Abstract
Background
In this study, we aimed to evaluate the effects of the transition from the 6th edition of the Tumor, Node, Metastasis (TNM) staging system to the 7th edition, and from the 7th edition to the 8th edition by comparing the stage migrations. We also aimed to externally validate the 8th edition of the TNM staging system.
Methods
Between September 2005 and June 2015, a total of 1,077 patients (986 males, 91 females; mean age: 59.6±8.3 years; range, 35 to 84 years) with non-small cell lung cancer who underwent lung resection were retrospectively analyzed. We re-staged patients according to 6th, 7th, and 8th TNM staging and compared the stage migrations of cases among the three staging systems.
Results
Stage migration in the transition to the 7th edition of the TNM staging system was observed in 368 (34.1%) patients whereas it was observed in 541 (50.2%) patients in the transition to the 8th edition (p<0.001). The rate of upstaging in transition to the 7th edition staging system was 50.2% (n=185), whereas it was 98.1% (n=531) for the transition to the 8th edition (p<0.001). The survival rates of Stages 1B, 2B and 3A increased with transition to the 7th edition and the survival rates of Stages 1B, 2A, 2B, 3A, and 3B increased with the transition to the 8th edition. The best stratification in the survival curves in the 6th edition was between 1B-1A and 3B-3A. In the 7th edition, it occurred between 1B-1A, 3A-2B and 3B-3A and, in the 8th edition, between 1B-1A and 3B-3A.
Conclusion
Stratification according to the 7th edition showed better prognostic validity compared to the 6th edition; and that of the 8th edition was better compared to the 7th edition.
Keywords: 8th edition of TNM, external validation, non-small cell lung cancer, TNM classification
Introduction
Despite the continuous development in treatment strategies, lung cancer is still the most common cause of cancer deaths worldwide.[1] Asuccessful staging system is needed to determine appropriate treatment strategies and to predict the patient's prognosis. The primary goal of the staging systems is to provide survival curves that better illustrate the prognosis of patients.
Following the publication of the first edition of the Tumor, Node, Metastasis (TNM) staging system by the American Joint Committee in 1977, the first major revision of the system was made in 1997.[2,3] The major amendment was the reorganization of Stages 1 and 2 as 1A-1B and 2A-2B, respectively. Furthermore, while a satellite nodule(s) located in the same lobe was defined as T4, nodule(s) in the different lobes was classified as M1. In the 6th edition of t he TNM staging (6th TNM) published in 2002, no amendments were made regarding the staging of lung cancer.[4] The 6th TNM had some serious limitations, such as the collection of data from a single geographical area, inadequate studying of the subgroups, and the evaluation of only the cases undergoing surgery. These shortcomings were partly amended in the 7th edition of the TNM staging (7th TNM) published in 2009.[5] The most important m dification in the 7th T NM was accepting tumors greater than 3 cm to 7 cm as T2A-B, and moving those greater than 7 cm into the T3 category, and the stages of separate nodule(s) were also reinterpreted. A satellite nodule(s) located on the same lobe was downstaged to T3, while a nodule(s) in the ipsilateral, different lobe was downstaged to T4.[6,7] The most important limitations of the 7th TNM was that positron emission tomography (PET)-computed tomography (CT) was not yet being used in every center as a non-invasive staging tool, and although the geographical area was expanded, it was still not representative of the entire population. Furthermore, data on T3-4 tumors were not clear. Following the collection of data from 19 countries and 49 institutions, and based on the recommendations provided by the Staging and Prognostic Factors Committee (SPFC) between 2014 and 2016 and the International Association for the Study of Lung Cancer (IASLC), the much more comprehensive 8th edition of the TNM staging system (8th TNM) was published by the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) in 2017.[8,9] The 8th TNM provided several new categories and, for the first time, some prospective data were included. Non-surgically treated patients were more represented. A wider geography was represented. Tumor sizes, solid components, and metastases were regrouped. One of the most important changes took place following the observation that in tumors of up to 5 cm, the prognosis gets worse with each 1 cm increase in tumor size. Therefore, tumors of 5 to 7 cm were classified as T3, and those greater than 7 cm as T4.[10,11]
Although patient data from Türkiye were sent to the database of the 8th T NM, external validation of the 8th TNM with a large numbers of patient series has not been carried out in Türkiye. In general, several studies have compared the 6th TNM to the 7th TNM, or the 7th TNM to the 8th TNM; however, there are very few studies comparing three stagings. In the current study, we aimed to restage all patients according to the 6th, 7th, and 8th editions of the TNM system and to observe the evolution of the TNM staging system in non-small cell lung cancer (NSCLC) by comparing the stage migrations of the same 1,077 patients between successive TNM staging systems. Our objective was also to externally validate the 8th TNM.
