Abstract
The opportunistic pathogen Pneumocystis carinii causes pneumonia (P. carinii pneumonia, or PCP) in immunocompromised individuals such as AIDS patients. Rat-derived P. carinii carinii organisms have distinct sterols which are not synthesized by mammals and not found in other microbes infecting mammalian lungs. The dominant sterol present in the organism is cholesterol (which is believed to be scavenged from the host), but other sterols in P. carinii carinii have an alkyl group at C-24 of the sterol side chain (C28 and C29 24-alkylsterols) and a double bond at C-7 of the nucleus. Recently, pneumocysterol (C32), which is essentially lanosterol with a C-24 ethylidene group, was detected in lipids extracted from a formalin-fixed human P. carinii-infected lung, and its structures were elucidated by gas-liquid chromatography, mass spectrometry, and nuclear magnetic resonance spectrometry in conjunction with analyses of chemically synthesized authentic standards. The sterol composition of isolated P. carinii hominis organisms has yet to be reported. If P. carinii from animal models is to be used for identifying potential drug targets and for developing chemotherapeutic approaches to clear human infections, it is important to determine whether the 24-alkylsterols of organisms found in rats are also present in organisms in humans. In the present study, sterol analyses of P. carinii hominis organisms isolated from cryopreserved human P. carinii-infected lungs and from bronchoalveolar lavage fluid were performed. Several of the same distinct sterols (e.g., fungisterol and methylcholest-7-ene-3β-ol) previously identified in P. carinii carinii were also present in organisms isolated from human specimens. Pneumocysterol was detected in only some of the samples.
Pneumocystis carinii is an opportunistic eukaryotic microbe which does not cause life-threatening disease among immunocompetent individuals but can cause a type of pneumonitis (P. carinii pneumonia, or PCP) in immunocompromised or immunodeficient individuals. PCP is a major cause of mortality and morbidity in AIDS patients and P. carinii can also cause pneumonitis in recipients of transplanted organs and patients undergoing cancer therapy. P. carinii is a ubiquitous organism to which human populations are constantly exposed; most people test seropositive to P. carinii as young children (22, 23, 29).
The organism has close phylogenetic affinities with fungi but exhibits features that are unlike those that typify most fungi. An example is its resistance to common antifungal agents. Polyene antibiotics such as amphotericin B bind to ergosterol in fungal membranes, resulting in the formation of large pores and the dissipation of ion gradients, thus killing the cell. Unlike the case in most mammalian fungal pathogens, ergosterol was not detected in P. carinii, thus providing an explanation of the inefficacy of amphotericin B against PCP (3). However, it is now well documented that P. carinii carinii synthesizes a number of sterols which are distinct from those found in the mammalian host (11, 13, 15, 16, 17). The P. carinii-specific sterols include a number of Δ7 24-alkylsterols (C28 and C29 sterols).
Analysis of the total and free sterols in a formalin-fixed human P. carinii-infected lung (16) revealed two additional sterols that were absent or present only in trace amounts in P. carinii carinii (11, 13, 17, 27). These were characterized by their late elution times in gas-liquid chromatograms (GLC). The earlier-eluting minor component was tentatively identified as 24-methylenelanost-8-en-3β-ol (C31) by GLC-mass spectrometry (MS) (16). The larger component, which comprised approximately 50% of the noncholesterol sterols in that sample, was identified as C32 pneumocysterol [(24Z)-ethylidenelanost-8-en-3β-ol] (16). The structural identification of pneumocysterol was demonstrated by GLC-MS and nuclear magnetic resonance spectrometry of samples purified from P. carinii-infected lung by preparative GLC and compared to a chemically synthesized authentic standard of the sterol. In some organisms, lanosterol cannot serve as a substrate for Δ24-methyltransferase, and thus inhibition of demethylation of the lanosterol nucleus can inhibit the formation of 24-alkylsterols, which these organisms apparently require for growth. The C31 sterol and pneumocysterol are 24-alkylated lanosterol molecules, indicating that lanosterol can serve as a substrate for sterol Δ24-methyltransferase activity in P. carinii hominis. Pneumocysterol and Δ7 24-alkylsterols are relatively rare, and no other pathogen infecting human lungs has been reported to have them. Hence, when several of these sterols are detected in a sample, they can serve as signatures of P. carinii and hence provide a useful method for detecting and diagnosing the infection. Also, if P. carinii hominis and P. carinii carinii have the same major pathways for synthesizing sterols, experiments performed on P. carinii carinii can be used with greater confidence for designing chemotherapeutic approaches targeting sterol biosynthesis in P. carinii hominis. On the other hand, since Pneumocystis populations isolated from different mammalian host species as well as from the same host species have highly divergent genetic backgrounds (2, 9, 10, 19, 21, 26), the analysis of sterols may provide further insight into the biochemical nature of diverse groups within the Pneumocystis complex. This is the first report of sterol analyses performed on P. carinii hominis. The organisms studied were isolated from cryopreserved P. carinii-infected lungs and from bronchoalveolar lavage fluid (BALF) obtained from PCP patients.
MATERIALS AND METHODS
Isolation of P. carinii hominis from cryopreserved human P. carinii-infected lung tissue.
