Abstract
Background
Stress hyperglycemia is strongly associated with poor clinical outcomes in patients with acute coronary syndrome (ACS). Recently, the stress hyperglycemia ratio (SHR) has been proposed to represent relative hyperglycemia. Studies regarding the relationship between SHR and mortality in coronary artery disease (CAD) are limited. This study aimed to clarify the association between SHR and in-hospital mortality in patients with CAD.
Methods
A total of 19,929 patients with CAD who were hospitalized in Beijing Hospital were enrolled in this study. Patients with an estimated glomerular filtration rate < 30 ml/min, cancer, or missing blood glucose/HbA1c data were excluded; therefore, 8,196 patients were included in the final analysis. The patients were divided into three groups based on tertiles of SHR: T1 group (SHR < 0.725, n = 2,732), T2 group (0.725 ≤ SHR < 0.832, n = 2,730), and T3 group (SHR ≥ 0.832, n = 2,734). The primary endpoint was in-hospital mortality.
Results
The overall in-hospital mortality rate was 0.91% (n = 74). After adjusting for covariates, SHR was significantly associated with in-hospital mortality in patients with CAD [odds ratio (OR) = 17.038; 95% confidence interval (CI) = 9.668–30.027; P < 0.001], and the T3 group had a higher risk of in-hospital mortality (OR = 4.901; 95% CI = 2.583–9.297; P < 0.001) compared with T1 group. In the subgroup analysis, the T3 group had an increased risk of mortality among patients with pre-diabetes mellitus (pre-DM) (OR = 9.670; 95% CI = 1.886–49.571; P = 0.007) and diabetes mellitus (DM) (OR = 5.023; 95% CI = 2.371–10.640; P < 0.001) after adjustments for covariates. The relationship between SHR and in-hospital mortality among patients with ACS and chronic coronary syndrome was consistent with the main finding. SHR and in-hospital mortality exhibited a dose-response relationship, and the risk of in-hospital mortality increased when the SHR index was above 1.20. Moreover, the area under the curve of SHR for predicting in-hospital mortality in patients with CAD was 0.741.
Conclusion
SHR is significantly associated with in-hospital mortality in patients with CAD. SHR may be an effective predictor of in-hospital mortality in patients with CAD, especially for those with pre-DM and DM.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01645-y.
Keywords: Coronary artery disease, Diabetes, Mortality, Stress hyperglycemia, Stress hyperglycemia ratio
Background
Coronary artery disease (CAD) is the leading cause of mortality worldwide despite the development of prevention and treatment strategies [1, 2]. Metabolic disorders, including hyperglycemia, insulin resistance, and dyslipidemia that result from an unhealthy lifestyle and Western diet, lead to CAD [3]. Chronic hyperglycemia caused by diabetes mellitus (DM) is a well-defined risk factor for adverse cardiovascular events. Stress-induced glucose levels are associated with poor clinical outcomes among patients with acute coronary syndrome (ACS) [4–6], and this association is stronger in patients without DM than in those with DM [4, 7, 8]. Increased glucose levels at the time of hospital admission may be a result of acute stress reactivity or chronic hyperglycemia. Thus, the use of relative hyperglycemia to identify stress-induced hyperglycemia has been suggested [9, 10]. The stress hyperglycemia ratio (SHR) represents relative hyperglycemia and has been proven to be effective in predicting worsening prognosis in patients with severe acute disease [9]. Several studies have reported that SHR was significantly associated with adverse clinical outcomes in patients with ACS [11, 12]. However, this association may be a comprehensive effect of a heterogeneous population with ACS. Therefore, it is necessary to further assess this relationship under specific conditions. Studies regarding the relationship of SHR and mortality in patients with CAD are limited. This study aimed to clarify the association between SHR and in-hospital mortality in patients with CAD.
