphatic flow is mostly speculative and awaits testing in future studies.
Several studies discovered that body posture could affect the transport of CSF substances into the brain.50,51 For instance, Ooboshi et al. observed that the gene expression after intracisternal injection with viral vectors could be targeted by altering the head position of rats. When a viral suspension is injected while rats are in a prone position, the reporter gene is expressed mainly in the ventral brain. On the other hand, the inferior or lateral brain is stained primarily when rats are in a supine or lateral position. 51 However, it is worth noting that in their study, the viral vector was suspended in PBS with 20% sucrose. The high specific gravity of the sucrose suspension coupled with slow CSF flow could account for the selective delivery of the virus to the most dependent part of the SAS, which is mostly responsible for the gene expression targeted by altering head position.51,52 However, virus vectors and sucrose suspension are not suitable for mapping the glymphatic flow because CSF is mostly composed of water and small solutes. In contrast, small molecular tracers like Dex-3 (3 kDa), regularly dissolved in artificial CSF, are more suitable for tracing the CSF flow. Owing to high suspension stability, small tracers are not easy to settle to the dependent part of the SAS. Thus, it is unlikely that gravity has a significant effect on the delivery of small molecular tracers. The following study supports this speculation. Lee et al. showed that the glymphatic transport in the mouse brain is most efficient in the lateral position compared with the supine or prone positions. 50 However, they did not find the preferential delivery of CSF tracers (dextran, 3 kD; dextran, 2000 kD) to the most dependent part of the SAS when mice were in different body postures. More importantly, representative images of coronal sections showed that the ventral brain and the superficial tissue consistently had robust CSF tracer penetration when mice were placed in any of the three body postures (Figure 6(b)), 50 which is consistent with our observation.
Although the glymphatic influx pathway has been widely established,38,40 the evidence on the glymphatic efflux pathway is limited. In the initial study, Illif et al. found that fluorescent tracers are observed around the parenchymal veins at 1-3 h after intracisternal or intraparenchymal infusion. 2 In support of Illif’s observation, Thrane et al. discovered that both lipophilic and hydrophilic tracers are present in the venous PVSs 60–90 min after CM infusion. 17 However, other groups observed that tracers are cleared from the brain along the basement membranes of capillaries and arteriolar smooth muscle cells, without the involvement of the venous PVSs.53,54 Thus, the role of the venous PVSs in ISF drainage remains controversial. In this study, the PVSs around some large veins such as Rrhv, Achv and Crhv were observed to be involved in the clearance of parenchymal tracers, supporting the role of the venous PVSs playing in the ISF drainage.
Limitations
This study has a few limitations. Firstly, the imaging approach used here belongs to ex vivo fluorescence microscopy, which is not applicable to clinical work. Moreover, ex vivo imaging on specimens does not always reflect their real situation in the living body due to the potential effects of fixation and dehydration on tissues. For instance, Mestre et al. showed that transcardial perfusion with 4% PFA causes obvious PVSs collapse. The cross-sectional area of the PVS is about 1.4 times that of the adjacent artery, whereas fixation reduces this ratio to 0.14. As the PVSs shrink, CSF tracers move into the vessel wall including the smooth muscle layer and the basal lamina. 39 Thus, caution needs to be taken when translating the ex vivo observation. The second experimental limitation is the injection site, as the CM was namely used in the present study. Due to the adjacency to the injection site, a strong signal of CSF tracer was observed in the cerebellum and brainstem, which could be an artifact caused by the experimental procedure. Additionally, due to little CSF reflux into the ventricles during CM injection, the role of ventricles playing in CSF circulation was not analyzed. In recent work, Kulam et al. infused Gd-albumin into the lateral ventricle followed by ex vivo MRI. They obtained a unique PVSs network in the whole rat brain, suggesting that ventricles serve an important role in PVSs mediated solute clearance. 55 To explore CSF circulation, intracerebroventricular (ICV) injection of CSF tracer might be more suitable than CM injection, given that the choroid plexus physiologically produces CSF. To comprehensively understand the glymphatic pathway, ICV injection should be considered in future studies. The third limitation is that we failed to directly distinguish arteries from veins in the 3D demonstration. To precisely depict the role of PVSs along arterioles and venules during CSF circulation, transgenic reporter mice like Tie2-GFP: NG2-DsRed mice could be considered for further study.2,14 The final experimental limitation is the small sample size for fMOST. Due to the costly testing fee (approximately $4,650 per mouse brain), only one mouse per time-point was included in this study. As a complement, other methods including ex vivo imaging on the vibratome sections and the microplate assay were used to quantitatively determine the distribution of tracer in mice brain.
