ABSTRACT
The detection of antibodies against Histoplasma capsulatum remains a frequently relied-on approach to diagnose histoplasmosis. We retrospectively assessed the performances of complement fixation (CF) and immunodiffusion (ID) assays for anti-Histoplasma antibody detection in patients with culture-confirmed histoplasmosis at Mayo Clinic (Rochester, MN) over a 10-year period (2011 to 2020). Among 67 culture-confirmed patients who also had H. capsulatum CF/ID testing ordered, 51 (67.1%) were immunocompromised, 34 (50.7%) had localized disease, and 51 (76.1%) presented with <3 months of symptoms before testing. H. capsulatum CF and/or ID testing was positive in 47 (70.1%) patients, with both assays being positive in 39 cases. CF was positive in 44 (65.7%) patients, with reactivity against both H. capsulatum mycelial and yeast antigens in 30 (68.2%) cases, whereas 11 (25%) and 3 (6.8%) individuals had antibodies to the CF yeast or mycelial antigen only, respectively. H. capsulatum ID was positive in 42 (62.7%) patients, with the presence of the M-band only or the H- and M-bands in 27 (64.3%) and 15 (35.7%) cases, respectively. Among 18 serially tested patients, 12 remained ID and/or CF positive at the final time point (median, 154 days; range, 20 to 480 days). Serial CF testing showed that antibodies to the mycelial antigen serorevert to negative more frequently (6/11) than antibodies to the yeast antigen (2/13). There was no statistically significant difference in antibody positivity relative to patient immune status, degree of disease dissemination, or symptom duration. Serologic testing remains a valuable asset to support the diagnosis of histoplasmosis, particularly when direct detection methods fail to identify an infection.
KEYWORDS: Histoplasma capsulatum, serology, antibody, complement fixation, immunodiffusion
INTRODUCTION
Histoplasma capsulatum is a dimorphic fungal pathogen endemic to the Ohio and Mississippi River Valleys of North America, with a recently increasing incidence beyond these regions. Although exposure may lead to asymptomatic disease or limited, self-resolving symptoms in otherwise healthy individuals, morbidity and mortality are often more severe in older patients and those with impaired cellular immunity (1). As a result, the rapid and accurate diagnosis of infection with H. capsulatum is necessary to guide appropriate antifungal treatment for the best possible patient outcomes. The recovery of H. capsulatum in culture or tissue by histopathology remains the reference method for diagnosis. However, this method is associated with a number of limitations, including the need for invasive procedures to obtain optimal specimen types (which may be contraindicated in some patients), variable assay sensitivities depending on the extent of disease, and a long turnaround time for cultures depending on the inoculum and specimen type (2). In an effort to provide a timelier diagnosis, molecular methods have been developed, although these assays are also limited by the need for invasive specimen collection procedures and have been associated with variable sensitivities across studies (3–5). Finally, assessment for circulating H. capsulatum antigen in serum or urine offers a noninvasive means to directly detect infection, with a significantly shorter turnaround time than culture. Key limitations associated with this method, however, include cross-reactivity with other dimorphic pathogens and variable sensitivity depending on the disease state (2, 6, 7).
In addition to the above-mentioned methods, the detection of antibodies to H. capsulatum in serum via different serologic methods is also frequently relied on to assist in making the diagnosis. Serologic testing is particularly useful in patients for whom invasive specimen collection is contraindicated and those presenting with subacute or chronic forms of histoplasmosis, for which antigen detection is less sensitive (2, 8–10). Antibody detection, however, is associated with several limitations, including false positivity for some assays in patients infected with other dimorphic pathogens (i.e., cross-reactivity) and false-negative results in significantly immunocompromised patients and in those for whom sample collection occurs prior to the development of a detectable antibody response (i.e., seroconversion occurs between 4 and 6 weeks and as early as 2 weeks after infection) (2, 11–13). Available serologic methods for the detection of antibodies to H. capsulatum include enzyme immunoassays (EIAs), complement fixation (CF) assays, and immunodiffusion (ID) assays; the latter two classic methods were originally developed in the 1950s and clinically deployed in the 1970s and 1980s as part of outbreak investigations (14–16). EIAs offer high-throughput and automated testing; however, given their qualitative results and lower specificity (depending on the assay), some laboratories have opted to confirm positive results via CF/ID testing. In contrast to EIAs, CF and ID methods are labor-intensive, technically complicated, and typically performed manually, with increasingly limited reagent availability and challenges associated with the interpretation of the results. Due to these complexities and the significant technologist expertise required to maintain these assays, CF and ID methods are available primarily through reference laboratories. Interestingly, although frequently ordered, the majority of studies evaluating the performance characteristics of H. capsulatum CF/ID assays were conducted over 3 decades ago and used reagents developed in research laboratories or available through a single reference laboratory (2, 9, 17). Here, we present our institutional experience with H. capsulatum CF and ID testing using commercially available reagents, to provide a current assessment of the clinical sensitivity of these assays in patients with culture-confirmed histoplasmosis.
MATERIALS AND METHODS
Study design.
