Abstract
Objective
To examine the association of maternal hypertensive disorder of pregnancy (HDP) with overall and cause specific mortality in offspring from birth to young adulthood.
Design
Nationwide population based cohort study.
Setting
Danish national health registers.
Participants
All 2 437 718 individuals live born in Denmark, 1978-2018, with follow-up from date of birth until date of death, emigration, or 31 December 2018, whichever came first.
Main outcome measures
The primary outcome was all cause mortality. Secondary outcomes were 13 specific causes of death in offspring from birth to young adulthood (age 41 years). Cox regression was used to assess the association, taking into consideration several potential confounders. The role of timing of onset and severity of pre-eclampsia, maternal history of diabetes, and maternal education were also studied.
Results
102 095 mothers had HDP: 67 683 with pre-eclampsia, 679 with eclampsia, and 33 733 with hypertension. During follow-up to 41 years (median 19.4 (interquartile range 9.7-28.7) years), deaths occurred in 781 (58.94 per 100 000 person years) offspring born to mothers with pre-eclampsia, 17 (133.73 per 100 000 person years) born to mothers with eclampsia, 223 (44.38 per 100 000 person years) born to mothers with hypertension, and 19 119 (41.99 per 100 000 person years) born to mothers with no HDP. The difference in cumulative incidence in overall mortality between cohorts exposed and unexposed to maternal HDP was 0.37% (95% confidence interval 0.11% to 0.64%), and the population attributable fraction for maternal HDP was estimated as 1.09% (95% confidence interval 0.77% to 1.41%). Maternal HDP was associated with a 26% (hazard ratio 1.26, 95% confidence interval 1.18 to 1.34) higher risk of all cause mortality in offspring. The corresponding estimates for maternal pre-eclampsia, eclampsia, and hypertension were 1.29 (1.20 to 1.38), 2.88 (1.79 to 4.63), and 1.12 (0.98 to 1.28). Increased risks were also observed for several cause specific mortalities, such as deaths from conditions originating in the perinatal period (2.04, 1.81 to 2.30), cardiovascular diseases (1.52, 1.08 to 2.13), digestive system diseases (2.09, 1.27 to 3.43), and endocrine, nutritional, and metabolic diseases (1.56, 1.08 to 2.27). The increased risks were more pronounced among offspring of mothers with early onset and severe pre-eclampsia (6.06, 5.35 to 6.86) or with both HDP and diabetes history (1.57, 1.16 to 2.14) or HDP and low education level (1.49, 1.34 to 1.66).
Conclusion
Maternal HDP, particularly eclampsia and severe pre-eclampsia, is associated with increased risks of overall mortality and various cause specific mortalities in offspring from birth to young adulthood.
Introduction
Hypertensive disorder of pregnancy (HDP) is one of the leading causes of maternal and fetal morbidity and mortality, affecting up to 10% of pregnancies worldwide.1 2 In addition to adverse pregnancy and birth outcomes,3 4 maternal HDP has also been associated with several diseases in offspring in later life, such as metabolic syndrome, immune diseases, and neurodevelopmental and psychiatric disorders.5 6 7 Barker and colleagues proposed that an adverse intrauterine environment during pregnancy would lead to improper fetal growth and fetal programming, which in turn would lead to a predisposition to various diseases later in life.8 9 10 Specifically, maternal HDP might increase offsprings’ susceptibility to disease through multiple pathways, such as placental dysfunction, an hypoxic-ischaemic environment during pregnancy, abnormal inflammatory levels, and epigenetic changes.7 8 9 10 11
However, the empirical evidence from large scale prospective studies on the association between maternal HDP and long term mortality risk in offspring remains scarce, except for one retrospective study with a limited sample size in which offspring born to mothers with HDP had excess risks of overall and cardiovascular mortality in mid-adulthood.12 Work on the role of specific types of maternal HDP in cause specific mortality in the early decades of life is also lacking, and the effect of timing of onset and severity of maternal HDP on mortality in offspring remains to be elucidated. Also, women with diabetes and low education level might have an excess risk of HDP,13 14 15 16 therefore evaluating the joint effect of maternal history of diabetes or education and HDP would be important.
Using data from Danish national health registers, we established a large population based cohort study with follow-up for up to four decades to investigate the association between overall and specific maternal HDPs with overall and cause specific mortality in offspring from birth to young adulthood. We also examined whether maternal diabetes and maternal education would have an additive effect on mortality in offspring, and whether the timing of onset and severity of HDP, specifically for pre-eclampsia, has an influence.
Methods
Study population
Since the late 1960s, when population registration systems started in Denmark, all live births and new residents have been assigned a unique individual personal identification number—the central personal register number.17 18 This number can be used for linkage between different national health registers.17 18 After obtaining approval from the Data Protection Agency, researchers can access the anonymized data from nationwide registers and obtain information on diseases, deaths, and other covariates.17 We first identified all 2 537 421 live births in Denmark between 1978 and 2018 using the Danish Medical Birth Register.17 Individuals with a birth weight <500 g and gestational age at birth <22 weeks (n=97 128) were excluded according to the World Health Organization criteria.19 We further excluded individuals who died on the day of birth (n=2575), because death on this day is probably attributable to birth defects, extreme preterm birth, or low birth weight, and our aim was to evaluate the short term and long term effects of HDP on mortality in offspring up to young adulthood. The final cohort comprised 2 437 718 liveborn individuals (see supplementary figure S1). Follow-up started from the date of birth and ended until the date of death, emigration, or 31 December 2018, whichever came first. A total of 66 832 offspring (2.7%) were censored owing to emigration. Supplementary appendix 1 provides detailed descriptions of the registers used in this study.
Exposure status
Based on information from the Danish Medical Birth Register and Danish National Patient Register, we identified HDP among mothers using ICD-8 (international classification of diseases, eighth revision) codes from 1978 to 1993 and ICD-10 (10th revision) codes from 1994 onwards (see supplementary table S1). HDP was classified as eclampsia, pre-eclampsia, or hypertension. At each hospital contact, doctors were responsible for the registration of diagnoses using ICD codes.20 The diagnostic criteria for pre-eclampsia was the development of hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or both) and proteinuria after 20 weeks of gestation.21 Eclampsia was the new onset of seizures or coma in a pregnant woman with pre-eclampsia.21 According to severity, pre-eclampsia was categorised into unspecified, moderate, or severe, and HELLP (haemolysis, elevated liver enzymes, and low platelets) syndrome. Hypertension was categorised into pregestational (hypertension before pregnancy) and gestational (new onset hypertension after 20 weeks of gestation).21 If women had multiple HDP diagnoses, we categorised them according to the hierarchy: eclampsia, pre-eclampsia, pregestational hypertension, and gestational hypertension.
