Abstract
Luteal phase support (LPS) is crucial in assisted reproductive technology (ART) cycles when the luteal phase has been found to be defective. Such deficiency is most likely related to the supraphysiological steroid levels that usually occurr in stimulated cycles which, in turn, could severely affect luteinizing hormone (LH) secretion and function, thereby negatively influencing the luteal phase. A number of different medications and routes have been successfully used for LPS in ART. Although an optimal protocol has not yet been identified, the existing plethora of medications offer the opportunity to personalize LPS according to individual needs. Subcutaneous administration progesterone has been proposed for LPS and could represent an alternative to a vaginal and intramuscular route. The aim of the present systematic review is to summarize the evidence found in the literature concerning the application of subcutaneous progesterone in ARTs, highlighting the benefits and limits of this novel strategy. With this aim in mind, we carried out systematic research in the Medline, ISI Web of Knowledge, and Embase databases from their inception through to November 2020. Randomized controlled trials (RCTs) were preferred by the authors in the elaboration of this article, although case-control and cohort studies have also been considered. According to our findings, evidence exists which supports that, in women with a good prognosis undergoing a fresh in vitro fertilization (IVF) cycle, subcutaneous Pg is not inferior to vaginal products. In the Frozen-thawed embryo transfer (FET) cycle, data concerning efficacy is mixed with an increased miscarriage rate in women undergoing a subcutaneous route in oocyte donor recipients. Data concerning the acceptance of the subcutaneous route versus the vaginal route are encouraging despite the different scales and questionnaires which were used. In addition, a cost-effective analysis has not yet been conducted.
Keywords: progesterone, subcutaneous progesterone, assisted reproductive technology, in-vitro fertilization, luteal phase support, luteal phase defect, ovulation induction, systematic review
Introduction
The importance of luteal phase support (LPS) is widely recognized in assisted reproductive technology (ART) and an adequate luteal phase is mandatory for embryo implantation. Under physiological conditions, the luteal phase is mainly sustained by a luteinizing hormone (LH) and a follicle-stimulating hormone (FSH) surge during ovulation, which induces the “luteinization” of the granulosa cells, thereby promoting the formation of the corpus luteum (1). In particular, LH is important, not only during the late phase of folliculogenesis (2–6) and for the support of the corpus luteum (7), but also in the early stage of embryo implantation (8–10). During LP, the corpus luteum sustains the endometrium, preparing it for implantation that usually occurs 6 days after fertilization (2). In medically assisted reproduction, a luteal phase defect is observed. Indeed, the supraphysiological production of sexual steroids during ovarian stimulation (OS) significantly suppresses LH levels, thereby causing impairment of LPS (11). Clinically, the latest Cochrane meta-analysis which included 94 randomized controlled trials (RCTs) (26,198 infertile women undergoing ART) confirmed that LPS is associated with significantly higher live births and ongoing pregnancy compared with placebo or no treatment (12). LPS is carried out mainly with progesterone (Pg) and/or other hormones that promote its production, such as human chorionic gonadotropin (hCG) and the gonadotropin-releasing hormone agonist (GnRHa). Pg supplementation is by far the most common strategy adopted worldwide (11, 13). Pg can be administrated vaginally, rectally, intramuscularly, and orally (12). Oral Pg seems to be more effective, using synthetic Pg rather than natural micronized Pg (11, 14, 15). Notably, among oral products, dydrogesterone seems to offer better results in terms of live births and pregnancy rates in fresh cycles compared with vaginal micronized Pg (16); however, vaginal administration of Pg is still the most preferred route among clinicians (17, 18). Conversely, <6% of practitioners opted for an intramuscular or oral route (13, 17).
Recently, a water-soluble formulation of Pg for subcutaneous administration has been introduced (19, 20). A preliminary study demonstrated that this route can induce endometrial decidualization and present bioavailability similar to oil-based products (19–21). In the present review, we summarize the clinical studies that have adopted subcutaneous Pg for LPS in an ART setting.
Materials and Methods
Search Strategy
A systematic search was undertaken in Medline, ISI Web of Knowledge, and EMBASE databases from their inception through to November 2020. Specifically, studies in which subcutaneous Pg was adopted for LPS in women undergoing in vitro fertilization (IVF) or intrauterine inseminations (IUI) were selected. RCTs were preferred by the authors in the elaboration of this search, although case-control and cohort studies have been also considered. The following keywords were adopted: LPS, ART, IVF, and assisted reproduction.