Five cryopreserved autopsied samples of human P. carinii-infected lungs (coded 288, 313, 293, 480, and 438) were obtained from M. T. Cushion (V.A. Medical Center, Cincinnati, Ohio). After being thawed, the organisms were isolated by modifications of a protocol used to obtain purified organisms from infected rat lungs (18). Approximately 100 g of the lung was minced in a buffer solution containing 25 mM HEPES buffer at pH 7.4, 5 mM EDTA, and 0.85% NaCl. In place of glutathione, a high concentration (10%) of the stronger mucolytic sulfhydryl agent dithiothreitol (24) was added to the buffer solution, since mucus in human lung samples is more difficult to liquify than that in rat lungs. After homogenization in a Stomacher lab blender (Tekmar, Cincinnati, Ohio), for 10 min at room temperature, the suspension was sieved (50 to 60 mesh), and then the sieved material was centrifuged at 925 × g for 15 min (high spin). The resultant pellet was treated with 0.85% NH4Cl, pH 6.8, at 37°C for 15 min to lyse host erythrocytes and then diluted threefold with the buffer solution. After a high spin, the resultant pellet was resuspended in the buffer solution and centrifuged at 60 × g for 10 min (low spin). The low-spin supernatant was recovered and centrifuged at a high spin, and then the cycle of low- and high-spin steps was repeated with the buffer solution to remove lung contaminants associated with the organisms. The final pellet was resuspended and passed through tandemly attached membrane holders (Millipore Corp., Bedford, Mass.) equipped with 25-mm-diameter polycarbonate membranes. The pores in the microfiltration units had straight channels; the first membrane had 8-μm pores and the second had 5-μm pores (Poretics Corp., Livermore, Calif., or Nuclepore, Pleasanton, Calif.). After filtration, the membranes were rinsed once with the buffer, and then the pooled filtrates were subjected to a high spin to concentrate the P. carinii hominis organisms.
One P. carinii hominis preparation (herein designated Italy-1) was isolated from a cryopreserved autopsied lung sample from an HIV-positive P. carinii-infected patient who was not treated before death. The organisms in this preparation were isolated by the methods developed by Walzer et al. (30) and then lyophilized. The lyophilized sample was then sent to the Department of Biological Sciences, University of Cincinnati, Cincinnati, Ohio, for sterol analysis.
One P. carinii hominis sample (herein designated as Denmark-1; originally coded Pch 1/97) was prepared from a cryopreserved lung from an HIV-negative, P. carinii-infected cancer patient who had been treated with sulfamethoxazole-trimethoprim (Bactrim). The organisms in this sample were isolated by homogenization in a Stomacher unit, filtered through gauze, washed twice with phosphate-buffered saline, and then purified on a Percoll gradient. The band collected contained both cystic and trophic forms. This and three other organism preparations (herein designated Denmark-2, -3, and -4; originally coded BH crude 21/12-94, PC NY 7/7, and G crude 1/9, respectively) that had been isolated by the Walzer et al. (30) protocol were shipped on dry ice to the Department of Biological Sciences, University of Cincinnati, for sterol analysis.
P. carinii hominis organisms isolated from BALF.
Bronchoscopy of HIV-positive, PCP-positive patients treated at the University Hospital, Cincinnati, Ohio, was performed with Olympus bronchoscopes (models P10 and P20DO; New Hyde Park, N.Y.). The bronchoscope was inserted intranasally or intraorally after treatment with the local anesthetic lidocaine; then after examination of the bronchial tree, the instrument was wedged into the distal airway of the middle lobe, lingula, or the most involved segment as identified by the chest roentgenogram (4, 5). With 60 ml of 0.9% normal saline for each round (a total of 120 to 240 ml for each patient), the liquid was gently instilled and aspirated with a handheld syringe. The aspirated BALF samples were pooled, and an aliquot of 100 to 200 μl was immediately prepared for microscopic examination.
Cells in the BALF aliquot were concentrated onto a glass slide with a cytocentrifuge (Cytospin II; Shandon Southern Instruments, Sewickley, Pa.), and the sample was stained with Diff-Quik (Baxter Healthcare Corporation, McGaw Park, Ill.). Pathogen load was quantified by counting clusters of P. carinii hominis organisms (6). The number of organisms per cluster varied. The BALF sample was centrifuged at 400 × g for 5 min. Due to the highly viscous nature of human BALF, P. carinii clusters were present in both the pellet and supernatant fractions. Aliquots of both the pellet and supernatant fractions were cryopreserved at −80°C.
Organisms were isolated from 10-ml aliquots of cryopreserved BALF samples. The samples were thawed, and then 30 ml of the same buffer solution used for homogenizing P. carinii-infected lungs to isolate organisms (see above) was added to the BALF. The suspension was mixed and incubated at room temperature for 10 min. The cells were recovered by centrifugation at 3,000 × g for 20 min. The pelleted material was resuspended in 5 ml of the buffer solution and total lipids were extracted.
Extraction and purification of lipids and isolation of sterols.
Total lipids were extracted with a monophasic neutral system according to the method of Bligh and Dyer (7) by the addition of chloroform (CHCl3) and methanol (MeOH) to the final organism preparation and concentrated into a packed pellet in a final ratio of CHCl3:MeOH:organism suspension (1:2:0.8, vol/vol/vol). In the case of the lyophilized sample (Italy-1), water was added to extract the lipids by this monophasic system. All solvents, except for that used to dissolve the sterols for GLC analysis, contained the antioxidant butylated hydroxytoluene to prevent lipid degradation. After being stirred for at least 2 h at room temperature in a 15-ml glass centrifuge tube or in a 60-ml culture tube, CHCl3 and H2O were added to form a biphasic system (12) for the purification of lipids from other compounds. The lower organic layer containing lipids was removed and dried under N2.
