Highlights
-
•
The first study describing the cross-reactivity of antibodies elicited by a Chinese smallpox vaccine against MPXV.
-
•
Mice immunized with vaccinia virus Tiantan strain yield antibodies cross-reactive with MPXV protective antigens.
-
•
Cross-reactivities of VTT-elicited antibodies against monkeypox protective antigens are ranging from 33% to 94%.
Dear Editor,
Since the global eradication of smallpox in the 1980s, monkeypox has become the most severe infectious disease caused by pathogenic orthopoxvirus (OPXV) infection. Monkeypox virus (MPXV) is the causative pathogen of monkeypox and was classified as a category of human infectious pathogen by the Ministry of Health of the People's Republic of China in 2006. Monkeypox has traditionally been endemic in West and Central Africa since it was discovered nearly 70 years ago (Breman et al., 1980). However, beginning in early May 2022, a sudden outbreak of monkeypox has taken place and remains ongoing in more than 100 nonendemic countries and territories located at Europe, Americas, and Asia (https://worldhealthorg.shinyapps.io/mpx_global/). This is the first time that human infection of MPXV has been concurrently reported in widely disparate geographical areas (https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html). The World Health Organization therefore declared that the global monkeypox outbreak represented a public health emergency of international concern on July 23, 2022 (https://www.who.int/news-room/speeches/item/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022).
Several smallpox vaccines have been shown to be effective in preventing MPXV infection (Earl et al., 2004; Edghill-Smith et al., 2005; Saijo et al., 2006; Marriott et al., 2008). In China, the vaccinia virus (VACV) Tiantan strain (VTT) has historically served as a smallpox vaccine and played an essential role in the eradication of smallpox (Fang et al., 2005; Parrino and Graham, 2006). To prevent possible monkeypox outbreaks in China, one of the most urgent tasks is to determine the protective efficacy of VTT immunization against MPXV infection. However, since monkeypox has never emerged in China, vaccine evaluation through normal methods is hampered by the lack of live MPXV and an animal infection model. Therefore, an alternative evaluation method must be employed as an emergency solution.
OPXVs possess two major types of infectious particles, designated mature virions (MVs) and enveloped virions (EVs) (Moss, 2012). MVs are the basic infectious viral particles and contain all viral proteins required for viral cell entry. EVs are derived from MVs and enveloped with an additional lipoprotein membrane responsible for cell-to-cell viral spread (Pickup, 2015). Several surface proteins of MVs and EVs have been demonstrated to be essential for OPXV infectivity and serve as major targets for protective immunity (Moss, 2011). These protective antigens (PAs) include MV surface proteins A27 (Kaever et al., 2016), L1 (Kaever et al., 2014), D8 (Sakhatskyy et al., 2006), and H3 (McCausland et al., 2010), as well as EV surface proteins B5 (Benhnia et al., 2009) and A33 (Matho et al., 2015). Monoclonal antibodies against these PAs were shown to effectively cross-neutralize various OPXVs, including MPXV, and their combination could successfully protect animal models from lethal challenge with vaccinia virus (Gilchuk et al., 2016). In addition to the aforementioned PAs, other antigens including A4, A56, and F9 were also reported to induce protective immunity against MPXV challenge (Hirao et al., 2011).