Bias Evaluation
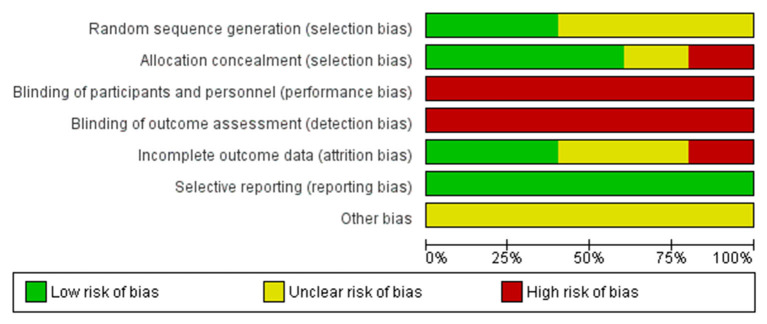
Three authors (AC, VM, and FC) independently evaluated the risk of bias. Senior authors solved conflicts (CA and IS). Eligible RCTs were assessed using the Cochrane risk of bias assessment tool (12, 22). The following issues were assessed in detail: (1) random sequence generation; (2) allocation concealment; (3) binding of participants and personnel; (4) incomplete outcome data; and (5) selective reporting. The risk of bias was graded per consideration as low, unclear, or high.
Non-RCTs were assessed using the Newcastle–Ottawa scale (NOS) (23) score according to three issues: selection of study group, comparability between groups, and exposure/outcome.
Results
A total of 100 items were identified through database research. Fourteen full-text papers were assessed for eligibility. Five of them did not report data concerning clinical outcomes or compliance of patients and were excluded (17, 19–21, 24). Five RCTs, two retrospectives, and two cohort studies were selected (Figure 1). In two RCTs and one non-RCT study, data were extracted from conference abstracts (25–27). All characteristics of the studies included are illustrated in Table 1 (fresh cycle) and Table 2 (frozen cycle). The risk of bias in non-RCTs is stated in Table 3, while the risk of bias in RCTs is illustrated in Figure 2.
Figure 1.
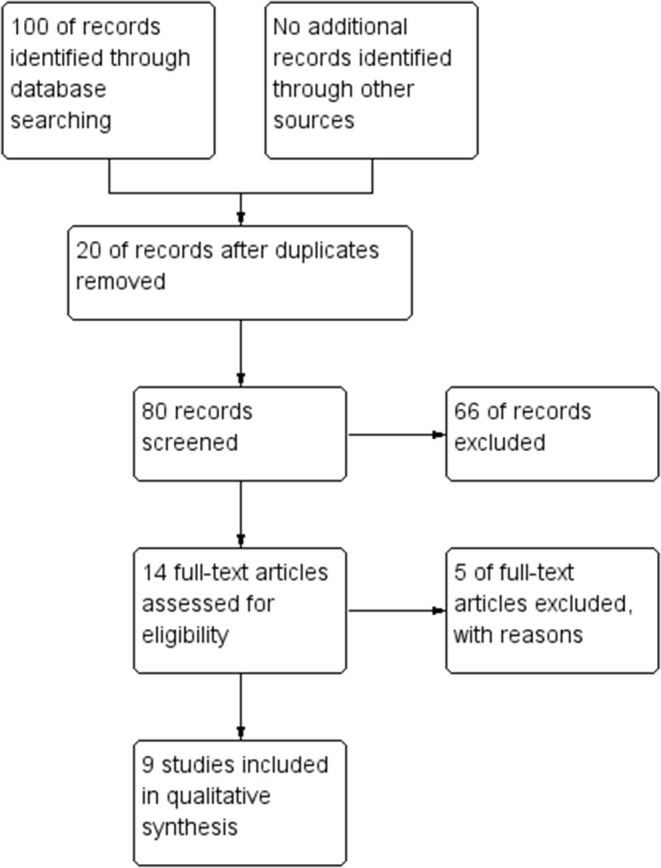
Flow chart.
Table 1.
Characteristics of studies included (fresh cycle).
| Reference | Study type | Period of observation | Country | Cycle | Population | Intervention | Comparison | Results |
|---|---|---|---|---|---|---|---|---|
| Baker et al. (28) | RCT | 2009–2011 | USA (8 centers) | IVF/ICSI |
n = 782 Age 18-42 years BMI < 30 Kg/m2 FSH ≤ 15 IU/L Previous IVF < 3 |
n = 392 Subcutaneous Progesterone 25 mg/day |
n = 390 Vaginal progesterone 200 mg/day |
Comparable implantation rate, ongoing pregnancy rate, live birth rate. Comparable women satisfaction |
| Lockwood et al. (29) | RCT | 2009–2010 | Europe (13 centers) | IVF/ICSI | n = 683 Age 18-42 years BMI < 30 Kg/m2 FSH < 15 IU/L Previous IVF < 3 |
n = 339 Subcutaneous progesterone 25 mg/day |
n = 344 Vaginal progesterone gel 90 mg (8%)/ day |
Comparable ongoing pregnancy rate, live birth rate. Comparable women satisfaction |
| Mele et al. (30) | RCT | 2017 | Italy | IVF/ICSI |
n = 130 Age < 37 years BMI 20–25 Kg/m2 |
n = 65 Subcutaneous progesterone 25 mg/day |
n = 65 Intramuscular progesterone 33-50 mg/day |
Comparable hCGβ pregnancy test. Higher PRL and Cortisol in women with intramuscular progesterone |
| Venturella et al. (26) | RCT (abstract) | 2014–2016 | Italy | IUI |
n = 246 Age 18-38 years BMI 19-30 Kg/m2 |
n = 120 Subcutaneous progesterone 25 mg/day |
n = 126 Vaginal progesterone gel 90 mg (8%)/ day |
Comparable hCGβ pregnancy tes and clinical pregnancy rate Similar patients' satisfaction |
BMI, body mass index; FET, frozen embryo transfer; FSH, follicle stimulation hormone; IVF, in vitro fertilization; IUI, intrauterine insemination; n.a, not available; PRL, prolactin; RCT, randomized controlled trial.