The total lipids were hydrolyzed under alkaline conditions to release sterols from the steryl esters by adding 0.5 ml of 15% KOH in 100% MeOH; then they were heated at 65°C for 30 min. After saponification, 0.7 ml of H2O was added, the suspension was mixed, and then 2.5 ml of petroleum ether was added. After vortex mixing, the sample was centrifuged to obtain complete phase separation. After removing the petroleum ether upper phase that contained the total sterols, the lower phase was reextracted with petroleum ether. The pooled petroleum ether fraction was placed in a reaction vial with a conical bottom, and the sample was dried under N2.
GLC analysis.
The sterols were redissolved in hexane and analyzed in a Hewlett-Packard 5890 or 6890 Series II GLC equipped with a 3- by 0.32-mm-inner-diameter capillary column coated with 0.25 μm of DB-5 (Alltech Associates, Inc., Deerfield, Ill.), SPB-5 (Supelco Inc., Bellefonte, Pa.), or HP-5 (Hewlett-Packard, Wilmington, Del.). Isothermal analyses were performed with an oven temperature of 250°C; the injection temperature was set at 290°C and the flame ionization detector temperature was set at 305°C. Helium was used as the carrier gas at a flow rate of 1 ml/min.
Since the cholesterol GLC peak was very large compared to those of other sterols, the GLC column was overloaded to detect the minor sterols in these samples. Thus, the retention time for cholesterol was not always considered accurate, and relative retention times (RRT) for the other sterol peaks in the chromatograms were calculated with both cholesterol (peak 1) and fungisterol (methylcholest-7-en-3β-ol; peak 13) as references. Fungisterol was selected as a reference peak since it was a major distinct P. carinii carinii sterol that was detectable in most samples.
Individual sterol components were also identified by cochromatography of P. carinii sterols with known standards (15a). Authentic standards included cholesterol, campesterol, and β-sitosterol (all from Sigma Chemical Co., St. Louis, Mo.). Chemically synthesized fungisterol (methylcholest-7-en-3β-ol), ethylcholest-7-en-3β-ol, 24-ethylidenecholesta-7,24(28)-diene-3β-ol, 24-ethylidenecholesta-5,7-diene-3β-ol, 24-methylenelanost-8-en-3β-ol, and pneumocysterol [(24Z)-ethylidenelanost-8-en-3β-ol] were from E. Parish, Auburn University.
In some analyses of these small samples, sterols were derivatized to enhance volatilization in the GLC and hence the detection of minor components. Total sterols were converted to their trimethylsilyl (TMS) derivatives by adding 50 μl of bis(trimethylsilyl)trifluoroacetamide (Aldrich Co., Milwaukee, Wis.). The sample was heated for 30 min at 65°C. After derivatization, the volume was reduced to 6 to 7 μl under a stream of N2, and then 1 μl of the sample was analyzed by GLC. Since these derivatives are unstable, especially in the presence of water, GLC analyses were conducted immediately after the derivatization procedure, and the sterols in the reagent were not dried and redissolved but were injected onto the GLC column after concentration. Analyses of blank controls taken through the procedure indicated no detectable GLC peaks.
RESULTS
Assignments of GLC peaks of P. carinii hominis sterols.
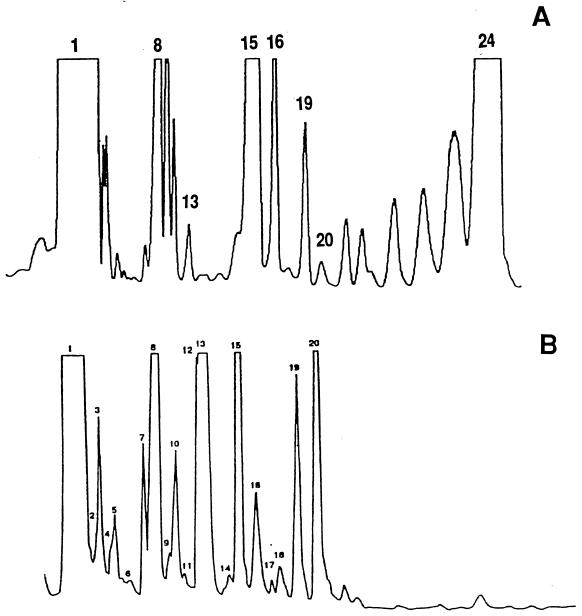
Tentative assignments of GLC peaks of P. carinii hominis sterols (Fig. 1) were made by comparing the RRTcholesterol obtained in analyses of purified P. carinii carinii sterols (17) and in a previous report of pneumocysterol and 24-methylenelanost-8-en-3β-ol from P. carinii hominis (16) (Fig. 2). In the report on P. carinii carinii sterols, the GLC peak that eluted last was designated peak 23 (17). This peak was found to have an elution time similar to that of pneumocysterol, which was designated peak 24 in P. carinii hominis sterols. Therefore, in this report, we designate this as peak 24 in organisms from both host species.
FIG. 1.
GLC tracings of the sterol composition of P. carinii hominis. Tentative peak assignments are based on those reported for sterols in P. carinii carinii (17) and the assignment of pneumocysterol to peak 24 (16). (A) P. carinii hominis organisms isolated from a cryopreserved HIV-positive, P. carinii-infected lung (Italy-1), analyzed as sterol TMS derivatives. (B) P. carinii carinii organisms isolated from the lungs of corticosteroid-immunosuppressed, infected rats. The analysis of underivatized sterols (modified from that reported in reference 17) is shown.
