Abstract
Elizabethkingia species often exhibit extensive antibiotic resistance and result in high morbidity and mortality, yet no systematic reviews exist that thoroughly characterize and quantify concerns for infected infants and children. We performed a review of literature and identified an initial 902 articles; 96 articles reporting 283 pediatric cases met our inclusion criteria and were subsequently reviewed. Case reports spanned 28 countries and ranged from 1944 to 2017. Neonatal meningitis remains the most common presentation of this organism in children, along with a range of other clinical manifestations. The majority of reported cases occurred as isolated cases, rather than within outbreaks. Mortality was high but has decreased in recent years, although neurologic sequelae among survivors remains concerning. Child outcomes can be improved through effective prevention measures and early identification and treatment of infected patients.
Keywords: Elizabethkingia, Chryseobacterium, Flavobacterium, children, emerging infectious diseases
Introduction
Elizabethkingia species were first described by Elizabeth O. King at the Communicable Disease Center (CDC, now Centers for Disease Control and Prevention) in 1959. The genus was previously classified as Flavobacterium and then reclassified in 1994 as Chryseobacterium before receiving its current taxonomic designation in 2005. They are non-glucose-fermenting, nonmotile, catalase- and oxidase-positive gram-negative rods ubiquitous in the environment that can colonize hospital environmental surfaces, and are known to be widely resistant to many classes of antibiotics. They can colonize human hosts but also cause symptomatic disease in adults such as pneumonia, meningitis, and endocarditis [1]. Adult disease typically occurs in people who are immunocompromised, with a high mortality rate reported [2]. E. meningoseptica (formerly F. meningosepticum and then C. meningosepticum) is particularly known to cause neonatal sepsis and meningitis, especially in premature newborns, sometimes leading to outbreaks in neonatal intensive care units (NICUs) [3]. The other named species of the genus—E. anophelis, E. endophytica, and E. miricola—are less common as reported sources of pediatric infection. However, recent literature suggest that many previous cases reported as E. meningoseptica were actually E. anophelis as these two species are difficult to differentiate by traditional microbiological methods [4]; whether E. endophytica is a distinct species is currently in question [5].
Two unrelated outbreak clusters of Elizabethkingia spp. occurring in the Midwest United States among adults were recently reported in Illinois and Wisconsin, shepherding new attention to this pathogen [6,7]. A neonatal case occurred in the same period and was described in news accounts yet was not associated with these outbreak clusters, as demonstrated by genetic sequencing [8]. Little knowledge exists regarding infants’ specific risks and needs, which is concerning given the elevated morbidity and mortality observed among neonatal cases of Elizabethkingia. Although a small number of reviews concerning this pathogen have been published in recent years, they have either not focused specifically on children [3] or did not employ a systematic review protocol [9]. In order to increase understanding of pediatric outbreak clusters and assist pediatric providers in being prepared for cases of this rare infection, we reviewed and characterized all cases of Elizabethkingia in children reported in the scientific literature dating back to the first instances of the bacterium’s isolation.
Methods
In order to identify studies that examine case reports of Elizabethkingia spp. in pediatric patients, we performed a systematic review [10] of the peer-reviewed and gray literature tailored to four electronic databases (PubMed, Scopus, Embase, and Global Health) using title, abstract, keyword, and Medical Subject Headings (MeSH) terms (see Supplemental Appendix 1 for search strategies and limits used). The reference lists of all papers were also reviewed for inclusion of additional reports. Following abstraction of the original set of papers in May 2016, we duplicated our search strategy in February 2017 to account for new papers published in the interim.
Inclusion/exclusion criteria
Papers were included if they reported on at least one human pediatric symptomatic infection (i.e., less than 18 years old) with bacteria currently classified as Elizabethkingia spp. Given the evolving taxonomy of Elizabethkingia spp. [5], we also examined studies that reported on cases of Flavobacterium meningosepticum and Chryseobacterium meningosepticum. Notably, we excluded studies in which the infectious organism was only specified to the genus level (e.g., Flavobacterium spp.) for genera other than Elizabethkingia. Only English-language papers were considered. No restrictions based on publication date or date of diagnosis were applied.