Table 2.
Characteristics of studies included (frozen cycle).
| Reference | Study type | Period of observation | Country | Cycle | Population | Intervention | Comparison | Results |
|---|---|---|---|---|---|---|---|---|
| Turkgeldi et al. (31) | Retrospective | 2017–2017 | Turkey | FET |
n = 214 Age ≤ 43 years Previous IVF failed ≤ 3 |
n = 107 Subcutaneous progesterone 25 mg twice a day |
n = 107 Vaginal progesterone gel 90 mg (8%) twice a day |
Comparable βhCG pregnancy test, ongoing pregnancy rate, live birth rate and miscarriage rate |
| Gosalvez Vega et al. (27) | Prospective cross-over study (abstract) | 2016 | Spain | FET | n = 45 | n = 45 Subcutaneous progesterone 25 mg/day |
n = 45 Vaginal progesterone 800 mg/day |
Subcutaneous progesterone was preferred to vaginal route |
| Ramos et al. (32) | Retrospective | 2019 | France | FET |
n = 320 Age 18-42 years BMI 18-30 Kg/m2 No recurrent pregnancy loss |
n = 160 serum progesterone ≤ 21.9 ng/ml (1-2 days before FET) subcutaneous progesterone 25 mg/day + vaginal progesterone 800 mg/day |
n = 160 serum progesterone > 21.9 ng/ml (1-2 days before FET) subcutaneous progesterone 25 mg/day + vaginal progesterone 800 mg/day |
Comparable implantation rate and clinical pregnancy rate Significantly lower miscarriage rate in women with serum progesterone above 21.9 ng/ml |
| Llacer et al. (25) | RCT (abstract) | n.a. | Spain | FET |
n = 120 Oocyte donation recipients |
n = 60 Subcutaneous progesterone 25 mg/day |
n = 60 Vaginal progesterone 800 mg/day |
Higher implantation rate, clinical pregnancy rate in women with vaginal progesterone Better compliance with subcutaneous progesterone |
| Venturella et al. (33) | Cohort study | 2016 | Italy | FET |
n = 69 Age 18–43 years |
n = 69 Subcutaneous progesterone 25 mg/day |
n = 69 Vaginal progesterone |
Better expectation and compliance with subcutaneous progesterone comparing with previous history with vaginal route |
BMI, body mass index; FET, frozen embryo transfer; FSH, follicle stimulating hormone; IVF, in vitro fertilization; IUI, intrauterine insemination; n.a., not available; PRL, prolactin; RCT, randomized controlled trial.
Table 3.
Newcastle-Ottawa scale for non RCTs.
Figure 2.
Bias assessment in RCTs.
Fresh Cycles
Two multicenter RCTs investigated the role of subcutaneous Pg in women undergoing fresh IVF cycles (28, 29). Lockwood et al. (29) designed a prospective, open-label, controlled, non-inferiority study involving 683 ART patients from 13 European centers randomized into two groups: subcutaneous Pg, 25 mg daily (n = 339); and vaginal Pg, 90 mg 8% gel daily (n = 344). Ongoing pregnancy rates at 10 weeks of treatment were 27.4% in women who underwent subcutaneous Pg and 30.5% in those who underwent vaginal gel, with a non-significant difference between the groups (−3.09%, 95% confidence interval [CI] 9.91–3.73). Consistently, live birth rate, miscarriage, and implantation rates were found to be similar between the groups. The overall incidence of non-serious adverse effects was also similar between the groups. While gastrointestinal and vaginal disorders (pruritus and discomfort) were more frequent with the use of vaginal gel, skin and subcutaneous reactions were more frequent using subcutaneous Pg. The second RTC was a prospective open-label non-inferiority trial conducted by Baker et al. (28) in 2014 and involved 800 women from eight clinics in the USA randomized to subcutaneous Pg (25 mg daily) vs. vaginal Pg (100 mg bid daily). The ongoing pregnancy rate was similar between the groups with a difference of −2.8% (41.6% subcutaneous Pg vs. 44.4% vaginal Pg). Comparable live birth rates, implantation rates, hCGβ, and clinical intrauterine pregnancies with fetal cardiac activity were observed. Both RCTs adopted similar eligibility criteria involving women with the retrieval of at least three oocytes aged between 18–42 years old (28, 29). Heterogenous OS was allowed in both trials in terms of pituitary suppression, gonadotropin regimen, and ovulation triggering. Furthermore, both RCTs used a −10% of non-inferiority margins for the lower limit of the two-sided 95% of CI. While Lockwood et al. (29) assumed an ongoing pregnancy rate of 30%, Baker et al. (28) considered an ongoing pregnancy rate of 43% in both groups.