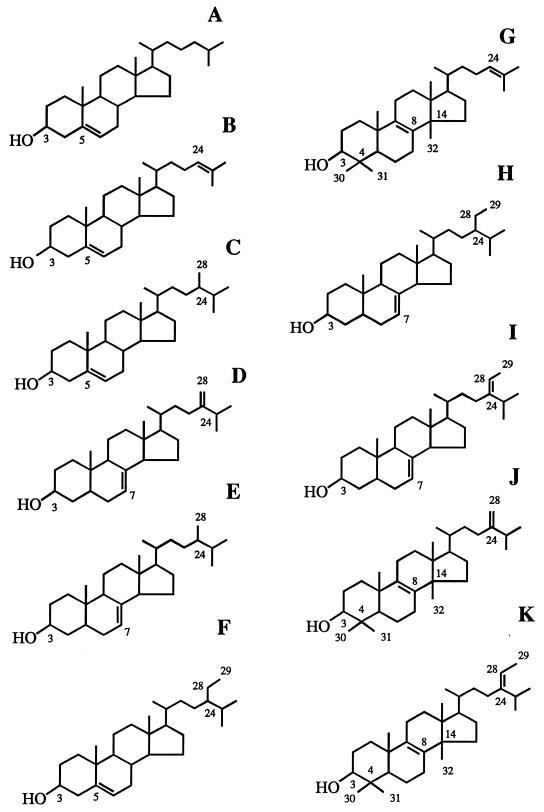
FIG. 2.
Structures of some sterols identified in P. carinii. Assignments of these sterols to some GLC peaks are considered tentative; GLC-MS analyses were not performed on the same P. carinii hominis sterols quantified by GLC. (A) Cholesterol, peak 1; (B) desmosterol (cholesta-5,24(25)-diene-3β-ol), peak 5; (C) campesterol (24-methylcholest-5-en-3β-ol), peak 8; (D) 24-methylenecholesta-7,24(28)-diene-3β-ol, peak 12; (E) fungisterol (24-methylcholest-7-en-3β-ol), peak 13; (F) β-sitosterol (24-ethylcholest-5-en-3β-ol), peak 15; (G) lanosterol, peak 17; (H) ethylcholest-7-en-3β-ol, peak 19; (I) 24-ethylcholesta-7,24(28)-diene-3β-ol, peak 20; (J) 24-methylenelanost-8-en-3β-ol, peak 23; (K) pneumocysterol [(24Z)-ethylidenelanost-8-en-3β-ol], peak 24.
The sterols in P. carinii carinii and/or P. carinii hominis were further identified by GLC cochromatography with authentic standards. The identities of sterols in the following GLC peaks were verified by these analyses: cholesterol (peak 1), cholesta-5,24(25)-diene-3β-ol (desmosterol; peak 5), 24-methylcholest-5-en-3β-ol (campesterol; peak 8), 24-methylenecholesta-7,24(28)-diene-3β-ol (peak 12), 24-methylcholest-7-en-3β-ol (fungisterol; peak 13), 24-ethylcholest-5-en-3β-ol (β-sitosterol; peak 15), lanosterol (peak 17), 24-ethylcholest-7-en-3β-ol (peak 19), 24-ethylcholesta-7,24(28)-diene-3β-ol (peak 20), 24-methylenelanost-8-en-3β-ol (peak 23), and (24Z)-ethylidenelanost-8-en-3β-ol (pneumocysterol; peak 24) (data not shown).
Since cholesterol is a dominant component of P. carinii total sterols in these samples, overloading the GLC column was necessary to detect minor components; hence, the cholesterol elution time was not always accurately estimated during peak integration. In this study, P. carinii-specific sterols were also identified by their GLC elution times with respect to fungisterol (peak 13; RRTfungisterol). The sterols were assigned from GLC peak 1 (cholesterol) to peak 24 (pneumocysterol) in the order of their elution from the column. Since peaks 2 and 3 were not well resolved from peak 1 in some GLC analyses, accurate quantitation of these components was not obtained for all samples. The structural assignments of GLC peaks are considered tentative since GLC-MS and nuclear magnetic resonance analyses have not been performed for all the sterol components of P. carinii hominis.
Sterol composition of isolated P. carinii hominis organisms isolated from cryopreserved P. carinii-infected lungs.
In this report, the sterols designated as signatures of the organism include pneumocysterol and those that were found in P. carinii carinii but were not detected in the lungs of healthy, untreated rats or the lungs of corticosteroid-treated, uninfected rats (17). Thus, these sterols (especially those represented by GLC peaks 13, 16, 19, and 20) are apparently synthesized by P. carinii carinii (11, 13, 17, 27). In the present study, most of these sterols were detected in P. carinii hominis organism preparations isolated from cryopreserved autopsied lungs (Fig. 1 and 2; Table 1). Pneumocysterol was detected in some, but not all, organism preparations isolated from human lung specimens.
TABLE 1.
Detection of P. carinii-specific signature sterols in P. carinii hominis preparations isolated from cryopreserved human P. carinii-infected lungsa
| Sample | GLC peak
|
||||
|---|---|---|---|---|---|
| 13 | 16 | 19 | 20 | 24 | |
| 288b | + | + | − | − | − |
| 480b | + | + | − | + | − |
| 438b | + | + | − | + | − |
| Denmark-1b | + | + | + | − | − |
| 293b | + | + | + | − | + |
| 313b | + | + | + | + | + |
| Denmark-4c | + | + | − | + | + |
| Denmark-2c | + | + | + | + | + |
| Denmark-3c | + | + | + | + | + |
| Italy-1bc | + | + | + | + | + |
+, detected; −, not detected.
Analyzed as underivatized sterols.
Analyzed as TMS sterol derivatives.
Sterol composition of P. carinii hominis organisms isolated from BALF of HIV-positive, P. carinii-infected patients.
