Abstract
Currently, diabetes mellitus (DM) is relevant problem, both for its prevalence and complications, including distal polyneuropathy (DPNP). At the same time, discussions continue on analgesic efficacy of transcutaneous electrical nerve stimulation (TENS) in DPNP. Aim of this study was to conduct a multi-faceted assessment of pain syndrome in these patients before and after TENS, taking into account levels of polyneuropathy, its severity and age of patients. The study was conducted in accordance with the research of the Federal State Budgetary Institution of the National Medical Research Center for Rehabilitation and Balneology of the Ministry of Health of the Russian Federation (CTR No. 121040100062-3) and with the permission of the Local Ethics Committee (IRB No. 2 dated 14.01.2021). The study included 75 patients with DM type II with DPNP, which are distributed into 3 groups of 25 people: Group 1a, patients received high–frequency TENS (HF); Group Ib, patients received low-frequency TENS (LF); as control, Group C received a standard method of pharmacological therapy without physiotherapy. Intensity of DPNP was evaluated before and after the course of treatment using a visual analog scale (VAS), the McGill Pain Questionnaire (MPQ), and a graphical linear analysis of pain on the neuropathic pain diagnostic questionnaire 4 (DN4) scale. TENS provides an analgesic effect that may exceed pharmacotherapy in terms of efficacy and safety. There was a 65.9% reduction in neuropathic pain according to VAS after a course of application, with the effects remaining up to 34% during the 6-month follow-up. HF TENS provided a higher significant analgesic effects than LF TENS, as it ensures the reduction of pain syndrome according to VAS by 25.8% (p <0.01), and total estimated characteristics - 35.5% (p <0.01), and touch - in at 58.1% (p = 0.001) and according to the scales of the MPQ (S) and DN4 - by 21% (p = 0.007). The observed differences in analgesic effects between HF TENS and LF TENS are based on analyses of pain in the immediate and long-term follow-up periods of type II DM patients with DPNP. These results, based on summation of the estimated parameters of the international pain scales support expectation of an expansion of the the use of analgesic TENS in aging patients suffering with DM of varying severity and extent of DPNP damage, a goal of great scientific and practical importance.
Key Words: Type II diabetes mellitus, distal polyneuropathy, neuropathic pain, high- and low-frequency TENS
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
It has been established that neuropathic pain develops in 10-50% of patients with distal polyneuropathy (DPNP) in type II diabetes mellitus (DM).1-7 At the same time, drug therapy, as a rule, provides insufficiently high analgesic results in the treatment of patients with severe neuropathic pain, which determines the importance of optimizing analgesic measures for DPNP by non-drug methods.8-10 Previously, it was proved that the use of transcutaneous electrical nerve stimulation (TENS) provides an increase in the effectiveness of analgesic drugs in patients with type II diabetes with DPND, potentiating the regression of negative sensory dysfunction by 1.5 times and positive sensory dysfunction by 2.1 times, directly affecting the clinical manifestations of neuropathic pain.11-19 It is important to note that the use of a single visual analog scale (VAS) provides only a frontal cross-section of pain regression, excluding multi-faceted aspects of pain syndrome in patients with DM and DPNP.20-24
The consensus recommendations of the Toronto Expert Group on Diabetic Neuropathy define TENS as an effective non-pharmacological treatment for neuropathic pain.25 TENS of peripheral nerves in patients with type II DM suffering with DPNP was first time applied in Russia in 2015,2,22 which allowed us to obtain new data and new scientific concept of the mechanisms of action of TENS: i) regional improvement of blood circulation in the nerve and the surrounding tissues;23 ii) an increase in axonal transport, leading to accumulation of growth factor in peripheral nerves;24 iii) formation of anastomoses between the intact and damaged nerve fibers;25 iv) reinnervation of muscle fibers;26 v) regeneration of nerve fibers;27 and vi) inhibition of pro-inflammatory cytokines.28
It should be noted that currently conducted clinical and experimental studies, including TENS, give a very contradictory assessment and interpretation of the results of immediate and long-term observation, both in relation to pulse modulations of high-frequency (HF TENS) and low-frequency (LF TENS) current of different shapes, as well as in terms of amplitude and frequency parameters (100-200 Hz) and (1-3 Hz).
