LETTER
The gold standard for diagnosing cholera is culture of fresh stool enriched in alkaline peptone water (APW) for 6 to 8 h with plating onto thiosulphate citrate bile salts (TCBS) agar, where Vibrio cholerae (Vc) appears as bright yellow colonies (1–3). Optimal methods for Vc recovery from frozen clinical samples have not been established (4).
We tested Vc recovery in stool and vomitus from patients with acute, severe cholera. Samples were collected from patients presenting to the International Center for Diarrheal Diseases Research, Bangladesh, prior to antibiotic treatment. A total of 50 mL vomitus and 50 mL stool from each participant was stored in 30% glycerol, and another 50 mL of each sample was stored without glycerol. Samples were immediately frozen at −80°C. Routine diagnostics were performed using fresh samples, including CFU counts of presumed Vc from samples plated on taurocholate-tellurite-gelatin agar (5). Among 20 individuals’ stool and vomitus samples, the mean recovery of Vc CFU/mL from fresh samples was 1.8 × 107 and 5.4 × 106, respectively. After shipping on dry ice and storage at −80°C for 1 year, samples were thawed and 100 μL was inoculated into Luria-Bertani broth (LB, Difco, Franklin Lakes, NJ) and APW (Oxoid, Basingstoke, United Kingdom) for enrichment, with subsequent plating onto LB and TCBS agar (Table 1). Next, 30 μL of each sample was also streaked directly onto LB, TCBS, and tryptic soy agar with 5% sheep’s blood (TSAb; Remel, Lenexa, KS). Direct plated and enrichment broths were incubated overnight at 37°C, and the next day, enrichment broths were plated onto agars listed in Table 1 for repeat overnight culture (2). Vc colonies isolated were confirmed by PCR amplification of cholera toxin subunit A and O1/O139 rfb regions (6). pH of frozen unpreserved samples was measured using a Sartorius meter (Gottingen, Germany), to determine if pH impacted ability to recover Vc. The icddr,b, Massachusetts General Hospital, and University of Washington approved this study.
TABLE 1.
Media used to isolate Vibrio cholerae from frozen, stored rice-water stool and vomitus from cholera patientsa
| VOMITUS |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No preservative |
Cultured from + glycerol |
|||||||||||||
| Patient | LB | TSAb | TCBS | LB > LB | APW > LB | LB > TCBS | APW > TCBS | LB | TSAb | TCBS | LB > LB | APW > LB | LB > TCBS | APW > TCBS |
| 1 | X | X | − | X | X | X | X | ND | ND | ND | ND | ND | ND | ND |
| 2 | − | − | − | − | − | − | − | − | − | − | − | X | − | X |
| 3 | − | − | − | − | − | − | − | − | − | − | − | − | − | X |
| 4 | − | − | − | − | − | − | − | X | X | − | − | X | − | X |
| 5 | − | − | − | − | − | − | − | X | X | − | − | X | − | X |
| 6 | − | X | − | X | X | − | − | ND | ND | ND | ND | ND | ND | ND |
| 7 | − | − | − | − | − | − | X | − | − | − | − | − | − | − |
| 8 | − | − | − | − | − | − | − | − | X | − | − | − | − | − |
| 9 | − | − | − | − | − | − | − | X | X | − | − | X | − | X |
| 10 | − | − | − | − | − | − | − | X | X | − | − | X | − | X |
| 11 | − | − | − | X | − | X | − | ND | ND | ND | ND | ND | ND | ND |
| 12 | − | − | − | X | X | − | X | − | − | − | − | − | − | − |
| STOOL | ||||||||||||||
| 1 | X | X | − | X | X | X | X | ND | ND | ND | ND | ND | ND | ND |
| 2 | X | X | − | X | X | X | X | ND | ND | ND | ND | ND | ND | ND |
| 3 | X | X | − | X | X | − | − | ND | ND | ND | ND | ND | ND | ND |
| 4 | − | − | − | X | X | X | X | ND | ND | ND | ND | ND | ND | ND |
| 5 | − | − | − | X | X | X | X | ND | ND | ND | ND | ND | ND | ND |
| 6 | − | − | − | X | X | − | − | ND | ND | ND | ND | ND | ND | ND |
| 7 | − | − | − | X | − | − | X | ND | ND | ND | ND | ND | ND | ND |
| 8 | − | − | − | − | − | − | − | X | X | − | X | X | − | X |
| 9 | − | − | − | − | − | − | − | X | X | − | − | X | − | − |
| 10 | − | − | − | − | − | − | − | X | X | − | − | − | − | − |
| 11 | − | − | − | − | − | − | − | X | − | − | − | − | − | − |
| 12 | − | − | − | − | − | − | − | − | X | − | X | − | X | − |
| 13 | − | − | − | − | − | − | − | − | − | − | − | − | X | − |
X represents successful isolation (the culture grew V. cholerae), “−” indicates a negative culture (V. cholerae did not grow). “>” denotes enrichment in broth (first set of initials) for 24 h prior to plating on agar (second set of initials). All direct plated samples were incubated at 37°C overnight, and enrichment samples underwent one overnight incubation in enrichment media and another after plating onto agar. Overnight enrichment was chosen because this interval was previously found to be equivalent to 6 to 8 h enrichment for Vc recovery (2). If samples stored without preservative did not yield Vc, glycerol-preserved samples were cultured. APW incubations were conducted in nonshaking culture, and LB incubations were in shaking culture at 225 rpm. Patient samples with no successful Vc isolations (7 stool samples and 8 vomitus samples) are not shown. ND, not done; LB, Luria-Bertani; TSAb, tryptic soy agar with 5% sheep’s blood; TCBS, thiosulphate citrate bile salts agar; APW, alkaline peptone water.
