Abstract
Hemoglobin (Hb) is a toxic molecule responsible for the extreme lethality associated with experimental Escherichia coli peritonitis, but the mechanism has yet to be elucidated. Hb, but not globin, showed toxic effects in a live E. coli model but not in a model using killed E. coli. Methemoglobin, hematin, and the well-known Fenton reagents iron and iron-EDTA demonstrated the same lethal effect in E. coli peritonitis as Hb, while the addition of the Fenton inhibitors desferrioxamine (DF) and diethylenetriamine pentaacetate removed most of the cytotoxic activity of iron. Administration of a combined dose of superoxide dismutase and catalase minimized the action of Hb and iron-EDTA, suggesting that both O2⋅− and H2O2 are involved in the toxic action of Hb in this rat model. The combination of the antioxidative enzymes and DF further suppressed iron-mediated lethality. An electron spin resonance technique with the spin-trapping reagent 5,5-dimethyl-1-pyroline-N-oxide (DMPO) showed O2⋅− generation in the peritoneal fluid of rats injected with E. coli alone or E. coli plus iron-DF, and ⋅OH generation was detected in the peritoneal fluid of the rats injected with iron-EDTA. Hb did not show any spin adduct of oxygen radicals, suggesting that Hb produces non-spin-trapping radical ferryl ion, which decayed the spin adduct of DMPO. In the presence of Hb or iron-EDTA, O2−-generating activity and viability of phagocytes decreased, whereas lipid peroxidation of peritoneal phagocytes increased. Generation of oxygen radicals and lipid peroxidation did not differ in the live and dead bacterial models. Bacterial numbers in the peritoneal cavity and blood were markedly increased in the live bacterial model with Hb and iron-EDTA. The Fenton inhibitor iron-DF prevented the loss of phagocyte function, lipid peroxidation, and bacterial proliferation. These results led us to conclude that the lethal toxicity of Hb in bacterial peritonitis is associated with a Fenton-type reaction, the products of which decrease phagocyte viability, through the induction of lipid peroxidation, allowing bacterial proliferation and resulting in mortality.
Phagocytes (macrophages and neutrophils) are primary host defense systems against peritoneal infection. They induce an important biochemical pathway, a respiratory burst, which results in generation of O2⋅− and H2O2, followed by the formation of more-toxic secondary oxidants such as hydroxyl radical (⋅OH) and HOCl. These oxidizing species are responsible for pathological events (29) such as destruction of biomolecules, killing of infected microorganisms, and autokilling of phagocytes. The hydroxyl radical is generated by transition metals, particularly iron, via the Fenton reaction in an O2⋅−-generating environment. This radical is thought to be a major species for oxygen toxicity in biological systems. Yamazaki and Piette (31) have stoichiometrically measured the formation of ⋅OH and confirmed the formation of not only ⋅OH but also ferryl ion from the Fenton reaction by using a spin-trapping technique.
Hemoglobin (Hb) may also act as a Fenton reagent in biological systems (22, 24). This possibility has been supported by many lines of evidences that show that Hb causes the oxidation of several organic molecules by producing ⋅OH and/or ferryl ion [Fe(IV)⩵O] in the presence of H2O2 (9, 23). Free Hb causes injury to the central nervous system through peroxidation of membrane lipid (26). We confirmed that neutrophils were inactivated by the oxidizing species, ferryl ion, produced from Hb in O2⋅−-generating systems (17).
Hb has been known as a potent toxic molecule responsible for the high lethality associated with experimental Escherichia coli peritonitis since the early 1960s, when Davis and Yull (3, 4) discovered a synergistic effect on mortality between bacteria and erythrocytes in the peritoneums of rats. This synergistic action is a perplexing problem caused by the combination of blood and bacteria during surgery. Recently, Kim et al. reported that erythrocytes scavenge both O2⋅− and nitric oxide and then inhibit the formation of the bactericidal molecule peroxynitrite (14). Although the lethal toxicity of purified Hb has been thought to be associated with various biochemical events in the peritoneal cavity (1, 7), the lethal effect of Hb in the animal model is not fully understood.
We have therefore examined the possibilities that Hb produces strong oxidizing species by reacting with O2⋅− and H2O2 generated by recruited neutrophils in the peritoneal cavity and that these oxidizing species cause deleterious effects on the host defense system. By comparing Hb toxicity with the effects of well-known Fenton reagents and inhibitors on the mortality of rats, reactive oxygen production, and the pathobiochemical changes in this animal model, we demonstrated that a Fenton-type reaction producing reactive oxygen species may be involved in the lethality of Hb in experimental E. coli peritonitis.
MATERIALS AND METHODS
Materials.
Catalase (CAT) (from bovine liver), superoxide dismutase (SOD) (from bovine erythrocytes), phorbal myristate acetate, cytochrome c (from horse heart), phosphate-buffered saline (PBS) (pH 7.4), 5,5-dimethyl-1-pyroline-N-oxide (DMPO), globin, and hemin were obtained from Sigma. DMPO was used after redistillation. Desferrioxamine B (deferoxamine) (DF) was from CIBA Pharmaceutical Co. Hematin solutions were prepared by dissolving hemin in a small volume of weak alkaline solution and diluting with PBS within 1 h of experiments. Hb was prepared from bovine erythrocytes by a one-step procedure with a DEAE-Sephadex A-50 column (30). Methemoglobin (MetHb) was prepared by oxidizing Hb with a slight excess of potassium ferricyanide and separated from the added components through a Sephadex G-25 column. Hb and MetHb were concentrated with a Centricon apparatus (Amicon). The Hb concentration was calculated as a heme concentration by spectrophotometry.
