Abstract
Background:
Deep vein thrombosis (DVT) and post-thrombotic syndrome (PTS) remain highly prevalent despite modern medical therapy. Contact activation is a promising target for safe antithrombotic anticoagulation. The anti-factor XI (FXI) mAB 14E11 reduces circulating levels of FXI without compromising hemostasis. The human recombinant analog, AB023, is in clinical development. The role of FXI in mediation of inflammation during DVT resolution is unknown.
Objectives:
Investigate the effects of pharmacological targeting of FXI with 14E11 in an experimental model of venous thrombosis.
Methods:
Adult wildtype CD1 mice were treated with subcutaneous anti-FXI antibody (14E11, 5 mg/kg) versus saline prior to undergoing surgical constriction of the inferior vena cava (IVC). Mice were evaluated at various time points to assess thrombus weight and volume, as well as histology analysis, ferumoxytol enhanced magnetic resonance imaging (Fe-MRI) and whole blood flow cytometry.
Results:
14E11-treated mice had reduced thrombus weights and volumes after IVC constriction on day 7 as compared to saline-treated mice. 14E11 treatment reduced circulating monocytes by flow cytometry and macrophage content within thrombi as evaluated by histologic staining and Fe-MRI. Collagen deposition was increased at day 3 while CD31 and smooth muscle cell actin expression was increased at day 7 in the thrombi of 14E11-treated mice as compared to saline-treated mice.
Conclusion:
Pharmacologic targeting of FXI enhances the early stages of experimental venous thrombus resolution in wildtype CD1 mice, and may be of interest for future clinical evaluation of the antibody in DVT and PTS.
Keywords: vein, thrombosis, contact activation, factor XI
INTRODUCTION:
Coagulation is a highly regulated process that produces clots in response to vascular insult or loss of integrity. While thrombus hemostatic blood clot formation is an essential response to limit blood loss at the vessel wall, aberrant activation of the coagulation cascade and the resulting pathologic thrombus formation results in negative health consequences. Deep vein thrombosis (DVT), the formation of thrombi in the large veins of the cardiovascular system, increases the risk of pulmonary embolism and can lead to the development of post-thrombotic syndrome [1]. Anticoagulants are prescribed to treat DVT with duration of treatment typically lasting several months. Notably, the anticoagulants that are currently approved to treat DVT function either through the inhibition of the common pathway, by inhibiting coagulation factor Xa (FXa) activity (e.g., apixaban or rivaroxaban), or through the inhibition of thrombin activity directly (e.g., dabigatran). While these treatments are successful in inhibiting thrombus extension and reoccurrences, they simultaneously increase the risk of developing bleeding complications [2].
While the common pathway has been the principal target for the development of anticoagulant therapies for DVT, evidence suggests that FXI contributes to pathologic thrombus formation [3]. Activation of factor XII (FXIIa) activates FXI as part of the contact activation system, which is composed of a series of serine protease zymogens that ultimately increase thrombin activity, resulting in fibrin clot formation [4]. Following activation by FXIIa as part of the contact pathway or by thrombin as part of a feedback mechanism, FIXa in turn initiates the common coagulation pathway with the activation of FX. Notably, several downstream coagulation factors, including FXI, increase FXII activation through a positive feedback loop [4]. Preclinical and early clinical data suggest that targeting FXI may be advantageous in reducing thrombus formation and DVT in particular [3]. A recent clinical trial has shown that the contact pathway inhibitor AB023 reduced clotting of dialyzer filters in patients with end stage renal disease on chronic hemodialysis [5], while genetic depletion of FXI in a flow restriction DVT model lacking endothelial disruption has been shown to decrease venous thrombus formation in mice [6].
The innate immune system has been shown to play a role in the progression and resolution of thrombi during DVT [7]. Previous studies have highlighted the roles of FXI and the contact pathway of coagulation in mediating inflammation in various models of disease [8–11]. In a mouse model of sepsis, contact pathway inhibition after cecal ligation and perforation was protective and increased survival [10]. Similarly, in a baboon model of Staphylococcus aureus induced sepsis, inhibition of FXI activation by FXIIa attenuated the inflammatory cytokine surge and reduced organ failure and mortality [9]. The anti-inflammatory effects of FXI inhibition may potentially be advantageous in settings of vascular inflammation and may represent an additional pathway through which targeting FXI may affect thrombus propagation and resolution during DVT.
In the present study, we examine the effects of targeting FXI with the anti-FXI monoclonal antibody 14E11, on the early stages of venous thrombus resolution and associated inflammatory responses using a mouse constriction model of DVT. While previous work has evaluated the role of FXI on thrombus initiation, our study will be among the first to evaluate the effect of pharmacological targeting of FXI on the early stages of venous thrombus resolution. In addition to using standard histology techniques to measure changes in cellular content of thrombi and post-thrombotic remodeling, we also applied a novel nanoparticle imaging technology (ferumoxytol enhanced magnetic resonance imaging (Fe-MRI)) that provides a non-invasive in vivo measurement of thrombus volumes as well as thrombus-associated macrophage content and phagocytic activity [12, 13]. This work implicates a role of FXI on peripheral monocyte expansion and recruitment to thrombi during the early stages of venous thrombus resolution.
METHODS:
Animals:
The experiments involving mice in this study were approved by the Oregon Health & Science University (OHSU) Committee on the Use and Care of Animals. Experiments were done in accordance to the national standards outlined by the National Institutes of Health. Wildtype CD1 mice (stock #550) of either female or male sex purchased from a commercial vendor (Charles River Laboratories, Wilmington, MA) were used for all experiments. All mice were housed under specific pathogen free conditions with controlled temperature and humidity in a 12 hour light/dark cycle with continuous access to food, water, and environmental enrichment.
FXI Antibody (14E11) derivation and dosing:
Derivation and activity of the murine anti-mouse FXI monoclonal antibody 14E11 used in this study have been described previously [14]. The antibody was generated by immunizing FXI-deficient mice with recombinant mouse FXI. FXI-deficient Balb-C mice were immunized with 25 mg of recombinant mouse FXI diluted 1:1 with Freund adjuvant (200 mL total) by intraperitoneal injection. Two 25-mg booster doses in incomplete Freund adjuvant were given 3 and 7 weeks later and hybridomas were generated by standard protocols. Media were tested for capacity to recognize mouse FXI by enzyme-linked immunosorbent assay and to prolong the aPTT of mouse and human plasmas. Clones of interest were subcloned twice by limiting dilution. Clone 14E11 was expanded in a CL1000 bioreactor (Integra Biosciences), and immunoglobulin G (IgG) was purified by cation exchange and thiophilic agarose chromatography.
The 5 mg/kg dosage of 14E11 was chosen based on previous work that showed that 14E11 decreased murine FXI levels below the limit of detection [8]. In brief, the dose-finding experiment was performed wherein C57BL/6 mice were injected with a single subcutaneous (SC) dose of 14E11 (5 mg/kg) and activated partial thromboplastin time (aPTT) was monitored over a period of 10 days. Mice in this study were injected with a single subcutaneous (SC) dose of 14E11 (5 mg/kg) immediately before IVC constriction surgery. To confirm the degree of pharmacological inhibition on the contact pathway in our current model, the detectable levels of plasma FXI and aPTT were measured in surgically naïve CD1 wildtype mice injected with a single subcutaneous dose of 14E11 (5 mg/kg) at 0, 2, and 18 hours after administration, which correlates to the time period of venous thrombosis induction and thrombus formation.
aPTT Measurements:
Whole blood was collected from a single direct puncture of inferior vena cava after midline laparotomy into sodium citrate (0.32% w/v). Platelet-poor plasma (PPP) was isolated by centrifuging blood samples at 2000 g for 10 min at room temperature. PPP was mixed 1:1 with aPTT reagent and incubated for 3 min at 37°C. CaCl2 (8.3 mM final concentration) was added in equal volume to PPP and aPTT reagent. The time (seconds) to thrombus formation was measured using a KC4 coagulation analyzer (Trinity Biotech, Jamestown, NY).
Western Blotting:
Whole blood was collected from the inferior vena cava into sodium citrate (0.32% w/v) and isolated PPP was used to measure changes in detectable levels of circulating FXI. PPP was size-fractionated under non-reducing conditions on a 7.5% polyacrylamide-sodium dodecyl sulfate gel. Proteins were transferred to a nitrocellulose membrane and FXI was detected using biotin-conjugated anti-mouse IgG 14E11. Western blots were developed using streptavidin-horseradish peroxidase and chemiluminescence.
Measurement of Endotoxins Levels in Prepared 14E11, FXI Antibody:
A chromogenic endotoxin quantification kit (Thermo Fisher Scientific/Pierce, Waltham, MA) was used per manufacturer instructions to measure endotoxin levels in batches of prepared 14E11 antibody. The preclinical upper threshold of approximately 5 EU/kg for a 30 g mouse was used as an acceptable endotoxin dose level [15].
