Abstract
The ability of kidney transplant candidates to travel outside of their usual place of care varies by sociodemographic factors, potentially exacerbating disparities in access. We used Transplant Referral Regions (TRRs) to overcome previous methodological barriers of using geographic distance to assess the characteristics and outcomes of patients listed for kidney transplant at centers in neighboring TRR or beyond neighboring TRRs. Among listed kidney transplant candidates, 20.9% traveled to a neighbor and 5.6% beyond a neighbor. A higher proportion of travelers were White, had some college education, and lived in ZIP codes with lower poverty. Travel to a neighbor was associated with a 7% increase in likelihood of deceased donor transplant (cHR: 1.07, 95% CI: 1.05, 1.09) and traveling beyond a neighbor with a 19% increase (cHR: 1.19, 95% CI: 1.15, 1.24). Travelers had similar rates of living donor transplant and waitlist mortality as patients who did not travel; those who traveled beyond a neighbor had slightly lower posttransplant mortality (HR: 0.91, 95% CI: 0.83, 0.99). In conclusion, the ability to travel outside of the recipient’s assigned TRR increases access to transplantation and improves long-term survival.
Keywords: disparities, health services and outcomes research, kidney transplantation/nephrology, patient characteristics, registry/registry analysis, Scientific Registry for Transplant Recipients (SRTR)
1 |. INTRODUCTION
The rate of kidney transplantation varies substantially across transplant centers in the United States.1,2 This variation is driven by factors at multiple levels including organ availability in the geographic region,3 organ acceptance practices at the center, and demographic characteristics of the patient.2,4 Transplant rate among waitlisted patients is now being used as a quality metric to assess center performance and to inform patients who are selecting a transplant center.5,6 However, the ability of patients to select and travel to centers with higher transplant rates may vary by sociodemographic factors and contribute to pre-existing disparities in transplant access.7–9
Prior studies have used one of two main approaches to define travel: geographic distance10–12 or travel to a different donor service area (DSA).13 Defining travel using distance, often the distance from a patient’s home ZIP code to their transplant center, may conflate the effects of barriers in the physical distance that a patient lives from a center with other barriers, including the cost and burden of travel. Furthermore, the effect of distance on access may vary by geographic context, such as degree of rurality, absolute drive time, and availability of public transportation. Studies focused on travel to a different DSA often focus on organ availability as the driver of patient decisions. However, previous studies have shown that probability of transplant varies substantially at centers within a DSA,2 indicating that center behaviors such as organ acceptance practices may also drive decisions to travel.
An alternative way to study transplant travel is by identifying deviations from similar patients’ usual place of care, which can be defined using geographic catchment areas. Transplant referral regions (TRRs) are geographic catchment areas recently developed for transplant centers14 that assign patients to a usual place of care based on historical listing patterns for patients in their zip code. Travel outside of a patient’s home TRR, especially to a TRR that is not adjacent to their home TRR, suggests that a patient has selected a center where they would not ordinarily be expected to seek care. The purpose of this study was to identify factors associated with ‘traveling’ for a kidney transplant, and to describe the impact of traveling for kidney transplant on waitlist and posttransplant outcomes.
2 |. METHODS
2.1 |. Study population and data sources
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Patients were included if they were adults (age ≥ 18) listed for a kidney-only transplant in the United States between December 5, 2014 (after the implementation of the new Kidney Allocation System [KAS]), and December 31, 2020. Patients were excluded if they were missing a permanent ZIP code (n = 997), were listed in Puerto Rico (n = 953), or lived outside of the United States (n = 669). Among patients with multiple transplants in the study period, only the first was considered in the posttransplant analyses. This project was determined to be exempt from review by the Emory Institutional Review Board (IRB).
2.2 |. Variables
Patients were assigned to a ‘home’ transplant center, or group of centers, based on the transplant referral region (TRR) that contained the ZIP code of their permanent residence. Transplant referral regions were developed to represent geographic catchment areas for transplant centers or groups of centers co-located within 10 miles of each other.14 We determined that TRRs defined using 10-mile grouping distances for centers more accurately represented prevailing care patterns in comparison to TRRs that used smaller distances (Table S1). We defined neighboring TRRs as those that shared an edge or a vertex with an index TRR (queen contiguity). We identified three categories of traveling: (1) no travel—patients were listed at a center in their TRR of residence, (2) travel to a neighbor—patients were listed at a center whose TRR was geographically contiguous with their TRR of residence, (3) travel, beyond neighbors—patients were listed at a center outside of their TRR of residence that was not geographically contiguous with their TRR of residence. For patients who had multiple listings, travel was considered a time-varying exposure in which travel status was updated for each new listing, and survival time was prospectively re-assigned to the new exposure category. For posttransplant outcomes, travel category was assigned based on the center where the patient received their transplant, regardless of whether the patient had multiple listings.
Outcomes included time to deceased donor transplant, time to living donor transplant, waitlist mortality, and posttransplant survival. For each patient, time to transplant was defined as the time between listing date and transplant date or study end (December 31, 2020). Time to waitlist mortality was defined as time until death or delisting for being too sick or study end, and posttransplant survival was calculated as the time between transplant and death or graft failure, censored at loss to follow up or study end.
A directed acyclic graph (DAG) was used to identify potential confounders.15 Confounders included age at listing, gender, race and ethnicity, educational attainment (high school diploma or less vs. some college or more), primary payer (private, Medicaid, Medicare, or other), lived in a high-poverty ZIP code (defined as >20% of the ZIP code below the federal poverty line), body mass index (BMI) (< or ≥35 kg/m2), diabetes, and time on dialysis prior to first listing (none [pre-emptive listing], <1 year, 1–3 years, 3–5 years, or >5 years). For posttransplant outcomes, we included covariates described above, donor type (deceased vs. living), and a random intercept to account for outcome clustering within transplant centers. Donor characteristics (donor age, cold ischemic time, high risk donor classification, and donation after cardiac death) were considered to be on the causal pathway between travel and posttransplant outcomes.