Reviews, commentaries, and other papers that described only non-primary data sources were excluded from data extraction to reduce the threat of duplicate publication bias, but we did examine their reference lists for additional articles. In some instances, papers contained some cases that we included because they were novel and others that we excluded because they had already been reported elsewhere; reviewers extracted data from the oldest reference.
Cases of colonization with Elizabethkingia spp. bacteria without clinical symptoms of infection were excluded, and only symptomatic cases from those papers, if any, were abstracted. Adult case reports of Elizabethkingia infection and non-human studies (e.g., analyses of microbiological isolates, animal studies) were all excluded.
Study selection
Once all identified bibliographic records from the four electronic databases were consolidated, with duplicates removed, the list of papers was divided evenly between two reviewers (MS and JLF) and another reviewer (EJD) separately reviewed all of them. These reviewers independently screened titles and abstracts using the aforementioned eligibility criteria, and iteratively discussed points of confusion. Finally, additional papers identified from reference lists during the data extraction process described below or additional searches were also subject to screening for inclusion criteria.
Data extraction
For the included papers, two reviewers (EJD and MS, JLF, or DB) independently extracted data by a standardized process, with discussions to resolve discrepancies on study parameters. The following information was extracted from each paper: publication year, country (and state, if U.S.), number of pediatric cases, age and sex of cases, clinical presentation, bacterial species, and outcomes of cases (recovered, died, or unknown). Reports were studied to identify which cases were documented as outbreak clusters and further describe those settings. For children who recovered from infection, reviewers noted documentation and descriptions of complications. For children who did not recover from infection, the number of days from onset of symptoms until death was recorded, if reported. If patients were reported to leave against medical advice, their outcome was classified as unknown. Notably, it was sometimes not possible to link unique cases with their respective outcomes due to how findings were reported in each paper. We opted not to assess information pertaining to antibiotic treatment, as this would likely be more of a function of evolving treatment options over time rather than information of clinical value.
No individual or cumulative assessments for risk of bias (e.g., selection, reporting, performance biases) among included papers were conducted.
Analytic approach
All included studies were entered into a database, and basic descriptive statistics were generated. We did not perform extensive analyses to determine statistically meaningful differences between groups, due to inconsistencies in reporting observations. The two exceptions were determining whether there were differences in mortality before vs. after 1990 (which was evaluated with Wilcoxon rank-sum tests) and whether there were differences in likelihood of death across age groups among patients with known outcomes (evaluated using Fisher’s exact tests). These tests were selected after determining that the dataset was not normally distributed.