We performed a 10-year retrospective chart review of all adult patients with culture-confirmed histoplasmosis at Mayo Clinic (Rochester, MN) from January 2011 to December 2020. Using these criteria, 76 patients with culture-confirmed histoplasmosis were identified, among whom 67 had at least one H. capsulatum serology order as part of the disease episode and were included in this analysis. H. capsulatum serologic testing at Mayo Clinic includes the performance of both complement fixation (CF) and immunodiffusion (ID) assays as a panel (i.e., CF and ID assays cannot be ordered individually). Each patient chart was reviewed for demographic information (i.e., age at diagnosis, sex, race, comorbidities, and immune status), clinical presentation (i.e., disease severity and duration of symptoms prior to presentation), the highest level of care received (i.e., outpatient, inpatient, or intensive care unit), and H. capsulatum serologic test results from CF and ID assays. Study data were collected and managed using Research Electronic Data Capture, which is a secure, Web-based application designed to support data capture for research studies at Mayo Clinic (18). The study was approved by the Mayo Clinic institutional review board.
Histoplasma capsulatum immunodiffusion.
Immunodiffusion testing was performed using 4- or 8-rosette-pattern immunodiffusion agar plates from either Gibson Biosciences (Lexington, KY) or IMMY (Norman, OK). H. capsulatum immunodiffusion antigen and antiserum (i.e., positive control) were acquired from either Gibson Biosciences or IMMY, depending on the availability over the 10-year time frame. The immunodiffusion assay was performed according to previously described methods (19). Briefly, for each rosette, positive-control antiserum was added to wells 1 and 4, negative-control serum was added to well 3, and 3 unique patient serum samples were added to wells 2, 5, and 6 (Fig. 1). Sera were allowed to prediffuse for 30 min in a room-temperature moisture chamber, prior to the addition of H. capsulatum antigen in the center well. Agar plates were subsequently incubated at room temperature in a moisture chamber for 48 h prior to visual inspection for the presence or absence of a precipitation reaction (i.e., development of a band). Results were reported as negative or M-band, H-band, or H- and M-band positive, as appropriate (Fig. 1).
FIG 1.
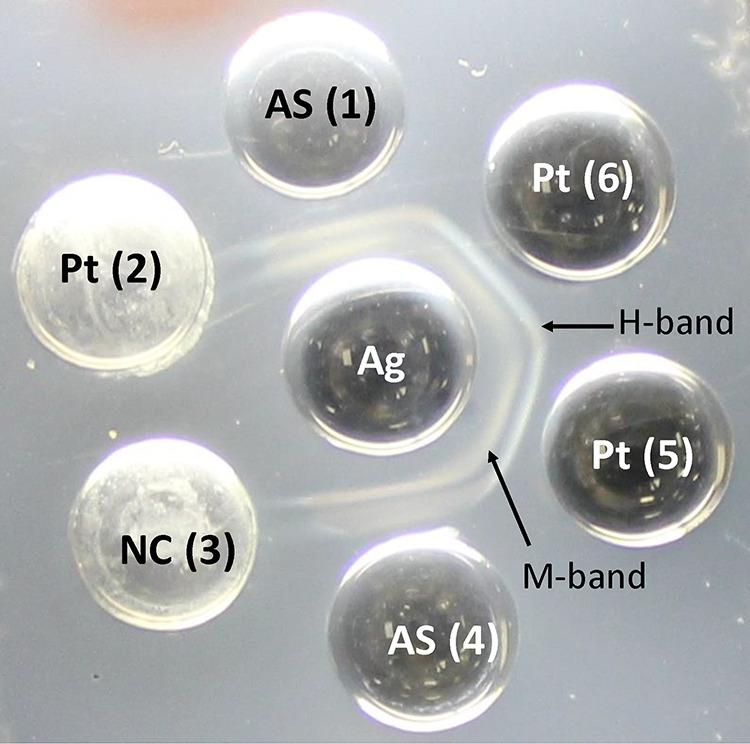
H. capsulatum immunodiffusion assay. H- and M-bands are visible in both control wells with positive-control antisera (AS) and patient serum (Pt) wells 5 and 6. Both the negative-control (NC) well (well 3) and patient well 2 are negative for antibodies to H. capsulatum. Ag, H. capsulatum antigen.
Histoplasma capsulatum complement fixation.
Complement fixation was performed as previously described, with a few modifications (19). H. capsulatum mycelial (Myc) and yeast (Yst) CF antigens were purchased from IMMY (Normal, OK), while guinea pig complement, sheep blood, and anti-sheep hemolysin were acquired from Colorado Serum Company (Denver, CO). Briefly, sera are heat inactivated at 56°C for 30 min, cooled, and serially diluted (1:2 to 1:256) using veronal buffer diluent (VBD) into three separate 96-well microtiter plates using the Hamilton (Reno, NV) Microlab STARlet system. H. capsulatum yeast or mycelial antigen is added to two of the three 96-well microtiter plates containing serially diluted sera. VBD is added instead of antigen to the third plate containing serially diluted sera and serves as the control plate to assess anticomplementary activity. Plates are incubated at room temperature for 20 min prior to the addition of titrated guinea pig complement to all three replicate plates, after which the plates are covered and refrigerated at 2°C to 4°C for 15 to 18 h. Following acclimation to room temperature, hemolysin-sensitized sheep red blood cells (RBCs) are added to each 96-well microtiter plate, which is subsequently gently mixed, sealed, and incubated in a 37°C water bath for 30 min. Plates are briefly centrifuged and refrigerated until they are ready to be visually read for percent hemolysis relative to a hemolysis color standard, freshly prepared on each day of testing. The reciprocal of the highest serum dilution at which 0% to 30% hemolysis occurs is considered the endpoint titer. For samples with an endpoint titer of ≥1:256, the original sample is diluted 1:8 before repeating the assay. The serum control plate (i.e., with no H. capsulatum antigen added) is checked to ensure the absence of anticomplementary activity before reporting the results. H. capsulatum yeast or mycelial CF titers of 1:8 or higher are considered positive and are reported. Sera exhibiting 35% to 100% hemolysis are considered negative.