The aim of this study was to conduct a multi-faceted assessment of pain syndrome in patients with type II DM suffering with DPNP before and after use of pulsed modulated current of different frequency and amplitude in the form of TENS, adding scientific data on the mechanism of action and developing optimizing analgesic techniques taking into account the levels and severity of polyneuropathy and age of patients based on analytical structural details of this pain syndrome.
Materials and Methods
The study is a prospective randomized study, with patients divided into groups by stratification method and is controlled in nature according to the data of the immediate and long-term results of the study, with a single-center and sample assessment based on the longitudinal collection of statistical material. Enrolled patients were informed in advance and signed informed consent. The study was conducted in accordance with the research rules of the Federal State Budgetary Institution NMC of the Ministry of Health of the Russian Federation (CTR No. 121040100062-3) and with the permission of the Local Ethics Committee (IRB No. 2 dated 14.01.2021). Patient data bases were generated using spreadsheets Excel MS Office 2007, utilizing the application data package STATISTICA 7.0. Qualitative variables were compared using the t-Student criteria, as well as quantitative variables, based on the normal distribution of the trait. The differences were considered significant at p < 0.05.
A course of TENS-therapy was performed in patients with type II diabetes with DPNP four weeks after standard drug therapy: alpha-lipoic acid 600 mg / day for 2 months, pentoxifylline 100 mg / 3 times a day for 1 month, vitamin B12 1000 mcg / m for 10 days, with pain syndrome - gabapentin 300 mg / 3 times a day.
Technic TENS: TENS was performed on a CE0434 BTL-4000 certified smart / premium device (BTL Industries Ltd., Hertfordshire, UK). HF TENS: single-phase pulses with a frequency of 100 Hz and a duration of 100 microseconds, where the pulse amplitude was adjusted individually from 5 to 40 mA to achieve painless sensory sensations. The fibular and tibial nerves were stimulated for 5 minutes each. LF-TENS is based on single-phase pulses with a frequency of 1 Hz and a duration of 300 microseconds. The pulse amplitude was individually adjusted from 5 to 40 mA until a painless muscle contraction was achieved. The wrist and ulnar nerves were stimulated for 5 minutes each. A total of 15 procedures were performed daily or every other day. Tools for assessing neuropathic pain: the VAS system, where pain intensity was assessed using a 10-point visual scale,28 including the area of its projection on skin tissues on the MPQ scale (MPQs) according to McGill,28 evaluation of spatial modeling of pain syndrome with analysis of localization, pain projection in the form of graphical analysis,28 which made it possible to visualize the pain syndrome according to the system (PPA) defined by a special two-dimensional transparent grid (Douleur Neuropathique.4 Questions- DN4).28 The complexity of the structural assessment of pain syndrome allowed to improve the system of differentiation of neuropathic pain in DPNP in patients with type II diabetes.
Results and Discussion
The study included 75 (38 women, 37 men) patients with type II diabetes complicated by DPNP. The average age of patients was 48.5 ± 3.6 years (p <0.05). In the patients included in the study, the duration of diabetes mellitus was 9.3 ± 0.46 years (p <0.05) with no significant difference between the control patients and the main groups where HF TENS and LF TENS were used (p > 0.05).
Table 1.
Dynamics of sensory and affective characteristics of DPNP before/after application of different methods of TENS.
| Sensory characteristics | Before treatment | Immediately after the course of treatment | Distant periods | |
|---|---|---|---|---|
| 2 months | 6 months | |||
| Control | 16.2±0.41 | 12.4±0.56* | 11.4±04* | 14.1±0,49 |
| HF TENS | 15.3±0.42 | 6.10±0.43***## | 7.22±0.43***## | 10.3±0,36**# |
| LF TENS | 15.2±0.43 | 9.42±0.42***## | 7.51±0.42***## | 11.1±0,45*** |
| Affective characteristics | Before treatment | Immediately after the course of treatment | Distant periods | |
|
|
|
|
2 months | 6 months |
| Control | 10.2±0.39 | 6.4±0.28** | 7.2±0.27* | of 9.82±0.4 |
| HF TENS | 9.10±0.35 | 5.0±0.21** | 5.5±0.11*# | 6.9±0.29*## |
| LF TENS | of 10.3±0.36 | 3.28±0.27**### | 2.9±0.17***### | 4.5±0.34**## |
Note: *, p <0.05 - Reliability of differences in the results compared to the baseline values before treatment according to the paired Student 's criterion; #, p <0.05 - compared to similar indicators of the control according to the paired Student's criterion.
