Abstract
Per- and polyfluoroalkyl substances (PFAS) are emerging contaminants widely used in a variety of industrial and consumer applications. Due to phasing out legacy PFAS, some manufacturers developed short-chain alternatives like perfluoroalkyl ether carboxylic acids (PFECA). Published liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods cover a wide range of these replacement chemicals including PFMPA (perfluoro-3-methoxypropanoic acid) and PFMBA (perfluoro-4-methoxybutanoic acid). However, many methods do not monitor for their branched isomers, PMPA (perfluoro-2-methoxypropanoic acid) and PEPA (perfluoro-2-ethoxypropanoic acid), respectively. Although these isomers are chromatographically separable under certain conditions, using the common MS/MS transitions for PFMPA (m/z 229 → 85) and PFMBA (m/z 279 → 85) can yield low or no detection signals for PMPA and PEPA, thus leading to underestimated values or nondetects. We compared various MS/MS transitions for these isomers and determined the optimal transitions for PMPA (m/z 185 → 85) and PEPA (m/z 235 → 135). We applied the developed method to water sampled near two chemical manufacturing plants and observed these analytes, plus a suspected third isomer. Using these MS/MS transitions will ensure all isomers are detected and will lead to better monitoring and exposure estimates of PFECA in humans and the environment.
Keywords: Per- and polyfluoroalkyl substances (PFAS), Perfluoroalkyl ether carboxylic acid (PFECA), Tandem mass spectrometry (MS/MS), Method development, Isomers
Graphical Abstract

INTRODUCTION
Per- and polyfluoroalkyl substances (PFAS), a large family of synthetic fluorinated chemicals, are frequently used in industrial and consumer applications (e.g., fire-fighting foams, cosmetics, household products).1 These emerging contaminants persist ubiquitously in various environmental media including water, soil, and air due to their physiochemical properties that make them difficult to biodegrade. Additionally, some PFAS are bioaccumulative and have shown evidence of adverse health effects.2
In the 21st century, major manufacturing companies began to voluntarily phase out legacy PFAS as part of the PFOA Stewardship Program.3 Subsequently, the phase out of these legacy long-chain PFAS led some companies to develop short-chain alternatives like perfluoroalkyl ether carboxylic acids (PFECA), which contain ether bond(s) in the perfluorinated carbon backbone.4 Their presence in the environment was first reported in the literature in 2015 by Strynar et al., in which 10 novel PFECAs were detected in the Cape Fear River system, including hexafluoropropylene oxide dimer acid (HFPO-DA) or GenX.5 Thereafter, PFECAs have been detected elsewhere in the environment6–8 and in wildlife9–11 and their toxicities studied.12–16 D’Ambro et al. described how HFPO-DA can result from the hydrolysis of the acyl fluoride, hexafluoropropylene oxide dimer acid fluoride (HFPO-DAF), as seen in the emissions from a fluoropolymer manufacturing facility and following release.17 While some acyl fluorides are intermediates synthesized purposefully during the production of perfluoro vinyl ethers, additional unintended acyl fluorides are produced and released as well.
While published liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods cover some of these alternative chemicals including PFMPA and PFMBA, many methods do not monitor for their branched isomers, PMPA and PEPA, respectively (see Table 1 for full chemical names, CASRNs, and structures).18–20
Table 1.
Perfluoroalkyl Ether Carboxylic Acids (PFECA) Isomer Pairs

|
If the common MS/MS transitions for PFMPA (m/z 229 → 85) and PFMBA (m/z 279 → 85) are used, then PMPA could be misidentified, and PEPA could be missed in analyses, especially since 85 m/z is not a possible fragment for PEPA (Figure 1). Additionally, while these isomers can be chromatographically resolved under certain conditions, the branched isomers may go undetected if the retention time window is too narrow. It is important to monitor for all isomers to avoid misidentification and to correctly quantitate concentrations. Therefore, a targeted MS/MS analytical method is needed to adequately monitor both branched and linear isomers, which laboratories can incorporate easily into their routine workflows.