Preparation of bacterial solution.
E. coli (ATCC 25922) was incubated on a blood agar plate at 37°C for 17 h, and a single colony was cultured in tryptic soy broth for an additional 18 h to produce a suspension of approximately 109 cells/ml. The bacteria were harvested by centrifugation, washed three times with saline, and resuspended in saline. The cell concentration was measured by spectrophotometry at 550 nm. The bacterial solution (absorbance was ∼1.0 at 550 nm) was diluted in PBS by 106- and 107-fold, inoculated on agar plates, and incubated at 37°C for 18 h (17). The number of viable bacteria was measured by counting developed colonies. Killed E. coli was prepared by treatment with formalin (5).
Animal mortality.
Male Sprague-Dawley rats (180 to 200 g) were given standard rat pellets and water ad libitum. Experimental peritonitis was induced in rats by intraperitoneal (i.p.) injection of E. coli (4 × 109 CFU/kg of body weight) with or without iron complexes (12.4 μmol of iron/kg of body weight). The injection mixtures were prepared immediately before injection, and the injection volume was 5 ml/kg of body weight. Mortality of rats was determined at 24 h after administration. The effects of SOD and CAT were examined by two i.p. injections of 2,600 and 11,000 U/kg of body weight, respectively, at 0 and 8 h following injection of bacteria.
Measurement of O2⋅− production in the peritoneal fluid.
The rats injected i.p. with viable E. coli with or without an iron complex were anesthetized with an intramuscular injection of Ketamine (44 mg/kg) at various time points. The rats were i.p. injected with 1 ml of saline and then physically shaken for 1 min to ensure adequate mixing of peritoneal contents. A midline laparotomy was performed, and the fluid was obtained from all regions of the peritoneal cavity. O2⋅− generation in an ex vivo system was measured by SOD (100 U/ml)-inhibitable cytochrome c reduction, monitored at 550 nm (Δɛ550 = 21 mM−1 cm−1 for reduced oxidized cytochrome c), in PBS (1 ml) containing cytochrome c (100 μM), peritoneal fluid (250 μl), and CAT (100 U).
Isolation, quantitation, and viability of peritoneal phagocytes.
All peritoneal fluid was obtained from the rats injected i.p. with viable E. coli with or without an iron complex. Peritoneal phagocytes were prepared by centrifugation at 300 × g for 8 min and washed twice with ice-cold PBS. The cell pellets were resuspended in PBS. Total phagocytes (macrophages and neutrophils) were counted by light microscopy with trypan blue staining. For counting the macrophage population, a cell suspension was plated on 24-well plates and incubated for 2 h at 37°C in a CO2 incubator. The plates were washed three times with PBS, and the adhering cells were counted by light microscopy. Cell viability was determined by trypan blue dye exclusion.
Bacterial counts in peritoneal fluid and blood.
Blood was collected by heart puncture and collected in a Vacutainer (Becton Dickinson; 7-ml capacity) containing 10.5 mg of EDTA. Peritoneal fluid and blood samples were diluted with ice-cold sterile water. The number of viable bacteria was measured as described above.
Lipid peroxidation.
Peritoneal exudate cells were prepared by centrifugation at 500 × g for 10 min and suspended in a small volume of PBS. Lipid peroxidation was assayed by measuring thiobarbituric acid (TBA)-reactive substance (TBARS) at 532 nm (2). A sample (0.25 ml) was mixed with 0.5 ml of trichloroacetic acid-TBA-HCl (15%, 0.375%, and 0.25 N, respectively) containing 0.05% butylated hydroxytoluene. The mixture was heated in a boiling water bath for 20 min. After being cooled with tap water, the mixture was centrifuged at 2,200 × g and 4°C for 20 min. The absorbance of the supernatant was measured at 532 nm. TBARS as a lipid peroxide level was calculated by using an extinction coefficient of 1.56 × 105 M−1 cm−1.
ESR spectroscopy of DMPO spin adducts.
Peritoneal fluid was collected from the rats 4 h after the i.p. injection of E. coli with or without iron complexes. The fluid (460 μl) was mixed with 40 μl of 1 M DMPO and incubated at 37°C for 8 min. The mixture was immediately transferred into an electron spin resonance (ESR) flat cell, and ESR spectra were recorded with a Varian E-109 spectrometer. Spectrometer settings were as follows: microwave power, 20 mW; modulation frequency, 100 kHz; modulation amplitude, 1.0 G; time constant, 0.25 s; receiver gain, 1.25 × 104. For decay of DMPO-OH by ferryl ion, DMPO-OH was formed by incubation of DMPO (10 mM), 100 μM Fe2+-EDTA, and 200 μM H2O2 for 1 min and then addition of CAT (100 U/ml) to remove excessive H2O2. The reaction mixture was incubated with or without ferryl ion, which is generated by mixing Hb (0.4 mM) with H2O2 (0.4 mM) for 4 min and then incubating with 100 U of CAT/ml for 1 min. After 8 min, ESR spectra were recorded with a Varian E-109 spectrometer with a receiver gain of 2 × 102.