2.3 |. Statistical analyses
We described demographic and clinical characteristics for kidney candidates, stratified by baseline travel status, and reported rates of our outcomes of interest. For waitlist outcomes, including transplant and mortality, we calculated cause-specific hazards ratios accounting for covariates described above. For posttransplant outcomes, we calculated Cox proportional hazard ratios accounting for covariates as described above, with a random intercept to account for outcome clustering within transplant centers. The proportional hazards assumption was checked by examining log-log survival curves. We evaluated potential effect modification of the impact of travel by race/ethnicity, education, ZIP-code poverty, and multiple listings by including interaction terms in the model. We performed likelihood ratio tests to assess the significance of each interaction term, adjusting for clinical and sociodemographic covariates. While we considered donor characteristics to be potential mediators of the association between travel and posttransplant outcomes and did not adjust for them in our model, we did evaluate whether there were significant differences by travel status among patients who received a deceased donor transplant using Chi-square tests for categorical variables and ANOVA for continuous variables.
To facilitate comparison of our result to prior studies that have examined similar outcomes using geographic distance, rather than TRR catchment areas, we conducted a sensitivity analysis using excess travel distance (ETD) as the travel metric, as has been previously described.10 We calculated the distance from the patient’s home ZIP code to the closest active adult transplant center (>25 waitlist registrations for patients older than 18 in the study period) and the distance to the center at which the patient was listed. To calculate ETD, we subtracted the distance to the closest center from the distance traveled. ETD was categorized as in the previous paper into <30 miles, 30–55 miles, 55–117 miles, and >117 miles. Patients whose home or center ZIP could not be matched to a valid ZIP code tabulation area (ZCTA) either in the ZIPcodeR package or using the USPS ZIP to ZCTA crosswalk were excluded (n = 2637; 1.2% of cohort). We compared ETD across our TRR-based travel categories, and calculated rates and adjusted cause-specific HRs for all outcomes. All analyses were conducted in R.
3 |. RESULTS
3.1 |. Demographic characteristics by travel category
We included 194,303 transplant candidates residing in 110 TRRs (median TRR area: 12 4742 miles; IQR: 6067 to 31 9292 miles). Approximately one quarter of candidates were listed for transplant outside of their home TRR (26.5%). Overall, 20.9% of candidates (78.8% of travelers) were listed in a neighboring TRR and 5.6% of candidates (21.2% of travelers) were listed beyond a neighboring TRR. Demographic and clinical characteristics varied by travel category (Table 1). There was a higher proportion of non-Hispanic White recipients among those who traveled in comparison to those who did not travel. A higher proportion of travelers had at least some college education, and a lower proportion lived in a high-poverty ZIP code. There was a lower proportion of patients with Medicaid among patients who traveled than patients who did not travel, and a higher proportion of patients with insurance other than private, Medicaid, or Medicare among patients who traveled beyond a neighbor. While the proportion of patients who were listed with no time on dialysis was similar across travel categories, the proportion of patients listed for the first time with over 5 years on dialysis was higher among those who traveled beyond a neighbor.
TABLE 1.
Demographic and clinical characteristics of kidney candidates by travel category, December 5, 2014–December 31, 2020
| No travel (N = 143 383) |
Travel, to neighbor (N = 40 128) |
Travel, beyond neighbor (N = 10 792) |
Overall (N = 194 303) |
|
|---|---|---|---|---|
| Gender | ||||
| Male | 88271 (61.6%) | 24936 (62.1%) | 7114 (65.9%) | 120321 (61.9%) |
| Female | 55112 (38.4%) | 15192 (37.9%) | 3678 (34.1%) | 73982 (38.1%) |
|
| ||||
| Race/Ethnicity | ||||
| Non-Hispanic White | 57486 (40.1%) | 20072 (50.0%) | 5658 (52.4%) | 83216 (42.8%) |
| Black | 42530 (29.7%) | 10320 (25.7%) | 2792 (25.9%) | 55642 (28.6%) |
| Hispanic | 29612 (20.7%) | 6090 (15.2%) | 1100 (10.2%) | 36802 (18.9%) |
| Asian | 10764 (7.5%) | 2812 (7.0%) | 911 (8.4%) | 14487 (7.5%) |
| Other/Missing | 2991 (2.1%) | 834 (2.1%) | 331 (3.1%) | 4156 (2.1%) |
|
| ||||
| Educational attainment | ||||
| High school diploma or less | 63845 (44.5%) | 16089 (40.1%) | 3145 (29.1%) | 83079 (44.1%) |
| Some college or more | 75580 (52.7%) | 22729 (56.6%) | 7191 (66.6%) | 105500 (55.9%) |
| Missing | 3958 (2.8%) | 1310 (3.3%) | 456 (4.2%) | 5724 (2.9%) |
|
| ||||
| Primary payer | ||||
| Private | 61930 (43.2%) | 17827 (44.4%) | 4009 (37.1%) | 83766 (43.2%) |
| Medicaid | 15193 (10.6%) | 2761 (6.9%) | 349 (3.2%) | 18303 (9.4%) |
| Medicare | 64008 (44.6%) | 18371 (45.8%) | 4559 (42.2%) | 86938 (44.8%) |
| Other | 2067 (1.4%) | 1101 (2.7%) | 1856 (17.2%) | 5024 (2.6%) |