H. capsulatum direct detection methods.
Fungal culture and H. capsulatum real-time PCR (RT-PCR) from blood, respiratory specimens, and tissue were performed as part of routine clinical care, as described previously (5). H. capsulatum antigen testing in urine was performed at Mayo Clinic, as previously described, using analyte-specific reagents manufactured by IMMY (Norman, OK), or samples were submitted to MiraVista Diagnostics (Indianapolis, IN) (7). H. capsulatum serum antigen testing was performed at MiraVista Diagnostics.
Statistical analysis.
Descriptive statistics were used to evaluate the data using BlueSky Statistics software v.7.20 (BlueSky Statistics LLC, Chicago, IL, USA) and Social Sciences Statistics (20). Continuous variables were compared using the Mann-Whitney U test, while chi-square analysis was used for statistical analysis of categorical variables. Analyses were considered statistically significant if a P value of <0.05 was achieved.
RESULTS
Patient demographics.
H. capsulatum infection was confirmed by culture in 76 adult patients between 2011 and 2020 at our institution, of whom 67 (88%) had at least one H. capsulatum serology order during the disease episode. The median age of these 67 patients was 59 years (range, 19 to 80 years), 44 (65.7%) were male, 52 (77.6%) had underlying comorbidities (diabetes mellitus, hypertension, cardiovascular disease, or chronic kidney disease, etc.), and 51 (76.1%) had an underlying immunocompromising condition (none were HIV positive), including solid-organ transplant (n = 4), hematologic malignancy (n = 8), solid-organ malignancy (n = 2), and immunosuppressive therapy (steroids or tumor necrosis factor alpha [TNF-α] inhibitors, etc.) (n = 37) (Table 1 and data not shown). A total of 33 (49.3%) patients had disseminated disease, defined as the involvement of more than one organ system and/or a blood culture positive for H. capsulatum, whereas 34 (50.7%) had localized organ or site involvement. Among patients with disseminated histoplasmosis, eight were blood culture positive, with involvement of the lungs, bone marrow or oropharynx in six, two and one patients, respectively. For the remaining 25 patients with disseminated histoplasmosis, the lungs (15/25), bone marrow (9/25), liver (7/25), and spleen (6/25) were most frequently involved, with less frequent involvement of the lymph nodes (3/25), prosthetic valves (2/25), oropharynx (2/25), central nervous system (1/25), and adrenal glands (1/25). Organs or sites involved in localized histoplasmosis included the lungs (n = 23), musculoskeletal sites (n = 7), oropharyngeal sites (n = 3), and one case of ocular histoplasmosis. The diagnosis of H. capsulatum infection was made in the inpatient setting for 50 (74.6%) patients, 18 (36.0%) of whom were admitted to the intensive care unit and 13 (26%) of whom required mechanical ventilation.
TABLE 1.
Comparison of H. capsulatum CF and ID results among patients with culture-confirmed histoplasmosis (n = 67)
| Parameter | Value |
P value | ||
|---|---|---|---|---|
| Overall (n = 67) |
H. capsulatum CF/ID result |
|||
| Positive (n = 47)a | Negative (n = 20)b | |||
| Median age (yrs) at diagnosis (range) | 59 (19–80) | 58 (19–77) | 64 (46–80) | 0.023 |
| No. (%) of male patients | 44 (65.7) | 30 (63.8) | 14 (70.0) | 0.626 |
| No. (%) of immunocompromised patientsc | ||||
| Present | 51 (76.1) | 36 (76.6) | 15 (75.0) | 0.889 |
| Steroid use | 20 (39) | 16 (34) | 4 (20) | |
| TNF-α inhibitorsf | 11 (21.5) | 11 (23.4) | 0 (0) | |
| Malignancy | 10 (19.6) | 5 (10.6) | 5 (25) | |
| Solid-organ transplant | 4 (7.8) | 1 (2.1) | 3 (15) | |
| Other immunocompromising conditions | 6 (11.7) | 3 (6.4) | 3 (15) | |
| Absent | 16 (23.9) | 11 (23.4) | 5 (25.0) | |
| No. (%) of patients with comorbiditiesd | ||||
| Present | 52 (77.6) | 37 (78.7) | 15 (75.0) | 0.738 |
| Absent | 15 (22.4) | 10 (21.3) | 5 (25.0) | |
| Duration of Symptoms (No. [%] of patients) | ||||
| <1 month | 29 (43.3) | 20 (42.6) | 9 (45.0) | 0.76 |
| 1–3 months | 22 (32.8) | 17 (36.2) | 5 (25.0) | |
| >3 month | 14 (20.9) | 9 (19.1) | 5 (25.0) | |
| Asymptomatic | 2 (3.0) | 1 (2.1) | 1 (5.0) | |
| Extent of disease (No. [%] of patients) | ||||
| Localized | 34 (50.7) | 21 (44.7) | 13 (65.0) | 0.128 |
| Disseminatede | 33 (49.3) | 26 (55.3) | 7 (35.0) | |
| No. (%) of hospitalized patients | ||||
| Yes | 50 (74.6) | 35 (74.5) | 15 (75.0) | 0.963 |
| No | 17 (25.4) | 12 (25.5) | 5 (25.0) | |
A positive result includes CF and/or ID positivity.
