Abstract
The recent outbreak of coronavirus disease 2019 (COVID-19) in Wuhan, China has spread rapidly around the world, leading to a widespread and urgent effort to develop and use comprehensive approaches in the treatment of COVID-19. While oral therapy is accepted as an effective and simple method, since the primary site of infection and disease progression of COVID-19 is mainly through the lungs, inhaled drug delivery directly to the lungs may be the most appropriate route of administration. To prevent or treat primary SARS-CoV-2 infections, it is essential to target the virus port of entry in the respiratory tract and airway epithelium, which requires rapid and high-intensity inhibition or control of viral entry or replication. To achieve success in this field, inhalation therapy is the most attractive treatment approach due to efficacy/safety profiles. In this review article, pulmonary drug delivery as a unique treatment option in lung diseases will be briefly reviewed. Then, possible inhalation therapies for the treatment of symptoms of COVID-19 will be discussed and the results of clinical trials will be presented. By pulmonary delivery of the currently approved drugs for COVID-19, efficacy of the treatment would be improved along with reducing systemic side effects.
Graphical abstract
Keywords: COVID-19, Inhalation therapy, Lung diseases, Pulmonary drug delivery
Introduction
Inhalation therapy
Medical inhalation therapy dates back at least 4000 years, the Indian traditional therapies of Ayurvedic and the ancient Egyptians and Persian prescription to treat respiratory disorders have been documented; however, the modern contemporary inhalation therapy began in the nineteenth century [1, 2].
Pulmonary delivery is the preferred route of administration for treatment of lung diseases especially in asthma and chronic obstructive pulmonary disease (COPD). Direct drug delivery to the lungs may offer a rapid onset of action with higher efficacy by lower doses which leads to the reduced systemic adverse effects [2].
For inhalation therapy, drug particle size is considered the most important parameter since drugs should reach the entire bronchial tree and in particular the small airways (or bronchioles). The inhaled particles with diameter between 5 and 10 μm may settle in the trachea and main bronchi while particles with diameter larger than 10 μm are trapped in mouth and pharynx and they can be absorbed systematically, especially if swallowed. Particles with smaller diameter between 2 and 5 μm can actually reach the small airways [1–3]. Mass median aerodynamic diameter (MMAD) is a key factor in optimizing the particle deposition in the lung [4, 5]. This is the major challenge in formulating pulmonary devices as reducing the size requires energy which can directly affects the cost and stability of formulation. The applicability to different drugs should also be concerned.
Inhaled particle characteristics
Patient physiological condition (breathing pattern, lung general health, and adherence to treatment) as well as aerosol physicochemical properties (shape, size, density, and hygroscopisity) are essential factors affecting inhaled aerosol particle deposition in the lungs. Inhaled particle aerodynamic diameter is one of the most important parameters to determine their deposition in different parts of the respiratory tract. Particles with diameter of 1–5 μm showed better lung deposition and consequently, the greater potential efficacy [6–9].
Aerosol generator devices
To provide drug delivery devices that are efficient for delivery of drugs to the respiratory tract and convenient to use for patients, different pulmonary devices have been designed and developed. The most commonly used inhaler devices are classified into three groups: nebulizers, pressurized metered-dose inhalers (pMDIs), and dry powder inhalers [10–12]. Choosing between different options depends on many factors including the drug physicochemical properties, ease of use, age of patient, treatment options, and industrial scale production concerns. Therefore, simplicity, easy use, reproducibility, clinical outcome, and patient preferences should be considered when designing a pulmonary drug delivery system [10].
Small volume nebulizers (SVNs)
Small volume nebulizers (SVNs) are designed to deliver medication liquids to the lungs. Preliminary nebulizers were first introduced to the market in mid 1800s and then electric and hand-bulb ones were developed between 1930 and 1950 [2]. Nebulizers are categorized to jet (or pneumatic), ultrasonic, and mesh nebulizers. The most remarkable characteristic of nebulizers which gained a wide popularity is their ease of use. Nebulizers need tidal breathing without requiring patient education and cooperation. Therefore, nebulizers are not only suitable for emergencies but also are useful for geriatrics and pediatrics with cognitive and physical impairments [11]. There is a major concern about using nebulizers in infectious respiratory diseases which mainly was encountered during SARA-CoV-2 pandemic. Nebulizers, especially the jet types with face mask, can be source of disseminating the infectious aerosols to the health care providers because of entrance to the nebulizer reservoir. It is recommended to use nebulizers with mouthpiece and filters on the large tube of the nebulizer as well as use of mesh nebulizers with mouthpiece and one-way filters [13].
Pressurized metered dose inhalers (p-MDIs)
Pressurized metered-dose inhalers (pMDI) are devices that deliver medication to the lungs using a pressurized flow which is produced by an inert propellant. MDIs are attractive since they are portable, hand-held drug delivery system and can deliver precise medication amounts in every actuation. pMDIs are resistant to bacterial contamination and humidity and are cost-effective. However, their effectiveness is highly dependent to patient breathing pattern and requires patient coordination between actuation of the device and the breath pattern. Children and patients with disability need spacers and face masks and assistance to use this device. A major drawback of this device is high deposition in the oropharyngeal region which requires loading high amounts to provide therapeutic levels of drug in the site of action [11, 14]. Compatibility of drug with the propellant and the metal canister and actuator, solubility or suspendability of drug in the propellant, possibility of nozzle obstruction, and very advanced design of the device are other concerns that may be challenging [11].
Dry powder inhalers (DPIs)
DPIs are aerosol generator devices that can deliver fine and micronized dry powders to the lungs. The market for this pulmonary device is increasing and many single and multi-dose DPIs have been developed. DPIs are free of propellants and do not need actuation-breath coordination [14]. No external force is required to use this device and their drug delivery is extremely dependent to the patient inhalation power. Therefore, DPIs are less useful for young, elderly, and neuromuscularly weak or altered mental patients [3, 11]. Size, morphology, density, hygroscopicity, and moisture content are important determinants of particle deposition in the respiratory tract [8, 15]. Dry powder inhalers are marketed as single-dose and multidose easy-to-use devices without the drawbacks of pMDIs. DPIs are suitable devices for delivery of drugs prone to hydrolysis or degradation in humid environment. Therefore, high stability of drug product is achieved. DPIs can be used for delivery of various drugs regardless of their solubility by applying efficient carrier particles [3, 15].
Inhalation therapy for lung diseases
Inhalation drug delivery, compared with other routes, is a promising non-invasive strategy and may be the most appropriate treatment for patients suffering from lung disorders. The market appeal for inhaled medications to treat lung disorders could confirm these advantages. Inhalation drug delivery to the lungs would provide rapid action with lower side effects especially in emergency situations such as asthma and COPD [11]. Although pulmonary drug delivery systems were first introduced for acute and chronic obstructive and allergic lung diseases, the need to locally treat pulmonary infections also improved development of these dosage forms. For example, fluconazole large porous particles for pulmonary cryptococcosis [7], inhaled zanamivir for influenza, inhaled ribavirin for respiratory syncytial virus infection [16], and pulmonary delivery systems for anti-tuberculosis drugs [6, 17] have been developed.
Choice of the device for pulmonary delivery of drugs is essentially related to the physicochemical and stability of the drug. Patient compliance and wish should also be noted, since it would affect the device efficiency as well [10]. Nebulized medications are frequently administered in both acute and chronic conditions especially in acute asthma, COPD, and cystic fibrosis [18].