| Missing | 185 (0.1%) | 68 (0.2%) | 19 (0.2%) | 272 (0.1%) |
|
| ||||
| Living in a high-poverty ZIP | ||||
| No | 98893 (69.0%) | 29635 (73.9%) | 8378 (77.6%) | 136906 (71.1%) |
| Yes | 43229 (30.1%) | 10078 (25.1%) | 2262 (21.0%) | 55569 (28.9%) |
| Missing | 1261 (0.9%) | 415 (1.0%) | 152 (1.4%) | 1828 (0.9%) |
|
| ||||
| Age at listing | ||||
| Mean (SD) | 52.7 (13.0) | 52.6 (13.0) | 52.9 (13.3) | 52.7 (13.0) |
| Median (Min, Max) | 55.0 (19.0, 90.0) | 54.0 (19.0, 89.0) | 55.0 (19.0, 86.0) | 55.0 (19.0, 90.0) |
|
| ||||
| Diabetes type | ||||
| None | 78288 (54.6%) | 22749 (56.7%) | 6841 (63.4%) | 107878 (56.0%) |
| Type 1 | 5005 (3.5%) | 1685 (4.2%) | 428 (4.0%) | 7118 (3.7%) |
| Type II | 58864 (41.1%) | 15268 (38.0%) | 3401 (31.5%) | 77533 (40.3%) |
| Missing | 1226 (0.9%) | 426 (1.1%) | 122 (1.1%) | 1774 (0.9%) |
|
| ||||
| Candidate BMI | ||||
| Mean (SD) | 29.0 (5.56) | 29.2 (5.65) | 28.3 (5.58) | 29.0 (5.58) |
| Median (Min, Max) | 29.0 (12.0, 70.0) | 29.0 (12.0, 68.0) | 28.0 (12.0, 68.0) | 29.0 (12.0, 70.0) |
|
| ||||
| BMI ≥ 35 | ||||
| No | 124007 (86.8%) | 34160 (85.5%) | 9587 (89.2%) | 167754 (86.7%) |
| Yes | 18852 (13.2%) | 5797 (14.5%) | 1156 (10.8%) | 25805 (13.3%) |
|
| ||||
| Time on dialysis prior to listing | ||||
| None | 51988 (36.3%) | 12978 (34.8%) | 3752 (34.8%) | 69718 (35.9%) |
| <1 year | 36384 (25.4%) | 9680 (24.1%) | 2179 (20.2%) | 48243 (24.8%) |
| 1–3 years | 33502 (23.4%) | 9546 (23.8%) | 2339 (21.7%) | 45387 (23.4%) |
| 3–5 years | 10442 (7.3%) | 3326 (8.3%) | 1092 (10.1%) | 14860 (7.7%) |
| >5 years | 11067 (7.7%) | 3598 (9.0%) | 1430 (13.3%) | 16095 (8.3%) |
3.2 |. Rates of transplant and waitlist mortality by travel category
Patients who traveled had a higher rate of deceased donor transplant (to a neighbor: 18.78 per 100 PY, 95% CI: 18.45, 19.10; beyond a neighbor: 22.62 per 100 PY, 95% CI: 21.96, 23.27) than those who did not (none: 16.44 per 100 PY, 95% CI: 16.27, 16.60) (Table 2). Living donor transplant was more common among those who traveled to a neighbor (8.95 per 100 PY, 95% CI: 8.73, 9.18) and those who traveled beyond a neighbor (9.24 per 100 PY, 95% CI: 8.82, 9.66) than among those who did not travel (8.53 per 100 PY, 95% CI: 8.41, 8.64). Waitlist mortality was slightly higher among those who traveled to a neighbor (7.79 per 100 PY, 95% CI: 7.58, 8.00) and those who traveled beyond a neighbor (8.16 per 100 PY, 95% CI: 7.77, 8.55) than among those who did not travel (7.41 per 100 PY, 95% CI: 7.30, 7.52). Posttransplant mortality was slightly lower among patients who traveled to a neighbor (4.56 per 100 PY, 95% CI: 4.16, 4.96) compared to those who did not travel (4.95 per 100 PY, 95% CI: 4.81, 5.09).
TABLE 2.
Rates of pre- and posttransplant events by travel status among kidney candidates in the United States, 2010-2020
| Number of events | Total person-time, in years | Rate per 100 PY (95% CI) | Crude HR or cHR (95% CI) | |
|---|---|---|---|---|
| Deceased donor transplant | ||||
| No travel | 39 453 | 240 032 | 16.44 (16.27, 16.60) | Ref |
| Travel, to neighbor | 12 665 | 67 455 | 18.78 (18.45, 19.10) | 1.14 (1.12, 1.17) |
| Travel, beyond neighbor | 4568 | 20 197 | 22.62 (21.96, 23.27) | 1.39 (1.34, 1.44) |
|
| ||||
| Living donor transplant | ||||
| No travel | 20 465 | 240 032 | 8.53 (8.41, 8.64) | Ref |
| Travel, to neighbor | 6040 | 67 455 | 8.95 (8.73, 9.18) | 1.06 (1.03, 1.09) |
| Travel, beyond neighbor | 1867 | 20 197 | 9.24 (8.82, 9.66) | 1.16 (1.10, 1.21) |
|
| ||||
| Died, pre-transplant | ||||
| No travel | 17 778 | 240 032 | 7.41 (7.30, 7.52) | Ref |
| Travel, to neighbor | 5257 | 67 455 | 7.79 (7.58, 8.00) | 1.05 (1.02, 1.08) |
| Travel, beyond neighbor | 1648 | 20 197 | 8.16 (7.77, 8.55) | 1.06 (1.00, 1.11) |
|
| ||||
| Died, posttransplant | ||||
| No travel | 4852 | 98 097 | 4.95 (4.81, 5.09) | Ref |
| Travel, to neighbor | 1557 | 30 236 | 5.15 (4.89, 5.41) | 1.04 (0.97, 1.12) |
| Travel, beyond neighbor | 496 | 10 873 | 4.56 (4.16, 4.96) | 0.92 (0.83, 1.02) |
3.3 |. Adjusted incidence of transplant, waitlist mortality, and posttransplant survival
After adjustment for clinical and demographic factors, kidney candidates who traveled to a neighbor had a 7% higher adjusted rate of deceased donor transplant (cause-specific hazard ratio [cHR]: 1.07, 95% CI: 1.05, 1.09) and those who traveled beyond a neighbor had a 19% higher adjusted rate of deceased donor transplant (cHR: 1.19, 95% CI: 1.15, 1.24) than those who did not travel (Table 3). Similarly, the adjusted rate of living donor transplant was slightly higher among patients who traveled beyond a neighbor (cHR: 1.05, 95% CI: 1.00, 1.10) compared to those who did not travel. Other factors associated with higher transplant rates, both living and deceased, included White race, higher educational attainment, private insurance, and pre-emptive listing. Increasing ZIP code poverty and diabetes were associated with lower transplant rates. Length of time on dialysis prior to listing was positively associated with receipt of deceased donor transplant, and negatively associated with receipt of living donor transplant. Travelers had similar waitlist mortality rates to those who did not travel.