Negative results are defined as CF and ID negative.
Immunocompromise includes solid-organ transplantation, hematologic malignancy, and the use of immunosuppressive therapy.
Comorbidities include diabetes mellitus, hypertension, cardiovascular disease, and chronic kidney disease, etc., excluding an immunocompromised status.
Disseminated disease is defined as the involvement of more than one organ system and/or blood cultures positive for H. capsulatum.
TNF-α, tumor necrosis factor alpha.
All patients had multiple diagnostic tests ordered for the assessment of H. capsulatum infection, and the initial diagnosis was established based on results from fungal culture, histopathology, H. capsulatum serology, H. capsulatum urine antigen testing, or H. capsulatum PCR testing in 22, 15, 12, 9, and 6 cases, respectively (data not shown). In two cases, the diagnosis was made by a combination of two tests with results finalized on the same date (i.e., positive PCR and serum H. capsulatum antigen test or positive histopathology and serology), and a diagnosis in one case was made based on the presence of intracellular inclusions consistent with H. capsulatum in a peripheral blood smear. H. capsulatum serologic testing was ordered for 29 patients presenting with <1 month of symptoms, 22 patients with 1 to 3 months of symptoms, and 14 patients presenting with >3 months of symptoms (Table 1). Two additional patients were asymptomatic at the time of presentation; however, due to abnormal findings on imaging performed for other purposes, both patients had H. capsulatum serologic testing ordered and underwent either lymph node biopsy or bronchoalveolar lavage (BAL) fluid collection for culture.
Performance of H. capsulatum serologic assays in different patient cohorts.
Among the 67 patients with culture-confirmed histoplasmosis, H. capsulatum CF and/or ID seropositivity was observed in 69% (20/29) of patients with acute symptoms (<1 month), 77.3% (17/22) of individuals with a subacute presentation (1 to 3 months), and 64.3% (9/14) of patients presenting with more than 3 months of disease; the difference in positivity rates between these groups was not statistically significant (P = 0.76) (Table 1). Likewise, CF and/or ID positivity rates were similar between immunocompromised and otherwise healthy individuals (70.6% versus 68.8% [P = 0.889]) (Table 1) in our patient cohort. Interestingly, H. capsulatum serologic testing was positive in all 11 patients treated with a TNF-α inhibitor, compared to positivity in 62.5% (25/40) of individuals with other immunocompromising conditions (P < 0.05) (data not shown). Finally, although we observed a trend toward low H. capsulatum CF/ID sensitivities in patients with localized versus disseminated infection (61.8% versus 78.8%), these rates were not statistically significant (P = 0.128).
Performance of H. capsulatum immunodiffusion and complement fixation assays.
H. capsulatum CF and/or ID assays were positive in 47 of the 67 patients with culture-confirmed histoplasmosis, for an overall combined sensitivity of 70.1% (Table 2). The individual sensitivities of the CF and ID assays in these patients were 65.7% (44/67) and 62.7% (42/67), respectively, and did not differ significantly (P = 0.719) (Table 2). Among the 47 H. capsulatum-seropositive patients, both the CF and ID assays were positive in 39 (83%) cases, while 5 (10.6%) and 3 (6.4%) patients were positive only by CF or ID, respectively (Table 3).
TABLE 2.
Clinical sensitivity of H. capsulatum immunodiffusion and complement fixation assays in culture-confirmed cases (n = 67)a
| Result | No. of assays positive in culture-confirmed cases (% of the total positive) |
Clinical sensitivity (%) |
|---|---|---|
| ID positive | ||
| Total | 42 | 62.7 |
| H-band only | 0 (0) | NA |
| M-band only | 27 (64.3) | NA |
| H- and M-bands | 15 (35.7) | NA |
| CF positive | ||
| Total | 44 | 65.7 |
| Ab to mycelial Ag | 3 (6.8) | NA |
| Ab to yeast Ag | 11 (25) | NA |
| Ab to mycelial and yeast Ag | 30 (68.2) | NA |
| Total CF and/or ID positive | 47 | 70.1 |
ID, immunodiffusion; CF, complement fixation; Ab, antibody; Ag, antigen; NA, not applicable.
TABLE 3.
Comparison of H. capsulatum CF and ID results among patients with culture-confirmed histoplasmosis (n = 67)a
| ID result | No. (%) of patients with result |
||||
|---|---|---|---|---|---|
| CF positive (n = 44) |
CF negative (n = 23) | ||||
| Mycelial Ab | Yeast Ab | Yeast + mycelial Ab | Total | ||
| Positive (n = 42) | |||||
| H- + M-bands | 1 | 0 | 13 | 14 | 1 |
| M-band | 2 | 7 | 16 | 25 | 2 |
| Total | 3 | 7 | 29 | 39 | 3 |
| Negative (n = 25) | 0 | 4 | 1 | 5 | 20 |
ID, immunodiffusion; CF, complement fixation; Ab, antibody.
(i) H. capsulatum complement fixation.
Among the 44 culture-confirmed patients with a positive H. capsulatum CF result, antibodies to both the yeast (Yst) and mycelial (Myc) antigens were detected in 30 (68.2%) cases, 29 of whom were also positive by the H. capsulatum ID assay (Tables 2 and 3). CF antibody titers ranged from 1:8 to 1:2,048, with a median of 1:128 for both antigens. H. capsulatum CF positivity rates in culture-confirmed patients did not differ significantly between individuals based on disease dissemination, immune status, or duration of symptoms prior to presentation (Table S1).