A recent individual patient meta-analysis was carried out merging data from these two RCTs (34). The pooled risk difference in terms of ongoing pregnancy rates for subcutaneous Pg vs. vaginal Pg was −0.03 (95% CI −0.08–0.02). Consistently, the pooled risk difference regarding live birth rates was −0.02 (95% CI −0.07–0.03). In addition, the pooled odds ratio (OR) regarding OHSS risk was similar between the groups (OR 1.04, 95% CI 0.40–1.81).
A comparison between the intramuscular and subcutaneous routes was recently carried out by Mele et al. in 2020 (30). In detail, 130 women undergoing a first IVF cycle were randomized to intramuscular Pg (33 mg/day from ovum pick up and 50 mg/day from embryo transfer) and subcutaneous Pg (25 mg/day). The authors did not observe any difference in terms of the hCGβ pregnancy test between groups. Conversely, what was observed was significantly higher prolactin and cortisol levels measured seven days after ovum pick up in women who had undergone intramuscular Pg compared with those who had undergone a subcutaneous route. These data could suggest that the subcutaneous route might represent a solution to reduce stress and anxiety compared with the more painful intramuscular route.
Frozen-Thawed Embryo Transfer (FET)
Frozen–thawed embryo transfer is widely used in ART. In contrast with a conventional fresh cycle, the transfer of embryos did not occur immediately after OS. This approach dramatically reduced the risk of OHSS and, at the same time, offered the possibility to transfer the embryos in a more physiological environment. Recent meta-analyses suggest that the transfer of cryopreserved embryos is associated with favorable perinatal outcomes (35–38).
Five studies explored the effects of subcutaneous Pg in a FET cycle (25, 27, 31–33). Specifically, a retrospective cohort study (31) involving 214 age-matched women compared 107 women who had undergone subcutaneous Pg (25 mg bid daily) with 107 women who had undergone vaginal Pg (90 mg 8% Pg vaginal gel bid daily). Only women <43-years-old and with <3 failed ART cycles were included (31). In both groups, only vitrified blastocysts reaching at least an expansion grade were transferred. Both groups showed comparable body mass index, antral follicle count, and the number of oocytes retrieved. Ongoing pregnancy rates (RR 1.11; 95% CI 0.78–1.56) and miscarriage rates (RR 1.08; 95% CI 0.76–1.55) were similar between groups.
Another retrospective trial explored the combination of both vaginal and Pg routes for LPS following FET (32). Two hundred thirteen women under 42-years-old, BMI between 18–30 kg/m2, and no history of recurrent miscarriage were included. Patients were stratified according to serum Pg measured 1–2 days before embryo transfer. LP was supported using vaginal formulation at a dose of 800 mg/day plus subcutaneous injections of Pg at a dose of 25 mg/day. Women with Pg levels above 21.9 ng/ml showed significantly lower miscarriage rates compared with those below these cut-off values. On the other hand, implantation and clinical pregnancy rates (gestational sac with heart activity) were similar, irrespective of Pg levels.
In a pilot randomized study, 120 oocyte recipients were randomized to receive subcutaneous Pg (25 mg daily) vs. vaginal Pg (200 mg bid daily) (25). The main aim of the investigators was to compare patients satisfaction through the administration of a questionnaire on comfort, hygiene, and sexual relations. Significantly better satisfaction was recorded using the subcutaneous route, although the ongoing pregnancy rate at 12 weeks of gestation was higher in women who had undergone a vaginal route (33.3 vs. 50%, p = 0.086) (25). In addition, significantly lower clinical pregnancy rates (36.8 vs. 59.3%, p = 0.022), implantation rates (32.5; 53.7% p = 0.017), and biochemical pregnancy per embryo transfer (26.3 vs. 5.6%, p = 0.004) were observed in women who had undergone a subcutaneous route compared with those who had undergone a vaginal route (25). In an open-label crossover study, Gosalvez-Vega et al. confirmed that subcutaneous Pg is better tolerated than the vaginal route in a FET cycle (27). Another study in the FET cycle was reported in an Italian prospective study involving 69 women with previous experience of vaginal Pg (33). In detail, patients were asked to complete three questionnaires (33). The first one regarded previous experience with a vaginal device, the second was at the time of transfer, and the third questionnaire was 8 days later evaluating their experience with subcutaneous progesterone. The authors reported better acceptance of the subcutaneous route compared with vaginal products.