When 1-ml BALF samples from HIV-positive, PCP-positive patients were analyzed, the P. carinii signature sterols were not always detectable (1). This was mainly due to the reduced levels of sterols in these samples (8), which is consistent with other studies demonstrating reduced levels of total lipids (14) and phospholipids (14, 20, 25) in BALF from P. carinii-infected lungs. In most analyses of organism preparations isolated from 1 ml of BALF, only cholesterol was detected. The organism yield from some samples was insufficient such that even cholesterol was undetectable by the procedures used in the present study.
Sufficient numbers of organisms were isolated from 10 ml of BALF, which allowed the detection of minor sterol components in these preparations. The P. carinii signature sterols were present (Table 2). Thus, qualitatively, P. carinii carinii and P. carinii hominis appear to have the same sterols.
TABLE 2.
Detection of P. carinii-specific signature sterols in P. carinii hominis preparations isolated from HIV-positive P. carinii-positive BALFa
| Sample | GLC peak
|
||||
|---|---|---|---|---|---|
| 13 | 16 | 19 | 20 | 24 | |
| J858 | + | + | + | + | + |
| J7128 | + | + | + | − | + |
| J7128 | + | + | + | − | + |
| B7228 | + | + | + | + | + |
| B7228 | + | + | + | + | + |
| D10228 | + | + | + | − | + |
| J1168 | + | + | + | + | + |
| W1188 | + | + | + | + | + |
| G10278 | + | + | + | − | + |
| K9118 | + | + | + | + | + |
Ten milliliters of BALF supernatant fractions was used for these analyses. Sterols were analyzed as TMS derivatives. +, detected; −, not detected.
Sterol profiles of isolated P. carinii hominis.
Sterol compositional data from organism preparations from cryopreserved lungs and BALF were compared and were also compared to those previously reported for purified P. carinii carinii organisms (17) (Table 3).
TABLE 3.
Composition of sterols in P. carinii hominis organisms isolated from cryopreserved human P. carinii-infected lungs and P. carinii-positive BALF
| GLC peak | Wt % ± SD (SEM) of
sterolsa
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
P. carinii
hominis isolated from:
|
P. carinii
cariniic
|
|||||||
|
P.
carinii-infected lungs
|
BALF
|
|||||||
| Total | Excluding cholesterol | Only signature sterolsb | Total | Excluding cholesterol | Only signature sterols | Total | Only signature sterols | |
| 1 | 83.8 ± 13.5 (4.3) | 99.0 ± 0.5 (0.2) | 78.4 | |||||
| 2 | 3.8 ± 8.0 (5.2) | 12.7 | ND | Trace | ||||
| 3 | 0.5 ± 1.0 (0.3) | 5.8 | ND | 0.6 | ||||
| 4 | 0.2 ± 0.6 (0.2) | 0.8 | ND | 0.1 | ||||
| 5 | 0.5 ± 0.6 (0.2) | 3.6 | ND | 0.2 | ||||
| 6 | 0.0 ± 0.1 (0.0) | 0.1 | ND | Trace | ||||
| 7 | 0.2 ± 0.4 (0.1) | 2.7 | ND | 0.6 | ||||
| 8 | 3.5 ± 4.3 (1.4) | 28.0 | 0.1 ± 0.1 (0.0) | 5.7 | 3.7 | |||
| 9 | 0.1 ± 0.2 (0.1) | 0.8 | ND | 0.2 | ||||
| 10 | 0.2 ± 0.3 (0.1) | 2.8 | ND | 1.5 | ||||
| 11 | 0.0 ± 0.1 (0.0) | 0.2 | ND | 0.0 | ||||
| 12 | ND | ND | ND | 1.2 | ||||
| 13 | 0.9 ± 0.9 (0.3) | 8.6 | 24.8 | 0.1 ± 0.0 (0.0) | 12.0 | 14.6 | 4.5 | 41.4 |
| 14 | 0.0 ± 0.0 (0.0) | 0.6 | ND | 0.1 | ||||
| 15 | 0.7 ± 1.8 (0.6) | 3.8 | 0.1 ± 0.0 (0.0) | 11.7 | 2.1 | |||
| 16 | 1.2 ± 2.0 (0.6) | 10.8 | 31.2 | 0.0 ± 0.0 (0.0) | 0.4 | 0.4 | 0.9 | 8.6 |
| 17 | 0.1 ± 0.2 (0.1) | 0.85 | ND | 0.1 | ||||
| 18 | 0.0 ± 0.0 (0.0) | 0.3 | ND | 0.3 | ||||
| 19 | 0.2 ± 0.2 (0.1) | 1.6 | 4.5 | 0.7 ± 0.4 (0.1) | 67.4 | 82.3 | 1.8 | 17.1 |
| 20 | 0.3 ± 0.3 (0.1) | 2.5 | 7.2 | 0.0 ± 0.0 (0.0) | 0.1 | 0.1 | 3.5 | 32.0 |
| 21 | 0.1 ± 0.2 (0.1) | 0.6 | 0.0 ± 0.0 (0.0) | 0.1 | ||||
| 22 | 0.1 ± 0.1 (0.0) | 0.2 | 0.0 ± 0.0 (0.0) | 0.0 | ||||
| 23 | 0.0 ± 0.1 (0.0) | 0.2 | ND | ND | ||||
| 24 | 1.8 ± 3.9 (1.2) | 11.2 | 32.2 | 0.0 ± 0.0 (0.0) | 2.1 | 2.7 | 0.1 | 0.9 |
Organism preparations isolated from P. carinii-infected lungs, n = 10; organism preparations isolated from BALF, n = 10. ND, not detected.
Includes only the five major P. carinii-specific signature sterols.
Values are from reference 17.