Statistical analysis.
Data were analyzed by the chi-square test for animal mortality and by the Student t test. Statistical significance was established at a P value of <0.05.
RESULTS
As shown by Simmons’ group (5, 6, 10, 11, 19, 22), purified Hb administered simultaneously with E. coli into the peritoneal cavity results in a potentiated lethal effect (Fig. 1). Viable E. coli at 8 × 108 bacteria/200 g of body weight, which is a nonlethal dose, resulted in more than 80% mortality when Hb was administered within 24 h of injection (Fig. 1A). However, formalin-killed E. coli, showing a similar dose-response mortality curve at a concentration about 100 times higher than that for live bacteria, did not show a synergistic effect on mortality at 5 × 1010 bacteria/200 g of body weight when concomitantly injected with Hb (Fig. 1B), indicating that the viability of E. coli is an important factor in the toxic effect of Hb in experimental peritonitis.
FIG. 1.
Effect of Hb on mortality in experimental E. coli peritonitis. Six or seven animals were studied in each group. Mortality was measured at 24 h after i.p. injection of live E. coli (A) or formalin-killed E. coli (B) with or without Hb (12.4 mmol/kg of body weight [BW]). The total volume of the injection was 5 ml/kg of body weight.
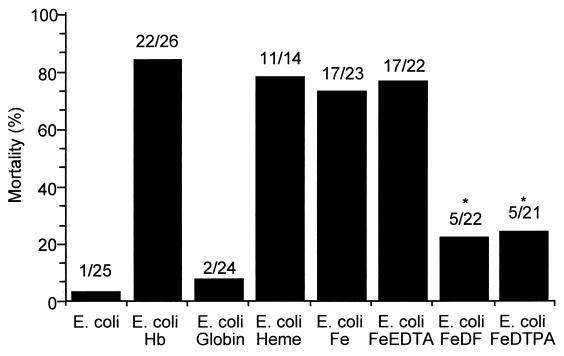
It is well known that iron from a variety of sources, including Hb, can enter into Fenton-type chemistry (25) and that neutrophils infiltrating the infectious site produce O2⋅− (13). It is therefore possible that Hb as a Fenton reagent produces a highly toxic oxidant in the peritoneal cavity during bacterial peritonitis. To test the hypothesis that a Fenton-type reaction is responsible for the lethal effect of Hb in bacterial peritonitis, we investigated the effects of globin, hematin, and several iron(II) chelates on mortality in the rat peritonitis model (Fig. 2). Hematin, iron itself, and iron-EDTA showed effects on mortality similar to those of Hb, whereas globin did not show a toxic effect. The initial valence state of iron, either ferrous or ferric, did not change the mortality (data not shown). Unlike EDTA, DF and diethylenetriamine pentaacetate (DTPA) significantly reduced the toxic effect of iron itself in experimental peritonitis. These data suggest that a Fenton-type reaction may be involved in the toxic effect of Hb in this animal model.
FIG. 2.
Effects of Hb, globin, hematin, and iron chelates on mortality in experimental E. coli peritonitis. Rats were injected i.p. with E. coli (4 × 109 cells/kg of body weight) and iron complex or globin (12.4 mmol/kg of body weight), and mortality was determined at 24 h after injection. The injection mixtures were prepared immediately before injection. The total volume injected was 5 ml/kg of body weight. The molar ratio of iron(II) to chelator was 1:2. The ratio of dead rats to treated rats is indicated above each bar. Statistically significant mortality reductions (asterisks) with chelators, DF, and DTPA versus iron ion itself (P < 0.001) were determined by the chi-square test. MetHb and iron(III) ion had the same adjuvant effects as Hb and Fe(II) ion, respectively.
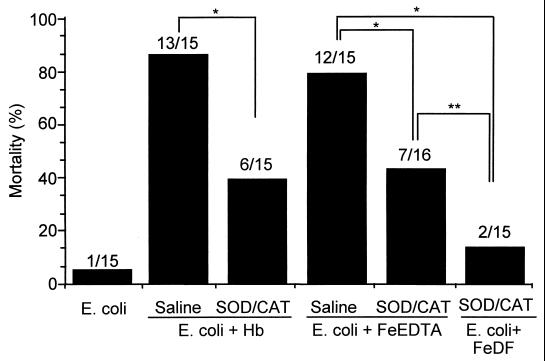
To test whether iron complexes play a role as a Fenton reagent in the rat peritonitis model, the effects of SOD and CAT on mortality and on generation of O2⋅− and H2O2 in the peritoneal cavity (since these oxygen metabolites are important reagents in Fenton chemistry) were investigated. A mixture of SOD and CAT was injected twice i.p. with E. coli and Hb or iron-EDTA into rats, simultaneously and 8 h after injection of bacteria. The toxic effect of the iron complexes on mortality was examined at 24 h after treatment. Administration of an SOD-CAT mixture reduced the toxic action of Hb (P < 0.01) and iron-EDTA (P < 0.01), though not completely (Fig. 3). Furthermore, the combination of the antioxidative enzymes and DF strongly suppressed iron-mediated lethality. The results suggest that phagocytes (resident macrophages and recruited neutrophils) produced activated oxygen species (O2⋅− and H2O2), which react with Hb and iron-EDTA to generate more-toxic species such as ferryl ion and ⋅OH.