When evaluating the severity and timing of onset of pre-eclampsia, we compiled pre-eclampsia as a binary variable for severity and categorised it as moderate or severe (including severe pre-eclampsia and HELLP syndrome). According to the timing of diagnosis, we categorised pre-eclampsia into early onset (<34 weeks of gestation) and late onset (≥34 weeks of gestation).22
Outcomes
The primary outcome of interest was all cause mortality identified in the Danish Register of Causes of Death.17 As secondary outcomes, we further examined 13 specific causes of death: cardiovascular diseases; cancer; infectious and parasitic diseases; endocrine, nutritional, and metabolic diseases; mental and behavioural disorders; diseases of the nervous system and sense organs; diseases of the respiratory system; diseases of the digestive system; diseases of the musculoskeletal system or connective tissue; diseases of the genitourinary system; certain conditions originating in the perinatal period; congenital malformations and chromosomal abnormalities; and external causes of morbidity and mortality. Causes of death were determined based on ICD codes according to the European Shortlist for Causes of Death and used information on underlying cause in the Danish Register of Causes of Death.17 Supplementary table S2 provides the ICD codes for causes of deaths.
Covariates
Covariates included sex of offspring (male, female); singleton birth (yes, no); parity (1, 2, ≥3 children); birth year of the child (1977-80, and five year intervals in 1981-2015 and 2016-18); maternal age (<20, 20-24, 25-29, 30-34, ≥35 years), smoking during pregnancy (yes, no), living arrangements (single, cohabitating), residence (Copenhagen, big cities (≥100 000 inhabitants), and other places), country of origin (Denmark, non-Denmark), education before pregnancy (low: 0-9 years, medium: 10-14 years, high: ≥15 years), income at birth of the child (no income, three tertiles), pre-pregnancy body mass index (BMI) (underweight: <18.5, normal: 18.5-24.9, overweight: 25.0-29.9, obese: ≥30.0), and history of diabetes (yes, no); and parental history of cardiovascular disease before birth of the child (yes, no), which were selected by directed acyclic graphs (see supplementary figure S2). To deal with missing values we used a multiple imputation procedure with fully conditional specification (SAS Institute) to impute 10 replications (greater than the percentage of data missing).23 Supplementary appendix 2 describes the covariates and multiple imputation methods in detail.
Statistical analysis
Cumulative incidence of all cause mortality was computed according to maternal HDP status after adjusting for covariates. We examined the association of maternal HDP with all cause mortality and cause specific mortality in offspring using a Cox regression model with age as the time scale. For cause specific mortality, we considered deaths from other causes as competing events and censored those participants at the date of death from other causes. Adjusted hazard ratios and corresponding 95% confidence intervals were calculated after adjusting for calendar year; sex; singleton; parity; birth year of the child; maternal age, smoking, cohabitation, country of origin, residence, education, income at birth, pre-pregnancy BMI, and history of diabetes; and parental history of cardiovascular disease before birth of the child. The proportional hazard assumptions for all cause and cause specific mortality were not violated according to the log-minus-log plot (see supplementary figures S3 and S4). We examined if the association between maternal HDP and all cause mortality in offspring differed by the timing of diagnosis (early and late onset) and severity (moderate and severe) of pre-eclampsia. To assess the role of maternal diabetes or education level on the association, we investigated the joint effect of maternal HDP and maternal diabetes or maternal HDP and maternal education before pregnancy on mortality in offspring.
We conducted several sensitivity analyses. Firstly, to account for the influence of genetic or familial factors, we carried out sibship analysis for the association between maternal HDP and all cause mortality using the stratified cox regression model. Half sibling and full sibling were defined as offspring born to the same mother and offspring born to both the same mother and the same father, respectively. Secondly, as the relationship between maternal HDP or paternal hypertension and mortality in offspring may partially be confounded by genetic or familial factors, we considered paternal hypertension before pregnancy as the control exposure to investigate the influence of underlying genetic or familial factors on the association between maternal HDP and mortality in offspring. Thirdly, we divided offspring according to small for gestational age (defined as infants with birth weight below the 10th centile for infants of the same gestational age, sex, and birth year) or not, and performed analysis stratified by small for gestational age to investigate whether fetal growth restriction would affect the association between maternal HDP and mortality in offspring. Fourthly, we investigated the association between maternal HDP and all cause mortality in offspring according to sex and parity. Fifthly, we split offspring into different age groups (0-18, ≥19 years old) to assess the impact of HDP on mortality by offspring age. Sixthly, we further conducted several subgroup analyses: additionally adjusting for paternal hypertension; analyses restricting to singleton births; analyses restricting to offspring born after 1991, 1994, and 2004, owing to the change in ICD code and availability of data on confounders; complete cases analysis; excluding individuals exposed to maternal pregestational hypertension; additional adjusting for Charlson comorbidity index scores; including all live births from 1977 to 2018. Seventhly, to account for the influence of delayed diagnoses, we changed the cutoff points of early onset and late onset pre-eclampsia to 35 weeks or 36 weeks of gestation and reanalysed the impact of timing of diagnosis and severity of pre-eclampsia on mortality in offspring. Eighthly, we performed additional analysis using restricted cubic splines rather than categorisation for covariates (maternal age, parity, maternal income, and calendar year) in the regression models. Ninthly, we further estimated hazard ratios using a time varying Cox model, which allowed the effect of maternal HDP on mortality in offspring to vary over time. Finally, we calculated a series of averaged hazard ratios according to follow-up time (0-5, 0-10, 0-15, 0-20, 0-25, 0-30, 0-35, and 0-41 years).24 All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and Stata 15.1 (StataCorp, College Station, TX). Supplementary appendix 3 presents the SAS codes for the main analysis.
Patient and public involvement
This study used data from large population based registers that were not specifically designed for the current study’s aim of examining the association of HDP with mortality in offspring from birth to young adulthood. Therefore, no patients were involved in the study design, study implementation, setting the research question, or the outcome measures.
Results
Among a total of 2 437 718 offspring, 102 095 (4.2%) were prenatally exposed to maternal HDP, including 68 362 (2.8%) exposed to pre-eclampsia or eclampsia and 33 733 (1.4%) exposed to hypertension. Compared with unexposed offspring, exposed offspring were more likely to be born to mothers with lower parity, not cohabiting, younger age at childbirth, higher pre-pregnancy BMI, or a history of diabetes or cardiovascular disease (table 1).
Table 1.