As in P. carinii carinii, cholesterol was the dominant sterol in the P. carinii hominis preparations isolated from infected human lungs, constituting approximately 84% of total sterols. In P. carinii hominis isolated from BALF, cholesterol comprised 99% of total sterols.
Campesterol (peak 8), cholest-5-en-3-one (peak 10), and β-sitosterol (peak 15), which were detected in the lungs of healthy, untreated rats and immunosuppressed, uninfected rats (17), were also present in these P. carinii hominis preparations. Plant sterols such as campesterol and β-sitosterol that are present in the lung and in P. carinii very likely originated in the host’s diet, since it is known that plant sterols appear in the blood and vary with dietary intake (28). Thus, it is most likely that these sterols, as well as cholesterol, were scavenged by the organisms from the host lung. The identity of peak 10 as cholest-5-en-3β-one was further verified in samples analyzed as both underivatized sterols and their TMS derivatives. The TMS attaches to the 3-hydroxyl group of sterols, and since this component was not derivatized, its peak area remained the same while that of other components in the sample increased upon derivatization.
To compare P. carinii sterol profiles obtained from different preparations, components that may not be synthesized de novo by the pathogen were omitted, and then the weights percent were recalculated. Omitting cholesterol alone helps in focusing on the sterols that may be synthesized by P. carinii (Table 3); however, the metabolism of each sterol component detected in these preparations has not been evaluated. Sterols found in the organism could be scavenged from the mammalian lung, produced by de novo biosynthesis by P. carinii, or (as precursors) scavenged and further metabolized. Therefore, only five major P. carinii sterols (signatures of the organism; Table 1 and 2) were considered in the calculations shown in Table 3. These values were compared with similar calculations of sterol compositions previously reported for P. carinii carinii.
The most dramatic difference noted between individual P. carinii hominis samples was in the magnitude of peak 24, pneumocysterol. This sterol was undetectable in some samples, whereas it accounted for up to 53% of noncholesterol components in GLC analyses of some samples, e.g., Italy-1 (Fig. 1). Such high proportions of pneumocysterol were not seen in any of the analyses of organisms isolated from BALF.
The relative proportions of the five major signature sterols of P. carinii were different in P. carinii hominis organisms isolated from cryopreserved lungs and from BALF (Table 3). Also, the proportions of these sterols in P. carinii hominis from both types of human source materials were different from that found in rat-derived P. carinii carinii.
DISCUSSION
Purity of isolated organisms and assignments of sterol structures.
Since organisms from human sources are difficult to obtain, the purity of these preparations was not as extensively characterized by biochemical and immunochemical criteria as those isolated from infected rat lungs (18). The purity and composition of these preparations were determined only by microscopic analysis, which indicated that P. carinii was the major, if not the only, microbe identifiable. However, since most of the same sterol components (identified as GLC peaks) were similar to those reported for P. carinii carinii, it is apparent that at least the major signature sterols are present in organisms isolated from both humans and rats.
The sterol profiles in P. carinii hominis.
In our study, cholesterol constituted 78% of total sterols in freshly isolated P. carinii carinii derived from rats (17). Most, if not all, of the cholesterol in the organism is scavenged from the host and is presumed to form the bulk of the lipid bilayer of the pathogen’s membranes. Many organisms take up nutrients from their environments; parasites are particularly efficient in scavenging host molecules and incorporating them into their cellular structures. In some parasites where cholesterol fully satisfies the structural requirements of their membrane functions, cholesterol may be the only sterol detected. In other parasites, despite scavenging and incorporating cholesterol into the bulk phase of membrane lipid bilayers distinctive sterol compounds may need to be synthesized if cholesterol does not fulfill the precise structural requirements for specialized functions of the membrane (e.g., enzyme activity).
The higher proportion of cholesterol in preparations isolated from cryopreserved human lungs and especially from BALF can in part be explained by the inability to detect minor sterols that are probably present in the samples. If minor components were not detected by the GLC flame ionization detector, their peak areas would not be integrated, similar to the analyses performed on 1-ml samples of BALF from HIV-positive, PCP-positive patients in which only cholesterol was detected (100%). It cannot be ruled out that higher cholesterol values were obtained from these samples of P. carinii hominis because these preparations were not as pure as the P. carinii carinii preparations obtained from the lungs of corticosteroid-immunosuppressed rats. In that case, cholesterol in lung tissue or lung surfactant may be present in higher amounts in the human-derived samples than in the rat-derived samples. However, it should be noted that the Denmark-1 sample was purified by Percoll gradient centrifugation, but due to sample size (and analysis of underivatized sterols), only seven GLC peaks were integrated and the cholesterol weight percent value was 95%. Hence, sample size appears to be the main reason for the apparent, but probably inaccurate, higher cholesterol values obtained from human-derived samples of the organism.
Sterol profiles in organisms vary according to physiological differences (e.g., different life cycle stages); thus, the variations observed in the relative proportions of different sterol components are not surprising, since these preparations consist of mixed life cycle stages. When a specific GLC peak in these analyses was not detected in a sample, it may reflect different proportions of different life cycle stages (e.g., trophic and cystic stages) in different organism preparations. Also, the organism population recovered by homogenization of the lungs is expected to differ from that aspirated during the lavage procedure. It is known that trophic forms, with extensive elaborations of their cell surfaces (tubular extensions), are tightly adherent to type I epithelial cells lining the alveolus, as well as to adjacent organisms (18). In contrast, cystic forms have fewer tubular extensions. However, both life cycle forms are found in the clusters of P. carinii hominis retrieved from BALF, which is performed by gentle washing which would not be expected to dislodge or detach those organisms attached to type I pneumocytes. The observations that trophic forms are found in BALF and that the organisms in these clusters are difficult to disperse suggest that these organisms are adhered to each other and not to type I pneumocytes. Although the percentage of trophic versus cystic forms in P. carinii hominis clusters was not quantified in the present study, it is likely that the population of organisms isolated from BALF differed from that isolated from infected lungs.