FIG. 3.
Effect of both SOD and CAT on the adjuvant effect of Hb or iron-EDTA in experimental E. coli peritonitis. Experimental conditions were as described in the legend to Fig. 2, except that SOD and CAT were injected i.p. twice, at 2,600 and 11,000 U/kg, respectively, at 0 and 8 h after i.p. injection of E. coli and adjuvant. The ratio of dead rats to treated rats is indicated above each bar. Statistically significant mortality reductions versus each control were determined by the chi-square test (∗, P < 0.01; ∗∗, P < 0.05).
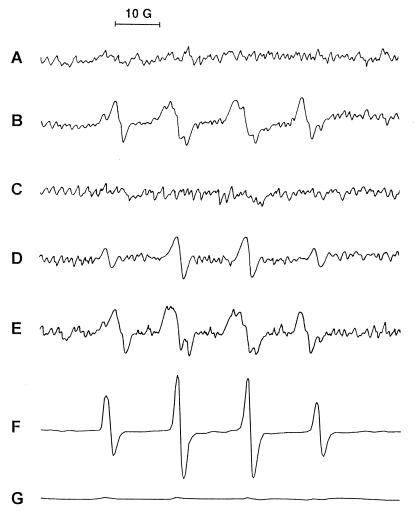
To establish definitively the generation of reactive oxygen species in our experimental model, we used ESR spectroscopy to detect spin adducts of DMPO with oxygen radicals which were generated in the peritoneal fluid collected from rats at 4 h after injection with bacteria alone or bacteria plus iron chelates. As shown in Fig. 4A, DMPO spin adducts were not found in the peritoneal fluid of rats which received saline as a control. The mixtures of DMPO spin adducts of O2⋅− (DMPO-OOH) and ⋅OH (DMPO-OH), but mostly DMPO-OH, were detected directly in the peritoneal fluid of rats injected with bacteria alone (Fig. 4B). The spectrum obtained for the system of bacteria plus iron-DF was not different from that obtained for rats injected with bacteria alone (Fig. 4E). Under the same experimental conditions, the spectrum of DMPO-OH disappeared with the addition of SOD (data not shown), indicating that DMPO-OH was generated by decay of DMPO-OOH, as previously reported (20). However, the spectrum of only DMPO-OH was identified in the peritoneal fluid of rats injected with bacteria plus iron-EDTA (Fig. 4D), but no spin adduct was found in rats injected with bacteria plus Hb (Fig. 4C). Since DMPO spin adducts can be rapidly decayed by the reaction product of Hb and H2O2, the disappearance of the DMPO adduct of ⋅OH was examined. An ESR spectrum of DMPO-OH was detected in the mixture of iron-EDTA and H2O2 (Fig. 4F), and this spectrum disappeared with the addition of ferryl ion generated from the reaction of Hb with H2O2 (Fig. 4G).
FIG. 4.
ESR spectrum of DMPO spin adducts of oxygen radical generated in peritoneal fluid. DMPO spin adducts in peritoneal fluids collected from rats injected with saline (A), E. coli (B), E. coli plus Hb (C), E. coli plus iron-EDTA (D), or E. coli plus iron-DF (E) were measured. In vitro formation of DMPO-OH was detected in the reaction mixture of Fe2+-EDTA and H2O2 (F) in the presence of ferryl ion (G), which is formed by incubation of Hb and H2O2, by ESR spectroscopy. Spectrometer settings are described in Materials and Methods. The spectra were from one of three experiments, which showed similar results.
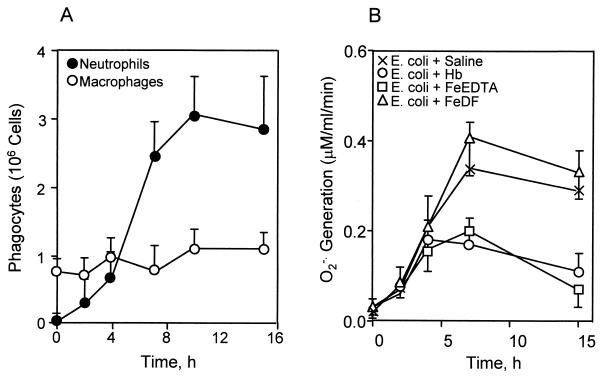
Our previous data showed that neutrophils are inactivated by toxic oxidants generated from Fenton-type reagents, including Hb in an O2⋅−-generating system (17). To test if peritoneal phagocytes are inactivated by iron complexes in experimental bacterial peritonitis, we examined the phagocyte population and its O2⋅−-generating activity in the peritoneal cavity (Fig. 5). The numbers of peritoneal macrophages were nearly unchanged, while the neutrophil population was significantly increased after 4 h of bacterial injection (Fig. 5A). The O2⋅−-generating activity of the peritoneal phagocytes isolated from rats injected with E. coli alone or E. coli plus iron-DF increased after 2 h and reached maximum levels after 7 h (P < 0.01) (Fig. 5B). Under the same conditions, however, treatment with iron-EDTA or Hb attenuated the O2⋅−-generating activity of peritoneal phagocytes.