Murine Stenosis Model of Deep Vein Thrombosis:
Wildtype, female or male CD1 mice were anesthetized with isoflurane vapor anesthesia and subcutaneously injected with either saline or 14E11 antibody (5 mg/kg) before undergoing laparotomy for constriction of the infra-renal inferior vena cava (IVC). Endothelial injury was induced with clamping of the IVC twice for a duration of 15 s and large lumbar veins were cauterized. In this stenosis model of DVT, a thrombus forms up to day 3 post-surgery and more than 50% of the thrombus resolves over the proceeding 7–14 days [16, 17]. On days 6 and 7 post-surgery, thrombi were evaluated using magnetic resonance imaging (MRI) as described below. A separate cohort of animals underwent whole blood flow cytometry and hematological analysis on day 7. Animals were euthanized at day 7 after IVC constriction and tissue collected for immunohistochemical analysis.
Thrombus Weight Analysis:
Thrombi were excised at the time of sacrifice at serial time points (days 3 and 7) from each mouse. Thrombi were blotted with delicate task wipers to absorb and remove excess fluid and then weighed in milligrams using an analytical balance.
Ferumoxytol Enhanced Magnetic Resonance Imaging (Fe-MRI):
Magnetic resonance imaging (MRI) with ferumoxytol (Fe) was performed to evaluate thrombus volume and macrophage content using 24 hour delayed MRI scanning after Fe injection. Fe is an iron nanoparticle with a half-life of 2 hours in rodents that is used as a blood pool contrast agent for MRI [18]. A horizontal bore 11.75T MR scanner (Bruker Scientific Instruments) was equipped with isoflurane anesthesia, oxygen delivery and a custom mouse body coil. Monitoring was used to evaluate the abdomen and lower extremities at days 6 and 7 after IVC constriction. The intravenous Fe contrast (7 mg/kg) was administered on day 6 (immediately before scanning) via tail vein catheter allowing for injection without repositioning the animal. High resolution T1 and T2-relaxation mapping sequences were obtained before and after Fe injection to evaluate the IVC after thrombus formation. Axial T1 weighted sequences were obtained to calculate thrombus volumes. For the axial T2 mapping sequence, a 2D RARE spin-echo sequence was used with repetition time (TR)=2000ms, echo times (TEs) = [9.0, 18.0, 27.0, 36.0ms], field of view (FOV) = 32.1mm, 192×192 matrix, 1 signal average, slice thickness = 1mm, and fat suppression. The same sequence was acquired 24 hours after Fe injection to evaluate intra- and peri-vascular inflammation.
Thrombi and adjacent vein walls were segmented on the first echo of the T2 mapping sequence (TE=9.7 ms) for each slice where either was visible, with care to avoid partial volume artifacts. Each pixel in the segmentations was fit for T2 relaxation times using a nonlinear least squares 2-parameter fit (M0, T2) of the T2 weighted images in each pixel.
Penetration of Fe through the thrombus is estimated by measuring T2 values before Fe injection and immediately after Fe injection on day 6. Macrophage content of thrombi and vein walls is estimated by measuring T2 values before Fe injection on day 6 and 24 hours after Fe injection on day 7 (delta T2 analysis). Injection of Fe and immediate measurement of T2 signal allows for assessment of tissue perfusion. After 24 hours, Fe is cleared from the peripheral circulation and engulfed by tissue macrophages. Thus, T2 analysis after 24 hours can be used as a direct measure of macrophage content in the thrombi and vein walls and Fe penetration into the tissue. Since Fe is engulfed by macrophages after 24 hours, the change in T2 values in the 24 hours delayed Fe-MRI imaging, estimates macrophage content [12, 13].
Immunohistochemistry:
After euthanasia at day 7, mice underwent necropsy and the IVC with residual thrombus were carefully dissected from surrounding tissues. Thrombi and IVC specimens were excised en bloc and fixed in formalin before they were embedded in paraffin blocks for sectioning. Histological analysis and immunohistochemical markers were performed using standard techniques on 5 μm sections. After blocking sections with normal serum to decrease nonspecific staining, tissue sections were incubated with primary antibodies to Iba1 (rabbit polyclonal, 1:5000/0.1 μg/μl, Fujifilm Wako Chemicals, Richmond, VA), CD31 (rabbit multiclonal, 1:250/2 μg/μl, Abcam, Cambridge, MA); smooth muscle actin (SMA; anti-mouse, 1:1000/0.07 μg/μl, DAKO, Agilent Technologies, Santa Clara, CA), and MMP-2 (rabbit polyclonal MMP-2, 1:2500/0.13 μg/μl, ProteinTech, Rosemont, IL). An ABC peroxidase kit was used according to the manufacturer’s instructions (Vector Laboratories Inc., Burlingame, California). The slides were counterstained with dilute hematoxylin and eosin. To stain for collagen (blue) and muscle/keratin (red), a Masson’s Trichrome (Aniline Blue Kit, Newcomer Supply, Middleton, WI) staining protocol was used per manufacturer instructions.
Immunostained slides were scored in a blinded fashion. Each section was scored by estimating the percent area stained using the following scoring rubric: 0= no staining, 1= <25%, 2=25–50%, 3=50–75%, and 4= >75% staining. Scores for each section were then averaged for each animal.
Hematological Analysis:
Animals were anesthetized with isoflurane vapor and whole blood collected from the retro-orbital sinus. Blood was diluted 1:1 with 10 mM EDTA and analyzed using a ScilVet ABC hematology analyzer (ScilVet, Viernheim, Germany). A complete blood count (CBC) with differentials (percentage lymphocytes, monocytes and granulocytes) was performed on surgically naïve mice 0 and 7 days following a single subcutaneous dose of 14E11 (5 mg/kg) as well as on saline- and 14E11-treated mice at day 7 post IVC constriction.
Flow Cytometry:
Whole blood was collected from the retro-orbital sinus into EDTA for a final concentration of 5 nM EDTA and incubated for 30 min at room temperature (RT) with fluorescent-conjugated antibodies. Red blood cells were lysed for 10 min at RT using 1× FACs Lysis Buffer (BD Bioscience) and remaining blood cells were washed with 1×PBS before being fixed in 1% paraformaldehyde. All samples were analyzed using a BD LSR II flow cytometer and FLOWJO. Monocytes were identified using PE-CD11b (553311, BD Bioscience) while T-lymphocytes, B-lymphocytes, NK Cells, and granulocytes were excluded from analysis through the negative selection of FitC-Thy-1(CD90) (555595, BD Bioscience), FitC-CD335 (NKp46) (560756, BD Bioscience), FitC-B220 (CD45R) (553087, BD Bioscience), and FitC- Ly6G (561105, BD Bioscience). Platelet monocyte aggregates were identified using BV421-CD41 (133912, BioLegend). Neutrophils/granulocytes were stained with PE-CD11b and identified as FitC-Ly6G high and BV421-Ly6C intermediate. All gates were set using fluorescence minus one controls.
Statistical Analysis:
The number of animals tested were based on power calculation to allow for 80% power and detection of a 30% difference in thrombus weights between groups. Normality of all data was tested using the Shapiro-Wilk test and normality was confirmed for DVT weights, thrombus volume, and flow cytometry data. Data of control and treatment groups were reported as averages with standard deviations. Data, which were parametric and normal, were compared using an unpaired Student’s t-test (DVT weights, thrombus volume, and flow cytometry). Non-parametric or non-normal data were compared using the Mann-Whitney U test (all histology data). Significance level was pre-set at P≤0.05.
RESULTS:
Pharmacological targeting of FXI with 14E11
Wildtype CD1 female or male mice were administered 14E11 (5 mg/kg) subcutaneously and plasma was analyzed for aPTT. Activated PTT levels were significantly elevated 2 hours and 18 hours after administration (Figure 1A). Consistent with previous reports, plasma FXI was not detectable by Western blot analysis at 2 or 18 hours post-14E11 treatment (Figure 1B). Endotoxin levels in the 14E11 antibody preparations used in this study ranged from 0.0029 to 0.0078 EU/kg (data not shown), which is well below the acceptable threshold of 5 EU/kg for a 30 g mouse [15].
Figure 1.