Pneumocysterol.
Although there were quantitative differences in the percentages of individual sterols in organisms isolated from human lungs, human BALF, and rat-derived P. carinii carinii, the major Δ7 24-alkylsterol components were generally similar. In contrast the Δ8 24-alkylsterol pneumocysterol, which was undetected in some samples, comprised 50% or more of the noncholesterol sterols in other samples. It is consistently only a minor component in P. carinii carinii, which is obtained from an experimentally controlled animal model (17). The broad range of values for this sterol in human samples is not understood. However, it cannot be ruled out that the patients from whom organisms with high pneumocsyterol had been isolated had taken a drug or food that influences sterol metabolism in the organisms. It would seem reasonable to predict that inhibitors of lanosterol nucleus demethylation enhance the production of pneumocysterol, since this molecule has an alkyl group added to C-24 of the side chain but apparently retains the three methyl groups attached to the sterol nucleus.
It cannot be ruled out that organisms from different patients represent different species or strains. There is now strong evidence that P. carinii organisms infecting a single host species are genetically diverse (2, 9, 10, 19, 21, 26), although not as divergent as those infecting other mammalian hosts. Thus, it is possible that different P. carinii hominis species or strains differ in their ability to biosynthesize the Δ8 24-alkylsterol pneumocysterol, and the broad range of values observed in this sterol in human-derived samples reflects different proportions of distinct P. carinii species or strains among different mixed populations.
This is the first report of the sterol composition of Pneumocystis organisms isolated from human material. These human-derived organisms and those isolated from corticosteroid-immunosuppressed rats (3, 15, 17, 27) have in common several Δ7 24-alkylsterols, which are not found in any other microbe infecting human lungs. Thus, although sterols have been analyzed in organisms only from rats and humans, it is likely that these sterols will be found in all strains of Pneumocystis proliferating in other mammals. On the other hand, pneumocysterol may represent a biochemical difference between different genetic populations of Pneumocystis organisms proliferating in humans, and thus it cannot be ruled out that this sterol naturally occurs in organisms proliferating in other mammalian host species.
ACKNOWLEDGMENTS
We thank M. T. Cushion for specimens of cryopreserved P. carinii-infected lungs, R. Smith for specimens of formalin-fixed lung controls, M. Perreira for a formalin-fixed P. carinii-infected lung, O. Settnes for preparing purified organisms (Denmark-1), and B. Kleykamp, M. Swonger, and M. A. Wyder for technical assistance.
This study was supported by NIH grants RO1 AI38758 and RO1 AI29316 (E.S.K.) and PO1 HL56387 (R.P.B.) and a fellowship from the Universiti Malaysia Sarawak (Z.A.).
REFERENCES
- 1.Amit, Z., R. P. Baughman, and E. S. Kaneshiro. Pneumocystis carinii sterols in human bronchoalveolar lavage fluid. Submitted for publication.
- 2.Banerji S, Lugli E B, Wakefield A E. Identification of two genetically distinct strains of Pneumocystis carinii in infected ferret lungs. J Eukaryot Microbiol. 1994;41:73S. [PubMed] [Google Scholar]
- 3.Bartlett M S, Eichboltz R, Smith J W. Antimicrobial susceptibility of Pneumocystis carinii in culture. Diagn Microbiol Infect Dis. 1985;3:381–387. doi: 10.1016/0732-8893(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 4.Baughman R P, Dohn M N, Frame P T. The continuing utility of bronchoalveolar lavage to diagnose opportunistic infection in AIDS patients. Am J Med. 1994;97:515–522. doi: 10.1016/0002-9343(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 5.Baughman R P, Dohn M N, Loudon R G, Frame P T. Bronchoscopy with bronchoalveolar lavage in tuberculosis and fungal infections. Chest. 1991;99:92–97. doi: 10.1378/chest.99.1.92. [DOI] [PubMed] [Google Scholar]
- 6.Baughman R P, Strohofer S, Colangelo G, Frame P T. Semiquantitative technique for estimating Pneumocystis carinii burden in the lung. J Clin Microbiol. 1990;28:1425–1427. doi: 10.1128/jcm.28.6.1425-1427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 8.Chandra J, Amit Z, Baughman R P, Kleykamp B, Kaneshiro E S. Pneumocystis infection is correlated with a reduction of the total sterol content of human bronchoalveolar lavage fluid. J Eukaryot Microbiol. 1999;46:146S–148S. [PubMed] [Google Scholar]
- 9.Cushion M T. Pneumocystis carinii. In: Collier L, Balows A, Sussman M, editors. Topley and Wilson’s microbiology and microbial infections. Medical mycology. 9th ed. Vol. 4. N.Y: Arnold Publishers, Oxford University Press, New York; 1998. pp. 645–683. [Google Scholar]
- 10.Cushion M T, Zhang J, Kaselis M, Giuntoli D, Stringer S L, Stringer J R. Evidence for two genetic variants of Pneumocystis carinii coinfecting laboratory rats. J Clin Microbiol. 1993;31:1217–1223. doi: 10.1128/jcm.31.5.1217-1223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florin-Christensen M, Florin-Christensen J, Wu Y P, Zhou L, Gupta A, Rudney H, Kaneshiro E S. Occurrence of specific sterols in Pneumocystis carinii. Biochem Biophys Res Commun. 1994;198:236–242. doi: 10.1006/bbrc.1994.1033. [DOI] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloane-Stanley G H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 13.Furlong S T, Samia J A, Rose R M, Fishman J A. Phytosterols are present in Pneumocystis carinii. Antimicrob Agents Chemother. 1994;38:2534–2540. doi: 10.1128/aac.38.11.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman A G D, Lawrence M G, Ognibene F P, Suffredini A F, Lipschik G Y, Kovacs J A, Masur H, Shelhamer J H. Reduction of pulmonary surfactant in patients with human immunodeficiency virus infection and Pneumocystis carinii pneumonia. Chest. 1992;102:1730–1736. doi: 10.1378/chest.102.6.1730. [DOI] [PubMed] [Google Scholar]
- 15.Kaneshiro E S. The lipids of Pneumocystis carinii. Clin Microbiol Rev. 1998;11:27–41. doi: 10.1128/cmr.11.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Kaneshiro, E. S., et al. Unpublished data.