Results
Overall findings
A total of 902 articles were retrieved from the initial May 2016 search (Figure 1). Five additional papers were identified during the abstraction process by examining the reference lists of included articles, and 14 papers were identified during the February 2017 iteration of the original search.
Figure 1.
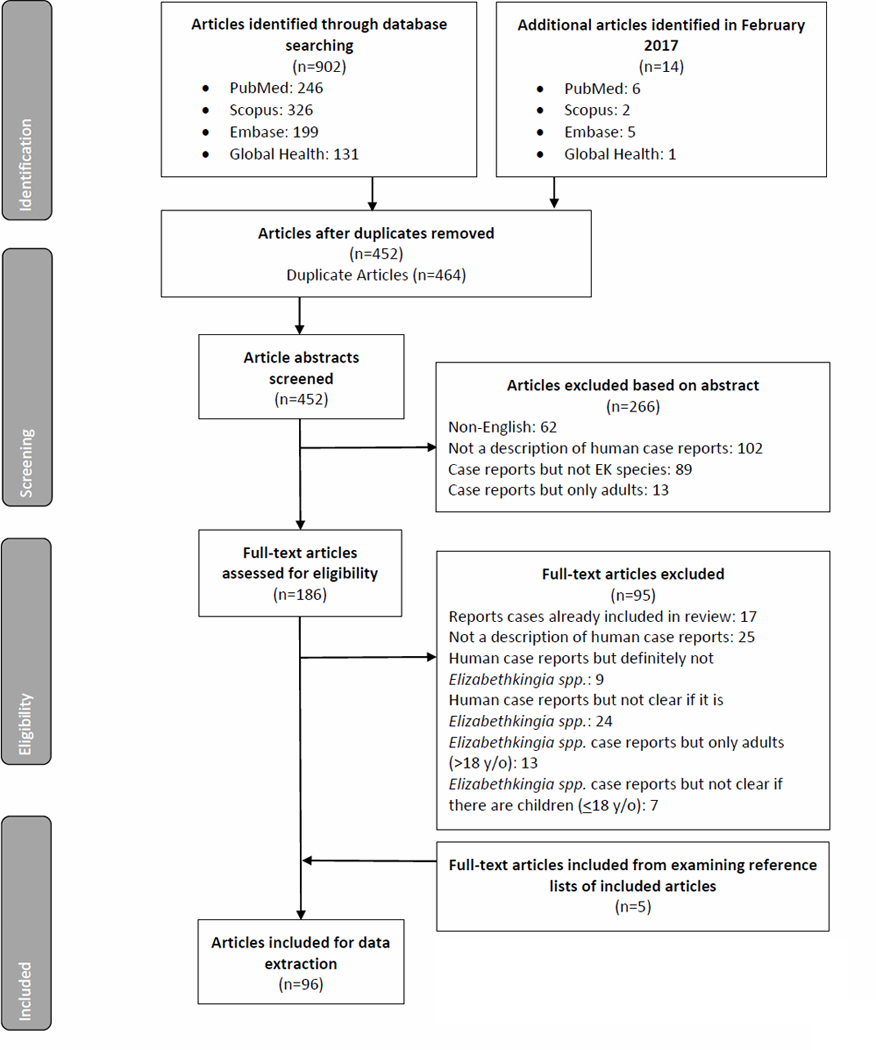
PRISMA flowchart for review on Elizabethkingia spp. Infection in pediatric patients [10]
Note: Several articles fit multiple exclusion criteria, but only one main exclusion criterion was applied in accordance with a pre-determined exclusion hierarchy.
Ninety-six reports describing 283 cases of Elizabethkingia infection in children met our inclusion criteria and were subsequently analyzed (Supplemental Appendix 2). Over three-quarters of cases were in neonates (less than one month of age), with smaller numbers in infants (one month to less than one year of age), children (one year to less than 18 years of age), and children of unknown age (Table 1). Of the 178 (62.9%) cases for which sex was reported, 97 (54.5%) were in males and 81 (45.5%) in females.
Table 1.
Reported outcomes of Elizabethkingia spp. case reports among patients aged 0–17 years reported in the peer-reviewed literature,a 1944–2017.
| Patient Outcomes, N (%) |
Total | |||
|---|---|---|---|---|
| Died | Recovered | Unknown Outcome | ||
|
| ||||
| Neonates (0–1 Months) | 81 (37.7) | 100 (46.5) | 34 (15.8) | 215 (76.0) |
| Infants (1–12 Months) | 0 (0.0) | 17 (94.4) | 1 (5.6) | 18 (6.4) |
| Older Children (1–17 Years) | 6 (17.1) | 18 (51.4) | 11 (31.4) | 35 (12.4) |
| Children of Unknown Age a | 2 (13.3) | 3 (20.0) | 10 (66.7) | 15 (5.3) |
| Total | 89 (31.4) | 138 (48.8) | 56 (19.8) | 283 |
For 98 cases (34.6%), there was reported evidence demonstrating that cases were part of an outbreak cluster. In total, 19 different outbreak clusters were described across 15 published reports, some exclusively in children and others including adults as well. Some clusters included colonized patients; however, the majority of the literature did not distinguish exposures or risk factors between colonized and symptomatic cases. Thirteen of the 15 reports (86.7%) describe cases in NICU or related settings (e.g., postnatal hospital nurseries). Another report described three outbreak clusters in the same pediatric hospital, with an index case from the NICU and spreading across five different units [11]. The remaining report described an outbreak in an intensive care unit where all affected patients (all but one being adults) were on bedside hemodialysis [12]. The remaining 185 (65.4%) cases occurred sporadically, or at least did not have reported evidence of connection to any outbreak cluster. In one case there was evidence of perinatal or intrauterine transmission [13].