FIG. 5.
Quantitation of peritoneal phagocytes and their O2⋅−-generating activity in experimental E. coli peritonitis. Numbers of total peritoneal phagocytes were determined by light spectroscopy with the trypan blue staining method. Macrophages were counted from adhered cells on culture plates. Subpopulations of neutrophils and macrophages (A) were calculated by the subtraction method. O2⋅− production (B) was measured by SOD-inhibitable cytochrome c reduction in peritoneal phagocytes obtained from the rats injected with bacteria in the absence of iron complex and in the presence of Hb, iron-EDTA, or iron-DF. Each point represents the mean ± standard deviation for three individual rats.
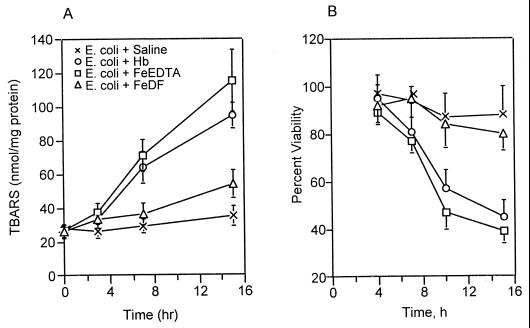
Toxic oxidants produced from a Fenton-type reaction cause oxidative injury through lipid peroxidation (28), resulting in cell death and inactivation of neutrophil function (17). To address this concern, lipid peroxidation and phagocyte viability were measured in the peritoneal phagocytes of rats injected with E. coli and iron complexes. Figure 6A shows that injection of E. coli alone into the peritoneal cavity did not significantly increase lipid peroxidation in peritoneal phagocytes, but its injection with Hb or iron-EDTA markedly increased lipid peroxidation in peritoneal phagocytes (P < 0.02 at 15 h). Iron-DF had no effect on lipid peroxidation. Phagocyte viability in the peritoneal cavity decreased (P < 0.01 at 15 h) following injection with Hb or iron-EDTA but did not decrease significantly (P > 0.1 at 15 h) when bacteria were injected alone or with iron-DF (Fig. 6B). Furthermore, we examined the effects of live and dead E. coli on reactive oxygen generation and lipid peroxidation in the peritoneal cavity. The formation of DMPO spin adducts and lipid peroxidation in the peritoneal cavity were not significantly different between these peritonitis models with or without Hb (Table 1). These results suggest that bacterial proliferation through phagocyte inactivation is a critical factor for Hb toxicity in E. coli peritonitis.
FIG. 6.
Effects of Hb and iron chelates on lipid peroxidation (A) and viability (B) of peritoneal phagocytes during E. coli peritonitis. Peritoneal phagocytes were prepared by centrifugation immediately after collection of the peritoneal fluids and washed with PBS. Data represent the means ± standard deviations for three individual rats, with results obtained in duplicate for each experiment.
TABLE 1.
Effects of live and dead E. coli on reactive oxygen generation and lipid peroxidation in bacterial peritonitis
| E. coli | Injection | DMPO spin adductsa (mean ± SD) | Lipid peroxidationb (mean ± SD) |
|---|---|---|---|
| Live | Saline | 100.0 ± 10.2 | 35.8 ± 5.3 |
| Hb | 5.6 ± 2.1 | 76.5 ± 6.3 | |
| Dead | Saline | 84.2 ± 11.5 | 28.8 ± 5.3 |
| Hb | 4.2 ± 1.7 | 62.5 ± 7.4 |
DMPO spin adducts were measured 4 h after peritoneal injection of E. coli and Hb. Data are relative intensities of reactive oxygen spin adducts for three animals.
Lipid peroxidation was measured 12 h after injection. Data are nanomoles of TBARS per milligram of protein.
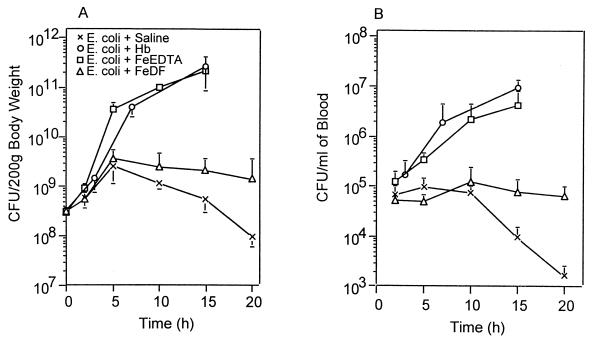
From work performed by Dunn et al. (5) and O’Brien (21), one might conclude that an important role for Hb or any iron source in experimental peritonitis is to cause uncontrolled bacterial proliferation in the peritoneal cavity. Although for E. coli alone the number of cells in the peritoneal cavity began to decrease after an initial weak increase, the number of E. coli cells injected with Fenton-type reagents, Hb, or iron-EDTA rapidly increased in the peritoneal cavity, by about 103-fold within 15 h (P < 0.01) (Fig. 7A). The number of viable bacteria in blood was lower than that in the peritoneal cavity but its time dependence was identical to that in the peritoneal cavity (Fig. 7B). When Fe-DF (ratio of 1 to 2) was injected, the numbers of viable bacteria in the peritonal cavity and blood were similar to those in animals injected with bacteria alone until 10 h and thereafter were a little higher (P < 0.05 at 15 h), probably due to the suppression of phagocyte-dependent bacterial killing by the excessive amount of DF (27).