Methods
Study design and population
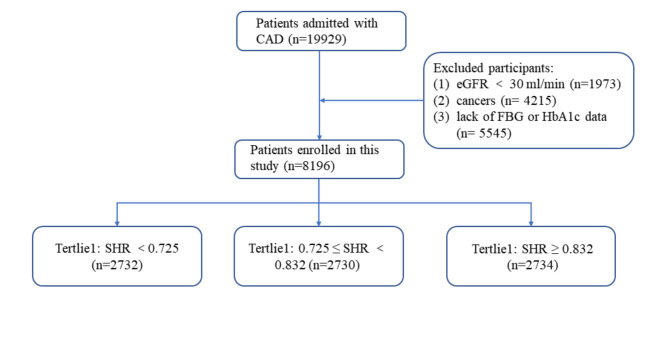
This retrospective cohort study was conducted at Beijing Hospital in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Hospital. Written informed consent was obtained from all patients. A total of 19,929 patients diagnosed with CAD who were hospitalized at Beijing Hospital between January 1, 2016 and December 30, 2021 were enrolled in this study. However, 1,973 patients with an estimated glomerular filtration rate (eGFR) < 30 ml/min; 4,215 patients with cancer; and 5,545 patients with missing blood glucose or HbA1c data were excluded (Fig. 1). A total of 8,196 patients were included in the final analysis. The patients were divided into three groups according to the tertiles of SHR: T1 group (SHR < 0.725, n = 2,732), T2 group (0.725 ≤ SHR < 0.832, n = 2,730), and T3 group (SHR ≥ 0.832, n = 2,734). The primary endpoint was in-hospital mortality rate.
Fig. 1.
Patient flowchart
Data measurement and definitions
Patient demographics (age, sex, height, weight, smoking status, and drinking status), medical history (hypertension, diabetes mellitus, and cancer), and laboratory test results were obtained from the medical records at Beijing Hospital. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) on hospital admission were recorded, as was the patient’s use of antihypertensive, antiplatelet, or antilipidemic medications. In-hospital death was defined as all-cause death that occurred in-hospital within 30 days of admission and was collected via medical records. The admission blood glucose (ABG) referred to the first-measured random serum glucose within the first 24 h of admission. Blood samples for testing HbA1c, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and eGFR were obtained from the cubital vein after at least eight hours of fasting. Blood glucose, eGFR, TC, TG, HDL-C, and LDL-C were measured using a LABOSPECT 008 system (Hitachi, Tokyo, Japan), and the HbA1c level was determined using high-performance liquid chromatography (G8, TOSOH, Tokyo, Japan) at the central laboratory of Beijing Hospital. Body mass index (BMI) was calculated as weight (kg) divided by the squared height (m), and eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration creatinine equation [13].
SHR was calculated using the following equation: SHR = ABG (mmol/L) / [1.59 × HbA1c(%) − 2.59] [9].
CAD was defined as ≥ 50% lumen stenosis in at least one major coronary artery (left anterior descending, left circumflex, or right coronary arteries). Henceforth, diabetes refers to type 2 diabetes and was defined as a history of type 2 diabetes or an HbA1c > 6.5% [14]. Pre-diabetic status was defined as patients without a history of diabetes but with HbA1c ranging from 5.7 to 6.4%. Patients with no history of diabetes or an HbA1c ≤ 5.7% were regarded as normoglycemia (NGR).
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median and interquartile range (25–75%), as appropriate. Categorical variables are presented as numbers (n) and percentages (%). A one-way analysis of variance or Kruskal–Wallis test was conducted to compare the baseline variables between the SHR groups. Chi-square tests were performed to compare the categorical variables.
To analyze the association between SHR and in-hospital deaths, odds ratios (OR) and 95% confidence intervals (CI) were calculated using a logistic regression analysis. In the current study, model 1 was unadjusted; model 2 was adjusted for age and sex; and model 3 was further adjusted for BMI, SBP, DBP, eGFR, TC, smoking status, and drinking status. Restricted cubic splines (RCS) were used to examine the shape of the association between SHR and in-hospital mortality. Receiver operating curves (ROC) were used to calculate the area under the curve (AUC) of the SHR for predicting in-hospital mortality in patients with CAD. Further, we also did subgroup analysis according to the ACS and chronic coronary syndromes (CCS) groups and different diabetes status by using logistic regression analysis.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R language version 4.0.4. Statistical significance was set at P < 0.05.
Results
Baseline characteristics
The mean patient age was 68 ± 11 years. A total of 5,266 (64.25%) patients were men, and 4412 (53.83%) had DM. Patient age, sex, BMI, HR, DBP, smoking status, drinking status, hypertension, NGR, pre-DM, DM, ACS, glucose, HbA1c, HDL-C, LDL-C, TC, TG, eGFR, use of antiplatelets, and use of antilipidemic drugs were significantly different between the three groups (all, P < 0.05) (Table 1).
Table 1.