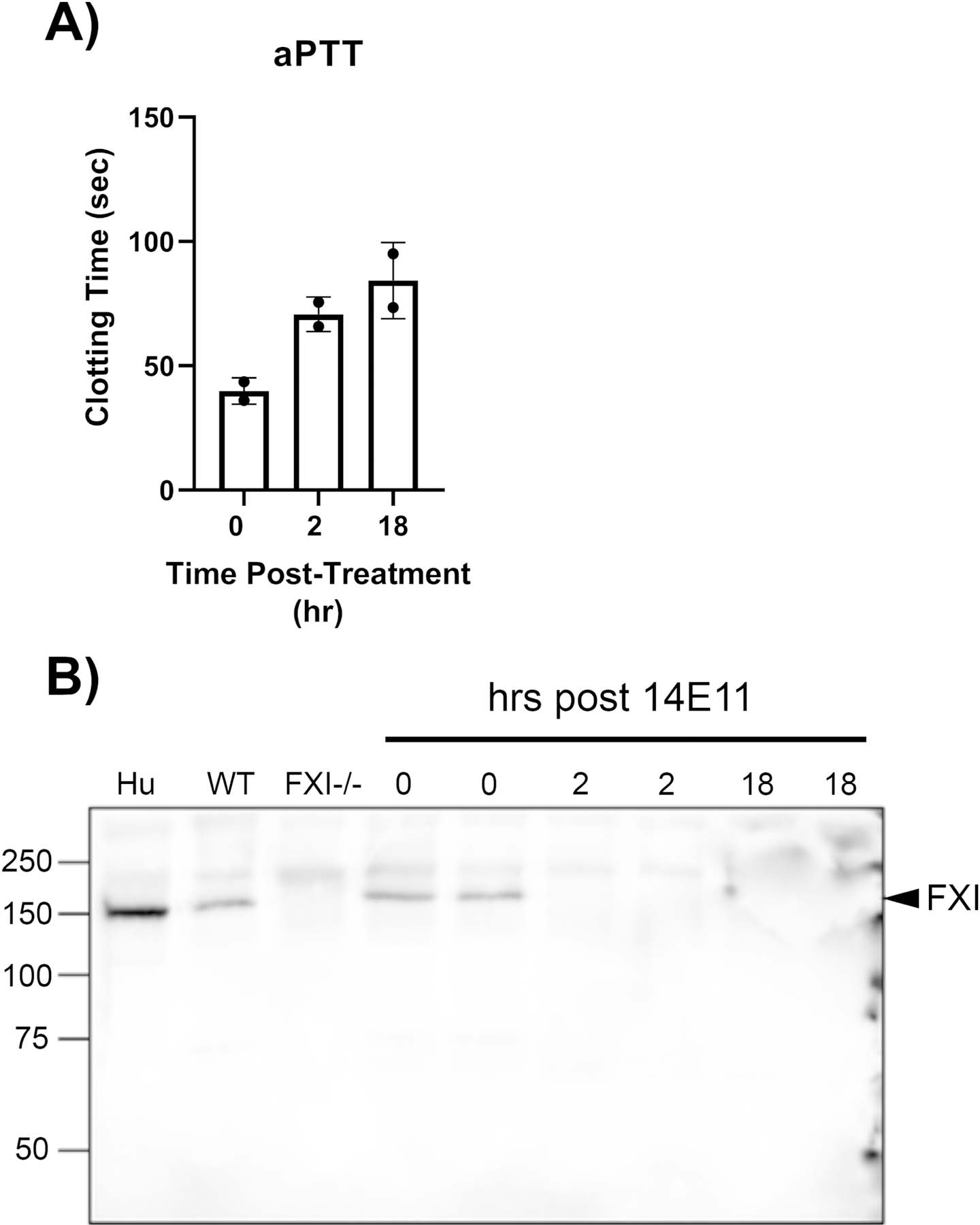
Effect of 14E11 on activated partial thromboplastin time (aPTT) and plasma FXI levels. aPTT measurements 0, 2, and 18 hrs after administration of 14E11 (5 mg/kg) (n=2) (A). Representative western blot of FXI plasma levels 0, 2, and 18 hrs after administration of 14E11 (5 mg/kg) (n=2) (B).
Effect of pharmacological targeting of FXI on the early stages of venous thrombus resolution
We first evaluated the effect of the anti-FXI monoclonal antibody 14E11 on the early stages of thrombus resolution following IVC constriction as a function of time. Thrombus weights were quantified and compared between saline-treated and 14E11-treated cohorts after 3 and 7 days to evaluate thrombus formation and the early stages of thrombus resolution, respectively. Our results show that pharmacological inhibition of FXI with 14E11 significantly reduced thrombus weights relative to body weight by day 7 following IVC constriction (Figure 2A). We next confirmed thrombus resolution by quantifying in vivo thrombus volumes in the early stages of thrombus resolution using T2 weighted magnetic resonance imaging (MRI) (Figure 2B). Thrombus volumes were significantly decreased on day 7 after IVC constriction in mice treated with 14E11 as compared to saline controls.
Figure 2:
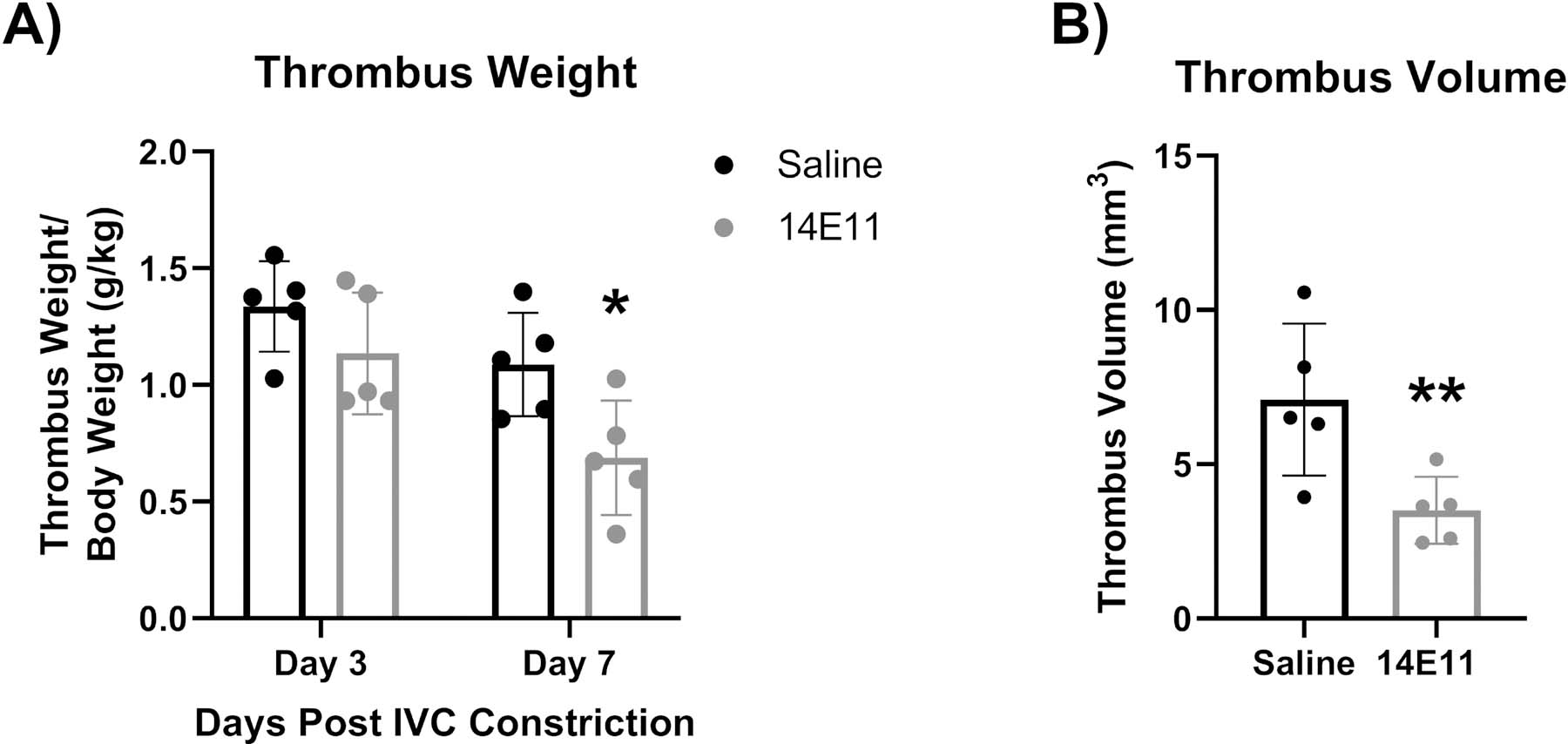
Effects of pharmacologic targeting of FXI by 14E11 on thrombus resolution following IVC constriction (stenosis model). Thrombus weights 3 and 7 days post IVC constriction (n=5, 1.09±0.1 versus 0.69±0.2, *P=0.03) (A). Representative data of a single experiment that was repeated 2 times. Thrombus volumes 7 days post IVC constriction, as measured by MRI (n=5, 7±2 versus 3.5±1, **P=0.02) (B). Data was analyzed using volumetric analysis of T1 images of thrombi that were compared using an unpaired Student’s t-test and presented as mean±SD.
Effect of pharmacological targeting of FXI on blood perfusion and macrophage content during the early stages of thrombus resolution
Changes in blood perfusion is a hallmark of thrombus resolution [7, 19, 20]. We therefore utilized quantitative Fe-MRI as a radiographic marker to study the effect of pharmacologically targeting FXI with 14E11 on blood perfusion at day 7 post IVC constriction. Interrogation of thrombus blood perfusion showed a trend towards decreasing delta T2 values at the vein wall of 14E11 as compared to saline-treated mice that was not statistically significant, while no change was observed within the thrombus (Figure 3A). Thus, while 14E11 treatments reduced thrombus weights and volumes at day 7, there was overall no change in overall blood perfusion.