Intrauterine Insemination
Only one trial was developed to investigate the effect of subcutaneous Pg in women undergoing IUI (26). A total of 246 women were randomized to receive subcutaneous Pg (n = 126, 25 mg daily) or vaginal Pg gel (n = 120, 90 mg daily). Ongoing pregnancy rates per cycle (11.9 vs. 12.7 %) and hCGβ test (14.4 vs. 14%) were comparable between the groups, as was tolerability measured by a satisfactory score.
Discussion
Subcutaneous Pg represents one of the most recent innovations proposed for LPS in IVF. This formulation was obtained by combining Pg with hydroxypropyl-b-cyclodextrin, which in turn increases the solubility of Pg (21). A preliminary RCT demonstrated that 25 mg/day or 50 mg/day of subcutaneous Pg can induce decidualization in 24 volunteers, whose ovarian function was suppressed with GnRH agonist and subsequently estrogenized using transdermal route (20). Sator et al. demonstrated that subcutaneous formulation was bioequivalent to intramuscular formulation in terms of the extent of exposure (19). In detail, the subcutaneous formulation is promptly absorbed and could achieve progesterone peak serum levels at an earlier time than the intramuscular route (19). In 24 donors undergoing OS and triggered with GnRHa, randomized to subcutaneous or intramuscular Pg, endometrial histology and endometrial receptivity array (ERA) transcriptomic expression showed non-significant differences in the secretory dating (24). Subcutaneous administration can be self-administered and is associated with lower injection site reactions, such as pain and irritation, compared with the intramuscular route (28). In addition, Mele et al. reported a significantly reduced level of stress-related hormones, such as Prolactin and Cortisol (30).
As an alternative to the vaginal route, subcutaneous Pg could be proposed to women who are reluctant to use vaginal medication for cultural or religious reasons. In addition, a vaginal route could be associated with vaginal irritation and discomfort (29), and the insertion of a vaginal device might be potentially associated with an increased risk of genital tract infection. Despite this belief, clinical data concerning genital (herpes virus, vaginal bacterial infection, vulvovaginal mycotic infection) and urinary tract infections seem to be similar when comparing vaginal and subcutaneous products (29). The potentially better compliance of subcutaneous vs. vaginal administration was investigated in several trials with mixed results. The most robust RCT trials (28, 29) conducted during fresh cycles did not demonstrate any relevant differences in terms of tolerability or adverse side-effects when comparing vaginal and subcutaneous administration. Conversely, two unpublished RCTs conducted in FET cycles reported higher satisfaction using the subcutaneous route. Similarly, Venturella et al. (33) observed better compliance with the subcutaneous route in a prospective study involving women with a previous experience of vaginal Pg; however, in FET cycles higher miscarriage and lower clinical pregnancy rates of the subcutaneous vs. the vaginal approach were reported in one RCT, involving oocytes donor recipients (25). This effect may be linked to the fact that the appropriate dosage in these populations is mainly unknown. So far, only one study compared intramuscular and subcutaneous Pg with no differences in terms of pregnancy rate between groups, but significantly lower prolactin and cortisol levels in the women who had received the subcutaneous route (30).
However, more evidence is necessary to better understand the application of subcutaneous Pg in ART. To the best of our knowledge, no RCT compared a subcutaneous Pg vs. oral dydrogesterone which could be a valuable approach for LPS in women undergoing ART (16, 39). In addition, so far no studies have been developed in women with a low prognosis to ART (40–46) or in women at risk of a hyper-response, such as those affected by PCOS (47–49). As yet, no cost-effective analysis has been conducted.
To conclude, a fair amount of evidence exists to support the hypothesis that in women with a good prognosis undergoing fresh IVF cycles, subcutaneous Pg is not inferior to vaginal products. In the FET cycle, data concerning efficacy are mixed with one RCT conducted in oocyte donor recipients which observed reduced clinical pregnancy rates and increased miscarriage rates in women undergoing a subcutaneous route. This data should be interpreted with caution considering that there is still too much uncertainty about the dosages to be used in women undergoing the FET cycle.
Data concerning the acceptance of a subcutaneous vs. vaginal route are encouraging despite the different scales and questionnaires used to test the acceptance of women among trials. Therefore, subcutaneous Pg could be proposed to women who are against vaginal administration and, in contrast with the intramuscular route, may be associated with better tolerability and reduced injection site reaction.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AC idealized the article and wrote the first draft. All authors participated in the literature research and paper editing. All authors listed have made an intellectual contribution to the work and approved the final version.
