Abstract
Mechanisms causing the post-acute sequelae of SARS-CoV-2 (long COVID) remain elusive, but the clinical phenotype is consistent with cardiac deconditioning. We report a case series of patients with long COVID whose symptoms improved/resolved with exercise and present exercise training as a novel therapeutic strategy for management of long COVID syndrome. (Level of Difficulty: Intermediate.)
Key Words: cardiac deconditioning, exercise training, long COVID, post acute sequelae of SARS-CoV-2
Abbreviations and Acronyms: BP, blood pressure; CFS, chronic fatigue syndrome; HR, heart rate; POTS, postural orthostatic tachycardia syndrome; Vo2, oxygen consumption
Central Illustration
Mechanisms accounting for the post-acute sequelae of SARS CoV-2 (long COVID) are unclear. However, long COVID is consistent with cardiac deconditioning,1 causing exercise intolerance, brain fog/impaired cognition, tachycardia, and orthostatic intolerance—all characteristic of long COVID.2 Furthermore, cardiac deconditioning may occur with as little as 20 hours of bed rest.3
Learning Objectives
-
•
To understand that the long COVID clinical phenotype is similar to symptoms encountered with cardiac deconditioning.
-
•
To characterize the positive effect of exercise on symptoms among patients with long COVID.
-
•
To describe a tailored exercise prescription as a novel therapeutic strategy for patients with long COVID.
Conditions such as postural orthostatic tachycardia syndrome (POTS), chronic fatigue syndrome (CFS),4 and debility following bed rest improve with exercise.5 We have developed an exercise prescription (Figure 1) similar to that recently proposed by the American College of Cardiology for patients with long COVID6 and found that exercise training provides clinically meaningful improvement in symptoms. Herein, we highlight 3 illustrative examples of patients who have benefited from completing this program.
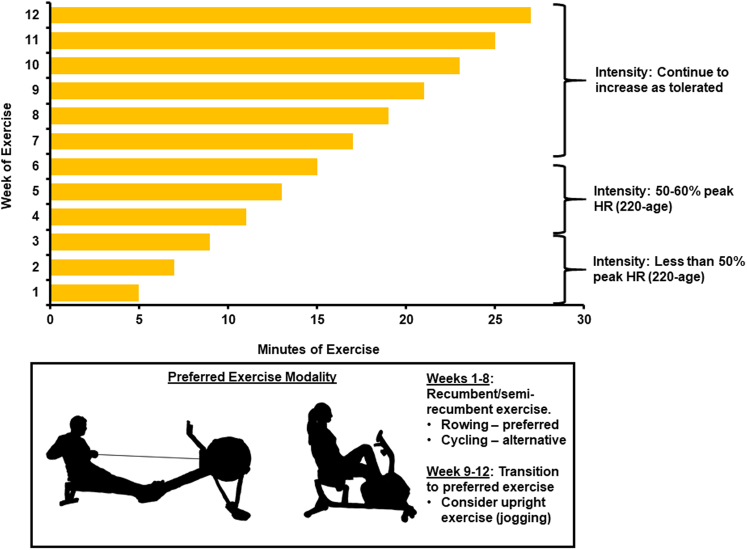
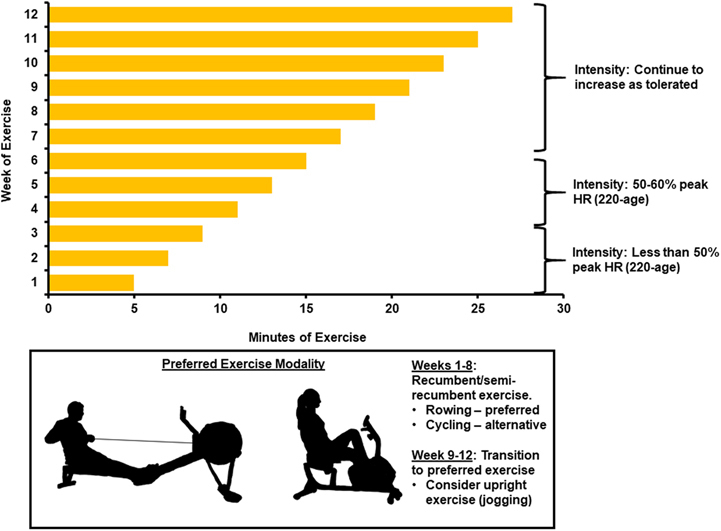
Figure 1.
12-Week Exercise Training Regimen for Management of Long COVID
Recumbent/semirecumbent exercise is prescribed. Participants begin exercise 5 min/d, 5 times per week, and increase exercise duration by 2 min/d each week. Intensity is limited to <50% peak heart rate (HR) for first 3 weeks, then 50%-60% peak HR for weeks 4-6, and then increase gradually as tolerated. At weeks 9-12, patients begin transition from recumbent exercise to an exercise modality of their choice.