Patient baseline characteristics according to tertiles of stress hyperglycemia ratio
| Total (n = 8196) | T1 (n = 2732) | T2 (n = 2730) | T3 (n = 2734) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 68 ± 11 | 69 ± 10 | 68 ± 11 | 67 ± 11 | <0.001* |
| Male (n, %) | 5266 (64.25%) | 1659 (60.72%) | 1726 (63.22%) | 1881 (68.80%) | <0.001* |
| BMI (Kg/m2) | 25.57 ± 3.41 | 25.39 ± 3.42 | 25.57 ± 3.34 | 25.72 ± 3.45 | <0.001* |
| HR (beats/min) | 78 ± 13 | 77 ± 13 | 77 ± 13 | 79 ± 14 | <0.001* |
| SBP (mmHg) | 136 ± 19 | 136 ± 19 | 136 ± 18 | 137 ± 20 | 0.138 |
| DBP (mmHg) | 77 ± 12 | 76 ± 13 | 77 ± 12 | 77 ± 12 | <0.001* |
| Smoking (n, %) | 3524 (43.00%) | 1113 (40.74%) | 1163 (42.60%) | 1248 (45.65%) | 0.001* |
| Drinking (n, %) | 4672 (57.00%) | 1466 (53.66%) | 1565 (57.33%) | 1641 (60.02%) | <0.001* |
| Hypertension (n, %) | 5894 (71.91%) | 1992 (72.91%) | 1909 (69.93%) | 1993 (72.90%) | 0.018* |
| NGR (n, %) | 1395 (17.02%) | 149 (5.45%) | 567 (20.77%) | 679 (24.84%) | <0.001* |
| Pre-DM (n, %) | 2389 (29.15%) | 842 (30.82%) | 1016 (37.22%) | 531 (19.42%) | <0.001* |
| DM (n, %) | 4412 (53.83%) | 1741 (63.73%) | 1147 (42.01%) | 1524 (55.74%) | <0.001* |
| ACS (n, %) | 3001 (36.62%) | 906 (33.16%) | 1004 (36.78%) | 1091 (39.90%) | <0.001* |
| Glucose (mmol/l) | 6.55 ± 2.39 | 5.52 ± 1.30 | 5.96 ± 1.32 | 8.17 ± 3.12 | <0.001* |
| HbA1c (%) | 6.80 ± 1.38 | 7.22 ± 1.53 | 6.46 ± 1.06 | 6.71 ± 1.40 | <0.001* |
| HDL-C (mg/dL) | 1.06 ± 0.27 | 1.08 ± 0.28 | 1.07 ± 0.26 | 1.03 ± 0.27 | <0.001* |
| LDL-C (mg/dL) | 2.17 ± 0.83 | 2.10 ± 0.80 | 2.21 ± 0.85 | 2.21 ± 0.83 | <0.001* |
| TC (mg/dL) | 3.81 ± 0.98 | 3.72 ± 0.95 | 3.85 ± 0.99 | 3.87 ± 0.99 | <0.001* |
| TG (mg/dL) | 1.43 ± 0.94 | 1.31 ± 0.82 | 1.44 ± 0.89 | 1.57 ± 1.07 | <0.001* |
| eGFR (ml/min) | 84.68 ± 17.49 | 82.98 ± 17.88 | 86.22 ± 16.04 | 84.84 ± 18.32 | <0.001* |
| Medications | |||||
| Antiplatelets (n, %) | 6844 (83.60%) | 2284 (83.60%) | 2318 (84.91%) | 2242 (82.00%) | 0.015* |
| Antihypertensive drugs (n, %) | 6200 (76.65%) | 2086 (76.35%) | 2071 (75.86%) | 2043 (74.73%) | 0.355 |
| Antilipidemic drugs (n, %) | 6901 (84.20%) | 2306 (84.41%) | 2362 (86.52%) | 2233 (81.68%) | <0.001* |
* Statistically significant P values
Patients in the T3 group were more likely to have higher glucose and HbA1c, dyslipidemia, and a history of DM, hypertension, and a diagnosis of ACS.
Clinical outcomes
The overall in-hospital mortality rate was 0.91% (n = 74). SHR was significantly associated with the risk of in-hospital deaths (OR = 22.309; 95% CI = 13.194–37.723; P<0.001) (Table 2). SHR was identified as an independent risk factor for in-hospital mortality in patients with CAD after adjusting for potential risk factors in model 2 (OR = 20.177; 95% CI = 11.485–35.444; P < 0.001) and model 3 (OR = 17.038; 95% CI = 9.668–30.027; P < 0.001). Highest value of SHR group (T3 group) was correlated with a higher risk of in-hospital mortality (OR = 4.481; 95% CI = 2.389–8.404; P < 0.001) in model 1. After adjusting for age, sex, BMI, SBP, DBP, smoking, drinking, ACS, TC, eGFR, the T3 group had a higher risk of in-hospital mortality by 4.9-fold than the T1 group (OR = 4.901; 95% CI = 2.583–9.297; P < 0.001).