Figure 1.
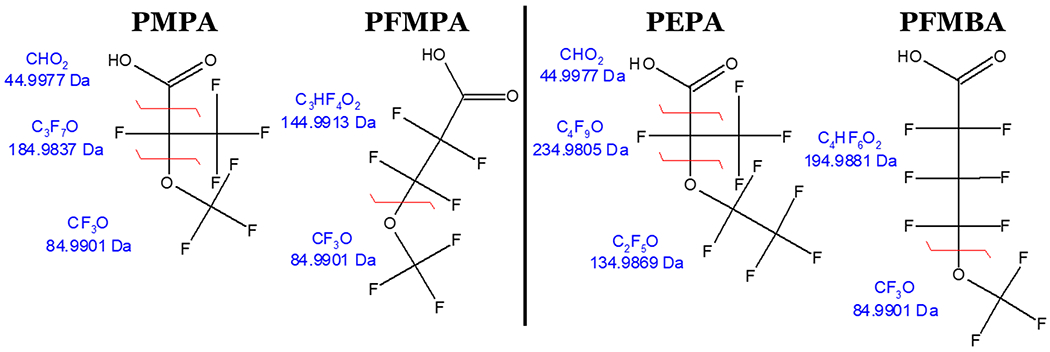
MS/MS fragments of isomers and their exact monoisotopic masses.
Herein, we describe an investigation that determined the optimal mass transitions for monitoring all four analytes. First, the top three product ions for each analyte using [M-H]− and [M-CO2H]− as precursor ions were chosen by compound optimization on a triple quadruple mass spectrometer (QQQ). Next, each analyte was monitored at those six mass transitions to determine which mass transitions provided optimal analytical response. Additionally, standards were prepared with different water/methanol ratios to investigate enhanced chromatographic resolution. Last, the finalized method was applied to field samples. One sample was well water collected near a chemical manufacturing plant, and the second sample was chemical manufacturer effluent.
MATERIALS AND METHODS
LC-MS grade acetonitrile was purchased from Fisher Scientific (Fair Lawn, NJ) and methanol from Honeywell - Burdick & Jackson (Muskegon, MI). ACS reagent grade ammonium acetate was purchased from Sigma-Aldrich (St. Louis, MO). In-house deionized water was used. PFECA standards (PMPA (99%) and PEPA (99%)) were donated by The Chemours Company (Wilmington, DE), and PFMPA (98%) and PFMBA (97%) were purchased from Synquest Laboratories (Alachua, FL).
For direct infusion, solutions of each standard were prepared in methanol for a final concentration of 1 ng/μL. Standards were directly infused at a flow rate of 16 μL/min into the QQQ, and the compound optimization software produced the top three product ions for each analyte using [M-H]− and [M-CO2H]− as the precursor ions (Table S1 details the direct infusion parameters).
For direct injection LC-MS/MS, standard solutions containing all four isomers were prepared at a final concentration of 1 pg/μL in an autosampler vial. Standard solutions were prepared at three varying deionized water/methanol ratios (20/80, 50/50, 75/25 v/v). Targeted analyses were performed using a Thermo Vanquish Horizon ultrahigh performance liquid chromatograph (UHPLC) coupled to a Thermo TSQ Quantis triple-quadrupole (QQQ) mass spectrometer operated in negative ion mode. A reversed-phase separation occurred on a Phenomenex Gemini C18, 2 mm × 50 mm, 3.0 μm silica with TMS end-capping column (Torrance, CA) at 55 °C. A Thermo Scientific Hypersil GOLD C18, 1.9 μm, 3 mm × 50 mm was used as a delay column (Waltham, MA) as part of in-house standard practice. A binary gradient was used for all analyses as shown in Table S2. Chromatographic conditions were modeled after a previously validated method.18 This investigation focused only on the development of MS/MS transitions that could be incorporated easily into existing workflows; thus, limited chromatographic development occurred to keep within the confines of the validated method. Table S3 details the QQQ parameters and acquisition settings, and Table S4 lists each analyte’s mass transitions. Each standard solution was injected three times with an injection volume of 10 μL.