- 16.Kaneshiro E S, Amit Z, Swonger M M, Kreishman G P, Brooks E E, Kreishman M, Jayasimhulu K, Parish E J, Sun H, Kizito S A, Beach D H. Pneumocysterol [(24Z)-ethylidenelanost-8-en-3β-ol], a rare sterol detected in the opportunistic pathogen Pneumocystis carinii hominis: structural identity and chemical synthesis. Proc Natl Acad Sci USA. 1999;96:97–102. doi: 10.1073/pnas.96.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneshiro E S, Ellis J E, Jayasimhulu K, Beach D H. Evidence for the presence of “metabolic sterols” in Pneumocystis: identification and initial characterization of Pneumocystis carinii sterols. J Eukaryot Microbiol. 1994;41:78–85. doi: 10.1111/j.1550-7408.1994.tb05938.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaneshiro E S, Wyder M A, Zhou L H, Ellis J E, Voelker D R, Langreth S G. Characterization of Pneumocystis carinii preparations developed for lipid analysis. J Eukaryot Microbiol. 1993;40:805–815. doi: 10.1111/j.1550-7408.1993.tb04479.x. [DOI] [PubMed] [Google Scholar]
- 19.Keely S P, Stringer J R, Baughman R P, Linke M J, Walzer P D, Smulian A G. Genetic variation among Pneumocystis carinii hominis isolates in recurrent pneumocystosis. J Infect Dis. 1995;172:595–598. doi: 10.1093/infdis/172.2.595. [DOI] [PubMed] [Google Scholar]
- 20.Kernbaum S, Masliah U, Alcindor L G, Bouton C, Cristol D. Phospholipase activities of bronchoalveolar lavage fluid in rat Pneumocystis carinii pneumonia. Br J Exp Pathol. 1983;64:460–470. [PMC free article] [PubMed] [Google Scholar]
- 21.Latouche S, Roux P, Poirot J L, Lavrard I, Hermelin B, Bertrand V. Preliminary results of Pneumocystis carinii strain differentiation by using molecular biology. J Clin Microbiol. 1994;32:3052–3053. doi: 10.1128/jcm.32.12.3052-3053.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peglow S L, Smulian A G, Linke M J, Pogue C L, Nurre S, Crisler J, Phair J, Gold J W, Armstrong D, Walzer P D. Serologic responses to Pneumocystis carinii antigens in health and disease. J Infect Dis. 1990;161:296–306. doi: 10.1093/infdis/161.2.296. [DOI] [PubMed] [Google Scholar]
- 23.Pifer L L, Hughes W T, Stagno S, Woods D. Pneumocystis carinii infection: evidence for high prevalence in normal and immunosuppressed children. Pediatrics. 1978;61:35–41. [PubMed] [Google Scholar]
- 24.Sarić M, Clarkson A B., Jr Ornithine decarboxylase in Pneumocystis carinii and implications for therapy. Antimicrob Agents Chemother. 1994;38:2545–2552. doi: 10.1128/aac.38.11.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehan P M, Stokes D C, Yeh Y-Y, Hughes W T. Surfactant phospholipids and lavage phospholipase A2 in experimental Pneumocystis carinii pneumonia. Am Rev Respir Dis. 1986;134:526–531. doi: 10.1164/arrd.1986.134.3.526. [DOI] [PubMed] [Google Scholar]
- 26.Stringer J R, Walzer P D. Molecular biology and epidemiology of Pneumocystis carinii infection in AIDS. AIDS. 1996;10:561–571. doi: 10.1097/00002030-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Urbina J A, Visbal G, Contreras L M, McLaughlin G, Docampo R. Inhibitors of Δ24(25) sterol methyltransferase block sterol synthesis and cell proliferation in Pneumocystis carinii. Antimicrob Agents Chemother. 1997;41:1428–1432. doi: 10.1128/aac.41.7.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanhanen H T, Miettinen T A. Effects of unsaturated and saturated dietary plant sterols on their serum contents. Clin Chim Acta. 1992;205:97–107. doi: 10.1016/s0009-8981(05)80004-x. [DOI] [PubMed] [Google Scholar]
- 29.Wakefield A E, Stewart T J, Moxon E R, Marsh K, Hopkin J M. Infection with Pneumocystis carinii is prevalent in healthy Gambian children. Trans R Soc Trop Med Hyg. 1990;84:800–802. doi: 10.1016/0035-9203(90)90087-u. [DOI] [PubMed] [Google Scholar]
- 30.Walzer P D, Rutledge M E, Yoneda K. Pneumocystis carinii: new separation method from lung tissue. Exp Parasitol. 1979;47:356–368. doi: 10.1016/0014-4894(79)90088-2. [DOI] [PubMed] [Google Scholar]