Table 2.
Associations between stress hyperglycemia ratio and in-hospital mortality
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| SHR index | 22.309 | 13.194–37.723 | <0.001 | 20.177 | 11.485–35.444 | <0.001 | 17.038 | 9.668–30.027 | <0.001 |
| T1 | Reference | Reference | Reference | ||||||
| T2 | 0.750 | 0.315–1.782 | 0.514 | 0.913 | 0.383–2.181 | 0.838 | 0.937 | 0.390–2.246 | 0.883 |
| T3 | 4.481 | 2.389–8.404 | <0.001 | 5.012 | 2.657–9.453 | <0.001 | 4.901 | 2.583–9.297 | <0.001 |
Model 1: Original model
Model 2: Adjusted for age and sex
Model 3: Adjusted for age, sex, BMI, SBP, DBP, smoking, drinking, ACS, TC, and eGFR.
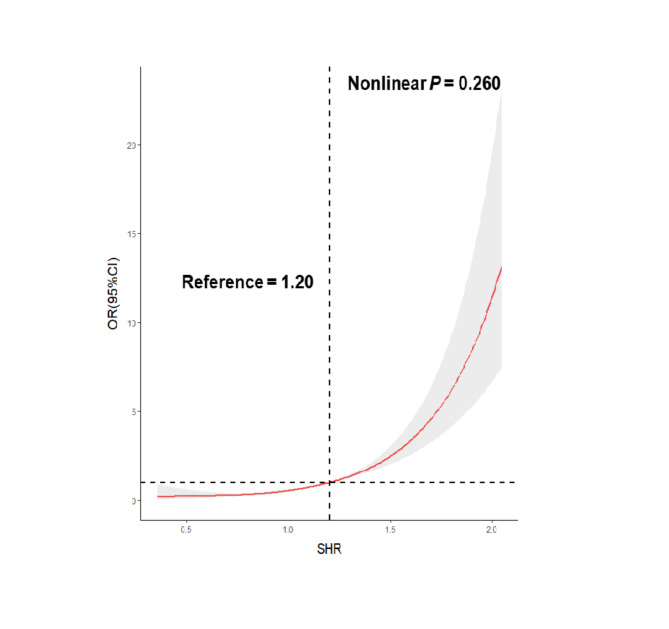
A dose-response relationship between SHR and in-hospital mortality was observed (nonlinear P value = 0.260) (Fig. 2). The ORs of in-hospital mortality significantly increased when the SHR index was above 1.20. The AUC of SHR for predicting in-hospital deaths was 0.741, which is considered a strong predictive ability (Fig. 3). Additionally, both the ABG (AUC = 0.757) and SHR (AUC = 0.741) showed a strong predictive power in the total study population (Supplementary material Figure S1a); however, SHR (AUC = 0.766) was slightly better than ABG (AUC = 0.728) and better than HbA1c (AUC = 0.594) in predicting in-hospital mortality among patients with CAD and diabetes (Figure S1b). In patients with CCS, the AUC of ABG, HbA1c, and SHR was 0.750, 0.563 and 0.694, respectively (Figure S1c). For patients with ACS, the AUC of SHR (AUC = 0.779) for predicting in-hospital mortality was better than ABG (AUC = 0.757) and HbA1c (AUC = 0.528) (Figure S1d).
Fig. 2.
Restricted cubic splines for the odds ratio of in-hospital mortality
Fig. 3.
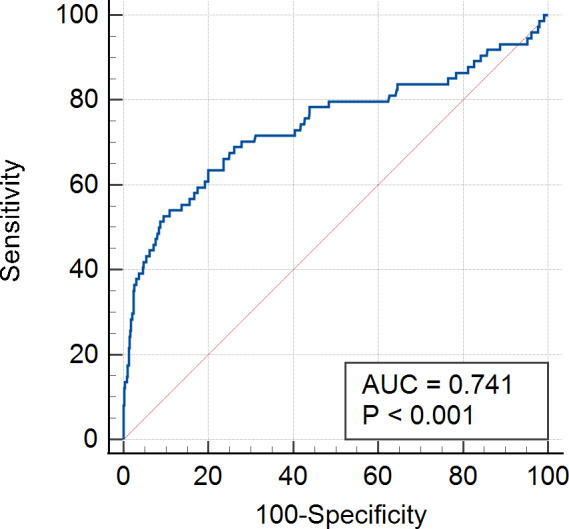
Receiver operating curves of stress hyperglycemia ratio for predicting in-hospital mortality
Subgroup analyses
Table 3 shows the associations between SHR and in-hospital mortality in patients with ACS and CCS. The multivariable logistic regression model (model 3) illustrated that SHR was an independent predictor for in-hospital mortality in both ACS group (OR = 19.351; 95% CI = 8.397–44.592; P <0.001) and CCS group (OR = 15.453; 95% CI = 6.924–34.488; P <0.001). Moreover, the T3 group in patients with ACS had a higher risk of in-hospital mortality by 7.9-fold than that of the T1 group (OR = 7.939; 95% CI = 2.723–23.151; P <0.001). In those with CCS, the T3 group had a higher risk of in-hospital mortality by 3.6-fold than that of the T1 group (OR = 3.564; 95% CI = 1.567–8.107; P = 0.002).