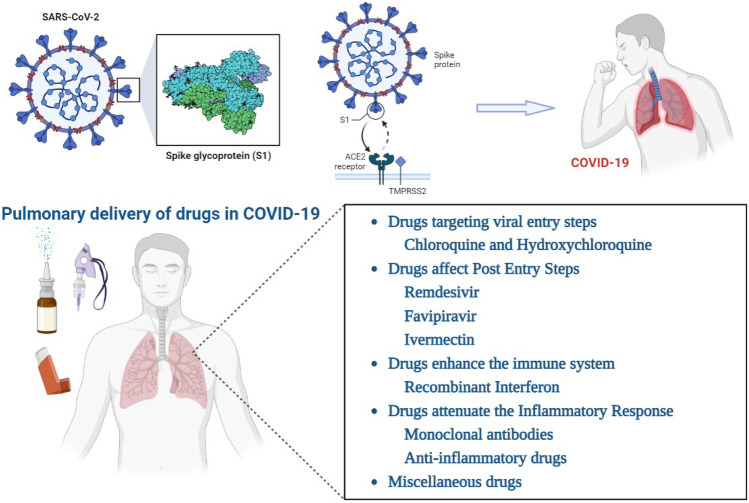
Since the symptoms of COVID-19 are mainly presented in the respiratory system, in this review, we aimed to summarize the studies related to pulmonary delivery of drugs used and tested in vitro, in animal, or in trials for COVID-19 pandemic. At first, the disease and its viral source are briefly reviewed. Then, the main targets of the virus cycle that could be used for drug therapy are explained and reports of pulmonary delivery of the drugs are presented.
Figure 1 shows the major benefits of pulmonary drug delivery for treatment of respiratory diseases.
Fig. 1.
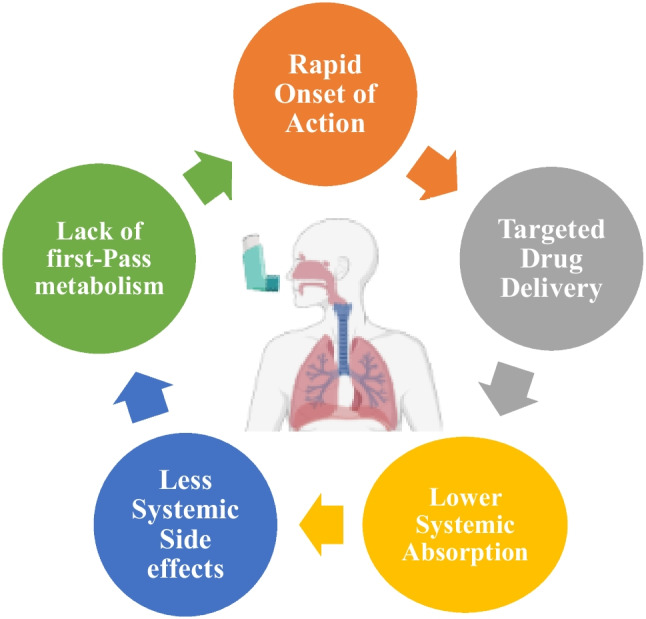
Advantages of pulmonary drug delivery
COVID-19
COVID-19 (coronavirus disease 2019) is a severe acute respiratory syndrome induced by a new RNA coronavirus from the same family of viruses causing SARS and MERS in 2002 and 2012, respectively. This new virus which was called SARS-CoV-2 was originated in mainland China which rapidly spread worldwide and soon posed a global life-threatening pandemic respiratory infection [19–21]. Current evidence suggests that COVID-19 spreads between people through direct, indirect, or close contact with infected people via mouth and nose secretions. These include saliva, respiratory secretions, or secretion droplets. The ingestion of droplets in the lungs leads to different degree of lower respiratory tract infections, ranging from mild respiratory infections to acute respiratory syndrome. Severity of symptoms presentation in COVID-19 patients is very different. The most common and prevalent symptoms are similar to other coronaviruses typical signs including flu-like syndrome such as fever and chills, cough, dyspnea, chest pain, muscular weakness, malaise, and fatigue. Meanwhile, in severe conditions, it can lead to viral pneumonia and finally, severe acute respiratory distress syndrome (ARDS) and even death [22–24]. Different respiratory, olfactory and gustatory, gastrointestinal, ophthalmic, neurological, dermatological, cardiac, renal, and rheumatologic manifestations as well as metabolic impairments and coagulopathy have also been reported in COVID-19 patients [22, 25]. Lung injuries were presented severely in high risk individuals such as elderly patients and those with chronic respiratory disease, cardiovascular disease, diabetes, cancer which may lead to progressive pneumonia and acute respiratory distress syndrome, multi-organ failure, and finally high death rates [21, 23, 26–28]. There is a huge number of co-infection with other viruses, fungi, and bacteria which complicates the symptoms and prognosis of the disease [29]. Based on the report by WHO, as of 26 May 2022, 524,878,064 confirmed cases of COVID-19, including 6,283,119 deaths have been recorded [30].
So far, it is evident that lungs are the major target of COVID-19 infection. Infected lungs are involved by severe complications including consolidation, gray-white viscous fluids, edema, and presence of colored hemorrhage, intra-alveolar fibrin accumulation surrounded by fibroblasts, hyaline membrane formation, infiltration of neutrophils, lymphocytes and macrophages into the alveoli, and lymphocytic inflammation [16, 28]. Because the route of infection and disease progression is primarily through the lungs, inhaled delivery of the drug directly to the lungs is the most appropriate prescription for COVID-19 treatment. Since the symptoms of COVID-19 are mainly manifested in the respiratory system, the International Association of Aerosols in Medicine (ISAM) has also called for development of inhalation therapies for treatment of COVID-19 [31].
SARS-CoV-2 virus structure and morphology
SARS-CoV-2 is a positive-sense RNA virus from the Coronaviridae family with a crown-like morphology results from the surface glycoprotein called spike protein (S-protein). S-protein constitutes of two units: the receptor binding unit (S1) and the membrane-fusion unit (S2). S-protein interacts with different receptors in the host cells and induces immune responses in the host [21, 24].
SARS-CoV-2 cell entrance mechanism
Coronaviruses have shown various mechanisms to enter host cells after receptor binding, including membrane fusion activities. Recent studies have reported in vitro binding ability of SARS-CoV-2 to the angiotensin converting enzyme 2 (ACE2) by viral surface spike glycoprotein (S protein). Transmembrane serine protease 2 (TMPRSS2) was shown to be essential for viral spike protein priming. Therefore, the level of cellular expression of virus receptors (ACE2 and TMPRSS2) plays an important role in virus entry and replication [28, 32, 33]. Due to the high expression rate of ACE2 in the respiratory tract and epithelial cells, COVID-19 can clearly damage the lungs by entry through this receptor [28, 34].
SARS-CoV-2 life cycle
The SARS-COV-2 life cycle consists of two distinct stages: virus entry and post-entry. The stages of virus entry include receptor recognition, endocytosis, and viral-membrane fusion. Post-entry stages include translation and replication of genomic RNA, virion assembly, maturation, and exocytosis [35, 36].