Conclusion
Herein, we have obtained the first high-resolution 3D atlas of the glymphatic pathway in the entire mouse brain using fMOST (N = 1, per time-point). Together with other methods, we discovered that the glymphatic flow exhibits a regional preference. These cerebral regions with large penetrating arteries and/or adjacent to the SAS display robust CSF flow, while those brain regions with small penetrating arteries and/or located in the deep brain exhibit little CSF flow. Moreover, based on the 3D visualization of the glymphatic architecture in the entire brain, we found that PVSs along large arteries mostly connecting the CW constitute the preferential glymphatic influx vessels, and PVSs around large-caliber veins constitute the main glymphatic efflux vessels. Our observation can be used as a guide for the normal anatomy of the glympathic system and for identifying deviation from normal patterns caused by neurological disorders.
Supplemental Material
Supplemental material, sj-jpg-1-jcb-10.1177_0271678X221109997 for High-resolution 3D demonstration of regional heterogeneity in the glymphatic system by Xu-Zhong He, Xin Li, Zhen-Hua Li, Jing-Cai Meng, Rui-Ting Mao, Xue-Ke Zhang, Rong-Ting Zhang, Huai-Liang Huang, Qian Gui, Guang-Yin Xu and Lin-Hui Wang in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-jpg-2-jcb-10.1177_0271678X221109997 for High-resolution 3D demonstration of regional heterogeneity in the glymphatic system by Xu-Zhong He, Xin Li, Zhen-Hua Li, Jing-Cai Meng, Rui-Ting Mao, Xue-Ke Zhang, Rong-Ting Zhang, Huai-Liang Huang, Qian Gui, Guang-Yin Xu and Lin-Hui Wang in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-3-jcb-10.1177_0271678X221109997 for High-resolution 3D demonstration of regional heterogeneity in the glymphatic system by Xu-Zhong He, Xin Li, Zhen-Hua Li, Jing-Cai Meng, Rui-Ting Mao, Xue-Ke Zhang, Rong-Ting Zhang, Huai-Liang Huang, Qian Gui, Guang-Yin Xu and Lin-Hui Wang in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We would like to acknowledge the efforts of Wuhan OE-Bio Co.,Ltd in preparing and imaging the specimens.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (31871167, 81920108016 and 31730040), Shanghai Key Laboratory of Psychotic Disorders Open Grant (13dz2260500), Suzhou Science and Technology Research Project (SKJY2021121), China Postdoctoral Science Foundation (2016M601882), Postdoctoral Science Foundation of Jiangsu Province, China (1601083 C), and Priority Academic Program Development of Jiangsu Higher Education Institutions.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: LHW and GYX designed the experiments, XZH, ZHL, JCM, RTM, XKZ, RTZ, HLH and QG performed the experiments, XZH and XL analyzed the data and made figures, LHW wrote the manuscript.
Supplemental material: Supplemental material for this article is available online.