For field samples, the well water sample was collected in July 2017 and the chemical manufacturer effluent collected in November 2013. The well water sample was concentrated by weak anion exchange solid-phase extraction as detailed in Strynar et al.5 The effluent sample was cleaned to remove any particulate matter by filtering through a 0.45 μm, 25 mm GD/XP, polypropylene medium disposable syringe filter from Whatman (Florham Park, NJ) and concentrating 10 times (from 10 to 1 mL) at 40 °C with a TurboVap LV nitrogen evaporator from Caliper Life Sciences (Hopkinton, MA). Both field samples were prepared in autosampler vials at a sample composition of 75/25 water/methanol (v/v), and 50 μL was injected. A larger injection volume was used compared to the standard solutions to achieve increased signal.
After data collection, chromatograms were processed and peak areas integrated in Thermo Scientific Xcalibur Quan Browser 4.3. Chromatographic resolution was calculated accordingly: twice the difference of two peaks’ retention times divided by the summation of their baseline peak widths. Statistical analyses were conducted using Microsoft Excel 2016’s Data Analysis. A two-tailed t test compared mean chromatographic resolutions between different sample compositions, and an α of 0.05 was used to denote significance.
RESULTS AND DISCUSSION
After each analyte was directly infused into the QQQ, the compound optimization software provided the top three product ions from precursor ions [M-H]− and [M-CO2H]− with their optimal collision energies and RF lens values (Table S4). Table S5 lists the six product ions for each analyte in order from highest detected signal to lowest detected signal. The product ion corresponding to the fragment CF3O, 85 m/z, was commonly seen among the analytes, except PEPA as expected (Figure 1). Some product ions were most likely source artifacts and not real chemical fragments of the analyte. Nonetheless, these transitions were still monitored during the next step.
After generating six mass transitions from the compound optimization software, the isomers were monitored with a direct injection LC-MS/MS method. Figures S1–S4 display extracted ion chromatograms of each analyte monitored at six mass transitions. As expected, the MS/MS transitions pertaining to the source artifacts listed in Table S4 provided no detectable peaks. The linear isomers, PFMPA and PFMBA, were detected only using [M-H]− as the precursor ion, while the branched isomers, PMPA and PEPA, did not respond well using the [M-H]− precursor ion likely due to abundant insource decarboxylation. Both PEPA and PFMBA were observed, and the peaks overlapped when mass transition m/z 279 → 235 was utilized. As seen in Table 2, the mass transitions for PFMPA and PFMBA that provided the largest responses were m/z 229 → 85 and m/z 279 → 85, respectively, which matches what is published in the literature.
Table 2.
Mean Peak Area ± Standard Deviation (SD) for MS/MS Transitions That Gave the Highest Response for Standards Prepared in 75/25 Water/Methanol (v/v), n = 3
| Analyte | MS/MS transition (m/z) | Mean peak area ± SD |
|---|---|---|
| PFMPA, PFMOPrAa | 229 → 85 | 130,000 ± 2500 |
| PFMBA, PFMOBAa | 279 → 85 | 230,000 ± 4000 |
| PMPAb | 185 → 119 | 170,000 ± 2700 |
| 185 → 85 | 210,000 ± 3000 | |
| PEPAb | 235 → 135 | 280,000 ± 2500 |
| 235 → 119 | 200,000 ± 4700 |
Precursor ion [M-H]−.
Precursor ion [M-CO2H]−.
For branched isomer detection, the highest peak area observed involved mass transitions from the [M-CO2H]− precursor ion: PMPA (m/z 185 → 85) and PEPA (m/z 235 → 135). PMPA and PEPA had additional transitions with appreciable signals (m/z 185 → 119 and m/z 235 → 119) that could serve as confirmation transitions (or could be the main transition depending on source conditions).