Patients were divided into treatment groups: Ia (Main group - 1; n = 25 patients; women: 15, men: 10), in which HF TENS currents were used; patients of group Ib (Main group-2; n = 25 patients; women: 13, men: 12) - received low-frequency LF TENS; control (C; n = 25 patients; 15 women, 10 men) received a standard method of pharmacological therapy without physiotherapy. Data on regression of sensory and Table 1 presents manifestations of neuropathic pain syndrome in patients with type II diabetes with DPNP after using various TENS methods, including a long-term follow-up period (6 months).
When assessing the intensity of pain syndrome in patients with type II diabetes with DPNP, the maximum VAS indicator was set: 5 or more than 5 points according to the DN4 rating system. In 4 (5.3%) control patients, dorsiflexia paresis of the foot complicated by DPNP was detected (strength on a 5-point scale: 3.5, 4, 3, 3.5 points); in 5 (6.6%) patients who received HF TENS, dorsiflexia paresis of the foot complicated by DPNP was presented: 3,5, 3, 3, 4, 4 in 4 (5.3%) patients who received LF TENS, respectively: 3, 3, 3.5, 4 points. Paresis of the plantar flexion of the foot was diagnosed in one patient (1.3%) in the control group (4.0 points), one (1.3%) in the HF TENS group (4.5 points) and two (2.6%) in the LF TENS group (4.0 points).
During the study, negative sensory symptoms were evaluated according to the prevalence on a 5-point VAS scale: control patients - 2.7 ± 0.17 points, patients after HF TENS - 2.8 ± 0.16 points and after LF TENS-2.8 ± 0.17 points (respectively, p = 0.004, p = 0.009, р <0.01).
Positive sensory symptoms, according to the prevalence on a 10-point VAS scale: in control patients-6.8 ± 0.27 points, patients after HF TENS-6.8 ± 0.26 points, patients after LF TENS-6.9 ± 0.34 points (respectively, p <0.05, p = 0.005, р <0,001).
Positive and negative sensory symptoms were evaluated immediately after TENS and after 2, 4, and 6 months of follow-up, using both the VAS system and the DN4 scale (Figure 1).
After the course of treatment and after 6 months at the level of 29.5% (for all indicators, p < 0.05). The decrease in PPA in patients after LF TENS was 21,1% higher, both immediately after the procedure, and after 2 months of follow-up (by 16%) and 6 months (by 8.5%), than in patients after HF TENS (for all indicators, p <0.05). In control patients, similar analgesic effects were observed immediately after drug treatment. When assessing the pain syndrome on the DN4 scale, the total score averaged 7.52 ± 0.07 points (p = 0.004) in all patients with type II DM with LDL. Complete resolution of neuropathic pain was observed in 16 (21.5%) (p <0.01) patients after HF TENS and in 32 (42.6%) patients after LF TENS (p <0.05). In patients who received only standard drug therapy, complete resolution of the pain syndrome was not observed.
Fig 1.
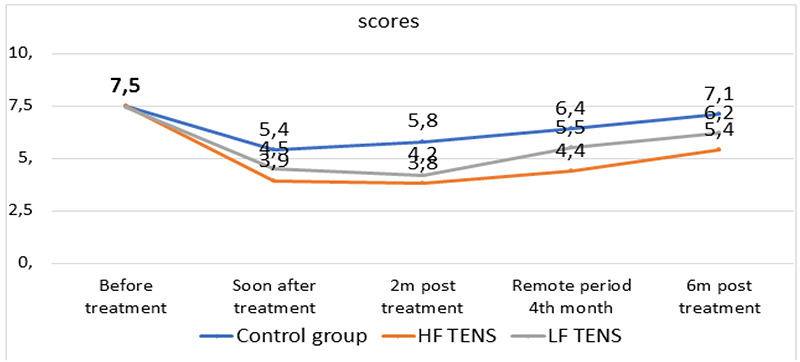
Pain assessment on the DN4 scale immediately after TENS and in the long-term follow-up period.