Antibodies to only the CF Yst antigen were detected in 11 (25%) patients, with titers ranging from 1:8 to 1:128 (median, 1:16), and notably, 4 of these patients were negative by ID (Table 3). The majority of the CF Yst-only-positive individuals presented with <1 month of symptoms (n = 7/11), were immunocompromised (n = 8/11), and approximately one-half (n = 5/11) had localized disease. Three (6.8%) of the 44 CF-positive patients were reactive for antibodies to the Myc antigen only, with a median titer of 1:32 (range, 1:8 to 1:256), and all 3 were also positive by H. capsulatum ID (Table 3). One patient each presented with either <1 month, 1 to 3 months, or >3 months of symptoms, and all three patients were immunocompromised. Overall, antibody reactivity to the CF Myc and/or Yst antigens was positive in five patients who were ID negative, and this positivity was the basis for the initial diagnosis for one individual (CF Yst titer of 1:8).
(ii) H. capsulatum immunodiffusion.
Among the 42 culture-confirmed patients with a positive H. capsulatum ID result, 15 (35.7%) showed H and M precipitation bands, and 27 (64.3%) had an M-band only (Table 2). Slightly more than one-half of the patients with either H- and M-bands or M-bands only presented with disseminated disease (8/15 and 15/27, respectively). A notably higher proportion of ID-seropositive patients presenting with <1 month of symptoms were M-band-only positive (16/18), whereas the H- and M-band and M-band-only positivity rates were equivalent in individuals with 1 to 3 months (8/15 and 7/15, respectively) or >3 months (4/8 and 4/8, respectively) of symptoms. Despite these trends, there was no statistically significant difference in ID positivity rates based on patient immune status, duration of symptoms prior to testing, or the extent of disease (Table S1).
Among the 15 patients with H- and M-band positivity, 14 were also CF positive, with all but one sample showing reactivity to both the Yst (median titer, 1:128) and Myc (median titer, 1:256) antigens (Table 3 and Table S2). Of the 27 samples with M-band-only precipitation, 16 were CF positive for antibodies to both Yst and Myc antigens, 7 were CF positive for antibodies to Yst antigen only, and 2 were reactive to the CF Myc antigen only (Table 3). The median CF Yst and Myc titers in the M-band-only ID-positive patients were both 1:32 and significantly lower than the CF antibody titers in the H- and M-band-positive samples (P < 0.05) (Table S2). Finally, three patients were positive by ID only (n = 2 for the M-band only, and n = 1 for the H- and M-bands), without any reactivity to either CF antigen, including one of the two patients for whom histoplasmosis was an incidental finding (Table 3).
(iii) H. capsulatum CF- and ID-negative patients.
H. capsulatum CF and ID testing was negative for 20 culture-confirmed patients, all but 2 of whom were immunocompromised or had a defined, underlying comorbidity. While histoplasmosis was an incidental finding for 1 asymptomatic patient, among the remaining 19 patients, 9 had <1 month of symptoms, and 5 each had either 1 to 3 months or >3 months of symptoms. Notably, 13 of the 20 (65.0%) patients had localized disease, including lung infection in 9 patients, 2 cases of tenosynovitis, and 1 case each of ocular infection and prosthetic knee joint infection. The initial diagnosis of these cases occurred by growth in culture or by histopathology in eight cases each, RT-PCR of BAL fluid in two patients, and antigen testing or peripheral blood smear in one case each.
(iv) Serial H. capsulatum serologic testing.
Eighteen of the 67 culture-confirmed patients (26.9%) had repeat serologic testing ordered within 18 months of diagnosis (median number of serial tests, 2; range, 1 to 4). All 18 patients were initiated on antifungal treatment at the time of diagnosis with either itraconazole alone (n = 11) or liposomal amphotericin B and itraconazole (n = 7), for a median duration of treatment of 18 months (range, 1 month to indefinite). Among the 18 serially tested patients, 6 were initially seronegative by both H. capsulatum CF and ID. Four remained seronegative upon repeat testing a median of 192 days after the initial assessment, and two eventually seroconverted. One patient became ID (M-band) and CF (titers, 1:32 for Myc antigen and 1:256 for Yst antigen) positive 37 days following the initial serologic assessment, while the second patient converted to CF Yst-antigen-only positive (titer, 1:32) 80 days following initial testing (data not shown).