Case 1
A previously healthy 24-year-old woman developed SARS-CoV-2, which was managed conservatively at home with 3 weeks of flu-like symptoms. She was referred to cardiology 5 months later because she was experiencing debilitating fatigue, lightheadedness, and brain fog, along with recurrent syncope with orthostasis. At baseline she hiked regularly and jogged 3 miles a day several days per week, but she was unable to participate in any of these activities since her index infection. Physical exam was notable for orthostatic tachycardia during 10-minute bedside orthostatic challenge: supine resting heart rate (HR) 67 beats/min and blood pressure (BP) 116/73 mm Hg, which increased to 103 beats/min and 121/84 mm Hg, respectively, after 10-minute stand. Echocardiography demonstrated a left ventricular ejection fraction of 56% with normal structure/function. Pulmonary function testing showed normal volumes with no obstruction (forced expiratory volume-1 3.9 L, 100% predicted) and a diffusion capacity for carbon monoxide of 95% predicted. Cardiopulmonary exercise testing showed normal hemodynamic responses to exercise with peak HR and BP of 200 beats/min and 200/64 mm Hg, respectively, and a peak oxygen consumption (peak Vo2) of 41.7 mL/kg/min (127% predicted) and minute ventilation–carbon dioxide production (ventilatory efficiency) slope of 32.5. The exercise program was prescribed and after 2 months of rowing, she completed a 7-day backpacking trip in the Sangre de Cristo mountains, and after 3 months, she climbed the summit of Mt Democrat in Colorado (14,155 feet).
Case 2
An 18-year-old woman tested positive for SARS-CoV-2 in July 2020 during pretravel screening despite having no symptoms. One month later, she developed severe fatigue, lightheadedness, brain fog, and palpitations. Physical examination was significant only for orthostatic tachycardia after standing for 10 minutes during bedside orthostatic assessment: supine resting HR and BP: 56 beats/min and 102/70 mm Hg, respectively, and HR and BP after 10-minute standing: 88 beats/min and 106/72 mm Hg, respectively. Echocardiography demonstrated a left ventricular ejection fraction of 59% and there was no evidence of myocarditis on cardiac magnetic resonance. Computed tomography of the chest was negative for pulmonary embolism or intrinsic lung disease. Cardiopulmonary exercise testing demonstrated normal hemodynamic responses to exercise with no hypoxia, a peak Vo2 of 40.1 mL/kg/min (112% predicted) and a minute ventilation–carbon dioxide production slope of 35.5. The exercise program was prescribed and after 12 weeks, she reported significant improvement in symptoms with near resolution of fatigue and brain fog. Her symptoms returned after a non-sports-related injury necessitated temporary discontinuation of training. However, after 3 weeks of recovery, she resumed rowing, and on follow-up 4 weeks later, her symptoms significantly improved.
Case 3
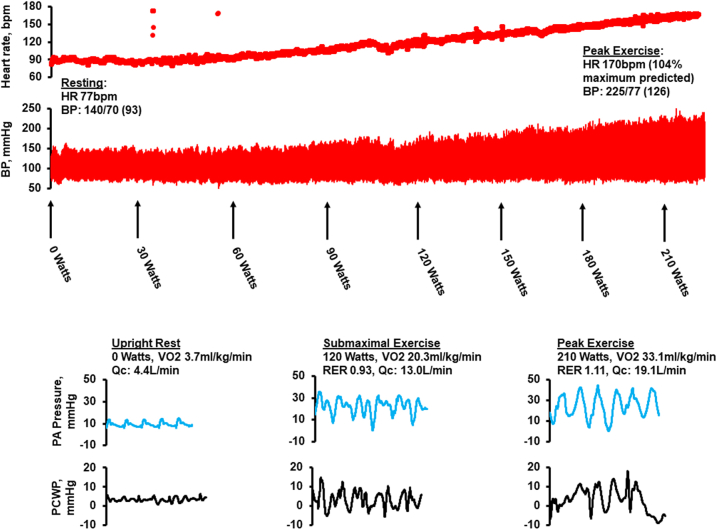
A healthy 56-year-old male athlete (peak Vo2 62.5 mL/kg/min) contracted SARS-CoV-2 and was hospitalized for 48 hours because he was experiencing dyspnea, which was treated with remdesivir, dexamethasone, and 2-3 L of supplemental oxygen. Over the next 8-10 weeks, he suffered from dyspnea, coughing paroxysms, along with an increase in resting HR by 10-15 beats/min (verified by home monitor) and tachycardia with HR >30 beats/min for any given level of work compared to exertional HR prior to infection. After 3 months of persistent symptoms, he was referred for evaluation. Echocardiogram demonstrated normal cardiac size/function with left ventricular ejection fraction of 67% and no structural abnormalities. Cardiac magnetic resonance was negative for myocarditis. Pulmonary function testing showed a forced expiratory volume-1 of 4.46 L (113% predicted) and diffusion capacity for carbon monoxide of 134% predicted. The patient completed invasive cardiopulmonary exercise testing (Figure 2). Supine resting hemodynamics included a right atrial pressure of 5 mm Hg, systolic, diastolic, and mean pulmonary arterial pressure of 20, 10, and 13, respectively, and a pulmonary capillary wedge pressure of 8 mm Hg. Fick method and thermodilution, respectively, were used to determine cardiac output of 4.6 and 2.4 L/min and cardiac index of 5.4 and 2.9 L/min/m2. During exercise on upright stationary cycle ergometry, he had a normal cardiovascular response to exercise along with normal exertional hemodynamics, a peak Vo2 of 33.1 mL/kg/min (111% predicted), and a respiratory exchange ratio of 1.11 with minute ventilation–carbon dioxide production slope of 34. BP during testing was assessed with radial arterial catheterization and arterial oximetry remained >90% throughout exercise. Exercise training was prescribed following our protocol. After approximately 8 weeks, the patient transitioned from rowing to running (his preferred modality) and on follow-up 4 weeks later, he was running ∼5 times per week and his only major complaint was a persistent cough.