TABLE 3.
Adjusted waitlist and posttransplant outcomes, by travel category
| Deceased donor transplant |
Living donor transplant |
Pre-transplant mortality |
Posttransplant mortality |
|||||
|---|---|---|---|---|---|---|---|---|
| Kidney | cHR | 95% CI: | cHR | 95% CI | cHR | 95% CI | HR | 95% CI |
| Travel | ||||||||
| None | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| To neighbor | 1.07 | 1.05, 1.09 | 0.97 | 0.94, 1.00 | 1.00 | 0.97, 1.04 | 1.03 | 0.97, 1.09 |
| Not to neighbor | 1.19 | 1.15, 1.24 | 1.05 | 1.00, 1.10 | 1.00 | 0.93, 1.05 | 0.91 | 0.83, 0.99 |
|
| ||||||||
| Age at listing | 1.00 | 1.00, 1.00 | 0.98 | 0.98, 0.98 | 1.04 | 1.04, 1.04 | 1.02 | 1.01, 1.02 |
|
| ||||||||
| Female | 1.08 | 1.06, 1.10 | 0.91 | 0.89, 0.93 | 1.00 | 0.98, 1.03 | 0.88 | 0.83, 0.94 |
|
| ||||||||
| Race | ||||||||
| NHW | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| NHB | 0.77 | 0.75, 0.79 | 0.32 | 0.31, 0.34 | 0.67 | 0.65, 0.69 | 1.02 | 0.96, 1.10 |
| Hispanic | 0.71 | 0.69, 0.73 | 0.67 | 0.65, 0.70 | 0.63 | 0.60, 0.65 | 0.86 | 0.79, 0.94 |
| Asian | 0.68 | 0.66, 0.71 | 0.43 | 0.41, 0.46 | 0.55 | 0.52, 0.58 | 0.68 | 0.59, 0.78 |
| Other | 0.84 | 0.79, 0.90 | 0.47 | 0.43, 0.53 | 0.75 | 0.68, 0.82 | 1.01 | 0.84, 1.22 |
|
| ||||||||
| Education | ||||||||
| HS diploma or less | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Some college or more | 0.98 | 0.96, 1.00 | 1.42 | 1.38, 1.46 | 0.99 | 0.96, 1.02 | 0.99 | 0.93, 1.05 |
|
| ||||||||
| High poverty zip code | 0.97 | 0.95, 0.99 | 0.77 | 0.74, 0.79 | 1.05 | 1.02, 1.08 | 0.98 | 0.93, 1.04 |
|
| ||||||||
| Insurance | ||||||||
| Private | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Medicaid | 0.94 | 0.91, 0.98 | 0.53 | 0.50, 0.56 | 1.10 | 1.04, 1.16 | 1.19 | 1.08, 1.31 |
| Medicare | 1.13 | 1.11, 1.15 | 0.72 | 0.70, 0.74 | 1.20 | 1.16, 1.25 | 1.22 | 1.15, 1.31 |
| Other | 0.93 | 0.87, 0.99 | 0.59 | 0.53, 0.64 | 1.08 | 0.99, 1.17 | 0.95 | 0.76, 1.18 |
|
| ||||||||
| BMI ≥ 35 | 0.88 | 0.86, 0.90 | 0.80 | 0.77, 0.83 | 0.91 | 0.87, 0.95 | 1.14 | 1.05, 1.23 |
|
| ||||||||
| Diabetes type | ||||||||
| None | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Type I | 0.60 | 0.57, 0.63 | 0.74 | 0.70, 0.79 | 2.53 | 2.38, 2.69 | 1.38 | 1.20, 1.57 |
| Type II | 0.78 | 0.77, 0.80 | 0.64 | 0.63, 0.66 | 1.73 | 1.68, 1.79 | 1.38 | 1.30, 1.46 |
|
| ||||||||
| Time on dialysis prior to listing | ||||||||
| None | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| <1 year | 1.35 | 1.30, 1.37 | 0.93 | 0.90, 0.95 | 1.54 | 1.49, 1.60 | 1.18 | 1.09, 1.27 |
| 1–3 years | 1.62 | 1.58, 1.66 | 0.51 | 0.49, 0.53 | 1.77 | 1.70, 1.84 | 1.29 | 1.20, 1.40 |
| 3–5 years | 2.95 | 2.86, 3.05 | 0.38 | 0.35, 0.41 | 2.16 | 2.04, 2.28 | 1.38 | 1.25, 1.52 |
| >5 years | 5.73 | 5.55, 5.93 | 0.27 | 0.24, 0.30 | 2.97 | 2.79, 3.15 | 1.36 | 1.23, 1.50 |
|
| ||||||||
| Donor type | ||||||||
| Deceased | Ref | Ref | ||||||
| Living | 0.50 | 0.46, 0.54 | ||||||
Among those who received a kidney transplant, patients who traveled beyond a neighbor had lower posttransplant mortality compared to those who did not travel (HR: 0.91, 95% CI: 0.83, 0.99). Female gender, Hispanic ethnicity, Asian race, private insurance, pre-emptive transplant, and receipt of a living versus deceased donor were all associated with improved posttransplant survival, while age at listing, diabetes, BMI ≥ 35, and time on dialysis prior to listing were all associated with decreased posttransplant survival (Table 3). We evaluated donor characteristics among patients who received a deceased donor transplant (Table S2), and found that patients who traveled received organs with slightly less cold ischemic time than those who did not (17.6 h vs. 18.0 h, p = .003) and were less likely to receive an organ donated after cardiac death (22.4% vs. 24.1%, p = .04).