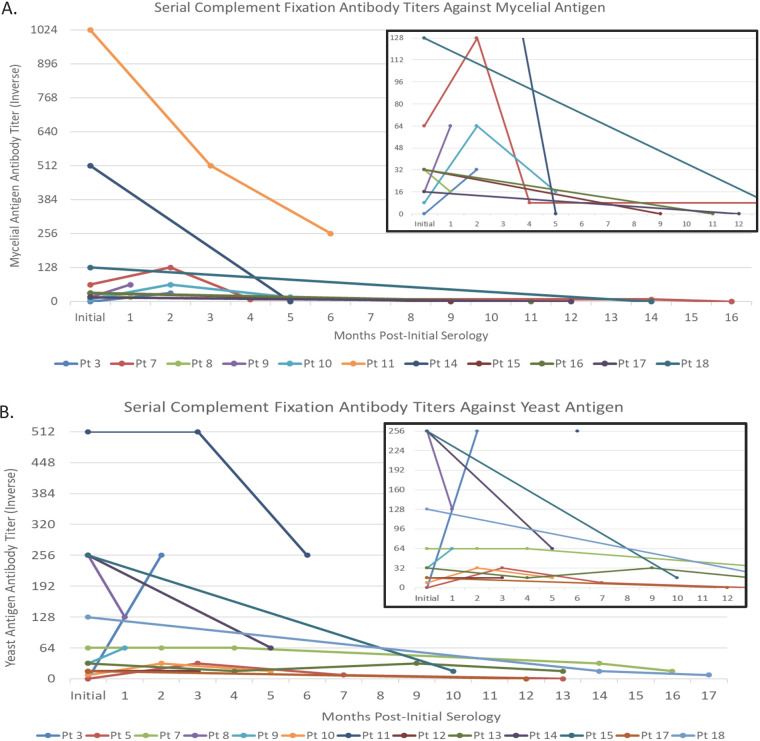
Among the 12 initially seropositive patients, all were positive by ID (n = 10 for the M-band only; n = 2 for the H- and M-bands). One patient with H- and M-band positivity remained positive for both bands 176 days after initial testing, while the second H- and M-band-positive patient converted to ID negative by day 397 after initial testing. Among the initially M-band-only-positive patients, one converted to H- and M-band positive by 60 days but reverted to M-band-only positive at 395 days and remained positive at the final repeat testing date 480 days after the initial diagnosis. The remaining nine M-band-only-positive patients remained positive at the last time point tested, which ranged from 20 days to 341 days after the initial diagnosis (median, 212 days). Evaluation of CF antibody titer trends in serially tested patients showed that antibodies to the Myc antigen reverted to negative more frequently (n = 6/11) than did anti-Yst antigen antibodies (n = 2/13) over time (Fig. 2). Among nine individuals with serial serology performed ≥3 months after initial testing, six remained Yst antibody positive (titer range, 1:8 to 1:64) but seroreverted to negative for antibodies to the Myc antigen.
FIG 2.
H. capsulatum complement fixation antibody titer trends among serially tested patients. Trends in antibody titers to the mycelial (A) and yeast (B) antigens are shown over time in serially tested patients (Pt). The insets in both panels A and B show zoomed-in views for patients with antibody titers of less than 1:256 and 1:512, respectively, to allow better resolution.
DISCUSSION
Serologic testing for histoplasmosis remains a frequently used approach for supporting the diagnosis of H. capsulatum infections, and although high-throughput EIAs are now available, the reference methods for antibody detection remain complement fixation (CF) and immunodiffusion (ID). These classic assays have been used for over 7 decades in clinical laboratories, and are currently performed primarily in large academic medical centers or reference laboratories due to their complexity. The performance characteristics that are frequently cited for these methods were initially established between the 1950s and 1980s. While the overarching CF/ID methods have remained unchanged during this time frame, there has been significant evolution in these techniques to allow for higher testing throughput, as well as differences in the manufacture and availability of assay reagents, which are now offered through a variety of different in vitro diagnostic companies (10, 14, 21–23). Therefore, in an effort to assess the present-day performance characteristics of these assays, with a focus on sensitivity, we performed a retrospective review of H. capsulatum CF and ID results in patients with culture-confirmed histoplasmosis over a 10-year period at our institution.
We report an overall CF/ID sensitivity of 70.1% among this patient cohort, which is notably lower than previously reported sensitivity rates for these methods, ranging from 72% to 94% among culture-confirmed patients (10, 22, 24). Additionally, we observed similar individual positivity rates for the CF (65.7%) and ID (67.7%) assays in our cohort, which is in contrast to original studies showing CF positivity rates nearly double those of ID (10). These differences are important to be aware of with respect to contemporary Histoplasma serologic testing and are likely due to a combination of variables, including differences in study design, the evaluated patient populations (especially as these studies are from many decades ago), the CF/ID reagents used (the use of different Histoplasma isolates for antigen isolation, hemolysin, sheep red blood cells, or agar gel plates, etc.), and the procedures used by the performing laboratories. Histoplasma CF and ID sensitivities are also known to vary depending on the extent of the disease, with higher rates of positivity among patients with subacute or chronic pulmonary infections (83% to 95%) than among those with acute pulmonary infections or progressive, disseminated disease (64% to 75%) (2, 10). While our study did not categorize patients into these same four groups, we did not find a significant difference in sensitivity between patients with localized and those with disseminated histoplasmosis, although the majority of negative results occurred in patients with focal infection.
The Histoplasma ID assay was first described by D. C. Heiner in 1958, who utilized an H. capsulatum mycelium-phase culture filtrate (i.e., histoplasmin) as the antigen to evaluate the presence of precipitin bands in agar gels after incubation with patient sera (14). While multiple different precipitin bands may form, two were more consistently associated with histoplasmosis, the H- and M-bands, which correlate to Histoplasma β-glucosidase and surface catalase, respectively (13, 21, 23). The M-band typically develops prior to the H-band, is often the only precipitin detected, and can persist for up to 3 years following infection, making a sole positive M-band result difficult to interpret (25). In contrast, H precipitins form after the M-band and disappear sooner, making the presence of H- and M-bands strong evidence for recent infection (10, 21). Consistent with these classic interpretive dogmas, we show that M-band-only patterns continue to be the ones most frequently observed in patients presenting within 1 month of symptoms, whereas M-band-only and H- and M-band patterns appear at similar rates in patients with a longer duration of symptoms.