Figure 3:
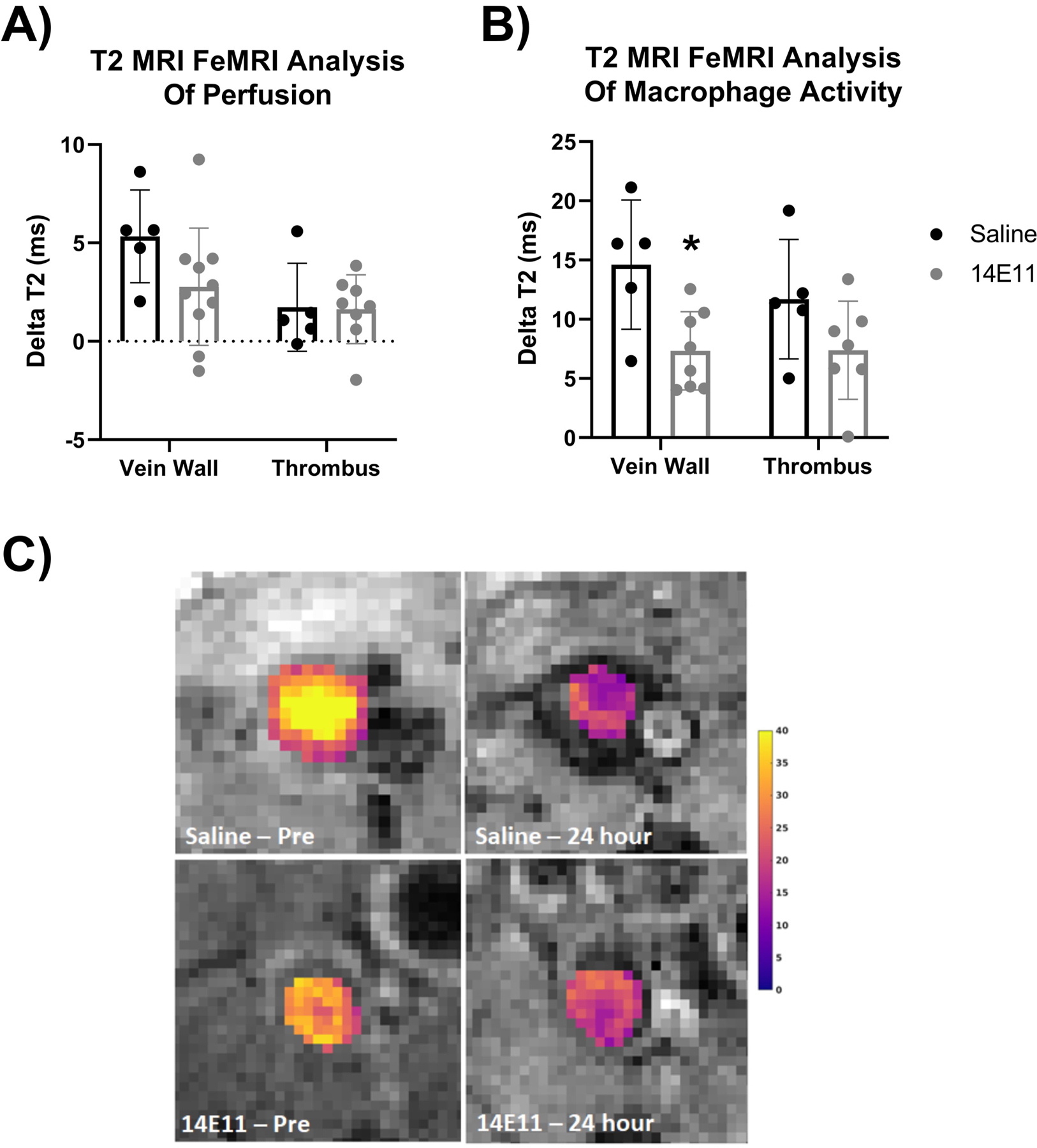
Effects of pharmacologic targeting of FXI by 14E11 on thrombus and vein wall ferumoxytol (Fe) penetration and macrophage content, as determined by ferumoxytol enhanced MRI (Fe-MRI). T2 Fe-MRI analysis of Fe tissue penetration 7 days after IVC constriction shows no significant difference between saline- versus 14E11-treated mice (P=0.94) (A). T2 Fe-MRI analysis of macrophage content in the vein wall 7 days post IVC constriction in saline- and 14E11-treated mice (n=5–7, 14.6±5 versus 7.3±3, *P=0.01) as well as macrophage content in the thrombus 7 days post IVC constriction (n=5–7, 6.7±6 versus 4.43±4, P=0.74) (B). Representative images of T2 maps of thrombi before and after ferumoxytol injection in saline- versus 14E11-treated mice (C). Data was analyzed using a region of interest T2 mapping of axial images of thrombi, unpaired Student’s t-test comparison and presented as mean±SD.
As inflammation and the innate immune system are linked with venous thrombosis, Fe-MRI as a radiographic marker was used to assess macrophage content 7 days post IVC constriction. Twenty-four hour delayed Fe-MRI analysis shows a significant difference in T2 value (delta T2) in both saline- and 14E11-treated mice that is associated with presence of macrophage content or Fe penetration in the thrombi and at the vein walls (Figure 3B). While macrophages were present in the veins and thrombi of both treatment groups, 14E11-treated mice had significantly lower delta T2 values at the vein wall, suggesting a decrease in macrophage activity compared to saline controls (Figure 3B). No significant difference in delta T2 values were observed within the thrombus. Representative images of T2 mapping shows significant changes between pre-ferumoxytol images and 24 hour-post injection images with saline-treated mice after IVC constriction correlating with greater macrophage content (Figure 3C).
To corroborate the in vivo MRI measurements of macrophage content, immunohistochemical analysis of the macrophage specific marker, ionized calcium binding adaptor molecule 1 (Iba1), was performed on paraffin embedded thrombus/IVC sections. Immunostaining demonstrated a significant decrease in the percent area of Iba1 staining in both the thrombus and at the vein wall of 14E11-treated mice as compared to saline-treated mice on day 7 (Figure 4A and 4B). There was no significant difference in macrophage content at day 3 between saline- and 14E11-treated mice (Figure 4A and 4B). Representative images of Iba1 staining in the thrombus and vein wall of saline- versus 14E11-treated mice on days 3 and 7 are shown in Figure 4C.
Figure 4:
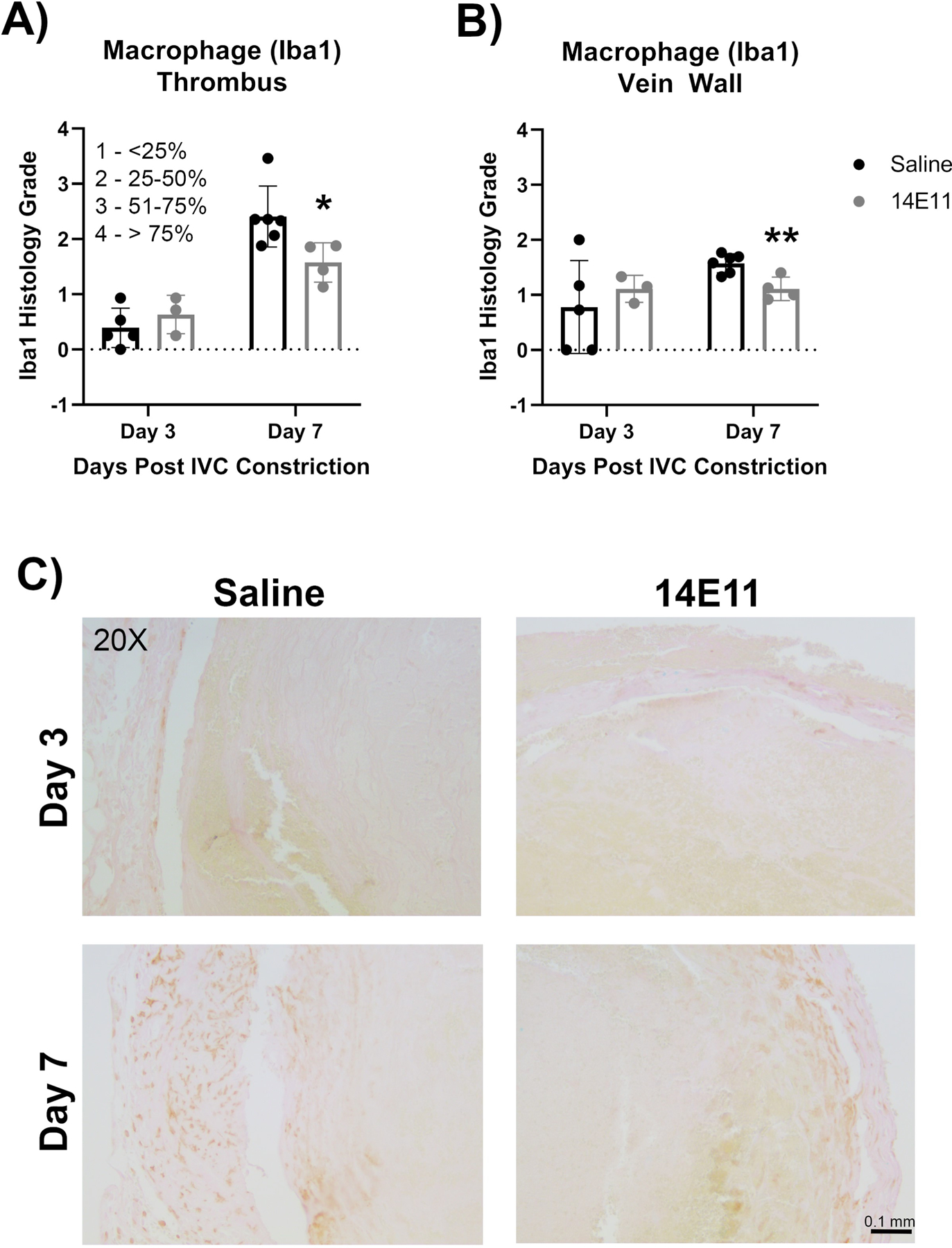
Effects of pharmacologic targeting of FXI by 14E11 on macrophage recruitment following IVC constriction. Quantification of the percent area of Iba1 in the thrombus on days 3 and 7 post IVC constriction in saline- versus 14E11-treated mice (n=4–5, Day 3: 0.4±0.4 versus 0.6±0.4, P=0.41 and Day 7: 2.4±0.6 versus 1.6±0.4, *P=0.029) (A). Quantification of the percent area of Iba1 immunostaining along the vein wall 3 and 7 days post IVC constriction in saline- versus 14E11-treated mice (n=4–5, Day 3: 0.78±0.8 versus 1.1±0.2, P=0.52 and Day 7: 1.6±0.2 versus 1.1±0.2, **P=0.01) (B). Representative images of Iba1 immunostaining of the thrombus and vein wall at 20x (scale bar=0.5 mm) (C). Data were analyzed using Mann-Whitney test comparison and presented as mean±SD.