We explored the potential effect modification of travel by race/ethnicity, insurance, educational attainment, and ZIP code poverty on receipt of deceased donor transplant (Table 4). Non-Hispanic Black patients experienced less benefit from traveling beyond a neighbor for deceased donor transplant compared to those who did not travel (cHR: 1.06, 95% CI: 0.99, 1.13) than non-Hispanic White (NHW) patients (cHR: 1.19, 95% CI: 1.13, 1.26), Asian patients (cHR: 1.43, 95% CI: 1.28, 1.61) or Hispanic patients (cHR: 1.44, 95% CI: 1.29, 1.59; p-value for interaction <.001). The benefit of traveling beyond a neighbor relative to not traveling also varied by insurance type, with Medicare patients experiencing less benefit (cHR: 1.15, 95% CI: 1.09, 1.21) than privately-insured (cHR: 1.38, 95% CI: 1.30, 1.47) or Medicaid patients (cHR: 1.48, 95% CI: 1.22, 1.80; p-value for interaction <.001). Among patients with other insurance, traveling was associated with lower rates of deceased donor transplant, particularly traveling beyond a neighbor (cHR: 0.74, 95% CI: 0.65, 0.85). Patients who were multiply-listed experienced a greater degree of benefit from traveling (to a neighbor cHR: 1.11, 95% CI: 1.04, 1.18; beyond a neighbor cHR: 1.25, 95% CI: 1.16, 1.35) than patients who were listed at a single center (to a neighbor cHR 1.02, 95% CI: 1.00, 1.05; beyond a neighbor cHR: 1.05, 95% CI: 1.00, 1.10; p-value for interaction <.001). There was no meaningful variation in the impact of travel by educational attainment or ZIP-code poverty.
TABLE 4.
Effect modification of the impact of travel for deceased donor mortality and posttransplant survival, adjusted for clinical and sociodemograph characteristics
| DDTx |
|||||
|---|---|---|---|---|---|
| Travel, to neighbor |
Travel, beyond neighbor |
||||
| cHR | 95% CI | cHR | 95% CI | LR test p-value | |
| Race | <0.001 | ||||
| NHW | 1.09 | 1.06, 1.13 | 1.19 | 1.13, 1.26 | |
| NHB | 1.06 | 1.02, 1.10 | 1.06 | 0.99, 1.13 | |
| Hispanic | 1.05 | 1.00, 1.11 | 1.44 | 1.29, 1.59 | |
| Asian | 0.95 | 0.87, 1.03 | 1.43 | 1.28, 1.61 | |
| Other | 1.20 | 1.04, 1.40 | 1.00 | 0.81, 1.24 | |
|
| |||||
| Insurance | <0.001 | ||||
| Private | 1.07 | 1.04, 1.11 | 1.38 | 1.30, 1.47 | |
| Medicaid | 1.13 | 1.04, 1.23 | 1.48 | 1.22, 1.80 | |
| Medicare | 1.06 | 1.03, 1.09 | 1.15 | 1.09, 1.21 | |
| Other | 0.90 | 0.78, 1.04 | 0.74 | 0.65, 0.85 | |
|
| |||||
| Education | 0.65 | ||||
| HS diploma or less | 1.06 | 1.03, 1.10 | 1.17 | 1.10, 1.25 | |
| Some college or more | 1.07 | 1.04, 1.11 | 1.20 | 1.15, 1.26 | |
|
| |||||
| High poverty zip code | 0.17 | ||||
| No | 1.06 | 1.03, 1.08 | 1.20 | 1.15, 1.25 | |
| Yes | 1.10 | 1.05, 1.14 | 1.17 | 1.09, 1.27 | |
|
| |||||
| Multiply listed | < 0.001 | ||||
| No | 1.02 | 1.00, 1.05 | 1.05 | 1.00, 1.10 | |
| Yes | 1.11 | 1.04, 1.18 | 1.25 | 1.16, 1.35 | |
3.4 |. Geographic distribution of travel
Figure 1 provides the proportion of patients traveling to or beyond a neighbor by TRR of patient residence (number of waitlisted patients traveling/number of waitlisted patients residing in a TRR). Traveling to a neighbor was more prevalent in the Northeast and Southeast than in the West, although certain TRRs had a very high proportion of patients traveling to a neighbor (Figure 1A). Traveling beyond a neighbor was more common in Hawaii, in the upper Midwest, and in New England (Figure 1B).
FIGURE 1.