FIG. 7.
Effects of Hb and iron chelates on bacterial proliferation in the peritoneal cavity (A) and blood (B) during E. coli peritonitis. Experimental conditions were the same as described in the legend to Fig. 5. Each point represents the mean ± standard deviation of CFU from two individual rats, with results obtained in triplicate, at each indicated time point after i.p. inoculation with E. coli.
DISCUSSION
As reported previously (6, 18), Hb increased lethality in experimental bacterial peritonitis. The most prominent characteristic reported so far regarding the toxic effect of Hb in experimental peritonitis seems to be the significantly increased rate of bacterial proliferation in vivo (7, 11). Hb did not increase the lethality in a peritonitis model of killed E. coli, supporting a role for Hb in increasing bacterial growth. We previously reported that Hb diminished the microbicidal activity of neutrophils in an O2⋅−-generating system (17), and we postulated that a Fenton reaction producing toxic radicals played an important role in neutrophil inactivation. We here report that Hb and the Fenton reagent iron-EDTA, but not the Fenton inhibitor iron-DF, increased lethal toxicity by decreasing phagocyte viability and increasing bacterial growth in experimental bacterial peritonitis.
Peritoneal phagocytes such as resident macrophages and infiltrating neutrophils are the first line of defense against bacterial peritonitis. They produce O2⋅− and H2O2, which are implicated in bacterial killing. O2⋅− supplied by phagocytes interacts with oxidized iron to generate the reduced form of iron. The reduced forms of iron ion and its chelate complexes are well-known Fenton reagents that react with H2O2 to produce strong oxidizing species such as ⋅OH and Fe(IV)⩵O, which oxidize several cellular components (17, 24).
Many investigators (9, 23, 25, 26, 30) have reported that Hb produces oxidizing species, ⋅OH and/or Fe(IV)⩵O, in the presence of H2O2 through a Fenton-type reaction. It has been suggested that these oxidizing species are responsible for oxygen toxicity, including neutrophil inactivation (17) and protein degradation (16), in various biological systems. Although iron(II)-DF and iron(II)-DTPA produce ⋅OH in the presence of H2O2, DF and DTPA, probably because of the slow oxidation-reduction cycle of these iron chelates, are known inhibitors of the Fenton reaction. Here, we will first focus our discussion on the possibility that the lethal effects of Hb and iron-EDTA in our peritonitis model are associated with the production of oxidizing species through a Fenton-type reaction (Fig. 2). Dead bacteria or lipopolysaccharide can also stimulate phagocytes and produce reactive oxygen species (12). The oxygen radicals inactivate the O2⋅−-generating activity of neutrophils through lipid peroxidation in the presence of a Fenton reagent in vitro (17). Although Hb also increases peroxidation through oxygen radical generation in dead E. coli peritonitis to a level similar to that in live E. coli peritonitis (Table 1), Hb did not enhance the lethal effect (Fig. 1B). These results suggest that bacterial proliferation through decreased phagocyte function in the peritoneal cavity is one critical factor for Hb-mediated adjuvant mortality in bacterial peritonitis.
SOD and CAT are powerful scavengers of O2⋅− and H2O2. Therefore, they are effective in inhibiting a Fenton reaction (18) and neutralizing neutrophil-mediated tissue damage (8). In our peritonitis model, administration of SOD and CAT reduced the lethal effect of Hb and iron-EDTA (Fig. 3), and the Fenton inhibitors DF and DTPA also suppressed the lethal effect of iron (Fig. 2). Furthermore, the combination of SOD, CAT, and DF strongly inhibited iron-mediated mortality in our peritonitis model. These results suggest that O2⋅− and H2O2 are involved in the production of oxidizing species from the Fenton reaction of Hb and cause the toxic effect of Hb in our peritonitis model.
Phagocytes, i.e., macrophages and neutrophils, are known to produce O2⋅− and H2O2, which normally are involved in the killing of infectious bacteria, and such was the case when E. coli alone was injected. These oxygen species can interact with Fenton reagents such as iron-EDTA and Hb and produce stronger oxidants such as ⋅OH and ferryl ion, which are involved in tissue injury through lipid peroxidation (17). ESR spectroscopy showed direct evidence that peritoneal phagocytes produce reactive oxygen species during bacterial peritonitis, followed by the formation of more-autotoxic secondary oxidants such as ⋅OH or ferryl ion in the presence of Fenton reagents. Neither O2⋅− nor ⋅OH was detected in the peritoneal cavities of the rats injected with bacteria plus Hb. A major reactive species produced by the reaction of Hb with H2O2 is thought to be ferryl ion (17), which is not directly trapped by DMPO (31). The ferryl species rapidly decayed DMPO spin adducts to other products that were not detected by ESR spectroscopy (15). This evidence may explain why no ESR spectrum was detected when Hb was injected into the peritoneal cavity. These results indicate that the large number of neutrophils which migrated into the peritoneal cavity 4 h after bacterial injection appear to be the major source of O2⋅− and H2O2, participating in lipid peroxidation, inactivation of phagocytic function, and bacterial proliferation via a Fenton reaction.