SARS-CoV-2 treatment options
Due to the lack of standard effective treatment for COVID-19, several known therapeutic agents which are in development process or approved for another indication, have been “repurposed,” and evaluated in COVID-19 patients [34]. Common categories are as follows:
Drugs targeting viral entry steps
Blocking virus–cell membrane fusion (receptor neutralizing agents)
Receptor neutralizing agents block virus–cell membrane fusion. Angiotensin-converting enzyme 2 (ACE2) is a pathway for COVID-19 to enter the human cells. Therefore, compounds which can directly bind to ACE2 receptor with high affinity can be suggested for competition with virus. There are some reports on using rhACE2 or angiotensin 1–9 and angiotensin 1–7 (anti-inflammatory angiotensin) to revert the impaired renin-angiotensin system back to the normal function [34, 37]. Opioid pain killers such as morphine and codeine can also impair virus entry through binding to ACE2 receptor [38].
Meanwhile, some evidences showed that SARS-CoV-2 may also use DPP4 (dipeptidyl peptidase 4 or CD26) to enter cells of the respiratory system and this interaction is very effective in the virulence of the virus. It seems that both DPP4 and ACE2 play a vital role in the virus cell entry mechanism. It will be concluded that DPP4 inhibitors, like gliptins, can be a strategy to combat COVID-19 inflammation in respiratory cells [39]. Yet, there is no report on pulmonary administration of these agents for treatment of COVID-19.
Chloroquine (CQ) and hydroxychloroquine (HCQ)
are antimalarial agents which are thought to impair terminal glycosylation of the virus ACE2, but not affecting the mammalian cell ACE2 and therefore, could be theoretically used in the treatment of COVID-19. Oral CQ and HCQ were examined in different clinical trials for COVID-19 and showed reducing load of the virus and reduced symptoms of pneumonia as well as improved lung function and imaging results [40]. Since effectiveness of these two drugs in treating COVID-19 is achieved in higher oral doses than usual, various gastrointestinal adverse effects (dysgeusia, dyspepsia, and stomachache) and toxicities (especially cardiac) may appear after oral administration [41, 42].
Pulmonary delivery of CQ and HCQ would provide the chance of lowering dose, reducing adverse effects and increasing the alveolar concentration. HCQ-induced cardiac toxicity after high oral doses was remarkable, while no cardiac adverse effect or significant electrocardiogram (ECG) changes were reported after administration of nebulized inhaled HCQ simple solution to healthy volunteers with 40-fold lower dose for COVID-19 treatment [43]. Another study confirmed that inhaled HCQ dry powder was safe and well tolerated in healthy volunteers except cough and bitter taste, thus, inhaled HCQ therapy may be suggested with a positive prognosis in COVID-19 treatment [44]. An inhalable dry powder of HCQ was formulated by jet-milling of HCQ sulfate. The particles showed successful inhalable characteristics in vitro which could be a potential treatment for COVID-19 [41]. Another potential formulation developed by Tai et al. was a mesh-nebulized HCQ aerosol which provided suitable properties for preclinical and clinical studies [45].
Drugs affect post entry steps
RNA-dependent RNA polymerase inhibitors
Remdesivir — a broad-spectrum antiviral agent — is a monophosphoramide prodrug that is metabolized intracellularly to an adenosine analog that can inhibit viral RNA polymerases and reduce viral RNA production. Previous studies have shown in vitro activity of remdesivir against coronarviruses including SARS-CoV-2. Appropriate clinical safety profiles of remedisivir were reported in approximately 500 healthy volunteers and patients treated for acute Ebola virus infection [46, 47]. Remdesivir is one of the two FDA approved medications for COVID-19 treatment [48]. Previous studies have reported remdesivir efficacy in shortening the recovery time and reducing respiratory infection in hospitalized adults with COVID-19 [49]. Remdesivir also reduced viral replication in nasal and bronchial epithelial cells [50]. Remdesivir is administered via intravenous (IV) injection. Intramuscular injection (IM) is not appropriate for remdesivir, due to the release variation from muscle after IM injection and oral administration of the drug is not successful, because of poor oral bioavailability and high hepatic metabolism. The problem with IV dosage form is that remdesivir is practically insoluble in water and requires an auxiliary solvent in its formulation. The solubility enhancer of remdesivir is sulfobutylether-beta-cyclodextrin (SBECD), which is cleared by the kidneys, so remdesivir use is limited in renal insufficiency. Some studies have also shown that IV administration could not provide effective lung concentration of remdesivir in both prodrug and metabolized forms [46, 48, 51]. In addition, remdesivir hepatotoxicity limits its use in high doses [48]. Remdesivir exhibited a 24-h residence time in rats’ lung tissue after a single dose of dry powder insufflation [46]. Both nebulizer and dry inhalation powder were hypothesized to be successful alternatives for pulmonary distribution compared to IV injection form [47, 48].
It was suggested that remdesivir in its current lyophilized formulation could be used for nebulization but the period of nebulizing should be short to assure the stability of drug in aqueous media. However, the efficacy and safety of this formulation still need to be confirmed in human. Dry powder inhaler of remdesivir can overcome problems and limitations of safety and stability [51]. A dry powder formulation of remdesivir has been developed by thin film freezing which provided aerosolized particles with high stability and sufficient distribution and dissolution in the lung fluid [46]. Another study used liposome as a nanocarrier for inhalation delivery of remdesivir. The liposomes made from FDA approved phospholipids provided an optimum MMAD and fine particle fraction (FPF) and sufficient stability for pulmonary delivery of remdesivir [52, 53]. It should be noted that pulmonary remdesivir could only be used in patients who have adequate respiratory function not ventilated cases. Two clinical trials are examining inhalable formulations of remdesivir for COVID-19 [48]. In one of these trials, remdesivir in an inhaled formulation with or without NA-831 (a neuroprotective and neurogenesis drug) was compared with placebo in healthy volunteers to study safety and tolerability of remdesivir. Results of this trial have not been published, yet [54]. In another one, remdesivir in an aerosolized solution was compared with a placebo in patients with early stage COVID-19 to evaluate safety, efficacy, and pharmacokinetics of remdesivir. Outcome measure was viral load in saliva from baseline through day 7. Adverse effects were also investigated as the secondary outcome. Results showed decrease in viral load through 7 days without any mortality. Serious adverse effects were rare; one patient with acute myocardial infarction and one with COVID-19 pneumonia in the remdesivir group and two patients with COVID-19 pneumonia in placebo group were diagnosed [55]. Pharmacokinetics parameters were not reported in the study results and data has not yet been published in scientific journals.
Favipiravir is another competitive inhibitor of RNA polymerase which reduces replication and transcription of viruses’ RNA. It showed efficacy against influenza viruses resistant to standard treatments, Ebola virus, and SARS-CoV-2 as well. Studies on efficacy of favipiravir in COVID-19 patients are very controversial. Some clinical trials revealed satisfactory effects of favipiravir on reducing disease period, accelerating virus clearance and recovery, especially in mild to moderate cases, while others showed limited efficacy and no observed changes in mortality [56].
A solid lipid nanoparticle (SLN) formulation of favipiravir was developed for pulmonary delivery by hot evaporation method. The particle size and FPF were suitable for aerosol performance and the formulation showed promising in vitro antiviral activity against SARS-CoV-2 [57]. Focused on increasing drug solubility and reducing the dose, a co crystal of favipiravir and theophylline was produced by spray drying method. MMAD and FPF were suitable for pulmonary delivery and the crystals presented no toxic effects on A549 cell line [58]. There is also a hypothesis that combined pulmonary delivery of favipiravir and tocilizumab (antibody against interleukin-6 receptor that will be covered in the next pages) in a mucoadhesive nanostructured carrier would prolong drug retention in the alveolar region and control drug release in the disease site leading to higher efficacy [59].