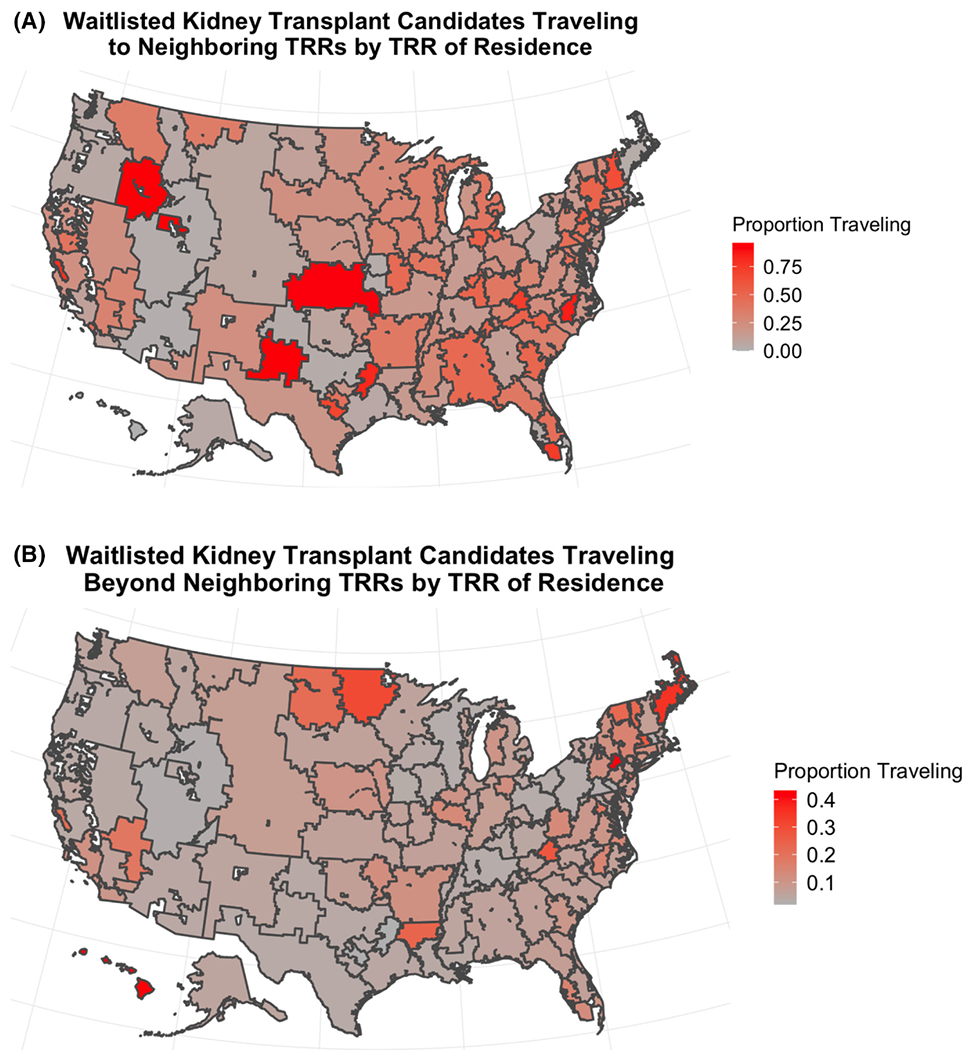
Proportion of patients traveling to a neighbor (A) and beyond a neighbor (B) by TRR [Color figure can be viewed at wileyonlinelibrary.com]
3.5 |. Sensitivity analysis
We present results of a sensitivity analysis of our findings using excess travel distance (distance beyond the nearest center) instead of our TRR-based classification of travel in Table S3. The median ETD for patients designated as not traveling in our classification was 0 miles (IQR: 0, 4), while the median for patients designated as traveling to a neighbor was 27 miles (IQR: 3, 70) and 350 miles for those traveling beyond a neighbor (IQR: 138, 860). Among patients who traveled to a neighbor, 49% fell into the first ETD category of <30 miles, 15% fell into the second ETD category of 30–55, 24% fell into the third ETD category of 55–117, and 12% fell into the fourth ETD category of >117 miles. Among patients who did not travel, 95% fell in the first ETD category (<30 miles), and among patients who traveled beyond a neighbor, 79% had an ETD of >117 miles. In both unadjusted and adjusted analyses (Table S4), we found that ETD >30 was associated with higher rates of deceased donor transplant, with the greatest increase among patients with ETD >117 (adjusted cHR: 1.23, 95% CI: 1.19, 1.26), and that ETD >117 was associated with higher rates of living donor transplant (adjusted cHR: 1.06, 95% CI: 1.01, 1.11).
4 |. DISCUSSION
In this study, we used geographic catchment areas for transplant centers to identify patients who traveled beyond their usual place of care for kidney transplant. We described characteristics of travelers and compared outcomes among travelers to those who did not travel. Approximately one-quarter of the transplant population traveled outside their home catchment area. Patients who traveled were disproportionately White, had some college education, and lived in higher income ZIP. Those who traveled beyond a neighbor had a 19% increased rate of deceased donor kidney transplant in comparison to those who did not travel. This benefit was modified by patient race and ethnicity, insurance, and whether they were multiply listed.
Traveling for care is not unique to transplantation, and as in our study, long travel distances have been associated with increased access to care among some patient populations. For example, Turner et al. identified a ‘cancer geography paradox’ among patients in the United Kingdom, in which patients who traveled longer distances for care had quicker diagnosis and time to treatment.16 In the United States, patients with colorectal cancer who traveled long distances to their diagnosing facility were more likely to be diagnosed with metastatic disease, but also more likely to initiate therapy more quickly.17 Findings are more mixed in terms of outcomes—while Turner et al. identified travel as associated with poorer outcomes,16 in a study of US head and neck cancer patients, patients who traveled long distances for care were more likely to be treated at academic and high volume centers, and travel was associated with improved survival.18 In many of these studies, racial and ethnic minority patients and patients with Medicaid were less likely to travel for care, which we also observed. Because of the unique nature of transplantation, which involves a degree of resource scarcity not seen in other fields, the impact of travel must be considered not just in the context of individual patients but how it affects the system as a whole. In this case, travel provides increased access to the organ supply, further exacerbating differences in access to the organ supply as a result of socioeconomic factors. A key remaining question is whether travel for some patients results in increased wait times for other transplant candidates at their destination, or serves as a method to reduce differences in wait times across centers by decreasing differences in supply/demand ratios.