Table 3.
Associations between stress hyperglycemia ratio and in-hospital mortality in patients with acute coronary syndromes and chronic coronary syndromes
| Subgroup | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| ACS | |||||||||
| SHR | 19.547 | 9.008–42.417 | <0.001 | 21.057 | 9.180-48.299 | <0.001 | 19.351 | 8.397–44.592 | <0.001 |
| T1 | Reference | Reference | Reference | ||||||
| T2 | 1.129 | 0.302–4.216 | 0.857 | 1.308 | 0.348–4.911 | 0.691 | 1.558 | 0.410–5.917 | 0.515 |
| T3 | 6.158 | 2.157–17.582 | 0.001 | 6.858 | 2.387–19.702 | <0.001 | 7.939 | 2.723–23.151 | <0.001 |
| CCS | |||||||||
| SHR | 23.664 | 11.547–48.495 | <0.001 | 17.841 | 8.099–39.299 | <0.001 | 15.453 | 6.924–34.488 | <0.001 |
| T1 | Reference | Reference | Reference | ||||||
| T2 | 0.528 | 0.159–1.756 | 0.298 | 0.685 | 0.204–2.303 | 0.541 | 0.664 | 0.197–2.238 | 0.509 |
| T3 | 3.369 | 1.509–7.519 | 0.003 | 3.770 | 1.668–8.521 | 0.001 | 3.564 | 1.567–8.107 | 0.002 |
Model 1: Original model
Model 2: Adjusted for age and sex
Model 3: Adjusted for age, sex, BMI, SBP, DBP, smoking, drinking, ACS, TC, and eGFR.
In Table 4, among patients with pre-DM, the univariable logistic regression model (model 1) suggested that the T3 group had a higher risk of in-hospital mortality than the T1 group (OR = 7.241; 95% CI = 1.559–33.645; P = 0.012). This higher risk remained in model 2 (OR = 8.073; 95% CI = 1.701–38.315; P = 0.009) and model 3 (OR = 9.670; 95% CI = 1.886–49.571; P = 0.007). Among patients with CAD and DM, a higher SHR was associated with an increased risk of in-hospital mortality in model 1 (OR = 4.788; 95% CI = 2.304–9.954; P < 0.001), model 2 (OR = 4.974; 95% CI = 2.377–10.406; P < 0.001), and model 3 (OR = 5.023; 95% CI = 2.371–10.640; P < 0.001).
Table 4.
Associations between stress hyperglycemia ratio and in-hospital mortality in patients with and without diabetes mellitus
| Diabetes status | Events/n | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | ||
| NGR | ||||||||||
| T1 | 1/149 | Reference | Reference | Reference | ||||||
| T2 | 0/567 | - | - | - | - | - | - | - | - | - |
| T3 | 7/679 | 1.542 | 0.188–12.625 | 0.687 | 2.215 | 0.257–19.111 | 0.470 | 1.020 | 0.071–14.581 | 0.988 |
| Pre-DM | ||||||||||
| T1 | 2/842 | Reference | Reference | Reference | ||||||
| T2 | 2/1016 | 0.828 | 0.116–5.893 | 0.851 | 0.996 | 0.139–7.142 | 0.997 | 1.036 | 0.140–7.685 | 0.973 |
| T3 | 9/531 | 7.241 | 1.559–33.645 | 0.012 | 8.073 | 1.701–38.315 | 0.009 | 9.670 | 1.886–49.571 | 0.007 |
| DM | ||||||||||
| T1 | 9/1741 | Reference | Reference | Reference | ||||||
| T2 | 7/1147 | 1.182 | 0.439–3.182 | 0.741 | 1.363 | 0.503–3.693 | 0.543 | 1.458 | 0.532–3.995 | 0.464 |
| T3 | 37/1524 | 4.788 | 2.304–9.954 | <0.001 | 4.974 | 2.377–10.406 | <0.001 | 5.023 | 2.371–10.640 | <0.001 |
Model 1: Original model
Model 2: Adjusted for age and sex
Model 3: Adjusted for age, sex, BMI, SBP, DBP, smoking, drinking, ACS, TC, and eGFR.