Baseline characteristics according to offspring’s exposure to maternal hypertensive disorder of pregnancy. Values are numbers (row percentages) of offspring
| Characteristics | No hypertensive disorders | Pre-eclampsia* | Eclampsia | Hypertension | Total |
|---|---|---|---|---|---|
| Overall mortality | |||||
| No | 2 316 504 (99.2) | 66 902 (98.8) | 662 (97.5) | 33 510 (99.3) | 2 417 578 (99.2) |
| Yes | 19 119 (0.8) | 781 (1.2) | 17 (2.5) | 223 (0.7) | 20 140 (0.8) |
| Singleton | |||||
| No | 73 251 (3.1) | 6208 (9.2) | 47 (6.9) | 1466 (4.3) | 80 972 (3.3) |
| Yes | 2 262 372 (96.9) | 61 475 (90.8) | 632 (93.1) | 32 267 (95.6) | 2 356 746 (96.7) |
| Sex of offspring | |||||
| Male | 1 197 594 (51.3) | 35 207 (52.0) | 355 (52.3) | 17 451 (51.7) | 1 250 607 (51.3) |
| Female | 1 136 971 (48.7) | 32 447 (47.9) | 323 (47.6) | 16 273 (48.2) | 1 186 014 (48.7) |
| Unknown† | 1058 (0.05) | 29 (0.04) | - | 9 (0.03) | 1097 (0.0) |
| Maternal parity | |||||
| 1 | 1 039 964 (44.5) | 44 517 (65.8) | 476 (70.1) | 15 948 (47.3) | 1 100 905 (45.2) |
| 2 | 875 642 (37.5) | 15 697 (23.2) | 138 (20.3) | 11 407 (33.8) | 902 884 (37.0) |
| ≥3 | 420 017 (18.0) | 7469 (11.0) | 65 (9.6) | 6378 (18.9) | 433 929 (17.8) |
| Maternal age at childbirth (years) | |||||
| <20 | 51 443 (2.2) | 1953 (2.9) | 32 (4.7) | 297 (0.9) | 53 725 (2.2) |
| 20-24 | 396 615 (17.0) | 13 650 (20.2) | 142 (20.9) | 3573 (10.6) | 413 980 (17.0) |
| 25-29 | 849 734 (36.4) | 24 226 (35.8) | 225 (33.1) | 9836 (29.2) | 884 021 (36.3) |
| 30-34 | 712 235 (30.5) | 17 855 (26.4) | 183 (26.9) | 11 345 (33.6) | 741 618 (30.4) |
| ≥35 | 325 596 (13.9) | 9999 (14.8) | 97 (14.3) | 8682 (25.7) | 344 374 (14.1) |
| Maternal smoking during pregnancy‡ | |||||
| No | 1 311 663 (78.0) | 38 860 (81.3) | 403 (80.3) | 23 664 (84.3) | 1 374 590 (78.2) |
| Yes | 312190 (18.6) | 6742 (14.1) | 66 (13.1) | 3488 (12.4) | 322 486 (18.3) |
| Unknown | 57 337 (3.4) | 2207 (4.6) | 33 (6.6) | 922 (3.3) | 60 499 (3.4) |
| Maternal education at childbirth (years) | |||||
| Low | 598 048 (25.6) | 18 726 (27.7) | 194 (28.6) | 6697 (19.9) | 623 665 (25.6) |
| Medium | 1 000 397 (42.8) | 30 175 (44.6) | 301 (44.3) | 15 029 (44.6 | 1 045 902 (42.9) |
| High | 698 445 (29.9) | 18 013 (26.6) | 174 (25.6) | 11 620 (34.4) | 728 252 (29.9) |
| Unknown | 38 733 (1.7) | 769 (1.1) | 10 (1.5) | 387 (1.1) | 39 899 (1.6) |
| Maternal cohabitation at childbirth | |||||
| No | 1 069 832 (45.8) | 35 080 (51.8) | 374 (55.1) | 15 781 (46.8) | 1 121 067 (46.0) |
| Yes | 1 264 965 (54.2) | 32 585 (48.1) | 304 (44.8) | 17 943 (53.2) | 1 315 797 (54.0) |
| Unknown† | 826 (0.04) | 18 (0.03) | - | 9 (0.03) | 854 (0.0) |
| Parental medical history before birth of child | |||||
| Maternal CVD: | |||||
| No | 2 273 674 (97.3) | 65 420 (96.7) | 655 (96.5) | 31 926 (94.6) | 2 371 675 (97.3) |
| Yes | 61 949 (2.7) | 2263 (3.3) | 24 (3.5) | 1807 (5.4) | 66 043 (2.7) |
| Paternal CVD: | |||||
| No | 2 235 720 (95.7) | 64 380 (95.1) | 644 (94.9) | 31 852 (94.4) | 2 332 596 (95.7) |
| Yes | 77 595 (3.3) | 2399 (3.5) | 24 (3.5) | 1462 (4.3) | 81 480 (3.3) |
| Unknown | 22 308 (1.0) | 904 (1.3) | 11 (1.6) | 419 (1.2) | 23 642 (1.0) |
| Maternal diabetes mellitus | |||||
| No | 2 296 701 (98.3) | 64 769 (95.7) | 646 (95.1) | 31 332 (92.9) | 2 393 448 (98.2) |
| Yes | 38 922 (1.7) | 2914 (4.3) | 33 (4.9) | 2401 (7.1) | 44 270 (1.8) |
| Maternal income | |||||
| No income | 422 287 (18.8) | 10 407 (15.9) | 118 (18.0) | 5024 (15.2) | 437 836 (18.6) |
| Less than lower tertiles | 608 422 (27.0) | 18 700 (28.6) | 201 (30.7) | 8067 (24.4) | 635 390 (27.0) |
| Lower and higher tertiles | 609 553 (27.1) | 18 633 (28.5) | 173 (26.4) | 10 044 (30.4) | 638 403 (27.2) |
| More than higher tertiles | 611 273 (27.1) | 17 652 (27.0) | 162 (24.8) | 9903 (30.0) | 638 990 (27.2) |
| Unknown† | 433 (0.02) | - | - | - | 442 (0.0) |
| Pre-pregnancy maternal BMI§ | |||||
| <18.5 | 36 933 (4.3) | 567 (2.2) | 8 (3.1) | 396 (2.0) | 37 904 (4.2) |
| 18.5-24.9 | 521 731 (60.4) | 11 475 (45.4) | 120 (47.2) | 8405 (43.1) | 541 731 (59.6) |
| 25.0-29.9 | 174 229 (20.2) | 6442 (25.5) | 64 (25.2) | 4779 (24.5) | 185 514 (20.4) |
| ≥30.0 | 98 718 (11.4) | 6067 (24.0) | 47 (18.5) | 5360 (27.5) | 110 192 (12.1) |
| Unknown | 31 840 (3.7) | 747 (3.0) | 15 (5.9) | 556 (2.9) | 33 158 (3.6) |
BMI=body mass index; CVD=cardiovascular disease.
Includes all pre-eclampsia diagnoses (moderate, severe, HELLP (haemolysis, elevated liver enzymes, low platelets) syndrome, and unspecified).
<6 patients therefore not allowed to report owing to data protection in Denmark.
Data available in Denmark 1991-2018.
Data available in Denmark 2004-18.