Previous research on the impact of traveling for transplant has yielded mixed findings, likely due to differences in defining travel. In a study of SRTR data from 1999 to 2009, Axelrod et al. found that inter-DSA travel was associated with higher rates of deceased donor kidney transplant.13 As in our study, travelers were more likely to be White and of high SES. A more recent study using United States Renal Data System (USRDS) data from 1995 to 2015 also found that travel was more likely among non-Black patients and patients with private insurance. However, in their study, travel was associated with a higher rate of living donor transplant, but a lower likelihood of deceased donor transplant, which is in contrast to our findings.10 In a sensitivity analysis, we calculated ETD as was done in that study and found that a higher ETD was associated with a higher likelihood of deceased donor transplant in our cohort, consistent with our main analyses. One likely explanation for the differences observed in our study compared to the Whelan et al study is the different eras of each study. We included only patients in the post-KAS era (December 2014–2020), which encompasses a new era of kidney allocation in which organs are allocated based on time on dialysis, not time on the waitlist, and Whelan et al largely examined historical trends (1995–2015).
Another potential explanation for the increased access to deceased donor transplant attained by travelers in the post-KAS era is the way that we handled multiple listings in analyses. We considered travel to be a time-varying exposure and allowed travel status to update if patients were listed at subsequent centers. While current OPTN policy allows patients to register at the transplant program or programs of their choice, multiple listing has been controversial, resulting in lawsuits and challenges.19 The underlying principle permitting multiple listing is patient autonomy over treatment decisions, while detractors point out that advantages conferred by multiple listing result at the expense of patients who are not multiply listed. These arguments similarly apply to traveling for transplant. However, it is unclear that policy changes that ban multiple listing or travel would result in improved equity. For example, a previous ban on multiple listing in New York resulted in small, mixed effects on equity in transplant access.20 Additionally, blanket bans may result in unintended consequences for certain patient populations with unique needs (e.g., highly sensitized patients). Removing opportunities for patients to aggressively pursue transplant is an attempt to improve equity by bringing the “ceiling” of access down. An alternate, more population-focused approach to policy is to bring the “floor” of access up, improving access for all patients through policy that promotes increased referral to transplant, streamlined evaluation and listing processes, and maximized organ availability and utilization. A critical next step to informing policy is to determine how travel and multiple listing impacts waiting time and likelihood of transplant for local patients on a center-by-center basis. While patients should be allowed to pursue care at the centers most likely to offer them transplant, they should not be prioritized above patients living in the areas served by those centers.
Our findings suggest that traveling for transplant is associated with greater access to deceased donor transplant, indicating a potential benefit of travel. We also found that patient characteristics, including race and insurance, affected both propensity to travel and benefit from traveling. In particular, Black patients were less likely to travel for transplant and experienced less benefit from traveling than patients of other racial and ethnic groups. Potential mechanisms for lower likelihood to travel among Black kidney transplant candidates include differences in access to information about travel and multiple listing, provider referral patterns, insurance network coverage, social support, and structural barriers to travel (e.g., transportation, lodging). However, the factors contributing to a lower benefit from travel are less clear. If travel is beneficial to patients and permitted through OPTN policy, then promoting greater equity in these benefits should be prioritized. For example, patients should have consistent access to travel reimbursement benefits, similar to the support provided to living kidney donors by the National Living Donor Assistance Center (NLDAC) and some private insurance plans.21 HRSA could also consider offering grants or funding to transplant centers to reimburse travel and other expenses incurred by patients.
Increased deceased donor transplant rates among traveling patients could result from traveling to DSAs with higher organ availability, to centers with better organ utilization or innovative clinical programs, residual confounding by the factors influencing the ability to travel, or some combination of these mechanisms. The geographic distribution of travel does not suggest that patients are predominantly traveling to regions thought to have higher relative organ availability, such as the Southeast.22 Instead, “destination” TRRs appear to be relatively dispersed, indicating that center-specific characteristics, such as reputation for good outcomes, increased organ utilization, insurance network requirements, or more relaxed listing criteria, may drive travel. A previous qualitative study of kidney transplant recipients found patients prioritized reputation, comfort, and convenience when selecting a transplant center, and often felt that they underestimated differences in listing and transplant rates, indicating a complex decision-making process beyond with additional priorities beyond simply getting an organ quickly.23
Our findings must be interpreted in the context of their limitations. First, we are likely to have residual confounding by sociodemographic and clinical factors that impact a patient’s ability to travel. While we adjusted for several of these potential factors, many remain unknown or unmeasured by current administrative data, and due to data limitations we are unable to account for time-varying confounding such as change in clinical status over time that may influence access. Second, we chose to use queen contiguity as opposed to a fixed distance buffer to define neighboring TRRs. While this was chosen to account for patients living along the border of two TRRs, in some parts of the country—especially the Northeast—there could be nearby TRRs that are not necessarily geographically contiguous. Changing the definition of a ‘neighbor’ may impact the prevalence of travel in this region. Third, it is unclear how allocation systems involving broader organ sharing may affect the need for or impact of travel. Finally, private insurance network coverage requirements may mandate travel to alternative centers. The impact of this factor on transplant care is likely to grow as patients move from traditional fee-for-service Medicare to Medicare Advantage plans which utilize narrow networks.