Conflict of Interest
AC and CA decleare fees from Merck Serono Italia outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.McNeilly AS, Crawford JL, Taragnat C, Nicol L, McNeilly JR. The differential secretion of FSH and LH: regulation through genes, feedback and packaging. Reprod Camb Engl Suppl. (2003) 61:463–76. [PubMed] [Google Scholar]
- 2.Alviggi C, Clarizia R, Mollo A, Ranieri A, De Placido G. Who needs LH in ovarian stimulation? Reprod Biomed Online. (2006) 12:599–607. 10.1016/S1472-6483(10)61186-8 [DOI] [PubMed] [Google Scholar]
- 3.Alviggi C, Mollo A, Clarizia R, De Placido G. Exploiting LH in ovarian stimulation. Reprod Biomed Online. (2006) 12:221–33. 10.1016/S1472-6483(10)60865-6 [DOI] [PubMed] [Google Scholar]
- 4.Santi D, Casarini L, Alviggi C, Simoni M. Efficacy of follicle-stimulating hormone (FSH) alone, FSH + luteinizing hormone, human menopausal gonadotropin or FSH + human chorionic gonadotropin on assisted reproductive technology outcomes in the “Personalized” medicine era: a meta-analysis. Front Endocrinol. (2017) 8:114. 10.3389/fendo.2017.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kol SLH. Supplementation in ovarian stimulation for IVF: the individual, LH deficient, patient perspective. Gynecol Obstet Invest. (2020) 85:307–11. 10.1159/000509162 [DOI] [PubMed] [Google Scholar]
- 6.Conforti A, Esteves SC, Di Rella F, Strina I, De Rosa P, Fiorenza A, et al. The role of recombinant LH in women with hypo-response to controlled ovarian stimulation: a systematic review and meta-analysis. Reprod Biol Endocrinol RBE. (2019) 17:18. 10.1186/s12958-019-0475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomsen LH, Kesmodel US, Andersen CY, Humaidan P. Daytime variation in serum progesterone during the mid-luteal phase in women undergoing in vitro fertilization treatment. Front Endocrinol. (2018) 9:92. 10.3389/fendo.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humaidan P, Engmann L, Benadiva C. Luteal phase supplementation after gonadotropin-releasing hormone agonist trigger in fresh embryo transfer: the American versus European approaches. Fertil Steril. (2015) 103:879–85. 10.1016/j.fertnstert.2015.01.034 [DOI] [PubMed] [Google Scholar]
- 9.Sugino N, Kashida S, Takiguchi S, Karube A, Kato H. Expression of vascular endothelial growth factor and its receptors in the human corpus luteum during the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. (2000) 85:3919–24. 10.1210/jcem.85.10.6888 [DOI] [PubMed] [Google Scholar]
- 10.Freis A, Germeyer A, Jauckus J, Capp E, Strowitzki T, Zorn M, et al. Endometrial expression of receptivity markers subject to ovulation induction agents. Arch Gynecol Obstet. (2019) 300:1741–50. 10.1007/s00404-019-05346-y [DOI] [PubMed] [Google Scholar]
- 11.Fatemi HM. The luteal phase after 3 decades of IVF: what do we know? Reprod Biomed Online. (2009) 19 Suppl 4:4331. 10.1016/S1472-6483(10)61065-6 [DOI] [PubMed] [Google Scholar]
- 12.van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. (2015) 2015:D009154. 10.1002/14651858.CD009154.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conforti A, Strina I, Mollo A, Amoroso R, Marrone V, Alviggi C, et al. The efficacy of modified luteal phase support with intramuscular progesterone in IVF/ICSI cycles: a retrospective observational study. Eur Rev Med Pharmacol Sci. (2017) 21:657–61. [PubMed] [Google Scholar]
- 14.McAuley JW, Kroboth FJ, Kroboth PD. Oral administration of micronized progesterone: a review and more experience. Pharmacotherapy. (1996) 16:453–7. [PubMed] [Google Scholar]
- 15.Labarta E, Rodríguez C. Progesterone use in assisted reproductive technology. Best Pract Res Clin Obstet Gynaecol. (2020) 69:74–84. 10.1016/j.bpobgyn.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 16.Griesinger G, Blockeel C, Kahler E, Pexman-Fieth C, Olofsson JI, Driessen S, et al. Dydrogesterone as an oral alternative to vaginal progesterone for IVF luteal phase support: A systematic review and individual participant data meta-analysis. PLoS ONE. (2020) 15:e0241044. 10.1371/journal.pone.0241044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Guardo F, Midassi H, Racca A, Tournaye H, De Vos M, Blockeel C. Luteal phase support in IVF: comparison between evidence-based medicine and real-life practices. Front Endocrinol. (2020) 11:500. 10.3389/fendo.2020.00500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaisbuch E, de Ziegler D, Leong M, Weissman A, Shoham Z. Luteal-phase support in assisted reproduction treatment: real-life practices reported worldwide by an updated website-based survey. Reprod Biomed Online. (2014) 28:330–5. 10.1016/j.rbmo.2013.10.022 [DOI] [PubMed] [Google Scholar]