⋅OH and ferryl ion are highly reactive species and react with a variety of biological molecules such as protein, membrane lipid, and nucleic acids. Peroxidation of membrane lipid is a major cellular injury caused by these oxidizing species. This markedly decreased the O2⋅− production in the peritoneal cavity in the presence of Hb or iron-EDTA (Fig. 5), probably because of the decreased phagocyte viability through Fenton-type reactions (Fig. 6B). Inactivation of phagocyte function allowed increased bacterial proliferation (Fig. 7). The protective effect of DF on O2⋅− production suggests that the Fenton-type reaction causes inactivation of the peritoneal phagocytes. The results are in accordance with in vitro neutrophil inactivation by Fenton-type iron complexes in the O2⋅−-generating system (17).
Hb can interact with NO, synthesized from l-arginine by any of the three isotypes of NO synthase and oxidized to nitrate, which is a nontoxic stable product. Hb is known to be a biological scavenger of NO. NO interacts with O2⋅− and then produces highly toxic peroxynitite, which reacts against bacteria and host cells. Recent studies have reported that erythrocytes inhibit the formation of peroxynitrite and allow bacteria to proliferate in the peritoneal cavity, resulting in increased mortality (14). Purified Hb is very much different from erythrocytes, because erythrocytes contain many different types of antioxidative enzymes and antioxidants. The toxic mechanism of purified Hb may be different from that of Hb in erythrocytes. Like Hb, the Fenton inhibitor Fe-DTPA can interact with NO and neutralize the biological and pathological functions of NO (13). However, Fe-DTPA did not show a synergistic effect on mortality with bacteria in our animal model. The production of reactive oxygen species, but not NO, in the peritoneal cavity was detected by ESR 4 h after bacterial injection, because NO is produced in vivo by the induction of inducibe NO synthase 8 to 10 h after injection of lipopolysaccharide or bacteria. These results suggest that inactivation of phagocyte function may be due to a reactive oxygen-dependent Fenton reaction.
The products formed by the Fenton reaction, ferryl ion and ⋅OH, may indiscriminately destroy biomolecules to kill not only host cells but also microorganisms in the infectious site. In our peritonitis model, however, apparently the host cells are destroyed and the numbers of viable bacteria in the peritoneal cavity and blood increase (Fig. 6). This finding could be explained by assuming that the phagocytes are more susceptible to the oxidizing species than the bacteria because of differences in the cell wall structures or proximity of the oxidant formation, resulting in bacterial proliferation. These phenomena are enhanced by the addition of Hb or iron-EDTA but not iron-DF, supporting the hypothesis that Hb in this rat model produces oxidizing species which decrease phagocyte viability in the peritoneal cavity to allow bacterial proliferation, resulting in lethality.
ACKNOWLEDGMENT
This work was supported by Korea Science and Engineering Foundation grant 981-0714-100-2 and Korea Research Foundation grant 1998-021-F00050.
REFERENCES
- 1.Bornside G H, Bouis P J, Jr, Cohn I., Jr Enhancement of Escherichia coli infection and endotoxic activity by hemoglobin and ferric ammonium citrate. Surgery. 1970;68:350–355. [PubMed] [Google Scholar]
- 2.Buege J A, Aust S D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 3.Davis J H, Yull A B. A possible toxic factor in abdominal injury. J Trauma. 1962;2:291–300. doi: 10.1097/00005373-196205000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Davis J H, Yull A B. A toxic factor in abdominal injury. II. The role of the red cell component. J Trauma. 1964;4:84–90. [PubMed] [Google Scholar]
- 5.Dunn D L, Barke R A, Lee J T, Jr, Condie R M, Humphrey E W, Simmons R L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. VII. Hemoglobin does not inhibit clearance of Escherichia coli from the peritoneal cavity. Surgery. 1983;94:487–493. [PubMed] [Google Scholar]
- 6.Dunn D L, Nelson R D, Condie R M, Simmons R L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. VI. Effects of stroma-free hemoglobin and red blood cell stroma on mortality and neutrophil function. Surgery. 1983;93:653–659. [PubMed] [Google Scholar]
- 7.Filler R M, Sleeman H K. Pathogenesis of peritonitis. I. The effect of Escherichia coli and hemoglobin on peritoneal absorption. Surgery. 1967;61:385–392. [PubMed] [Google Scholar]
- 8.Grisham M B, Granger D N. Neutrophil-mediated mucosal injury. Dig Dis Sci. 1988;33(Suppl.):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- 9.Gutteridge J M C. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986;201:291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- 10.Hau T, Nelson R D, Fiegel V D, Levenson R, Simmons R L. Mechanisms of the adjuvant action of hemoglobin in experimental peritonitis. 2. Influence of hemoglobin on human leukocyte chemotaxis in vitro. J Surg Res. 1977;22:174–180. doi: 10.1016/0022-4804(77)90131-7. [DOI] [PubMed] [Google Scholar]