Effect of pharmacological targeting of FXI on endothelial cell CD31 expression in thrombi after IVC constriction
Immunohistochemical analysis of cellular CD31 expression was performed to identify endothelial cells within the thrombi as the presence of thrombus-associated endothelial cells are associated with the formation of neochannels and recanalization to restore flow. CD31 immunostaining was performed on thrombus/IVC sections and CD31 staining associated with a nucleus was analyzed to measure differences in endothelial cell content at days 3 and 7 post IVC constriction. Cellular CD31 staining was significantly increased in 14E11-treated mice as compared to saline-treated mice on day 7, suggesting increased endothelial cell recruitment or differentiation within thrombi in mice in which FXI was pharmacologically targeted (Figure 5B). No differences in CD31 staining were observed at day 3. Representative images of CD31 staining are shown in Figure 5A and 5C demonstrating increased CD31 expression preferentially localized at the periphery of thrombi, which may suggest endothelial cell expression associated with formation of neo-channels.
Figure 5:
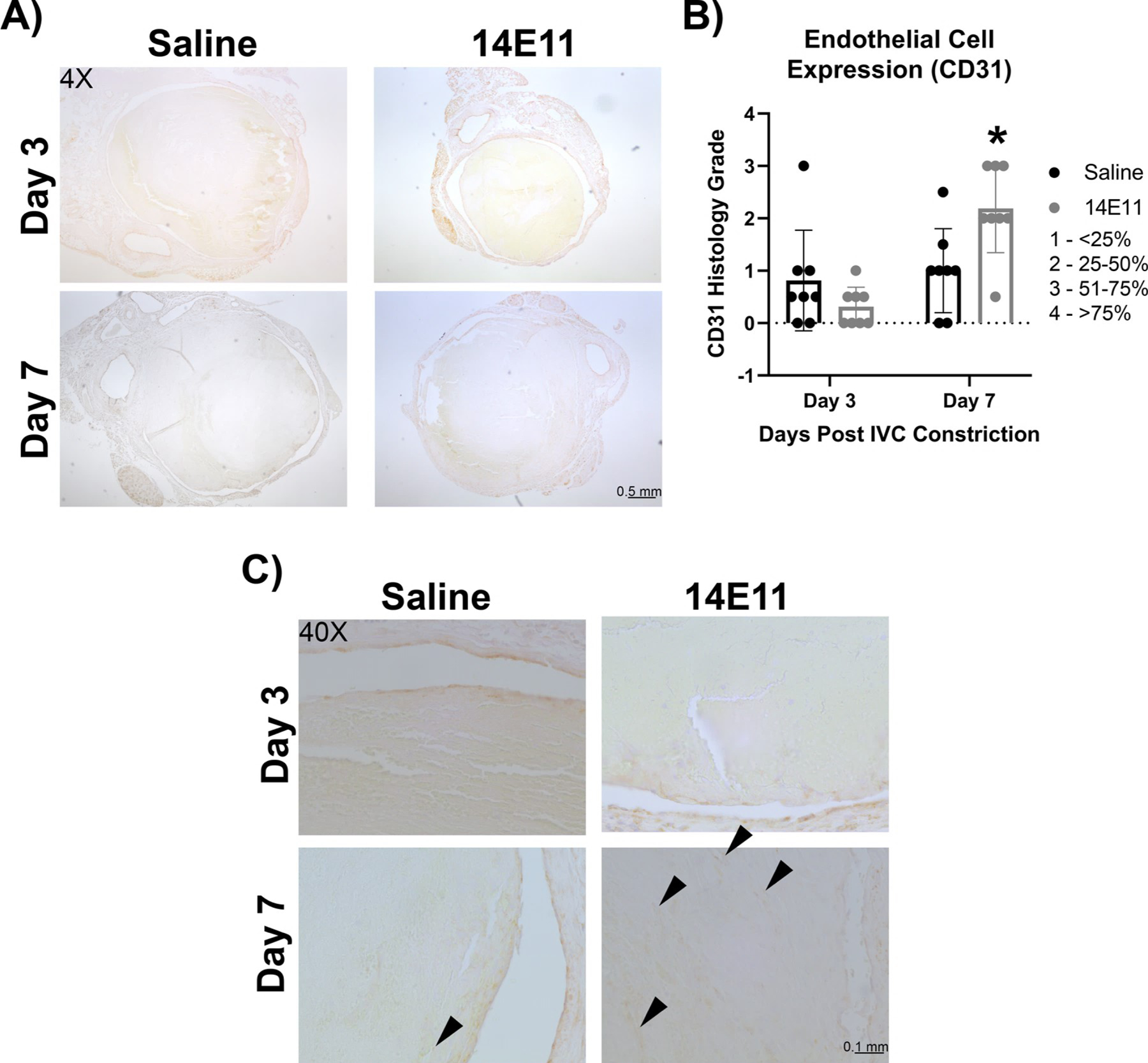
Effects of pharmacologic targeting of FXI by 14E11 on CD31 staining. CD31 immunostaining of saline- vs 14E11-treated animals on days 3 and 7 post IVC constriction at 4x (scale bar=0.5 mm) (A). Quantification of cellular CD31 immunostaining (n=8) (Day 3: 1.1±0.9 versus 1±0.4, P=0.27 and Day 7: 1.0±0.8 versus 1.8±1.4, *P=0.02) (B). Representative images of CD31 immunostaining at 40x (scale bar=0.05 mm) (C). Data were analyzed using a Mann-Whitney test comparison and presented as mean±SD.
Analysis of complete blood counts (CBC) after pharmacological targeting of FXI in mice after IVC constriction
Complete blood counts (CBC) with differentials were evaluated at day 0 (baseline) and day 7 after saline or 14E11 treatment in mice that had not undergone any operations to confirm that 14E11 treatments did not affect the composition of circulating immune cells. Notably, white blood cell count (2.48±1.0 vs. 2.97±1.4, P=0.50), percentage of lymphocytes (81%±3 vs. 82%±7, P=0.90), percentage of monocytes (4.5%±1 vs. 4.8%±1, P=0.60), percentage of granulocytes (14.3%±2 vs. 13.6%±6, P=0.79), and platelet counts (684±110 vs. 621±270, P=0.61), were similar in both groups. On day 7 post IVC constriction, complete blood counts with differentials were also not significantly different between saline- or 14E11-treated mice, indeed, both treatment groups had similar white blood cell counts (2.18±0.6 vs. 2.13±0.9, P=0.94) and platelet counts (621±270 vs. 753±110, P=0.37).
Effect of pharmacological targeting of FXI on circulating monocytes after IVC constriction
Since macrophage content was decreased in the thrombus and along the vein wall at day 7 in 14E11-treated mice, we next assessed whether 14E11 treatments affected the percentage of circulating monocytes. Interestingly, the decrease in macrophage content within the thrombi and vein walls of 14E11-treated mice was concurrent with a significant decrease in percent of circulating monocytes after IVC constriction. Analysis of whole blood by flow cytometry demonstrates a significant decrease in circulating monocytes as a percentage of total cells at day 7 post IVC constriction (Figure 6B, P=0.04). The gating strategy used to evaluate circulating monocytes is represented in Figure 6A. Briefly, monocytes were defined as CD11b+ CD90.2- CD335- CD45R- Ly6G- to exclude T-cells, B-cells, natural killer cells and neutrophils from analysis. Platelet-monocyte aggregates (CD41+ monocytes) and granulocytes (CD11b+Ly6cintLy6ghi) were also assessed and no differences between treatment groups was found (Figure 6C and D). Since the bone marrow is a primary source of circulating immune cells, we measured the percentage of monocyte and neutrophils within the bone marrow compartment and saw no differences between treatment groups (Supplementary Figure 1). Lastly, there was no significant changes in circulating monocytes of unoperated wildtype CD1 mice treated with control vehicle as compared to those treated with 14E11 (Supplemental Figure 2).