ORCID iD
Lin-Hui Wang https://orcid.org/0000-0001-5295-324X
References
- 1.Jessen NA, Munk ASF, Lundgaard I, et al. The glymphatic system: a beginner's guide. Neurochem Res 2015; 40: 2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iliff JJ, Wang MH, Liao YH, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4: 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 2018; 17: 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mestre H, Mori Y, Nedergaard M. The brain's glymphatic system: current controversies. Trends Neurosci 2020; 43: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013; 123: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mestre H, Hablitz LM, Xavier ALR, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 2018; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie LL, Kang HY, Xu QW, et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munk AS, Wang W, Bechet NB, et al. PDGF-B is required for development of the glymphatic system. Cell Rep 2019; 26: 2955–2969.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bechet NB, Shanbhag NC, Lundgaard I. Glymphatic pathways in the gyrencephalic brain. J Cereb Blood Flow Metab 2021; 41: 2264–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017; 140: 2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Leon MJ, Li Y, Okamura N, et al. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J Nucl Med 2017; 58: 1471–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringstad G, Valnes LM, Dale AM, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018; 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edeklev CS, Halvorsen M, Lovland G, et al. Intrathecal use of gadobutrol for glymphatic MR imaging: prospective safety study of 100 patients. AJNR Am J Neuroradiol 2019; 40: 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 2014; 34: 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundgaard I, Lu ML, Yang E, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 2017; 37: 2112–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achariyar TM, Li BM, Peng WG, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener 2016; 11: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thrane VR, Thrane AS, Plog BA, et al. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep 2013; 3: 2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin BJ, Smith AJ, Verkman AS. Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 2016; 148: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AJ, Yao XM, Dix JA, et al. Test of the ‘glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 2017; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holter KE, Kehlet B, Devor A, et al. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A 2017; 114: 9894–9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schain A, Melo-Carrillo A, Strassman A, et al. Cortical spreading depression closes the paravascular space and impairs glymphatic flow: implications for migraine headache and treatment. Cephalalgia 2017; 37: 301–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kress BT, Iliff JJ, Xia MS, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014; 76: 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei F, Zhang C, Xue R, et al. The pathway of subarachnoid CSF moving into the spinal parenchyma and the role of astrocytic aquaporin-4 in this process. Life Sci 2017; 182: 29–40. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Lin J, Wei F, et al. Characterizing the glymphatic influx by utilizing intracisternal infusion of fluorescently conjugated cadaverine. Life Sci 2018; 201: 150–160. [DOI] [PubMed] [Google Scholar]
- 25.Wei F, Song J, Zhang C, et al. Chronic stress impairs the aquaporin-4-mediated glymphatic transport through glucocorticoid signaling. Psychopharmacology (Berl) 2019; 236: 1367–1384. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Song J, He XZ, Xiong J, et al. Quantitative determination of glymphatic flow using spectrophotofluorometry. Neurosci Bull 2020; 36: 1524–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng T, Feng Z, Wang XJ, et al. Review of micro-optical sectioning tomography (MOST): technology and applications for whole-brain optical imaging [invited]. Biomed Opt Express 2019; 10: 4075–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li AA, Gong H, Zhang B, et al. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain. Science 2010; 330: 1404–1408. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XC, Yin XZ, Zhang JJ, et al. High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer's disease mice. Natl Sci Rev 2019; 6: 1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JJ, Sun P, Lv XH, et al. Divergent projection patterns revealed by reconstruction of individual neurons in orbitofrontal cortex. Neurosci Bull 2021; 37: 461–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hablitz LM, Vinitsky HS, Sun Q, et al. Increased glymphatic influx is correlated with high EEG Delta power and low heart rate in mice under anesthesia. Sci Adv 2019; 5: eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benveniste H, Lee H, Ding F, et al. Anesthesia with dexmedetomidine and low-dose isoflurane increases solute transport via the glymphatic pathway in rat brain when compared with high-dose isoflurane. Anesthesiology 2017; 127: 976–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu J, Nelson LE, Franks N, et al. Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol 2008; 508: 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musizza B, Stefanovska A, McClintock PV, et al. Interactions between cardiac, respiratory and EEG-Delta oscillations in rats during anaesthesia. J Physiol 2007; 580: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xavier ALR, Hauglund NL, von Holstein-Rathlou S, et al. Cannula implantation into the cisterna magna of rodents. JoVE 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson RT, Levine ST, Haynes SM, et al. Use of labeled tomato lectin for imaging vasculature structures. Histochem Cell Biol 2015; 143: 225–234. [DOI] [PubMed] [Google Scholar]
- 37.Battistella R, Kritsilis M, Matuskova H, et al. Not all lectins are equally suitable for labeling rodent vasculature. Int J Mol Sci 2021; 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iliff JJ, Wang MH, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 2013; 33: 18190–18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mestre H, Tithof J, Du T, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 2018; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaberel T, Gakuba C, Goulay R, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI a new target for fibrinolysis? Stroke 2014; 45: 3092–3096. [DOI] [PubMed] [Google Scholar]
- 41.Takano K, Yamada M. Contrast-enhanced magnetic resonance imaging evidence for the role of astrocytic aquaporin-4 water channels in glymphatic influx and interstitial solute transport. Magn Reson Imaging 2020; 71: 11–16. [DOI] [PubMed] [Google Scholar]
- 42.Bedussi B, van der Wel NN, de Vos J, et al. Paravascular channels, cisterns, and the subarachnoid space in the rat brain: a single compartment with preferential pathways. J Cereb Blood Flow Metab 2017; 37: 1374–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bechet NB, Kylkilahti TM, Mattsson B, et al. Light sheet fluorescence microscopy of optically cleared brains for studying the glymphatic system. J Cereb Blood Flow Metab 2020; 40: 1975–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang LJ, Kress BT, Weber HJ, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med 2013; 11: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020; 16: 137–153. [DOI] [PubMed] [Google Scholar]
- 46.Pollock H, Hutchings M, Weller RO, et al. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anatomy 1997; 191: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown R, Benveniste H, Black SE, et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 2018; 114: 1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mestre H, Kostrikov S, Mehta RI, et al. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 2017; 131: 2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison IF, Ismail O, Machhada A, et al. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain 2020; 143: 2576–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H, Xie L, Yu M, et al. The effect of body posture on brain glymphatic transport. J Neurosci 2015; 35: 11034–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ooboshi H, Welsh MJ, Rios CD, et al. Adenovirus-mediated gene transfer in vivo to cerebral blood vessels and perivascular tissue. Circ Res 1995; 77: 7–13. [DOI] [PubMed] [Google Scholar]
- 52.Christenson SD, Lake KD, Ooboshi H, et al. Adenovirus-mediated gene transfer in vivo to cerebral blood vessels and perivascular tissue in mice. Stroke 1998; 29: 1411–1415; discussion 1416. [DOI] [PubMed] [Google Scholar]
- 53.Morris AWJ, Sharp MM, Albargothy NJ, et al. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol 2016; 131: 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albargothy NJ, Johnston DA, MacGregor-Sharp M, et al. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol 2018; 136: 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magdoom KN, Brown A, Rey J, et al. MRI of whole rat brain perivascular network reveals role for ventricles in brain waste clearance. Sci Rep 2019; 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-jcb-10.1177_0271678X221109997 for High-resolution 3D demonstration of regional heterogeneity in the glymphatic system by Xu-Zhong He, Xin Li, Zhen-Hua Li, Jing-Cai Meng, Rui-Ting Mao, Xue-Ke Zhang, Rong-Ting Zhang, Huai-Liang Huang, Qian Gui, Guang-Yin Xu and Lin-Hui Wang in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-jpg-2-jcb-10.1177_0271678X221109997 for High-resolution 3D demonstration of regional heterogeneity in the glymphatic system by Xu-Zhong He, Xin Li, Zhen-Hua Li, Jing-Cai Meng, Rui-Ting Mao, Xue-Ke Zhang, Rong-Ting Zhang, Huai-Liang Huang, Qian Gui, Guang-Yin Xu and Lin-Hui Wang in Journal of Cerebral Blood Flow & Metabolism
Supplemental material, sj-pdf-3-jcb-10.1177_0271678X221109997 for High-resolution 3D demonstration of regional heterogeneity in the glymphatic system by Xu-Zhong He, Xin Li, Zhen-Hua Li, Jing-Cai Meng, Rui-Ting Mao, Xue-Ke Zhang, Rong-Ting Zhang, Huai-Liang Huang, Qian Gui, Guang-Yin Xu and Lin-Hui Wang in Journal of Cerebral Blood Flow & Metabolism