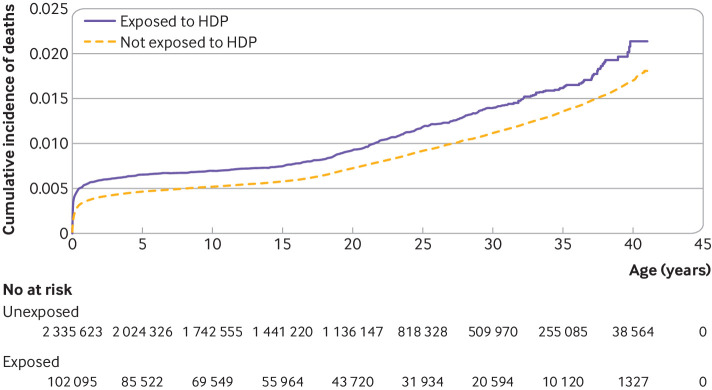
During the follow-up of up to 41 years (median 19.4 (interquartile range 9.7-28.7) years), deaths occurred in 781 (58.94 per 100 000 person years) offspring exposed to pre-eclampsia, 17 (133.73 per 100 000 person years) exposed to eclampsia, 223 (44.38 per 100 000 person years) exposed to hypertension, and 19 119 (41.99 per 100 000 person years) not exposed (table 2). The cumulative incidence of all cause mortality was higher in the exposed cohort (2.06%, 95% confidence interval 1.82% to 2.34%) compared with non-exposed cohort (1.69%, 1.65% to 1.73%), and the difference was 0.37% (0.11% to 0.64%) (fig 1). The population attributable fraction for maternal HDP on mortality in offspring was estimated as 1.09% (95% confidence interval 0.77% to 1.41%). Offspring exposed to maternal HDP had a 26% higher risk (hazard ratio 1.26, 95% confidence interval 1.18 to 1.34) of all cause mortality than non-exposed offspring (table 2). The associated increased risk for pre-eclampsia, eclampsia, and hypertension was 29% (1.29, 1.20 to 1.38), 188% (2.88, 1.79 to 4.63), and 12% (1.12, 0.98 to 1.28), respectively. For severity of pre-eclampsia, we observed a dose-response association: moderate 0.90 (0.82 to 0.99), severe 2.99 (2.66 to 3.37), and HELLP syndrome 4.79 (3.34 to 6.86).
Table 2.
Hazard ratios for associations between maternal hypertensive disorder of pregnancy (HDP) and all cause (main outcome) and cause specific (secondary outcomes) mortality in offspring
| Outcome and exposure | No of deaths | Follow-up (person years) | Rate (per 100 000 person years) | Crude hazard ratio (95% CI) | Adjusted hazard ratio* (95% CI) |
|---|---|---|---|---|---|
| Overall mortality | |||||
| No maternal HDP | 19 119 | 45 534 378.72 | 41.99 | 1.0 (Reference) | 1.0 (Reference) |
| Maternal HDP | 1021 | 1 840 136.80 | 55.49 | 1.28 (1.21 to 1.37) | 1.26 (1.18 to 1.34) |
| Pre-eclampsia or eclampsia | 798 | 1 337 690.23 | 59.66 | 1.42 (1.32 to 1.52) | 1.30 (1.21 to 1.40) |
| Pre-eclampsia | 781 | 1 324 978.47 | 58.94 | 1.40 (1.30 to 1.50) | 1.29 (1.20 to 1.38) |
| Severity of pre-eclampsia: | |||||
| Moderate | 423 | 1 013 731.05 | 41.73 | 1.00 (0.91 to 1.10) | 0.90 (0.82 to 0.99) |
| Severe | 279 | 208 803.94 | 133.62 | 3.10 (2.75 to 3.49) | 2.99 (2.66 to 3.37) |
| HELLP syndrome | 30 | 18 671.24 | 160.67 | 3.11 (2.18 to 4.45) | 4.79 (3.34 to 6.86) |
| Unspecified | 49 | 83 772.24 | 58.49 | 1.44 (1.09 to 1.91) | 1.30 (0.98 to 1.72) |
| Eclampsia | 17 | 12 711.76 | 133.73 | 3.17 (1.97 to 5.10) | 2.88 (1.79 to 4.63) |
| Hypertension: | |||||
| Overall | 223 | 502 446.57 | 44.38 | 0.96 (0.84 to 1.10) | 1.12 (0.98 to 1.28) |
| Pregestational | 81 | 181 836.48 | 44.55 | 0.90 (0.72 to 1.12) | 1.27 (1.02 to 1.58) |
| Gestational | 142 | 320 610.09 | 44.29 | 1.00 (0.85 to 1.18) | 1.06 (0.90 to 1.25) |
| Cause specific mortality | |||||
| Cardiovascular diseases: | |||||
| No HDP | 572 | 45 534 378.72 | 1.26 | 1.0 (reference) | 1.0 (reference) |
| HDP | 36 | 1 840 136.80 | 1.96 | 1.57 (1.12 to 2.20) | 1.52 (1.08 to 2.13) |
| Malignant neoplasms: | |||||
| No HDP | 1819 | 45 534 378.72 | 3.99 | 1.0 (reference) | 1.0 (reference) |
| HDP | 78 | 1 840 136.80 | 4.24 | 1.07 (0.85 to 1.34) | 1.04 (0.83 to 1.31) |
| Infectious and parasitic diseases: | |||||
| No HDP | 578 | 45 534 378.72 | 1.27 | 1.0 (reference) | 1.0 (reference) |
| HDP | 29 | 1 840 136.80 | 1.58 | 1.18 (0.81 to 1.72) | 1.26 (0.86 to 1.83) |
| Endocrine, nutritional, and metabolic diseases: | |||||
| No HDP | 498 | 45 534 378.72 | 1.09 | 1.0 (reference) | 1.0 (reference) |
| HDP | 30 | 1 840 136.80 | 1.63 | 1.45 (1.00 to 2.09) | 1.56 (1.08 to 2.27) |
| Mental and behavioural disorders: | |||||
| No HDP | 134 | 45 534 378.72 | 0.29 | 1.0 (reference) | 1.0 (reference) |
| HDP† | - | 1 840 136.80 | 0.11 | 0.38 (0.09 to 1.53) | 0.36 (0.09 to 1.48) |
| Nervous system and sense organs diseases: | |||||
| No HDP | 955 | 45 534 378.72 | 2.10 | 1.0 (reference) | 1.0 (reference) |
| HDP | 41 | 1 840 136.80 | 2.23 | 1.04 (0.76 to 1.42) | 1.01 (0.74 to 1.38) |
| Respiratory diseases: | |||||
| No HDP | 467 | 45 534 378.72 | 1.03 | 1.0 (reference) | 1.0 (reference) |
| HDP | 23 | 1 840 136.80 | 1.25 | 1.17 (0.77 to 1.77) | 1.22 (0.80 to 1.86) |
| Digestive diseases: | |||||
| No HDP | 210 | 45 534 378.72 | 0.46 | 1.0 (reference) | 1.0 (reference) |
| HDP | 17 | 1 840 136.80 | 0.92 | 1.99 (1.21 to 3.26) | 2.09 (1.27 to 3.43) |
| Musculoskeletal system or connective tissue diseases: | |||||
| No HDP | 51 | 45 534 378.72 | 0.11 | 1.0 (reference) | 1.0 (reference) |
| HDP† | - | 1 840 136.80 | 0.11 | 0.99 (0.24 to 4.07) | 1.08 (0.26 to 4.48) |
| Genitourinary diseases: | |||||
| No HDP | 34 | 45 534 378.72 | 0.07 | 1.0 (reference) | 1.0 (reference) |
| HDP† | - | 1 840 136.80 | 0.16 | 2.16 (0.66 to 7.03) | 2.34 (0.71 to 7.72) |
| Conditions originating in perinatal period: | |||||
| No HDP | 2768 | 45 534 378.72 | 6.08 | 1.0 (reference) | 1.0 (reference) |
| HDP | 310 | 1 840 136.80 | 16.85 | 2.57 (2.28 to 2.89) | 2.04 (1.81 to 2.30) |
| Congenital malformations and chromosomal abnormalities: | |||||
| No HDP | 3514 | 45 534 378.72 | 7.72 | 1.0 (reference) | 1.0 (reference) |
| HDP | 153 | 1 840 136.80 | 8.31 | 1.01 (0.86 to 1.19) | 1.04 (0.88 to 1.22) |
| External causes of morbidity and mortality: | |||||
| No HDP | 4345 | 45 534 378.72 | 9.54 | 1.0 (reference) | 1.0 (reference) |
| HDP | 184 | 1 840 136.80 | 10.00 | 1.07 (0.93 to 1.25) | 1.05 (0.90 to 1.21) |
| Other causes: | |||||
| No HDP | 3174 | 45 534 378.72 | 6.97 | 1.0 (reference) | 1.0 (reference) |
| HDP | 113 | 1 840 136.80 | 6.14 | 0.84 (0.70 to 1.02) | 0.94 (0.78 to 1.13) |
CI=confidence interval.