Figure 6:
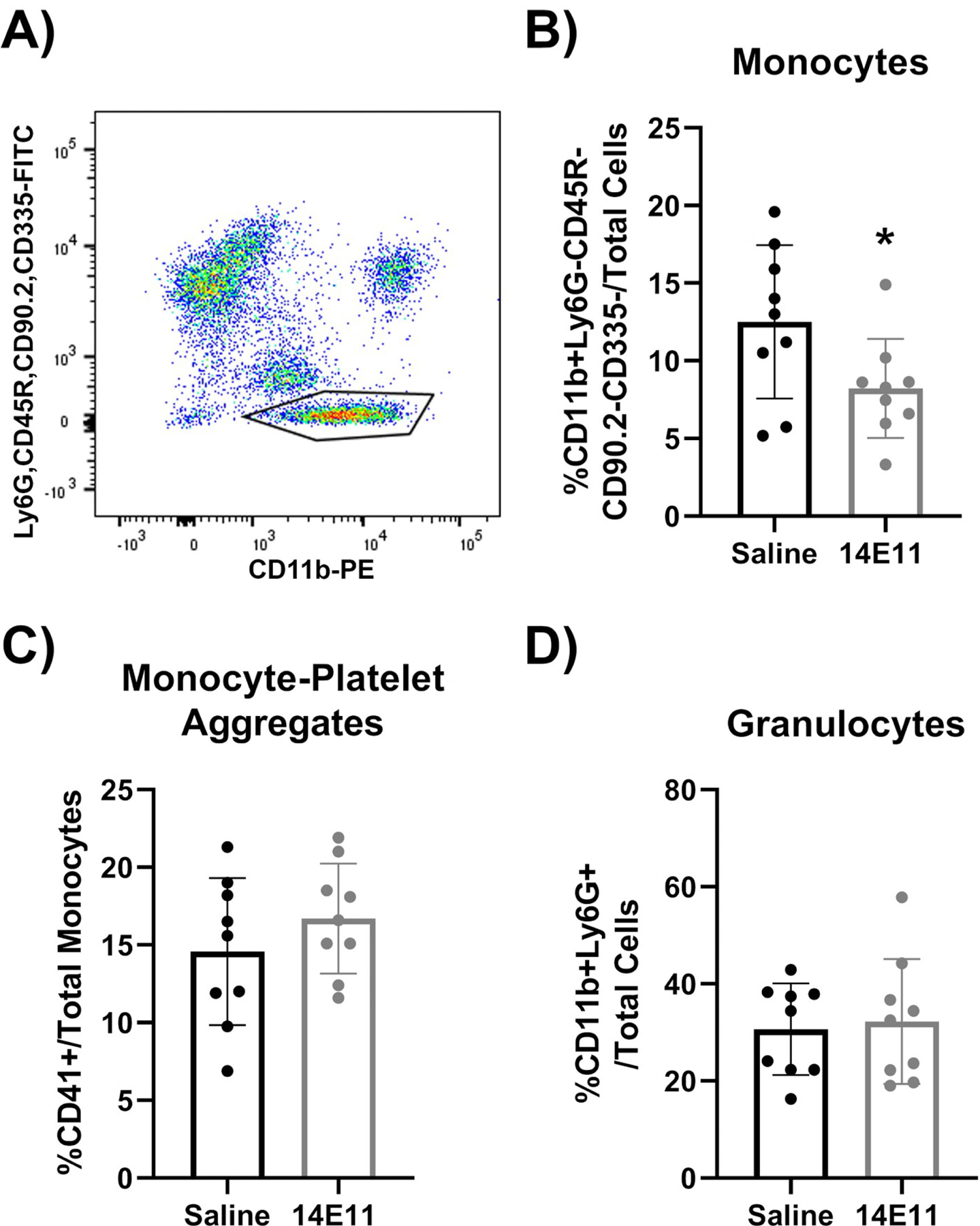
Effects of pharmacologic targeting of FXI by 14E11 on circulating leukocytes following IVC constriction, as determined by Flow Cytometry. Representative image of the gating strategy used to identify CD11b+CD90/CD335/B220/Ly6G- monocytes (A). Quantification of circulating monocytes as a % of total cells, 7 days post IVC constriction (12.5±5 versus 8.2±3, *P=0.04, n=9) (B). Quantification of monocyte-platelet aggregates shown as a %CD41+ of total monocytes, 7 days post IVC constriction (14.6±5 versus 16.7±4, P=0.30, n=9) (C). Quantification of circulating granulocytes as a % of total cells, 7 days post IVC constriction (30.7±9 versus 33.3±13, P=0.77, n=9) (D). Data were analyzed using an unpaired Student’s t-test comparison and presented as mean±SD.
Effect of pharmacological targeting of FXI on post-thrombotic tissue remodeling after IVC constriction
Structural analysis of the thrombi were evaluated in saline- and 14E11-treated mice that underwent IVC constriction. Massons’ Trichrome staining was performed to measure changes in collagen deposition within the thrombus and along the vein wall. At day 3 post IVC constriction, 14E11-treated mice had a significant increase in collagen deposition within the thrombus compared with saline-treated animals. Collagen deposition within the thrombus increased over time in both groups. While 14E11-treated animals trended towards an increase in collagen deposition within the thrombus as compared with saline controls, no significant differences were found (Figure 7B). Collagen deposition at the vein wall remained consistent over time in all animals and no differences were detected between 14E11- and saline-treated animals at any time point (Figure 7B). Representative images of Massons’ trichrome staining demonstrate changes in collagen deposition between treatment groups over time are shown in Figure 7A.
Figure 7:
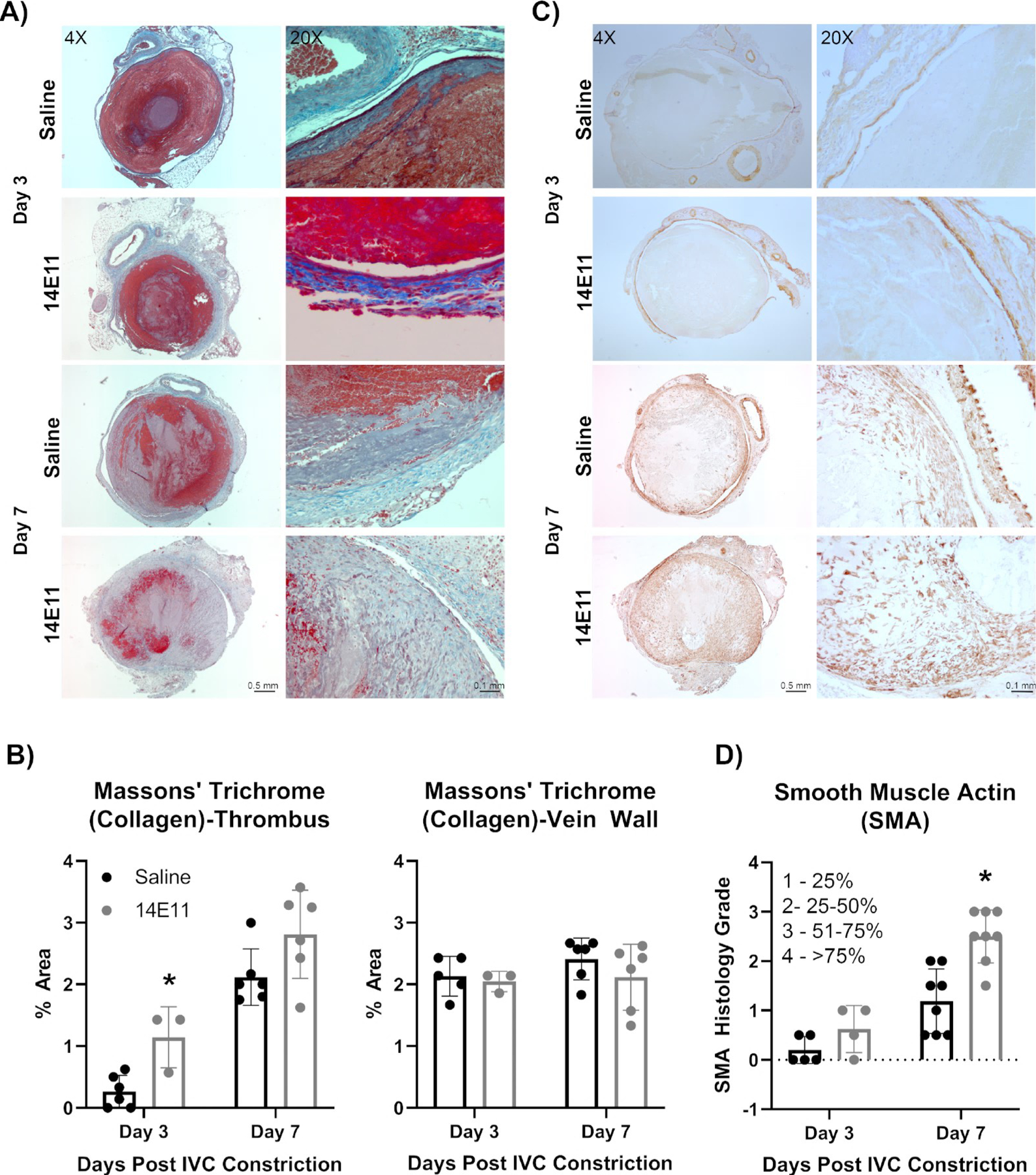
Effects of pharmacologic targeting of FXI by 14E11 on vein wall collagen deposition and expression of Smooth muscle actin in resolving thrombi. Representative images of vein wall collagen deposition on days 3 and 7 post IVC constriction as determined by Masson’s Trichrome staining at 4x (scale bar=0.5 mm) and 20x (scale bar=0.1 mm) (A). Quantification of vein wall collagen deposition on days 3 and 7 post IVC constriction by Masson’s Trichrome staining (n=3–6). Day 3: 0.3±0.3 versus 1.1±0.5, P=0.008 and Day 7: 2.1±0.5 versus 2.8±0.7, *P=0.07 (B). Representative images of smooth muscle actin (SMA) staining in thrombi on days 3 and 7 post IVC constriction at 4X (scale bar=0.5 mm) and 20X (scale bar=0.1 mm) (C). Quantification of smooth muscle cell actin (SMA) staining within resolving thrombi on days 3 and 7 post IVC constriction (n=3–8). Day 3: 0.42±0.5 versus 0.67±0.3, P=0.48 and Day 7: 2.9±0.2 versus 1.1±0.7, *P=0.002) (D). Data were analyzed using a Mann-Whitney test comparison and presented as mean±SD.