New criteria for pain syndrome verification in patients with type II DM with DPND were obtained depending on the localization (upper and lower extremities), prevalence, and projection of the pain syndrome. Thus, pain assessment using MPQ allowed us to establish the average PRI parameter in patients with type II DM with DPND: 16.2 ± 0.41 points in the sensory dimension, 10.2 ± 0.39 points in the affective dimension, and 4.35 ± 0.19 points in the estimated MPQ dimensions (for all indicators, р <0,05). Sensory PRI size after HF TENS was 35.2% lower relative to analgesic effects after LF TENS (р <0.001). The affective PRI size after LF TENS was 34.4% lower than after HF TENS (р <0.001).
New scientific evidence has been obtained that the resolution of pain syndrome in patients with type II DM with DPNP in the lower extremities primarily depends on the modality of the applied pulse current TENS, including those based on electroneuromyography (ENMG) indicators.28 Thus, positive sensory pain symptoms increased by 23.9% (р <0.05). Negative sensory pain symptoms, primarily in the lower extremities, are more actively stopped by low-frequency parameters (1-3 Hz, sending a pause – 100 ms) TENS. The obtained differences in the assessment of pain syndrome were noted only after its multi-system analysis, including based on the assessment of the acid-oxidative reserve of blood in surrounding tissues in the projection of peripheral nerve stimulation (Table 2).
Table 2.
Average values of the acid-oxidative reserve of blood and surrounding tissues in the projection of nerve stimulation on the upper/lower extremities in patients with type II DM with DPNP of different ages before and after TENS.
| Age norm Art/Ven Age norm Art/Ven |
K-Patients DM II DM II patients DPNP | Patients DM with DPNP extremities DM II patients DPNP extremities | ||||||
|---|---|---|---|---|---|---|---|---|
| Before TENS Before TENS |
After TENS After TENS |
Before TENS Before TENS |
After TENS After TENS |
|||||
| Art | Ven | Upper extremity Upper extremity | Lower limb Bottom extremity | |||||
| <40 years/ <40 years 120,9±2,1/3 7,6±2,2 |
96,9 ±3,51 |
to 103.3± 3,6* |
30,9 ±2,1 |
33,5 ±2,3* | 21,9 ±2,3 |
22,5± 2,1# | 29,1 ±1,83 | of 30.3 ±1,9# |
| > 40 years > 40 years of 103.5±1,92 4,4±1,3 | 90,4 ±3,14 | 93,9 ±3,9* | 23,5 ±2,5 | 28,5± 2,2** | 19,7 ±2.3 years | was 21.9±2,1## | 26,5 ±1,83 | 30,4±1,7## |
Note: *, p <0.05 - Reliability of mean values before/after TENS; # - p <0.05 - the difference of the average analogue values on the upper and lower extremities of the DPNP according to the paired Student 's criterion.
The obtained results demonstrate that the use of TENS, according to various indicators of validated pain scales (VAS, MPQ, DN4, graphical pain assessment), provides pronounced analgesic effects, the activity of which in relation to positive and negative sensory symptoms of pain activity, affective and sensory values of PRI, in the upper and lower extremities, significantly depends on the modulations of the pulsed current used for electroneurostimulation, which confirms previously published literature data.29 The use of sinusoidal modulated current of different frequencies and pulse feed provides "multiphase" effects based on the mismatch of pulses in the phase of current modulations, which forms the maximum amplitudes (Um) currents associated with ion reactions and the action potential.29 Taking into account the fact that the frequency of physiological repetition of bioelectric activity pulses in neuromuscular tissues lies in the range of 1-2 to 400 Hz,29 it follows that these frequencies potentiate the formation of a physiological impulse in the nerve fiber, which is more significant at low pulse frequencies, providing activation of neurohormone synthesis and normalization of the acid-oxidative reserve in surrounding tissues. At the same time, elastic waves with a high amplitude are formed at the cellular level under the pulsed action of TENS the amount (from units of microvolts to tens of millivolts) in the muscles, the spread of which along the damaged nerve fiber ensures their effective recovery.30 For example, the process of nerve impulse propagation along the sciatic nerve is accompanied by pulsing along the entire surface of the nerve fiber with an amplitude of up to 30 nm and a pulse frequency of up to 4 Hz, which affects the speed of nerve impulses (from 1 to 100 m/s-1), reducing the pain syndrome in the damaged nerve [30]. We believe that the developmental changes in nerve fibers in DPNP affect mitochondrial cells and nuclear structures, protein macromolecules, which is accompanied by the release of endogenous opioids and neurotransmitters, changing the acid-oxidative reserve of blood and surrounding tissues in the projection of nerve stimulation,30 which is of interest in further research. In this regard, it can be stated that despite the widespread and rather successful application of methods for recording the bioelectric activity of nerve and muscle tissues, many mechanisms of generating biopotentials are not fully understood, including from the point of view of frequency-dependent stimulation effects of modulated and amplitude-pulse parameters. Pain assessment using VAS system shows that before treatment, pain intensity in type II DM patients with DPND in the main groups and in the control group averaged 7.68 ± 0.14 points. As result of treatment, a significant reduction in pain intensity was observed in the main groups by 65.3% (p <0.05), versus 35.3% (p <0.05) in control patients.