- 11.Hau T, Hoffman R, Simmons R L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. I. In vivo inhibition of peritoneal lekocytosis. Surgery. 1978;83:223–229. [PubMed] [Google Scholar]
- 12.Jersmann H P, Rathjen D A, Ferrante A. Enhancement of lipopolysaccharide-induced neutrophil oxygen radical production by tumor necrosis factor alpha. Infect Immun. 1998;66:1744–1747. doi: 10.1128/iai.66.4.1744-1747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazmierski W M, Wolberg G, Wilson J G, Smith S R, Williams D S, Thorp H H, Molina L. Iron chelates bind nitric oxide and decrease mortality in an experimental model of septic shock. Proc Natl Acad Sci USA. 1996;93:9138–9141. doi: 10.1073/pnas.93.17.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y M, Hong S J, Billiar T R, Simmons R L. Counterprotective effect of erythrocytes in experimental bacterial peritonitis is due to scavenging of nitric oxide and reactive oxygen intermediates. Infect Immun. 1996;64:3074–3080. doi: 10.1128/iai.64.8.3074-3080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y M, Jeong S H, Yamazaki I, Piette L H, Han S, Hong S J. Decay studies of DMPO-spin adducts of free radicals produced by reactions of metmyoglobin and methemoglobin with hydrogen peroxide. Free Radic Res. 1995;22:11–21. doi: 10.3109/10715769509147524. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y M, Kim S S, Kang G, Yoo Y M, Kim K M, Lee M E, Han J A, Hong S J. Comparative studies of protein modification mediated by Fenton-like reaction of iron, hematin, and hemoglobin: generation of different reactive oxidizing species. J Biochem Mol Biol. 1998;31:161–169. [Google Scholar]
- 17.Kim Y M, Yamazaki I, Piette L H. The effect of hemoglobin, hematin, and iron on neutrophil inactivation in superoxide generating system. Arch Biochem Biophys. 1994;309:308–314. doi: 10.1006/abbi.1994.1118. [DOI] [PubMed] [Google Scholar]
- 18.Kuppusamy P, Zweier J L. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem. 1989;264:9880–9884. [PubMed] [Google Scholar]
- 19.Lee J T, Jr, Ahrenholz D H, Nelson R D, Simmons R L. Mechanisms of the adjuvant effect of hemoglobin in experimental peritonitis. V. The significance of the coordinated iron component. Surgery. 1979;86:41–48. [PubMed] [Google Scholar]
- 20.Lioyd R V, Mason R P. Evidence against transition metal-independent hydroxyl radical generation by xanthine oxidase. J Biol Chem. 1990;265:16733–16736. [PubMed] [Google Scholar]
- 21.O’Brien A D. Innate resistance of mice to Salmonella typhi infection. Infect Immun. 1982;38:948–952. doi: 10.1128/iai.38.3.948-952.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pruett T L, Rotstein O D, Fiegel V D, Sorenson J J, Nelson R D, Simmons R L. Mechanism of the adjuvant effect of hemoglobin in experimental peritonitis. VIII. A leukotoxin is produced by Escherichia coli metabolism in hemoglobin. Surgery. 1984;96:375–383. [PubMed] [Google Scholar]
- 23.Puppo A, Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Is haemoglobin a biological Fenton reagent? Biochem J. 1988;249:185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rush J D, Koppenol W H. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c. J Biol Chem. 1986;261:6730–6733. [PubMed] [Google Scholar]
- 25.Sadrzadeh S M H, Graf E, Panter S S, Hallaway P E, Eaton J W. Hemoglobin. A biologic Fenton reagent. J Biol Chem. 1984;259:14354–14356. [PubMed] [Google Scholar]
- 26.Sadrzadeh S M H, Eaton J W. Hemoglobin-mediated oxidant damage to the central nervous system requires endogenous ascorbate. J Clin Investig. 1988;82:1510–1515. doi: 10.1172/JCI113759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sane A, Manzi S, Perfect J, Herzberg A J, Moore J O. Desferoxamine treatment as a risk factor for zygomycete infection. J Infect Dis. 1989;159:151–152. doi: 10.1093/infdis/159.1.151. [DOI] [PubMed] [Google Scholar]
- 28.van-Bebber I P, Boekholz W K, Goris R J, Schillings P H, Dinges H P, Bahrami S, Redl H, Schlag G. Neutrophil fuction and lipid peroxidation in a rat model of multiple organ failure. J Surg Res. 1989;47:471–475. doi: 10.1016/0022-4804(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 29.Weiss S J, LoBuglio A F. Biology of disease. Phagocyte-generated oxygen metabolites and cellular injury. Lab Investig. 1982;47:5–18. [PubMed] [Google Scholar]
- 30.Winterbourn C C. Reaction with superoxide with hemoglobin. In: Greenwald R A, editor. CRC handbook of methods for oxygen radical research. Boca Raton, Fla: CRC Press Inc.; 1985. pp. 137–141. [Google Scholar]
- 31.Yamazaki I, Piette L H. ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J Biol Chem. 1990;265:13589–13594. [PubMed] [Google Scholar]