- 19.Sator M, Radicioni M, Cometti B, Loprete L, Leuratti C, Schmidl D. Garhöfer G. Pharmacokinetics and safety profile of a novel progesterone aqueous formulation administered by the sc route. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. (2013) 29:205–8. 10.3109/09513590.2012.736560 [DOI] [PubMed] [Google Scholar]
- 20.de Ziegler D, Sator M, Binelli D, Leuratti C, Cometti B, Bourgain C, et al. Randomized trial comparing the endometrial effects of daily subcutaneous administration of 25 mg and 50 mg progesterone in aqueous preparation. Fertil Steril. (2013) 100:860–6. 10.1016/j.fertnstert.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 21.Zoppetti G, Puppini N, Pizzutti M, Fini A, Giovani T, Comini S. Water soluble progesterone–hydroxypropyl-β-cyclodextrin complex for injectable formulations. J Incl Phenom Macrocycl Chem. (2007) 57:283–8. 10.1007/s10847-006-9174-2 [DOI] [Google Scholar]
- 22.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons. (2019). 10.1002/9781119536604 [DOI] [Google Scholar]
- 23.Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:45. 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lara EE, Díaz-Gimeno P, Casas AB, Garrigás V, Ballesteros A. Is luteal phase support successfully performed using 25 mg/day of subcutaneous progesterone in controlled ovarian stimulation cycles with GnRH agonist triggering? Hum Reprod. (2016) 31:i283. 10.26226/morressier.573c1512d462b80296c9856b [DOI] [Google Scholar]
- 25.Llacer J, Garcia-Hernandez EM, Moliner B, Luque L, Ten J, Bernabeu R. Subcutaneous progesterone for endometrial preparation in substituted cycles for oocyte donation recipients: A randomized controlled trial. Hum Reprod. (2017) 32:i58. [Google Scholar]
- 26.Venturella R, Mocciaro R, Lico D, Rocca M, Di Cello A, Piccione F, et al. Subcutaneous aqueous versus vaginal progesterone gel for luteal phase support in intrauterine insemination cycles: A pilot randomized, controlled trial. Hum Reprod. (2016) 31:i304–5. 10.26226/morressier.573c1513d462b80296c98741 [DOI] [Google Scholar]
- 27.Gosalvez-Vega A, Ninchritz E, Fernandez-Sanchez E. Patients prefer subcutaneous progesterone over vaginal administration. Unexpected results of a prospective trial. Fertil Steril. (2016) 106:e323. 10.1016/j.fertnstert.2016.07.915 [DOI] [Google Scholar]
- 28.Baker VL, Jones CA, Doody K, Foulk R, Yee B, Adamson GD, et al. A randomized, controlled trial comparing the efficacy and safety of aqueous subcutaneous progesterone with vaginal progesterone for luteal phase support of in vitro fertilization. Hum Reprod Oxf Engl. (2014) 29:2212–20. 10.1093/humrep/deu194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockwood G, Griesinger G, Cometti B, De Placido G, Alviggi C, Ranieri A, et al. Subcutaneous progesterone versus vaginal progesterone gel for luteal phase support in in-vitro fertilization: a noninferiority randomized controlled study. Fertil Steril. (2014) 101. 10.1016/j.fertnstert.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 30.Mele D, Caprio F. D'eufemia MD, Schiattarella A, Labriola D, Schettino MT, et al. In vitro fertilization and psychological stress: New insight about different routes of progesterone administration. Ital J Gynaecol Obstet. (2020) 32:119–25. 10.36129/jog.32.02.04 [DOI] [Google Scholar]
- 31.Turkgeldi E, Hanege BY, Yildiz S, Keles I, Ata B. Subcutaneous versus vaginal progesterone for vitrified-warmed blastocyst transfer in artificial cycles. Reprod Biomed Online. (2020) 41:248–53. 10.1016/j.rbmo.2020.04.007 [DOI] [PubMed] [Google Scholar]
- 32.Ramos NN, Pirtea P, Benammar A, Ziegler D, de Jolly E, Frydman R, et al. Is there a link between plasma progesterone 1-2 days before frozen embryo transfers (FET) and ART outcomes in frozen blastocyst transfers? Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. (2020) 1–4. 10.1080/09513590.2020.1825669 [DOI] [PubMed] [Google Scholar]
- 33.Venturella R, Vaiarelli A, Buffo L. D'alessandro P, Colamaria S, Pedri S, et al. Progesterone for preparation of the endometrium for frozen-thawed blastocyst transfer in vitro fertilization cycles: a prospective study on patients' opinions on a new subcutaneous formulation. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. (2018) 34:766–71. 10.1080/09513590.2018.1451508 [DOI] [PubMed] [Google Scholar]