Offspring age as time scale, adjusted for calendar year; sex; singleton; parity; birth year of child; maternal age, smoking, cohabitation, country of origin, residence, education, income at birth, pre-pregnancy body mass index, and history of diabetes; and parental history of cardiovascular disease before birth of the child.
<6 cases therefore not allowed to report owing to data protection in Denmark.
Fig 1.
Cumulative incidence of all cause mortality among offspring prenatally exposed or not exposed to maternal hypertensive disorder of pregnancy (HDP)
The increased risk of cause specific mortality among offspring exposed to maternal HDP reached more than twofold for deaths from digestive diseases (2.09, 1.27 to 3.43) and from conditions originating in the perinatal period (2.04, 1.81 to 2.30), followed by 56% for deaths from endocrine, nutritional, and metabolic diseases (1.56, 1.08 to 2.27) and 52% for cardiovascular diseases (1.52, 1.08 to 2.13). We did not observe a significant association between maternal HDP and cancer mortality in offspring (1.04, 0.83 to 1.31) (table 2).
We observed a much higher increased risk of all cause mortality in offspring prenatally exposed to early onset pre-eclampsia (2.71, 2.45 to 3.00) compared with offspring exposed to late-onset pre-eclampsia (0.80, 0.72 to 0.89). When both timing of diagnosis and severity of maternal pre-eclampsia were considered, the strongest association was observed in offspring prenatally exposed to early onset and severe pre-eclampsia (6.06, 5.35 to 6.86) (table 3).
Table 3.
Risk of all cause mortality in offspring according to timing and severity of maternal pre-eclampsia*
| Variables for pre-eclampsia | No of deaths | Follow-up (person years) | Rate (per 100 000 person years) | Crude hazard ratio (95% CI) | Adjusted hazard ratio† (95% CI) |
|---|---|---|---|---|---|
| No HDP | 19 119 | 45 534 378.72 | 41.99 | 1.0 (reference) | 1.0 (Reference) |
| Timing: | |||||
| Late onset | 338 | 913 226.04 | 37.01 | 0.90 (0.81 to 1.01) | 0.80 (0.72 to 0.89) |
| Early onset | 394 | 327 980.19 | 120.13 | 2.63 (2.38 to 2.90) | 2.71 (2.45 to 3.00) |
| Severity: | |||||
| Moderate | 423 | 1 013 731.05 | 41.73 | 1.00 (0.91 to 1.10) | 0.90 (0.82 to 0.99) |
| Severe and HELLP syndrome | 309 | 227 475.18 | 135.84 | 3.10 (2.77 to 3.47) | 3.10 (2.77 to 3.47) |
| Timing and severity interaction: | |||||
| Late onset: | |||||
| Moderate | 287 | 786 320.06 | 36.50 | 0.90 (0.80 to 1.01) | 0.78 (0.70 to 0.88) |
| Severe and HELLP syndrome | 51 | 126 905.99 | 40.19 | 0.92 (0.70 to 1.22) | 0.90 (0.68 to 1.18) |
| Early onset: | |||||
| Moderate | 136 | 227 410.99 | 59.80 | 1.29 (1.09 to 1.53) | 1.33 (1.12 to 1.57) |
| Severe and HELLP syndrome | 258 | 100 569.19 | 256.54 | 5.79 (5.12 to 6.54) | 6.06 (5.35 to 6.86) |
CI=confidence interval; HDP=hypertensive disorder of pregnancy; HELLP=haemolysis, elevated liver enzymes, and low platelets.
Includes moderate pre-eclampsia, severe pre-eclampsia, and HELLP syndrome.
Offspring age as time scale, adjusted for calendar year; sex; singleton; parity; birth year of child; maternal age, smoking, cohabitation, country of origin, residence, education, income at birth, pre-pregnancy body mass index, and history of diabetes; and parental history of cardiovascular disease before birth of the child.
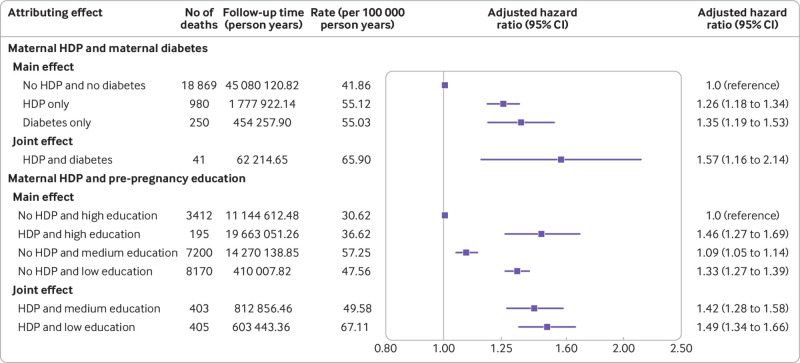
Moreover, we found that the offspring of mothers with both HDP and a history of diabetes had a 57% higher risk of all cause mortality (1.57, 1.16 to 2.14) compared with offspring of mothers with HDP alone (1.26, 1.18 to 1.34) (fig 2). Offspring of mothers with both HDP and low education had a 49% higher risk of all cause mortality (1.49, 1.34 to 1.66), and offspring of mothers with both HDP and medium education had a 42% higher risk of all cause mortality (1.42, 1.28 to 1.58) compared with offspring of mothers with no HDP and high education (fig 2).
Fig 2.