Patients who received LF TENS showed a more significant reduction in the pain intensity of PPA by 2,1% (p <0.001), compared with HF TENS (p =0.002), including in relation to the acid-oxidative reserve of blood and surrounding tissues in the projection of stimulated nerve. There was no significant increase in pain syndrome on the VAS scale and affective pain sizes after the 6th month of the long-term follow-up period in patients who received LF TENS, in relation to the effects after HF TENS, both immediately after treatment (by 35.2%, p <0.001), and in the long-term period after 6 months - by 34.4% (p <0.001).
Therefore, our data confirm the efficacy and safety of LF and HF TENS in type II DM patients suffering with DPND-related pain.
Acknowledgments
The authors express their gratitude to patients and everyone who participated in their examination.
List of acronyms
- DM
diabetes mellitus
- DN4
analog pain assessment using a questionnaire
- DPNP
distal polyneuropathy
- HF TENS
high frequency current
- LF TENS
low-frequency current
- MRQ
pain assessment based on McGill
- PPA
graphic description of pain
- PRC
area of pain on the skin
- VAS
visual pain assessment scale
Funding Statement
Funding: Source of funding for scientific work and the process of collecting material of a state organization - FGBU NMIC of the Ministry of Health of the Russian Federation.
Contributor Information
Natalia Kulikova, Email: kulikova.natalya.moscow@gmail.com.
Al-Zamil Mustafa Khalilovich, Email: alzamil@mail.ru.
Tatiana Konchugova, Email: umc-rnc@mail.ru.
Andrey Rachin, Email: RachinАP@nmicrk.ru.
Tinatin Chkheidze, Email: Tinatin@mail.ru.
Detelina Kulchitskaya, Email: deti_ku@mail.ru.
Fesyun Anatoliy, Email: fad68@yandex.ru.
Natalia P. Sanina, Email: nataliasanina2@yandex.ru.
References
- 1.Didangelos T, Doupis J, Veves A. Painful diabetic neuropathy: clinical aspects. Handb Clin Neurol. 2014;126:53-61. doi: 10.1016/B978-0-444-53480-4.00005-9. [DOI] [PubMed] [Google Scholar]
- 2.Al Zamil MKh. Results of a comparative analysis between percutaneous electroneurostimulation and acupuncture in the treatment of 548 patients with diabetic distal polyneuropathy of the lower extremities. Clinical Neurology. 2019; 4(1):9-17. (In Russ.). [Google Scholar]
- 3.Minenko IA, Al-Zamil MKh. Dynamics of quality of life of patients with diabetic neuropathic pain syndrome against the background of complex application of percutaneous electrical nerve stimulation and acupuncture. Siberian Scientific Medical Journal. 2017; 37(5): 62-67. (In Russ.). [Google Scholar]
- 4.Singh-Franco D, Jacobs RJ. Patient perspectives on peripheral neuropathic pain experience within the community. Diabetes Metab Syndr. 2017. Nov;11 Suppl 1:S243-S246. doi: 10.1016/j.dsx.2016.12.038. Epub 2016 Dec 22. [DOI] [PubMed] [Google Scholar]
- 5.Sergeenko EYu, Romashina OM, Lobysheva AA, Zhitareva IV, Barysheva OV. Combined use of low-frequency pulse current and vacuum exposure in the rehabilitation of patients with diabetic polyneuropathy. Bulletin of Restorative Medicine. 2019; 5(93):40-46. (In Russ.). [Google Scholar]
- 6.Tesfaye S, Vileikyte L, Rayman G, Sindrup SH, Perkins BA, Baconja M, Vinik AI, Boulton AJ; Toronto Expert Panel on Diabetic Neuropathy. Painful diabetic peripheral neuropathy: consensus recommendations on diagnosis, assessment and management. Diabetes Metab Res Rev. 2011. Oct;27(7):629-38. doi: 10.1002/dmrr.1225 [DOI] [PubMed] [Google Scholar]