Nearly all cases (277 [97.9%], from 92 reports) were described as E. meningoseptica. We identified only three reports of E. anophelis in children, representing five cases, all in reports since 2013. All but one occurred in Hong Kong; the other occurred in the Central African Republic, which also had a second case report of E. anophelis in a child but which was not included because the report was not in English [14,15]. All included cases of E. anophelis were in newborns and two (40%) died. There was only one identified pediatric case of E. miricola: a two-year-old child in Switzerland with a urinary tract infection whose outcome was not reported [16].
Time and location
Cases were described starting with a 1959 article identifying the new E. meningoseptica species (then F. meningosepticum), and retrospective analysis identified cases published back to 1944. A report of neonatal meningitis from 1922 was later postulated to potentially be Elizabethkingia species, but was not included because this identification was never confirmed [17,18]. Every decade from the 1940s onward included reported cases, with more pediatric cases reported after 2010 than in any previous decade despite the fact that our analysis included only papers published through February 2017. Analyzing the 47 reports (165 children) published prior to 1990 compared to the 49 reports (118 children) following, there is higher reported mortality prior to 1990 among all children with known outcomes (47.1% vs. 26.4%, p<0.001), with a similar result when limiting the comparison to neonates only (51.2% vs. 31.0%, p=0.004) or when including all children with any known or unknown outcome in the denominator (40.0% vs. 19.5%, p<0.001).
Reports originated in 28 countries. The United States had the largest number (71 cases from 23 reports), followed by India (35 cases from 14 reports), Taiwan (35 cases from nine reports), and Malaysia (32 cases from four reports). There were cases from all six inhabited continents, with the majority (165, 58.3%) arising from Asia. The 71 U.S. cases originated from 16 states and Puerto Rico. Local context of cases and outbreak clusters were described for some reports, including several originating from healthcare settings, but these data were reported inconsistently and could not be adequately quantified for this review.
Clinical presentation
Clinical presentation was described for 275 (97.2%) cases. Two hundred nine (73.9%) presented with meningitis. Sixty-seven (23.7%) presented with sepsis (with some overlap with diagnoses of meningitis), with 20 (7.1%) other cases having report of bacteremia. Symptoms at presentation included expected findings of meningitis or sepsis, including fever, lethargy, cyanosis, and apneic episodes. Forty-four (15.5%) were noted to have seizures and 19 (6.7%) with jaundice. Nineteen (6.7%) were reported to present with pneumonia, and seven (2.5%) with gastroenteritis or diarrhea, with bloody diarrhea in one case [19]. Other presentations less frequently reported were ventriculitis, pneumothorax, cellulitis, septic arthritis, urinary tract infection, peritonitis, sinusitis, and subdural abscess. Only one report described a rash, which was pustular and covered the genital region of the neonate, but it is unclear whether this was related to the Elizabethkingia infection [20]. Three cases had inflammatory eye findings described (conjunctivitis, discharge) while another had keratitis and corneal ulcer as the presenting infection in a teenaged wearer of contact lenses [21]. A number of cases, particularly nonneonatal ones, occurred in children with pre-existing medical conditions leading to immune suppression (e.g., leukemia, liver transplant), exposure to extensive invasive procedures (e.g., abdominal surgeries, shunt placements, peritoneal dialysis), or prolonged hospitalizations that increased exposure time to hospital-acquired infections, and some attributed the source of infection to hospital water supplies or medical equipment [22,23]. However, some cases were noted to be community-acquired [24–26] or even foodborne [27] infections, and a number of cases occurred in previously healthy children without known risk factors.
Outcomes
Among the 283 cases, 56 (19.8%) had an unknown outcome. Of the remainder, 138 were reported to have survived: 48.8% of all cases, and 60.8% of those with known outcome. Of these 138 known survivors, 66 (47.8%) were reported to have recovered completely with typical development, although length of follow-up was varied. Of the remaining surviving children, forty-two (30.4% of all survivors) were reported to have developed hydrocephalus following their infection, excluding a small number of cases where hydrocephalus was present prior to Elizabethkingia infection. Some additional cases had further details reported about the sequelae of infection, such as motor or cognitive deficits, spasticity, or ongoing seizures. At least nine (6.5%) surviving children had some degree of hearing loss specifically reported as a sequela. Nineteen (13.8%) surviving children did not have any further information on their outcomes.