Inhibiting nuclear transport
Ivermectin is an anthelmintic agent with known efficacy against some single strain RNA viruses, e.g., HIV-1 and dengue virus. Ivermectin can significantly reduce SARS-CoV-2 in-vitro replication by inhibiting the shuttle protein responsible for nucleocytoplasmic transport of the virus [60–62]. Oral ivermectin has been shown to cause lower mortality in COVID-19 patients [60], but effective plasma concentrations are not achievable with safe oral doses. Meanwhile, due to the potential benefits of ivermectin in hospital mortality, it is extensively used in COVID-19 treatment or prevention regimens and there are different registered clinical trials which have tested oral, parenteral, and nasal ivermectin alone or in combination with other medications. It was also hypothesized that its anti-inflammatory and immunomodulatory effects could be helpful [61, 62]. The greatest advantage of inhalation therapy is that the effective drug concentration is produced instantly in the lung tissue. Nebulized ivermectin showed a detectable concentration in rats’ lung within 7 days [62]. According to the previous reports on the safety of inhaled ivermectin without any adverse effects in animals, inhalation therapy appears to be a suitable drug delivery technique for COVID-19 [61]. Currently, one study is testing a nasal formulation of ivermectin for COVID-19 infection in human. In this parallel clinical study, ivermectin was administered as nasal spray (1 ml in each nostril two times daily) and compared with oral ivermectin against standard care (which was oxygen via mask or ventilator). Participants were Egyptian patients with mild to moderate symptoms of COVID-19 and positive PCR test. The primary outcome considered was negative PCR test [52]. Unfortunately, the results of this clinical trial have not been published, yet and the efficacy is not fully recognized.
Amantadine
approved antiviral drug for treatment of H1N1 influenza, has shown beneficial effects against SAR-CoV-2 infection. It inhibits transport of genetic material of the virus to the host cell nucleus [64, 65]. Islam et al. have reviewed possible positive outcome of pulmonary delivery of amantadine alone or in combination with other drugs in controlling the symptoms of COVID-19 patients especially in cases with Parkinson’s disease [66]. In a clinical study on patients with influenza A infection, amantadine at a dose of 1 g/100 ml distilled water was administered through a glass bulb nebulizer. Nebulizer produced a mist with a MMAD about 3 µm. Drug treatment was compared with placebo (distilled water). In amantadine-treated group, symptoms of respiratory illness were significantly decreased by day 2 of the study. Patients experienced very mild local side effects such as rhinitis and rhinorrhea without any pulmonary dysfunction or decreased function [67].
Enhancing the innate immune system
Recombinant interferon
Interferons are innate immune system response to viral infections. Interferon-α and interferon-β are in subgroup of interferon type I which are mainly produced by macrophages and bronchial epithelial cells, respectively, in viral infection [68–70]. Since localized inhalation therapy can reduce systemic adverse effects, while increasing medication concentration in the infected epithelium, the impact of inhaled interferon type I on COVID-19 Chinese patients has been studied. Nebulized IFN-α-2b was used in pneumonia associated with SARS-CoV in China and newer data also showed decreased mortality in COVID-19 patients. Indeed, encouraging results indicated that there was a significant association between IFN-β-1a use and patient survival, alone or in combination therapy [69, 71]. An open-label non-randomized clinical trial on moderately ill COVID-19 patients showed that inhalation aerosol of IFN-κ (a mild type-1 IFN) along with TFF2 (trefoil factor 2) which is a protective polypeptide with positive effects on airway repair, could present satisfactory effects in COVID-19 patients [26].
Attenuating the inflammatory response
Monoclonal antibodies (mAbs)
Recently, antibody-based therapy potential to mitigate virus spread and disease severity has been considered and two monoclonal antibodies are approved for the treatment of viral infections [72]. Based on huge promising data on using neutralizing antibodies against SARS-CoV and MERS-CoV, it seems that m-Abs could provide promising treatment results in COVID-19 as well [73]. Low lung concentrations and stability issues are main obstacles for using mAbs in respiratory diseases. Pulmonary delivery of mAbs may overcome common challenges in systemic administration of these agents [72, 73].
Immunomodulation therapy showed beneficial effects in the inflammatory phases of severe COVID-19. Tocilizumab, a recombinant human mAb against Interleukin-6 receptor (IL-6R), was first approved by FDA for cytokine release syndrome [59, 74]. Tocilizumab could turn-off the cytokine storm of COVID-19 by inhibiting IL-6R. IL-6 is one of the key factors in initiating cytokine storm [19]. A hypothesis on simultaneous pulmonary delivery of favipiravir and tocilizumab by mucoadhesive nanovesicles is being investigated for long-term retention of drugs in the lungs [59]. A major issue encountered in inhalation delivery of Abs is stability of Ab molecule during stresses of the nebulization process [75].
Anti-inflammatory drugs
Inhaled corticosteroids (ICSs) are the basis of anti-inflammatory therapy for chronic obstructive pulmonary disease (COPD) and asthma [76]. There are controversial logics behind the use of corticosteroids in viral infections. Corticosteroids may reduce the anti-viral immunity and increase the risk of pneumonia [77]. Studies reported that ICSs could decrease the inflammatory reactions in admitted patients of COVID-19; however, worsening of outcomes has been reported in some patients. Meanwhile, ICS could reduce replication of SARS-CoV-2 virus through suppressing ACE2 expression [77, 78]. Beneficial effects of ICSs especially ciclesonide inhalation have been observed on virus replication as well as pulmonary implications in admitted patients [78, 79]. It was also mentioned that ICS administration in COPD may lead to lower SARS-CoV-2 entry receptor, ACE2, expression, and may change COPD patients’ susceptibility to COVID-19 [80]. It is also should be considered that CSs have shown in vitro inhibitory effects on IL-6, which may help in preventing lung fibrosis in COVID-19 patients [78]. Taking all together, ICSs should be continued for patients who were on these treatments before onset of COVID-19 and changing drug regimens should be with strict controlling of the patient condition [76].
Miscellaneous drugs
Many different classes of herbal and traditional drugs have been considered and tested for use as inhalation therapy in COVID-19 [27]. One of these classes is essential oils. Essential oils have shown beneficial effects in controlling the symptoms of inflammation in SARS-CoV-1 infection. It is assumed that because of similarity between SARS-CoV-1 and 2, nebulized essential oils such as T. orientalis, J. oxycedrus, L. nobilis, Rosemary, Ravensara, Ravintsara, Tea Tree, Bergamot, Eucalyptus, Lemon balm, Thyme, Oregano, Fennel, Peppermint, Cinnamon, and Clove could be helpful in prevention and treatment of respiratory symptoms of COVID-19 [81, 82]. The precise mechanism of action of essential oils is not known, but it seems that a combination of different effects provides the benefits of these agents. Essential oils are lipophilic agents and this nature provides the opportunity for them to enter viral membranes and disrupt the membrane. They also present phagocytic activity, anti-inflammatory, bronchodilatory, and mucolytic effects that all together can alleviate the respiratory symptoms of COVID-19 [81]. Meanwhile, the ultimate beneficial and adverse effects of these therapeutics should be confirmed in clinical trials. An in-silico study on Indian herbal steam inhalation therapy (which is used for common cold) has shown positive effects of three plants; Vitex negundo, Justicia adhatoda, and Eucalyptus globules, against COVID-19 [83].