Joint effect of maternal hypertensive disorder of pregnancy (HDP) and maternal history of diabetes or maternal education before pregnancy on all cause mortality in offspring. *Offspring age as time scale, adjusted for calendar year; sex; singleton; parity; birth year of child; maternal age, smoking, cohabitation, country of origin, residence, education, income at birth, and pre-pregnancy body mass index; and parental cardiovascular disease before birth of child. †Offspring’s age as time scale, adjusted for calendar year; sex; singleton; parity; birth year of child; maternal age, smoking, cohabitation, country of origin, residence, income at birth, pre-pregnancy body mass index, and diabetes; and parental cardiovascular disease before birth of child. CI=confidence interval
The sibling matched analysis yielded results for all cause mortality similar to those of the main analyses in both half siblings and full siblings (see supplementary table S3). A weaker association between paternal hypertension and mortality in offspring (1.20, 0.94 to 1.54) was observed when using paternal hypertension before pregnancy as the control exposure (see supplementary table S4). When performing analyses stratified by small for gestational age, we found that the association between maternal HDP and all cause mortality was stronger in the offspring who were small for gestational age (1.53, 1.36 to 1.73) compared with other offspring (1.12, 1.04 to 1.21) (see supplementary table S5). Similar associations were observed across different sex and parity (see supplementary figure S5). Similar results to the primary analyses were observed in subgroup analyses additionally adjusted for paternal hypertension; and when restricting to offspring born after 1991, 1994, or 2004; restricting to singleton offspring; restricting to complete cases analyses; excluding individuals exposed to maternal pregestational hypertension; additionally adjusting for Charlson comorbidity index scores; including all live births in 1977-2018; and performing analysis using restricted cubic spline for continuous covariates (see supplementary tables S6 and S7). Results from analyses using different cutoff points of 35 weeks or 36 weeks for early onset and late onset pre-eclampsia were similar to those obtained in the primary analysis (see supplementary table S8). Allowing the effect of maternal HDP on mortality in offspring to vary with offspring age, we observed a peak of increased mortality risk shortly after birth among offspring exposed to maternal HDP, followed by a declining trend in childhood and adolescence. From early adulthood, mortality risk showed a slight upward trend in offspring exposed to maternal HDP compared with non-exposed offspring (see supplementary figure S6). A series of averaged hazard ratios from 0 to 41 years of follow-up suggested that HDP was associated with an increased risk of mortality in offspring (see supplementary table S9). Increased mortality risk was observed in offspring aged both 0-18 years and ≥19 years (see supplementary table S10). Supplementary figures S7 and S8 show cumulative incidence curves and incidence rates for cause specific mortality in offspring.
Discussion
In this population based cohort study among 2.4 million live births in Denmark, offspring prenatally exposed to maternal pre-eclampsia, eclampsia, and hypertension had 29%, 188%, and 12% increased risks of all cause mortality, respectively, which were independent of several maternal and offspring factors. The increased risks were also found in several cause specific deaths, including deaths from digestive diseases; conditions originating in the perinatal period; endocrine, nutritional, and metabolic diseases; and cardiovascular disease, but not from cancer. In particular, the observed risk was among the highest for offspring exposed to early onset and severe maternal pre-eclampsia. Furthermore, a strong association was also observed among offspring of mothers with HDP and a history of diabetes or low education level.
Comparison with other studies
Previous studies of maternal HDP and mortality in offspring mainly focused on the negative impacts of maternal HDP in the fetal and neonatal periods,3 25 26 27 with one exception in mid-adulthood,12 whereas no evidence has been presented for mortality in childhood, adolescence, and young adulthood. In our large study we explored the association between maternal HDP and mortality in offspring from birth to young adulthood and also investigated specific types of maternal HDP and cause specific mortality in offspring. Our findings are in line with those of the retrospective US study of 11 039 adults born from 1947 to 1967 and aged 49-69 years at the end of follow-up, which reported a higher risk of all cause mortality (hazard ratio 1.19, 95% confidence interval 1.07 to 1.32) and cardiovascular mortality (1.57, 1.16 to 2.12) in offspring exposed to maternal HDP.12 This study also showed an increased risk of cancer mortality (1.37, 1.04 to 1.81), which was different from our study, probably as a result of the heterogeneities of epidemiological profiles of cancer across different countries.12 28 Also, the US study suggested that the impact of HDP on cardiovascular mortality differed by sex and increased with higher birth order.12 Nevertheless, our study observed similar associations in both sexes and among different parity. The inconsistent results in sex and parity between the US study and our study could be related to differences in the study period, sample size, and population sociodemographic characteristics—in particular age distribution.12
The underlying physiological mechanisms between maternal HDP and mortality in offspring remain unclear, although some potential pathways have been proposed.8 9 10 29 30 31 32 33 34 35 Maternal HDP, a disease related to placental disorder, might result in placental lesions, DNA methylation in the placenta, and changes in gene expression.30 31 32 HDP related placental dysfunction is associated with impaired fetal development and could have a negative long term effect on health outcomes in offspring.33 34 Furthermore, the epigenetic changes resulting from exposure to maternal HDP in utero, or shared environment or lifestyle behavioural factors of offspring after delivery, may also play an important role.30 35 For the increased risk of cause specific mortality, numerous studies have found maternal HDP to be associated with a broad range of health outcomes in offspring beginning from birth, including cardiovascular, neurodevelopmental, mental, metabolic, and behavioural outcomes,7 36 37 38 39 which may further increase the risk of corresponding cause specific mortality over a long period in later life.
The present study also adds to existing evidence by providing the findings of severity and timing of diagnosis of pre-eclampsia. Offspring whose mothers had severe and early onset pre-eclampsia had more than six times the mortality risk than offspring whose mothers had no HDP, whereas moderate and late onset pre-eclampsia seemed to have a weak protective effect on all cause mortality in offspring. This indicates that severe HDP would substantially increase the risk of mortality in offspring and should be given more consideration in clinical evaluation and management during pregnancy.40 Neutrophil gene expression profiles in women who developed severe pre-eclampsia differed greatly from those who developed moderate pre-eclampsia,41 which may partly explain the different impact of moderate and severe pre-eclampsia on mortality in offspring. Besides, early onset pre-eclampsia is caused by defective placentation, whereas late onset pre-eclampsia is most often a result of the interaction between normal senescence of the placenta and maternal genetic susceptibility to cardiovascular and metabolic diseases.30 The distinct pathophysiological mechanisms between early and late onset pre-eclampsia might help to explain our findings.30 Further investigation is needed to examine the mechanisms of severity and timing of diagnosis of pre-eclampsia and the protective mechanisms of moderate and late onset pre-eclampsia on mortality in offspring.
We found that offspring of mothers with HDP and a history of maternal diabetes or lower maternal education before pregnancy are at higher risk of all cause mortality. A study showed that women with overt diabetes might have an altered risk of HDP, which could act together to increase the risk of mortality in offspring.13 Previous evidence has suggested a socioeconomic based disparity in the risk of maternal morbidities, including HDP.14 42 The risk of mortality in offspring might also vary by maternal education, a proxy of socioeconomic status, along with maternal HDP.14 16 The pathophysiology of the joint effect of maternal HDP and history of diabetes or lower education level on mortality in offspring remains unclear, and further research for the underlying mechanisms is warranted.