Figure 2.
Hemodynamic Tracings During Invasive Cardiopulmonary Exercise Testing
Hemodynamic tracings during invasive cardiopulmonary exercise testing in a 56-year-old man with long COVID, demonstrating normal hemodynamics during exercise. BP = blood pressure; HR = heart rate; PA = pulmonary arterial; PCWP = pulmonary capillary wedge pressure; RER = respiratory exchange ratio; Qc = cardiac output; Vo2 = oxygen consumption.
Discussion
Exercise prescriptions improve quality of life and functional capacity when applied to patients with POTS,7 CFS,4 and cardiac deconditioning.8 Based on these observations, we developed a structured exercise prescription specifically for patients with long COVID (Figure 1). This prescription incorporates recumbent/semirecumbent exercise—preferably with rowing or cycling, for the following reasons. First, orthostatic intolerance associated with long COVID prohibits meaningful upright exercise. Second, rowing leads to concentric and eccentric remodeling,9 which is ideal for patients with cardiac deconditioning, who have reduced cardiac size and stroke volume.5 Third, rowing is a highly dynamic and highly static form of exercise,6,9 strengthening large skeletal muscle groups in addition to the aforementioned cardiac benefits. Fourth, long COVID has been compared to CFS and results, at least partially, from low cardiac preload.10 Exercise training effectively improves chronic fatigue syndrome4 and also increases total blood volume, offsetting the reduced preload reported among patients with long COVID on invasive hemodynamic assessment.10 Finally, in the absence of contraindications, this program can be incorporated into management of patients irrespective of their baseline functional capacity—sedentary versus active—because the level of intensity and duration of exercise are very low initially and gradually increase over time.
Conclusions
Exercise prescriptions improve quality of life and functional capacity among patients with POTS, CFS, and cardiac deconditioning. Our case series demonstrates that exercise may also lead to clinically meaningful improvement in symptom severity of long COVID. Further research is necessary but in the absence of contraindications, exercise training should be considered for management of these patients to assist with improvement in quality of life and functional capacity.
Funding Support and Author Disclosures
Dr Cornwell has received funding by a National Institutes of Health/National Heart, Lung, and Blood Institute Mentored Patient-Oriented Research Career Development Award (1K23HLI32048), as well as the National Institutes of Health/National Center for Advancing Translational Sciences (UL1TR002535). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Hasser E.M., Moffitt J.A. Regulation of sympathetic nervous system function after cardiovascular deconditioning. Ann N Y Acad Sci. 2001;940:454–468. doi: 10.1111/j.1749-6632.2001.tb03698.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayes L.D., Ingram J., Sculthorpe N.F. More than 100 persistent symptoms of SARS-CoV-2 (long COVID): a scoping review. Front Med. 2021;8 doi: 10.3389/fmed.2021.750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaffney F.A., Nixon J.V., Karlsson E.S., et al. Cardiovacular deconditioning produced by 20 hours of bedrest with head-down tilt (−5 degrees) in middle-aged healthy men. Am J Cardiol. 1985;56(10):634–638. doi: 10.1016/0002-9149(85)91025-2. [DOI] [PubMed] [Google Scholar]
- 4.Larun L., Brurberg K.G., Odgaard-Jensen J., et al. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2017;4(4):CD003200. doi: 10.1002/14651858.CD003200.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata S., Fu Q., Bivens T.B., et al. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol. 2012;590(15):3495–3505. doi: 10.1113/jphysiol.2012.233858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Writing Committee. Gluckman T.J., Bhave N.M., et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;79(17):1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Q., Vangundy T.B., Galbreath M.M., et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55(25):2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saltin B., Blomqvist G., Mitchell J.H., et al. Response to exercise after bed rest and after training. Circulation. 1968;38(5 suppl):VII1–VII78. [PubMed] [Google Scholar]
- 9.Pelliccia A., Maron B.J., Spataro A., et al. The upper limit of physiologic cardiac hypertrophy in highly trained athletes. N Engl J Med. 1991;324(5):295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 10.Mancini D.M., Brunjes D.L., Lala A., et al. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. J Am Coll Cardiol HF. 2021;9(12):927–937. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]