Among all cases, 89 (31.4%) children were reported to die prior to recovery from Elizabethkingia infection; rates of death were different across age groups with highest mortality observed among neonates relative to all other age categories (37.7%; p=0.006) (Table 1). Of 45 (50.6%) cases where time from onset of symptoms to death was reported, the range was one to 192 days, with a mean of 27.7 days and median of 16 days.
Discussion
This review captures all identified cases of Elizabethkingia infection in children published in the English language literature, providing the most current source of information regarding the clinical aspects of this rare yet emerging infection. Numbers of reported cases are increasing in recent years, possibly associated with improved diagnostic capabilities in low-resource areas, and geographic distribution is widespread. Our results show that neonatal meningitis remains the most common presentation of this infection in children, but a variety of other clinical manifestations were also reported. Elizabethkingia infections in children are often fatal, and higher mortality than seen in this analysis has been described in earlier reviews [28,29]. Deaths were reported most commonly in our review among infected neonates, although some occurred among infected older children as well. The decreased mortality in more recent cases may be due to newer antibiotic options and increasing use of antimicrobial susceptibility testing, given the widespread antimicrobial resistance that frequently occurs in this genus; improved conditions of intensive care units; or differences in delay to diagnosis over time. However, the frequent reports of severe morbidity among survivors, especially hydrocephalus, developmental deficits, and hearing loss, also reinforce the importance of early identification and treatment before progression of neurologic damage can occur [3,26,30].
While outbreaks of Elizabethkingia spp. are concerning and can lead to poor outcomes, it is worth noting that the majority of cases reported in children occurred sporadically. Cases of Elizabethkingia spp. infection are typically avoidable, regardless of whether they are sporadic or in a cluster. Many cases were hospital-acquired infections from sources such as water supplies or medical equipment, making this an important nosocomial infection. Many children in hospitals have immune systems weakened by intensive medical interventions, malnourishment, prematurity, or any of a number of other chronic or infectious conditions, and are vulnerable to opportunistic pathogens. Proper infection control protocols are essential to avoid contaminated sources introducing these bacteria to children who may be at greatest risk of disease.
Retrospective analysis of isolates from cases described as E. meningoseptica have shown many infections to have been caused by E. anophelis. Using newer advanced molecular identification techniques may provide further insights into clinical differences between these species and possibly identify E. anophelis as the causative agent in more pediatric infections [31]. The single case of E. miricola in a two-year old child with a urinary tract infection did not have a reported outcome, and more experience with this species will be necessary to determine its relative pathogenicity [16].
Several unusual presentations of E. meningoseptica in children have been reported that may be useful for clinicians to keep in mind. Gunnarsson, et al. reported a rare case in Iceland of a 17-year-old with septic arthritis following a puncturing injury to the knee [32]. A rare case of keratitis in Singapore was presented in a 14-year-old contact lens user, a condition previously reported in an adult [33]. Other unusual reports often occurred in immune-compromised children, such as sinusitis and bacteremia in a 16-year-old in the U.S. with Shwachman-Diamond syndrome [34] and a two-year-old in Singapore with acute lymphoblastic leukemia who developed septic shock from the bacteria after eating sushi [27]. Ratnamani and Rao described E. meningoseptica as an emerging nosocomial pathogen among patients on hemodialysis, including a three-year-old child [12].
Limitations and Strengths
Several limitations to this review merit consideration. As with any review, our selected protocol may have limited the full breadth of articles that could conceivably contain relevant case reports. Specifically, exclusion of studies written in non-English languages could result in our findings representing geographic bias and an undercount (we excluded 62 non-English articles out of 452 total reviewed reports). Another source of underestimation may have arisen from the several articles that we did not include due to a lack of explicit statement of our species of interest—for instance, those that described Flavobacterium spp. without specifying beyond the genus level.