Patients and Methods
This retrospective study was conducted at Yedikule Chest Diseases and Thoracic Surgery Training and Research Hospital, Department of Thoracic Surgery between September 2005 and June 2015. The data of patients who underwent surgery due to NSCLC were reviewed using the database that was formed prospectively by collecting lung cancer data from our institution. The data of 1,418 patients were evaluated. Forty-eight patients who died within the first 30 days following surgery were excluded. Fifty patients with histological subtypes other than adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and large cell carcinoma were also excluded. Thirty-two cases, with lesions smaller than 2 cm, who underwent wedge resection or segmentectomy due to insufficient lung capacity, eight patients due to incomplete resection (R1-R2), 38 cases with inadequate lymph node sampling (
Pulmonary function testing and diagnostic bronchoscopy were routinely performed for each patient who was a candidate for surgery. The patients were required to have adequate lung capacity and a general condition to tolerate the width of resection (pneumonectomy, lobectomy, bilobectomy) necessary for the complete resection of the tumor. In preoperative clinical staging, routine CT of the thorax and upper abdomen were requested until 2008, while PET-CT was requested routinely in the following years. Mediastinoscopy for staging was routinely performed except for patients with negative PET-CT findings, those with a mediastinal lymph node of less than 1 cm on thoracic CT, and cT1N0M0 patients diagnosed with squamous cell carcinoma. Postoperative chemotherapy and/or radiotherapy was applied to all patients with the decision of the Multidisciplinary Council.
Clinical follow-up of the patients was performed once every three months for the first two years, once every six months at two to five years, and once a year after the fifth year. Non-contrast thoracic CT was performed once every six months. The PET-CT was requested, when recurrence or metastasis was suspected.
For the purpose of the study, clinical files and pathology reports were reevaluated and the cases were restaged according to the 6th, 7th, and 8th TNM. In the new stage, the pathological stage was named upstage if it had increased, downstage if it had decreased, and same stage if it had stayed the same.
The effects of factors such as age, sex, histological type, type of resection, pN status, and tumor size on overall survival (OS) were analyzed. Overall survival was assessed from the day of resection to death from any cause.
Statistical analysis
Statistical analysis was performed using the IBM SPSS for Windows version 23.0 software (IBM Corp., Armonk, NY, USA). Descriptive data were expressed in mean ± standard deviation (SD), median (min-max) or number and frequency. The Kaplan-Meier estimate was used for survival analysis, and comparison of survival between the groups was done using the log-rank test. Differences in the stage-specific survival rates of each of the three staging systems were analyzed. Survival rates of the same stages were compared. Stages within each of the TNM systems were compared to the one higher stage. Comparison among the 6th, 7th, and 8th editions of the TNM systems was made for the same stages. A p value of <0.05 was considered statistically significant.
Results
The most common type of operation was lobectomy (n=741, 68.8%). The mean tumor size was 5.00±2.64 cm, and the most common type of tumor was squamous cell carcinoma. A total of 780 (72.4%) patients received adjuvant therapy, including chemotherapy, radiotherapy, or concurrent chemoradiation therapy. The median overall survival was 76.0±5.5 months for the entire patient population, and the five-year survival rate was 55.0%. The mean follow-up was 63.9 (range, 24 to 144) months. Follow-ups were ceased after 144 months. The overall five-year survival rate was 63.1% for N0, 50.7% for N1 and 34.3% for N2 (p<0.001). Demographic and pathological features, T and N status of the patients are given in Table 1.