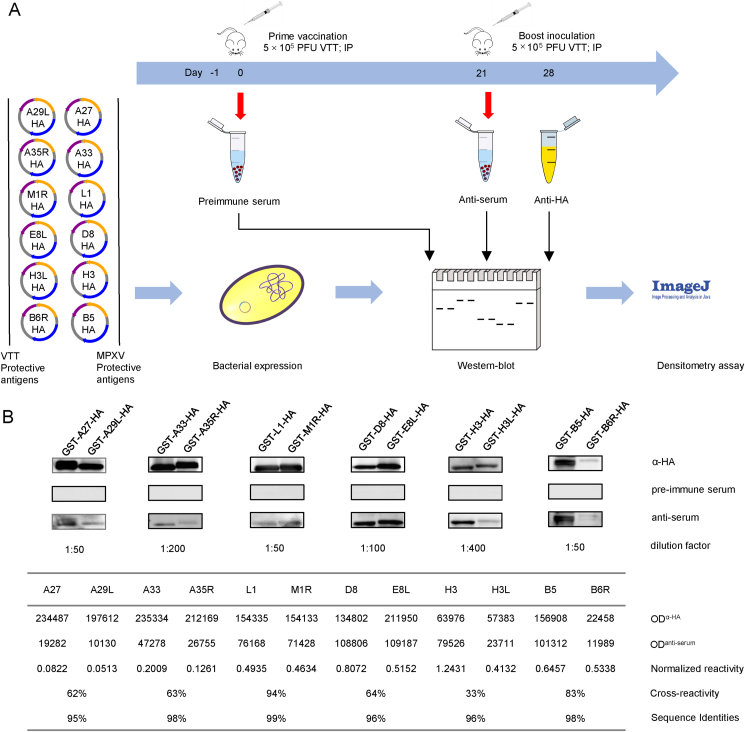
Since antibodies against the aforementioned PAs can provide sufficient cross-protection to various OPXV infections, we aimed to investigate whether VTT immunization could elicit antibodies that cross-react with orthologous PAs of MPXV. The experimental design is diagramed in Fig. 1A. Six-week-old female BALB/c mice (n = 6) were intraperitoneally vaccinated with VTT of 5 × 105 plaque forming units (PFUs). At 21 days after the prime vaccination, mice received a boost inoculation. Mice were euthanized at Day 28 post vaccination and their sera were collected and pooled.
Fig. 1.
VTT-elicited mouse anti-serum cross-reacts with MPXV protective antigens. A Diagram of the experiment. Mice were immunized with VTT, and the sera were collected. Protective antigens (PAs) of VTT and MPXV were expressed as HA-fusion proteins and were subjected to a Western-blot assay using either anti-HA or anti-serum. The cross-reactivity of VTT-elicited anti-serum was calculated through densitometry of PA immunoblots. B Western-blot assay of MPXV and VTT PAs. The immune-blots of VTT and MPXV PAs were probed with anti-HA antibody, pre-immune serum, and VTT-elicited anti-serum. The dilution factors of the anti-serum are indicated in the figure. The optical density (OD) of each immunoblot was determined by ImageJ software. The normalized reactivity was calculated as the ratio of OD of each PA blot probed with anti-serum to that probed with anti-HA antibody. The cross-reactivity of VTT-elicited anti-serum against MPXV PAs is presented as the percentage of the normalized reactivity of MPXV PA to that of the VTT ortholog. The amino acid sequence identities of each pair of orthologous PAs were calculated by the Blast2seq online program.
Six MPXV PA coding sequences, including A29L (A27 ortholog), M1R (L1 ortholog), E8L (D8 ortholog), H3L (H3 ortholog), B6R (B5 ortholog), and A35R (A33 ortholog), were chemically synthesized with an HA tag at their 3′-end. Six VTT PA coding sequences were amplified by PCR. These sequences were then cloned into the pGEX-KG vector for expression as glutathione S-transferase (GST) fusion proteins. The resulting plasmids were transformed into BL21 (DE3) E. coli cells, and GST fusion proteins were expressed after isopropyl-β-D-thio-galactoside (IPTG) induction. Cleared cell lysates were collected, and total protein was subjected to a Western-blot assay (Fig. 1A). All the orthologous PAs of MPXV and VTT were loaded in pairs at comparable levels (except for B5 and B6R), as the immunoblots probed with anti-HA antibody were similar in terms of optical density (Fig. 1B). In parallel, the same amounts of total protein were probed with a serum mixture collected from pre-immune and immunized mice. Both VTT and MPXV PAs could be specifically recognized by VTT-elicited anti-serum but not by pre-immune serum (Fig. 1B). A densitometry assay using ImageJ software (v1.8.0_172; National Institutes of Health, USA) was employed to quantitatively analyze the immunoblots. The optical density of each PA blot probed with anti-serum was compared to that probed with anti-HA, thus generating a normalized reactivity value for each PA. As expected, VTT-elicited anti-serum showed higher normalized reactivity against VTT PAs than their MPXV orthologs (Fig. 1B). To further quantify the cross-reactivity of VTT-elicited anti-serum against MPXV PAs, the percentage of the normalized reactivity of MPXV PA to that of the VTT ortholog was calculated (Fig. 1B). Among the six MPXV PAs, M1R possessed the highest cross-reactivity of 94%, while H3L showed the lowest cross-reactivity of 33% against VTT-elicited anti-serum (Fig. 1B). The cross-reactivity level appeared to be loosely correlated with the amino acid sequence identities between the MPXV PAs and their VTT orthologs (Fig. 1B). The detailed sequence alignment of PAs were shown in Supplementary Fig. S1.