The heterogeneity in reporting of clinical presentation and outcomes of patients may be a function of a variety of factors that is challenging to quantify, such as journal word limits or outcome reporting bias; patients may have experienced symptoms and sequelae that were not reported, and we often could not determine the temporality of events (e.g., pre-existing conditions). This limitation precluded us from investigating questions, such as the proportion of infected patients that were born premature or sustained permanent neurologic sequelae. Consistently reporting these types of features in future case reports of Elizabethkingia in children will improve understanding of these infections. Relatedly, it was not always possible to causally link outcomes with Elizabethkingia infection [35]. While clinical disease from Elizabethkingia infection in children is often severe, there were also reported cases of asymptomatic colonization, even among neonates with E. meningoseptica, requiring no treatment and causing no sequelae [22,23]; this review was not intended to describe those cases. Finally, we acknowledge that included case reports may disproportionately represent locations with more resources (e.g., academic medical centers, NICUs, etc.) that could be more likely to have laboratory capacity to diagnose these infections and to seek dissemination of results in peer-reviewed outlets.
Although these limitations are notable, any other review protocol would be vulnerable to similar ones. To our knowledge, this is the first review that has investigated the literature on Elizabethkingia cases in pediatric patients using a systematic, reproducible protocol. Further, we note the strength of comprehensively including reported cases without restricting our search by geography, time, or evolving taxonomic classification. Further, although we were unable to identify gray literature that fulfilled our inclusion criteria, this review was not purposefully limited to peer-reviewed articles.
Conclusions
Elizabethkingia infections in children are often of high consequence and are associated with substantial morbidity and mortality, especially among neonates. In this review we identified pediatric cases, mostly sporadic but also in outbreak clusters, that go back over 70 years and span the globe, presenting predominantly as neonatal meningitis. We observed lower mortality in recent years, although neurologic sequelae among survivors remains concerning. More understanding of the evolving taxonomy of this genus is required to better characterize risks of individual species and target interventions appropriately.
Supplementary Material
Key Points:
A comprehensive literature review of cases of Elizabethkingia infection in children characterizes the epidemiology, demographics, clinical presentation, and outcomes. Cases were reported from 1944–2017 (n=283) demonstrating high mortality that decreased in recent decades, and substantial morbidity among survivors, especially neonates.
Acknowledgements:
We thank Bill Thomas, MLIS and Onnalee Gomez, MS for their guidance in the literature search. This research was supported in part by an appointment to the Research Participation Program at the CDC by the Oak Ridge Institute for Science and Education, through an interagency agreement between the U.S. Department of Energy and CDC.
Footnotes
Conflict of Interest Disclosures: None.
Disclaimers: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. All authors reviewed the final manuscript and approved submission.
References
- 1.Werthamer S, Weiner M. Subacute Bacterial Endocarditis Due to Flavobacterium meningosepticum. Am. J. Clin. Pathol. 1972; 57:410–412. [DOI] [PubMed] [Google Scholar]
- 2.Bloch KC, Nadarajah R, Jacobs R. Chryseobacterium meningosepticum: An Emerging Pathogen Among Immunocompromised Adults Report of 6 Cases and Literature Review. Medicine (Baltimore). 1997; [DOI] [PubMed] [Google Scholar]
- 3.Jean SS, Lee WS, Chen FL, Ou TY, Hsueh PR. Elizabethkingia meningoseptica: An important emerging pathogen causing healthcare-associated infections. J. Hosp. Infect. 2014; 86:244–249. [DOI] [PubMed] [Google Scholar]
- 4.Breurec S, Criscuolo A, Diancourt L, et al. Genomic epidemiology and global diversity of the emerging bacterial pathogen Elizabethkingia anophelis. Sci. Rep. 2016; 6:30379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doijad S, Ghosh H, Glaeser S, Kämpfer P, Chakraborty T. Taxonomic reassessment of the genus Elizabethkingia using whole-genome sequencing: Elizabethkingia endophytica Kämpfer et al. 2015 is a later subjective synonym of Elizabethkingia anophelis Kämpfer et al. 2011. Int. J. Syst. Evol. Microbiol. 2016; 66:4555–4559. [DOI] [PubMed] [Google Scholar]
- 6.Cluster Of Elizabethkingia Cases Identified In Illinois. Illinois Dep. Public Heal. 2016; Available at: http://dph.illinois.gov/news/cluster-elizabethkingia-cases-identified-illinois. Accessed 9 May 2017. [Google Scholar]
- 7.Navon L, Clegg WJ, Morgan J, et al. Notes from the Field : Investigation of Elizabethkingia anophelis Cluster — Illinois, 2014–2016. MMWR. Morb. Mortal. Wkly. Rep. 2016; 65:1380–1381. Available at: http://www.cdc.gov/mmwr/volumes/65/wr/mm6548a6.htm. Accessed 11 April 2017. [DOI] [PubMed] [Google Scholar]
- 8.Elizabethkingia: Recent Outbreaks. 2016. Available at: https://www.cdc.gov/elizabethkingia/outbreaks/.