We observed that the effect of maternal HDP on all cause mortality in offspring was strongest shortly after birth, and maternal HDP was also associated with an increase in the risk of mortality in offspring in young adulthood. These findings highlight the importance of preventing and managing maternal HDP in women of childbearing age—for example, tightly controlling for maternal blood pressure during pregnancy and treatment for blood pressure in a monitored setting for consistently high readings.43 Also, offspring prenatally exposed to maternal HDP require early evaluation and clinical attention to reduce their risk of mortality, particularly infants born to mothers with maternal HDP as they are in a vulnerable period with a greater risk of mortality during infancy.44 With the upward trend of mortality risk observed starting from early adulthood, offspring born to mothers with HDP should also be more health conscious from an early age, as death could result from long term health impairments from prenatal exposure to maternal conditions such as HDP.45
Strengths and limitations of this study
This study has several strengths. Firstly, our study was based on a large population of more than two million individuals, which allowed us to examine the association between most types of HDP and all cause and 13 types of cause specific mortality in offspring. Secondly, this prospective cohort from Danish national registers comprised almost all liveborn individuals in Denmark with complete follow-up over the study period, which helps to minimise recall bias and selection bias. Thirdly, we performed sibling analysis to consider the effect of genetic factors and some unmeasured family factors, such as diet or lifestyle. Fourthly, the validity of diagnoses in Danish registers was high for exposure to maternal HDP and outcome cause of death in our study.46
Our study also has several limitations. Firstly, we could not rule out the influence of some unmeasured confounders, such as smoking, alcohol use, poor diet quality, obesity, and sedentary lifestyle in offspring, which should be considered as time varying covariates. However, the positive and consistent results from the sibling analysis and subgroup analysis adjusted for many important potential confounders lend some support to the findings of our study.47 48 In addition, as shown in supplementary figure S2, some of these unmeasured confounders would be present in the same confounding pathway with maternal characteristics, such as maternal income, maternal education, and maternal BMI. The influence of these unmeasured confounders can be partially controlled by adjusting for these maternal factors. Secondly, we did not include stillbirths or early pregnancy losses in our study, which potentially could result in selection bias. However, the risk of fetal deaths in pregnancies with HDP was higher than those in normotensive pregnancies, which would lead to underestimated hazard ratios of mortality in offspring when considering stillbirths and early pregnancy losses.49 Thirdly, Denmark has universal health coverage with high quality health services, which might limit the generalisability of our findings.17 Fourthly, we could have missed some individuals with mild HDP who were identified only in the primary care system but not registered in the Danish National Patient Register.50 However, a validity study that evaluated diagnoses of HDP in this register from 1998 to 2000 indicated that the validity of overall HDP is high, especially for pre-eclampsia.50 In addition, results from analysis excluding individuals exposed to maternal pregestational hypertension remained similar to the overall estimates in main analysis. In any case, mild HDP misclassified as no HDP would mostly lead to an effect estimate biased towards the null.
Conclusions
This large cohort study provides strong evidence that maternal HDP, especially severe pre-eclampsia, HELLP syndrome, and eclampsia, are associated with increased risks of all cause and some cause specific mortality in offspring, including deaths from cardiovascular diseases, digestive diseases, conditions originating in the perinatal period, and endocrine, nutritional, and metabolic diseases from birth to young adulthood.
What is already known on this topic
Hypertensive disorder of pregnancy (HDP) is one of the leading causes of maternal and fetal morbidity and mortality
Maternal HDP has been associated with several conditions in offspring in later life, such as metabolic syndrome, immune diseases, and neurodevelopmental and psychiatric disorders
Evidence of maternal HDP on long term mortality in offspring from birth to adolescence and beyond is lacking
What this study adds
Maternal HDP, especially severe pre-eclampsia, HELLP (haemolysis, elevated liver enzymes, and low platelets) syndrome, and eclampsia, is associated with increased risk of all cause and cause specific mortality in offspring from birth to young adulthood
Deaths include those from conditions originating in the perinatal period, cardiovascular diseases, digestive diseases, and endocrine, nutritional, and metabolic diseases
Web extra.
Extra material supplied by authors
Supplementary information: appendices S1-S3, tables S1-S10, and figures S1-S8
Contributors: YY (yu@fudan.edu.cn), GQ (gyqin@fudan.edu.cn), and JL (jl@clin.au.dk) are corresponding authors and senior authors who contributed equally to this study. CH and KW are joint first authors with equal contribution. JL and PMYL had full access to all the data in this study and take full responsibility for the integrity of the data and the accuracy of the data analysis. YY, GQ, and JL conceived and designed the study. CH, JL, and PMYL undertook the statistical analysis. CH and KW drafted the manuscript. YY, GQ, and JL are the guarantors. All authors provided critical input to the analyses, interpreted the data, and revised the manuscript critically. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by the National Natural Science Foundation of China (No 82173612 and 82073570), Shanghai Rising-Star Program (21QA1401300), Shanghai Municipal Natural Science Foundation (22ZR1414900), Shanghai Municipal Science and Technology Major Project (ZD2021CY001), Independent Research Fund Denmark (grant No DFF-6110-00019B, 9039-00010B, and 1030-00012B), Nordic Cancer Union (R275-A15770 and R278-A15877), Karen Elise Jensens Fond (2016), and Novo Nordisk Fonden (NNF18OC0052029). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Natural Science Foundation of China, Shanghai Rising-Star Program, Shanghai Municipal Natural Science Foundation, Shanghai Municipal Science and Technology Major Project, Independent Research Fund Denmark, Nordic Cancer Union, Karen Elise Jensens Fond, and Novo Nordisk Fonden for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead authors (YY, GQ, and JL) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: We plan to disseminate the results of this study to the general public mainly through press releases and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the Data Protection Agency (record No 2013-41-2569). By Danish law, no informed consent is required for a register based study based on anonymised data.
Data availability statement
No additional data available.
References
- 1. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet 2021;398:341-54. 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- 2. Behrens I, Basit S, Melbye M, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ 2017;358:j3078. 10.1136/bmj.j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrazzani S, Luciano R, Garofalo S, et al. Neonatal outcome in hypertensive disorders of pregnancy. Early Hum Dev 2011;87:445-9. 10.1016/j.earlhumdev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 4. Li F, Wang T, Chen L, Zhang S, Chen L, Qin J. Adverse pregnancy outcomes among mothers with hypertensive disorders in pregnancy: A meta-analysis of cohort studies. Pregnancy Hypertens 2021;24:107-17. 10.1016/j.preghy.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 5. Dachew BA, Mamun A, Maravilla JC, Alati R. Association between hypertensive disorders of pregnancy and the development of offspring mental and behavioural problems: A systematic review and meta-analysis. Psychiatry Res 2018;260:458-67. 10.1016/j.psychres.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 6. Huang C, Li J, Qin G, et al. Maternal hypertensive disorder of pregnancy and offspring early-onset cardiovascular disease in childhood, adolescence, and young adulthood: A national population-based cohort study. PLoS Med 2021;18:e1003805. 10.1371/journal.pmed.1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinheiro TV, Brunetto S, Ramos JG, Bernardi JR, Goldani MZ. Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J Dev Orig Health Dis 2016;7:391-407. 10.1017/S2040174416000209. [DOI] [PubMed] [Google Scholar]