Table 1. Demographic and pathological features of patients.
| n | % | Mean±df | |
| Age (year) | 59.6±8.3 | ||
| Sex | |||
| Male | 986 | 91.6 | |
| Female | 91 | 8.4 | |
| Operation type | |||
| Lobectomy | 741 | 68.8 | |
| Pneumonectomy | 336 | 31.2 | |
| Histological type | |||
| Squamous cell carcinoma | 623 | 57.8 | |
| Adenocarcinoma | 411 | 38.2 | |
| Other | 43 | 4.0 | |
| Tumor diameter (cm±df) | 5.0±2.6 | ||
| 0-1 | 26 | ||
| 0-2 | 109 | ||
| 2-3 | 167 | ||
| 3-4 | 195 | ||
| 4-5 | 178 | ||
| 5-7 | 197 | ||
| >7 | 205 | ||
| PL0 (No pleural invasion) | 773 | 71.8 | |
| PL1 (Invasion beyond the elastic layer) | 64 | 5.9 | |
| PL2 (Invasion to the surface of the visceral pleura) | 137 | 12.7 | |
| PL3 (Invasion of the parietal pleura) | 103 | 9.6 | |
| df: Degree of freedom. | |||
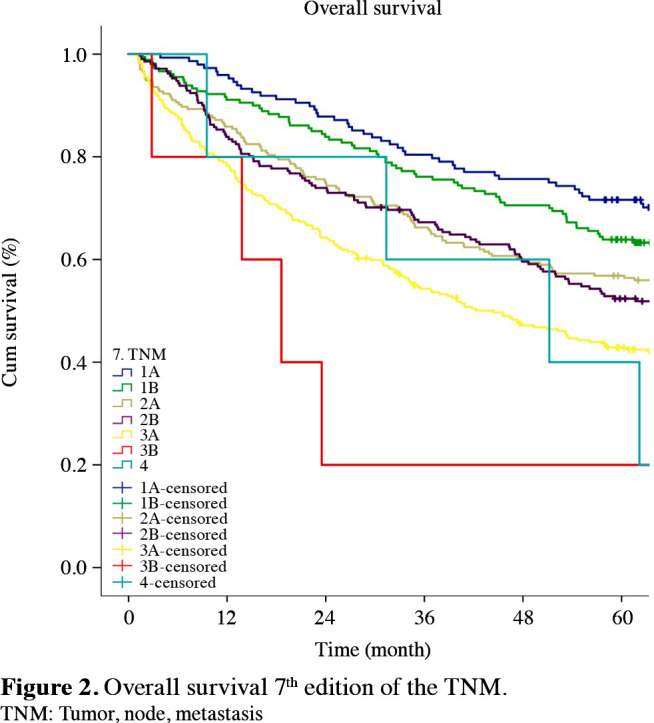
The staging systems showed distinctive survival rates between groups (p<0.001) (Figures 1-3). The stages were better distributed in the 7th TNM compared to the 6th T NM; the stages in the 8th TNM showed better stratification in terms of prognostic validity than the 7th T NM. By the 8th TNM staging, deteriorations of hazard ratios (HRs) across the stages were slightly more linear than the previous stagings.
Figure 1. Overall survival 6<sup>th</sup> edition of the TNM. TNM: Tumor, node, metastasis.
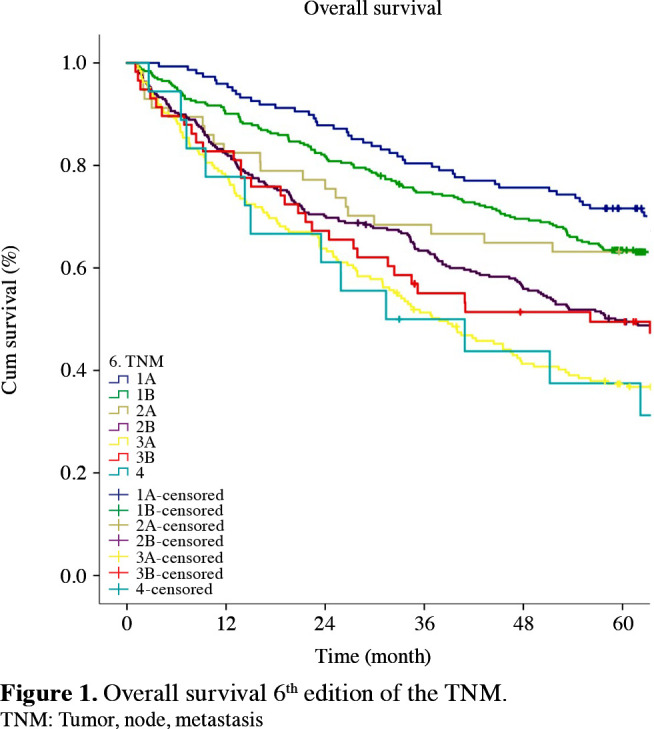
Figure 3. Overall survival 8<sup>th</sup> edition of the TNM. TNM: Tumor, node, metastasis.