- 9.Ceyhan M, Celik M. Elizabethkingia meningosepticum ( Chryseobacterium meningosepticum ) Infections in Children. Int. J. Pediatr. 2011; 2011:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman D. Preferred Reporting Items for Systematic Reviews and MetaAnalyses: The PRISMA Statement. PLoS Med 2009; 6 (7):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceyhan M A Chryseobacterium meningosepticum outbreak observed in 3 clusters involving both neonatal and non-neonatal pediatric patients. Am. J. Infect. Control 2008; 36:453–457. Available at: http://www.sciencedirect.com/science/article/pii/S0196655308000473. Accessed 13 October 2017. [DOI] [PubMed] [Google Scholar]
- 12.Ratnamani MS, Rao R. Elizabethkingia meningoseptica: Emerging nosocomial pathogen in bedside hemodialysis patients. Indian J. Crit. Care Med. 2013; 17:304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau SKP, Wu AKL, Teng JLL, et al. Evidence for Elizabethkingia anophelis transmission from mother to infant, Hong Kong. Emerg. Infect. Dis. 2015; 21:232–41. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25625669. Accessed 13 October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bobossi-Serengbe G, Gody JC, Beyam NE, Bercion R. First documented case of Chryseobacterium meningosepticum meningitis in Central African Republic. Médecine Trop. 2006; 66:182–184. [PubMed] [Google Scholar]
- 15.Frank T, Gody JC, Nguyen LBL, et al. First case of Elizabethkingia anophelis meningitis in the Central African Republic. Lancet 2013; 381:1876. [DOI] [PubMed] [Google Scholar]
- 16.Colapietro M, Endimiani A, Sabatini A, et al. BlaB-15, a new BlaB metallo-??-lactamase variant found in an Elizabethkingia miricola clinical isolate. Diagn. Microbiol. Infect. Dis. 2016; 85:195–197. [DOI] [PubMed] [Google Scholar]
- 17.Cooke J V, Bell HH. The incidence of meningitis in early infancy, with a description of two cases due to unusual organisms. Arch. Pediatr. Adolesc. Med. 1922; 24:387. [Google Scholar]
- 18.Ferlauto JJ, Wells DH. Flavobacterium meningosepticum in the neonatal period. South. Med. J. 1981; 74:757–9. [DOI] [PubMed] [Google Scholar]
- 19.Nadarajah M, Tan TH. Flavobacterium Meningosepticum Infections. Singapore Med. J. 1979; 20. [PubMed] [Google Scholar]
- 20.Senquiz AL. Neonatal meningitis by flavobacterium meningosepticum. Bol. Asoc. Méd. P. R 1987; 79:464–466. [PubMed] [Google Scholar]
- 21.Ray M, Lim DK. A Rare Polymicrobial Keratitis Involving Chryseobacterium meningosepticum and Delftia acidovorans in a Cosmetic Contact Lens Wearer. Eye Contact Lens 2013; 39:192–193. [DOI] [PubMed] [Google Scholar]
- 22.Hazuka BT, Dajani AS, Talbot K, Keen BM. Two outbreaks of Flavobacterium meningosepticum type E in a neonatal intensive care unit. J. Clin. Microbiol. 1977; 6:450–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoque SN, Graham J, Kaufmann ME, Tabaqchali S. Chryseobacterium (Flavobacterium) meningosepticum outbreak associated with colonization of water taps in a neonatal intensive care unit. J. Hosp. Infect. 2001; 47:188–192. [DOI] [PubMed] [Google Scholar]
- 24.Lin PY, Chu C, Su LH, Huang CT, Chang WY, Chiu CH. Clinical and microbiological analysis of bloodstream infections caused by Chryseobacterium meningosepticum in nonneonatal patients. J. Clin. Microbiol. 2004; 42:3353–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung P-P, Lin Y-H, Lin C-F, Liu M-F, Shi Z-Y. Chryseobacterium meningosepticum infection: antibiotic susceptibility and risk factors for mortality. J. Microbiol. Immunol. Infect. 2008; 41:137–44. [PubMed] [Google Scholar]