- 7.Al-Zamil MKh, Kulikova NG, Bezrukova OV, Volkova IV, Stakhurlova VE. Effectiveness of percutaneous electrical neurostimulation for the treatment of diabetic distal polyneuropathy. European Journal of Neurology. 2019; 26(1): 552-744. (In Russ.). [Google Scholar]
- 8.Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D; American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011. May 17;76(20):1758-65. doi: 10.1212/WNL.0b013e3182166ebe. Epub 2011 Apr 11. Erratum in: Neurology. 2011. Aug 9;77(6): 603. Dosage error in article text. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moisset X, Bouhassira D, Avez Couturier J, Alchaar H, Conradi S, Delmotte MH, Lanteri-Minet M, Lefaucheur JP, Mick G, Piano V, Pickering G, Piquet E, Regis C, Salvat E, Attal N. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Rev Neurol (Paris). 2020. May;176(5):325-352. doi:10.1016/j.neurol.2020.01.361. Epub 2020 Apr 7 [DOI] [PubMed] [Google Scholar]
- 10.Al-Zamil MH, Kulikova NG, Minenko IA, Vasilyeva ES. Direct percutaneous electroneurostimulation in the treatment of pathologies of the peripheral nervous system. Physical therapist. 2020; 3(1): 57-69. (In Russ.). [Google Scholar]
- 11.Gibson W, Wand BM, Meads C, Catley MJ, O'Connell NE. Transcutaneous electrical nerve stimulation (TENS) for chronic pain - an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2019. Apr 3;4(4):CD011890. doi: 10.1002/14651858.CD011890.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gozani SN. Remote Analgesic Effects Of Conventional Transcutaneous Electrical Nerve Stimulation: A Scientific And Clinical Review With A Focus On Chronic Pain. J Pain Res. 2019. Nov 26;12:3185-3201. doi: 10.2147/JPR.S226600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokhtari T, Ren Q, Li N, Wang F, Bi Y. Transcutaneous electrical nerve stimulation in the relief of neuropathic pain: basic mechanisms and clinical application. Curr Pain Headache Rep. 2018; 24(4):14. doi: 10.1007/s11916-020-0846-1. [DOI] [PubMed] [Google Scholar]
- 14.Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009. Sep;219(1):258-65. doi: 10.1016/j.expneurol.2009.05.034. Epub 2009 Jun 3. [DOI] [PubMed] [Google Scholar]
- 15.Davoudi M, Rezaei V, Rajairamsheh F, Ahmadi S, Taheri AA. Predicting quality of life based on pain measurements and psychiatric symptoms in patients with painful diabetic neuropathy: a cross-sectional prevalence study in Iranian patients. Health-Life. Results. 2021; 9.19(1):49. doi: 10.1186/s12955-021-01697-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocyigit F, Akalin E, Gezer NS, Orbay O, Kocyigit A, Ada E. Functional magnetic resonance imaging of the effects of low-frequency transcutaneous electrical nerve stimulation on central pain modulation: a double-blind, placebo-controlled trial. Clin J Pain. 2012. Sep;28(7):581-8. doi: 10.1097/AJP.0b013e31823c2bd7. [DOI] [PubMed] [Google Scholar]