In conclusion, we found that patients who traveled outside of their home TRR had higher deceased donor kidney transplant rates than those who stayed in their home TRR, particularly for patients who traveled beyond their neighboring TRRs. This association varied by patient race and ethnicity, insurance type, and multiple listing status. Key remaining questions include whether the patient who traveled outside of their local TRR increased wait times for transplant candidates at their destination or displaced organs for this population, and how updated allocation systems such as continuous distribution may impact travel in the future. Future research is needed to understand how centers can optimize transplant access for local and distant patients.
Supplementary Material
ACKNOWLEDGMENTS
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. Dr. Ross-Driscoll is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378 and KL2TR002381. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ANOVA
analysis of variance
- BMI
body mass index
- cHR
cause-specific hazard ratio
- DAG
directed acyclic graph
- DSA
donor service area
- ETD
excess travel distance
- HR
hazard ratio
- HRSA
Health Resources and Services Administration
- IQR
interquartile range
- KAS
Kidney Allocation System
- NLDAC
National Living Donor Assistance Center
- OPTN
Organ Procurement and Transplantation Network
- PY
person-years
- SRTR
Scientific Registry of Transplant Recipients
- TRR
transplant referral region
- USRDS
United States Renal Data System
- VA
Veteran’s Administration
- ZCTA
ZIP code tabulation area
- ZIP
Zone Improvement Plan
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. DA is a consultant for CareDx and Talaris.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Zhou S, Massie AB, Luo X, et al. Geographic disparity in kidney transplantation under KAS. Am J Transplant. 2018;18(6):1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King KL, Husain SA, Schold JD, et al. Major variation across local transplant centers in probability of kidney transplant for wait-listed patients. J Am Soc Nephrol. 2020;31(12):2900–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King KL, Husain SA, Mohan S. Geographic variation in the availability of deceased donor kidneys per wait-listed candidate in the United States. Kidney Int Rep. 2019;4(11):1630–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garonzik-Wang JM, James NT, Weatherspoon KC, et al. The aggressive phenotype: center-level patterns in the utilization of sub-optimal kidneys. Am J Transplant. 2012;12(2):400–408. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, Salkowski N, Wey A, Israni AK, Snyder JJ. Scientific registry of transplant recipients program-specific reports: where we have been and where we are going. Curr Opin Organ Transplant. 2019;24(1):58–63. [DOI] [PubMed] [Google Scholar]
- 6.Schaffhausen CR, Bruin MJ, Chu S, et al. The importance of transplant program measures: surveys of three national patient advocacy groups. Clin Transplant. 2018;32(12):e13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart DE, Wilk AR, Toll AE, et al. Measuring and monitoring equity in access to deceased donor kidney transplantation. Am J Transplant. 2018;18(8):1924–1935. [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, Klarenbach S, Rose C, Wiebe N, Gill J. Access to kidney transplantation among remote-and rural-dwelling patients with kidney failure in the United States. JAMA. 2009;301(16):1681–1690. [DOI] [PubMed] [Google Scholar]
- 9.Patzer RE, Perryman JP, Schrager JD, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. Am J Transplant. 2012;12(2):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelan AM, Johansen KL, Brar S, et al. Association between longer travel distance for transplant care and access to kidney transplantation and graft survival in the United States. J Am Soc Nephrol. 2021;32(5):1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg DS, French B, Forde KA, et al. Association of distance from a transplant center with access to waitlist placement, receipt of liver transplantation, and survival among US veterans. JAMA. 2014;311(12):1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherson LJ, Barry V, Yackley J, et al. Distance to kidney transplant center and access to early steps in the kidney transplantation process in the southeastern United States. Clin J Am Soc Nephrol. 2020;15(4):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5(12):2276–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross-Driscoll K, Axelrod D, Lynch R, Patzer RE. Using geographic catchment areas to measure population-based access to kidney transplant in the United States. Transplant. 2020;104(12):e342–e350. [DOI] [PubMed] [Google Scholar]
- 15.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiol. 1999;37–48. [PubMed] [Google Scholar]
- 16.Turner M, Fielding S, Ong Y, et al. A cancer geography paradox? Poorer cancer outcomes with longer travelling times to healthcare facilities despite prompter diagnosis and treatment: a data-linkage study. Br J Cancer. 2017;117(3):439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massarweh NN, Chiang Y-J, Xing Y, et al. Association between travel distance and metastatic disease at diagnosis among patients with colon cancer. J Clin Oncol. 2014;32(9):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graboyes EM, Ellis MA, Li H, et al. Racial and ethnic disparities in travel for head and neck cancer treatment and the impact of travel distance on survival. Cancer. 2018;124(15):3181–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ardekani MS, Orlowski JM. Multiple listing in kidney transplantation. Am J Kidney Dis. 2010;55(4):717–725. [DOI] [PubMed] [Google Scholar]
- 20.White A, Ozminkowski R, Hassol A, Dennis JM, Murphy M. The effects of New York state’s ban on multiple listing for cadaveric kidney transplantation. Health Serv Res. 1998;33(2 Pt 1):205. [PMC free article] [PubMed] [Google Scholar]
- 21.Mathur AK, Stewart Lewis ZA, Warren PH, et al. Best practices to optimize utilization of the National Living Donor Assistance Center for the financial assistance of living organ donors. Am J Transplant. 2020;20(1):25–33. [DOI] [PubMed] [Google Scholar]
- 22.Sheehy E, O’Connor KJ, Luskin RS, et al. Investigating geographic variation in mortality in the context of organ donation. Am J Transplant. 2012;12(6):1598–1602. [DOI] [PubMed] [Google Scholar]
- 23.Schaffhausen CR, Bruin MJ, McKinney WT, et al. How patients choose kidney transplant centers: a qualitative study of patient experiences. Clin Transplant. 2019;33(5):e13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