PMPA and PFMPA resolved well chromatographically and separated by roughly 0.5 min in all standard samples. These two isomers easily were distinguished chromatographically and mass spectrally. Conversely, the PEPA peak was hard to discern from the PFMBA peak given its poor chromatographic resolution. To investigate improving resolution, the standard solution was composed at three water/methanol ratios (20/80, 50/50, 75/25 v/v) as illustrated in Figure S5. While none of the ratios provided baseline resolution, 50/50 gave the highest resolution of 0.42 ± 0.01 (n = 3) and 20/80 gave the lowest resolution 0.35 ± 0.01 (Table S6); there was a significant difference in resolution (P value = 7.7 × 10−3). An intermediate resolution of 0.39 ± 0.02 was achieved with 75/25. No significant difference in resolution was determined between 50/50 and 75/25 (P value = 0.26) or 20/80 and 75/25 (P value = 0.24). Using a different column with a smaller particle diameter could improve resolution; however, distinct mass transitions are the best way to differentiate PEPA and PFMBA no matter the chromatographic situation.
Once the optimal mass transitions were determined for the isomers, field samples were analyzed. All four isomers were detected in a well water sample collected near a chemical manufacturing plant as displayed in Figure 2a. Additionally, while monitoring for PFMBA using the m/z 279 → 85 transition, another peak was detected. We suspect this peak to be another branched isomer with two possible configurations (Figure 2b) that both have the same monoisotopic mass as PEPA and PFMBA and could fragment into CF3O (85 m/z). Given that this isomer eluted before both PEPA and PFMBA, we speculate that this PFECA is more highly branched than PEPA. We believe this would be the first time this isomer has been reported in the literature. In the second field sample (effluent from a chemical manufacturer), only PMPA and PEPA were detected as featured in Figure 2c.
Figure 2.

(a) Extracted ion chromatogram of a well water sample near a chemical manufacturing plant. All isomers were detected in addition to what is speculated to be a second branched isomer of C5HF9O3 eluting before PEPA. (b) Chemical structures of suspected branched isomer seen in well water chromatogram. (c) Extracted ion chromatogram of manufacturing plant effluent. Only PMPA and PEPA were detected.
If common commercially available methods were used, PMPA and PEPA could have been misidentified or missed completely, thus demonstrating the importance of including these transitions in future analyses. EPA Method 533 already monitors for the linear isomers (PFMPA and PFMBA). Since both linear isomers rely on the [M-H]− peaks (228 and 278 m/z, respectively) and PMPA and PEPA generally manifest as [M-CO2H]− (185 and 235 m/z, respectively), but not exclusively, the isomers could be misidentified. To the authors’ knowledge, isotope labeled standards are not available for any of these isomers for retention time confirmation. To accurately quantify linear and branched PFECA isomers, it is important that isotope labeled standards are created and subsequently used for both types to account for their different fragmentation patterns.
Furthermore, monitoring for all isomers can assist with environmental fingerprinting since these two samples provided different PFECA isomer profiles. For example, the branched isomers (PMPA and PEPA) have been reported in the literature to be present in wastewater and surface water nearby a fluorochemical manufacturing plant in Fayetteville, NC that makes the polymer processing aid (PPA), HFPO-DA (also known as GenX).5,21 Additionally, Zhang et al. demonstrated that the PPA from another fluorochemical manufacturer, known as ADONA, can be oxidized to the terminal product PFMPA when conducting the total oxidizable precursor (TOP) assay.22 It is unknown if the natural breakdown of ADONA in the environment leads to the same terminal PFMPA. Since both PMPA and PFMPA could exist in environmental samples and can potentially aid with industrial source attribution, it is imperative that analysts understand and can distinguish between these branched and linear isomers.