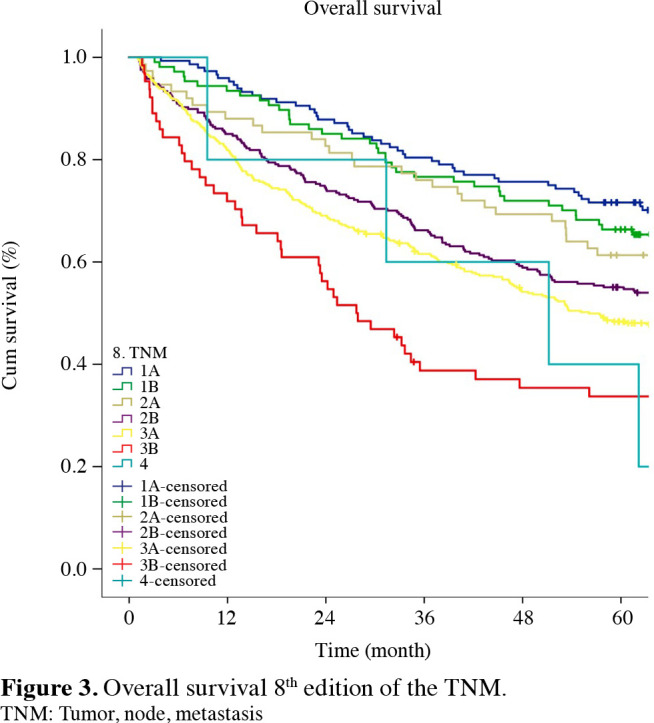
Figure 2. Overall survival 7<sup>th</sup> edition of the TNM. TNM: Tumor, node, metastasis.
Stage migrations between the 6th,7th, and 8th TNM
With the emergence of the 7th TNM, 368 (34.1%) patients were redistributed to different stages (Table 2). Of these patients, 185 (50.2%) were moved to the higher stages and 183 (49.8%) to the lower stages. Within the entire patient population, 17.1% were upstaged, and 16.9% downstaged. The most frequent transitions to the upper stages were from Stage 1B to Stages 2A and 2B (61/313=19.4%; 72/313=23.0%), and from Stage 2B to 3A (52/298=17.4%). The most frequent transitions to the lower stages were from Stage 3B to 3A, and from Stage 2B to 2A (45/58=77.5%; 116/298=38.9%).
Table 2. Migrations of tumor-stage between 6<sup>th</sup> and 7<sup>th</sup> TNM.
| 7th edition | ||||||||
| 1A (n=148) | 1B (n=180) | 2A (n=234) | 2B (n=211) | 3A (n=294) | 3B (n=5) | 4 (n=5) | ||
| 6thedition | 1A (n=148) | 148† | - | - | - | - | - | - |
| 1B (n=313) | - | 180† | 61* | 72* | - | - | - | |
| 2A (n=57) | - | - | 57† | - | - | - | - | |
| 2B (n=298) | - | - | 116‡ | 130† | 52* | - | - | |
| 3A (n=185) | - | - | - | - | 185† | - | - | |
| 3B (n=58) | - | - | - | 9‡ | 45‡ | 4† | - | |
| 4 (n=18) | - | - | - | - | 12‡ | 1‡ | 5† | |
| * Up-staging; † Same-staging; ‡ Down-staging. | ||||||||
With the emergence of the 8th TNM, 541 (50.2%) patients were redistributed to different stages (Table 3). Of those patients, 531 (98.1%) were moved to higher stages, whereas only 10 (1.9%) were moved to lower stages. Within the entire patient population, 49.3% were upstaged, and 0.9% downstaged. The most frequent transitions to the upper stages were from Stage 2A, which was upstaged to 2B (233/233=100%), Stage 2B to 3A (163/212=76.8%), from Stage 1B to 2A (75/180=41.6%), and from Stage 3A to 3B (59/294=20%).