Lidocaine
is an anti-arrhythmic and local anesthetic agent, which showed cough suppression and anti-inflammatory response in asthmatic patients. There is a hypothesis that nebulized lidocaine may be useful in adjunctive therapy of COVID-19, but the supporting clinical data should be provided [84].
Furosemide
is a loop diuretic structurally related to tryptophan metabolite and shows anti-inflammatory effects through inhibition of cytokines [85]. Furosemide has been used previously in many pulmonary disorders and shows a broad spectrum inhibitory effects on different cytokines such as IL-6, IL-8, and TNF-α. In local therapy, it also presents some antiviral activity. It seems that furosemide inhalation could reveal beneficial effects in COVID-19, but the efficacy should be addressed by clinical trials [86].
Fenretinide
is a retinoid derivative with strong anti-tumor, antiviral, and anti-inflammatory effects. It shows antiviral activity against zika virus, dengue virus, RSV, HCV, and HIV and could inhibit acute lung injury and respiratory distress syndrome in pulmonary disorders. By these different anti-inflammatory and antiviral activities, it could be an effective agent against SARS-CoV-2 in the pulmonary formulations especially if be used with other therapeutic agents of this disease. Pulmonary formulations of fenretinide can provide high local drug concentration in alveoli and hence present rapid anti-inflammatory effects. Indeed, anti-viral effects of fenretinide in lower respiratory tract would enhance its efficacy. To be effective, pulmonary formulations of fenretinide need to provide particle sizes less than 2 µm and penetrate aqueous environment of the lower airways. This is the limitation of fenretinide, as it is hydrophobic in nature. Therefore, formulating in inclusion complexes with cyclodextrines or loading into nanomicelles could be an alternative solution [87].
GSH and NAC
These two anti-oxidant agents, which act in redox imbalance, could inhibit oxidative stress and the resulting inflammatory cascade which leads to lung injury in COVID-19. Oral delivery of these agents is not very successful because of degradation in GI tract. Pulmonary delivery may provide therapeutic levels in the lungs, though clinical impact should be proved by evidences [88].
Nitric oxide gas (NO)
is a pulmonary vasodilator which can be used in COVID-19 patients in the case of refractory hypoxemia. NO gas inhalation has been tested in some clinical trial studies and beneficial effects have been confirmed in some patients but not all the cases [40, 89].
Adenosine
Inhaled adenosine is an off-label treatment in patients with severe lung injury to compensate the depletion of endogenous adenosine. A short-term analysis showed that inhaled adenosine is safe and effective in reducing inflammation in lungs affected by SARS-CoV-2; however, controlled clinical trials are required for further investigations [90].
After all, some other novel and encouraging therapeutic results also have been reported or hypothesized, e.g., hydrogen therapy [91], exosomes derived from allogenic adipose mesenchymal stem cells in the aerosol form, and DAS181 (a fusion protein) [40].
Tables 1 and 2 summarize the list of drugs designed or used for pulmonary delivery in COVID-19, their mechanism of action, and the phase of development.
Table 1.
List of drugs used in human or animals for pulmonary delivery in COVID-19 and their phase of development
| Drug formulation | Mechanism of action | Phase of development (Ref no.) |
|---|---|---|
| Hydroxychloroquin DPI | Receptor neutralization | Open-label phase 1a single ascending dose study [44] |
| Remdesivir DPI | RNA polymerase inhibition | Pharmacokinetics study in rats [46] |
| Ivermectin nebulized aerosol | Nuclear transport inhibition | Pharmacokinetics study in rats [62] |
| Ivermectin nasal spray | Nuclear transport inhibition | Phase 2 study, Tanta University, Egypt (NCT04510233) [52, 63] |
| IFN-β-1a nebulized aerosol | Innate immunity enhancement | Phase 2 RCT (NCT04385095) [68] |
| IFN-κ -TFF2 inhalation aerosol | Innate immunity enhancement | Open-label non-randomized CT [26] |
| Ciclesonide inhalation aerosol | Inflammatory response attenuation | Case report study [79] |
| NO gas | May improve oxygenation index | Case report [89] |
| Adenosine inhalation | May control inflammatory storm and virus replication | Short-term analysis in patients [90] |
Table 2.
List of drugs in the in vitro studies or hypothesis phase for pulmonary delivery in COVID-19 and their phase of development
| Drug formulation | Mechanism of action | Phase of development (Ref no.) |
|---|---|---|
| Hydroxychloroquin DPI | Receptor neutralization | In vitro characterization of delivery system [41] |
| Hydroxychloroquin nebulized aerosol | Receptor neutralization | In vitro characterization of delivery system [45] |
| Remdesivir liposomal aerosol | RNA polymerase inhibition | In vitro characterization of delivery system [53] |
| Favipiravir nebulized SLN | RNA polymerase inhibition | In vitro characterization of delivery system and toxicity study [57] |
| Favipiravir DPI | RNA polymerase inhibition | In vitro characterization of delivery system and toxicity study [58] |
| Favipiravir-tocilizumab nanovesicles | RNA polymerase inhibition — inflammatory response attenuation | Hypothesis [59] |
| IFN-α-2b nebulized aerosol | Innate immunity enhancement | In vitro characterization of delivery system and case report of 4 patients [69] |
| Essential oils inhalation | Not exactly determined (May control inflammatory symptoms) | Hypothesis and in-silico studies [81, 82] |
| Lidocaine nebulized aerosol | Not exactly determined (May control inflammatory response) | Hypothesis [84] |
| Furosemide inhalation | Not exactly determined (May control inflammatory response) | Hypothesis [86] |
| Fenretinide inhalation | May control inflammatory response | Hypothesis [87] |
| GSH & NAC inhalation | May control inflammatory response | Hypothesis [88] |
There are a huge number of studies and reports on therapeutic agents repurposed and examined or are under clinical trials for COVID-19 as main or adjunctive treatment via different administration routs. Only studies were included in this review that aimed at pulmonary drug delivery for treatment of COVID-19. Therefore, approved drugs for treatment of COVID-19 such as nirmatrelvir were not discussed, as there is no report on pulmonary delivery of this agent [92].
Conclusion and future prospects
Inhalation drugs have been developed as a suitable treatment option for lung diseases as well as many other infectious and non-infectious diseases and syndromes. Over the past decade, existing approaches have evolved significantly, opening up new avenues for the use of inhalation technology to effectively fight lung diseases. Despite the help of the international medical and scientific community and the recent decline in hospitalization, COVID-19 remains a major problem in the community health system.
Currently, most of the treatments used to treat COVID-19 are systemic, but recently, there has been a strong interest in establishing a non-invasive nasopharyngeal route for inhalation and delivery by inhalation to treat SARS-CoV-2 infection. It has been proposed as a promising and non-invasive treatment with unique benefits.
Several inhalation methods in phases one and two of the clinical trials are currently under investigation. Drug treatment by direct inhalation into the airways is a very valuable solution because it is the main route of infection and early transmission of the virus through the respiratory tract.
In addition, it is important to discover alternative ways to reduce the health risks associated with SARS-CoV-2, as well as to conduct further research on inhaled drugs and to develop innovative vaccines for other respiratory pathogens.
Acknowledgements
The authors would like to appreciate Shiraz University of Medical Sciences for providing institutional subscription access to scientific databases and publications.