Discussion
In this study, SHR was independently associated with in-hospital mortality in patients with CAD, especially in the pre-DM and DM group. Patients with the highest SHR (T3 group) had an increased risk of in-hospital mortality, regardless of their DM status. SHR and in-hospital mortality had a dose-response relationship, and the ORs for in-hospital mortality increased when the SHR index was above 1.20. In addition, the AUC of the SHR for predicting in-hospital deaths in patients with CAD was 0.741, which is interpreted a strong predictive ability.
The hypothalamic-pituitary-adrenal axis and sympathoadrenal system are activated and the release of proinflammatory cytokines is increased in response to stress [15]. These processes act synergistically to induce stress hyperglycemia [15]. Moderate stress hyperglycemia is a protective response to stress [16]. In an animal model of shock, external highly permeable glucose increased cardiac output and improved survival [17]. However, several studies have reported that stress hyperglycemia is strongly associated with adverse clinical outcomes among patients with critical illnesses, including myocardial infarction, stroke, sepsis, and COVID-19 [11, 18, 19]. Stress hyperglycemia has been identified as an independent risk factor for worsened prognoses in patients with ACS [4–6, 20]. Furthermore, accumulating evidence indicates that stress hyperglycemia was associated with a larger myocardial necrosis and poorer short and long-term prognosis in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA) [21, 22]. The mechanism of this association may be related to a decrease in endothelium-dependent vasodilation, impaired platelet anti-aggregatory effects, and hyperactivation of sympathetic nerves with a proinflammatory pathway [23–25].
The threshold should be different in individuals with and without diabetes if the blood glucose level on admission is used to define stress hyperglycemia. Thus, stress-induced hyperglycemia cannot simply be represented by the ABG level but should be considered in conjunction with the status of chronic hyperglycemia. Robert et al. proposed the SHR index to represent relative hyperglycemia using the ratio of ABG to estimated chronic blood glucose [9]. Nathan et al. further suggested using HbA1c to estimate average glucose (AG) using the following equation: [AG (mg/dl) = 28.7 x HbA1c − 46.7] [26]. Several studies including patients with ACS have been conducted to verify the predictive value of SHR for adverse outcomes (Supplementary material Table S1). Marenzi et al. found that SHR was a more accurate predictor of in-hospital morbidity and mortality than ABG in patients with acute myocardial infarction [10]. Sia et al. found that SHR is an independent predictor of one-year mortality in patients with ST-segment elevation myocardial infarction (STEMI) with and without diabetes mellitus [27]. Xu et al. reported that SHR was significantly associated with 30-day mortality in patients with STEMI and that the predictive efficiency of the TIMI risk score can be improved after adding SHR as one point [12]. Yang et al. observed a J-shaped relationship between SHR and in-hospital cardiac death in patients with ACS who underwent the implantation of a drug-eluting stent [11]. Chen et al. reported a dose-response relationship between SHR and in-hospital deaths in patients > 75 years of age with acute myocardial infarction [28].
Consistent with these previous studies, the results of the current study indicate that SHR is significantly associated with in-hospital mortality in patients with CAD, especially in those with pre-DM and DM. Moreover, a dose-response association was observed between SHR and in-hospital mortality in the RCS analysis. A previous study reported a 7.9% mortality rate among patients with CAD, stable angina, and ACS who underwent percutaneous coronary intervention (PCI) over a median follow-up period of 2.5 years, suggesting that SHR is an effective marker of major cardiovascular and cerebrovascular events (MACCE) in patients after PCI, especially in patients with STEMI and without diabetes mellitus [29]. However, patients with the highest SHR in the current study (T3 group) had an increased risk of in-hospital mortality both in the ACS and CCS population. This difference may be attributed to different study populations. The proportion of patients with ACS in the previous study (48.3%) was higher than that in the current study (36.6%). In addition, in-hospital mortality within 30 days of admission was measured in the current study, which represents short-term mortality. As previously reported, SHR may not be effective in predicting long-term mortality [30]. This may be due to the fact that stress hyperglycemia represents only a transient stress-induced response during critical severe illness, while the factors affecting long-term mortality may be related to age and medical complications [30]. In the current study, the long-term mortality rate of patients with CAD was not measured. Therefore, the relationship between SHR and long-term mortality in patients with CAD should be validated in future studies.