- 8. Barker DJ. Fetal origins of coronary heart disease. BMJ 1995;311:171-4. 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barker DJ. In utero programming of cardiovascular disease. Theriogenology 2000;53:555-74. 10.1016/S0093-691X(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 10. Barker DJ, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2006;2:700-7. 10.1038/ncpneph0344. [DOI] [PubMed] [Google Scholar]
- 11. Davis EF, Newton L, Lewandowski AJ, et al. Pre-eclampsia and offspring cardiovascular health: mechanistic insights from experimental studies. Clin Sci (Lond) 2012;123:53-72. 10.1042/CS20110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammad IA, Meeks H, Fraser A, et al. Risks of cause-specific mortality in offspring of pregnancies complicated by hypertensive disease of pregnancy. Am J Obstet Gynecol 2020;222:75.e1-9. 10.1016/j.ajog.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 13. Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012;8:639-49. 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sole KB, Staff AC, Laine K. The association of maternal country of birth and education with hypertensive disorders of pregnancy: A population-based study of 960 516 deliveries in Norway. Acta Obstet Gynecol Scand 2018;97:1237-47. 10.1111/aogs.13393. [DOI] [PubMed] [Google Scholar]
- 15. Knorr S, Stochholm K, Vlachová Z, et al. Multisystem Morbidity and Mortality in Offspring of Women With Type 1 Diabetes (the EPICOM Study): A Register-Based Prospective Cohort Study. Diabetes Care 2015;38:821-6. 10.2337/dc14-2907. [DOI] [PubMed] [Google Scholar]
- 16. Yu Y, Liew Z, Wang A, et al. Mediating roles of preterm birth and restricted fetal growth in the relationship between maternal education and infant mortality: A Danish population-based cohort study. PLoS Med 2019;16:e1002831. 10.1371/journal.pmed.1002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563-91. 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541-9. 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 19. Gissler M, Mohangoo AD, Blondel B, et al. Euro-Peristat Group . Perinatal health monitoring in Europe: results from the EURO-PERISTAT project. Inform Health Soc Care 2010;35:64-79. 10.3109/17538157.2010.492923. [DOI] [PubMed] [Google Scholar]
- 20. Arendt LH, Henriksen TB, Lindhard MS, Parner ET, Olsen J, Ramlau-Hansen CH. Hypertensive Disorders of Pregnancy and Genital Anomalies in Boys: A Danish Nationwide Cohort Study. Epidemiology 2018;29:739-48. 10.1097/EDE.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 21. Mammaro A, Carrara S, Cavaliere A, et al. Hypertensive disorders of pregnancy. J Prenat Med 2009;3:1-5. [PMC free article] [PubMed] [Google Scholar]
- 22. van der Merwe JL, Hall DR, Wright C, Schubert P, Grové D. Are early and late preeclampsia distinct subclasses of the disease--what does the placenta reveal? Hypertens Pregnancy 2010;29:457-67. 10.3109/10641950903572282. [DOI] [PubMed] [Google Scholar]
- 23. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 24. Hernán MA. The hazards of hazard ratios. Epidemiology 2010;21:13-5. 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura N, Ushida T, Nakatochi M, et al. Neonatal Research Network of Japan . Mortality and neurological outcomes in extremely and very preterm infants born to mothers with hypertensive disorders of pregnancy. Sci Rep 2021;11:1729. 10.1038/s41598-021-81292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haavaldsen C, Strøm-Roum EM, Eskild A. Temporal changes in fetal death risk in pregnancies with preeclampsia: Does offspring birthweight matter? A population study. Eur J Obstet Gynecol Reprod Biol X 2019;2:100009-09. 10.1016/j.eurox.2019.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmad AS, Samuelsen SO. Hypertensive disorders in pregnancy and fetal death at different gestational lengths: a population study of 2 121 371 pregnancies. BJOG 2012;119:1521-8. 10.1111/j.1471-0528.2012.03460.x. [DOI] [PubMed] [Google Scholar]
- 28. Fitzmaurice C, Abate D, Abbasi N, et al. Global Burden of Disease Cancer Collaboration . Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989;298:564-7. 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 2019;366:l2381. 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- 31. Yung HW, Atkinson D, Campion-Smith T, Olovsson M, Charnock-Jones DS, Burton GJ. Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J Pathol 2014;234:262-76. 10.1002/path.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herzog EM, Eggink AJ, Willemsen SP, et al. Early- and late-onset preeclampsia and the tissue-specific epigenome of the placenta and newborn. Placenta 2017;58:122-32. 10.1016/j.placenta.2017.08.070. [DOI] [PubMed] [Google Scholar]
- 33. Ganguly E, Hula N, Spaans F, Cooke CM, Davidge ST. Placenta-targeted treatment strategies: An opportunity to impact fetal development and improve offspring health later in life. Pharmacol Res 2020;157:104836. 10.1016/j.phrs.2020.104836. [DOI] [PubMed] [Google Scholar]
- 34. Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 2019;15:275-89. 10.1038/s41581-019-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Davis EF, Newton L, Lewandowski AJ, et al. Pre-eclampsia and offspring cardiovascular health: mechanistic insights from experimental studies. Clin Sci (Lond) 2012;123:53-72. 10.1042/CS20110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maher GM, O’Keeffe GW, Kearney PM, et al. Association of Hypertensive Disorders of Pregnancy With Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018;75:809-19. 10.1001/jamapsychiatry.2018.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lahti-Pulkkinen M, Girchenko P, Tuovinen S, et al. Maternal Hypertensive Pregnancy Disorders and Mental Disorders in Children. Hypertension 2020;75:1429-38. 10.1161/HYPERTENSIONAHA.119.14140. [DOI] [PubMed] [Google Scholar]
- 38. Lazdam M, de la Horra A, Pitcher A, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension 2010;56:159-65. 10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 39. Lawlor DA, Macdonald-Wallis C, Fraser A, et al. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J 2012;33:335-45. 10.1093/eurheartj/ehr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sibai BM, Publications Committee, Society for Maternal-Fetal Medicine . Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol 2011;205:191-8. 10.1016/j.ajog.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 41. Walsh SW, Al Dulaimi M, Archer KJ, Strauss JF, 3rd. Patterns of Maternal Neutrophil Gene Expression at 30 Weeks of Gestation, but Not DNA Methylation, Distinguish Mild from Severe Preeclampsia. Int J Mol Sci 2021;22:12876. 10.3390/ijms222312876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Choe SA, Min HS, Cho SI. The income-based disparities in preeclampsia and postpartum hemorrhage: a study of the Korean National Health Insurance cohort data from 2002 to 2013. Springerplus 2016;5:895. 10.1186/s40064-016-2620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown MA, Magee LA, Kenny LC, et al. International Society for the Study of Hypertension in Pregnancy (ISSHP) . Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018;72:24-43. 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 44. Herschkowitz N, Kagan J, Zilles K. Neurobiological bases of behavioral development in the first year. Neuropediatrics 1997;28:296-306. 10.1055/s-2007-973720. [DOI] [PubMed] [Google Scholar]
- 45. Généreux P, Piazza N, Alu MC, et al. VARC-3 WRITING COMMITTEE . Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J 2021;42:1825-57. 10.1093/eurheartj/ehaa799. [DOI] [PubMed] [Google Scholar]
- 46. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449-90. 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pan A, Wang Y, Talaei M, Hu FB. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation 2015;132:1795-804. 10.1161/CIRCULATIONAHA.115.017926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 2019;366:l4570. 10.1136/bmj.l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahmad AS, Samuelsen SO. Hypertensive disorders in pregnancy and fetal death at different gestational lengths: a population study of 2 121 371 pregnancies. BJOG 2012;119:1521-8. 10.1111/j.1471-0528.2012.03460.x. [DOI] [PubMed] [Google Scholar]
- 50. Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. Am J Epidemiol 2007;166:117-24. 10.1093/aje/kwm139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: appendices S1-S3, tables S1-S10, and figures S1-S8
Data Availability Statement
No additional data available.