Author contribution
All authors contributed to the study conception and design. Data collection and review were performed by Fatemeh Ahmadi, Shohreh Alipour, and Laleh Mahmoudi. The first draft of the manuscript was written by Shohreh Alipour and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The work was financially supported by Shiraz University of Medical Sciences Grant for review papers (grant no. 26525).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ information
The authors of this paper, Dr Fatemeh Ahmadi, Dr Laleh Mahmoudi and Dr Shohreh Alipour (Shiraz University of Medical Sciences, Iran) have several publications in the field of Pharmaceutics, Drug Delivery (specifically pulmonary drug delivery), and Clinical Pharmacy.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed with the content and all gave explicit consent to submit the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rogliani P, Calzetta L, Coppola A, Cavalli F, Ora J, Puxeddu E, et al. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med. 2017;124:6–14. doi: 10.1016/j.rmed.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Sorino C, Negri S, Spanevello A, Visca D, Scichilone N. Inhalation therapy devices for the treatment of obstructive lung diseases: the history of inhalers towards the ideal inhaler. Eur J Intern Med. 2020;75:15–8. doi: 10.1016/j.ejim.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Virchow JC, Crompton GK, Dal Negro R, Pedersen S, Magnan A, Seidenberg J, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10–9. doi: 10.1016/j.rmed.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Alipour S, Montaseri H, Khalili A, Tafaghodi M. Non-invasive endotracheal delivery of paclitaxel-loaded alginate microparticles. Journal of Chemotherapy. 2016;28(5):411–6. doi: 10.1080/1120009X.2015.1105624. [DOI] [PubMed] [Google Scholar]
- 5.Alipour S, Montaseri H, Tafaghodi M. Preparation and characterization of biodegradable paclitaxel loaded alginate microparticles for pulmonary delivery. Colloids and Surfaces B: Biointerfaces. 2010;81(2):521–9. doi: 10.1016/j.colsurfb.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 6.Parhizkar E, Sadeghinia D, Hamishehkar H, Yaqoubi S, Nokhodchi A, Alipour S. Carrier effect in development of rifampin loaded proliposome for pulmonary delivery: a quality by design study. Adv Pharm Bull. 2022;12(2):336–45. doi: 10.34172/apb.2022.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alipour S, Shirooee A, Ahmadi F. POROGEN EFFECTS ON AEROSOLIZATION PROPERTIES OF FLUCONAZOLE LOADED PLGA LARGE POROUS PARTICLES. Int J Appl Pharm. 2020;12(4):258–63. doi: 10.22159/ijap.2020v12i4.37453. [DOI] [Google Scholar]
- 8.Chaurasiya B, Zhao Y-Y. Dry powder for pulmonary delivery: a comprehensive review. Pharmaceutics. 2021;13(1). [DOI] [PMC free article] [PubMed]
- 9.Alipour S, Montaseri H, Tafaghodi M. Inhalable, large porous PLGA microparticles loaded with paclitaxel: preparation, in vitro and in vivo characterization. Journal of Microencapsulation. 2015;32(7):661–8. doi: 10.3109/02652048.2014.944949. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen S. Inhalers and nebulizers: which to choose and why. Respir Med. 1996;90(2):69–77. doi: 10.1016/S0954-6111(96)90201-2. [DOI] [PubMed] [Google Scholar]
- 11.Gregory KL, Wilken L, Hart MK. Pulmonary disease aerosol delivery devices. 3rd ed: Am Assoc Resp Care. 2017.
- 12.Sanchis J, Corrigan C, Levy ML, Viejo JL. Inhaler devices – from theory to practice. Respir Med. 2013;107(4):495–502. doi: 10.1016/j.rmed.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Sethi S, Barjaktarevic IZ, Tashkin DP. The use of nebulized pharmacotherapies during the COVID-19 pandemic. Ther Adv Respir Dis. 2020;14:1753466620954366. doi: 10.1177/1753466620954366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cazzola M, Cavalli F, Usmani OS, Rogliani P. Advances in pulmonary drug delivery devices for the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Deliv. 2020;17(5):635–46. doi: 10.1080/17425247.2020.1739021. [DOI] [PubMed] [Google Scholar]
- 15.Hamishehkar H, Pourtahmaseb M, Babazadeh A, Alipour S. Physicochemical and aerosolization assessment of inhalable spray dried fluconazole powder. Pharm Sci. 2018;24(3):219–26. doi: 10.15171/PS.2018.32. [DOI] [Google Scholar]
- 16.Abdellatif AAH, Tawfeek HM, Abdelfattah A, El-Saber Batiha G, Hetta HF. Recent updates in COVID-19 with emphasis on inhalation therapeutics: nanostructured and targeting systems. J Drug Deliv Sci Technol. 2021:102435. [DOI] [PMC free article] [PubMed]
- 17.Pham D-D, Fattal E, Tsapis N. Pulmonary drug delivery systems for tuberculosis treatment. International journal of pharmaceutics. 2015;478(2):517–29. doi: 10.1016/j.ijpharm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Swarnakar R, Gupta NM, Halder I, Khilnani GC. Guidance for nebulization during the COVID-19 pandemic. Lung India. 2021;38(Suppl 1). [DOI] [PMC free article] [PubMed]
- 19.Smetana K, Brabek J. Role of interleukin-6 in lung complications in patients with COVID-19: therapeutic implications. In Vivo. 2020;34 Suppl 3:1589–92. [DOI] [PMC free article] [PubMed]
- 20.Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, et al. Coronavirus disease 2019-COVID-19. Clin Microbiol Rev. 2020;33(4). [DOI] [PMC free article] [PubMed]
- 22.Lovato A, de Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J. 2020;99(9):569–76. doi: 10.1177/0145561320920762. [DOI] [PubMed] [Google Scholar]
- 23.Baj J, Karakuła-Juchnowicz H, Teresiński G, Buszewicz G, Ciesielka M, Sitarz E, et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9(6). [DOI] [PMC free article] [PubMed]
- 24.Fierabracci A, Arena A, Rossi P. COVID-19: a review on diagnosis, treatment, and prophylaxis. Int J Mol Sci. 2020;21(14). [DOI] [PMC free article] [PubMed]
- 25.Franchini M, Marano G, Cruciani M, Mengoli C, Pati I, Masiello F, et al. COVID-19-associated coagulopathy. Diagnosis (Berl). 2020;7(4):357–63. doi: 10.1515/dx-2020-0078. [DOI] [PubMed] [Google Scholar]
- 26.Fu W, Liu Y, Xia L, Li M, Song Z, Hu H, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478. doi: 10.1016/j.eclinm.2020.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silveira D, Prieto-Garcia JM, Boylan F, Estrada O, Fonseca-Bazzo YM, Jamal CM, et al. COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Frontiers in Pharmacology. 2020;11(1479). [DOI] [PMC free article] [PubMed]
- 28.Calabrese F, Pezzuto F, Fortarezza F, Hofman P, Kern I, Panizo A, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477(3):359-72. [DOI] [PMC free article] [PubMed]
- 29.Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, et al. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020;104(18):7777–85. doi: 10.1007/s00253-020-10814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/: WHO; 2022 [Updated 26 May 2022].