Table 3. Migrations of tumor-stage between 7<sup>th</sup> and 8<sup>th</sup> TNM.
| 8th edition | ||||||||
| 1A (n=148) | 1B (n=107) | 2A (n=75) | 2B (n=287) | 3A (n=391) | 3B (n=64) | 4 (n=5) | ||
| 7thedition | 1A (n=148) | 148† | - | - | - | - | - | - |
| 1B (n=180) | - | 105† | 75* | - | - | - | - | |
| 2A (n=233) | - | - | - | 233* | - | - | - | |
| 2B (n=212) | - | 2‡ | - | 46† | 164* | - | - | |
| 3A (n=294) | - | - | - | 8‡ | 227† | 59* | - | |
| 3B (n=5) | - | - | - | - | - | 5† | - | |
| 4 (n=5) | - | - | - | - | - | - | 5† | |
| * Up-staging; † Same-staging; ‡ Down-staging. | ||||||||
When the same stages were compared in the 6th, 7th, and 8th staging systems, the survival rates of Stages 1B, 2B and 3A increased with the transition to the 7th TNM. Furthermore, survival rates of Stages 1B, 2A, 2B, 3A, and 3B increased with the transition to the 8th TNM (Table 4).
Table 4. Survival comparisons between 6th, 7th, and 8th TNM staging systems for each same stage.
| 6th TNM system | 7th TNM system | 8th TNM system | 6th TNM vs. 7th TNM | 7th TNM vs. 8th TNM | |||
| 5-year survival | 5-year survival | 5-year survival | Hazard ratio | Hazard ratio | |||
| % | % | % | p | 95% CI | p | 95% CI | |
| Stage 1A | 70.9 | 70.9 | 70.9 | 1.000 | - | 1.000 | - |
| Stage 1B | 63.1 | 63.3* | 66.4‡ | 0.939 | 0.989 (0.760-1.288) | 0.776 | 0.951 (0.673-1.343) |
| Stage 2A | 61.3 | 56.4 | 61.3‡ | 0.303 | 1.242 (0.843-1.828) | 0.274 | 0.816 (0.577-1.153) |
| Stage 2B | 49.5 | 51.9* | 54.7‡ | 0.255 | 0.877 (0.697-1.098) | 0.842 | 1.026 (0.812-1.298) |
| Stage 3A | 36.8 | 42.5* | 48.0‡ | 0.287 | 0.887 (0.710-1.109) | 0.03 | 0.815 (0.673-0.987) |
| Stage 3B | 47.3 | 20.0 | 33.7‡ | 0.02 | 2.682 (0.652-11.037) | 0.218 | 0.531 (0.137-2.054) |
| Stage 4 | 31.2 | 20.0 | 20.0 | 0.877 | 0.918 (0.306-2.747) | 1.000 | - |
| TNM: Tumor, node, metastasis; CI: Confidence interval; * The 7th TNM staging system showed better survival rate than the 6th TNM staging system in same stage in both staging systems; ‡ The 8th TNM staging system showed better survival rate than the 7th TNM staging system in each same stage in both staging systems; HR >1 for all comparisons of each stage. | |||||||
According to the Cox proportional hazard analysis, all HRs between adjacent staging groups were higher than 1.0 for almost every stage, indicating gradual deterioration in prognosis according to the staging groups. Furthermore, according to the Cox proportional hazards regression analysis that calculated the HRs between each pair of adjacent stages, the best stratifications were as follows: for the 6th TNM, between 1B-1A and 3B-3A (HR: 1.551, 1.419; p values: 0.006, 0.001); for the 7th TNM, between 1B-1A, 3A-2B and 3B-3A (HR: 1.513, 1.419, 2,309. p values: 0.01, 0.002, 0.04); and for the 8th TNM, between 1B-1A and 3B-3A (HR: 1.457, 1.702. p values: 0.048, 0.0005) (Table 5).
Table 5. Cox proportional hazards regression model output for the 6th, 7th, and 8th TNM staging systems.