As shown with the field samples, both the linear and branched isomers are present in the environment, and methods could be modified to ensure all isomers can be resolved and quantitated. Different source conditions and instrumentation may provide slightly different results, but this study provides the foundation for further in-house method development. Our results demonstrate how analytes from the same subclass possess dissimilar fragmentation patterns and highlight that assumptions cannot be made that one mass transition will yield the same response for isomers. The proposed optimized MS/MS transitions for PMPA and PEPA have the potential to improve detection of these additional analytes and lead to better monitoring and exposure estimates of PFECA. With the application of nontargeted analysis (NTA) and suspect screening for PFAS in complex environmental samples, it is likely additional isomers will be uncovered in future studies. Prospective unresolved PFAS isomers will require similar approaches shown in this study and certain considerations should be acknowledged when developing quantitative methods.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank The Chemours Company for their donation of select PFECA standards. We also thank Jacqueline Bangma and Marci Smeltz for reviewing this manuscript. The views expressed in this publication are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names, manufacturers or products does not imply an endorsement by the United States Government or the U.S. Environmental Protection Agency. EPA and its employees do not endorse any commercial products, services, or enterprises.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.2c00509.
Additional experimental details including UHPLC and QQQ conditions, parameters, and acquisition settings. Additional results including chromatograms of PMPA, PFMPA, PEPA, and PFMBA at various mass transitions. (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.estlett.2c00509
The authors declare no competing financial interest.
Contributor Information
Kelsey E. Miller, Office of Research and Development, Center for Environmental Measurement and Modeling, U.S. Environmental Protection Agency, Research Triangle Park, North Carolina 27709, United States; Present Address: Kelsey E. Miller: Office of Chemical Safety and Pollution Prevention, Office of Pollution Prevention and Toxics, U.S. Environmental Protection Agency, Washington, DC 20460, United States.
Mark J. Strynar, Office of Research and Development, Center for Environmental Measurement and Modeling, U.S. Environmental Protection Agency, Research Triangle Park, North Carolina 27709, United States.
REFERENCES
- (1).Wang Z; DeWitt JC; Higgins CP; Cousins IT; Never-Ending A A Never-Endin Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol 2017, 51 (5), 2508–2518. [DOI] [PubMed] [Google Scholar]
- (2).Fenton SE; Ducatman A; Boobis A; DeWitt JC; Lau C; Ng C; Smith JS; Roberts SM Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem 2021, 40 (3), 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).USEPA 2010/15 PFOA Stewardship Program. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/201015-pfoastewardship-program-guidance-reporting (accessed January 13, 2022).
- (4).Wang Z; Cousins IT; Scheringer M; Hungerbuhler K Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int 2013, 60, 242–248. [DOI] [PubMed] [Google Scholar]
- (5).Strynar M; Dagnino S; McMahen R; Liang S; Lindstrom A; Andersen E; McMillan L; Thurman M; Ferrer I; Ball C Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol 2015, 49 (19), 11622–11630. [DOI] [PubMed] [Google Scholar]
- (6).Pan Y; Zhang H; Cui Q; Sheng N; Yeung LWY; Guo Y; Sun Y; Dai J First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ. Sci. Technol 2017, 51 (17), 9553–9560. [DOI] [PubMed] [Google Scholar]