Vascular and tissue remodeling was assessed by histology for smooth muscle actin (SMA) staining. As compared with saline-treated mice, the vein walls of 14E11-treated mice showed no significant deposition of smooth muscle cell actin in the vein walls and thrombi on day 7 (1.1±0.8 versus 1.2±0.8, P=0.82). 14E11-treated mice trended towards an increase in thrombus-associated SMA staining at day 3 post IVC constriction and had a significant increase in SMA % area at day 7 post IVC constriction (Figure 7C, 7D). Metalloproteinase-2 (MMP-2) expression by macrophages was examined by immunostaining. Overall, on day 3, there was significant expression of MMP-2 that decreased by day 7 in both control mice and those treated with 14E11. However, there were no significant differences in MMP-2 expression between saline- and 14E11-treated mice at either day 3 or day 7 post-IVC constriction (day 3: 0.2±0.1 versus 0.1±0.1, P=0.54; day 7: 0.7±0.4 versus 0.8±0.2, P=0.77).
DISCUSSION:
This study shows the effects of targeting factor XI on the early stages of venous thrombus resolution, post-thrombotic remodeling, and associated monocyte/macrophage responses. In summary, pharmacological targeting of FXI with 14E11 reduced levels of circulating FXI to non-detectable levels and accelerated early-stage thrombus resolution following IVC constriction. 14E11 treatments reduced the percent of circulating monocytes as well as thrombus- and vein wall-associated macrophages. Vein wall-associated macrophage content was similarly reduced in 14E11-treated animals. Finally, pharmacological targeting of FXI affected post-thrombotic remodeling as CD31 and SMA expression were increased in 14E11-treated mice. While collagen deposition was increased at day 3 post IVC, during thrombus progression, no significant change in collagen deposition was observed at day 7 post IVC constriction.
DVT resolution involves the activation of the innate immune system which influences vascular and tissue remodeling, neovascularization, and thrombus involution [7]. Fe-MRI imaging and immunostaining demonstrated that FXI depletion following treatment with 14E11 significantly reduced thrombus- and vein wall-associated macrophage content. The percentage of circulating monocytes out of total cells was also significantly decreased in 14E11-treated mice, suggesting FXI may play a role in peripheral monocyte expansion and recruitment. These effects may be specific to the period of thrombus resolution, as no difference in Iba1 staining was detected at day 3 post IVC.
While previous studies have demonstrated that monocyte depletion impairs thrombus resolution, the reduction in monocytes in our study was concurrent with a reduction in thrombus weight and volume [21], therefore a decreased but not complete inhibition of the monocyte response may be beneficial in promoting thrombus resolution. The kallikrein-kinin system has been shown to influence immune responses through the production of inflammatory bradykinins as well as by modulating activation of the complement system; additional studies are needed to determine if these mechanisms underlay the findings in this study [22]. In addition, further studies are needed to understand how reducing FXI levels with 14E11 affects monocyte differentiation to macrophages, monocyte recruitment to the site of thrombosis, and whether accelerated thrombus resolution in this model is associated with a change in macrophage phenotype and function.
Prior studies have shown evidence that neovascularization plays an important role in venous thrombus resolution [19]. While the increase in CD31 and SMA expression within the thrombus of 14E11-treated mice is suggestive of neo-channel formation and increased neovascularization, no changes in blood perfusion was detected by Fe-MRI.
This discrepancy may be reflective of a limitation with the imaging modality. Furthermore, the increase in CD31 and SMA expression identified by histology may not significantly improve overall flow restoration. Although FXI depletion following 14E11 treatment increased collagen deposition at day 3, during progression, at day 7 thrombus collagen deposition was not significantly different between groups. However, 14E11-treated animals trended towards an increase in thrombus collage deposition, suggesting a potential for increased fibrosis and risk of vessel stenosis associated with pharmacological targeting of FXI. Additional studies are warranted to verify whether FXI activity plays a role in increases collagen deposition and fibrosis at later time points during thrombus resolution.
To date, several clinical trials have demonstrated a therapeutic benefit to FXI depletion or inhibition on thrombosis. Importantly, targeting FXI has been shown to be effective in prolonging aPTT and reducing incidence of thrombosis in clinical cohorts without increasing the detectable risk of bleeding or resulting in adverse events [23]. The human analog of 14E11, 3G3/AB023, has been shown to provide efficacy in reducing incidence of device-associated thrombosis in patients undergoing chronic hemodialysis [5]. Importantly, in humans and non-human primates, the mechanism of action of 3G3/AB023 appears to be inhibition of FXI activation by FXIIa as opposed to depletion or reduction of FXI levels. Therefore, the results presented in this study may be more mechanistically related to clinical trials investigating the FXI antisense oligonucleotide ISIS 416858, whose mechanism of action is reduction of FXI levels. FXI depletion by ISIS 416858 has been shown to safely reduce incidences of thromboembolism in patients undergoing elective primary unilateral total knee arthroplasty at comparable rates to enoxaparin; moreover, ISIS 41685 treatment reduced incidence of hemorrhage when compared to enoxaparin [24].
Limitations:
There are some limitations in this study. While we test 14E11 in comparison to vehicle as our control, we did not use an isotype control antibody and note this as a limitation of this study. The surgical model selected for this study is the IVC stenosis model which may less accurately represent acute thrombus formation in humans where the initial thrombus is often occlusive, larger, and more severe. Analysis of the effect of pharmacological targeting of FXI in the caval stasis model may allow for evaluation of thrombus resolution in the acute thrombosis setting where the thrombus is completely occlusive. Furthermore, the nidus for DVT formation often occurs at the venous valve cusp. Valve anatomy and flow patterns contribute to human thrombus initiation, but there are currently no well-established or characterized murine models that reflect the contribution of valves. Thus, more costly, larger animal models, including porcine and non-human primate models, are potential alternatives for future studies that permit the evaluation and analysis of venous valves effects.
CONCLUSION:
In summary, pharmacological targeting of FXI with 14E11 results in improved thrombus resolution that is associated with a decrease in circulating monocytes and thrombus macrophage content, as well as accelerated tissue remodeling with decreased vein wall fibrosis. Further investigation of the effects of FXI on the innate immune system may help us better understand its role in inflammation, fibrosis, and thrombus resolution after DVT. Overall, further research regarding the role of FXI and FXII in venous thrombosis resolution may lead to novel treatments for and preventions of DVT and its chronic sequelae, post-thrombotic syndrome.
Supplementary Material
Supplementary Figure 1: Effects of pharmacologic targeting of FXI by 14E11 on bone marrow cells on day 7 post IVC constriction. Quantification of CD11b+CD90/CD335/B220/Ly6G- monocytes (15±1.1 versus 15±1.7, n=5–6, P=0.95) (A) and Ly6GhiLy6CintCD11b+ Granulocytes (67±4.4 versus 68±3.6, n=5–6, P=0.74) (B). Data were analyzed using an unpaired Student’s t-test comparison and presented as mean±SD.
Supplementary Figure 2: Effects of pharmacological targeting of FXI by 14E11 on immune composition in naïve mice without surgical intervention or thrombus formation. Quantification of circulating CD11b+CD90/CD335/B220/Ly6G- monocytes (A) Ly6CLo monocytes (20±6 versus 17±5, n=9) and Ly6CHi monocytes (60.5±8.4 versus 16.9±4.9, n=9) (B), monocyte-platelet aggregates (14.6±4.7 versus 15.1±5.2, n=9) (C), and neutrophils (30.7±9.4 versus 31.1±14.5, n=9) (D). Data were analyzed using an unpaired Student’s t-test comparison and presented as mean±SD.