| Stages comparisons | 6th TNM system | 7th TNM system | 8th TNM system | |||
| Hazard ratio | p | Hazard ratio | p | Hazard ratio | p | |
| 95% CI | 95% CI | 95% CI | ||||
| 1B vs. 1A | 1.551 (1.160-2.073) | 0.006 | 1.513 (1.096-2.139) | 0.01 | 1.457 (0.979-2.169) | 0.048 |
| 2A vs. 1B | 0.989 (0.658-1.488) | 0.961 | 1.254 (0.961-1.636) | 0.099 | 1.086 (0.707-1.666) | 0.702 |
| 2B vs. 2A | 1.443 (1.012-2.058) | 0.072 | 1.017 (0.794-1.303) | 0.899 | 1.274 (0.915-1.775) | 0.180 |
| 3A vs. 2B | 1.419 (1.125-1.791) | 0.001 | 1.419 (1.138-1.769) | 0.002 | 1.129 (0.928-1.374) | 0.226 |
| 3B vs. 3A | 0.758 (0.536-1.071) | 0.143 | 2.309 (0.610-8.734) | 0.04 | 1.702 (1.177-2.463) | 0.0005 |
| 4 vs. 3B | 1.387 (0.693-2.774) | 0.309 | 0.506 (0.132-1.936) | 0.289 | 0.779 (0.311-1.915) | 0.629 |
| TNM: Tumor, node, metastasis; CI: Confidence interval. | ||||||
The number of patients migrating from the 7th TNM to the 8th TNM was statistically significantly higher than that of those migrating from the 6th TNM to the 7th TNM (50.2% vs. 34.1%; p<0.05). In the 8th TNM, the number of upstages were statistically significantly higher than the upstages in the 7th TNM, and the number of downstages were significantly lower (upstage: 98.1% vs. 50.2%; p<0.0001).
Discussion
Treatment of lung cancer is determined according to stages. Therefore, a patient"s stage is important for the patient to receive the appropriate treatment and to evaluate their life expectancy. In the current study, we attempted to observe the evolution in the staging systems on anatomically resected patients with NSCLC, and indirectly validate the 8th T NM system. Our aim was to compare the stage migrations between consecutive staging systems and also to investigate whether patients were represented in their correct stages according to their survival with the 8th TNM staging system.
In an appropriate staging system, successive survival curves, whether increasing or decreasing, should be distinct for each group, and should not intersect. When we restaged the patients according to the 6th, 7th, and 8th TNM, we observed that, in the 6th TNM, the Kaplan-Meier survival curves for Stages 1B-2A and 2B-3A were intertwined. In the 7th TNM, distinction was maintained between Stages 1B-2A and 2B-3A; however, Stages 2A-2B were not entirely distinct and crossed at numerous points. Nonetheless, we observed that the survival curves did not intersect in the 8th TNM, they were distinct and showed a slightly clearer distribution. Also, in a successful staging system, the prognosis of any stage group should be statistically different from the others. Although there were differences in the survival rates between the stages in all staging systems, a statistically significant difference was not seen, possibly as the validation set contains relatively fewer series than the proposed studies with large series.
The survival curves show that the excessive difference in survival rates particularly between Stages 2A-2B and 2B-3A in the 6th TNM was decreased in the 7th TNM; however, continued for Stages 2B-3A. The difference between the survival curves decreased forming a close distribution in the 8th TNM. Successive survival curves do not intersect and, as a result, overall survival curves showed gradual deterioration for each group.
It is thought that each new staging system suggestions should increase survival rates in all of the stages compared to the previous system. As every patient would migrate to their appropriate stage, it would be meaningful to have increased survival rates in all stages.[12] Therefore, having a greater number of upstages in a staging system means that system is a better prognostic indicator than the previous. The number of upstages were statistically significantly higher in the 8th TNM than the upstages in the 7th TNM, and the number of downstages were significantly lower, showing the better stratification ability of the 8th TNM than the 7th TNM. The study by Chansky et al.[13] showed similar results to our study, given the entire patient population of 43.5% were upstaged in the 8th TNM. Upstages, which are most frequent between Stages 2A-2B and 2B-3A, correlate with our results.
The most important modification brought by the 7th TNM is the narrowing of the group of patients classified as T2 with tumors greater than 3 cm, and regrouping them as T2A for 3 to 5-cm tumors, T2B for 5 to 7-cm tumors, and T3 for tumors greater than 7 cm. Consequently, 5 to 7-cm tumors have been upstaged from Stage 1B to Stage 2A, and many cases with tumors greater than 7 cm were upstaged to Stage 2B. As a result, a 57% decrease in the patient population of Stage 1B was observed with the transition to the 7th TNM, leading to an increase in survival rates. Despite this change, the gap between Stages 1B and 1A in the survival curve seen in the 6th TNM was not narrowed as much as desired. After the subgrouping in the T2 patient group, the T2AN1 patient group, which appeared to show better survival rates, was transferred from Stage 2B to 2A. The most striking point here is the increase in the number of patients in Stage 2A in the 7th T NM. H owever, although not statistically significant, survival rates in Stage 2A decreased compared to the 6th TNM. The 7th TNM showed increased survival rates for Stages 1B, 2B, and 3A; however, it did not show an increase in the survival rates for Stage 2A. In the study by Fukui et al.,[14] the increase in the number of patients in Stage 2A was emphasized, and similar to our study, there was no significant increase in the life expectancy of the patients. In regard to the 6th TNM, the survival rates of our group of patients in Stage 3B were better than those in Stage 3A. We believe that the reason for this is because, in Stage 3B, there were six patients with N2, whereas, in Stage 3A, 140 patients were N2. With the 7th TNM, T4 N0-1 cases were transferred from Stage 3B to 3A, which led to an increase in both the patient population and the survival rates of Stage 3A.