In summary, the current study demonstrated that VTT-elicited antibodies could effectively cross-react with MPXV PAs, implying that this traditional smallpox vaccine could still be valuable in MPXV prevention. Among the six MPXV PAs, M1R possessed the highest cross-reactivity of 94%, while H3L showed the lowest cross-reactivity of 33% against VTT-elicited anti-serum. The highest cross-reactivity of M1R could be attributed to its B-cell epitopes, including region 69–91 aa and 137–155 aa, which are identical to those of L1 (Heraud et al., 2006). In the case of H3L, it was reported that the substitution of residue 233A of H3 could lead to disruption of B-cell epitope to be unrecognizable by anti-VACV polyclonal antibodies (Khlusevich et al., 2022). After comparing the amino acid sequences between H3 and H3L, we found the 233rd residue of H3L is mutated from alanine to serine, which is probably responsible for the poor cross-reactivity of H3L against VTT-elicited anti-serum.
Currently, the spread of MPXV has become a global concern. According to previous studies, the VACV-based smallpox vaccine has a protection rate of about 85% in preventing monkeypox (https://www.who.int/news-room/fact-sheets/detail/monkeypox). These monkeypox-protective vaccines include MVA (Earl et al., 2004), LC16m8 (Saijo et al., 2006), ACAM2000 (Golden et al., 2012), and DryVAX (Edghill-Smith et al., 2005). For the current wave of MPXV, Syed Faraz Ahmed et al. reported that two commercially available smallpox vaccines JYNNEOS and ACAM2000 were expected to be capable of eliciting highly cross-reactive immunity (Ahmed et al., 2022). However, whether the Chinese smallpox vaccine VTT could function as efficiently as MVA-BN and ACAM200 remains undetermined.
Our research demonstrated that VTT-elicited antibodies could effectively cross-react with MPXV PAs. However, more experiments using live MPXV and animal infection models are required to determine whether these antibodies can neutralize and provide effective protection against MPXV infection. During the revision preparation, a monkeypox patient has been reported in Chongqing, China (https://weekly.chinacdc.cn/en/article/doi/10.46234/ccdcw2022.175). It is therefore of great emergence to investigate the efficiency of VTT in preventing MPXV.
Footnotes
This work was supported by the National Science and Technology Major Project of China (2018ZX10711001-006). The authors declare that they have no conflicts of interest. This study was approved by the ethical committees of the Wuhan Institute of Virology (WHIV) and the Chinese Academy of Sciences (CAS) (serial numbers WIVA02202201). Mice were raised at animal experiment center of Wuhan Institute of Virology, under specific pathogen-free conditions. All procedures were conducted in accordance with the “Guiding Principles in the Care and Use of Animals” (China) and requirements of ABSL-2 laboratory.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2022.10.004.
Contributor Information
Xinwen Chen, Email: chen_xinwen@gzlab.ac.cn.