CF testing for Histoplasma was similarly first described in the 1950s and has historically been associated with higher sensitivity than ID testing, although this is at the cost of specificity (70% to 80% versus >95%), with false-positive results being observed in patients with other dimorphic fungal infections, tuberculosis, and Legionella infections, among others (11, 13, 26). Both the classic and contemporary CF assays assess antibody reactivity against both Histoplasma mycelium-phase (i.e., histoplasmin) and yeast-phase antigens. Traditionally, the immune response to the Myc antigen (i.e., histoplasmin) has been associated with higher specificity (95% to 98% versus 85% to 95%), whereas anti-Yst phase responses have been associated with higher sensitivity (90% to 95% versus 70% to 75%) for histoplasmosis (2, 13, 27). Similar to previous studies, we documented higher CF anti-Yst antigen positivity rates, with 11 of the 44 CF-positive samples being reactive to the Yst antigen only, compared to 3 samples with only anti-Myc antigen reactivity. Additionally, while 4 of the 11 Yst-only CF-positive patients were ID negative, all 3 Myc-only-positive patients were ID positive. Conversely, among the 25 ID-negative sera, 5 were CF anti-Yst positive, with none showing anti-Myc antigen positivity. Collectively, these results bring into question the added value and role of CF testing for anti-Myc antibodies in the diagnosis of Histoplasma infections. This question is especially relevant as CF testing, in general, is highly manual, technically challenging, and subject to variable interpretations, with an increasing scarcity of quality reagents.
While serial Histoplasma serologic testing to monitor changes in CF titers or banding patterns is not routinely recommended following the initial diagnosis, nearly one-third of patients in our cohort (18/67) had at least one follow-up serologic test performed. Among these patients, the vast majority remained M-band positive at all serially tested time points, up to 16 months after the initial presentation, further confirming the limited role of serial ID testing after the initial diagnosis. CF Myc and Yst titers were also evaluated, and although both declined following the initiation of antifungal treatment, anti-Myc antibodies declined faster and seroreverted more frequently than did antibodies against the Yst antigen. Interestingly, while the original CF studies showed the rapid seroreversion of anti-Yst antibodies within 8 months of the initial diagnosis, the majority of patients in our cohort remained anti-Yst antibody positive beyond this time frame (26). Declining antibody titers have traditionally been considered indicative of disease resolution; however, to our knowledge, long-term follow-up with contemporary serologic assays to evaluate the significance of fluctuating titers has not been performed and remains of questionable clinical relevance (10).
Our study has several limitations that require acknowledgment. First, despite expanding our retrospective review to 10 years, the number of culture-confirmed patients with Histoplasma serologic testing available was low. This may have resulted in a failure to demonstrate significant differences in sensitivity when comparing different patient groups. Limiting our inclusion criteria to only patients with culture-confirmed Histoplasma infection may also have introduced bias, as not all patients with histoplasmosis had specimens obtained for culture, and alternative, noninvasive diagnostic methods are available (i.e., antigen and molecular). Furthermore, although considered the reference standard for the diagnosis of Histoplasma, culture is notoriously insensitive, and patients with culture-negative histoplasmosis diagnosed by serology or other means were excluded from this study. Additionally, the patient demographics were quite varied, limiting our ability to evaluate serologic assay performance in specific patient subgroups (disease extent, immune status, and symptom duration, etc.). Also, due to backorders and quality control issues, the reagents used for CF/ID varied during the study period; however, we did not observe any significant changes in assay positivity from year to year. Finally, our study focused only on the clinical sensitivity of CF/ID assays; we did not assess specificity in patients with alternative fungal or other infectious or noninfectious syndromes.
In conclusion, we provide a contemporary assessment of the sensitivity of Histoplasma CF and ID assays in patients with culture-confirmed histoplasmosis using materials and reagents that are commercially available to laboratories in North America. Compared to the original studies evaluating Histoplasma CF/ID sensitivity, which were performed largely between the 1950s and 1980s and continue to be referenced in present-day discussions, our assessment showed somewhat lower seropositivity rates in culture-confirmed patients. As outlined above, the reasons for this are multifold, but should be considered when evaluating seronegative patients for whom the suspicion for histoplasmosis remains high on the differential diagnosis. Ultimately, however, serologic testing, including acute- and convalescent-phase assessments, remains a valuable asset to support the diagnosis of histoplasmosis, particularly when direct detection methods fail to identify an infection.
Footnotes
Supplemental material is available online only.
Contributor Information
Elitza S. Theel, Email: theel.elitza@mayo.edu.