One of the important problems drawing attention in the 6th T NM was the significant difference in the survival rates between Stages 3A and 2B. With the transition to the 7th TNM, the increase in the survival rates of the cases in Stage 3A provided a much closer distribution of the stage groupings for 3A and 2B.
Following the removal of patients from Stage 3B with the transition to the 7th T NM, S tage 3 B w as limited to merely T4N2 and T1-4N3 cases. As we do not have an N3 patient group, we have very few patients in this stage.
As a result of the multivariate analyses performed, the T factor underwent many important changes with the 8th TNM. The previous T2 group of 3 to 7-cm tumors in the 7th TNM w s reorganized as T2A f r 3 to 4 cm, T2B for 4 to 5 cm, and T3 for 5 to 7-cm tumors. As a result of new regulations, 41.6% of the T2N0 patients in Stage 1B in the 7th T NM were upstaged to Stage 2A with the 8th TNM, which led to the increase in the survival rates of those in 1B, which was insufficient previously in the 7th TNM. Although there was an increase in the survival rates, there was no significant difference between the survival rates of the early stages. In their retrospective study of 1,316 patients, Jung et al.[15] had similar results to our study, and that were not able to detect a significant difference in the survival rates between the 7th TNM and 8th TNM in the early stages. By transferring the N1 patient group in Stage 2A to Stage 2B by the 8th TNM, the patient population in the 7th T NM s tacked in Stage 2 A was decreased and targeted survival rates were approached. Reorganization of 5 to 7-cm tumors as T3, and tumors greater than 7 cm as T4 led to an increased number of patients with T3N1 and T4N0-1; therefore, the number of patients in Stage 3A and their survival rates increased. The position of Stage 3A on the survival curve was provided by its better description from 2B and 3B, and the slightly clearer distribution of the survival curves. The 8th TNM appears to be superior in separating Stage 3A and 2B disease compared to the 7th TNM. The transfer of T3-4N2 patients from Stage 3A to 3B increased the number and variety of patients in Stage 3B. The increase in the number of patients with better prognosis in Stage 3B compared to the 7th TNM led to a significant increase in survival rates of 3B, and the gap between 3B and 3A was partially reduced. In 8th TNM, N3 patients were classified as 3C. We do not have N3 patients in present study (3C); however, Kanyılmaz et al.[16] studied 112 Stage 3 patients and reported that N3 disease was a poor prognostic factor and the 8th T NM successfully determined this by the significant survival difference of Stages 3A and 3C.
In general, the literature also draws attention to the gap between the advanced stages. In the latest IASLC database, the difference between the survival rates of Stages 2B, 3A, and 3B is portrayed. The five-year survival rates were reported as 56%, 41%, and 24%, respectively.[17] The survival rates of our group of patients regarding these stages are consistent with the literature. Survival rates of 3B seem to be better than those described in the literature. We believe that the reason for this is the low number of patients with a poor prognosis at this stage due to the lack of N3 patients in our cohort, and the exclusion of those who received neoadjuvant therapy.
As a result of our comparative analysis, we have seen that there was homogenous gradual deterioration in prognosis with the 8th TNM between the survival curves of adjacent staging groups until Stage 2A. However, with Stage 2B, this homogeneity degrades and similar to the literature, larger gaps are formed between survival rates.