- 26.Sztajnbok J, Troster EJ. Community-acquired Chryseobacterium meningosepticum pneumonia and sepsis in a previously healthy child [7]. J. Infect. 1998; 37:310–312. [DOI] [PubMed] [Google Scholar]
- 27.Lee ACW, Ong NDSP. Food-borne bacteremic illnesses in febrile neutropenic children. Hematol. Rep. 2011; 3:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eykens A, Eggermont E, Eeckels R, Vandepitte J, Spaepen J. Neonatal meningitis caused by flavobacterium meningosepticum. Helv. Paediatr. Acta 1973; 28:421–5. [PubMed] [Google Scholar]
- 29.Dooley JR, Nims LJ, Lipp VH, Beard A, Delaney LT. Meningitis of Infants Caused by Flavobacterium Meningosepticum: Report of a Patient and Analysis of 63 Infections. J. Trop. Pediatr. 1980; 26:24–30. [DOI] [PubMed] [Google Scholar]
- 30.Gokce IK, Oncel MY, Ozdemir R, et al. Trimethoprim–sulfamethoxazole treatment for meningitis owing to multidrug-resistant Elizabethkingia meningoseptica in an extremely low-birthweight, premature infant. Paediatr. Int. Child Health 2012; 32:177–179. Available at: http://www.tandfonline.com/doi/full/10.1179/2046905511Y.0000000008. Accessed 9 October 2017. [DOI] [PubMed] [Google Scholar]
- 31.Teo J, Tan SY-Y, Tay M, et al. First case of E anophelis outbreak in an intensive-care unit. Lancet 2013; 382:855–856. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0140673613618589. Accessed 13 October 2017. [DOI] [PubMed] [Google Scholar]
- 32.Gunnarsson G, Baldursson H, Hilmarsdottir I. Septic Arthritis Caused by Chryseobacterium meningosepticum in an Immunocompetent Male. Scand. J. Infect. Dis. 2002; 34:299–300. [DOI] [PubMed] [Google Scholar]
- 33.Ali NAM, Reddy SC. Bilateral simultaneous infectious keratitis secondary to contact lens wear: an unusual case report with rare organisms. Eye Contact Lens 2007; 33:338–40. [DOI] [PubMed] [Google Scholar]
- 34.Skapek SX, Scott Jones W, Hoffman KM KM. Sinusitis and bacteremia caused by Flavobacterium meningosepticum in a sixteen-year-old with Shwachman Diamond syndrome. Paediatr. Infect. Dis. J. 1992; [PubMed] [Google Scholar]
- 35.Humphreys H, Lovering A, White LO, Williams EW. Flavobacterium meningosepticum infection, in a 32-day-old child on acute peritoneal dialysis, treated with ciprofloxacin. J. Antimicrob. Chemother. 1989; 23:292–294. [DOI] [PubMed] [Google Scholar]
- 36.King EO. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am. J. Clin. Pathol. 1959; 31:241–247. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi T, Hill DE, Steele RW. The use of ceftizoxime in neonates. J. Antimicrob. Chemother. 1982; 10:297–301. [DOI] [PubMed] [Google Scholar]
- 38.Tsai M-H, Chu S-M, Hsu J-F, et al. Risk factors and outcomes for multidrug-resistant Gram-negative bacteremia in the NICU. Pediatrics 2014; 133:e322–9. [DOI] [PubMed] [Google Scholar]
- 39.Ayyagari A, Seghal R, Garg RK, Verma AD, Agarwal KC. Indian pediatrics : journal of the Indian Academy of Pediatrics. Indian Pediatr. 1988; 25:335–337. [PubMed] [Google Scholar]
- 40.De Oliveira Costa P, Atta EH, Da Silva ARA. Infection with multidrug-resistant gram-negative bacteria in a pediatric oncology intensive care unit: Risk factors and outcomes. J. Pediatr. (Rio. J). 2015; 91:435–441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