- 34.Doblinger J, Cometti B, Trevisan S, Griesinger G. Subcutaneous progesterone is effective and safe for luteal phase support in IVF: an individual patient data meta-analysis of the phase III trials. PloS ONE. (2016) 11:e0151388. 10.1371/journal.pone.0151388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alviggi C, Conforti A, Carbone IF, Borrelli R, de Placido G, Guerriero S. Influence of cryopreservation on perinatal outcome after blastocyst- vs cleavage-stage embryo transfer: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2018) 51:54–63. 10.1002/uog.18942 [DOI] [PubMed] [Google Scholar]
- 36.Sha T, Yin X, Cheng W, Massey IY. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: a meta-analysis. Fertil Steril. (2018) 109:330–42.e9. 10.1016/j.fertnstert.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 37.Conforti A, Picarelli S, Carbone L, La Marca A, Venturella R, Vaiarelli A, et al. Perinatal and obstetric outcomes in singleton pregnancies following fresh versus cryopreserved blastocyst transfer: a meta-analysis. Reprod Biomed Online. (2021) 42:401–12. 10.1016/j.rbmo.2020.09.029 [DOI] [PubMed] [Google Scholar]
- 38.Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer?. Hum Reprod Update. (2018) 24:35–58. 10.1093/humupd/dmx031 [DOI] [PubMed] [Google Scholar]
- 39.Tournaye H, Sukhikh GT, Kahler E, Griesinger G. A phase III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod Oxf Engl. (2017) 32:1019–27. 10.1093/humrep/dex023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteves SC, Alviggi C, Humaidan P, Fischer R, Andersen CY, Conforti A, et al. The POSEIDON criteria and its measure of success through the eyes of clinicians and embryologists. Front Endocrinol. (2019) 10:814. 10.3389/fendo.2019.00814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poseidon Group (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number), Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, et al. A new more detailed stratification of low responders to ovarian stimulation: from a poor ovarian response to a low prognosis concept. Fertil Steril. (2016) 105:1452–1453. 10.1016/j.fertnstert.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 42.Conforti A, Esteves SC, Cimadomo D, Vaiarelli A, Di Rella F, Ubaldi FM, et al. Management of women with an unexpected low ovarian response to gonadotropin. Front Endocrinol. (2019) 10:387. 10.3389/fendo.2019.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conforti A, Esteves SC, Picarelli S, Iorio G, Rania E, Zullo F, et al. Novel approaches for diagnosis and management of low prognosis patients in assisted reproductive technology: the POSEIDON concept. Panminerva Med. (2019) 61:24–9. 10.23736/S0031-0808.18.03511-5 [DOI] [PubMed] [Google Scholar]
- 44.Esteves SC, Roque M, Sunkara SK, Conforti A, Ubaldi FM, Humaidan P, et al. Oocyte quantity, as well as oocyte quality, plays a significant role for the cumulative live birth rate of a POSEIDON criteria patient. Hum Reprod Oxf Engl. (2019). 10.1093/humrep/dez181 [DOI] [PubMed] [Google Scholar]
- 45.Esteves SC, Roque M, Bedoschi GM, Conforti A, Humaidan P, Alviggi C. Defining low prognosis patients undergoing assisted reproductive technology: POSEIDON criteria-the why. Front Endocrinol. (2018) 9:461. 10.3389/fendo.2018.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picarelli S, Conforti A, Buonfantino C, Vallone R, De Rosa P, Carbone L, et al. Ivf during coronavirus pandemic: Who comes first? the poseidon viewpoint. Ital J Gynaecol Obstet. (2020) 32:223–8. 10.36129/jog.32.04.01 [DOI] [Google Scholar]
- 47.Alviggi C, Cariati F, Conforti A, De Rosa P, Vallone R, Strina I, et al. The effect of FT500 Plus® on ovarian stimulation in PCOS women. Reprod Toxicol. (2016) 59:40–4. 10.1016/j.reprotox.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 48.Alviggi C, Conforti A, De Rosa P, Strina I, Palomba S, Vallone R, et al. The Distribution of Stroma and Antral Follicles Differs between Insulin-Resistance and Hyperandrogenism-Related Polycystic Ovarian Syndrome. Front Endocrinol. (2017) 8:117. 10.3389/fendo.2017.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81:19–25. 10.1016/j.fertnstert.2003.10.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.