Since the 6th TNM, staging systems have focused on the T factor. Probably, the key to better stratification of the survival curves of Stage 2B and above in the 9th TNM, is the analysis of the N states. This was brought to attention in the 8th TNM and the issue of evaluating single station N1 (N1a), multiple N1 (N1b), skip N2 (N2 without N1=N2a), and the coexistence of N1 and N2 (N2b) was added to the agenda.[18]
Nonetheless, the present study has some limitations. Due to its retrospective nature, it has limited generalizability. Another limitation is that only the pathological stages were compared, and the clinical stages were not compared. As our study included only the patients who were operated, it is deemed insufficient regarding the comparison of the survival curves and the validation of advanced stages such as 3B and 4. One of the most important changes in the 8th edition is that the size criteria of tumors with radiological ground glass and solid attenuation that is regarded as pathological lepidic growth and invasive growth respectively is adopted. However, in our study, this detail regarding T1 tumors was not documented, since they accepted Stage 1 in all three staging systems. One of the advantages of our study is that our hospital is an experienced and successful hospital dealing with lung cancer. We believe that we perform a thorough preoperative evaluation, and meticulous intraoperative T and N staging of the patients; therefore, we believe that we have staged our patients accurately in terms of pathological staging. Another good aspect of our study is that it is one of the rare studies that compare all last three staging systems, in the NSCLC group. It is also one of the largest series from Türkiye to perform an external validation of the 8th staging system.
In conclusion, numerous intersections on the survival curves and the broad gap between the survival rates of the groups observed in the 6th T NM w ere partially mended with the 7th TNM and discrimination ability has reached its best level with the 8th TNM. Therefore, the rule of gradual deterioration in the prognosis between adjacent staging groups has been formed. With the transition to the 8th TNM, we found that the upstage migrations were significantly higher from the transition to the 7th TNM. Stratification according to 8th TNM is prognostically valid for patients in the study group.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Idea/concept: V.E., N.Ç.; Design: C.B.S.; Control/supervision: M.M.; Data collection and/or processing: E.Y.S., S.O.; Analysis and/or interpretation: N.Ç.; Literature review: Y.A., C.A.; Writing the article: V.E.; Critical review: M.V.D., Ö.S.; References and fundings: S.O.; Fundings materials: E.Y.E.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Olivre HB, Chairman DT, Carr PR, editors. American Joint Committee on Cancer. 1th ed. Philadelphia: J.B Lippincott Company; 1977. Manual for Staging of cancer. [Google Scholar]
- 3.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 4.Sobin LH, Christian W, editors . (UICC) Paperback. 2002 TNM classification of malignant tumours. [Google Scholar]
- 5.Sobin LH, Gospodarowicz MK, Christian WC, editors . International Union Against Cancer (UICC): TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- 6.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 7.Rami-Porta R, Ball D, Crowley J, Giroux DJ, Jett J, Travis WD, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 8.Sobin LH, Gospodarowicz MK, Christian WC, editors . Oxford: Wiley- Blackwell; 2017. International Union Against Cancer (UICC): TNM classification of malignant tumours. [Google Scholar]
- 9.Amin MB, editor. AJCC cancer staging manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 10.Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, et al. The IASLC Lung Cancer Staging Project: Proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:990–1003. doi: 10.1097/JTO.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 11.Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:138–155. doi: 10.3322/caac.21390. [DOI] [PubMed] [Google Scholar]
- 12.Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 13.Chansky K, Detterbeck FC, Nicholson AG, Rusch VW, Vallières E, Groome P, et al. The IASLC Lung Cancer Staging Project: External validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12:1109–1121. doi: 10.1016/j.jtho.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Fukui T, Mori S, Hatooka S, Shinoda M, Mitsudomi T. Prognostic evaluation based on a new TNM staging system proposed by the International Association for the Study of Lung Cancer for resected non-small cell lung cancers. J Thorac Cardiovasc Surg. 2008;136:1343–1348. doi: 10.1016/j.jtcvs.2007.12.085. [DOI] [PubMed] [Google Scholar]
- 15.Jung HS, Lee JG, Lee CY, Kim DJ, Chung KY. Validation of the T descriptor in the new 8th TNM classification for nonsmall cell lung cancer. J Thorac Dis. 2018;10:162–167. doi: 10.21037/jtd.2017.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanyılmaz G, Aktan M, Yavuz BB, Demir LS. Evre III küçük hücre dışı akciğer kanserli hastalarda sekizinci evreleme sisteminin prognostik etkileri. Ortadogu Tıp Derg. 2019;1:47–53. [Google Scholar]
- 17.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:1675–1684. doi: 10.1097/JTO.0000000000000678. [DOI] [PubMed] [Google Scholar]