- 31.Mitchell JP, Berlinski A, Canisius S, Cipolla D, Dolovich MB, Gonda I, et al. Urgent appeal from International Society for Aerosols in Medicine (ISAM) during COVID-19: clinical decision makers and governmental agencies should consider the inhaled route of administration: a statement from the ISAM regulatory and standardization issues networking group. J Aerosol Med Pulm Drug Deliv. 2020;33(4):235–8. doi: 10.1089/jamp.2020.1622. [DOI] [PubMed] [Google Scholar]
- 32.Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. Embo J. 2020;39(10):e105114-e. [DOI] [PMC free article] [PubMed]
- 33.Saheb Sharif-Askari N, Saheb Sharif-Askari F, Al Heialy S, Hamoudi R, Kashour T, Hamid Q, et al. Cardiovascular medications and regulation of COVID-19 receptors expression. International Journal of Cardiology Hypertension. 2020;6:100034. doi: 10.1016/j.ijchy.2020.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu Y-F, Chien C-S, Yarmishyn AA, Lin Y-Y, Luo Y-H, Lin Y-T, et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iacob S, Iacob DG. SARS-CoV-2 treatment approaches: numerous options, no certainty for a versatile virus. Frontiers in Pharmacology. 2020;11(1224). [DOI] [PMC free article] [PubMed]
- 36.Yang K-C, Lin J-C, Tsai H-H, Hsu C-Y, Shih V, Hu C-MJ. Nanotechnology advances in pathogen- and host-targeted antiviral delivery: multipronged therapeutic intervention for pandemic control. Drug Delivery and Translational Research. 2021;11(4):1420-37. [DOI] [PMC free article] [PubMed]
- 37.Muslim S, Nasrin N, Alotaibi FO, Prasad G, Singh SK, Alam I, et al. Treatment options available for COVID-19 and an analysis on possible role of combination of rhACE2, angiotensin (1-7) and angiotensin (1-9) as effective therapeutic measure. SN Compr Clin Med. 2020:1-6. [DOI] [PMC free article] [PubMed]
- 38.Roshanravan N, Ghaffari S, Hedayati M. Angiotensin converting enzyme-2 as therapeutic target in COVID-19. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(4):637–9. doi: 10.1016/j.dsx.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solerte SB, Di Sabatino A, Galli M, Fiorina P. Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol. 2020;57(7):779–83. doi: 10.1007/s00592-020-01539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colalto C. Volatile molecules for COVID-19: a possible pharmacological strategy? Drug Development Research. 2020;81(8):950–68. doi: 10.1002/ddr.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed H. Albariqi, Rachel Yoon Kyung Chang, Waiting Tai, Wei-Ren Ke, Michael Y.T. Chow, Patricia Tang, et al. Inhalable hydroxychloroquine powders for potential treatment of COVID-19. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2021;34:1-12. [DOI] [PubMed]
- 42.Klimke A, Hefner G, Will B, Voss U. Hydroxychloroquine as an aerosol might markedly reduce and even prevent severe clinical symptoms after SARS-CoV-2 infection. Med Hypotheses. 2020;142:109783. doi: 10.1016/j.mehy.2020.109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavanagh O, Marie Healy A, Dayton F, Robinson S, O'Reilly NJ, Mahoney B, et al. Inhaled hydroxychloroquine to improve efficacy and reduce harm in the treatment of COVID-19. Med Hypotheses. 2020;143:110110. [DOI] [PMC free article] [PubMed]
- 44.de Reus YA, Hagedoorn P, Sturkenboom MGG, Grasmeijer F, Bolhuis MS, Sibum I, et al. Tolerability and pharmacokinetic evaluation of inhaled dry powder hydroxychloroquine in healthy volunteers. medRxiv. 2020:2020.12.03.20243162. [DOI] [PMC free article] [PubMed]
- 45.Tai W, Chow MY, Chang RY, Tang P, Gonda I, MacArthur RB, et al. Nebulised isotonic hydroxychloroquine aerosols for potential treatment of COVID-19. Pharmaceutics. 2021;13(8). [DOI] [PMC free article] [PubMed]
- 46.Sahakijpijarn S, Moon C, Koleng JJ, Christensen DJ, Williams Iii RO. Development of remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics. 2020;12(11). [DOI] [PMC free article] [PubMed]
- 47.Contini C, Enrica Gallenga C, Neri G, Maritati M, Conti P. A new pharmacological approach based on remdesivir aerosolized administration on SARS-CoV-2 pulmonary inflammation: a possible and rational therapeutic application. Med Hypotheses. 2020;144:109876. doi: 10.1016/j.mehy.2020.109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gatti M, De Ponti F. Drug repurposing in the COVID-19 era: insights from case studies showing pharmaceutical peculiarities. Pharmaceutics. 2021;13(3). [DOI] [PMC free article] [PubMed]
- 49.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–26. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang C-J, Wei Y-J, Chang H-L, Chang P-Y, Tsai C-C, Chen Y-H, et al. Remdesivir use in the coronavirus disease 2019 pandemic: a mini-review. Journal of Microbiology, Immunology and Infection. 2020. [DOI] [PMC free article] [PubMed]
- 51.Sun D. Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer aditional benefit. Aaps j. 2020;22(4):77. doi: 10.1208/s12248-020-00459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eedara BB, Alabsi W, Encinas-Basurto D, Polt R, Ledford JG, Mansour HM. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutics. 2021;13(7):1077. doi: 10.3390/pharmaceutics13071077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vartak R, Patil SM, Saraswat A, Patki M, Kunda NK, Patel K. Aerosolized nanoliposomal carrier of remdesivir: an effective alternative for COVID-19 treatment in vitro. Nanomedicine. 2021;16(14):1187–202. doi: 10.2217/nnm-2020-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pharmacokinetics of inhaled nanoparticle formulation of remdesivir (GS-5734) and NA-831 [Internet]. 2020. Available from: https://clinicaltrials.gov/ct2/show/study/NCT04480333.
- 55.Study in participants with early stage coronavirus disease 2019 (COVID-19) to evaluate the safety, efficacy, and pharmacokinetics of remdesivir administered by inhalation [Internet]. 2020. Available from: https://clinicaltrials.gov/ct2/show/results/NCT045392622020 . Accessed March 2022.
- 56.Łagocka R, Dziedziejko V, Kłos P, Pawlik A. Favipiravir in therapy of viral infections. J Clin Med. 2021;10(2). [DOI] [PMC free article] [PubMed]
- 57.Tulbah AS, Lee W-H. Physicochemical characteristics and in vitro toxicity/anti-SARS-CoV-2 activity of favipiravir solid lipid nanoparticles (SLNs) Pharmaceuticals. 2021;14(10):1059. doi: 10.3390/ph14101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong SN, Weng J, Ip I, Chen R, Lakerveld R, Telford R, et al. Rational development of a carrier-free dry powder inhalation formulation for respiratory viral infections via quality by design: a drug-drug cocrystal of favipiravir and theophylline. Pharmaceutics. 2022;14(2):300. doi: 10.3390/pharmaceutics14020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thakur V, Ratho RK, Panda JJ. Respiratory delivery of favipiravir-tocilizumab combination through mucoadhesive protein-lipidic nanovesicles: prospective therapeutics against COVID-19. VirusDisease. 2021;32(1):131–6. doi: 10.1007/s13337-021-00679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elkholy KO, Hegazy O, Erdinc B, Abowali H. Ivermectin: a closer look at a potential remedy. Cureus. 2020;12(9):e10378. doi: 10.7759/cureus.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mittal N, Mittal R. Inhaled route and anti-inflammatory action of ivermectin: do they hold promise in fighting against COVID-19? Med Hypotheses. 2020:110364. [DOI] [PMC free article] [PubMed]
- 62.Chaccour C, Abizanda G, Irigoyen-Barrio Á, Casellas A, Aldaz A, Martínez-Galán F, et al. Nebulized ivermectin for COVID-19 and other respiratory diseases, a proof of concept, dose-ranging study in rats. Scientific Reports. 2020;10(1):17073. doi: 10.1038/s41598-020-74084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okasha K. Ivermectin nasal spray for COVID19 patients https://clinicaltrials.gov/ct2/show/study/NCT045102332020 [Available from: Available online: https://clinicaltrials.gov/ct2/show/NCT04510233 (Accessed on 19 June 2021).