Vc isolation was successful in 13 of 20 frozen stool and 12 of 20 frozen vomitus samples (25/40, 63%). For patients in which isolation was successful, Vc yield from direct plating was comparable to gold standard enrichment methods. Direct plating of stool (LB or TSAb) was successful in 8 of 13 stool samples and 9 of 13 using APW enrichment (X2 value 1.7, P = 0.68). Isolation was successful in 7 of 12 vomitus samples with direct plating, and 9 of 12 with APW enrichment (X2 value 0.8, P = 0.39). Vc recovery was independent of pH or CFU counts from the fresh sample (Fig. 1) (7). Potential reasons for failure of Vc recovery include freezing-related killing and the possibility that Vc enters a viable but nonculturable state when frozen for long time periods (8). In summary, we found that direct plating of stool and vomitus onto LB or TSAb agar was a successful method for Vc isolation from frozen clinical samples (stool and vomitus) and may reduce supplies and labor needed for Vc isolation. While direct plating of samples onto nonselective media such as LB or TSAb allows growth of other bacteria, we found that the morphology of Vc colonies were easily recognizable in these frozen samples from acute illness.
FIG 1.
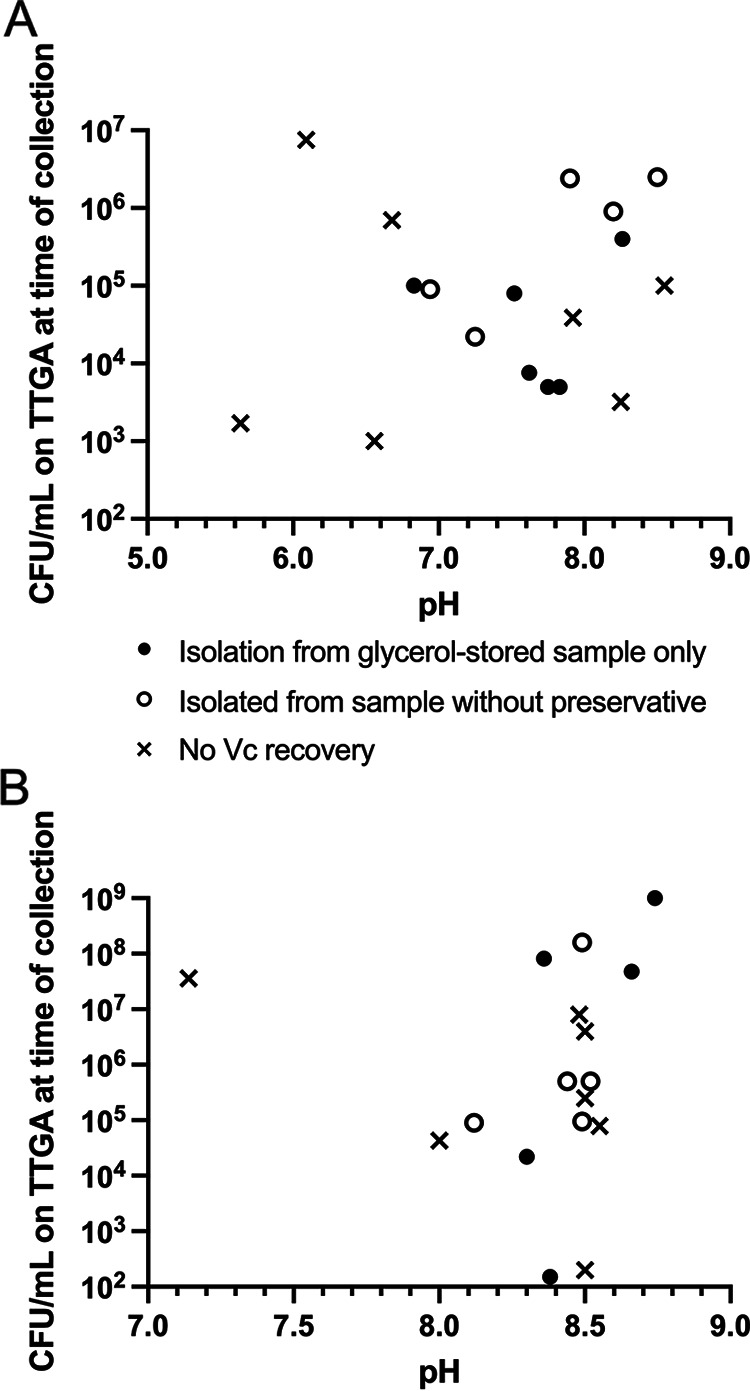
Vibrio cholerae (Vc) isolation and pH from a subset of frozen samples and the relationship between these factors and CFU count from the fresh sample; n = 18 for each sample type. Vc CFU were enumerated from fresh plating of stool selective tellurite taurocholate gelatin agar. (A) Vc isolation of vomitus and (B) stool samples. Each data point represents one sample. “Isolated from sample without preservative” indicates that Vc was isolated from samples stored in no preservative. If this method of isolation failed, samples stored in glycerol were attempted, and successful where “Isolation from glycerol-stored sample only” is indicated. If both glycerol-preserved samples and no preservative samples failed Vc isolation, “No Vc recovery” is shown. Three of 20 participants had one or both samples omitted from this figure: One study participant’s vomitus and stool samples are not included because only a glycerol culture for Vc was performed. One study participant’s fresh vomitus sample had no CFU on TTGA culture (and stool was TTGA Vc positive); thus, the vomitus sample was omitted from this figure. Another single study participant’s fresh stool sample had no CFU on TTGA culture (and vomitus was TTGA Vc positive); thus, the stool sample was omitted from this figure. One stool CFU count was “uncountable,” shown on this figure at 109.
ACKNOWLEDGMENTS