Strength and limitations
This study included a relatively large cohort of patients with CAD. This is the first report of a dose-response relationship between SHR and in-hospital mortality in patients with CAD using RCS analysis. However, this study is not without limitations. First, this was a single-center study and included only Asian patients. These results should be interpreted with caution. Second, the current study is limited by its retrospective design, and a causal relationship cannot be inferred in this study; further prospective multi-center studies are needed to validate these results. Third, this cohort study did not collect the information regarding hypoglycemic treatment of the participants; thus, further associations between SHR and the hypoglycemic treatment could not be evaluated.
Conclusion
SHR is significantly associated with in-hospital mortality in patients with CAD. SHR may be an effective predictor of in-hospital mortality in patients with CAD, especially for those with pre-DM and DM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Association of stress hyperglycemiaratioandin-hospital mortality in patients withcoronaryartery disease: Insights froma large cohortstudy
Acknowledgements
The authors thank all the members of the Beijing hospital for their contribution.
Abbreviations
- ACS
acute coronary syndrome.
- SHR
stress hyperglycemia ratio.
- CAD
coronary artery disease.
- OR
odds ratio.
- CI
confidence interval.
- DM
diabetes mellitus.
- eGFR
estimated glomerular filtration rate.
- SBP
systolic blood pressure.
- DBP
diastolic blood pressure.
- HR
heart rate.
- TC
total cholesterol.
- TG
triglycerides.
- HDL-C
high-density lipoprotein cholesterol.
- LDL-C
low-density lipoprotein cholesterol.
- BMI
body mass index.
- ABG
admission blood glucose.
- NGR
normoglycemia.
- SD
standard deviation.
- RCS
restricted cubic splines.
- ROC
receiver operating curves.
- AUC
area under the curve.
- CCS
chronic coronary syndromes.
- MINOCA
myocardial infarction with no obstructive coronary atherosclerosis.
- AG
average glucose.
- STEMI
st-segment elevation myocardial infarction.
- PCI
percutaneous coronary intervention.
- MACCE
major cardiovascular and cerebrovascular events.
Author contributions
WX, QS, and XW designed the study, FW and YZ reviewed and revised the manuscript. QS coded and analyzed the data. WX wrote the manuscript. ZZ, XM, and YX collected the data. CX, CY, and YG interpreted the data. All authors read and approved the final manuscript.
Funding
This work was supported by National Key R&D Program of China (No. 2020YFC2008100, No. 2020YFC2009000, and No. 2020YFC2009001).
Data availability
The datasets generated and analysed during the current study are not publicly available due privacy and ethical restrictions but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This cohort study conformed to the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Hospital. Written informed consent was obtained from all the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest regarding the submitted work.
Footnotes
†Wei Xu, Qirui Song and Xiang Wang contributed equally to this work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yatong Zhang, Email: 750826140@qq.com.
Fang Wang, Email: bjh_wangfang@163.com.
References
- 1.Global regional and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018;392(10159):1736–88. [DOI] [PMC free article] [PubMed]
- 2.Leong DP, Joseph PG, McKee M, Anand SS, Teo KK, Schwalm JD, et al. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circul Res. 2017;121(6):695–710. doi: 10.1161/CIRCRESAHA.117.311849. [DOI] [PubMed] [Google Scholar]
- 3.Ralapanawa U, Sivakanesan R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J Epidemiol Glob Health. 2021;11(2):169–77. doi: 10.2991/jegh.k.201217.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet (London England) 2000;355(9206):773–8. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara M, Kojima S, Sakamoto T, Asada Y, Tei C, Kimura K, et al. Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. Am Heart J. 2005;150(4):814–20. doi: 10.1016/j.ahj.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet (London England) 2009;373(9677):1798–807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–82. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 8.Khalfallah M, Abdelmageed R, Elgendy E, Hafez YM. Incidence, predictors and outcomes of stress hyperglycemia in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Diabetes Vasc Dis Res. 2020;17(1):1479164119883983. doi: 10.1177/1479164119883983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative Hyperglycemia, a Marker of Critical Illness: Introducing the Stress Hyperglycemia Ratio. J Clin Endocrinol Metab. 2015;100(12):4490–7. doi: 10.1210/jc.2015-2660. [DOI] [PubMed] [Google Scholar]