- (7).Pan Y; Zhang H; Cui Q; Sheng N; Yeung LWY; Sun Y; Guo Y; Dai J Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water. Environ. Sci. Technol 2018, 52 (14), 7621–7629. [DOI] [PubMed] [Google Scholar]
- (8).Yao J; Pan Y; Huan Y; Dai J Occurrence of Novel Perfluoroalkyl Ether Carboxylic Acids in River Water and Human Urine Quantified by a Simple Liquid–Liquid Microextraction Approach Coupled with LC–MS/MS. Environ. Sci. Technol. Lett 2021, 8 (9), 773–778. [Google Scholar]
- (9).Cui Q; Pan Y; Zhang H; Sheng N; Wang J; Guo Y; Dai J Occurrence and Tissue Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids and Legacy Per/Polyfluoroalkyl Substances in Black-Spotted Frog (Pelophylax nigromaculatus). Environ. Sci. Technol 2018, 52 (3), 982–990. [DOI] [PubMed] [Google Scholar]
- (10).Robuck AR; Cantwell MG; McCord JP; Addison LM; Pfohl M; Strynar MJ; McKinney R; Katz DR; Wiley DN; Lohmann R Legacy and Novel Per- and Polyfluoroalkyl Substances in Juvenile Seabirds from the U.S. Atlantic Coast. Environ. Sci. Technol 2020, 54 (20), 12938–12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zhang B; He Y; Yang G; Chen B; Yao Y; Sun H; Kannan K; Zhang T Legacy and Emerging Poly- and Perfluoroalkyl Substances in Finless Porpoises from East China Sea: Temporal Trends and Tissue-Specific Accumulation. Environ. Sci. Technol 2022, 56 (10), 6113–6122. [DOI] [PubMed] [Google Scholar]
- (12).Wang Z; Cousins IT; Scheringer M; Hungerbuehler K Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions. Environ. Int 2015, 75, 172–179. [DOI] [PubMed] [Google Scholar]
- (13).Guo H; Wang J; Yao J; Sun S; Sheng N; Zhang X; Guo X; Guo Y; Sun Y; Dai J Comparative Hepatotoxicity of Novel PFOA Alternatives (Perfluoropolyether Carboxylic Acids) on Male Mice. Environ. Sci. Technol 2019, 53 (7), 3929–3937. [DOI] [PubMed] [Google Scholar]
- (14).Conley JM; Lambright CS; Evans N; McCord J; Strynar MJ; Hill D; Medlock-Kakaley E; Wilson VS; Gray LE Jr. Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ. Int 2021, 146, 106204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Woodlief T; Vance S; Hu Q; DeWitt J Immunotoxicity of Per- and Polyfluoroalkyl Substances: Insights into Short-Chain PFAS Exposure. Toxics 2021, 9 (5), 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gebreab KY; Eeza MNH; Bai T; Zuberi Z; Matysik J; O’Shea KE; Alia A; Berry JP Comparative toxicometabolomics of perfluorooctanoic acid (PFOA) and next-generation perfluoroalkyl substances. Environ. Pollut 2020, 265, 114928. [DOI] [PubMed] [Google Scholar]
- (17).D’Ambro EL; Pye HOT; Bash JO; Bowyer J; Allen C; Efstathiou C; Gilliam RC; Reynolds L; Talgo K; Murphy BN Characterizing the Air Emissions, Transport, and Deposition of Per- and Polyfluoroalkyl Substances from a Fluoropolymer Manufacturing Facility. Environ. Sci. Technol 2021, 55 (2), 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Rosenblum L; Wendelken SC Method 533: Determination of Per- and Polyfluoroalkyl Substances in Drinking Water by Isotope Dilution Anion Exchange Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry. In Office of Ground Water and Drinking Water, Standards and Risk Management Division; U.S. Environmental Protection Agency: Cincinnati, OH, 2019. [Google Scholar]
- (19).Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DRU Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett 2016, 3 (12), 415–419. [Google Scholar]
- (20).Hopkins ZR; Sun M; DeWitt JC; Knappe DRU Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. - Am. Water Works Assoc 2018, 110 (7), 13–28. [Google Scholar]
- (21).McCord J; Strynar M Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ. Sci. Technol. 2019, 53 (9), 4717–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zhang C; Hopkins ZR; McCord J; Strynar MJ; Knappe DRU Fate of Per- and Polyfluoroalkyl Ether Acids in the Total Oxidizable Precursor Assay and Implications for the Analysis of Impacted Water. Environ. Sci. Technol. Lett 2019, 6 (11), 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