Yun Wang, Email: wangyun@wh.iov.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- Ahmed S.F., Sohail M.S., Quadeer A.A., McKay M.R. Vaccinia-virus-based vaccines are expected to elicit highly cross-reactive immunity to the 2022 monkeypox virus. Viruses. 2022;14:1960. doi: 10.3390/v14091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia M.R., McCausland M.M., Laudenslager J., Granger S.W., Rickert S., Koriazova L., Tahara T., Kubo R.T., Kato S., Crotty S. Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5. J. Virol. 2009;83:12355–12367. doi: 10.1128/JVI.01593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman J.G., Kalisa R., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull. World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., Eisenberg R.J., Hartmann C.J., Jackson D.L., Kulesh D.A., Martinez M.J., Miller D.M., Mucker E.M., Shamblin J.D., Zwiers S.H., Huggins J.W., Jahrling P.B., Moss B. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., Reimann K.A., Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Fang Q., Yang L., Zhu W., Liu L., Wang H., Yu W., Xiao G., Tien P., Zhang L., Chen Z. Host range, growth property, and virulence of the smallpox vaccine: vaccinia virus Tian Tan strain. Virology. 2005;335:242–251. doi: 10.1016/j.virol.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Gilchuk I., Gilchuk P., Sapparapu G., Lampley R., Singh V., Kose N., Blum D.L., Hughes L.J., Satheshkumar P.S., Townsend M.B., Kondas A.V., Reed Z., Weiner Z., Olson V.A., Hammarlund E., Raue H.P., Slifka M.K., Slaughter J.C., Graham B.S., Edwards K.M., Eisenberg R.J., Cohen G.H., Joyce S., Crowe J.E., Jr. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016;167:684–694 e689. doi: 10.1016/j.cell.2016.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J.W., Josleyn M., Mucker E.M., Hung C.F., Loudon P.T., Wu T.C., Hooper J.W. Side-by-side comparison of gene-based smallpox vaccine with MVA in nonhuman primates. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heraud J.M., Edghill-Smith Y., Ayala V., Kalisz I., Parrino J., Kalyanaraman V.S., Manischewitz J., King L.R., Hryniewicz A., Trindade C.J., Hassett M., Tsai W.P., Venzon D., Nalca A., Vaccari M., Silvera P., Bray M., Graham B.S., Golding H., Hooper J.W., Franchini G. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 2006;177:2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- Hirao L.A., Draghia-Akli R., Prigge J.T., Yang M., Satishchandran A., Wu L., Hammarlund E., Khan A.S., Babas T., Rhodes L., Silvera P., Slifka M., Sardesai N.Y., Weiner D.B. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 2011;203:95–102. doi: 10.1093/infdis/jiq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaever T., Matho M.H., Meng X., Crickard L., Schlossman A., Xiang Y., Crotty S., Peters B., Zajonc D.M. Linear epitopes in vaccinia virus A27 are targets of protective antibodies induced by vaccination against smallpox. J. Virol. 2016;90:4334–4345. doi: 10.1128/JVI.02878-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaever T., Meng X., Matho M.H., Schlossman A., Li S., Sela-Culang I., Ofran Y., Buller M., Crump R.W., Parker S., Frazier A., Crotty S., Zajonc D.M., Peters B., Xiang Y. Potent neutralization of vaccinia virus by divergent murine antibodies targeting a common site of vulnerability in L1 protein. J. Virol. 2014;88:11339–11355. doi: 10.1128/JVI.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khlusevich Y., Matveev A., Emelyanova L., Goncharova E., Golosova N., Pereverzev I., Tikunova N. New p35 (H3L) epitope involved in vaccinia virus neutralization and its deimmunization. Viruses. 2022;14:1224. doi: 10.3390/v14061224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott K.A., Parkinson C.V., Morefield S.I., Davenport R., Nichols R., Monath T.P. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26:581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matho M.H., Schlossman A., Meng X., Benhnia M.R., Kaever T., Buller M., Doronin K., Parker S., Peters B., Crotty S., Xiang Y., Zajonc D.M. Structural and functional characterization of anti-A33 antibodies reveal a potent cross-species orthopoxviruses neutralizer. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCausland M.M., Benhnia M.R., Crickard L., Laudenslager J., Granger S.W., Tahara T., Kubo R., Koriazova L., Kato S., Crotty S. Combination therapy of vaccinia virus infection with human anti-H3 and anti-B5 monoclonal antibodies in a small animal model. Antivir. Ther. 2010;15:661–675. doi: 10.3851/IMP1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Smallpox vaccines: targets of protective immunity. Immunol. Rev. 2011;239:8–26. doi: 10.1111/j.1600-065X.2010.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxvirus cell entry: how many proteins does it take? Viruses. 2012;4:688–707. doi: 10.3390/v4050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrino J., Graham B.S. Smallpox vaccines: past, present, and future. J. Allergy Clin. Immunol. 2006;118:1320–1326. doi: 10.1016/j.jaci.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup D.J. Extracellular virions: the advance guard of poxvirus infections. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo M., Ami Y., Suzaki Y., Nagata N., Iwata N., Hasegawa H., Ogata M., Fukushi S., Mizutani T., Sata T., Kurata T., Kurane I., Morikawa S. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J. Virol. 2006;80:5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhatskyy P., Wang S., Chou T.H., Lu S. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology. 2006;355:164–174. doi: 10.1016/j.virol.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.