- 10.Marenzi G, Cosentino N, Milazzo V, De Metrio M, Cecere M, Mosca S, et al. Prognostic Value of the Acute-to-Chronic Glycemic Ratio at Admission in Acute Myocardial Infarction: A Prospective Study. Diabetes Care. 2018;41(4):847–53. doi: 10.2337/dc17-1732. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The Impact of the Stress Hyperglycemia Ratio on Short-term and Long-term Poor Prognosis in Patients With Acute Coronary Syndrome: Insight From a Large Cohort Study in Asia. Diabetes Care. 2022;45(4):947–56. doi: 10.2337/dc21-1526. [DOI] [PubMed] [Google Scholar]
- 12.Xu W, Yang YM, Zhu J, Wu S, Wang J, Zhang H, et al. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: insights from a multi-center observational study. Cardiovasc Diabetol. 2022;21(1):48. doi: 10.1186/s12933-022-01479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilpatrick ES, Bloomgarden ZT, Zimmet PZ. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: response to the International Expert Committee. Diabetes Care. 2009;32(12):e159. doi: 10.2337/dc09-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Critical care. (London England) 2013;17(2):305. doi: 10.1186/cc12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soeters MR, Soeters PB. The evolutionary benefit of insulin resistance. Clin Nutr. 2012;31(6):1002–7. doi: 10.1016/j.clnu.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 17.McNamara JJ, Mills D, Aaby GV. Effect of hypertonic glucose on hemorrhagic shock in rabbits. Ann Thorac Surg. 1970;9(2):116–21. doi: 10.1016/S0003-4975(10)65784-0. [DOI] [PubMed] [Google Scholar]
- 18.Mi D, Li Z, Gu H, Jiang Y, Zhao X, Wang Y, et al. Stress hyperglycemia is associated with in-hospital mortality in patients with diabetes and acute ischemic stroke. CNS Neurosci Ther. 2022;28(3):372–81. doi: 10.1111/cns.13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvisi SL, Ramirez GA, Scavini M, Da Prat V, Di Lucca G, Laurenzi A, et al. Thromboembolism risk among patients with diabetes/stress hyperglycemia and COVID-19. Metab Clin Exp. 2021;123:154845. doi: 10.1016/j.metabol.2021.154845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujino M, Ishihara M, Honda S, Kawakami S, Yamane T, Nagai T, et al. Impact of acute and chronic hyperglycemia on in-hospital outcomes of patients with acute myocardial infarction. Am J Cardiol. 2014;114(12):1789–93. doi: 10.1016/j.amjcard.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Paolisso P, Foà A, Bergamaschi L, Donati F, Fabrizio M, Chiti C, et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol. 2021;20(1):33. doi: 10.1186/s12933-021-01222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paolisso P, Foà A, Bergamaschi L, Angeli F, Fabrizio M, Donati F, et al. Impact of admission hyperglycemia on short and long-term prognosis in acute myocardial infarction: MINOCA versus MIOCA. Cardiovasc Diabetol. 2021;20(1):192. doi: 10.1186/s12933-021-01384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, et al. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49(3):304–10. doi: 10.1016/j.jacc.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 24.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 25.Paolisso P, Bergamaschi L, Rambaldi P, Gatta G, Foà A, Angeli F, et al. Impact of Admission Hyperglycemia on Heart Failure Events and Mortality in Patients With Takotsubo Syndrome at Long-term Follow-up: Data From HIGH-GLUCOTAKO Investigators. Diabetes Care. 2021;44(9):2158–61. doi: 10.2337/dc21-0433. [DOI] [PubMed] [Google Scholar]
- 26.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–8. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sia CH, Chan MH, Zheng H, Ko J, Ho AF, Chong J, et al. Optimal glucose, HbA1c, glucose-HbA1c ratio and stress-hyperglycaemia ratio cut-off values for predicting 1-year mortality in diabetic and non-diabetic acute myocardial infarction patients. Cardiovasc Diabetol. 2021;20(1):211. doi: 10.1186/s12933-021-01395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen G, Li M, Wen X, Wang R, Zhou Y, Xue L, et al. Association Between Stress Hyperglycemia Ratio and In-hospital Outcomes in Elderly Patients With Acute Myocardial Infarction. Front Cardiovasc Med. 2021;8:698725. doi: 10.3389/fcvm.2021.698725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y, Jeong MH, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. 2017;241:57–63. doi: 10.1016/j.ijcard.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz T, Freuer D, Harmel E, Heier M, Peters A, Linseisen J, et al. Prognostic value of stress hyperglycemia ratio on short- and long-term mortality after acute myocardial infarction. Acta Diabetol. 2022;59(8):1019–29. doi: 10.1007/s00592-022-01893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Association of stress hyperglycemiaratioandin-hospital mortality in patients withcoronaryartery disease: Insights froma large cohortstudy
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due privacy and ethical restrictions but are available from the corresponding author on reasonable request.