Daniel J. Diekema, University of Iowa College of Medicine
REFERENCES
- 1.Kauffman CA. 2008. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 21:421–425. 10.1097/QCO.0b013e328306eb8d. [DOI] [PubMed] [Google Scholar]
- 2.Azar MM, Hage CA. 2017. Laboratory diagnostics for histoplasmosis. J Clin Microbiol 55:1612–1620. 10.1128/JCM.02430-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guedes HLDM, Guimarães AJ, Muniz MDM, Pizzini CV, Hamilton AJ, Peralta JM, Deepe GS, Jr, Zancopé-Oliveira RM. 2003. PCR assay for identification of Histoplasma capsulatum based on the nucleotide sequence of the M antigen. J Clin Microbiol 41:535–539. 10.1128/JCM.41.2.535-539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alanio A, Gits-Muselli M, Lanternier F, Sturny-Leclere A, Benazra M, Hamane S, Rodrigues AM, Garcia-Hermoso D, Lortholary O, Dromer F, Bretagne S, French Mycoses Study Group . 2021. Evaluation of a new Histoplasma spp. quantitative RT-PCR assay. J Mol Diagn 23:698–709. 10.1016/j.jmoldx.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Babady NE, Buckwalter SP, Hall L, Le Febre KM, Binnicker MJ, Wengenack NL. 2011. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J Clin Microbiol 49:3204–3208. 10.1128/JCM.00673-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Lei G-S, Lee C-H, Hage CA. 2015. Evaluation of two new enzyme immunoassay reagents for diagnosis of histoplasmosis in a cohort of clinically characterized patients. Med Mycol 53:868–873. 10.1093/mmy/myv062. [DOI] [PubMed] [Google Scholar]
- 7.Theel ES, Harring JA, Dababneh AS, Rollins LO, Bestrom JE, Jespersen DJ. 2015. Reevaluation of commercial reagents for detection of Histoplasma capsulatum antigen in urine. J Clin Microbiol 53:1198–1203. 10.1128/JCM.03175-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kauffman CA. 2007. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 20:115–132. 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheat LJ. 2009. Approach to the diagnosis of the endemic mycoses. Clin Chest Med 30:379–389. 10.1016/j.ccm.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Wheat J, French ML, Kohler RB, Zimmerman SE, Smith WR, Norton JA, Eitzen HE, Smith CD, Slama TG. 1982. The diagnostic laboratory tests for histoplasmosis: analysis of experience in a large urban outbreak. Ann Intern Med 97:680–685. 10.7326/0003-4819-97-5-680. [DOI] [PubMed] [Google Scholar]
- 11.Wheat J, French ML, Kamel S, Tewari RP. 1986. Evaluation of cross-reactions in Histoplasma capsulatum serologic tests. J Clin Microbiol 23:493–499. 10.1128/jcm.23.3.493-499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheat LJ, Kohler RB, Tewari RP. 1986. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. N Engl J Med 314:83–88. 10.1056/NEJM198601093140205. [DOI] [PubMed] [Google Scholar]
- 13.Guimaraes AJ, Nosanchuk JD, Zancope-Oliveira RM. 2006. Diagnosis of histoplasmosis. Braz J Microbiol 37:1–13. 10.1590/S1517-83822006000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiner DC. 1958. Diagnosis of histoplasmosis using precipitin reactions in agargel. Pediatrics 22:616–627. 10.1542/peds.22.4.616. [DOI] [PubMed] [Google Scholar]
- 15.Wheat LJ, Slama TG, Eitzen HE, Kohler RB, French ML, Biesecker JL. 1981. A large urban outbreak of histoplasmosis: clinical features. Ann Intern Med 94:331–337. 10.7326/0003-4819-94-3-331. [DOI] [PubMed] [Google Scholar]
- 16.Wheat LJ, Kohler RB, French ML, Garten M, Kleiman M, Zimmerman SE, Schlech W, Ho J, White A, Brahmi Z. 1983. Immunoglobulin M and G histoplasmal antibody response in histoplasmosis. Am Rev Respir Dis 128:65–70. 10.1164/arrd.1983.128.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Richer SM, Smedema ML, Durkin MM, Herman KM, Hage CA, Fuller D, Wheat LJ. 2016. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin Infect Dis 62:896–902. 10.1093/cid/ciw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsley MD. 2016. Serological and molecular diagnosis of fungal infections, p 503–534. In Detrick B, Schmitz JL, Hamilton RG (ed), Manual of molecular and clinical laboratory immunology, 8th ed. ASM Press, Washington, DC. [Google Scholar]
- 20.Stangroom J. 2022. Chi-square test calculator, on Social Science Statistics. https://www.socscistatistics.com/tests/chisquare2/default2.aspx. Accessed 2 June 2022.
- 21.Picardi JL, Kauffman CA, Schwarz J, Phair JP. 1976. Detection of precipitating antibodies to Histoplasma capsulatum by counterimmunoelectrophoresis. Am Rev Respir Dis 114:171–176. [DOI] [PubMed] [Google Scholar]
- 22.Bauman DS, Smith CD. 1975. Comparison of immunodiffusion and complement fixation tests in the diagnosis of histoplasmosis. J Clin Microbiol 2:77–80. 10.1128/jcm.2.2.77-80.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiggins GL, Schubert JH. 1965. Relationship of histoplasmin agar-gel bands and complement-fixation titers in histoplasmosis. J Bacteriol 89:589–596. 10.1128/jb.89.3.589-596.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sathapatayavongs B, Batteiger BE, Wheat J, Slama TG, Wass JL. 1983. Clinical and laboratory features of disseminated histoplasmosis during two large urban outbreaks. Medicine (Baltimore) 62:263–270. 10.1097/00005792-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Davies SF. 1986. Serodiagnosis of histoplasmosis. Semin Respir Infect 1:9–15. [PubMed] [Google Scholar]
- 26.Grayston JT. 1952. A study of the complement fixation reaction in histoplasmosis. J Lab Clin Med 40:90–101. [PubMed] [Google Scholar]
- 27.Wheat LJ. 2006. Improvements in diagnosis of histoplasmosis. Expert Opin Biol Ther 6:1207–1221. 10.1517/14712598.6.11.1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download jcm.01057-22-s0001.pdf, PDF file, 0.2 MB (159.2KB, pdf)