- 64.Cortés-Borra A, Aranda-Abreu GE. Amantadine in the prevention of clinical symptoms caused by SARS-CoV-2. Pharmacological reports : PR. 2021;73(3):962–5. doi: 10.1007/s43440-021-00231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grieb P, Świątkiewicz M, Prus K, Rejdak K. Amantadine for COVID-19. Journal of clinical pharmacology. 2021;61(3):412–3. doi: 10.1002/jcph.1802. [DOI] [PubMed] [Google Scholar]
- 66.Islam N, Rahman S. Novel pulmonary delivery of antiviral drugs for treating COVID-19 in patients with Parkinson’s disease. Curr Drug Deliv. 2022;19(3):260–5. doi: 10.2174/1567201818666210331121803. [DOI] [PubMed] [Google Scholar]
- 67.Hayden FG, Hall WJ, Douglas RG., Jr Therapeutic effects of aerosolized amantadine in naturally acquired infection due to influenza A virus. The Journal of Infectious Diseases. 1980;141(5):535–42. doi: 10.1093/infdis/141.5.535. [DOI] [PubMed] [Google Scholar]
- 68.Monk PD, Marsden RJ, Tear VJ, Brookes J, Batten TN, Mankowski M, et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet Respiratory Medicine. 2020;12. [DOI] [PMC free article] [PubMed]
- 69.Mary A, Hénaut L, Macq PY, Badoux L, Cappe A, Porée T, et al. Rationale for COVID-19 treatment by nebulized interferon-β-1b-literature review and personal preliminary experience. Frontiers in pharmacology. 2020;11:592543-. [DOI] [PMC free article] [PubMed]
- 70.Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral research. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peiffer-Smadja N, Yazdanpanah Y. Nebulised interferon beta-1a for patients with COVID-19. Lancet Respir Med. 2021;9(2):122–3. doi: 10.1016/S2213-2600(20)30523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parray HA, Shukla S, Perween R, Khatri R, Shrivastava T, Singh V, et al. Inhalation monoclonal antibody therapy: a new way to treat and manage respiratory infections. Appl Microbiol Biotechnol. 2021;105(16–17):6315–32. doi: 10.1007/s00253-021-11488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19) Asian Pac J Allergy Immunol. 2020;38(1):10–8. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 74.Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. The Oncologist. 2018;23(8):943–7. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mayor A, Thibert B, Huille S, Respaud R, Audat H, Heuzé-Vourc’h N. Inhaled antibodies: formulations require specific development to overcome instability due to nebulization. Drug Delivery and Translational Research. 2021;11(4):1625-33. [DOI] [PMC free article] [PubMed]
- 76.Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. European Respiratory Journal. 2020:2001009. [DOI] [PMC free article] [PubMed]
- 77.Singh D, Halpin DMG. Inhaled corticosteroids and COVID-19-related mortality: confounding or clarifying? The Lancet Respiratory Medicine. 2020;8(11):1065–6. doi: 10.1016/S2213-2600(20)30447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lipworth B, Kuo CR, Lipworth S, Chan R. Inhaled corticosteroids and COVID-19. Am J Respir Crit Care Med. 2020;202(6):899–900. doi: 10.1164/rccm.202005-2000LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases. Journal of Infection and Chemotherapy. 2020;26(6):625–32. doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Finney LJ, Glanville N, Farne H, Aniscenko J, Fenwick P, Kemp SV, et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol. 2021;147(2):510–9.e5. doi: 10.1016/j.jaci.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Asif M, Saleem M, Saadullah M, Yaseen HS, Al Zarzour R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology. 2020;28(5):1153–61. doi: 10.1007/s10787-020-00744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tushar P, Jayashri M, Pranali T. Inhalation of essential oils: could be adjuvant therapeutic steratey for COVID-19. Int J Pharm Sci Res. 2020;11(9).
- 83.Gowrishankar S, Muthumanickam S, Kamaladevi A, Karthika C, Jothi R, Boomi P, et al. Promising phytochemicals of traditional Indian herbal steam inhalation therapy to combat COVID-19 – an in silico study. Food and Chemical Toxicology. 2021;148:111966. doi: 10.1016/j.fct.2020.111966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali ZA, El-Mallakh RS. Nebulized lidocaine in COVID-19, an hypothesis. Med Hypotheses. 2020;144:109947. doi: 10.1016/j.mehy.2020.109947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee W-S, Lee S-M, Kim M-K, Park S-G, Choi I-W, Choi I, et al. The tryptophan metabolite 3-hydroxyanthranilic acid suppresses T cell responses by inhibiting dendritic cell activation. International Immunopharmacology. 2013;17(3):721–6. doi: 10.1016/j.intimp.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 86.Brennecke A, Villar L, Wang Z, Doyle LM, Meek A, Reed M, et al. Is inhaled furosemide a potential therapeutic for COVID-19? The American Journal of the Medical Sciences. 2020;360(3):216–21. doi: 10.1016/j.amjms.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orienti I, Gentilomi GA, Farruggia G. Pulmonary delivery of fenretinide: a possible adjuvant treatment in COVID-19. Int J Mol Sci. 2020;21(11). [DOI] [PMC free article] [PubMed]
- 88.Lana JFSD, Lana AVSD, Rodrigues QS, Santos GS, Navani R, Navani A, et al. Nebulization of glutathione and N-acetylcysteine as an adjuvant therapy for COVID-19 onset. Adv Redox Res. 2021;3. [DOI] [PMC free article] [PubMed]
- 89.Tavazzi G, Pozzi M, Mongodi S, Dammassa V, Romito G, Mojoli F. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Critical Care. 2020;24(1):508. doi: 10.1186/s13054-020-03222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Correale P, Caracciolo M, Bilotta F, Conte M, Cuzzola M, Falcone C, et al. Therapeutic effects of adenosine in high flow 21% oxygen aereosol in patients with Covid19-pneumonia. PLOS ONE. 2020;15(10):e0239692. doi: 10.1371/journal.pone.0239692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang M, Peng J, Hui J, Hou D, Li W, Yang J. Hydrogen therapy as an effective and novel adjuvant treatment against COVID-19. QJM: An International Journal of Medicine. 2020. [DOI] [PMC free article] [PubMed]
- 92.Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and nirmatrelvir-ritonavir: oral COVID antiviral drugs. Clinical Infectious Diseases. 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
The authors of this paper, Dr Fatemeh Ahmadi, Dr Laleh Mahmoudi and Dr Shohreh Alipour (Shiraz University of Medical Sciences, Iran) have several publications in the field of Pharmaceutics, Drug Delivery (specifically pulmonary drug delivery), and Clinical Pharmacy.