Author contributions
| Author | Concept & Design | Analysis | Data Interpretation | Critical Writing | Revising | Final Approval |
|---|---|---|---|---|---|---|
| M. Fallon | X | X | ||||
| D. Gailani | X | X | X | |||
| E. Tucker | X | X | ||||
| M. Hinds | X | X | X | X | X | |
| K. Jordan | X | X | X | X | X | |
| C. Lorentz | X | X | X | X | ||
| O. McCarty | X | X | X | |||
| E. Neuwelt | X | X | ||||
| K. Nguyen | X | X | X | X | X | X |
| R. Woltjer | X | X | X | |||
| C. Wyatt | X | X | X | X | ||
| Q. Cheng | X | X | ||||
ESSENTIALS.
Coagulation factor XI (FXI) of the contact activation system plays a role in thrombus formation and inhibition of FXI activity reduces markers of inflammation in rodent models of disease.
Detectable levels of circulating FXI were reduced in a mouse model of experimental deep vein thrombosis via administration of the anti-factor XI monoclonal antibody 14E11.
14E11 administration accelerated the resolution of experimental venous thrombi in mice.
Inflammatory monocyte counts in blood and macrophage counts in thrombi were reduced.
ACKNOWLEDGEMENTS:
This work was supported by a VA CSR&D Career Development Award [Grant Number: IK2CX001720] and grants from the National Institutes of Health [R01HL101972, R01AI157037, and R01HL144113].
The authors would like to acknowledge the technical assistance of Dr. Anh Ngo, Anh Le-Cook, Naly Setthavongsack, Barbara Smoody, and Leon Wong.
Footnotes
CONFLICT OF INTEREST:
Drs. Lorentz, Tucker, and the Oregon Health & Science University have a significant financial interest in Aronora, Inc., a company that may have a commercial interest in the results of this research. This potential conflict of interests have been reviewed and managed by the Oregon Health & Science University Conflict of Interest in Research Committee. The remaining authors declare no competing financial interests.
REFERENCES:
- 1.Thachil J Deep vein thrombosis. Hematology 2014; 19: 309–10. 10.1179/1024533214Z.000000000284. [DOI] [PubMed] [Google Scholar]
- 2.Kruger PC, Eikelboom JW, Douketis JD, Hankey GJ. Deep vein thrombosis: update on diagnosis and management. Med J Aust 2019; 210: 516–24. 10.5694/mja2.50201. [DOI] [PubMed] [Google Scholar]
- 3.Gailani D, Bane CE, Gruber A. Factor XI and contact activation as targets for antithrombotic therapy. J Thromb Haemost 2015; 13: 1383–95. 10.1111/jth.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puy C, Rigg RA, McCarty OJ. The hemostatic role of factor XI. Thromb Res 2016; 141 Suppl 2: S8–S11. 10.1016/S0049-3848(16)30354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorentz CU, Tucker EI, Verbout NG, Shatzel JJ, Olson SR, Markway BD, Wallisch M, Ralle M, Hinds M, McCarty OJT, Gailani D, Weitz JI, Gruber A. Contact Activation Inhibitor, AB023, in Heparin-Free Hemodialysis: Results of a Randomized Phase 2 Clinical Trial. Blood 2021. 10.1182/blood.2021011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012; 209: 819–35. 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 2008; 28: 387–91. 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 8.Ngo ATP, Jordan KR, Mueller PA, Hagen MW, Reitsma SE, Puy C, Revenko AS, Lorentz CU, Tucker EI, Cheng Q, Hinds MT, Fazio S, Monia BP, Gailani D, Gruber A, Tavori H, McCarty OJT. Pharmacological targeting of coagulation factor XI mitigates the development of experimental atherosclerosis in low-density lipoprotein receptor-deficient mice. J Thromb Haemost 2021; 19: 1001–17. 10.1111/jth.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silasi R, Keshari RS, Lupu C, Van Rensburg WJ, Chaaban H, Regmi G, Shamanaev A, Shatzel JJ, Puy C, Lorentz CU, Tucker EI, Gailani D, Gruber A, McCarty OJT, Lupu F. Inhibition of contact-mediated activation of factor XI protects baboons against. Blood Adv 2019; 3: 658–69. 10.1182/bloodadvances.2018029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tucker EI, Gailani D, Hurst S, Cheng Q, Hanson SR, Gruber A. Survival advantage of coagulation factor XI-deficient mice during peritoneal sepsis. J Infect Dis 2008; 198: 271–4. 10.1086/589514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tucker EI, Verbout NG, Leung PY, Hurst S, McCarty OJ, Gailani D, Gruber A. Inhibition of factor XI activation attenuates inflammation and coagulopathy while improving the survival of mouse polymicrobial sepsis. Blood 2012; 119: 4762–8. 10.1182/blood-2011-10-386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aghighi M, Theruvath AJ, Pareek A, Pisani LL, Alford R, Muehe AM, Sethi TK, Holdsworth SJ, Hazard FK, Gratzinger D, Luna-Fineman S, Advani R, Spunt SL, Daldrup-Link HE. Magnetic Resonance Imaging of Tumor-Associated Macrophages: Clinical Translation. Clin Cancer Res 2018; 24: 4110–8. 10.1158/1078-0432.CCR-18-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iv M, Samghabadi P, Holdsworth S, Gentles A, Rezaii P, Harsh G, Li G, Thomas R, Moseley M, Daldrup-Link HE, Vogel H, Wintermark M, Cheshier S, Yeom KW. Quantification of Macrophages in High-Grade Gliomas by Using Ferumoxytol-enhanced MRI: A Pilot Study. Radiology 2019; 290: 198–206. 10.1148/radiol.2018181204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renné T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood 2010; 116: 3981–9. 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malyala P, Singh M. Endotoxin limits in formulations for preclinical research. J Pharm Sci 2008; 97: 2041–4. 10.1002/jps.21152. [DOI] [PubMed] [Google Scholar]
- 16.Singh I, Burnand KG, Collins M, Luttun A, Collen D, Boelhouwer B, Smith A. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: rescue by normal bone marrow-derived cells. Circulation 2003; 107: 869–75. [DOI] [PubMed] [Google Scholar]
- 17.Humphries J, Gossage JA, Modarai B, Burnand KG, Sisson TH, Murdoch C, Smith A. Monocyte urokinase-type plasminogen activator up-regulation reduces thrombus size in a model of venous thrombosis. J Vasc Surg 2009; 50: 1127–34. 10.1016/j.jvs.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang SY, Kim CY, Miller MJ, Gupta RT, Lessne ML, Horvath JJ, Boll DT, Evans PD, Befera NT, Krishnan P, Chan JL, Merkle EM. Abdominopelvic and lower extremity deep venous thrombosis: evaluation with contrast-enhanced MR venography with a blood-pool agent. AJR Am J Roentgenol 2013; 201: 208–14. 10.2214/AJR.12.9611. [DOI] [PubMed] [Google Scholar]
- 19.Modarai B, Humphries J, Burnand KG, Gossage JA, Waltham M, Wadoodi A, Kanaganayagam GS, Afuwape A, Paleolog E, Smith A. Adenovirus-mediated VEGF gene therapy enhances venous thrombus recanalization and resolution. Arterioscler Thromb Vasc Biol 2008; 28: 1753–9. 10.1161/ATVBAHA.108.170571. [DOI] [PubMed] [Google Scholar]
- 20.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol 2004; 24: 1130–7. 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]
- 21.Kimball AS, Obi AT, Luke CE, Dowling AR, Cai Q, Adili R, Jankowski H, Schaller M, Holinstadt M, Jaffer FA, Kunkel SL, Gallagher KA, Henke PK. Ly6CLo Monocyte/Macrophages are Essential for Thrombus Resolution in a Murine Model of Venous Thrombosis. Thromb Haemost 2020; 120: 289–99. 10.1055/s-0039-3400959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y Contact pathway of coagulation and inflammation. Thromb J 2015; 13: 17. 10.1186/s12959-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLoughery EP, Olson SR, Puy C, McCarty OJT, Shatzel JJ. The Safety and Efficacy of Novel Agents Targeting Factors XI and XII in Early Phase Human Trials. Semin Thromb Hemost 2019; 45: 502–8. 10.1055/s-0039-1692439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buller HR, Gailani D, Weitz JI. Factor XI antisense oligonucleotide for venous thrombosis. N Engl J Med 2015; 372: 1672. 10.1056/NEJMc1503223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Effects of pharmacologic targeting of FXI by 14E11 on bone marrow cells on day 7 post IVC constriction. Quantification of CD11b+CD90/CD335/B220/Ly6G- monocytes (15±1.1 versus 15±1.7, n=5–6, P=0.95) (A) and Ly6GhiLy6CintCD11b+ Granulocytes (67±4.4 versus 68±3.6, n=5–6, P=0.74) (B). Data were analyzed using an unpaired Student’s t-test comparison and presented as mean±SD.
Supplementary Figure 2: Effects of pharmacological targeting of FXI by 14E11 on immune composition in naïve mice without surgical intervention or thrombus formation. Quantification of circulating CD11b+CD90/CD335/B220/Ly6G- monocytes (A) Ly6CLo monocytes (20±6 versus 17±5, n=9) and Ly6CHi monocytes (60.5±8.4 versus 16.9±4.9, n=9) (B), monocyte-platelet aggregates (14.6±4.7 versus 15.1±5.2, n=9) (C), and neutrophils (30.7±9.4 versus 31.1±14.5, n=9) (D). Data were analyzed using an unpaired Student’s t-test comparison and presented as mean±SD.