We thank the patients of the icddr,b where these samples were collected, and the staff at the icddr,b for data entry and sample collection. The icddr,b gratefully acknowledges the Government of the People’s Republic of Bangladesh; Global Affairs Canada; Swedish International Development Cooperation Agency and the Department for International Development. We also thank Jasneet Singh for his contributions to this project.
This work was supported in part by core support from the icddr,b (to F.Q.). This work was also supported by grants from the National Institutes of Health (AI106878 to E.T.R. and F.Q., AI058935 to E.T.R.; and AI123494 to A.A.W). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Ana A. Weil, Email: anaweil@uw.edu.
Nathan A. Ledeboer, Medical College of Wisconsin
REFERENCES
- 1.World Health Organization. 1987. Manual for the laboratory investigations of acute enteric infections. Program for Control of Diarrheal Diseases, World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Lesmana M, Richie E, Subekti D, Simanjuntak C, Walz SE. 1997. Comparison of direct-plating and enrichment methods for isolation of Vibrio cholerae from diarrhea patients. J Clin Microbiol 35:1856–1858. doi: 10.1128/jcm.35.7.1856-1858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rennels MB, Levine MM, Daya V, Angle P, Young C. 1980. Selective vs, nonselective media and direct plating vs. enrichment technique in Isolation of Vibrio cholerae: recommendations for clinical laboratories. J Infect Dis 142:328–331. doi: 10.1093/infdis/142.3.328. [DOI] [PubMed] [Google Scholar]
- 4.Dan M, Richardson J, Miliotis MD, Koornhof HJ. 1989. Comparison of preservation media and freezing conditions for storage of specimens of faeces. J Med Microbiol 28:151–154. doi: 10.1099/00222615-28-2-151. [DOI] [PubMed] [Google Scholar]
- 5.Khan AI, Rashid MM, Islam MT, Afrad MH, Salimuzzaman M, Hegde ST, Zion MMI, Khan AH, Shirin T, Habib ZH, Khan IA, Begum YA, Azman AS, Rahman M, Clemens JD, Flora MS, Qadri F. 2020. Epidemiology of cholera in Bangladesh: findings from nationwide hospital-based surveillance, 2014–2018. Clin Infect Dis 71:1635–1642. doi: 10.1093/cid/ciz1075. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino K, Yamasaki S, Mukhopadhyay AK, Chakraborty S, Basu A, Bhattacharya SK, Nair GB, Shimada T, Takeda Y. 1998. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol Med Microbiol 20:201–207. doi: 10.1111/j.1574-695X.1998.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 7.Huq A, West PA, Small EB, Huq MI, Colwell RR. 1984. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol 48:420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colwell RR, Huq A. 1994. Vibrios in the environment: viable but nonculturable Vibrio cholerae p 117–133. In Wachsmuth K, Blake PA, Olsvik O (ed), Vibrio cholerae and cholera: molecular to global perspectives. American Society for Microbiology. Washington, DC. [Google Scholar]