- 17.Koca Kutlu A, Ceçen D, Gürgen SG, Sayın O, Cetin F. A Comparison Study of Growth Factor Expression following Treatment with Transcutaneous Electrical Nerve Stimulation, Saline Solution, Povidone-Iodine, and Lavender Oil in Wounds Healing. Evid Based Complement Alternat Med. 2013;2013:361832. doi: 10.1155/2013/361832. Epub 2013 Jun 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naderi Nabi B, Sedighinejad A, Haghighi M, Biazar G, Hashemi M, Haddadi S, Fathi A. Comparison of Transcutaneous Electrical Nerve Stimulation and Pulsed Radiofrequency Sympathectomy for Treating Painful Diabetic Neuropathy. Anesth Pain Med. 2015. Oct 10;5(5):e29280. doi: 10.5812/aapm.29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nabi BN, Saberi A, Eghbali BB, Hosseininejad M, Gelareh Biazar G. Efficacy and safety of TENS and duloxetine in patients with painful diabetic neuropathy: a single blind randomized clinical trial. J Adv Med Biomed Res. 2021; 29(136): 286-292. [Google Scholar]
- 20.Forciniti L, Ybarra J 3rd, Zaman MH, Schmidt CE. Schwann cell response on polypyrrole substrates upon electrical stimulation. Acta Biomater. 2014. Jun;10(6):2423-33. doi: 10.1016/j.actbio.2014.01.030. Epub 2014 Feb 8. [DOI] [PubMed] [Google Scholar]
- 21.Shahanawaz SD. Effect of high TENS on neuropathic pain in patients with diabetic neuropathy. International Journal of Physical Therapy and Research. 2014; 2(4):604-07. [Google Scholar]
- 22.Kulikova NG, Deryagina LE, Bezrukova O V. Ecogenic risks of discogenic regeneration and modern ways of homestasiological physiotherapy correction. Human ecology. 2018; 2(1): 124-129. (In Russ.). [Google Scholar]
- 23.Gürgen SG, Sayın O, Cetin F, Tuç Yücel A. Transcutaneous electrical nerve stimulation (TENS) accelerates cutaneous wound healing and inhibits pro-inflammatory cytokines. Inflammation. 2014. Jun;37(3):775-84. doi: 10.1007/s10753-013-9796-7. [DOI] [PubMed] [Google Scholar]
- 24.Luo B, Huang J, Lu L, Hu X, Luo Z, Li M. Electrically induced brain-derived neurotrophic factor release from Schwann cells. J Neurosci Res. 2014. Jul;92(7):893-903. doi: 10.1002/jnr.23365. Epub 2014 Feb 19. [DOI] [PubMed] [Google Scholar]
- 25.Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther. 2013. Oct;93(10):1397-402. doi: 10.2522/ptj.20120281. Epub 2013 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upton GA, Tinley P, Al-Aubaidy H, Crawford R. The influence of transcutaneous electrical nerve stimulation parameters on the level of pain perceived by participants with painful diabetic neuropathy: A crossover study. Diabetes Metab Syndr. 2017. Apr-Jun;11(2):113-118. doi: 10.1016/j.dsx.2016.08.016. Epub 2016 Aug 23 [DOI] [PubMed] [Google Scholar]
- 27.Vieira PJ, Ribeiro JP, Cipriano G Jr, Umpierre D, Cahalin LP, Moraes RS, Chiappa GR. Effect of transcutaneous electrical nerve stimulation on muscle metaboreflex in healthy young and older subjects. Eur J Appl Physiol. 2012. Apr;112(4):1327-34. doi: 10.1007/s00421-011-2084-z. Epub 2011 Jul 28. [DOI] [PubMed] [Google Scholar]
- 28.Vakhitov BI, Raginov I.S, Vakhitov IKh, Bodrova RA, Izosimova AV. Features of changes in electromyography in patients with upper limb lesions during various exercises. Bulletin of Restorative Medicine. 2020; 2(96):54-58]. (In Russ.). [Google Scholar]
- 29.Reis F, Guimarães F, Nogueira LC, Meziat-Filho N, Sanchez TA, Wideman T. Association between pain drawing and psychological factors in musculoskeletal chronic pain: A systematic review. Physiother Theory Pract. 2019. Jun;35(6):533-542. doi: 10.1080/09593985.2018.1455122. Epub 2018 Apr 16. [DOI] [PubMed] [Google Scholar]
- 30.Konchugova TV, Kulikova НNG, Astakhova KA, Grushina TI. Methods of physiotherapy of peripheral polyneuropathy, induced by cytostatics. Questions of balneology, physiotherapy and physicaltherapy. 2021; 98:3(2): 94 (In Russ.). [Google Scholar]


